Abstract
We examined whether a Ca2+-K+ interaction was a potential mechanism operating during fatigue with repeated tetani in isolated mouse muscles. Raising the extracellular Ca2+ concentration ([Ca2+]o) from 1.3 to 10 mM in K+-depressed slow-twitch soleus and/or fast-twitch extensor digitorum longus muscles caused the following: 1) increase of intracellular K+ activity by 20–60 mM (raised intracellular K+ content, unchanged intracellular fluid volume), so that the K+-equilibrium potential increased by ∼10 mV and resting membrane potential repolarized by 5–10 mV; 2) large restoration of action potential amplitude (16–54 mV); 3) considerable recovery of excitable fibers (∼50% total); and 4) restoration of peak force with the peak tetanic force-extracellular K+ concentration ([K+]o) relationship shifting rightward toward higher [K+]o. Double-sigmoid curve-fitting to fatigue profiles (125 Hz for 500 ms, every second for 100 s) showed that prior exposure to raised [K+]o (7 mM) increased, whereas lowered [K+]o (2 mM) decreased, the rate and extent of force loss during the late phase of fatigue (second sigmoid) in soleus, hence implying a K+ dependence for late fatigue. Prior exposure to 10 mM [Ca2+]o slowed late fatigue in both muscle types, but was without effect on the extent of fatigue. These combined findings support our notion that a Ca2+-K+ interaction is plausible during severe fatigue in both muscle types. We speculate that a diminished transsarcolemmal K+ gradient and lowered [Ca2+]o contribute to late fatigue through reduced action potential amplitude and excitability. The raised [Ca2+]o-induced slowing of fatigue is likely to be mediated by a higher intracellular K+ activity, which prolongs the time before stimulation-induced K+ efflux depolarizes the sarcolemma sufficiently to interfere with action potentials.
Keywords: muscle fatigue, fiber-type, action potential, excitability, calcium, potassium
the notion that extracellular calcium concentration ([Ca2+]o) and/or transsarcolemmal Ca2+ fluxes have a role in muscle fatigue has existed for over 30 yr (6) and has been discussed in many reviews (3, 15, 19, 25, 40, 60). It is well established from studies of fatigue in isolated muscles that nominally Ca2+-free solutions accelerate the decline of isometric force during fatiguing stimulation (13, 24, 28, 56, 67, 68) and, conversely, that raised [Ca2+]o can provide some resistance to fatigue (13, 28). The cellular mechanisms to explain such effects are not fully understood, although several possibilities exist. First, a transsarcolemmal Ca2+ influx via any of several pathways, for example, voltage-activated L-type Ca2+ channels (41, 46, 68), store-operated Ca2+ channels (21, 24, 40, 51, 54, 65, 69, 71), stretch-activated Ca2+ channels (24, 54, 71), or Na+/Ca2+ exchanger proteins (28) may refill Ca2+ stores in the sarcoplasmic reticulum (SR) to better maintain Ca2+ release during fatigue (24, 40, 51, 54, 69, 71). Notably, such Ca2+ influx can lead to a fall in transverse (t-) tubular [Ca2+]o, as shown directly for repeated stimulation (41) or modeled with sustained depolarization (26). Second, raised [Ca2+]o and/or triadic intracellular Ca2+ concentration ([Ca2+]i) can stabilize the voltage sensor proteins and charge movement currents of excitation-contraction coupling in t-tubular membranes (4, 46, 66), which may also help to sustain SR Ca2+ release during fatigue. Third, nominally Ca2+-free solutions exacerbate the loss of sarcolemmal excitability during repeated stimulation of amphibian muscle fibers, by a mechanism independent of resting membrane potential (EM), and which is possibly related to lowered SR Ca2+ content (67). Fourth, raised [Ca2+]o may directly stabilize and/or reduce inactivation of voltage-dependent Na+ channels (46, 59, 61) to help promote sarcolemmal excitability. Fifth, raised [Ca2+]o can reverse the detrimental effects of a lowered transsarcolemmal K+ gradient in nonfatigued muscle and may also counteract K+-induced fatigue (13). Accordingly, a positive Ca2+-K+ interaction, which is mediated via recovery of the EM, may combat K+-induced depolarization and impairment of action potentials and excitability, thereby conferring protection against fatigue.
Our laboratory has recently provided indirect evidence that, with our stimulation protocol (repeated tetani evoked at high frequency and with short rest periods), a large component of the resulting severe fatigue involves impaired sarcolemmal excitability (16, 17), and overcoming it with stronger or longer stimulus pulses can increase Ca2+ release from the SR (17). Moreover, stimulation regimes comparable to ours induce considerable ionic disturbances (35, 36, 43) and large sarolemmal depolarizations (18, 35–37, 43), which render some fibers inexcitable (37). Thus it appears that the mechanisms underpinning the Ca2+ effect on fatigue are likely to involve changes to transsarcolemmal ion concentrations, resting EM, the action potential profile or sarcolemmal excitability, which clearly aligns with the notion of a Ca2+-K+ interaction. We consider that a likely physiological scenario involves a decline of [Ca2+]o, which acts in combination with a reduced transsarcolemmal K+ gradient to promote fatigue. To determine the consequence of these events, we investigated the Ca2+-K+ interaction in nonfatigued muscle.
Despite these developments, some recent studies report that altered [Ca2+]o has no effect on fatigue of fast-twitch mammalian muscle (18, 28, 47). In addition, nominally Ca2+-free conditions cause greater exacerbation of fatigue during repetitive tetanic stimulation in fatigue-resistant, than in easily fatigable, fibers from amphibian muscle (56). The density of several sarcolemmal Ca2+ entry pathways also varies with different muscle types (29, 39, 69), as does the extent of Ca2+ influx (29, 43). Therefore, it appears that any Ca2+ effect on fatigue may depend on the fiber-type composition of the muscle studied. Alternatively, revealing the Ca2+ effects may depend on the severity of fatigue, which relate to the different phases of the fatigue profile. To aid investigation of these aspects, our laboratory recently developed a double-sigmoid curve-fitting model that quantifies rates and extents of different phases of fatigue in mouse skeletal muscle (16).
In the present study, we chose to use a highly elevated [Ca2+]o of 10 mM in many experiments to investigate Ca2+ effects, since the influence of low [Ca2+]o on muscle processes appears to be smaller (13). Importantly, raised [Ca2+]o should also attenuate Ca2+ depletion in the t-tubules during fatigue. Our aims were to determine 1) the mechanism(s) for the Ca2+-K+ interaction in nonfatigued muscle and whether our model of fatigue is K+ dependent; and 2) whether there are different Ca2+ effects on the different phases of fatigue in fast-twitch and slow-twitch mammalian muscles. Together, this should allow evaluation of whether and how altered [Ca2+]o can influence K+-induced fatigue in mammalian skeletal muscle.
METHODS
Muscle Preparations and Solutions
The general experimental protocols and animal usage were approved by the Animal Ethics Committees of the Universities of Auckland and Otago (New Zealand), and the Animal Care Committee of the University of Ottawa (Canada). Adult mice (Swiss CD-1) of either sex (aged 1–3 mo, 20–35 g body wt) were killed by cervical dislocation in New Zealand or anesthetized with pentobarbital sodium in Canada (0.8 mg/10 g body wt ip, somontol, MTC Pharmaceuticals, Cambridge, Ontario, Canada) before muscle isolation and then killed by pentobarbital overdose. Intact whole fast-twitch extensor digitorum longus (EDL) or slow-twitch soleus muscles were dissected. These muscles have clearly different contractile properties, including fatigue resistance (10, 16, 17). Their fiber-type composition is heterogeneous, but shows that the EDL has virtually no slow-twitch type I fibers, and the soleus has no fast-twitch type IIB fibers (5, 16). Specifically, and based on myosin antibody assays, the fiber-type composition for mouse EDL is 13% type IIA-IIX, 24% type IIX, 42% type IIX-IIB, and 15% type IIB-IIX fibers and for mouse soleus is 27% type I, 7% type I-IIA, 33% type I-IIX, and 26% type IIA fibers (5). Muscles were equilibrated in the control (4K) Krebs solution of the following composition (in mM): 122.2 NaCl, 2.8 KCl, 1.2 KH2PO4, 25.1 NaHCO3, 1.2 MgSO4, 1.3 CaCl2, and 5 d-glucose, bubbled with 95% O2-5% CO2 at 25°C. The [Ca2+] in our control solution was 1.3 mM, which is close to that of normal plasma in humans (15, 49), whereas 1.8–2.5 mM [Ca2+] is commonly used elsewhere in control solutions (2, 4, 18, 21, 23, 24, 28, 35, 41, 54, 56, 64, 67–71). Experiments in Krebs solutions with raised [Ca2+] (2.5 or 10 mM) involved the addition of CaCl2 but without correction for change of osmolarity. In the Krebs solution with lowered [Ca2+] (0.5 mM), CaCl2 was replaced with equimolar MgCl2. Other Krebs solutions with 2–14 mM K+ concentration ([K+]), i.e., 2K–14K, were made with NaCl being exchanged for equimolar KCl.
Force Recording and Stimulation
Most contractile experiments involved muscles mounted vertically in a thermostatically controlled chamber (∼100 ml) containing Krebs solution (see Ref. 10 for a full description of the setup). Isometric contractions, one tendon was fixed and the other attached to a force transducer (KSP-2-E3, Kyowa, Japan), were elicited by electric field stimulation usually delivered via two parallel plate platinum electrodes (7.7 mm apart), which engulf the muscle. This procedure initiates action potentials simultaneously all along the length of the sarcolemma (10). A few experiments (indicated within the text) involved triggering action potentials, which propagate along the surface membrane by stimulating with two fine-wire electrodes (4–6 mm apart), which transverse the muscle (10). The standard stimulus pulses (20 V, 0.1 ms, supramaximal for the twitch) were initiated from an Apple Macintosh PowerPC 7100/80 using custom-written Labview software and delivered to the electrodes via a purpose-built power amplifier (MOSFET). Changes to stimulation frequency or contraction duration were achieved using Labview software. Twitch and tetanic contractions were recorded continuously on a chart recorder (Gould model 244) with selected contractions saved in digital form on the computer. Maximum force was elicited at 125 Hz for 2 s in soleus, and 200 Hz for 0.5 s in EDL.
Experimental protocol.
Muscles were stretched to the optimal length for the tetanus, then equilibrated at 4K for at least 30 min with one tetanus evoked every 5 min. Control nonfatigued contractile parameters were established before changing to different solutions (altered [Ca2+] or [K+]) and waiting for a steady-state response when the peak force was unchanged over three tetani; this usually exceeded 40–60 min. Steady-state effects were established first at raised extracellular K+ concentration ([K+]o) and then at raised [K+]o with altered [Ca2+]o in the same muscles. Fatigue runs were undertaken only after muscles had been equilibrated in each solution.
Analysis.
The contractile parameters measured included peak twitch and tetanic forces, resting force and fade, i.e., the decline of force from its peak within a short tetanus (12). The time for 50% of the force restoration with raised [Ca2+]o at elevated [K+]o was assessed by linear interpolation between the tetani, which were evoked once every 5 min. The fatigue profile is depicted as the peak force (evoked plus resting force) expressed relative to that of the first tetanus as a function of time. Changes of resting force during a fatigue run were expressed as a percentage of the peak tetanic force in fresh muscle. Those muscle preparations regarded as viable had a slow run-down of peak tetanic force, i.e., <0.2%/min, and recovered after fatigue to >80% initial in EDL or >90% initial in soleus. The average maximum tetanic stress of the muscles used was 275 ± 18 mN/mm2 (n = 19) for EDL and 268 ± 7 mN/mm2 (n = 42) for soleus.
