Abstract
Activation of the purinergic P2X7 receptor (P2X7R) has been associated with the development of experimental nephritis and diabetic and hypertensive nephropathy. However, its role in acute kidney injury (AKI) remains unknown. In this study, we examined the effects of P2X7R inhibition in a murine model of ischemia-reperfusion (I/R)-induced AKI using A438079, a selective inhibitor of P2X7R. At 24 h after I/R, mice developed renal dysfunction and renal tubular damage, which was accompanied by elevated expression of P2X7R. Early administration of A438079 immediately or 6 h after the onset of reperfusion protected against renal dysfunction and attenuated kidney damage whereas delayed administration of A438079 at 24 h after restoration of perfusion had no protective effects. The protective actions of A438079 were associated with inhibition of renal tubule injury and cell death and suppression of renal expression of monocyte chemotactic protein-1 and regulated upon expression normal T cell expressed and secreted (RANTES). Moreover, I/R injury led to an increase in phosphorylation (activation) of extracellular signal-regulated kinases 1/2 in the kidney; treatment with A438079 diminished this response. Collectively, these results indicate that early P2X7R inhibition is effective against renal tubule injury and proinflammatory response after I/R injury and suggest that targeting P2X7R may be a promising therapeutic strategy for treatment of AKI.
Keywords: acute kidney injury, purinergic receptors, renal tubular cells, apoptosis
acute kidney injury (AKI) after ischemia-reperfusion (I/R) is a common clinical problem complicated by substantial morbidity and mortality. The pathogenesis of kidney I/R is complex and generally involves direct induction of renal tubular cell death; renal endothelial injury; expression of many chemokines and cytokines such as interleukin-1β (IL-1β), monocyte chemotactic protein-1 (MCP-1), and regulated upon expression normal T cell expressed and secreted (RANTES); and infiltration of leukocytes (6, 14). Although recent advances in the use of biomarkers such as neutrophil gelatinase-associated lipocalin (NAGL) and kidney injury molecule 1 (Kim1) have helped in the early diagnosis of AKI, so far, there is no effective therapy for its treatment other than time and supportive measures or renal replacement therapy. Thus there is an urgent need to gain more mechanistic insights into I/R-induced AKI and develop novel approaches to treat this syndrome.
AKI leads to activation of various forms of cell death, including necrosis, apoptosis, or autophagy-associated cell death (6, 14). Apoptosis is the process of programmed cell death that involves the activation of enzymes (i.e., caspase-3) and activation of multiple intracellular signaling pathways including extracellular signal-regulated kinases 1/2 (ERK1/2), but cellular plasma membrane integrity is usually intact without release of intracellular contents. In contrast, necrosis is characterized by cell and organelle swelling with subsequent rupture of surface membranes and the spilling of their intracellular contents. This is particularly the case in the event of acute tissue injury such as I/R (14, 25). Renal epithelial cells contain a large amount of ATP (5–8 mM) (15). Along with other cellular contents, ATP is released into the environment surrounding damaged cells (15). ATP release is thought to lead to tissue inflammation and leukocyte infiltration, with the presence of inflammatory cells triggering additional cell damage and ATP release (7). Thus pharmacological strategies to block ATP release or ATP receptor signaling may have promise as a means to attenuate sterile inflammation and ameliorate the process of renal injury during I/R.
The biological actions of extracellular ATP are largely mediated by the purinergic P2X receptors, which include seven subtypes, P2X1–7 (15). Although all these members of the P2X receptor family form nonspecific cation channels on binding to a nucleotide ligand, the P2X7 receptor (P2X7R) has low ATP affinity, requiring 10–100 times higher concentrations of ATP for activation compared with other P2X receptors (34). Its sustained activation can induce membrane permeability or formation of large pores. The P2X7R-dependent cellular membrane changes are important in mediating ATP signaling in several pathophysiological processes such as membrane blebbing and microvesicle shedding (5). Irreversible membrane blebbing disrupts membrane integrity, leading to efflux of vital intracellular molecules and cell death. Microvesicle shedding can mediate ATP-induced IL-1β release from immune cells (5, 40). Altered P2X7R expression and function have been reported to be causally associated with numerous diseases such as cerebrovascular disease, rheumatoid arthritis, and multiple sclerosis (1, 21, 23).
Emerging evidence from animal studies indicates that P2X7R expression/activation has also been linked to renal diseases. For example, P2X7R mRNA increased in the kidneys of a rat model of proliferative glomerulonephritis and this coincided with the onset of proteinuria (38). P2X7R deficiency in mice or treatment with the selective P2X7 antagonist A438079 in rats prevented the development of glomerulonephritis (37). Pharmacological and/or genetic blockade of P2X7R also showed a renoprotective effect in a mouse model of accelerated nephrotoxic nephritis (41) and a rat model of lupus nephritis (43). Recent studies have further suggested that P2X7R contributes to the development of hypertensive and diabetic nephropathy as evidenced by albuminuria and renal interstitial fibrosis and increased serum creatinine levels in P2X7R knockout mice. In addition, unilateral obstruction induced upregulation of P2X7R in renal tubular epithelial cells, and P2X7R knockdown attenuated renal interstitial fibrosis and macrophage infiltration (12), suggesting that P2X7R expression is also associated with the pathogenesis of obstructive nephropathy. Even though P2X7R has been implicated in these forms of kidney injury, it role in AKI is still not well understood.
The present study was undertaken to examine the role of P2XR in a murine model of I/R-induced AKI. By administration of A438079, a selective P2X7 inhibitor at different time points (0, 6, 24 h) after starting reperfusion, we demonstrated that A438079 was effective in protecting against I/R-induced renal dysfunction and renal tubular damage when given within 6 h after onset of reperfusion.
