Abstract
In mammals, glycogen synthase kinase (GSK)3 comprises GSK3α and GSK3β isoforms. GSK3β has been shown to play a role in the ability of kidneys to concentrate urine by regulating vasopressin-mediated water permeability of collecting ducts, whereas the role of GSK3α has yet to be discerned. To investigate the role of GSK3α in urine concentration, we compared GSK3α knockout (GSK3αKO) mice with wild-type (WT) littermates. Under normal conditions, GSK3αKO mice had higher water intake and urine output. GSK3αKO mice also showed reduced urine osmolality and aquaporin-2 levels but higher urinary vasopressin. When water deprived, they failed to concentrate their urine to the same level as WT littermates. The addition of 1-desamino-8-d-arginine vasopressin to isolated inner medullary collecting ducts increased the cAMP response in WT mice, but this response was reduced in GSK3αKO mice, suggesting reduced responsiveness to vasopressin. Gene silencing of GSK3α in mpkCCD cells also reduced forskolin-induced aquaporin-2 expression. When treated with LiCl, an isoform nonselective inhibitor of GSK3 and known inducer of polyuria, WT mice developed significant polyuria within 6 days. However, in GSK3αKO mice, the polyuric response was markedly reduced. This study demonstrates, for the first time, that GSK3α could play a crucial role in renal urine concentration and suggest that GSK3α might be one of the initial targets of Li+ in LiCl-induced nephrogenic diabetes insipidus.
Keywords: glycogen synthase kinase 3α, glycogen synthase kinase 3β, urine concentration, vasopressin, lithium
regulation of water homeostasis is a critical function of the kidneys. When arginine vasopressin (AVP) binds to its V2 receptors on principal cells of renal collecting ducts, it leads to activation of adenylate cyclase, which triggers an elevation of cAMP. cAMP-mediated activation of PKA leads to increased trafficking of aquaporin (AQP)2 to the apical membrane, resulting in water reabsorption from the tubular fluid and the production of concentrated urine (1, 27, 47). Recent studies have demonstrated that the glycogen synthase kinase (GSK)3 family of serine/threonine protein kinases plays an important role in renal urine concentration (34). This concept originated from the observation that Li+, a common treatment for bipolar disorders that causes an AVP-insensitive form of urinary concentrating defect, is also an effective inhibitor of GSK3 (10, 30, 32, 37, 40, 42, 49).
The mammalian GSK3 isoforms, GSK3α and GSK3β, were thought to be redundant in their function because they share 98% sequence identity in their catalytic domains (17). Moreover, deletion of all four alleles of GSK3α and GSK3β was required to significantly increase β-catenin, a substrate negatively regulated by GSK3, suggesting that the isoforms could compensate for each other (7). However, recent studies have also shown that GSK3 isoforms could have unique functions. For example, in Drosophila carrying a mutation in ZW3/Shaggy, the fly ortholog of mammalian GSK3, GSK3β rescued the frizzled phenotype more efficiently than GSK3α (39). Similarly, in mice, GSK3β global knockout (GSK3βKO) causes lethality, whereas mice lacking GSK3α are viable and fertile, suggesting that GSK3 isoforms are not functionally redundant during embryonic development (13, 18, 26). Postnatally, GSK3α is critical for the heart's ability to respond to hemodynamic stress and aging in mice (51, 52). GSK3α and GSK3β isoforms also exhibit distinct functions in cardiac cell proliferation, glucose tolerance, glycogen synthesis, and regulation of transcription factors (18, 25, 26, 33). Thus, it is evident that GSK3α and GSK3β could have both overlapping as well as functionally distinct roles.
A major bottleneck in the study of GSK3 has been the absence of isoform-selective inhibitors, forcing analysis to genetic models. In the kidney, our previous studies and several other reports have demonstrated that LiCl treatment inhibits renal GSK3β activity and reduces AQP2 protein levels (5, 9, 19–21, 29, 35, 37), suggesting that GSK3β may be important for the regulation of renal urine concentration and Li+-induced nephrogenic diabetes insipidus (NDI) (34, 38, 46). However, Li+ is an isoform nonselective GSK3 inhibitor (41), and proof that inhibition of GSK3 is an essential mechanism by which Li+ causes NDI is lacking. Direct evidence for the role of the GSK3β isoform in renal urine concentration was provided by our study using renal collecting duct-specific GSK3βKO mice (36). The maximal urinary concentrating capacity of these mice in response to water deprivation or treatment with 1-desamino-8-d-arginine vasopressin (dDAVP; AVP analog) was reduced owing to reduced adenylate cyclase activity, cAMP generation, and AQP2 expression and trafficking. While it was evident that AVP signaling was impaired, GSK3βKO mice under normal conditions did not exhibit polyuria. This suggested that GSK3α may compensate for the loss of GSK3β in GSK3βKO kidneys and that GSK3α plays an unappreciated role in the regulation of renal urinary concentration and Li+-induced NDI. In the present study, we examined whether GSK3α is important for renal urine concentration. We show here that genetic depletion of GSK3α results in reduced urine concentrating capacity in mice.
MATERIALS AND METHODS
GSK3αKO mouse.
The GSK3αKO mouse has been previously described by MacAulay et al. (26). All animal experiments were approved and performed in accordance with guidelines of the University of Kansas Medical Center and Vanderbilt University Medical Center's Institutional Animal Care and Use Committee.
Metabolic cage experiments.
To measure baseline 24-h water and food intake and urine output, mice were housed in metabolic cages (Hatteras Instruments) with free access to food and water. Mice were acclimatized for 3 days. For LiCl treatment experiments, mice were injected with LiCl (4 mmol·kg−1·day−1) by daily intraperitoneal injections. For water deprivation experiments, water bottles were removed for 18 h (from 3:00 PM to 9:00 AM the next day), and spot urine samples were collected before and after water deprivation. Osmolality of urine and plasma was measured by the freezing point depression method using Osmett II (Precision Systems, Natick, MA). Plasma and urine concentrations of Na+, creatinine, urea, and plasma Li+ were measured using Vitros 950 (Johnson & Johnson) and a BWP XP flame photometer (BWB Technologies).
Immunoblot analysis.