Resting EM and Intracellular K+ Activity
Resting EM was measured using conventional microelectrodes filled with 3 M KCl and with tip resistances of 30–50 MΩ (12) or 5–15 MΩ (9, 10, 14), or with double-barreled ion-sensitive microelectrodes of tip resistance ∼150 MΩ (42). The EM was quantified as the maximum drop in potential to a steady level. Acceptable penetrations gave a sharp fall to a new stable value and returned close to 0 mV on electrode withdrawal. When obtained with action potentials (tip resistances <10 MΩ), the resting EM often depolarized slowly so that the value recorded was that immediately before the stimulus artifact (e.g., see Table 2). Measurements were made in both surface and layer 2 fibers because the EM and action potentials of deeper fibers can differ to surface fibers (14, 32) and thereby may contribute differently to contractile function. Fibers were usually impaled sequentially with the EM identified for each layer as described in Cairns et al. (14).
Table 2.
Influence of raised [Ca+]o (1.3 to 10 mM) on resting EM and the profile parameters of single action potentials at 10 mM [K+]o in surface fibers from soleus muscles
| Parameter | 4K | 10K | 10K + 10Ca |
|---|---|---|---|
| Resting EM, mV | −77.4 ± 1.6* | −56.2 ± 1.7* | −67.2 ± 1.2 |
| Overshoot, mV | 36.2 ± 1.2* | −20.3 ± 5.9* | 21.9 ± 3.0 |
| Amplitude, mV | 113.6 ± 2.1* | 35.3 ± 6.7* | 89.1 ± 2.6 |
| Width, ms | 0.765 ± 0.017* | 1.778 ± 0.120* | 0.954 ± 0.044 |
| Maximum +dV/dt, V/s | 524 ± 22* | 76 ± 18* | 250 ± 22 |
| Maximum −dV/dt, V/s | −174 ± 7* | −44 ± 9* | −115 ± 7 |
| n | 24/3 | 15/3 | 17/3 |
Values are means ± SE; n, no. of fibers/muscles. 4K data are pooled from before and after raised [K+]o solutions. Action potentials were initiated via fine platinum wire (0.3 ms pulses). Peak tetanic force (125 Hz, 2 s) recovered from 41 ± 15% of control at 10K to 83 ± 7% of control at 10K + 10Ca in these three soleus muscles. Stimulation was with large transverse wire electrodes and supramaximal (10 V, 0.3 ms) pulses. +dV/dt and −dV/dt, peak and trough of the first derivative of the action potential, respectively.
Significant difference between 4K and 10K, or 10K and 10K + 10Ca (P < 0.05, ANOVA).
Double-barreled K+-sensitive microelectrodes were used to measure intracellular K+ activity (aKi+), with an approach similar to that used previously in our laboratory (23, 42). This technique allows K+-equilibrium potential (EK) and EM to be determined for each fiber. Measurements of K+ activity (aK+) are lower than [K+] due to interactions between ions in solution (related by the activity coefficient), with intracellular K+ appearing to behave as in free solution without subcellular binding (23, 36). In brief, microelectrodes, constructed from glass capillary tubing (WPI-2B-150F-4), were silanized in the vapor of dichlorodimethyl silane for ∼40 s and then baked at 180°C for 2–3 h. One microelectrode barrel was backfilled with a K+-selective resin (liquid ion exchanger, Corning 477137) to its shank, and a fine chlorided silver wire inserted into the resin. The reference barrel for recording EM was filled with 3 M KCl. Each microelectrode was connected to the head stage of a differential amplifier (WPI F223A). The K+-sensitive electrode was calibrated in a series of pure KCl solutions ([K+] of 1, 0.1, 0.01, and 0.001 M), with the concentrations converted to aK+ using an activity coefficient of 0.76 (23), i.e., aK+ = [K+] × 0.76. For each electrode, its selectivity over Na+ was assessed using a solution containing 10 mM KCl and 150 mM NaCl. The mean empirically determined slope (S) for the K+-electrode response per decade change of aK+ was 56.1 mV, and its selectivity coefficient (k) for Na+ over K+ was 0.016. Calibrations were performed before and after each experiment to ensure that the electrode properties had not changed during the experiment. Changing [Ca2+] from 1.3 to 10 mM at 11K had no significant effect on the voltage signal from the K+-sensitive electrode.
Experimental protocol.
Loosely attached connective tissue was removed from the surface of isolated EDL muscles to assist with microelectrode penetration of fibers. Each muscle was then pinned via its tendons at approximately resting length into a 5-ml dish that was lined with Silastic rubber and mounted on an air table in a Faraday cage. Muscles were superfused with carbogenated Krebs solution under gravitational flow (1–2 ml/min) and equilibrated in the control 4K solution for 45–60 min before the first penetrations. The microelectrode was moved in small steps across the width of the muscle, with penetrations made first in a surface fiber, then with deeper penetration into a layer 2 fiber. Electrodes were held within each fiber long enough to get stable voltage signals from both K+ and reference barrels. When the solution was changed to 11K, and then 11K + 10Ca (raised K+ Krebs solution with 11 mM K+, 10 mM Ca2+), the muscles were equilibrated for >60 min in each solution before the first recordings. Penetrations were made at different locations along the length of the muscle in each solution. Experiments were done in solutions at room temperature (range 22.5–25°C).
Analysis.
The EM values accepted (in both surface and layer 2 fibers) at 4K were more negative than −65 mV, and at 8–11K were more negative than −45 mV. Fibers giving values lower than these, i.e., more depolarized, were considered to be damaged, and the values discarded. The aKi+ for each fiber was calculated using Eq. 1 (42, 62).
| (1) |
The values of extracellular aK+ (aKo+) and extracellular Na+ activity (aNao+) for each bathing solution are known, the electrode characteristics of S and k were determined from the calibration of each K+-sensitive electrode, and ΔEK and EM were measured for each fiber. ΔEK is the change of potential for the K+-sensitive electrode on penetration of each fiber (i.e., ΔEK = Ei − Eo), where Ei is intracellular electrical potential and Eo is extracellular electrical potential. The EK for each fiber was subsequently calculated using Eq. 2.
| (2) |
For this calculation, aKi+ was the measured value, and aKo+ was known for the bathing solution, while using the temperature (T) of the bathing solution during each experiment. R is the universal gas constant, and F is Faradays constant.
Intracellular fluid volume and intracellular [K+]
Experimental protocol.
Intracellular fluid volume (ICFV) and intracellular K+ content were assessed {and intracellular K+ concentration ([K+]i) calculated} using paired muscles from contralateral legs. Muscles were incubated under one of the following conditions for 30 min: 1) 4K; 2) 11K; 3) 11K + 10Ca; 4) 11K and then another 30 min at 11 + 10Ca. The ICFV and [K+]i determined for 3 and 4 were not different, so these data were pooled. Muscles had a final 10-min incubation under the same conditions but with the addition of 1 μCi/ml of 14C-sucrose, used as an extracellular marker.
Muscle weights.
Muscles were wiped using Kimwipe and weighed using an ultrasensitive (μg) Metler ME30 balance to obtain the total muscle wet weight. These values were mean (range) 10.8 mg (9.2–13.4 mg) for EDL and 8.5 mg (6.7–10.1 mg) for soleus. Muscles were freeze-dried overnight. At this point, paired EDL and soleus from the same mouse were combined. Fibers and tendons were separated and weighed individually to determine dry weights. We determined that tendon dry weight was on average 68.8 ± 1.1% (n = 10) of tendon wet weight using large gastrocnemius tendons. Using this value, EDL and soleus tendon wet weights were calculated. The true muscle fiber wet weights were calculated by subtracting the tendon wet weight from the total muscle wet weight.
14C-sucrose and K+ measurements.
Muscle 14C-sucrose and K+ were extracted from lyophilized dry muscle fibers by adding 1 ml of 100 mM NaCl, and the solution was sonicated for 15 min with a Fisher Scientific model 60 Sonic dismembrator (Canada). The [K+] was then measured in the extract solution using a K+-sensitive electrode (Lazar Research Laboratories). One hundred microliters of 60% perchloric acid were added, and the solution centrifuged for 10 min at 10,000 g to precipitate proteins. Supernatant was added to 10 ml of biodegradable counting scintillant (Amersham), and the 14C-sucrose count per minute (CPM) was obtained using a WinSpectral liquid scintillation counter (model 1414, PerkinElmer Life Sciences, Boston, MA).
Calculations.
The difference between muscle fiber wet and dry weights (in g) was converted to total water content (in ml) using a density of 1 g/ml (Eq. 3). 14C-sucrose was used to estimate the extracellular fluid volume (ECFV) (Eq. 4) with ICFV calculated as the difference between total water content and ECFV (Eq. 5).
| (3) |
| (4) |
| (5) |
The total muscle K+ content was calculated as the product of muscle extract solution [K+] and total volume (Eq. 6). The extracellular K+ content was determined as the product of the Krebs solution [K+] and ECFV (Eq. 7). Intracellular K+ content was calculated as the difference between total muscle K+ content and extracellular K+ content (Eq. 8), and normalized to μmol/g wet wt. [K+]i was calculated by dividing intracellular K+ content by the ICFV (Eq. 9).
| (6) |
| (7) |
| (8) |
| (9) |
Action Potential Profile and Sarcolemmal Excitability
For full details on intracellular recordings of single action potentials, see Refs. 9 and 10. In brief, muscles were mounted horizontally in a bath with a rapid flow-through system (∼15 ml/min). The recording microelectrode (tip resistance 7–10 MΩ, tip potential <5 mV) and a reference electrode (tip resistance 1 MΩ) were backfilled with 3 M KCl. Resting EM were recorded on both a chart recorder and computer. Action potential waveforms were recorded using a WPI electrometer and sampled at 200 kHz. In the first series of experiments on soleus muscles, action potentials were triggered using a fine platinum wire, largely enclosed within a glass microelectrode and moved adjacent to the surface of the muscle with micromanipulator adjustments. Stimulation involved low-voltage strengths (with 0.3-ms pulses) to activate only a few fibers near the stimulating electrode and thus minimize contraction, which may cause electrode dislodgement. With this approach, the action potential was completed before the start of the contraction, so that there was no need for stretching the muscle (9), which can sometimes lead to fiber damage and depolarization, and is difficult to distinguish from the depolarizing effects of K+. In the second series of experiments on EDL muscles, action potentials were triggered using large platinum wire electrodes with supramaximal pulses (10 V, 0.3 ms) to determine both sarcolemmal excitability and action potential profiles parameters. These experiments were done in the presence of 30 μM tubocurarine (i.e., curare) to ensure that sarcolemmal excitability was determined without any contribution via nerve twigs.
Experimental protocol.
Muscles were stretched to the optimal length for the tetanus and equilibrated in solutions as for the other contractile experiments. In soleus muscles, action potentials were recorded in single fibers in the surface layer and underlying layer 2. Recordings were made first at 4K, then under steady-state force conditions at 10K, followed by 10 mM [K+]o + 10 mM [Ca2+]o, (10K + 10Ca), before returning to 4K. The recording electrode was withdrawn before the tetanic stimulation that occurred every 5 min throughout the experiment. In EDL muscles, action potential waveforms and excitability were assessed only for surface fibers. Recordings were made first at 4K and then after equilibration for at least 30 min in various solutions containing curare. Hence repeat measurements were made at 4K + curare, 11K + curare, 11K + 10Ca + curare, then 4K + curare again, all in the same muscles.
Analysis.
The action potential profile was analyzed only when the criteria for measuring resting EM were met and when the stimulus artifact did not interfere with the upstroke of the action potential. We quantified action potential parameter values as follows: overshoot, difference between peak and 0 mV; amplitude, difference between peak and resting EM; width, time interval at 50% of amplitude; maximum +dV/dt and maximum −dV/dt, peak and trough of the first derivative of the action potential, respectively. Sarcolemmal excitability (%excitable fibers) was quantified as number of fibers that generated action potentials divided by total number of fibers penetrated (i.e., with and without action potentials) × 100, when using supramaximal pulse stimulation.