MATERIALS AND METHODS
Chemicals and antibodies.
Antibodies to P2X7R, poly(ADP-ribose)polymerase (PARP), and GAPDH and P2X7R siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-mouse NGAL antibody was purchased from R&D Systems (Minneapolis, MN). Antibodies to phospho-ERK1/2 and ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA). The terminal deoxynucleotidyl transferased UTP-mediated nick-end labeling (TUNEL) assay kit was obtained from Roche (Nutley, NJ). A438079 was purchased from Abcam (Cambridge, MA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Culture of renal proximal tubular cells and siRNA transfection.
Immortalized mouse renal proximal tubular cells (RPTC) were kindly provided by Dr. Elsa Bella-Reuss and were cultured in DMEM/F-12 with 5% FBS at 37°C in 5% CO2 as described in our previous study (33). The siRNA oligonucleotides targeted specifically to mouse P2X7R were used in this experiment. siRNA (750 pmol) was transfected into RPTC (2 × 106) using the Nucleofector Kit V and the Amaxa Nucleofector device according to the manufacturer's instructions (Gaithersburg, MD). In parallel, 750 pmol of scrambled siRNA were used to control for off-target changes in RPTC. After transfection, cells were cultured in DMEM/F-12 for 12 h and then switched to a serum-free medium for an additional 24 h. To examine the effect of H2O2 on the death of RPTC, 1 mM H2O2 was added to the culture for 5 h and then cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in our previous study (33).
Murine model of I/R-induced AKI and A438079 treatment.
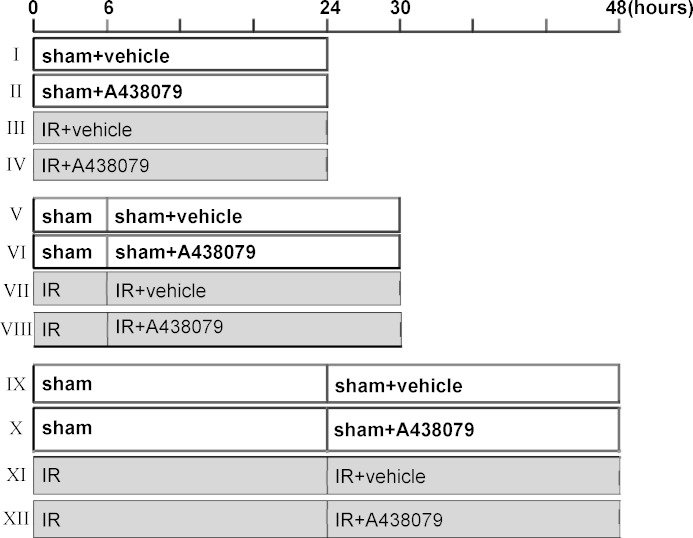
The murine model of I/R injury was established according to the procedures described in our recent studies (36). Mice were anesthetized by ketamine (75 mg/kg ip) and dexdomitor (50 mg/kg im). A flank incision was made on both left and right sides. The bilateral renal arteries and veins were isolated from the surrounding tissue by blunt dissection and then occluded with a nontraumatic vascular clamp (85 g of pressure; RobozSurg Instruments) for 30 min at 37°C. In the sham-operated kidney, used as a control, the renal pedicle was isolated but not clamped. All the mice were divided into three groups and treated with the same volume of either vehicle (DMSO) or A438079 (80 mg/kg ip) at different time points: immediately (I–IV), 6 (V–VIII), or 24 h (IX–XII) after the starting of reperfusion (Fig. 1). Animals were housed for an additional 24 h before being killed for collection of blood and kidneys; five to six animals were used in each group (Fig. 1). All experimental procedures were performed according to the US Guidelines to the Care and Use of Laboratory Animals and approved by the Lifespan Animal Welfare Committee.
Fig. 1.
Experimental design. Experiments were designed to evaluate the effect of A438079 on acute kidney injury (AKI) when it was given at 0 (I–IV), 6 (V–VIII), and 24 (IX–XII) h after onset of reperfusion. I/R, ischemia-reperfusion.
Immunofluorescent and histochemistry staining.
Tissues were fixed in 4.5% buffered formalin, dehydrated, and embedded in paraffin. Sections were stained with periodic acid-Schiff (PAS). For immunofluorescent staining, primary antibodies against NGAL (1:200), P2X7R (1:250), and fluorescent-conjugated secondary antibodies (1:500) were applied to the sections. Examination and scoring of sections from each kidney (n = 3–5 for each condition) were carried out in a blinded fashion. Morphological damage (epithelial necrosis, luminal necrotic debris, and tubular dilation) was quantified using the following scale: none = 0; <10% = 1; 11–25% = 2; 26–75% = 3; and >75% = 4. The histochemistry staining of MCP-1 and RANTES was conducted according to our previous protocol (27) and quantitatively measured using Image Pro-Plus software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of positive staining area and then calculating and graphing the average ratio of positive staining to each microscopic field (×200).
In situ TUNEL assays.
A TUNEL staining kit was used to detect DNA strand breaks according to the instructions provided by the manufacturer. The number of TUNEL-positive nuclei per field was evaluated in five fields per section and five sections per kidney.
Immunoblot analysis.
Immunoblot analysis for tissue samples was carried out according to our previous protocols (46). The densitometry analysis of immunoblot results was conducted by using NIH Image software (National Institutes of Health, Bethesda, MD).
Measurement of renal function.
Renal function was estimated by serum creatinine and blood urea nitrogen (BUN), measured using a colorimetric kit (Sigma Diagnostics) and enzymatic assay kit (Sigma Diagnostics), respectively, according to the protocol provided by the manufacture.
Statistical analysis.