Cortical and medullary tissues were homogenized in RIPA buffer and centrifuged at 1,000 g for 15 min at 4°C. Samples were run on 12% polyacrylamide gels (Bio-Rad Protean II). Proteins were transferred to a nitrocellulose membrane (Hybond ECL RPN 3032D, Amersham Pharmacia Biotech) and blocked with 5% nonfat dry milk in PBS with Tween 20 (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, and 0.1 Tween 20, adjusted to pH 7.4). After being washed in PBS with Tween 20, blots were incubated with primary antibodies overnight at 4°C. The antigen-antibody complex was visualized with horseradish peroxidase-conjugated secondary antibodies using the enhanced chemiluminescence system (Amersham Pharmacia Biotech). Immunolabeling controls were performed using peptide-absorbed antibody.
Immunostaining.
Kidneys were cut, fixed overnight in 4% paraformaldehyde at 4°C, washed in PBS, dehydrated in a graded alcohol series, and embedded in paraffin for histological analysis as previously described (14). For immunohistochemistry, 5-μm tissue sections were boiled in Target Retrieval solution (Dako, Carpinteria, CA) to unmask antigens. For immunofluorescence staining, slides were heated in a steamer for 20 min in citrate buffer (pH 6.0) to unmask antigens. Samples were blocked with 10% goat serum (Invitrogen) and then incubated overnight with primary antibody (GSK3α, GSK3β, and AQP2). After being washed with PBS, slides were incubated with appropriate fluorescence-labeled secondary antibody (Invitrogen). 4′,6-Diamidino-2-phenylindole was applied to visualize nuclei. Finally, slides were mounted with Fluoromount G (SouthernBiotech). Images were captured with a Nikon Eclipse E600 microscope and a Nikon DXM1200 digital camera and a Leica DMRE or Nikon 80i microscope. The observer was unaware of the protocol assignment of mice.
Primary antibodies.
Antibodies against GSK3α, GSK3β, cAMP response element-binding protein (CREB), and phosphorylated (p)CREB (Cell Signaling Technologies, Danvers, MA) as well as β-actin (Sigma-Aldrich, St. Louis, MO), AQP-2 (H7661) (31), pSer256-AQP2 (KO407) (3), and Tamm-Horsfall protein (Santa Cruz Biotechnology) were used.
Quantitative real-time PCR.
Quantitative PCR on RNA isolated from whole kidney samples was carried out as previously described (36) using an Applied Biosystems ViiA 7 Real-time PCR system. 18S, AQP2, GSK3α, and GSK3β probes were purchased from Applied Biosystems (Foster City, CA). To measure mRNA levels of the vasopressin type 2 receptor (V2R), the following primers were designed: forward 5′-GAGGGAGGAATGACAGGAAG-3′ and reverse 5′-GAGGAGAATAGGCAACAGAGG-3′, and β-actin used as a control.
Enzyme immunoassays for cAMP and vasopressin measurements.
Urine samples were centrifuged for 5 min at 13,000 rpm and diluted appropriately with enzyme immunoassay buffer. Urinary cAMP, creatinine (Cayman Chemical, Ann Arbor, MI), and vasopressin (Enzo Biochemical, Farmingdale, NY) were measured following the manufacturers' instructions.
cAMP measurements in acutely isolated inner medullary collecting ducts.
Ten-week-old wild-type (WT) or GSK3αKO mice were euthanized, and acutely isolated inner medullary collecting ducts (IMCDs) were obtained essentially as previously described (43). cAMP generation in response to 1 μM dDAVP was measured as previousy described with minor changes (36). The tubules obtained were suspended in prewarmed serum-free DMEM-F-12 containing 1 mM 3-isobutyl-1-methylxanthine and incubated at 37°C for 30 min followed by 1 μM dDAVP in a total reaction volume of 0.5 ml and incubated at 37°C for 10 min. The tubule suspension was centrifuged, and the tissue pellet was lysed in 0.1 M HCl, incubated at room temperature for 30 min, and centrifuged at 10,000 rpm for 10 min. The supernatant was directly used for cAMP estimation using a cAMP EIA Kit (Cayman Chemicals) according to the manufacturer's instruction. Total protein levels were measured in the supernatant, and cAMP levels were expressed per milligram of protein.
Measurement of urine PGE2.
Twenty-four-hour urine samples were collected from mice housed in metabolic cages as described above. Urine samples were centrifuged for 5 min at 10,000 rpm, and urinary PGE2 concentrations were measured using the Prostaglandin E2 EIA Kit (monoclonal) according to the manufacturer's instructions. Concentrations of PGE2 are expressed as picograms of PGE2 per 24-h urine volume per gram body wt.
mpkCCD-C14 cell lines stably transfected with doxycycline-inducible GSK3α short hairpin RNA.
Tripz GSK3α short hairpin (sh)RNA plasmid (Thermo Scientific), which encoded a doxycycline-inducible shRNA, and pMD2.g and psPAX2 lentiviral particle packaging plasmids were cotransfected using Lipofectamine 2000 transfection reagent in human embryonic kidney-293 cells according to the manufacturer's protocol. Lentiviral particles were collected after 48 h, filtered, and stored.
GSK3α shRNA-lentiviral transduction and stable mpkCCD cell line selection were conducted according to Tripz vector manufacturer's protocols. Mouse collecting duct cells [mpkCCD-C14 (clone 11)] (48) obtained from Dr. Mark Knepper were grown essentially as previously described (11). During normal growth, cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Transduction efficiency after 1 μg/ml doxycycline was evaluated after 24 h by checking fluorescence-TurboRFP expression. Stably transfected cell lines were selected using 2 μg/ml puromycin. To induce GSK3α shRNA and forskolin treatment, mpkCCD-C14 cells stably transfected with GSK3α shRNA were plated on six-well transwell plates. After 4 days, cells were treated with 1 μg/ml doxycycline for 3 days followed by forskolin treatment for 24 h.
Statistical analysis.
Values are expressed as means ± SE except for measurements of band density of Western blots, which is expressed as means ± SD. Data were analyzed by two-tailed unpaired t-tests with Welch's correction and F-test to compare variances. Bonferroni multiple comparisons were used to compare the effect of Li+ treatment. Graphpad Prism software was used (version 5.0d). P values of ≤0.05 were considered significant.
RESULTS
GSK3α is expressed in renal collecting ducts.