Fatigue and Curve-Fitting Procedures
Fatigue studies.
For the purpose of this study, we define fatigue as any reversible decline of peak tetanic force induced by repeated tetanic stimulation of an isolated muscle. The standard stimulation protocol (125 Hz for 500 ms, with one contraction every second for 100 s) involved standard supramaximal pulses delivered via parallel plate electrodes (10). The force recovery after each fatigue run was followed for ∼60 min. To examine whether prior exposure to altered [K+]o (2, 4, or 7 mM) influences the fatigue profile, we utilized repeated fatigue runs in soleus muscles since their fatigue profile is reproducible over several fatigue runs (16). Fatigue runs in soleus muscles with altered [Ca2+]o were repeated at 1.3 mM, then at 10 mM, then again at 1.3 mM [Ca2+]o in the same muscles. In EDL muscles, the effects of 1.3 and 10 mM [Ca2+]o on the fatigue profile were quantified in different but fresh muscles, because the EDL becomes slightly more fatigue resistant between the 5th and 20th tetanus with repeated fatigue runs (16). Fatigue runs at both 1.3 and 10 mM [Ca2+]o were performed in a few EDL to qualitatively check for Ca2+ effects.
Curve-fitting the fatigue profile.
The full details of curve-fitting procedures (double-sigmoid model) and its justification have been presented in Ref. 16, so it will be reviewed here only briefly. For individual muscles, fatigue runs comprised relative force (resting plus evoked) observed at 28 time points recorded once per second for the first 10 s (to capture the initial phase of fatigue) and once every 5 s thereafter, until the end of the stimulation period. These 28 points were then fitted (using the nonlinear regression module, NLIN, in SAS) by the double-sigmoidal expression given in Eq. 10. A six-parameter double-sigmoid model was used to describe the EDL fatigue data [F(t)].
| (10) |
The time constant (τ) denotes the time for each sigmoid to fall to one-half of its initial value; τ1 is for the first sigmoid (early fatigue), and τ2 is for the second sigmoid (late fatigue). The maximum slope for each sigmoid, i.e., S1 and S2, were obtained at τ1 and τ2, respectively, by differentiating Eq. 10 with respect to time. Fo is the intermediate asymptote shared by the two sigmoids, and Fmin is the minimum asymptote (plateau value) determined for the second sigmoid. For quantifying the fatigue profile in soleus, a five-parameter double-sigmoid model was used (Eq. 10 with Fo = 88.9). We acknowledge some uncertainty about fitting parameter values when applying the double-sigmoid model when fatigue is not severe (e.g., with soleus at 10 mM [Ca2+]o) as this requires considerable extrapolation for the second sigmoid (16). Nevertheless, we use the appropriate five- or six-parameter expression for graphical purposes.
Statistical Analyses
Data are presented throughout as the mean values ± SE for n, the number of muscles tested (i.e., contractile data) or number of fibers/muscles tested (i.e., resting EM, aKi+, action potential profile data). For K+ content (or [K+]i) and ICFV values, n represents the number of paired muscles from contralateral legs. Data were tested for significant differences using the Student's t-test or ANOVA.
The statistical significance of differences among best fit fatigue curves between experimental conditions (altered [K+]o or [Ca2+]o) was assessed using the Nonlinear Mixed module of SAS. In this approach, the parameters of best fit for a given muscle are treated as independent samples drawn from either a 5-variate (soleus) or a 6-variate (EDL) normal distribution. Data input to the module comprised the 28 measured values of relative force for each of the n muscles examined. The average line of best fit to the resulting n × 28 data points is achieved using the Maximum Likelihood principle, treating the factor “muscle” as random. The software returns standard errors of the estimates of each parameter, as well as the value of the Akaike Information Criterion (16). We report the goodness of fit for average double-sigmoid functions as both the squared correlation coefficient (r2) and the mean square error. The same software module tests for the difference of average regression lines between groups. The level of statistical significance was set at P < 0.05 throughout.
RESULTS
The Ca2+-K+ Interaction in Nonfatigued Fast-Twitch and Slow-Twitch Muscle
Isometric force.
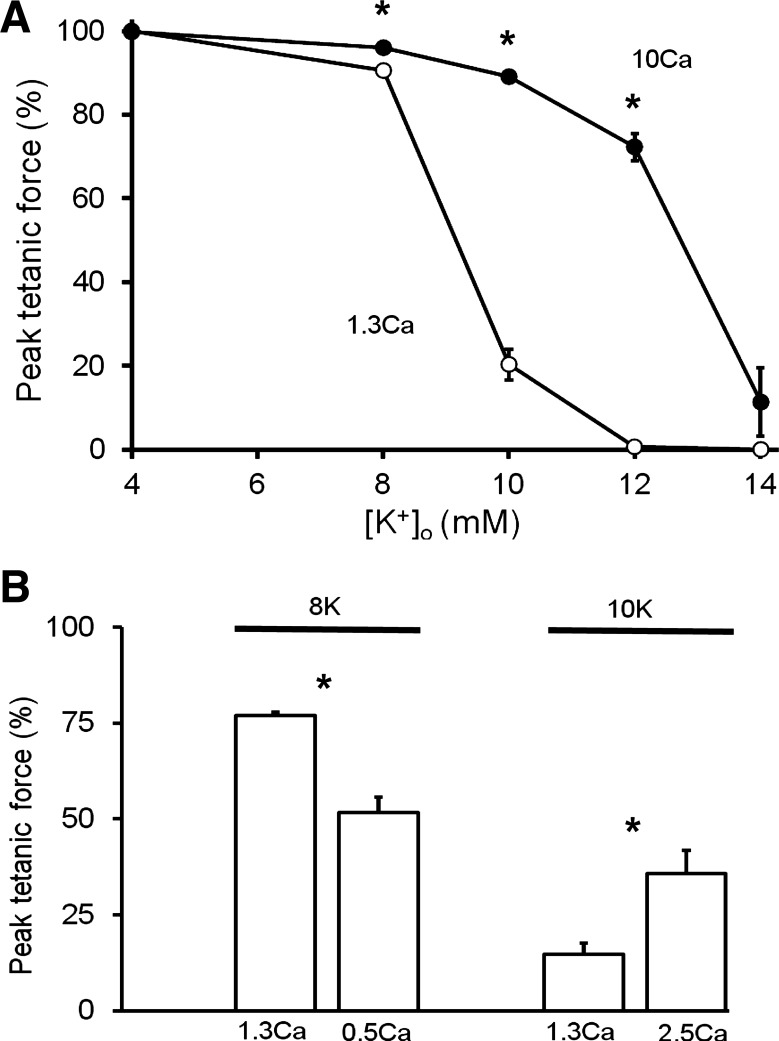
In soleus muscles, the steady-state peak tetanic force-[K+]o relationship was shifted rightwards toward higher [K+]o at raised [Ca2+]o (1.3 to 10 mM) (Fig. 1A). The force restoration was 5% of control at 8K, was much larger over the steep part of the curve at 10K or 12K, being ∼70% of control, but there was no significant restoration at 14K. Similarly, in five EDL muscles, the effect of 10 mM [Ca2+]o at 11 mM [K+]o (11K + 10Ca) was to increase peak tetanic force (200 Hz) by 47% of control, i.e., from 36 ± 10% at 11K to 83 ± 4% at 11K + 10Ca. Although the full force recovery with 10 mM [Ca2+]o took 40–60 min in both muscle types, the time for 50% force recovery was 10 ± 2 min at 11K in EDL (n = 5), and 4 ± 1 min at 10K in soleus (n = 5). We also tested for effects of smaller changes of [Ca2+]o in soleus muscles (Fig. 1B). When [Ca2+]o was raised from 1.3 to 2.5 mM at 10K, the peak tetanic force (125 Hz) increased by 21% of control. Moreover, when [Ca2+]o was lowered from 1.3 to 0.5 mM in four soleus muscles moderately depressed at 8K, the peak tetanic force (125 Hz) fell by a further 25% of control. When stimulating these same four muscles more physiologically with transverse wire electrodes, the 125-Hz tetanus was depressed from 64 ± 2% (1.3Ca) to 28 ± 6% (0.5Ca).
Fig. 1.
Influence of altered extracellular Ca2+ concentration ([Ca2+]o) on K+-depressed contractions in soleus muscles. A: steady-state effects of raised [Ca2+]o (1.3 to 10 mM) on the peak tetanic force-extracellular K+ concentration ([K+]o) relationship. Each point is mean value ± SE (n = 3–12). Curves differed over 8–12 mM (ANOVA, P < 0.05). B: steady-state effects of lowered [Ca2+]o (1.3 to 0.5 mM) at 8K (Krebs solutions with 8 mM K+ concentration) (n = 4) and raised [Ca2+]o (1.3 to 2.5 mM) at 10K (n = 4) on tetanic contractions (125 Hz). Similar effects were seen for the 50-Hz tetanus and peak twitch force at 8K and 10K. In both panels, the peak force was normalized (%) to that evoked at 125 Hz, at 4K with 1.3 Ca. *Significant difference (P < 0.05). Parallel plate electrodes were used with supramaximal (20 V, 0.1 ms) pulses.
Resting EM.
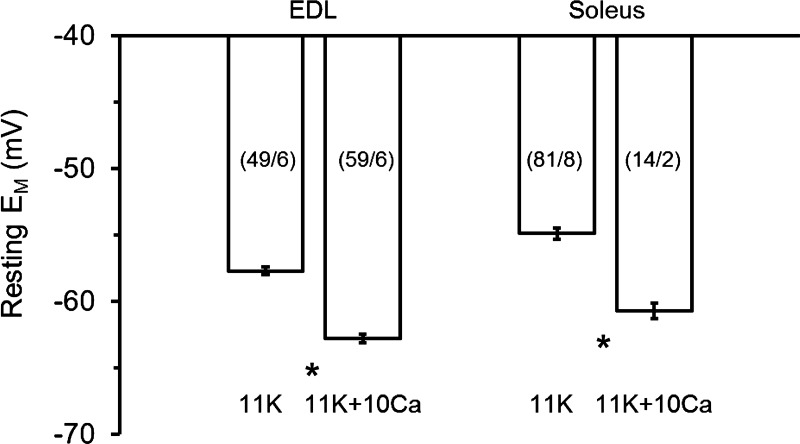
We compared the effect of raised [Ca2+]o (1.3 to 10 mM) on the resting EM of K+-depolarized fibers from different muscle types (Fig. 2). At 11K, a similar repolarization of surface fibers occurred with 11K + 10Ca, that is, 5.1 mV in EDL and 5.8 mV in soleus. Smaller changes of [Ca2+]o also influenced the EM in soleus fibers. Raising [Ca2+]o from 1.3 to 2.5 mM at 10K caused a significant repolarization of 2.2 mV in surface fibers; i.e., from −55.8 ± 1.1 mV (n = 37/7) at 10K to −58.0 ± 1.1 mV (n = 46/7) at 10K + 2.5Ca. We also tested the 10K + 2.5Ca intervention in layer 2 fibers, because the EM of deeper fibers differs from those of surface fibers at raised [K+]o (14, 32). A similar response occurred in layer 2 fibers, with the sarcolemma being significantly repolarized by 1.6 mV, i.e., from −66.7 ± 0.9 mV (n = 29/7) to −68.3 ± 1.2 mV (n = 35/7). Conversely, with lowered [Ca2+]o (1.3 to 0.5 mM) at 8K a significant further depolarization of 2.2 mV occurred in layer 2 fibers, but the 1.1-mV depolarization of surface fibers was not statistically significant. We also tested for effects of 10 mM [Ca2+]o at 4K, and there was no significant change of EM with repeated measures in surface fibers from two soleus muscles, i.e., −82.5 ± 1.9 mV (n = 14/2) at 4K and −85.3 ± 1.8 mV (n = 16/2) at 4K + 10Ca (P = 0.302), and similarly from one EDL muscle (data not shown).