Experiments were conducted at least three times. Data depicted in graphs represent the means ± SE for each group. Intergroup comparisons were made using one-way ANOVA. Multiple means were compared using Tukey's test. The differences between two groups were determined by Student t-test. Statistical significant difference between mean values was marked in each graph. P < 0.05 is considered significant.
RESULTS
Blocking P2X7R with A438079 protects against AKI induced by I/R in mice.
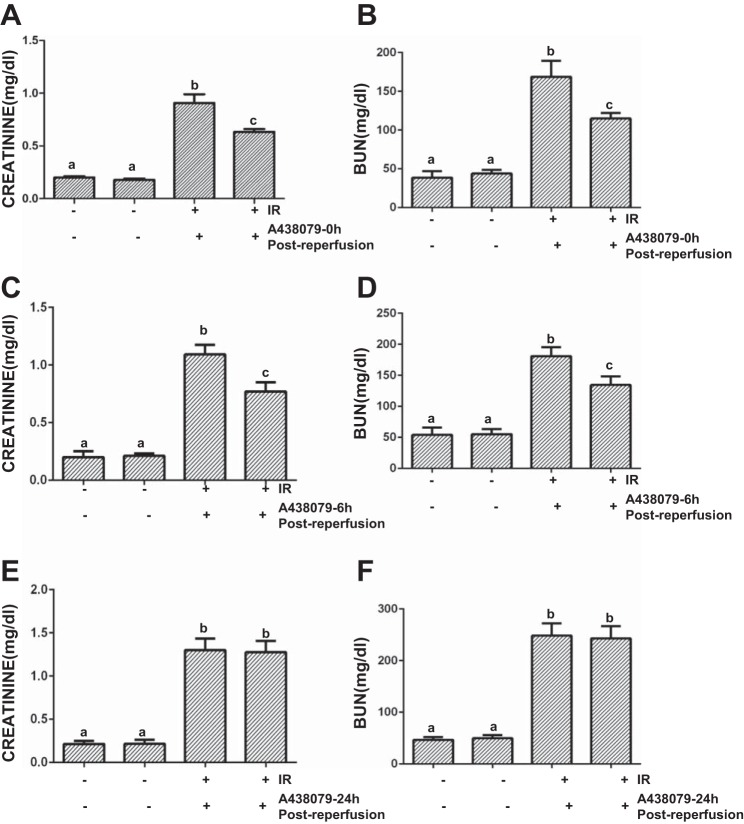
P2X7R activation has been reported to contribute to acute liver and spine cord injury (16, 31). To examine whether P2X7R also plays a role in AKI, we treated I/R-induced AKI in a murine model with A438079, a potent, selective, and competitive P2X7R antagonist (29). A438079 or vehicle was administrated immediately, 6, or 24 h after onset of reperfusion, and then blood and kidney were collected at 24 h after treatment with the antagonist or vehicle (Fig. 1). As shown in Fig. 2, A and B, the BUN and serum creatinine levels were significantly increased in I/R-injured mice compared with sham-operated animals. Administration of A438079 immediately or 6 h after the start of reperfusion significantly reduced blood BUN and serum creatinine levels (Fig. 2, C and D). However, A438079 did not affect blood BUN and creatinine levels when it was given at 24 h after reperfusion (Fig. 2, E and F). These data suggest that P2X7 inhibition can lessen renal dysfunction when A438079 is employed early during reperfusion after ischemia.
Fig. 2.
Inhibition of P2X7 by A438079 meliorates renal dysfunction in I/R-induced AKI in mice. Mice were subject to ischemia for 30 min followed by administration of A438079 or vehicle immediately (0), 6, or 24 h after onset of reperfusion. After treatments for an additional 24 h, blood was collected for measuring serum creatinine (A, C, and D) and blood urea nitrogen (BUN; B, D, and F). Data are represented as the mean ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Blocking P2X7R with A438079 attenuates I/R-induced renal tubular damage in mice.
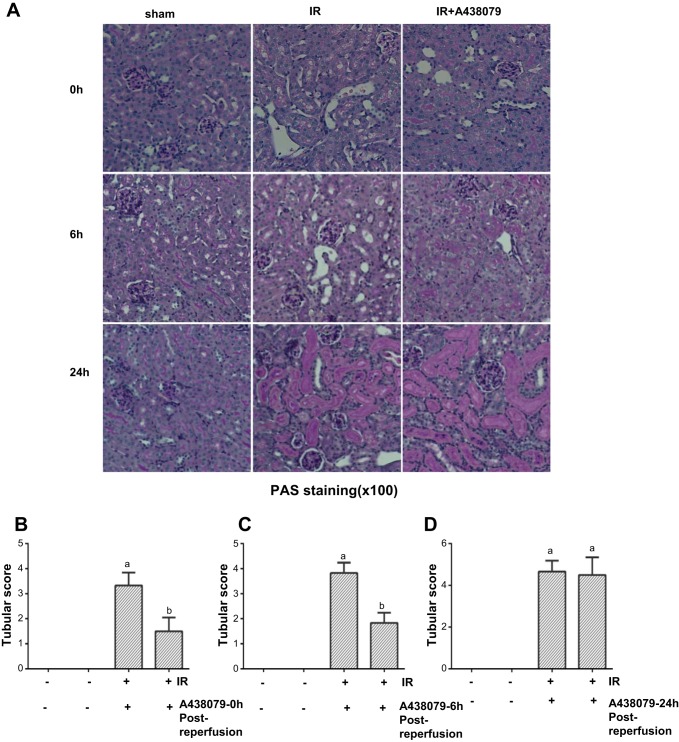
To examine the effect of P2X7R inhibition on renal tubular damage, we did PAS staining and analyzed the pathological changes. At 24 h after I/R injury, the loss of the PAS-positive brush border and scattered cell necrosis, sloughing of tubular epithelial cells, cast formation, and dilation of tubules were clearly observed in some tubules of the injured kidney without treatment of A438079, but only minimal damage of renal tubules was seen in animals given A438079 either immediately or 6 h (early treatments) after onset of reperfusion. After I/R for 48 h, kidneys displayed severe tubular damage with detached tubular cells and loss of brush border; delayed administration of A438079 at 24 h after reperfusion (late treatment) did not attenuate morphological damage of renal tubules (Fig. 3A). Using the semiquantitative scoring system described in materials and methods, we scored the degree of renal damage and also demonstrated a significant reduction in tubular damage after administration of A438079 at the early time compared with the treatment at late time (Fig. 3, B–D). These data illustrated that earlier treatment with A438079 after I/R can protect against renal injury and suggest that P2X7R is an important mediator of injury. Since administration of A438079 immediately or 6 h after reperfusion produced similar renoprotective effects and late treatment with A438079 at 48 h after reflow did not show such effects, we examined the renal protective mechanism of this agent by utilizing kidney tissue collected at 24 h in mice with immediate treatment after reperfusion in the following experiments.
