Abstract
We have previously demonstrated that estrogen (E2) downregulates phosphate transporter NaPi-IIa and causes phosphaturia and hypophosphatemia in ovariectomized rats. In the present study, we examined whether E2 directly targets NaPi-IIa in the proximal tubule (PT) and studied the respective roles of estrogen receptor isoforms (ERα and ERβ) in the downregulation of NaPi-IIa using both in vivo and an in vitro expression systems. We found that estrogen specifically downregulates NaPi-IIa but not NaPi-IIc or Pit2 in the kidney cortex. Proximal tubules incubated in a “shake” suspension with E2 for 24 h exhibited a dose-dependent decrease in NaPi-IIa protein abundance. Results from OVX rats treated with specific agonists for either ERα [4,4′,4″;-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol, PPT] or ERβ [4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol, DPN] or both (PPT + DPN), indicated that only the latter caused a sharp downregulation of NaPi-IIa, along with significant phosphaturia and hypophosphatemia. Lastly, heterologous expression studies demonstrated that estrogen downregulated NaPi-IIa only in U20S cells expressing both ERα and ERβ, but not in cells expressing either receptor alone. In conclusion, these studies demonstrate that rat PT cells express both ERα and ERβ and that E2 induces phosphaturia by directly and specifically targeting NaPi-IIa in the PT cells. This effect is mediated via a mechanism involving coactivation of both ERα and ERβ, which likely form a functional heterodimer complex in the rat kidney proximal tubule.
Keywords: inorganic phosphate, postmenopause, sex steroids, proximal tubule, hypophosphatemia.
several clinical studies have demonstrated that estrogen depletion (menopause) or repletion (hormone replacement therapy) is associated with changes in serum inorganic phosphate (Pi) levels in women. Indeed, clinical studies have shown that women treated with estrogen exhibit hypophosphatemia (2, 15, 18, 57, 59), and in some studies, a reduction in proximal tubular Pi reabsorption was observed (12, 17, 59). Conversely, estrogen-depleted patients showed elevated plasma levels of Pi and increased rate of proximal tubule Pi reabsorption (2).
Kidney and intestine play an important role in the control and maintenance of Pi homeostasis (8, 18, 20, 40). Pi enters the body through its absorption in the intestine via an apical sodium-dependent Pi transporters NaPi-IIb encoded by Slc34a2 (18, 20). The involvement of kidney is more efficient in controlling Pi metabolism, as it can reabsorb or excrete Pi depending on the circulating levels of Pi and the needs of the body. Indeed, most of filtered Pi is immediately reabsorbed in the proximal tubule via the activity of several Pi transporters. These transporters include NaPi-IIa (Slc34a1), NaPi-IIc (Slc34a3), and Pit-2 (Slc20a2), all of which are sodium-dependent (NaPi) and expressed in the brush-border membrane of the proximal tubule (see Refs. 9 and 40 for review). Recently, studies demonstrated that estrogen stimulates the expression of NaPi-IIb in the intestine (63) and inhibits phosphate uptake in brush border membrane vesicles harvested from kidneys of estrogen-treated rats (6). Subsequently, we demonstrated that estrogen downregulates NaPi-IIa and causes hypophosphatemia and renal Pi wasting in ovariectomized rats (23). We further demonstrated that the effect of estrogen on renal Pi handling results from the downregulation of NaPi-IIa and is independent of parathyroid hormone (PTH) levels or changes in food intake (23).
PTH and fibroblast growth factor 23 (FGF23) are known to regulate Pi balance through the downregulation of Pi transporters in the proximal tubule with subsequent phosphaturia in humans, as well as in experimental animals (7, 34, 52). Recent studies demonstrated that estrogen stimulates the synthesis and release of FGF23 from bone osteocytes, while decreasing the synthesis and secretion of PTH in the parathyroid gland of rat (11). The effect of estrogen on PTH appears to be indirect, as estrogen receptors ERα or ERβ are not expressed in the parathyroid cells and rather results from the increase in the circulating levels of FGF23 and its action in the parathyroid gland (11).