Fig. 2.
Steady-state effects of raised [Ca2+]o (1.3 to 10 mM) at 11 mM [K+]o on the resting membrane potential (EM) in surface fibers from fast-twitch extensor digitorum longus (EDL) and slow-twitch soleus muscles. Shown are mean values ± SE. The no. of fibers/muscles is given in parentheses. *Values at 11K (Krebs solutions with 11 mM K+ concentration) and 11K + 10Ca (raised K+ Krebs solution with 11 mM K+, 10 mM Ca2+) were significantly different (P < 0.05, t-test).
aKi+.
To enhance understanding of the Ca2+-induced repolarization at raised [K+]o, we measured both aKi+ and EM simultaneously in single EDL muscle fibers (Table 1). We tested whether the repolarization occurred via an increased K+ conductance, possibly by activation of a Ca2+-activated K+ channel (e.g., BKCa channel), which would bring the EM closer to the EK. The aKi+ in surface fibers at 4K was 124 mM, which is similar to that reported previously for mouse EDL muscle (23, 62), and is equivalent to a [K+]i of 163 mM. The aKi+ of layer 2 fibers was 45 mM greater than in surface fibers, and the EM was 7.8 mV more polarized. When the bathing solution was changed from 11K to 11K + 10Ca, a considerable increase of aKi+ occurred, i.e., 61 mM in surface fibers, 57 mM in layer 2 fibers (Table 1). Consequently, the EK of surface fibers increased by 10.4 mV (EM repolarized by 7.8 mV), and in layer 2 fibers the EK increased by 8.9 mV (EM repolarized by 6.6 mV). It is important to note that the EM failed to approach EK when the solution was changed from 11K to 11K + 10Ca. Rather the (EM − EK) increased by ∼2 mV, which was significant for layer 2 fibers. Thus the repolarization of resting EM appears to be related to an increased aKi+ and not to an increased K+ conductance.
Table 1.
Influence of raised [Ca2+]o (1.3 to 10 mM) on resting EM and intracellular K+ activity at 11 mM [K+]o in EDL muscle fibers
| Parameter | 4K | 11K | 11K + 10Ca |
|---|---|---|---|
| Surface fibers | |||
| EM, mV | −74.5 ± 1.7* | −55.0 ± 1.3* | −62.8 ± 1.1 |
| aKi+, mM | 124 ± 10 | 115 ± 4* | 176 ± 8 |
| EK, mV | −94.2 ± 1.9* | −66.9 ± 0.9* | −77.3 ± 1.1 |
| EM − EK, mV | 19.7 ± 1.3* | 12.0 ± 1.2 | 14.5 ± 1.1 |
| n | 11/4 | 20/4 | 35/4 |
| Layer 2 fibers | |||
| EM, mV | −82.2 ± 1.8* | −61.8 ± 1.6* | −68.4 ± 1.4 |
| aKi+, mM | 169 ± 13 | 144 ± 9* | 202 ± 10 |
| EK, mV | −101.7 ± 2.1* | −71.8 ± 1.7* | −80.7 ± 1.3 |
| EM − EK, mV | 19.6 ± 1.2* | 10.0 ± 0.5* | 12.2 ± 0.6 |
| n | 17/4 | 22/4 | 29/4 |
Values are means ± SE; n, no. of fibers/muscles. 4K, control Krebs solution with 4 mM K+, 1.3 mM Ca2+; 11K, raised K+ Krebs solution with 11 mM K+, 1.3 mM Ca2+; 11K + 10Ca, raised K+ Krebs solution with 11 mM K+, 10 mM Ca2+. Surface and layer 2 fibers were impaled from the same four extensor digitorum longus (EDL) muscles.
Significant difference (P < 0.05, unpaired t-test with Bonferroni correction) between 4K and 11K, or 11K and 11K + 10Ca. Changes of resting membrane potential (EM), intracellular K+ activity (aKi+), and K+-equilibrium potential (EK) were also statistically significant at the individual muscle level (4–14 fiber impalements in each solution).
ICFV and [K+]i.
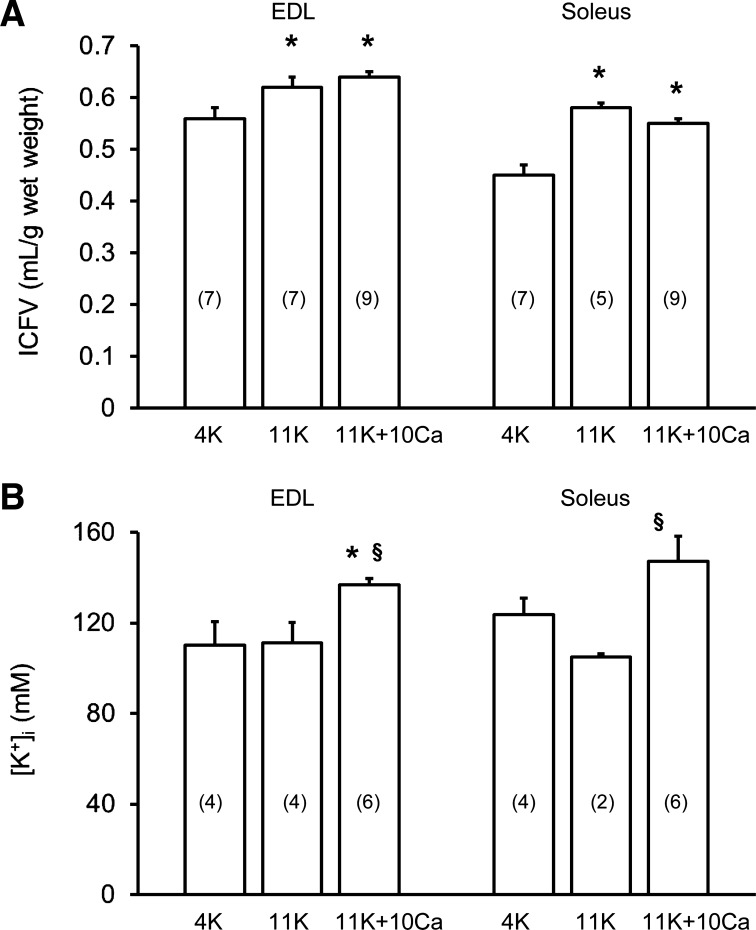
In another series of experiments on both muscle types, we examined whether the increase of aKi+ at 11K + 10Ca compared with 11K involved fiber shrinkage due to water efflux. To do this, we determined total water content (Eq. 3) and then calculated ICFV (Eq. 5), and intracellular K+ was quantified to calculate [K+]i (Eq. 9). Figure 3A shows that a change from 4K to 11K increased ICFV by 11% in EDL and 13% in soleus. The ICFV then remained elevated at 11K + 10Ca, but it did not differ from that at 11K, i.e., there was no fiber shrinkage with raised [Ca2+]o. The intracellular K+ content increased significantly from 11K to 11K + 10Ca for both muscle types. In EDL muscles, it increased from 66.2 ± 5.1 to 85.8 ± 2.4 μmol/g wet wt, and in soleus muscles it increased from 58.0 ± 1.3 to 72.2 ± 6.7 μmol/g wet wt. Figure 3B shows the calculated [K+]i using these data. When the bathing solution was changed from 11K to 11K + 10Ca, this resulted in an elevated [K+]i in EDL muscles by 26 mM (i.e., aKi+ increased by 20 mM) and in soleus muscles by 42 mM (i.e., aKi+ increased by 32 mM). Hence, the changes of [K+]i determined in this manner are consistent with the findings obtained using K+-sensitive microelectrodes.
Fig. 3.
Influence of raised [Ca2+]o (1.3 to 10 mM) at 11 mM [K+]o on intracellular fluid volume (ICFV; A) and intracellular K+ concentration ([K+]i; B) in EDL and soleus muscles. Data are mean values ± SE (no. of paired muscles in parentheses) obtained under steady-state conditions. [K+]i was calculated from K+ content and ICFV (see Eq. 8). *Significantly greater than 4K (P < 0.05, ANOVA). §Significantly greater than 11K (P < 0.05, ANOVA).
Action potential profile.
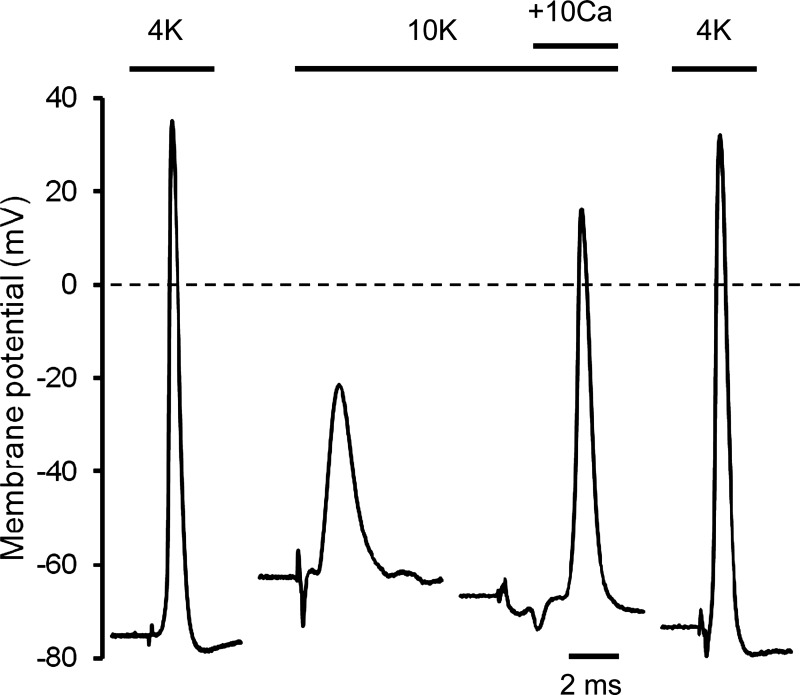
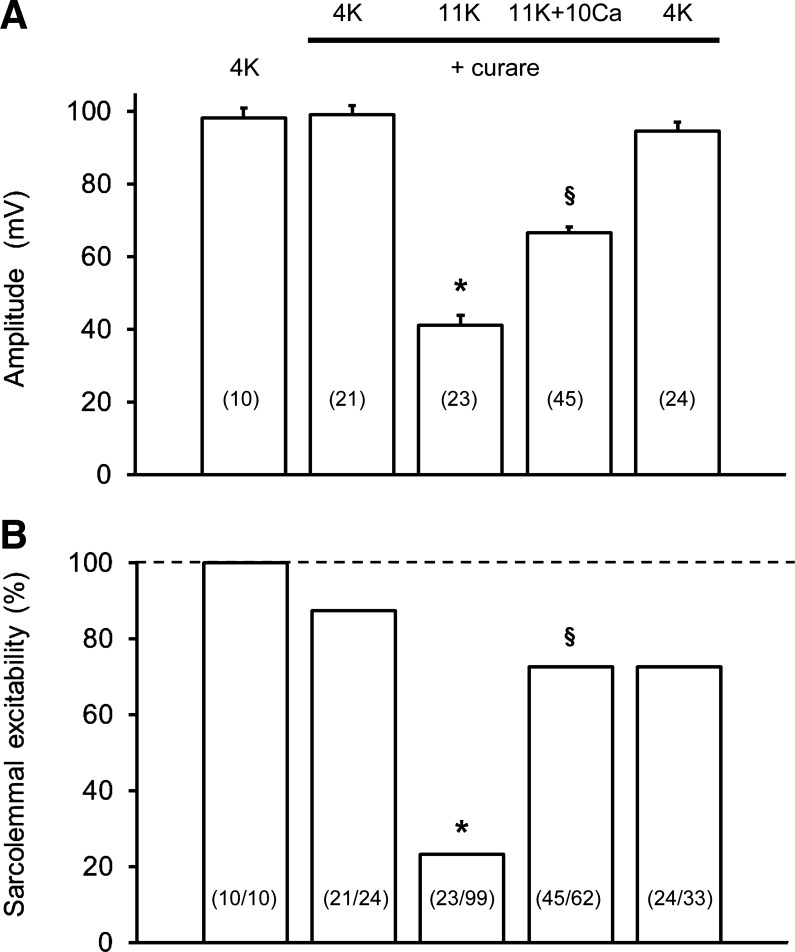
Since variations of resting EM can influence the action potential (55, 57, 58), we first tested for a Ca2+-K+ interactive effect on the single action potential profile in soleus muscle fibers. Figure 4 shows representative depressive effects of raised [K+]o (4K to 10K) and then restorative effects of raised [Ca2+]o (10K to 10K + 10Ca) on action potentials in soleus fibers. Mean data are summarized in Table 2. When [K+]o was increased to 10K around surface fibers, the mean resting EM depolarized by 21 mV, the overshoot disappeared with amplitude falling considerably by 80 mV, and the action potential profile became broader (with lower maximum rates of rise and fall of the action potential). With subsequent equilibration at 10K + 10Ca, there was a large reversal of all such effects. Most notably, the mean action potential amplitude increased by 54 mV so that the overshoot reappeared. All effects reversed on return to the 4K control solution (Fig. 4). In layer 2 fibers equilibrated at 10K and then exposed to 10K + 10Ca a repolarization of 3.5 mV occurred, and the action potential amplitude increased significantly by 16 mV, i.e., from 48.0 ± 5.2 mV (n = 22/3) to 63.7 ± 5.5 mV (n = 17/3). Figure 5A shows action potential amplitude data from similar experiments in surface EDL fibers but in the presence of curare. Curare had no significant effect on all action potential waveform parameters at 4K. At 11K + curare, the mean resting EM depolarized by 16 mV to −59 mV, and the peak of the action potential fell from +24 mV to −18 mV. When [Ca2+]o was raised to 10 mM (11K + curare to 11K + 10Ca + curare), a nonsignificant repolarization occurred (of excitable fibers), yet the mean action potential amplitude increased by 27 mV (Fig. 5A) with the overshoot returning to +7 mV. Also, a broadening of the action potential at raised [K+]o was partially restored with 10 mM [Ca2+]o, e.g., the width was 0.815 ± 0.026 ms (n = 21/5) at 4K + curare, 1.903 ± 0.102 ms (n = 23/5) at 11K + curare, and 1.189 ± 0.034 ms (n = 45/5) at 11K + 10Ca + curare.