Fig. 3.
A438079 protects against renal morphological damage in the murine model of I/R-induced AKI. After I/R and A43079 treatments as indicated in Fig. 2, the kidneys underwent periodic acid-Schiff (PAS) staining (A). Morphological changes were scored based on the scale described in materials and methods (B, C, and D). Data are represented as the means ± SE (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
I/R injury upregulates P2X7R expression in renal tubular cells and A438079 treatment reduces its expression.
P2X7R is barely expressed in normal kidneys, but its renal expression is increased in animal models of diabetic and hypertensive nephropathy (5). P2X7R expression in the kidney after acute injury is not clear. Figure 4, B and C, shows that a low level of P2X7R was detected in the sham-operated kidney and administration of A438079 did not affect its expression. However, renal P2X7R expression levels were significantly increased after I/R injury, and A438079 treatment reduced its expression. Immunofluorescent staining showed that P2X7R was primarily expressed in renal tubular cells (Fig. 4A). These data indicated that I/R injury can induce P2X7R expression in the kidney through a mechanism involved in self-regulation after I/R injury.
Fig. 4.
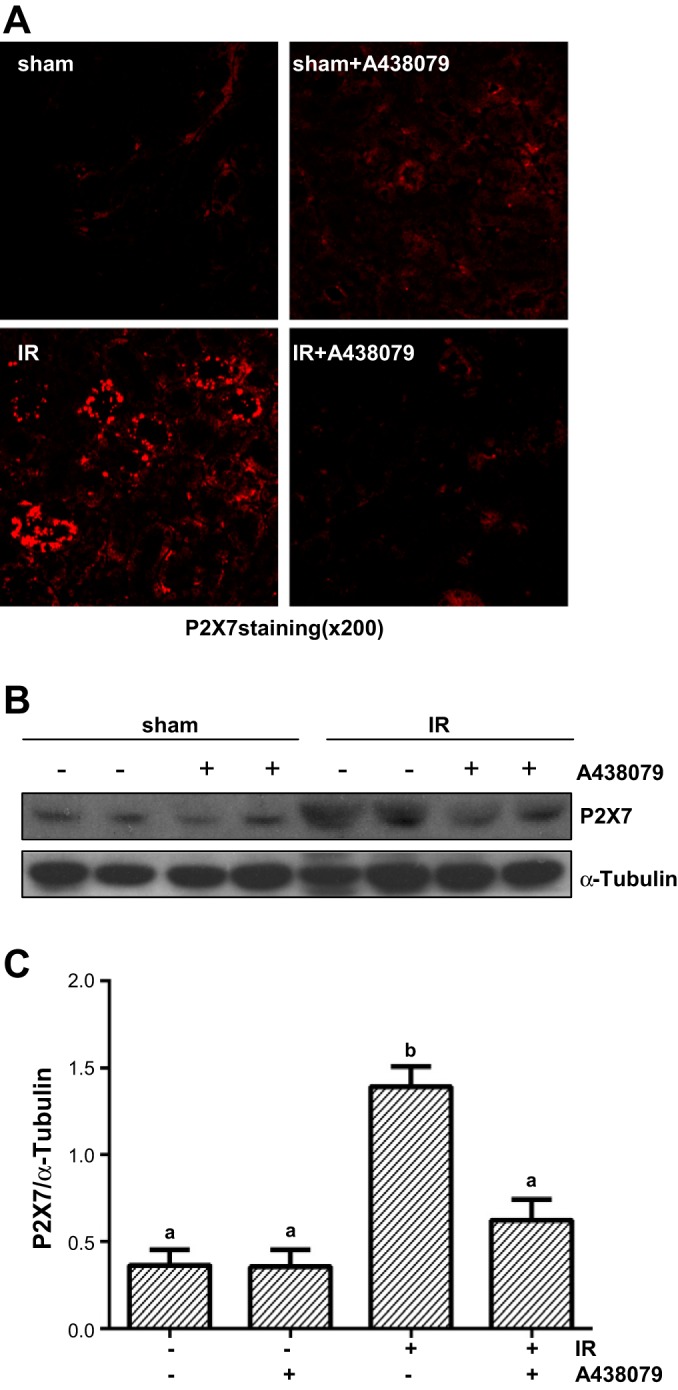
A438079 inhibits I/R-induced expression of P2X7 receptor (P2X7R) in renal tubular cells. Mice were subject to ischemia for 30 min followed by administration of A438079 immediately at onset of reperfusion. 24 h after treatments, kidneys were harvested for immunofluorescent staining of P2X7R (A) and the whole kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against P2X7R and α-tubulin (B). Representative immunoblots are 2 samples from 6 animals in each group. The expression level of P2X7R was quantified by densitometry and normalized with α-tubulin. Means with different symbols are significantly different from one another (P < 0.05; C).