The majority of estrogen response is mediated through two different isoforms of estrogen receptors (ER), usually referred to as ERα and ERβ, each encoded by a separate gene (33, 38, 39, 50, 58). Immunoblot studies have shown that ERα and ERβ are expressed in the kidney of rat (61) and mice (29). Immunohistochemistry studies demonstrated that ERα is expressed in both proximal tubule and distal tubule of both rat and mice kidney (29, 61). The segmental distribution of ERβ in rat kidney was not examined in this study (61). In mice, ERβ is also expressed in both proximal tubule and distal nephron segments (29). However, the specificity of the antibodies used in both studies was not demonstrated. Nevertheless, whether estrogen receptors mediate directly or indirectly the effect of estrogen on renal Pi transporters remains to be studied.
In the present studies, we examined the dose-response effect of estrogen on renal Pi handling and NaPi-IIa expression and determined the effects of estrogen on other apical Pi absorbing transporters in the kidney proximal tubule. Further, we examined whether the mRNA of ERα and ERβ is expressed in the kidney proximal tubule cells and studied whether they mediate a direct effect of estrogen on NaPi-IIa expression using proximal tubular suspensions. Lastly, we determined the respective roles of ERα and ERβ in estrogen-induced downregulation of NaPi-IIa using both in vivo and in vitro experiments.
MATERIALS AND METHODS
The experiments performed in these studies were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Ovariectomized Sprague-Dawley rats were purchased from Harlan (Harlan, Indianapolis, IN), housed two rats per cage with free access to rat chow and distilled water, and maintained in a temperature-controlled room regulated on a 12:12-h light-dark cycle for 1 wk before and during the following treatments.
Dose-Dependent Effects of Estrogen
OVX rats were placed in metabolic cages and allowed free access to food and water, as previously described in our laboratory (23). After 3 or 4 days of adjustment to metabolic cages, rats were randomly divided into several groups (n = 4 or 5 rats in each) and injected subcutaneously with different doses of 17β-estradiol (0.5, 5, 15, and 90 μg/100 g body wt/day) or vehicle (sesame oil). The animals were injected daily and euthanized after 3 days of treatment.
Respective Roles of Estrogen Receptors ERα or ERβ Using Pharmacological Agonists
OVX rats were housed in metabolic cages and fed rodent chow and distilled water and treated as follows: in a first set of the experiments, rats were divided into three groups (n = 4 rats in each) with two groups injected subcutaneously with either 150 or 500 μg/100 g body wt of PPT, a specific agonist of ERα (26, 27, 50, 54, 57), and the third group was injected with vehicle. Because it was expected that PPT would cause a reduction in food intake, as previously shown by others (49, 50), the access of vehicle group to food was restricted to the same amount consumed by PPT-treated rats at 500 μg/100 g body wt (pair-feeding). In a second set of experiments, rats were divided into three groups (four rats in each) and injected subcutaneously with either 150 or 500 μg/100 g body wt of DPN, a specific ERβ agonist (26, 27, 50, 54, 57), or vehicle (sesame oil). In the last experiment, a group of rats (n = 4) was injected subcutaneously with a combination of PPT + DPN at 150 μg/100 g body weight each. In all of these experiments, rats were injected daily and killed after 3 days of treatments. To confirm the findings of ER activation studies, the effect of PPT or DPN at 150 μg/100 g body wt vs. vehicle was reevaluated using another batch of four rats in each group, and NaPi-IIa protein abundance was reexamined in the kidney cortex of these animals.
Our choice of 150 μg/100 g body wt of PPT or DPN is estimated from studies that examined the dose-response effects of these compounds on food intake in OVX rats (26, 46, 50, 54, 57). Our doses are slightly overestimated to account for the difference in the vehicle of preference, i.e., sesame oil in our studies vs. DMSO in Santollo's work (50). To ascertain that PPT alone and DPN alone have no effect on renal phosphate handling, we have also studied the effects of a higher dose of 500 μg/100 g body wt of these compounds on food intake and renal Pi handling.
During each of the above studies, food and water intake and urine volume were measured daily. At the end of each experiment, rats were euthanized and kidneys were removed. Slices of kidney cortex were dissected and snap frozen in liquid nitrogen and stored at −80°C for total RNA and membrane protein isolation. Urine chloride concentration was measured using a chloridometer, and urine creatinine, Pi, and calcium concentrations were determined using colorimetric assays kits (BioAssay Systems, Hayward, CA; or BioVision, Milpitas, CA). These parameters were measured in urine collected on the last day of treatment. NH4+ concentration in culture media was measured as previously described and used in our laboratory (1).