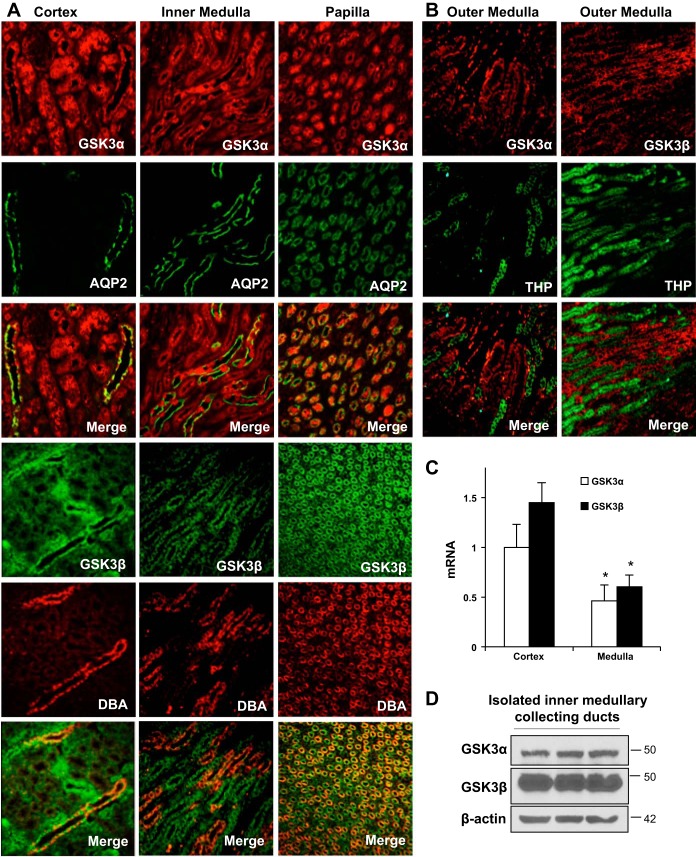
To determine the expression of GSK3α in the kidney relative to GSK3β, its expression pattern, protein, and mRNA levels were examined. In adult WT mouse kidneys, GSK3α and GSK3β were ubiquitously expressed in renal tubules and could be colocalized with AQP2 or Dolichos biflous agglutinin in collecting ducts in both the medulla and cortex (Fig. 1A). However, we did not see much colocalization of GSK3α or GSK3β with Tamm-Horsfall glycoprotein in the thick ascending limb of the loop of Henle in the outer medulla (Fig. 1B). In the renal inner medulla, GSK3α and GSK3β mRNA levels were not significantly different, but both isoforms were significantly lower in the inner medulla than the cortex (Fig. 1C). GSK3α and GSK3β protein levels were also detected in isolated IMCDs (Fig. 1D). Thus, both isoforms of GSK3 are expressed in the kidney, especially in the renal collecting ducts, an important site for water reabsorption and urine concentration.
Fig. 1.
Glycogen synthase kinase (GSK)3α and GSK3β are expressed in adult mouse collecting ducts. A and B: immunofluorescence staining for GSK3α and GSK3β in wild-type (WT) C57Bl/6J mouse kidneys. A: aquaporin 2 (AQP2; green) and Dolichos biflorus agglutinin (DBA; red) staining represent collecting ducts. B: Tamm-Horsfall glycoprotein (THP; green) staining represents the thick ascending limb (TAL). C: GSK3α and GSK3β mRNA relative to 18S. D: Western blot analysis for GSK3α and GSK3β in isolated renal inner medullary collecting ducts (IMCDs). n = 6. *P < 0.05 compared with the cortex.
GSK3αKO mice are polyuric under normal conditions.
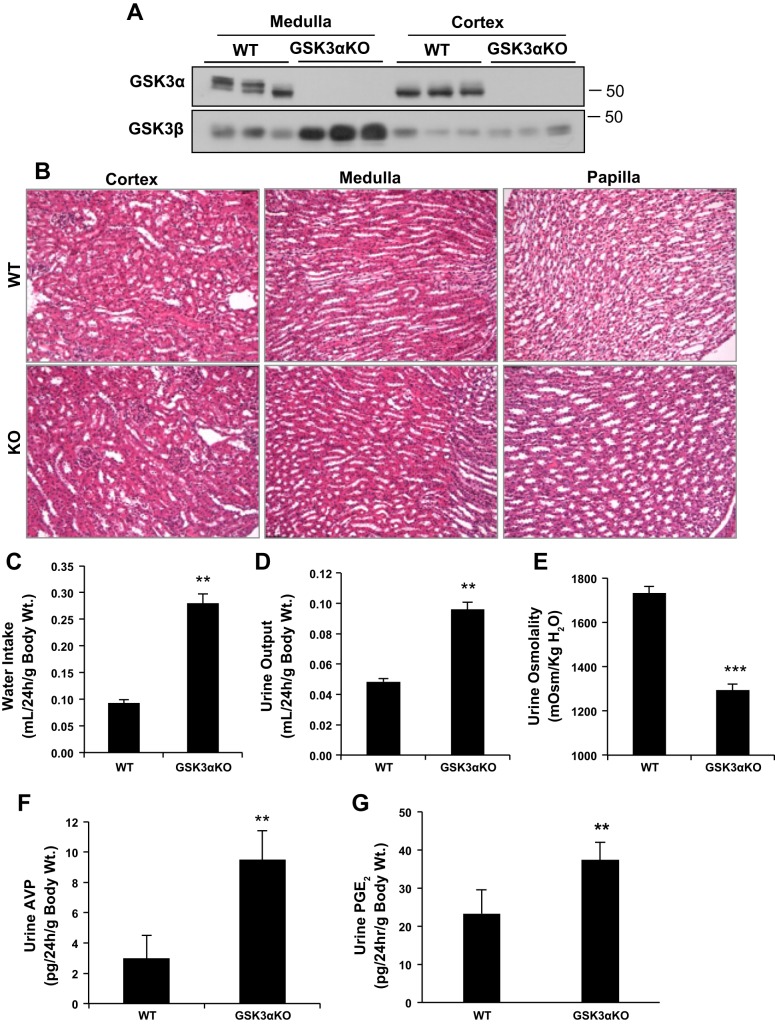
To determine the role of GSK3α in urine concentration, we examined the effect of its gene deletion in GSK3αKO mice. GSK3α was undetectable in the renal cortex and medulla, whereas GSK3β levels were increased in the medulla of GSK3αKO mice (Fig. 2A). No gross morphological abnormalities, such as cysts or dilated tubules, were detected in GSK3αKO kidneys (Fig. 2B). GSK3αKO mice weighed 13% more than WT mice (Table 1), although the reason for this increase is currently unclear. No significant differences in food intake, plasma osmolality, creatinine, urea levels, or urinary excretion of urea and Na+ were detected among the study groups (Table 1).