Inhibition of P2X7R with A438079 reduces NGAL expression in I/R-injured kidneys.
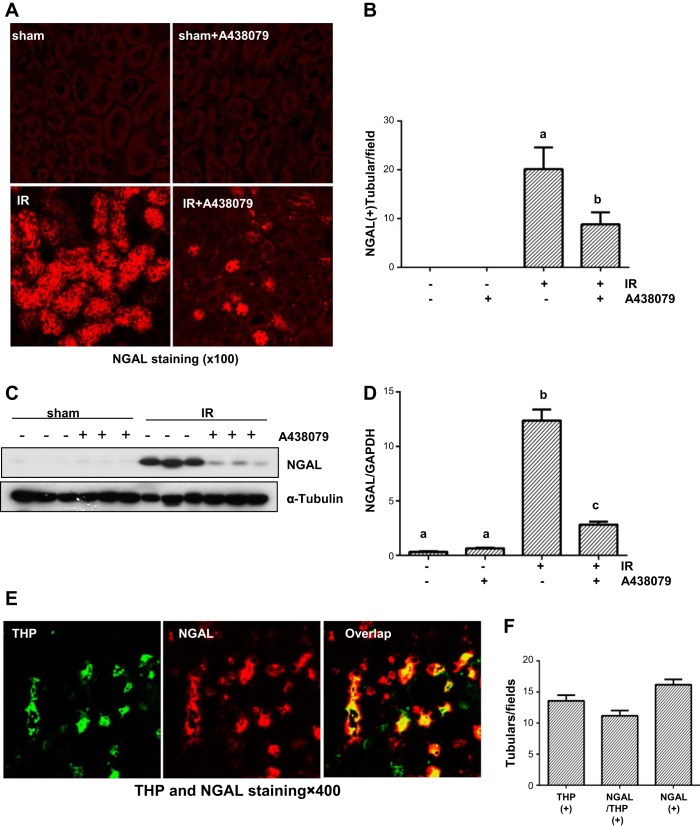
NGAL is a well-known early biomarker of renal tubular injury and is specifically expressed in injured renal tubular cells (39). To further demonstrate the role of P2X7R in renal tubular injury, we further examined NGAL expression in the P2X7R model. Immunostaining showed that NGAL was expressed in the most of renal tubular cells of the injured kidney and treatment with A438079 significantly reduced its expression. As expected, its expression was not detected in the sham-operated kidney with and without administration of A438079 (Fig. 5, A and B). The same results were also observed by Western blot analysis (Fig. 5, C and D). It has been reported that NGAL is primarily expressed in the thick ascending limbs and distal tubules of the nephron (30). By costaining of NGAL with Tamm-Horsfall protein (THP), a marker of thick ascending limb (10), we confirmed an abundant expression of NGAL in this segment of renal tubules after I/R injury (Fig. 5, E and F). However, there were also some tubules expressing NGAL without THP, suggesting that NGAL is also expressed in the distal tubules. Collectively, these data suggest that P2X7R blockade with A438079 is effective in suppressing renal tubular injury.
Fig. 5.
A438079 inhibits I/R-induced expression of neutrophil gelatinase-associated lipocalin (NAGL) in renal tubular cells. Mice were subject to ischemia for 30 min followed by administration of A438079 immediately at onset of reperfusion. Twenty-four hours after treatments, kidneys were harvested for immunofluorescent staining of NGAL (A) and tubules expressing NGAL were counted in 3 fields (1 × 100) of each kidney from 3 kidneys (B). The whole kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against NGAL and α-tubulin (C). Representative immunoblots are 3 samples from 6 animals in each group. The expression level of NGAL was quantified by densitometry and normalized with α-tubulin (D). Means with different symbols are significantly different from one another (P < 0.05). The section of I/R-injured kidney was costained with antibodies to Tamm-Horsfall protein (THP) and NGAL (E). Tubular cells with positive staining for both THP and NGAL, or THP, or NGAL alone were counted in 5 high-power fields and expressed as means ± SE (F).
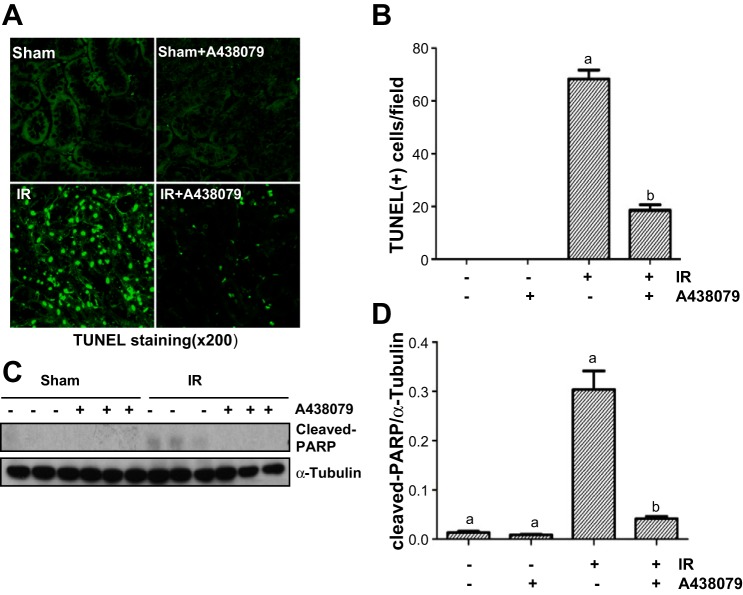
A438079 treatment inhibits renal tubular death in I/R-injured kidneys.