Fig. 4.
Representative traces of single action potentials showing the steady-state effects of 10K and then with elevated [Ca2+]o (1.3 to 10 mM) in surface fibers of soleus muscles. Action potentials were elicited with single 0.3-ms pulses using fine platinum electrodes. Exposure time in each solution was 4K (220 min), 10K (73 min), 10K + 10Ca (93 min), and 4K recovery (43 min). Horizontal dashed line represents 0 mV. The deflection before the upstroke of each action potential is the stimulus artifact.
Fig. 5.
Steady-state effects of raised [Ca2+]o (1.3 to 10 mM) at 11 mM [K+]o on single action potential amplitude (A) and sarcolemmal excitability (B) in surface fibers of EDL muscles. Data in A are mean values ± SE (no. of excitable fibers in parentheses) and in B are the level of excitability as % (no. of excitable fibers/total number of fibers penetrated in parentheses), from the same experiments as in A. Experiments were done in the presence of 30 μM curare (n = 5 muscles) with at least 30-min exposure to each solution before action potentials were triggered. Data at 4K without curare were from two of these muscles. *11K + curare significantly less than 4K + curare (P < 0.05, ANOVA). §11K + 10Ca + curare is significantly greater than 11K + curare (P < 0.05, ANOVA).
Sarcolemmal excitability.
Figure 5B depicts an inhibitory effect of 11K and then restorative effect of 11K + 10Ca on the percentage of excitable fibers in EDL muscles, in the presence of curare. At 4K, exposure to curare resulted in a small but nonsignificant loss of excitability. At 11K + curare, 74% of the excitable fibers at 4K + curare were rendered inexcitable. Then with raised [Ca2+]o (11K + 10Ca + curare), a huge restoration of excitability occurred (∼50% of total fibers) to a level that was only 13% below that at 4K + curare.
Influence of Altered [K+]o on Fatigue with Repeated Tetani
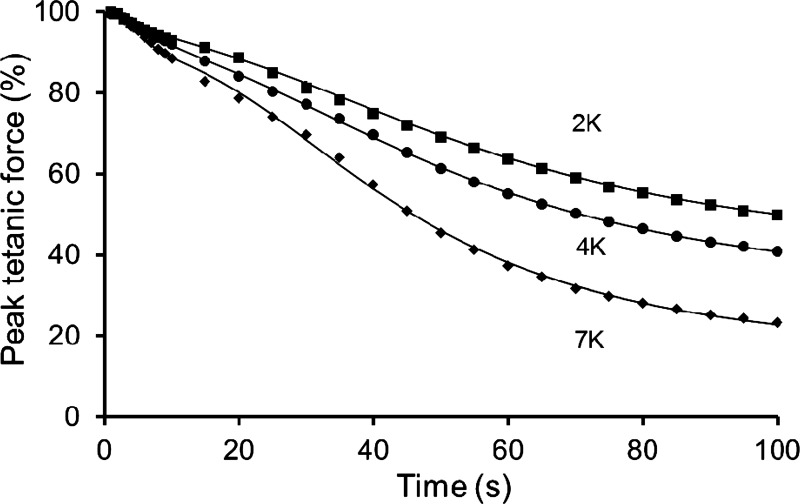
For a Ca2+-K+ interaction to occur during fatigue, a necessary prerequisite is that K+ is also involved in our model of fatigue with repeated tetani. We therefore, hypothesized that prior exposure to altered [K+]o would influence the fatigue profile, i.e., an increase from 4 to 7 mM [K+]o (7K) would exacerbate, and a decrease from 4 to 2 mM [K+]o (2K) would attenuate, the rate of fatigue. When soleus muscles were equilibrated at 7K, the peak tetanic force (125 Hz) fell slightly to 91 ± 2% (n = 5). When fatigue was subsequently induced at 7K, the rate and extent of peak force decline was exacerbated compared with that at 4K (Fig. 6). The fatigue profiles were well fitted by a double-sigmoid model with the largest effects of 7K seen for the second sigmoid, i.e., the late phase of fatigue (Table 3). The fitting parameters show that its time constant (τ2) was abbreviated by 8 s, the maximum rate of peak force decline (S2) was steepened by ∼60%, and the final plateau of force (Fmin) was further depressed by ∼50%. A feature of this K+ effect was a significant reduction of both evoked and resting forces. At 100 s of stimulation, the evoked force of 31 ± 1% initial (4K) fell to 20 ± 2% initial (7K), and the resting force of 9 ± 1% (4K) fell to 4 ± 1% (7K). After equilibration of soleus muscles at 2K, the peak tetanic force was 100 ± 2% initial (n = 3). The subsequent fatigue profile showed that both the rate and extent of late fatigue (second sigmoid) were attenuated at 2K compared with 4K (Table 3, Fig. 6). These effects involved a small increase of both evoked and resting forces.
Fig. 6.
Influence of prior exposure to different [K+]o (2, 4, 7 mM) on fatigue induced with repeated tetani in soleus muscles. Mean data are shown for peak tetanic force (%) during fatiguing stimulation as a function of time. A double-sigmoid function was fitted to all data points for each condition [5-parameter, intermediate asymptote shared by the two sigmoids (Fo) = 88.9]. Fatigue protocol: 125 Hz for 500 ms, evoked once every second for 100 s. Supramaximal pulses (20 V, 0.1 ms) were delivered via parallel plate electrodes. n = 5 muscles at 7K, n = 3 muscles at 2K, and n = 8 muscles at 4K. All fatigue curves differed significantly from one another (P < 0.05, ANOVA). For fitting parameters, see Table 3.
Table 3.
Parameter estimates obtained by fitting double-sigmoid models to fatigue profiles evoked with repeated tetani at lowered (2 mM), normal (4 mM), and raised (7 mM) [K+]o in soleus muscles
| Parameter | 2K | 4K | 7K |
|---|---|---|---|
| First sigmoid | |||
| τ1, s | 7.3 ± 3.4 | 7.1 ± 0.2* | 5.3 ± 0.3 |
| S1, %/s | −0.1 ± 0.6 | −0.2 ± 0.7 | −0.2 ± 1.3 |
| Second sigmoid | |||
| τ2, s | 58.0 ± 3.3* | 53.6 ± 1.0* | 45.3 ± 0.9 |
| S2, %/s | −0.50 ± 0.03* | −0.63 ± 0.04* | −0.98 ± 0.06 |
| Fmin, % | 36.9 ± 3.4* | 28.9 ± 1.1* | 13.6 ± 1.5 |
| Statistics | |||
| r2 | 0.9928 | 0.9785 | 0.8883 |
| MSε, % | 0.03 | 0.02 | 0.70 |
| n | 3 | 8 | 5 |
Values are means ± SE; n, no. of muscles. Stimulation protocol: 125 Hz for 500 ms, once every second for 100 s. Supramaximal pulses (20 V, 0.1 ms) were delivered via parallel plate electrodes. S1 and S2, maximum slope for each sigmoid; τ1 and τ2, time for each sigmoid to fall to one-half of its initial value [τ1 is for the first sigmoid (early fatigue), and τ2 is for the second sigmoid (late fatigue)]; Fmin, minimum asymptote (plateau value) determined for the second sigmoid; MSε, mean square error. The five parameter estimates (τ1, S1, τ2, S2, Fmin) and fitting statistics (r2, MSε) were obtained from fitting to all data. The intermediate asymptote shared by the two sigmoids (Fo) was set at 88.9% (16). For each 2K and 7K experiment, the test run at the specified value of [K+]o was bracketed by standard runs at 4K. The before and after values at 4K were averaged for each of the 8 test runs.
Significant difference between 2K and 4K, or 4K and 7K (P < 0.05).