Effect of Estrogen on NaPi-IIa Expression In Vitro Using Proximal Tubular Suspension
Isolation of renal proximal tubule suspension.
Proximal tubule suspensions (PTS) were prepared from 200- to 250-g ovariectomized Sprague-Dawley rats using collagenase digestion protocol that has been described and extensively used by others (14, 28, 31, 45, 46). This method was used to study the regulation of Na+/H+ exchanger (NHE3) activity in freshly isolated proximal tubule suspensions (14, 28, 31, 45, 46). We have also used PTS in our laboratory to study the activity of basolateral Na+:HCO3− cotransporter in the kidney proximal tubule of control and K+-depleted rats (3). The passage of the final suspension through a 70-μm opening nylon mesh eliminates small fragments of distal tubules and isolated cells, while enriching the suspension with proximal tubule fragments, as shown in our RT-PCR data depicted below (Fig. 3).
Fig. 3.
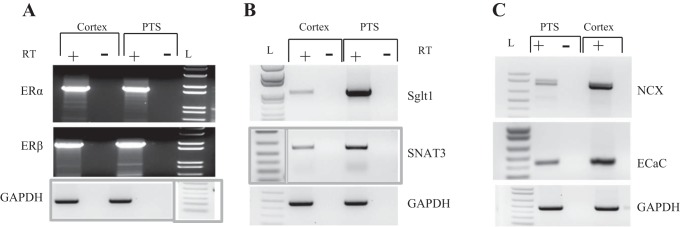
Expression of ERα and ERβ in the kidney proximal tubule. Kidneys were harvested from OVX rats, and total RNA was isolated from the cortex or proximal tubular suspension (PTS). RNA was reverse transcribed, and resulting cDNA was PCR amplified using specific primers for ERα (A, top) or ERβ (A: bottom). Specific markers of proximal tubule cells SGLT1 (B, top) and SNAT3 (B: bottom) and distal tubule cells NCX (C, top) and ECaC1 (C, bottom) were used to evaluate the enrichment of proximal tubule fragments in the tubular suspensions. GAPDH is used as a control for cDNA loading. In negative samples, the reverse transcriptase was omitted in the reverse transcription reaction (RT). L: 1 kb Plus DNA ladder. The ladders for GAPDH in A and for SNAT3 in B were rearranged to maintain consistency in the position of the ladder in the entire graph.
Expression of estrogen receptor isoforms in the proximal tubule cells.
Total RNA isolated from proximal tubule suspensions and cortex slices was reverse transcribed and used as a DNA template to examine the expression of ERα and ERβ by PCR. Primers for cDNA synthesis and PCR amplification of ERα are 5′-CCA TGACCATGACCCTTCACAC-3′ (forward) and 5′-GAGCCTGGGAGTTCTCAG ATG G-3′(reverse), corresponding to nucleotides 208 to 2026 of rat ERα (accession no. NM_012689). The primers for ERβ are 5′-GGCTGAGCGACAACCAGTGGCTG-3′ (forward) and 5′-GCGTGTGAGCATTCAGCATCTC-3′ (reverse) corresponding to nucleotides 90 to 1779 of rat ERβ (accession no. NM_012754). The primers for sodium glucose cotransporter SGLT1 are 5′-ATGGTGTGGTGGCCGATTGG-3′ (forward) and 5′-GTGTAGATGTCCATGGTGAAGAG-3′ (reverse) corresponding to nucleotides 362 to 1379 of rat SGLT1 (accession no. NM_013033). The primers for neutral amino acid transporter SNAT3 or SN1 are 5′-CTGAAGACGCCCAACACTG-3′ (forward) and 5′-CAGAATGATGATGACGGATA-3′ (reverse) corresponding to nucleotides 201 to 700 of rat SNAT3 (accession no. 145776). The primers for epithelial calcium channel (ECaC1 or TRPV5) are 5′-CCTCAAGTTCCGTGATGCC-3′ (forward) and 5′-CATTAGCCAGCAGAAGCG-3′ (reverse) corresponding to nucleotides 773 to 1211 of rat ECaC1 (accession no. XM_006236376.2). The primers for the Na+/Ca2+ exchanger (NCX) are 5′-AATGAGCTTGGTGGCTTCACA-3′ (forward) and 5′-CCGCCGATACAGCAGCAC-3′ (reverse) corresponding to nucleotides 2113 to 2958 of the rat NCX (accession no. XM_008764435.1). Lastly, the primers used for GAPDH are 5′-CCCATCACCATCTTCCAGGACC-3′ (forward) and 5′-CCAGTGAGCTTCCCGTTCAGC-3′ (reverse) corresponding to nucleotides 286 to 758 of rat GAPDH (accession no. NM_017008.4). PCR products were resolved on 1.2% agarose gel and visualized with ethidium bromide staining using Kodak Gel Logic 100 Imaging instrument and UV light transilluminator.