After I/R injury, renal tubular cells die by necrosis and apoptosis (14, 25). To examine whether P2X7R is necessary for regulation of renal tubular cell death, we conducted TUNEL staining to investigate the effect of A438079 on cell death. Figure 6, A and B, shows that the I/R-injured kidney displayed a large number of TUNEL-positive tubular cells; A438079 treatment significantly reduced this population of cells. To further demonstrate the effect of P2X7 inhibition on necrosis, we performed immunoblot analysis to examine the effect of A438079 on the expression of PARP at 55-kDa fragment, which is an hallmark of necrotic cell death (8, 11). As shown in Fig. 6, C and D, A438079 was also effective in suppressing cleavage of PARP to this fragment, suggesting that P2X7R plays a critical role in cell death of renal tubules after I/R-induced AKI. In line with this observation, we also demonstrated that either inhibition of P2X7R with A438079 or silencing of P2X7R with its specific siRNA protected cultured RPTC against oxidant injury. P2X7R protein levels were successfully downregulated in cells treated with its siRNA (Supplemental Fig. S1; Supplemental Material for this article is available online at the Journal website).
Fig. 6.
A438079 inhibits I/R-induced renal epithelial cell death and cleavage of poly(ADP-ribose)polymerase-1 (PARP-1). Mice were subject to ischemia for 30 min followed by administration of A438079 immediately at onset of reperfusion. Twenty-four hours after treatments, kidneys were harvested for terminal deoxynucleotidyl transferased UTP-mediated nick-end labeling (TUNEL) staining (A). Positive TUNEL staining cells were counted in 10 high-power fields and expressed as means ± SE (B). The whole kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against the PARP-1 fragment at 55 kDa and α-tubulin (C and D). The expression level of cleaved PARP-1 was quantified by densitometry and normalized with α-tubulin. Data are means ± SE (n = 6). Representative immunoblots are 2 samples from 6 animals in each group. Means with different symbols are significantly different from one another (P < 0.05).
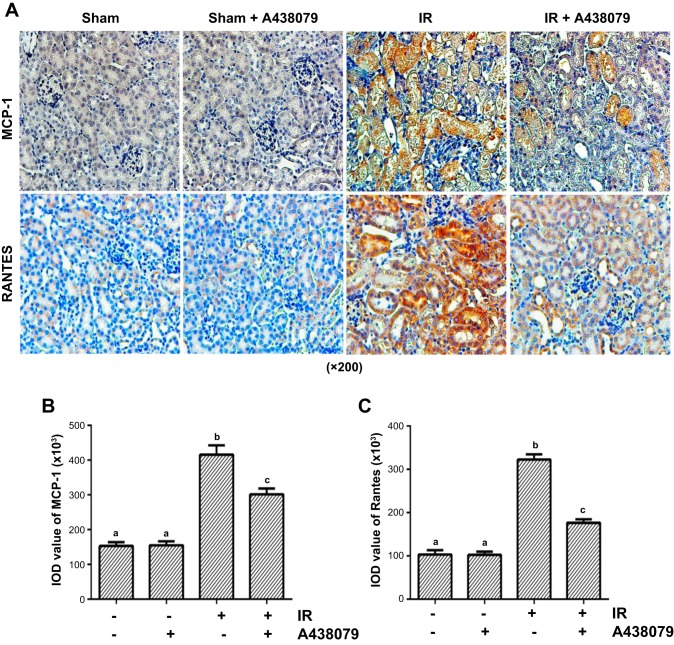
Administration of A438079 reduces expression of MCP-1 and RANTES in renal tubular cells after I/R injury.
Sterile inflammation during I/R is characterized by accumulation of inflammatory cells (6). Previous studies have revealed the role of P2X7R in mediating expression of interleukin-1β (5). To demonstrate whether this purinergic receptor is also involved in regulating expression of chemokines and accumulation of inflammatory cells, we first examined the effect of A438079 on the expression of MCP-1 and RANTES, two important chemokines in the kidney after I/R injury by histochemistry staining. I/R injury to the kidney resulted in increased expression of MCP-1 and RANTES in renal tubular cells and treatment with A438079 significantly reduced their expression when given immediately at the end of ischemia (Fig. 7) or 6 h after reperfusion (data not shown). However, A438079 did not show any inhibitory effect on the expression of these two chemokines when given 24 h after reperfusion (data not shown). Taken together, these data suggest that P2X7R activation is critically involved in the expression of chemokines in the kidney after I/R injury.
Fig. 7.
A438079 inhibits I/R-induced expression of monocyte chemotactic protein-1 (MCP-1) and regulated upon expression normal T cell expressed and secreted (RANTES) in the kidney. Mice were subject to ischemia for 30 min followed by administration of A438079 immediately at onset of reperfusion. At 24 h after treatments, kidneys were harvested for histochemical staining of MCP-1 and RANTES (A). The expression level of MCP-1 and RANTES was quantified by densitometry. Data are represented as the means ± SE (n = 6). Means with different symbols are significantly different from one another (P < 0.05; B and C).
Blocking P2X7R with A438079 inhibits I/R-induced ERK1/2 phosphorylation in the kidney.
Our previous study showed that ERK1/2 activation is implicated in both apoptotic and necrotic cell death in cultured RPTC (44, 45). As P2X7R activation also mediates these two types of cell death, we further examined the effect of P2X7R inhibition on I/R-induced activation of ERK1/2 in the kidney. A basal level of ERK1/2 phosphorylation was detected in the sham-operated kidney, and the expression levels were further increased in response to I/R injury (Fig. 8). A438079 treatment significantly reduced ERK1/2 phosphorylation. Therefore, P2X7R plays an essential role in the regulation of ERK1/2 activation and ERK1/2 may mediate, at least in part, actions of P2X7R.