Influence of Raised [Ca2+]o on Fatigue with Repeated Tetani
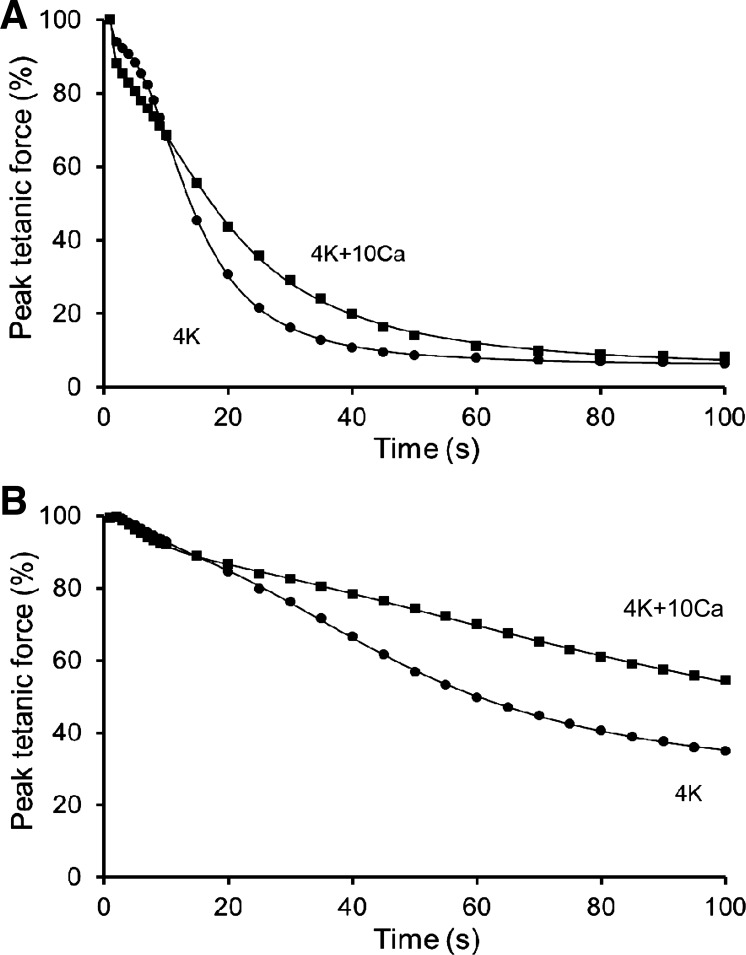
When [Ca2+]o was raised from 1.3 to 10 mM, the steady-state peak tetanic force (200 Hz) of nonfatigued EDL muscles was unchanged at 98 ± 2% initial (n = 7). The effect of 4K + 10Ca was then tested on fatigue in five EDL muscles, with the most notable feature being a slowing of late fatigue (Table 4, Fig. 7A). This protective Ca2+ effect occurred over 15–70 s of stimulation, with the largest attenuation being 16% of the peak force decline, which occurred at ∼20 s of stimulation. The fitting parameters show that, at 4K + 10Ca, τ2 was prolonged by 7 s, S2 was reduced by ∼60%, but Fmin was unaltered (Table 4). Moreover, resting force was unaltered during fatigue at either [Ca2+]o (data not shown). A second beneficial effect was that fade (relative decline of force within a tetanus) was delayed and first appeared at 75 ± 8 s (n = 5) at 4K + 10Ca, as opposed to 43 ± 8 s (n = 8) at 4K. The fade at 100 s of stimulation (125 Hz, 0.5 s tetanus) was attenuated by one-half from 0.61 ± 0.07 at 4K to 0.82 ± 0.07 at 4K + 10Ca. Unexpectedly, a small detrimental Ca2+ effect occurred at 4K + 10Ca during early fatigue, with an exacerbated peak force loss over the initial 10 tetani (ANOVA) (Fig. 7A). This effect manifested rapidly, with a significantly greater decline of peak force by the second tetanus, i.e., to 88 ± 1% initial at 4K + 10Ca (n = 5) compared with that to 94 ± 0.4% initial at 4K.
Table 4.
Parameter estimates obtained by fitting double-sigmoid models to fatigue profiles evoked with repeated tetani at 1.3 and 10 mM [Ca2+]o in EDL and soleus muscles
| EDL |
Soleus |
|||
|---|---|---|---|---|
| Parameter | 4K | 4K + 10Ca | 4K | 4K + 10Ca |
| First sigmoid | ||||
| τ1, s | 0.3 ± 0.9 | 0.9 ± 0.9 | 8.8 ± 1.3* | 6.5 ± 0.9 |
| S1, %/s | −3.2 ± 6.6 | −5.0 ± 10.0 | −0.2 ± 0.2 | −0.1 ± 0.0 |
| Fo, % | 89.9 ± 4.7 | 78.3 ± 7.2 | 88.9 | 88.9 |
| Second sigmoid | ||||
| τ2, s | 13.2 ± 0.2* | 20.1 ± 0.5 | 48.3 ± 1.9* | 105.6 ± 32.6 |
| S2, %/s | −4.5 ± 0.1* | −2.0 ± 0.4 | −0.8 ± 1.9 | −0.3 ± 0.0 |
| Fmin, % | 5.1 ± 2.1 | 4.0 ± 1.6 | 27.3 ± 3.1 | 15.4 ± 22.4 |
| Statistics | ||||
| r2 | 0.9975 | 0.9937 | 0.9991 | 0.9201 |
| MSε, % | 0.05 | 0.07 | 0.34 | 0.08 |
| n | 8 | 4 | 9 | 9 |
Parameter estimates are mean values ± SE; n, no. of muscles. Stimulation protocol: 125 Hz for 500 ms, once every second for 100 s. Supramaximal pulses (20 V, 0.1 ms) and plate electrodes were used. The parameter estimates (τ1, S1, τ2, S2, Fo, Fmin) and fitting statistics (r2, MSε) were determined by fitting to all data. Fo was fixed at 88.9% for soleus (16).
Significant difference between 4K and 4K + 10Ca (P < 0.05).
Fig. 7.
Influence of raised [Ca2+]o (1.3 to 10 mM) on fatigue induced with repeated tetani in EDL (A) and soleus muscles (B). Mean data are shown for peak tetanic force (%) during fatiguing stimulation as a function of time. A double-sigmoid function was fitted to all data points for each condition (6-parameter for EDL, 5-parameter for soleus, Fo = 88.9). Fatigue protocol: 125 Hz for 500 ms, evoked once every second for 100 s. Supramaximal pulses (20 V, 0.1 ms) were delivered via parallel plate electrodes. A: n = 4 EDL muscles at 4K + 10Ca and n = 8 EDL muscles at 4K. B: n = 9 soleus muscles exposed to 1.3 mM [Ca2+]o (average of before and after) and 10 mM [Ca2+]o. At least 60 min were allowed between fatigue runs. For both muscle types, the fatigue profile at 4K and 4K + 10Ca differed significantly (P < 0.05). For fitting parameters, see Table 4.
In soleus muscles, prior exposure to 4K + 10Ca also influenced the kinetics of late fatigue (Table 4, Fig. 7B), with the largest Ca2+ effect being an attenuated peak force decline by ∼16% initial from ∼60 s of stimulation onwards. There was a doubling of τ2 and S2 fell by ∼60% normal, but Fmin did not significantly differ (Table 4). In contrast to the EDL, the effect on late fatigue included a contribution from an elevated resting force, which at 100 s increased from 5 ± 2% of peak initial force at 4K to 14 ± 3% at 4K + 10Ca.
DISCUSSION
The main novel findings of the present study are that 1) raised [Ca2+]o induced a massive increase of aKi+ (or [K+]i) leading to sarcolemmal repolarization, and a striking recovery of action potential amplitude and percentage of excitable fibers in K+-depressed muscle; 2) prior exposure to higher or lower levels of [K+]o accelerated or slowed the late phase of fatigue during repeated tetani, respectively; and 3) raised [Ca2+]o slowed late fatigue during repeated tetani in fast-twitch muscle (consistent with the effect seen in slow-twitch muscle). We regard that a likely physiological scenario is that [Ca2+]o declines during fatigue and acts synergistically with a reduced K+ gradient to accelerate fatigue. In the following discussion, we first address the mechanism for the Ca2+-K+ interaction in nonfatigued muscle, leading to the physiological relevance of our findings during fatigue.
Mechanism for the Ca2+-K+ Interaction in Nonfatigued Muscle
Ca2+ effects on resting EM and aKi+ at raised [K+]o.
Raised [Ca2+]o (10 mM) induced an up to 10-mV repolarization of the sarcolemma in severely K+-depolarized fibers. This effect was of similar magnitude in fast-twitch and slow-twitch fibers (Fig. 2), which implies a common mechanism for both muscle types. Similar Ca2+-induced repolarizations have been shown for phospholipase-depolarized fibers (2), or small hyperpolarizations at normal [K+]o for intact fibers (2), cultured muscle cells (48), or loose-patch clamped fibers (59), while there are reports showing that Ca2+-free solutions promote depolarization (22, 67). It is also known that divalent cations, such as Ca2+, have the ability to screen surface charge so that the sarcolemma appears less depolarized without changing the EM (40, 46). However, the observed Ca2+-induced repolarization (Tables 1 and 2, Figs. 2 and 4) cannot be explained by such a screening mechanism. Interestingly, raised [Ca2+]o has been shown to elevate basal [Ca2+]i in skeletal muscle (48, 64), which may, in turn, influence various intracellular Ca2+ signaling pathways (50). Hence, the precise role for extra- or intracellular [Ca2+] in modulating the EM remains to be established.
A major new and unexpected finding was that 10 mM [Ca2+]o induced a massive increase of aKi+ by 20–60 mM at raised [K+]o in both EDL and soleus fibers (Table 1, Fig. 3B). The corresponding increase of EK is sufficient by itself to account for the entire increase of resting EM in EDL fibers (Table 1). Hence, the physiological basis of the Ca2+ effect on EM involves cellular K+ regulating processes. This rise of aKi+ (or [K+]i) cannot involve an increase in K+ conductance via activation of some K+ channels, such as Ca2+-activated K+ channels (34, 38, 39), because simple diffusion through such channels would result in K+ efflux and lowered aKi+ (31). Importantly, the EM did not approach EK, and the (EM − EK) was essentially unchanged, which argues against the hypothesis of an increased K+ conductance. The rise of aKi+ or intracellular K+ content may in principle involve enhanced transsarcolemmal K+ influx via some carrier-mediated process, impaired transsarcolemmal K+ efflux, or K+ release from some intracellular site. The possibility of greater K+ influx is unlikely to involve a direct Ca2+-induced stimulation of the Na+-K+ pump, since it appears that raised [Ca2+]o inhibits Na+-K+ pump function, at least in diaphragm muscle (8, 64). Also, our 20–60 mM elevation of aKi+ markedly exceeds the rise seen with Na+-K+ pump stimulation by β-agonists (36). We ruled out the possibility that this rise of aKi+ involves an osmotic effect with water efflux leading to cell shrinkage, since ICFV was unchanged in both muscle types (Fig. 3A). The increased ICFV at 11K compared with 4K (Fig. 3A), when K+ was exchanged for Na+ to maintain osmolarity but making the solution hypertonic, is consistent with the seminal work of Boyle and Conway (7), where an influx of KCl followed by water occurs so that [K+]i remains constant. The subsequent addition of CaCl2 to make a slightly hyperosmotic (and hypertonic) solution (11K + 10Ca) is expected to cause water efflux and fiber shrinkage. However, the ICFV was unchanged under steady-state conditions (Fig. 3A), which is consistent with protection due to a regulatory volume increase (30, 44). This latter process is known to involve activation of the sarcolemmal Na+-K+-2Cl− cotransporter (NKCC), which promotes K+ and Na+ influx along with Cl− and water (30, 38, 39, 44). Hence one proposal for the [Ca2+]o-induced rise in aKi+ is that the hypertonicity induces K+ influx via the NKCC, with the resulting elevation of aNai+, also stimulating the Na+-K+ pump (19, 20) to further increase K+ influx. Furthermore, Ca2+ may activate the NKCC, which is Ca2+ dependent in other tissues (63). These ideas also appear to be feasible during fatigue, since pharmacological manipulation of the NKCC or Na+-K+ pump alters fatigue kinetics during repeated tetani in normal solutions (36, 38). Another explanation for the increased aKi+ is that it results from inhibitory effects on K+ efflux, possibly via reduced K+ channel conductance, and this idea warrants investigation. Clearly, the potential contribution of a large number of cellular processes needs to be investigated with a detailed further study to establish the precise mechanism(s) involved.
Ca2+ effects on action potentials and sarcolemmal excitability at raised [K+]o.