Fig. 2.
GSK3α knockout (GSK3αKO) mice have polyuria and reduced urine concentrating ability. A: Western blot analysis showing no GSK3α protein in tissue lysates of the medulla and cortex of GSK3αKO mice. GSK3β levels increased in the medulla of GSK3αKO mice compared with WT mice. B: hematoxylin and eosin staining showed no gross morphological abnormalities in GSK3αKO kidneys. C–G: GSK3αKO mice showed increased water intake (C) and urine output (D), reduced urine osmolality (E), and increased urine vasopressin (F) and PGE2 (G) compared with WT mice. n = 8 mice/group. **P < 0.01 and ***P < 0.0001 compared with WT mice.
Table 1.
Baseline measurements in WT and GSK3αKO mice
| Wild-Type Mice | Glycogen Synthase Kinase 3α Knockout Mice | |
|---|---|---|
| Body weight, g | 22 ± 1 | 25 ± 0.7* |
| Food intake, g/24 h | 2 ± 0.3 | 2.7 ± 0.4 |
| Plasma creatinine, μM | 26.5 ± 3 | 22.3 ± 1 |
| Plasma urea, mM | 11.1 ± 1 | 10.2 ± 0.6 |
| Plasma Na+, mM | 155 ± 6 | 149 ± 0.5 |
| Plasma osmolality | 310 ± 6 | 316 ± 6 |
| Urinary urea, μmol·min−1·kg−1 | 28.2 ± 4 | 38.5 ± 5 |
| Urinary Na+, μmol·min−1·kg−1 | 4 ± 0.6 | 5.4 ± 0.3 |
Values are expressed as means ± SE; n = 8–12 mice/group.
P < 0.01.
Under normal conditions, with free access to food and water, water intake and urine output in GSK3αKO mice were elevated three- and twofold, respectively, and urine osmolality decreased by 25% compared with WT littermates (Fig. 2, C–E). Measurement of AVP levels in the urine has been previously reported as a reliable surrogate for circulating AVP levels (28, 50). In GSK3αKO mice, urinary AVP levels were threefold higher compared with WT mice (Fig. 2F), demonstrating that the urinary concentrating defect in these mice is not due to low vasopressin.
We also measured mRNA levels of the AVP receptor (V2R) and protein levels of urea transporter (UT)-A1, which is regulated by AVP (8). However, no significant differences in V2R or UT-A1 levels were observed between WT and GSK3αKO mice (data not shown). GSK3αKO mice had significantly higher urinary PGE2 levels compared with WT mice (Fig. 2G). PGE2 has been demonstrated to antagonize AVP-induced water reabsorption (12). Taken together, these results demonstrate that loss of renal GSK3α is associated with increased PGE2, polyuria, and urine concentrating defect.
Renal AQP2 levels are reduced in GSK3αKO mice.
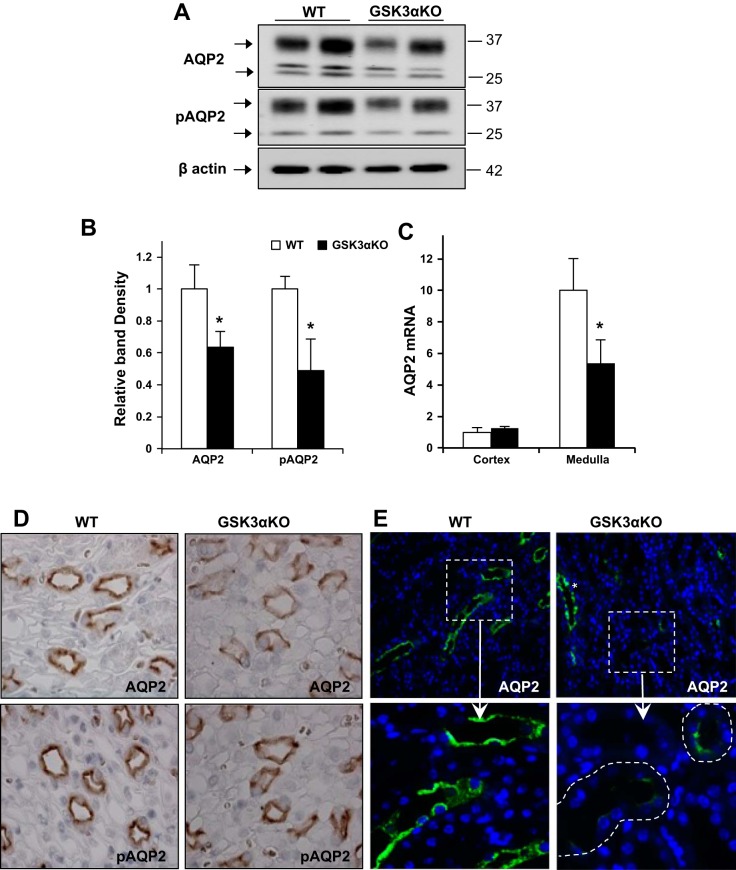
To determine if the impaired urine concentrating ability in GSK3αKO mice was due to changes in renal AQP2 abundance and/or localization, we examined renal AQP2 and pSer256-AQP2 (pAQP2), one of the phosphorylation sites crucial for trafficking (3). In the medulla, AQP2 and pAQP2 protein levels were significantly lower in GSK3αKO mice than in WT mice (Fig. 3, A and B). AQP2 mRNA levels in the renal medulla were also lower in GSK3αKO mice (Fig. 3C). In the cortex, however, AQP2 protein abundance, localization, and AQP2 mRNA levels were unchanged (data not shown). Consistent with Western blot analysis, immunohistochemical staining demonstrated weaker labeling of AQP2 and pAQP2 in medullary collecting ducts of GSK3αKO mice compared with WT mice (Fig. 3D). Importantly, many collecting ducts in the corticomedullary junction of GSK3αKO mice showed no staining for AQP2 (Fig. 3E).
Fig. 3.
AQP2 abundance is reduced in GSK3αKO mice. Renal medullary AQP2 and phosphorylated (p)AQP2 (pSer256 AQP2) levels were reduced in GSK3αKO mice, as demonstrated by Western blot analysis (A) and quantitation of band density (B). C: AQP2 mRNA relative to β-actin was reduced in GSK3αKO mice. n = 6 mice/group. D: immunostaining showing reduced AQP2 and pAQP2 staining in the renal papilla of GSK3αKO mice. E: in the cortico-medullary junction of GSK3αKO kidneys, many collecting ducts showed very limited AQP2 (green; inset). Collecting ducts with normal AQP2 expression could also be seen in the same area (*). Magnification: ×63. *P < 0.05 compared with WT mice.