Fig. 8.
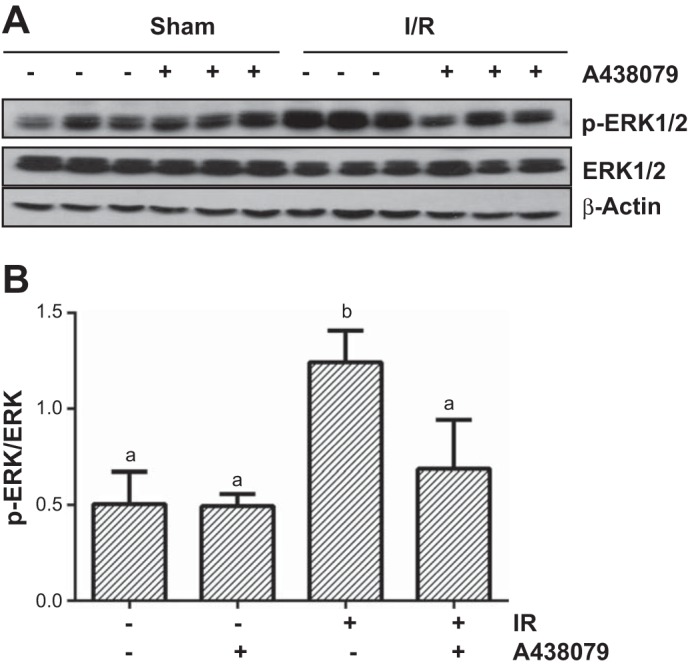
A438079 administration inhibits I/R-induced phosphorylation of ERK1/2 in the kidney. Mice were subject to ischemia for 30 min followed by administration of A438079 immediately at onset of reperfusion. At 24 h after treatments, kidneys were harvested and the whole kidney tissue lyates were used for immunoblot analysis with specific antibodies against p-ERK1/2 and ERK1/2 (A). Representative immunoblots are 3 samples from 6 animals in each group. The expression level of ERK1/2 was quantified by densitometry and normalized with β-actin. Means with different symbols are significantly different from one another (P < 0.0; B).
DISCUSSION
Previous studies have shown that P2X7R contributes to renal injury and inflammation in animal models of glomerular nephritis (37, 41, 43) and several chronic kidney diseases including diabetic, hypertensive, and ureteral obstructive nephropathy (12, 19, 20). However, its role in AKI remains unclear. In this study, we demonstrated that expression of P2X7R was increased in the kidney after I/R injury and administration of A438079, a selective inhibitor of P2X7R, within 6 h after onset of reperfusion improved renal function, attenuated renal tubule damage, and inhibited cell death. The early P2X7R inhibition also suppressed production of chemokines (i.e., MCP-1, RANTES) in the injured kidney. However, late treatment with this agent (24 h after reperfusion) did not show any beneficial effects on I/R-induced renal dysfunction and tubular damage. These results suggest that P2X7R activation plays an essential role in accelerating AKI by potentiating renal tubular cell death and inflammatory response and that P2X7R inhibition protects against ischemic AKI in mice. To our knowledge, this is the first time that P2X7R has been identified as a mediator of AKI.
The P2X7R is normally expressed in the cells of the immune system, and there is very little expression in normal kidney tissue (5). However, under pathological conditions, its expression is upregulated at sites of tissue damage and inflammation in the kidney. For example, a high level of the P2X7R was detected in the glomeruli of diabetic and hypertensive rats (19, 20) and in several cell types such as podocytes in response to stresses, cultured mesangial cells in response to TNF-α, and renal interstitial fibroblasts upon exposure to necrotic renal tubular cells (5, 15, 33). In this study, we demonstrated that I/R injury increased expression of P2X7R in the kidney; immunostaining indicated that it was localized in renal tubular cells, in particular, the thick ascending limbs. In line with our observations, P2XR was also mainly detected in the renal epithelial cells in the cortex of murine kidney after ureteral obstruction and in the outer medulla of kidney biopsy tissue from patients with lupus nephritis (12, 43). Currently, the mechanism that regulates expression of P2X7R is not fully understood. As P2X7 gene contains putative binding sites for Elk1, a transcriptional factor that is regulated by the MEK-ERK cascade (42), and we have recently revealed that ERK1/2-mediated phosphorylation of Elk1 is required for P2X7R expression in renal fibroblasts (32), it is possible that ERK1/2 might also play a role in regulating P2X7R expression in the kidney after I/R injury. Additional studies are needed to address this issue in vivo.
Our data indicate that P2X7 mediates renal tubular cell death and AKI. This was demonstrated by the following observations: 1) I/R injury increased P2X7R expression in renal tubule cells; 2) blocking P2X7R with A438079 attenuated renal tubular damage; 3) A438079 treatment suppressed expression of NGAL, a hallmark of acute tubule injury and inhibited renal epithelial cell death; and 4) siRNA-mediated knockdown of P2X7R protected cultured RPTC against oxidant injury. P2X7R-mediated cell death may be through activation of ERK1/2. This is suggested by our observations that activation of the ERK1/2 pathway leads to both renal tubular cell apoptosis (45) and necrosis (44) in response to oxidative stress. In addition, studies in animal models have also shown that blocking ERK1/2 signaling attenuates I/R and cisplatin-induced AKI and promotes renal function recovery (2, 22). As both apoptosis and necrosis of renal epithelial cells can be regulated in a programmed fashion (24, 26), ERK1/2 may act as the important signaling molecules that participate in modulation of these two types of cell death in the kidney. Although it is known that active ERK1/2 is required for the activation of caspase-3, a key signaling molecule in apoptotic pathway and induction of mitochondrial dysfunction in renal epithelial cells exposed to oxidants such as hydrogen peroxide (44, 45), it remains unclear whether ERK1/2 is associated with regulation of the necrotic machinery such as RIPK3-dependent assembly of the necrosome (24, 26). Future studies are needed to elucidate the possible role and mechanisms of ERK1/2 in the modulation of this and other identified necrotic pathways.