Chronic effect of estrogen on NaPi-IIa expression in vitro using proximal tubule suspensions.
The approach used for these studies was adapted from the method of shake medullary thick ascending limb (MTAL) suspension developed by Attmane-Elakeb et al. (4) and used for the long-term regulation of MTAL NKCC2/BSC1 (5, 30) and ROMK (24) in rat kidney. Briefly, Proximal tubule suspensions were suspended in aliquot of equal parts (volume) in 60-mm cell culture dishes containing phenol red- and serum-free DMEMF/12 medium supplemented with 400 UI/ml penicillin, 200 mg/ml streptomycin, 11 mM NaHCO3, 5 mM leucine, 15 mM HEPES, pH adjusted to 7.40 with tris(hydroxymethyl)-aminomethane (Tris). Dishes were then placed on the orbital shaker (Lab Line rotator orbital shaker, model no. 1314), which is housed in a water-jacketed CO2 cell culture incubator (model no. 2406; Shel Lab, Cornelius, OR) quipped with a path allowing the connection of the shaker to the electrical outlet. Tubule suspensions were, therefore, shaken at 37°C in a humid and 95% O2-5% CO2 atmosphere in the presence of different doses of estrogen (60, 300, or 600 nM) or its vehicle (ethanol) for up to 24 h. The viability of proximal tubule cells incubated in the above medium was verified using trypan blue exclusion method and by measuring their capacity to produce ammonium (NH4+) over a 24-h period (See results below). At the end of treatment, PT suspensions were washed twice with gentle centrifugation (50 g for 2 min) in the above Ringer solution and the suspension was then frozen in liquid nitrogen until used for membrane fractions isolation.
Cloning and Generation of Rat Full-Length NaPi-IIa Plasmid and Transfection Studies
The rat kidney full-length NaPi-IIa plasmid was generated using commercial services (GeneCopoeia, Rockville, MD). Briefly, rat full-length NaPi-IIa sequence was generated from kidney cDNA template by PCR using the forward primer 5′-CGAGCTGGAGCTGAGCCATAGTCAAGGAC-3′ and the reverse primer 5′-ATGATCAGGACAGATGCAACTTTTAAAACTTTTTC-3′ of rat NaPi-IIa mRNA sequence (GenBank accession no. NM_0.13030.1). The PCR product was subsequently constructed into the recombination scaffold of shuttle vector. The sequence in shuttle clone was verified through full-length sequencing using the following primers: forward 5′-CAGCCTCCGGACTCTAGC-3′ and reverse 5′-TAATACGACTCACTATAGGG-3′, and then transferred into the expression vector pEZ-M02 through recombination (GeneCopoeia). The plasmid containing full-length rat NaPi-IIa was amplified using Escherichia coli (Life Technologies, Carlsbad, CA) and purified using a plasmid Midi kit (Qiagen, Valencia, CA). For transient transfection studies, U20S cells grown in 60-mm dishes were transfected with 2 μg of the full-length rat NaPi-IIa cDNA construct using Lipofectamine (Life Technologies) or LyoVec (InvivoGen, San Diego, CA), according the manufacturer's instructions. The transfected cells were incubated and treated in appropriate media, as described below.
Effect of Estrogen on NaPi-IIa Expression In Vitro Using U20S Cells Expressing ERα and/or ERβ
We used human female U20S osteosarcoma cells stably expressing estrogen receptors ERα and/or ERβ under the control of doxycycline as previously described (37). U20S cells were cultured in DMEMF/12 medium containing 10% FBS, 1× antibiotic/antimycotic, 5 mg/l blasticidin, and 500 mg/l zeocin and maintained at 37°C in a 5% CO2-95% O2 air incubator. For estrogen treatment, cells (70–80% confluence) were switched to a phenol red-free medium supplemented with 10% charcoal-stripped serum and the same antibiotics described above and 24 h later, 100 ng/ml doxycycline was added to the cells. After an additional 24 h, cells were transfected with rat full-length NaPi-IIa plasmid, and treated in the same media with vehicle or 100 nM estrogen. After 24 h of treatment, cells were harvested in RIPA buffer containing 1× protease inhibitor cocktail and processed for total protein lysates extraction. The protein concentrations were then determined using BCA kit (Thermo Scientific, Rockford, IL).