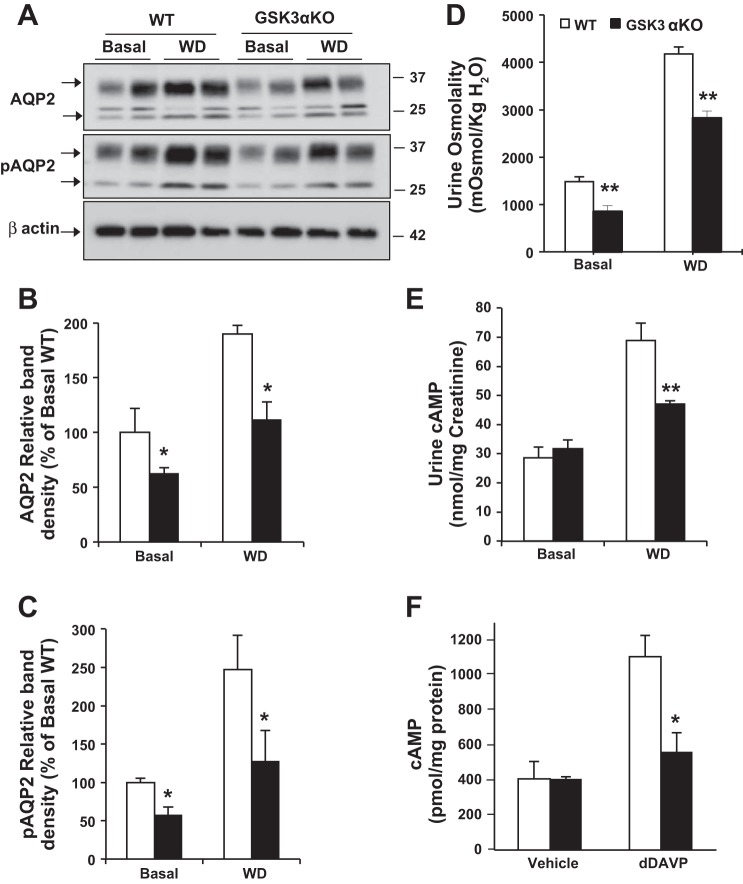
When water deprived for 18 h, medullary AQP2 and pAQP2 protein levels increased significantly in both WT and GSK3αKO mice. The fold increase in AQP2 levels due to water deprivation was not significantly different between WT and GSK3αKO kidneys (1.9- and 1.8-fold, respectively), although pAQP2 levels increased by 2.5-fold in WT kidneys and only 2.1-fold in GSK3αKO kidneys. However, both AQP2 and pAQP2 levels in water-deprived GSK3αKO mice were significantly lower than those in WT mice (Fig. 4, A–C). Consistently, urine osmolality and urinary cAMP levels (16) were lower by 33% and 40%, respectively, in GSK3αKO mice compared with WT mice (Fig. 4, D and E). Likewise, the addition of dDAVP to isolated IMCDs increased the cAMP response in WT mice, but this response was absent in GSK3αKO mice, suggesting a reduced response to AVP in GSK3αKO mice (Fig. 4F).
Fig. 4.
GSK3αKO mice have reduced urine concentrating ability in response to 18-h water deprivation (WD). A: Western blot analysis for AQP2 and pAQP2 in the renal medulla after 18-h WD. B and C: quantitation of band density for AQP2 (B) and pAQP2 (C) levels. D: urine osmolality values of spot urine samples collected before and after WD were lower in GSK3αKO mice. E: urinary cAMP levels were reduced in GSK3αKO mice. F: impaired desamino-8-d-arginine vasopressin (dDAVP)-induced cAMP response in freshly isolated IMCDs. n = 8 mice/group. *P < 0.05 and **P < 0.01 compared with WT mice.
Taken together, these data suggest that lower cAMP levels and AQP2 expression in GSK3αKO mice could have contributed to their reduced urine concentrating capacity.
Gene silencing of GSK3α in mpkCCD-C14 cells reduced AQP2 expression.
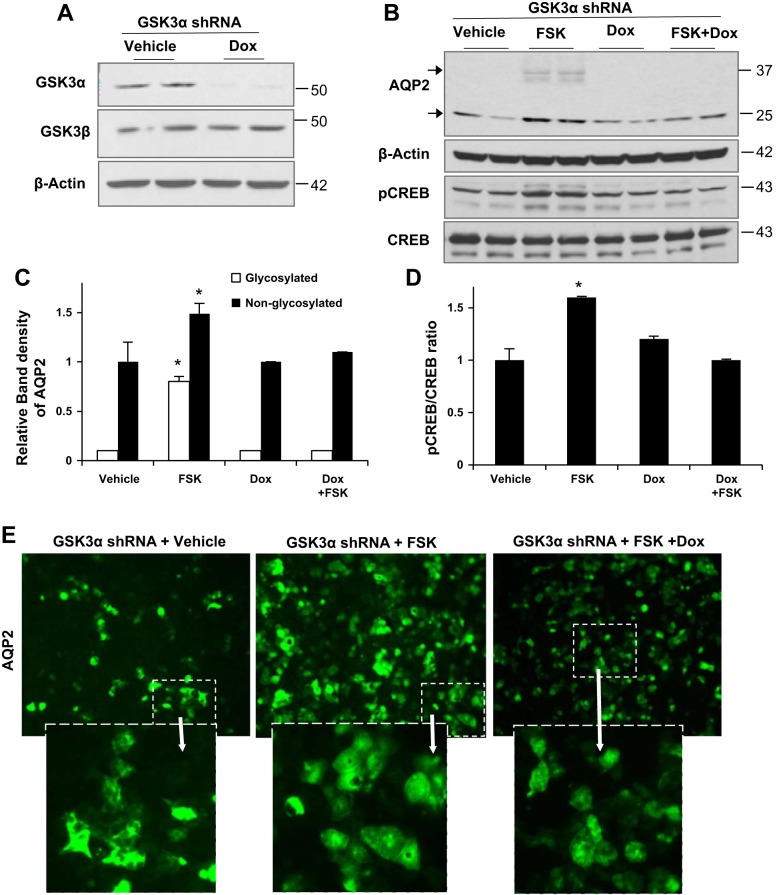
Previous studies have shown that pharmacological inhibition of GSK3 using LiCl, Zn, and 6-bromoindirubin-3′-oxime can reduce AQP2 levels in in vitro cell culture systems (20, 21, 24). However, these inhibitors are incapable of distinguishing between GSK3α and GSK3β isoforms. To test for isoform-specific roles of GSK3α in the regulation of AQP2, we used gene silencing. A cortical collecting duct cell line, mpkCCD-C14, was stably transfected with doxycycline-inducible shRNA for GSK3α. Polarized cells grown on transwells expressed significantly reduced GSK3α protein levels when treated with doxycycline, whereas GSK3β was unchanged (Fig. 5A).