Raised [Ca2+]o (10 mM) induced a striking recovery of action potential amplitude for severely depolarized fibers (68% recovery in soleus, 44% recovery in EDL) (Table 2, Figs. 4 and 5A), but this is not seen at normal EM (2). Our Ca2+ effect is likely to involve repolarization, since action potential amplitude increases markedly with small repolarizations of EM between −65 and −55 mV (55, 57, 58), but this is not the case when EM is more polarized than −70 mV (55). However, we cannot exclude that a non-EM-related mechanism contributes to this effect in EDL. Restoration of other action potential profile parameters with 10 mM [Ca2+]o at 10–11K in either muscle type (e.g., Table 2) are all consistent with effects of a lesser depolarization (55) as they resemble action potentials recorded at 8–9K, which have similar EM (S. P. Cairns and J.-M. Renaud, unpublished observations). A second functionally important effect of raised [Ca2+]o was a massive recovery of sarcolemma excitability at raised [K+]o (Fig. 5B), which presumably follows recovery of sarcolemmal action potential threshold. Indeed, a K+-induced increase of action potential threshold is shown with microelectrodes (2, 58) or inferred from a rightward shift of the twitch force-stimulation strength relationship (12, 13, 17). This effect is likely to make some fibers inexcitable at rest (12, 37, 57, 58), or cause action potential skipping and/or complete failure during action potential trains (57). A leftward shift of the twitch force-stimulation strength relationship at 10K + 10Ca compared with 10K in soleus muscles (13) is consistent with a recovery of action potential threshold. These combined effects are presumably a consequence of the Ca2+-induced repolarization, which causes reversal of slow inactivation of voltage-dependent Na+ channels (58, 59).
Ca2+ effects on force at raised [K+]o.
Raised [Ca2+]o (10 mM) caused a rightward shift of the peak tetanic force-[K+]o relationship toward higher [K+]o (Fig. 1A), comparable to that seen with β-agonists, lactate, or acidosis (45, 53). Smaller changes of [Ca2+]o (over 0.5–2.5 mM) influence contractile force over 8–10K (Fig. 1B), which strengthens the possibility that stimulation-induced changes of [Ca2+]o has a physiological role. We previously speculated that Ca2+-induced repolarizations likely cause the force recovery at raised [K+]o (13), since small repolarizations (i.e., a few millivolts) markedly influence force when the resting EM is between −65 and −55 mV, according to the peak tetanic force-resting EM relationship (12, 13, 15, 57). In this EM range, a 5-mV repolarization (e.g., 10K to 10K + 10Ca) is predicted to permit a recovery of force by 50–70% control, and a 2-mV repolarization (e.g., 10K to 10K + 2.5Ca) is predicted to increase force by ∼20% control. Both of these predictions match what we measured experimentally.
Taken together, raised [Ca2+]o induces an elevated aKi+ (or raised [K+]i) in K+-depressed muscle, which mediates repolarization of the sarcolemma, to restore action potential amplitude and sarcolemmal excitability and ultimately increases force. These changes should also permit better action potential propagation and pattern during train stimulation to attenuate the force loss during fatigue.
[K+]o Effects on Fatigue with Repeated Tetani
The present study is the first, to our knowledge, to demonstrate that prior exposure to physiologically low or high values of [K+]o (15, 19, 45, 49, 60) modulate the fatigue profile during repeated intermittent tetanic stimulation (Table 3, Fig. 6). The double-sigmoid curve-fitting analysis demonstrates that the major K+ effects occur when severe fatigue prevails during the late phase (i.e., the second sigmoid). When [K+]o was raised over 2–7 mM (Table 3), the kinetics of late fatigue are accelerated, and the extent of late fatigue is greater, so that K+ effects accounted for at least 27% of the eventual loss of force. Clearly, our model of fatigue is K+ dependent and supports the K+ hypothesis of fatigue (15, 19, 45, 60). Raised [K+]o has previously been shown to accelerate fatigue during prolonged continuous tetanic stimulation (11, 20, 70), which is a markedly different fatigue regime to what we use here. Notably, an observation that prior exposure to 10 mM [K+]o failed to modify fatigue during repeated short tetani (70) has been used to argue against a role for K+ in such fatigue. However, those findings are not surprising, given that the authors' stimulation regime has an impact on the interpretation (10), since it involved extended rest periods between tetani (i.e., 1.4 vs. 0.5 s) and longer stimulus pulses (i.e., 0.5 vs. 0.1 ms) than we used. These features are likely to attenuate transsarcolemmal K+ shifts and diminish the chances of detecting action potential impairments with K+ (12, 17).
Influence of Raised [Ca2+]o on Fatigue in Fast- and Slow-Twitch Muscle
The two beneficial effects of raised [Ca2+]o include a slowing of late fatigue in both muscle types (Table 4, Fig. 7), and a reduction in the extent of fade but only in fast-twitch EDL muscle. The largest improvement of relative force at any time point was ∼16% initial force in both muscle types and is attributed to a lower rate of peak force decline for late fatigue (the second sigmoid) (Table 4). This protective Ca2+ effect included an increased evoked force in both muscle types. However, in soleus muscles, an increased resting force contributed about one-third of the total effect, which may reflect an elevated basal [Ca2+]i between tetani (69). A small number of other studies have shown that raised [Ca2+]o, sarcolemmal Ca2+-channel activators, or Ca2+ ionophores cause ∼10–20% increase of relative force during various fatigue protocols in slow-twitch muscle (28, 54). The more common approach of using nominally Ca2+-free solutions (13, 24, 28, 51, 68), or muscles that are knockout for sarcolemmal stretch- or store-operated Ca2+ channels (54, 69), or pharmacological blockade of various Ca2+ channels or Na+/Ca2+ exchanger proteins (24, 28, 51, 71) show an exacerbated loss of force by 5–40% initial in different types of fatigue regime. These combined findings suggest that Ca2+ influx across the sarcolemma helps to sustain force during fatigue, and without this contribution performance is further impaired.
We show a [Ca2+]o dependence of late fatigue with repeated tetani in fast-twitch EDL muscle (Table 4, Fig. 7A), consistent with some studies (21, 69, 71), but in contradiction to others on fast-twitch muscle (18, 28, 47). The negative findings of others may be due to their experimental protocol. This includes inducing fatigue with a single prolonged continuous tetanus (28, 47), testing a partially lowered [Ca2+]o to ∼0.5 mM (18, 47), or utilizing short (15 min) equilibration times (18), given that it might take longer for Ca2+ effects to manifest (67). Furthermore, we showed a new Ca2+ effect in fast-twitch muscle, where an exacerbated peak force loss occurred during early fatigue (Fig. 7A). The mechanism for this detrimental effect is uncertain, but it may compete with and mask the positive Ca2+ effects on force production during fatigue, in some studies on fast-twitch muscle.
Is a Ca2+-K+ Interaction Involved in Our Model of Fatigue?
We provide several lines of evidence that suggest that a Ca2+-K+ interaction can operate in our model of fatigue. 1) Prior exposure to altered [K+]o influences late fatigue in a manner that confirms a K+ dependence (Table 3, Fig. 6), and it is also this phase of fatigue where raised [Ca2+]o exerts its effects (Table 4, Fig. 7). 2) Since fatigue-induced depolarization is mediated by raised extracellular aK+, together with lowered aKi+ (15, 19, 35, 43, 45, 60), our Ca2+ effect to increase aKi+ in K+-depressed muscle (Table 1, Fig. 3B) is also likely to provide resistance to depolarization during fatigue. Hence, the EM would takes longer to depolarize to the critical range for force depression (12, 13, 15, 57). 3) Raised [Ca2+]o restored action potential amplitude (Table 2, Figs. 4 and 5A) and sarcolemmal excitability (Fig. 5B), along with reversing the right shift of the twitch force-stimulation strength relationship, in K+-depressed muscles (13). Such actions may overcome the action potential impairments and right shift of the twitch force-stimulation strength relationship seen with our model of fatigue (17, 37). 4) Raised [Ca2+]o restored peak force, slowed the force decline, and attenuated fade in muscles exposed to raised [K+]o (Fig. 1, Ref. 13). Such Ca2+ effects on peak force were greater in magnitude than those seen during fatigue (Fig. 7). However, this is not surprising, since raised [Ca2+]o also impairs force production at lowered [Na+]o (13) and a reduced transsarcolemmal Na+ gradient occurs simultaneously with a lowered K+ gradient during fatigue (15, 35, 36, 43, 45). Importantly, we found previously (13, 15) that elevating or lowering [Ca2+]o in muscles depressed with both raised [K+]o and lowered [Na+]o restores or further depresses force production, respectively. We, therefore, suggest that a Ca2+-K+-Na+ interaction can occur during late fatigue. However, a water-tight assessment requires greater understanding of the processes responsible for this interaction and the use of a tool/intervention to block or antagonize such processes during fatigue.
Physiological Relevance
The present findings (Table 4, Fig. 7) are consistent with the notion that some Ca2+ depletion in interstitial and/or t-tubular compartments contributes to stimulation-induced fatigue, at least in isolated muscles. Moreover, our findings are also in line with studies proposing a role for Ca2+ influx via store-operated Ca2+ channels, stretch-activated Ca2+ channels, L-type Ca2+ channels, or Na/Ca exchanger proteins (21, 24, 28, 41, 51, 54, 65, 68, 69, 71), since these Ca2+ entry pathways can be linked to the Ca2+-K+ interaction. We, therefore, hypothesize that, during high-intensity exercise over 5–10 min, a lowered interstitial or t-tubular [Ca2+]o interacts with a reduced transsarcolemmal K+ gradient to further lower muscle aKi+ and contribute to fatigue. Arguments against this hypothesis include the high [Ca2+]o used in our study and the prolonged time course for maximum Ca2+-effects. However, smaller changes of [Ca2+]o (i.e., over 0.5 to 2.5 mM, rather than with 10 mM) can modulate K+ effects in nonfatigued muscle (Fig. 1B). Altered [Ca2+]o also causes notable changes to K+-depressed force in <10 min, which is consistent with the time needed for larger ionic changes during intense exercise (15, 45). Another consideration is that our fatigue model involves repeated high-frequency stimulation of isolated whole muscles (with known diffusion limitations), which may exacerbate transsarcolemmal ion fluxes and impair excitability (17, 35–37). Importantly, quantitatively similar ionic disturbances occur with intense exercise in humans (15, 19, 45, 60), which means that our whole muscle model has physiological relevance for testing the Ca2+-K+ interaction. Our data also lead to the intriguing possibility that artificially elevating plasma or interstitial [Ca2+]o before exercise in humans may be a useful intervention to increase and better maintain muscle aKi+, so that exercise performance is improved in events where large transsarcolemmal K+ disturbances occur (15, 45, 60).
Our findings on the mechanism for the Ca2+-K+ interaction (Tables 1 and 2, Figs. 1–5) also have physiological relevance for understanding a treatment for hyperkalemic periodic paralysis. In this clinical situation in humans, administration of calcium gluconate is used to alleviate the paralytic K+ attacks on muscle (27, 52) and is a modern day treatment (1). Also, altered [Ca2+]o has a major impact on the contractile performance of K+-depressed muscles isolated from mice suffering from hyperkalemic periodic paralysis (33). Therefore, further enhancing knowledge about the mechanisms underpinning this Ca2+ effect may assist with developing therapeutic procedures in the future.