Although there is growing appreciation for the role of P2X7R as a modulator of proinflammatory IL-1β processing, less studied is its role in the expression of chemokines. Numerous studies have shown that pharmacological and genetic inhibition of P2X7R leads to reduced infiltration of proinfilammatory cells in the kidney and other organs (3, 4), suggesting that P2X7R may play a role in regulating expression and production of chemokines. In this study, we examined the effect of P2X7R inhibition on the expression of MCP-1 and RANTES, two key chemokines, in the kidney after I/R injury and found that A438079 treatment significantly reduced their expression. Therefore, signaling via P2X7R may allow cells to respond to the events occurring in the extracellular environment, to modulate the transcription of genes involved in cellular inflammatory processes, and to thus regulate expression of cytokines/chemokines.
Drug discovery and preclinical studies have demonstrated the proof of concepts that the targeting P2XR is a promising therapeutics approach (4, 28). Drugs inhibiting P2X7R are currently in Phase 1 and 2 clinical trials to test the efficacy of P2X7R inhibitors in the treatment of osteoarthritis, chronic obstructive pulmonary disease, and inflammatory bowel disease (13, 28). As mentioned above, P2X7R inhibition has also been shown to be effective in treating glomerulonephritis and nephropathies induced by diabetes, hypertension, and ureteral obstruction in animal models. Our current studies have further suggested a pathologic role for P2X7R in AKI. It will be thus interesting to initiate clinical trials to test the efficacy of P2X7R blockade in treating various acute and chronic kidney diseases in the future.
In summary, this study demonstrates that P2X7R inhibition protects against AKI. The protective effect is associated with inhibition of renal tubule cell death and reduction of chemokine production. ERK1/2 may mediate the detrimental effect of P2X7R in the kidney. Thus blocking P2X7R with its selective antagonists would be a promising treatment for AKI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 2RO1DK08506505A1 (to S. Zhuang), National Natural Science Foundation of China Grants 81270778 and 81470920 (to S. Zhuang), and Key Discipline Construction Project of Pudong Health Bureau of Shanghai Grant PWZxk2014-6.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Y., X.Z., J.T., C.J., and E.T. performed experiments; Y.Y. and C.J. analyzed data; Y.Y. and S.Z. prepared figures; Y.Y. and S.Z. drafted manuscript; J.B. and S.Z. conception and design of research; G.B. and S.Z. edited and revised manuscript; R.G., T.C.Z., and S.Z. interpreted results of experiments; S.Z. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72: 2957–2969, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, van de Water B. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol 171: 452–462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves LA, Bezerra RJ, Faria RX, Ferreira LG, da Silva Frutuoso V. Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules 18: 10953–10972, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expet Opin Investig Drugs 20: 897–915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birch RE, Schwiebert EM, Peppiatt-Wildman CM, Wildman SS. Emerging key roles for P2X receptors in the kidney. Front Physiol 4: 262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol 78: 154–163, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8: 31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol 26: 499–513, 2006. [DOI] [PubMed] [Google Scholar]
- 10.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 8: 588–594, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Goncalves RG, Gabrich L, Rosario A Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M Jr. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70: 1599–1606, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Expert Opin Ther Pat 20: 625–646, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman KA, Burnstock G, Unwin RJ. The P2X7 ATP receptor in the kidney: a matter of life or death? Nephron Exp Nephrol 101: e24–30, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, Mehal WZ. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol 302: G1171–G1179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Yu W, Cui H, Wang Y, Zhang L, Han F, Huang T. P2X7 blockade attenuates mouse liver fibrosis. Mol Med Rep 9: 57–62, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys BD, Rice J, Kertesy SB, Dubyak GR. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem 275: 26792–26798, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res 35: 173–179, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Jiang LH, Baldwin JM, Roger S, Baldwin SA. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front Pharmacol 4: 55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int 67: 458–466, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kvist TM, Schwarz P, Jorgensen NR. The P2X7 receptor: a key player in immune-mediated bone loss? ScientificWorldJournal 2014: 954530, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant 27: 3412–3419, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Linkermann A, Green DR. Necroptosis. N Engl J Med 370: 455–465, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu N, He S, Tolbert E, Gong R, Bayliss G, Zhuang S. Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS One 7: e36194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Castejon G, Pelegrin P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Invest Drugs 21: 995–1007, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem 49: 3659–3666, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D'Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA 106: 12489–12493, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol 301: F650–F659, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem 272: 5482–5486, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Sun SH, Lin LB, Hung AC, Kuo JS. ATP-stimulated Ca2+ influx and phospholipase D activities of a rat brain-derived type-2 astrocyte cell line, RBA-2, are mediated through P2X7 receptors. J Neurochem 73: 334–343, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183: 160–172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22: 386–395, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 28: 254–273, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol 170: 5728–5738, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Vonend O, Turner CM, Chan CM, Loesch A, Dell'Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 23: 213–216, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum 65: 3176–3185, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Therap 325: 732–740, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol 292: F440–F447, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang S, Yan Y, Han J, Schnellmann RG. p38 kinase-mediated transactivation of the epidermal growth factor receptor is required for dedifferentiation of renal epithelial cells after oxidant injury. J Biol Chem 280: 21036–21042, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.