Fig. 5.
GSK3α gene silencing reduces forskolin (FSK)-induced AQP2 expression in mpkCCD-C14 cells. A: mpkCCD-C14 cells stably transfected with short hairpin (sh)RNA for GSK3α [doxycycline (Dox) inducible] showed significant decreases in GSK3α in cells treated with Dox. B: Western blots showing increases in AQP2 and activated pSer133 cAMP response element-binding protein (CREB) levels after FSK treatment (10 μM for 24 h) in vehicle-treated cells but not in Dox-treated cells. C and D: Quantitation of band density of AQP2 (C) and pCREB/total CREB (D). *P < 0.05; treatment and Western blots repeated for a total of n = 6 study replicates. E: immunofluorescence staining showing more AQP2 expression in FSK-treated cells than vehicle-treated or Dox-treated cells.
To examine if GSK3α gene silencing can reduce forskolin-induced AQP2 expression, we treated polarized cells with forskolin in the presence or absence of doxycycline. Forskolin is an agonist of adenylate cyclase and can increase intracellular cAMP levels. Forskolin treatment increased AQP2 in vehicle-treated but not in doxycycline-treated mpkCCD cells (Fig. 5, B and C). We also measured activated CREB (pSer133 CREB) (6). Increased intracellular cAMP and activation of PKA pathway activity can lead to CREB activation. Forskolin treatment increased pCREB levels in vehicle-treated cells but not in doxycycline-treated cells (Fig. 5, B and D). This suggests that cAMP/PKA-mediated pathways are not activated in GSK3α gene-silenced cells to the same extent as normal cells. Immunofluorescence staining revealed that compared with vehicle-treated cells, forskolin-treated cells showed marked increases in labeling of AQP2. Doxycycline-treated cells, on the other hand, showed weaker AQP2 labeling (Fig. 5E). These results suggest that GSK3α can regulate AQP2 expression, and this is consistent with the low AQP2 expression under basal and water-deprived conditions in GSK3αKO mice.
Effect of LiCl treatment on urine concentrating ability in GSK3αKO mice.
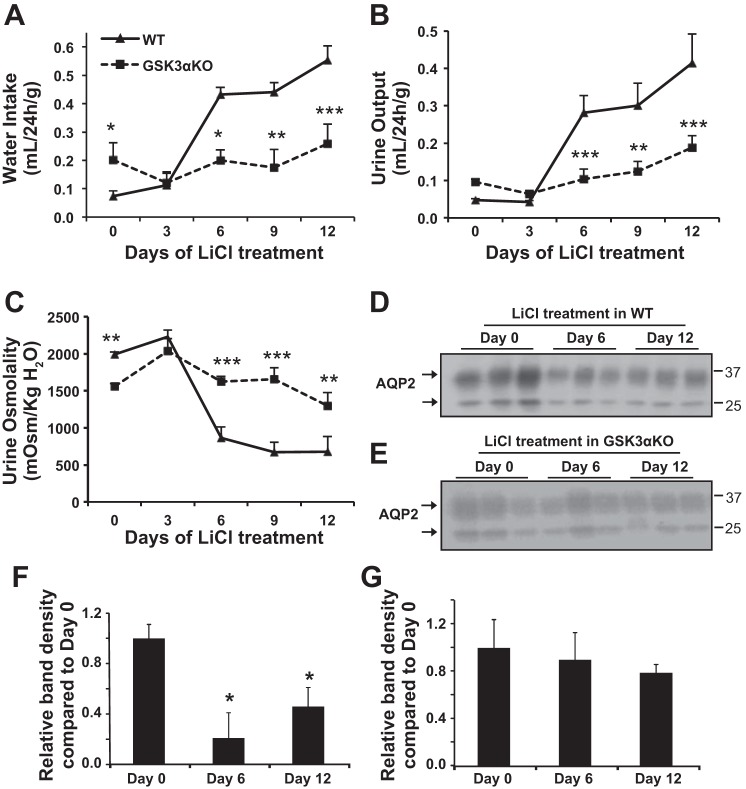
Although GSK3 inhibition has been implicated to be a major cause for LiCl-induced NDI, it is not clear if Li+ causes NDI primarily by inhibiting GSK3 or which GSK3 isoform is a preferential target of Li+. Hence, we examined the effect of LiCl treatment on GSK3αKO mice. LiCl treatment significantly increased water intake and urine output, accompanied by a decrease in urine osmolality in WT mice. In contrast, only small changes were observed in GSK3αKO mice (Fig. 6, A–C). For instance, urine output increased by 5-fold on day 6 and 8.6-fold on day 12 of LiCl treatment in WT mice, whereas in GSK3αKO mice, urine output was unchanged on day 6 and increased by only 2-fold on day 12 compared with baseline controls (Fig. 6B). Renal medullary AQP2 levels in WT mice treated with LiCl for 6 or 12 days were significantly lower than baseline controls (Fig. 6, D and F). In GSK3αKO mice, however, AQP2 levels did not reduce significantly by 6 or 12 days of LiCl treatment (Fig. 6, E and G). Plasma Li+ concentrations were not significantly different in WT and GSK3αKO mice on day 9 of LiCl treatment (WT mice: 2 ± 0.4 mmol/l vs. GSK3αKO mice: 1.7 ± 0.6 mmol/l, n = 6), suggesting that deletion of GSK3α does not alter the absorption of Li+. Plasma Li+ levels were similar to those from an earlier study (37) and in a range that could cause mild toxicity by human standards (45). However, mice did not show any symptoms such as lethargy, loss of body weight, ruffled fur, or diarrhea. Taken together, these data indicate that, in WT mice with intact GSK3α and GSK3β isoforms, LiCl treatment induces NDI, possibly by primarily inhibiting GSK3α. In GSK3αKO mice, which retain GSK3β and display polyuria at baseline, LiCl treatment does not induce NDI in the short term (6 or 9 days). These results demonstrate that GSK3 plays a major role in Li+-induced NDI and suggest that GSK3α could be a primary target of Li+ in the initial increase in polyuria and urinary concentrating defect.