GRANTS
We gratefully thank the funding support from Lotteries Health of New Zealand (to S. P. Cairns), and the Natural Sciences and Engineering Research Council of Canada (to J. M. Renaud).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.P.C. conception and design of research; S.P.C., J.P.L., D.S.L., A.H., W.L., and J.-M.R. performed experiments; S.P.C., J.P.L., D.S.L., A.H., W.L., and J.-M.R. analyzed data; S.P.C., J.P.L., D.S.L., A.H., W.L., and J.-M.R. interpreted results of experiments; S.P.C. prepared figures; S.P.C. and J.-M.R. drafted manuscript; S.P.C., J.P.L., D.S.L., A.H., W.L., and J.-M.R. edited and revised manuscript; S.P.C., J.P.L., D.S.L., A.H., W.L., and J.-M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. J.-C. Han for assistance with implementation of the NLMixed module in the SAS software.
REFERENCES
- 1.Ahmed J, Weisberg LS. Hyperkalemia in dialysis patients. Semin Dial 14: 348–356, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque EX, Thesleff S. The effect of calcium on the skeletal muscle membrane after treatment with phospholipase C. Acta Physiol Scand 72: 310–321, 1968. [DOI] [PubMed] [Google Scholar]
- 3.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Balog EM, Fitts RH. Effects of depolarization and low intracellular pH on charge movement currents of frog skeletal muscle fibers. J Appl Physiol 90: 228–234, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Banas C, Clow B, Jasmin J, Renaud JM. The KATP channel Kir6.2 subunit protein content is higher in glycolytic than in oxidative skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol 301: R916–R925, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi CP, Narayan S. Muscle fatigue and the role of transverse tubules. Science 215: 295–296, 1982. [DOI] [PubMed] [Google Scholar]
- 7.Boyle PJ, Conway EJ. Potassium accumulation in muscle and associated changes. J Physiol 100: 1–63, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breier A, Sulová Z, Vrbanová A. Ca2+-induced inhibition of sodium pump: noncompetitive inhibition in respect of magnesium and sodium cations. Gen Physiol Biophys 17: 179–188, 1998. [PubMed] [Google Scholar]
- 9.Cairns SP, Buller SJ, Loiselle DS, Renaud JM. Changes of action potentials and force at lowered [Na+]o in mouse skeletal muscle: implications for fatigue. Am J Physiol Cell Physiol 285: C1131–C1141, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cairns SP, Chin ER, Renaud JM. Stimulation pulse characteristics and electrode configuration determine site of excitation in isolated mammalian skeletal muscle: implications for fatigue. J Appl Physiol 103: 359–386, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cairns SP, Dulhunty AF. High-frequency fatigue in rat skeletal muscle: role of extracellular ion concentrations. Muscle Nerve 18: 890–898, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol Cell Physiol 273: C598–C611, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Role of extracellular [Ca2+] in fatigue of isolated mammalian skeletal muscle. J Appl Physiol 84: 1395–1406, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Cairns SP, Leader JP, Loiselle DS. Exacerbated potassium-induced paralysis of mouse soleus muscle at 37°C vis-à-vis 25°C: implications for fatigue. Pflügers Arch 461: 469–479, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Cairns SP, Lindinger MI. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586: 4039–4054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns SP, Robinson DM, Loiselle DS. Double-sigmoid model for fitting fatigue profiles in mouse fast- and slow-twitch muscle. Exp Physiol 93: 851–862, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Cairns SP, Taberner AJ, Loiselle DS. Changes of surface and t-tubular membrane excitability during fatigue with repeated tetani in isolated mouse fast- and slow-twitch muscle. J Appl Physiol 106: 101–112, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud JM. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol 93: 1126–1138, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Clausen T, Nielsen OB. Potassium, Na+,K+-pumps and fatigue in rat muscle. J Physiol 584: 295–304, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danieli-Betto D, Germinario E, Esposito A, Megighian A, Midrio M, Ravara B, Damiani E, Libera LD, Sabbadini RA, Betto R. Sphingosine 1-phosphate protects mouse extensor digitorum longus skeletal muscle during fatigue. Am J Physiol Cell Physiol 288: C1367–C1373, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Delbono O, Obejero Paz CA, Muchnik S. The effect of verapamil and Ca free solution on mechanical and electrical properties in fast twitch mammalian skeletal muscle. Acta Physiol Pharmacol Ther Latinoam 37: 423–435, 1987. [PubMed] [Google Scholar]
- 23.Donaldson PJ, Leader JP. Intracellular ionic activities in the EDL muscle of the mouse. Pflügers Arch 400: 166–170, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol 575: 913–924, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich O, Ehmer T, Uttenweiler D, Vogel M, Barry PH, Fink RHA. Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys J 80: 2046–2055, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamstorp I, Hauge M, Helweg-Larsen HF, Mjönes H, Sagild U. Adynamia Episodica Hereditaria. A disease clinically resembling familial periodic paralysis but characterized by increasing serum potassium during the paralytic attacks. Am J Med 23: 385–390, 1957. [DOI] [PubMed] [Google Scholar]
- 28.Germinario E, Esposito A, Midrio M, Peron S, Palade PT, Betto R, Danieli-Betto D. High-frequency fatigue of skeletal muscle: role of extracellular Ca2+. Eur J Appl Physiol 104: 445–453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gissel H, Clausen T. Excitation-induced Ca2+ influx in rat soleus and EDL muscle: mechanisms and effects on cellular integrity. Am J Physiol Regul Integr Comp Physiol 279: R917–R924, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Gosmanov AR, Lindinger MI, Thomason DB. Riding the tides: K+ concentration and volume regulation by muscle Na+-K+-2Cl− cotransport activity. News Physiol Sci 18: 196–200, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Grafe P, Quasthoff S, Strupp M, Lehmann-Horn F. Enhancement of K+ conductance improves in vitro the contraction force of skeletal muscle in hypokalemic periodic paralysis. Muscle Nerve 13: 451–457, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Harrison AP, Flatman JA. Measurement of force and both surface and deep M wave properties in isolated rat soleus muscles. Am J Physiol Regul Integr Comp Physiol 277: R1646–R1653, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Hayward LJ, Kim JS, Lee MY, Zhou H, Kim JW, Misra K, Salajegheh M, Wu FF, Matsuda C, Reid V, Cros D, Hoffman EP, Renaud JM, Cannon SC, Brown RH. Targeted mutation of mouse skeletal muscle sodium channel produces myotonia and potassium-sensitive weakness. J Clin Invest 118: 1437–1449, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquemond V, Allard B. Activation of Ca2+-activated K+ channels by an increase in intracellular Ca2+ induced by depolarization of mouse skeletal muscle fibres. J Physiol 509: 93–102, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflügers Arch 406: 458–463, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Juel C. The effect of β2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiol Scand 134: 209–216, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Juel C. Muscle action potential propagation velocity changes during activity. Muscle Nerve 11: 714–719, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen M, Hansen T, Juel C. Membrane proteins involved in potassium shifts during muscle activity and fatigue. Am J Physiol Regul Integr Comp Physiol 290: R766–R772, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen M, Juel C. Potassium-transporting proteins in skeletal muscle: cellular location and fibre-type differences. Acta Physiol (Oxf) 198: 105–123, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Launikonis BS, Murphy RM, Edwards JN. Towards the roles of store-operated Ca2+ entry in skeletal muscle. Pflügers Arch 460: 813–823, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Launikonis BS, Stephenson DG, Friedrich O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol 587: 2299–2312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leader JP, Bray JJ, Macknight ADC, Mason DR, McCaig D, Mills RG. Cellular ions in intact and denervated muscles of the rat. J Membr Biol 81: 19–27, 1984. [DOI] [PubMed] [Google Scholar]
- 43.Lindinger MI, Heigenhauser GJF. Ion fluxes during tetanic stimulation in isolated perfused rat hindlimb. Am J Physiol Regul Integr Comp Physiol 254: R117–R126, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Lindinger MI, Leung M, Trajcevski KE, Hawke TJ. Volume regulation in mammalian skeletal muscle: the role of sodium-potassium-chloride cotransporters during exposure to hypertonic solutions. J Physiol 589: 2887–2899, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl− disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol 104: 288–295, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1241: 59–116, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Mikkelsen UR, Fredsted A, Gissel H, Clausen T. Excitation-induced Ca2+ influx and muscle damage in the rat: loss of membrane integrity and impaired force recovery. J Physiol 559: 271–285, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naro F, De Arcangelis V, Coletti D, Molinaro M, Zani B, Vassanelli S, Reggiani C, Teti A, Adamo S. Increase in cytosolic Ca2+ induced by elevation of extracellular Ca2+ in skeletal myogenic cells. Am J Physiol Cell Physiol 284: C969–C976, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen HB, Bredmose PR, Strømstad M, Volianitis S, Quistorff B, Secher NH. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J Appl Physiol 93: 724–731, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Toda K. High extracellular Ca2+ stimulates Ca2+-activated Cl− currents in frog parathyroid cells through the mediation of arachidonic acid cascade. PLos One 6: e19158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol 4: 379–383, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Pearson CM. The periodic paralyses: differential features and pathological observations in permanent myopathic weakness. Brain 87: 341–354, 1964. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and β2-agonist. J Physiol 551: 277–286, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pritschow BW, Lange T, Kasch J, Kunert-Keil C, Liedtke W, Brinkmeier H. Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflügers Arch 461: 115–122, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Quiñonez M, González F, Morgado-Valle C, DiFranco M. Effects of membrane depolarization and changes in extracellular [K+] on the Ca2+ transients of fast skeletal muscle fibers. Implications for muscle fatigue. J Muscle Res Cell Motil 31: 13–33, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radzyukevich T, Lipska E, Pavelkova J, Zacharova D. Characterization of tension decline in different types of fatigue-resistant skeletal muscle fibres of the frog. Low extracellular calcium effects. Gen Physiol Biophys 12: 473–490, 1993. [PubMed] [Google Scholar]
- 57.Renaud JM, Light P. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implication for fatigue in vivo. Can J Physiol Pharmacol 70: 1236–1246, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol 547: 555–566, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruff RL, Simoncini L, Stühmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve 11: 502–510, 1988. [DOI] [PubMed] [Google Scholar]
- 60.Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80: 1411–1481, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Shah VN, Wingo TL, Weiss KL, Williams CK, Balser JR, Chazin WJ. Calcium-dependent regulation of the voltage-gated sodium channel hH1: intrinsic and extrinsic sensors use a common molecular switch. Proc Natl Acad Sci USA 103: 3592–3597, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shalton PM, Wareham AC. Intracellular activity of potassium in normal and dystrophic skeletal muscle from C57BL/6J mice. Exp Neurol 74: 673–687, 1981. [DOI] [PubMed] [Google Scholar]
- 63.Shin JH, Namkung W, Choi JY, Yoon JH, Lee MG. Purinergic stimulation induces Ca2+-dependent activation of Na+-K+-2Cl− cotransporter in human nasal epithelia. J Biol Chem 279: 18567–18574, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Stankovičovǎ T, Zemková H, Breier A, Amler E, Burkhard M, Vyskočil F. The effects of calcium and calcium channel blockers on sodium pump. Pflügers Arch 429: 716–721, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenburg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 10: 688–697, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stroffekova K, Heiny JA. Triadic Ca2+ modulates charge movement in skeletal muscle. Gen Physiol Biophys 16: 59–77, 1997. [PubMed] [Google Scholar]
- 67.Usher-Smith JA, Xu W, Fraser JA, Huang CLH. Alterations in calcium homeostasis reduce membrane excitability in amphibian skeletal muscle. Pflügers Arch 453: 211–221, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Williams JH, Ward CW. Dihydropyridine effects on skeletal muscle fatigue. J Physiol (Paris) 85: 235–238, 1991. [PubMed] [Google Scholar]
- 69.Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, Birnbaumer L, Gailly P. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol 298: C149–C162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol 572: 551–559, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handing properties of sarcalumenin knockout mice. Physiol Genomics 23: 72–78, 2005. [DOI] [PubMed] [Google Scholar]