Fig. 6.
Effect of LiCl treatment on GSK3αKO mice. Mice were treated with LiCl (4 mmol/kg body wt) by daily intraperitoneal injection. A–C: 24-h water intake (A), urine output (B), and urine osmolality (C) were measured. Data are means ± SE of n = 9 mice/group. *P < 0.05, **P < 0.01, and ***P < 0.001 for GSK3αKO vs. WT mice by Bonferroni multiple comparisons. D–G: Western blot analysis of renal medullary tissue lysate showing time-dependent changes in AQP2 resulting from LiCl treatment in WT (D) and GSK3αKO (E) mice and quantification of band density for WT (F) and GSK3αKO (G) mice on days 0, 6, and 12 of LiCl treatment. Data are means ± SE of n = 6 mice/group. *P < 0.05, WT vs. GSK3αKO mice.
DISCUSSION
The present study demonstrates, for the first time, that GSK3α is an important regulator of urine concentration by the kidneys and an essential target of LiCl in Li+-induced NDI. Gene deletion of GSK3α resulted in reduced AQP2 expression, polyuria, and urine concentrating defect in mice, and gene silencing of GSK3α reduced forskolin-induced AQP2 expression in cultured mpkCCD-C14 cells. A potential contributing factor to the reduction of AQP2 levels is the impaired cAMP generation in renal collecting ducts. This study also identified GSK3α as an important target for LiCl in Li+-induced NDI.
GSK3α is an important regulator of renal urine concentration.
Due to lack of isoform-selective GSK3 inhibitors and the assumption that GSK3β and GSK3α might be redundant in their functions, neither GSK3α expression nor its function in the kidney have been explored. The present study shows that GSK3α is expressed in renal tubular epithelial cells, especially in renal collecting ducts. Gene knockout of GSK3α leads to reduced AQP2, increased water intake, and the production of larger volumes of urine with low osmolality.
Urine concentrating defect could result from low physiological levels of AVP. However, GSK3αKO mice had higher urinary AVP levels and higher water intake (thirst) at baseline. Despite the higher AVP levels, they had lower urine osmolality, reduced AQP2, and were unable to concentrate their urine in response to water deprivation. These findings suggested that the urine-concentrating defect in GSK3αKO mice could be due to a defective renal response to vasopressin.
GSK3αKO mice had lower urinary cAMP levels after water deprivation, suggesting that they could have a reduced response to AVP. The importance of GSK3α in the cAMP-mediated signaling pathway was also supported by in vitro experiments. In mpkCCD cells, gene silencing of GSK3α reduced forskolin-induced pCREB levels. Since the cAMP-PKA-mediated pathway activates CREB (6), the low pCREB levels suggest reduced cAMP-mediated signaling in GSK3α gene-silenced cells. PKA-induced CREB activation is also known to regulate AQP2 expression (15, 27, 47). In a previous study (22) using mpkCCD cells, dDAVP treatment for 24 h increased AQP2 by a PKA- and pCREB-dependent mechanism, although 4 days of dDAVP treatment increased AQP2 by an alternate mechanism. In the present study, forskolin treatment for 24 h increased pCREB and AQP2 levels in normal mpkCCD cells but not in GSK3α gene-silenced cells. This further supports the observation in mice that gene KO of GSK3α can reduce cAMP levels and AQP2 expression.
The observation that baseline urine concentrating ability is disrupted in GSK3αKO mice is in contrast with our earlier observation in mice lacking GSK3β in collecting ducts, which were normal at baseline (36). This raises the possibility that GSK3α, rather than GSK3β, plays a more important role in renal urine concentration and that GSK3α could be able to compensate for the loss of GSK3β.
The possibility that GSK3αKO mice could have altered water or salt transport in other tubular segments of the nephron or have variations in the levels of hormones other than vasopressin cannot yet be discounted. However, we measured PGE2, and our data demonstrated increased urinary PGE2 excretion in GSK3αKO mice. PGE2 can interact with four different EP receptors (EP1–EP4) in collecting ducts (2), and the inhibitory effect of PGE2 on the AVP-induced increase in water permeability is likely to be mediated by EP1 and/or EP3 (12, 44). Thus, one could speculate that PGE2, possibly via EP1 or EP3, might mediate the inhibition of AVP action in the IMCD, leading to decreased cAMP and AQP2 expression in GSK3αKO mice. Nevertheless, our data indicate that GSK3αKO mice have a urinary concentrating defect of renal origin with the strong possibility that it is due to a reduced response to vasopressin.
Inhibition of GSK3α is key to the development of Li+-induced NDI.
Li+ is a common and effective therapy for bipolar disorders. NDI is the most frequently found undesirable effect of Li+ therapy, and it is found in up to 40% of patients (10, 42). The primary cause of Li+-induced NDI is the decrease in renal AQP2 expression (10), for which several mechanisms have been proposed, including altered AVP/cAMP and cyclooxygenase-2/PGE2 signaling (4, 21, 23, 24, 37).
Multiple studies have demonstrated that inhibition of GSK3β is involved in Li+-induced NDI. This is based on observations that 1) Li+ inhibits GSK3β kinase activity in mouse kidney and cortical collecting duct cells (19–21, 29, 35–37); 2) inhibition of GSK3β by LiCl or GSK3-specific inhibitors can reduce dDAVP-induced AQP2 protein levels in mpkCCD cells (21, 24); 3) inhibition of renal GSK3 coincides with the decrease in AQP2 expression as well as the increase in polyuria in LiCl-treated mice (29, 37); and 4) GSK3β in renal collecting ducts is important for urine concentration (36). However, clear proof that Li+ causes NDI, primarily by inhibiting GSK3, is lacking. Hence, we compared the effect of LiCl on WT and GSK3αKO mice.
The baseline NDI type of phenotype demonstrated by GSK3αKO mice combined with a compensatory increase in vasopressin levels and lower AQP2 are comparable to features of Li+-induced NDI (10, 38, 45), although only to a moderate level. LiCl treatment caused severe NDI and reduced renal AQP2 levels in WT mice within 6 days, consistent with earlier reports on Li+-induced NDI in mice (16, 29, 37). However, in GSK3αKO mice, the effect of LiCl treatment was abolished until 9 days after the start of LiCl treatment, and the onset of mild Li+-induced NDI was evident only by 12 days of treatment. As such, our data suggest that GSK3α could be an important target of LiCl.
Conclusions.
The results of the present study demonstrate that GSK3α is important for renal urine concentration and that this isoform may be relatively more important than GSK3β for the regulation of water homeostasis. The present study also revealed that GSK3 inhibition could be a major mechanism for Li+-induced NDI and GSK3α to be a preferential target for Li+. A better understanding of the distinct roles of GSK3α and GSK3β would be beneficial in designing isoform-selective drugs, which could avoid dehydration in patients on Li+ therapy.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-083525 and American Society of Nephrology-Carl W. Gottschalk Research Scholar grants to R. Rao, Canadian Institutes of Health Research Grant MOP 74711 to J. R. Woodgett and The Lundbeck Foundation, and the Helen and Ejnar Bjørnow Foundation, The NOVO Nordisk Foundation, and The AP Moller Foundation to R. Nørregaard.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.N. and R.R. conception and design of research; R.N., S.T., L.N., V.K., C.H., and R.R. performed experiments; R.N., S.T., V.K., and R.R. analyzed data; R.N. and R.R. drafted manuscript; S.T., V.K., and R.R. interpreted results of experiments; S.T., V.K., and R.R. prepared figures; J.R.W., A.S.Y., and R.R. edited and revised manuscript; R.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Knepper for the kind gift of mpkCCD C14 (clone 11) cells and Gitte Skou, Gitte Kall, Line Nielsen and Erin Suderman for expert technical assistance.
REFERENCES
- 1.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflügers Arch 456: 1005–1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyer MD, Breyer RM. Prostaglandin receptors: their role in regulating renal function. Curr Opin Nephrol Hypertens 9: 23–29, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278: F29–F42, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cogan E, Svoboda M, Abramow M. Mechanisms of lithium-vasopressin interaction in rabbit cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1080–F1087, 1987. [DOI] [PubMed] [Google Scholar]
- 5.de Groot T, Alsady M, Jaklofsky M, Otte-Holler I, Baumgarten R, Giles RH, Deen PM. Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol 25: 501–510, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal 17: 1343–1351, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell 12: 957–971, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Romero-Aleshire MJ, Cai Q, Price TJ, Brooks HL. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol 305: F1201–F1208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol 5: 270–276, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hebert RL. Cellular signalling of PGE2 and its selective receptor analogue sulprostone in rabbit cortical collecting duct. Prostaglandins Leukot Essent Fatty Acids 51: 147–155, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406: 86–90, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, Rao R. Specific deletion of glycogen synthase kinase-3β in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int 82: 1000–1009, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Jia Z, Wang H, Yang T. Mice lacking mPGES-1 are resistant to lithium-induced polyuria. Am J Physiol Renal Physiol 297: F1689–F1696, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 4: 40, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, Greytak S, Woulfe K, Trivedi CM, Woodgett JR, Epstein JA, Force T, Huggins GS. Deletion of GSK-3β in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest 118: 3609–3618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol 302: F455–F465, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Kortenoeven ML, Schweer H, Cox R, Wetzels JF, Deen PM. Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol 302: C131–C140, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Kortenoeven ML, Trimpert C, van den Brand M, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol 302: F1395–F1401, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Kotnik P, Nielsen J, Kwon TH, Krzisnik C, Frokiaer J, Nielsen S. Altered expression of COX-1, COX-2, and mPGES in rats with nephrogenic and central diabetes insipidus. Am J Physiol Renal Physiol 288: F1053–F1068, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Shaw S, Kamsteeg EJ, Vandewalle A, Deen PM. Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J Am Soc Nephrol 17: 1063–1072, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Liang MH, Chuang DM. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J Biol Chem 281: 30479–30484, 2006. [DOI] [PubMed] [Google Scholar]
- 26.MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, Nagy A, Woodgett JR. Glycogen synthase kinase 3α-specific regulation of murine hepatic glycogen metabolism. Cell Metab 6: 329–337, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Moses AM. Osmotic thresholds for AVP release with the use of plasma and urine AVP and free water clearance. Am J Physiol Regul Integr Comp Physiol 256: R892–R897, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen J, Kwon TH, Christensen BM, Frokiaer J, Nielsen S. Dysregulation of renal aquaporins and epithelial sodium channel in lithium-induced nephrogenic diabetes insipidus. Semin Nephrol 28: 227–244, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen J, Kwon TH, Frokiaer J, Knepper MA, Nielsen S. Lithium-induced NDI in rats is associated with loss of α-ENaC regulation by aldosterone in CCD. Am J Physiol Renal Physiol 290: F1222–F1233, 2006. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien WT, Klein PS. Validating GSK3 as an in vivo target of lithium action. Biochem Soc Trans 37: 1133–1138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens 21: 541–546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao R, Hao CM, Breyer MD. Hypertonic stress activates glycogen synthase kinase 3β-mediated apoptosis of renal medullary interstitial cells, suppressing an NFκB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem 279: 3949–3955, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Rao R, Patel S, Hao C, Woodgett J, Harris R. GSK3β mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Robben JH, Knoers NV, Deen PM. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 291: F257–F270, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature 362: 557–560, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 280: 720–725, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 12: 43–47, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Strait KA, Stricklett PK, Chapman M, Kohan DE. Characterization of vasopressin-responsive collecting duct adenylyl cyclases in the mouse. Am J Physiol Renal Physiol 298: F859–F867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamma G, Wiesner B, Furkert J, Hahm D, Oksche A, Schaefer M, Valenti G, Rosenthal W, Klussmann E. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J Cell Sci 116: 3285–3294, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol 23, Suppl 16: S43–S48, 2010. [PubMed] [Google Scholar]
- 47.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278: 33067–33077, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Morris KL, Sparrow SK, Dwyer KM, Enjyoji K, Robson SC, Kishore BK. Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am J Physiol Renal Physiol 303: F420–F430, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3α is a central regulator of age-related pathologies in mice. J Clin Invest 123: 1821–1832, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, Kerkela R, Doble BW, MacAulay K, DeCaul M, Koch WJ, Farber J, Woodgett J, Gao E, Force T. GSK-3α directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. J Clin Invest 120: 2280–2291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]