RNA Isolation and Northern Hybridization
Total cellular RNA was extracted from renal cortex by the method of Chomczynski and Sacchi (15), and as previously described in our laboratory (1, 3, 23). Total RNA samples (30 μg/lane) were fractionated on a 1.2% agarose-formaldehyde gel and transferred to Magna NT nylon membranes using 10× sodium chloride-sodium phosphate-EDTA as a transfer buffer. Hybridization was performed, according to Church and Gilbert (16) and as previously used in our laboratory (1, 3, 23). Specific probes for various Pi transporters were generated by RT-PCR using total RNA from rat kidney cortex.
Membrane Protein Isolation and Immunoblotting
Total cellular fraction containing plasma membrane and intracellular membrane proteins were prepared from the cortex, as previously described (1, 3, 23). Semiquantitative immunoblotting experiments were performed, as previously described and used in our laboratory (1, 3, 23). Actin was used as a control constitutive protein for the equity in protein loading in all gels. Rabbit polyclonal NaPi-IIa, NaPi-IIc, and Pit2 antiserum were generated, characterized, and used in Dr. Levi's laboratory, as described before (10, 64).
Immunofluorescence
U20S cells expressing both ERα and ERβ were grown to confluence in four-well chamber slides in the above culture medium, as previously described (43) with some modifications. Briefly, cells were transfected with rat full-length NaPi-IIa and treated with doxycycline in the presence of vehicle or 100 nM estrogen as described above. After washing with TBS and fixation with 1% paraformaldehyde, cells were blocked with TBS containing 10% normal goat serum (NGS) for 1 h. Cells were then incubated with anti-NaPi-IIa antiserum diluted at 1:50 in TBS containing 10% NGS overnight at 4°C. After washing with TBS-containing Tween-20 (TBST), cells were incubated with Alexa Fluor 594 goat anti-rabbit IgG in TBS containing 10% NGS for 1 h. Cells were then washed in TBST, and coverslips were mounted using Fluoromount-G. The immunofluorescence was then revealed by confocal microscopy at original magnification of ×20.
Materials
32P-dCTP was purchased from Perkin Elmer (Boston, MA). Paper blotting nitrocellulose membranes used for Northern hybridization were purchased from Midwest Scientific (St. Louis, MO). 17β-Estradiol was purchased from Cayman Chemical. PPT and DPN were obtained from Tocris (Tocris/R&D System, Minneapolis, MN). High prime DNA-labeling kit was purchased from Roche (Roche Diagnostics, Indianapolis, IN). NaPi-IIa, NaPi-IIc, and Pit2 were provided by Dr. Levi's laboratory. Actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Dallas, TX). Secondary antibodies donkey anti-rabbit and mouse anti-goat were purchased from Thermo Scientific (Thermo Scientific, Rockford, IL). Progesterone and all other chemicals were purchased from Sigma Chemical (St. Louis, MO). Alexa Fluor 594 goat anti-rabbit IgG and Fluoromount were purchased from Life Technologies (Grand Island, NY) and Southern Biotech (Birmingham, AL). NGS was purchased from Vector Laboratories (Burlingame, CA).
Statistical Analysis
Semi-quantification of immunoblots and northern hybridization band densities was determined by densitometry using a scanner (ScanJet ADF; Hewlett Packard, Palo Alto, CA) and UN-SCAN-IT gel software (Silk Scientific, Orem, UT) and Image Quant software (Molecular Dynamics, Sunnyvale, CA), respectively. Data were expressed as absolute values or % of control (vehicle). Results were presented as means ± SE. Statistical significance between control and experimental groups was determined by Student's unpaired t-test or one-way ANOVA, as needed using Sigma Plot software. P < 0.05 was considered significant.
RESULTS
Dose-Response Effects of Estrogen on Food Intake, Urinary Chloride, and Pi Excretion, and on the Expression of NaPi-IIa in the Kidney Cortex of OVX Rats
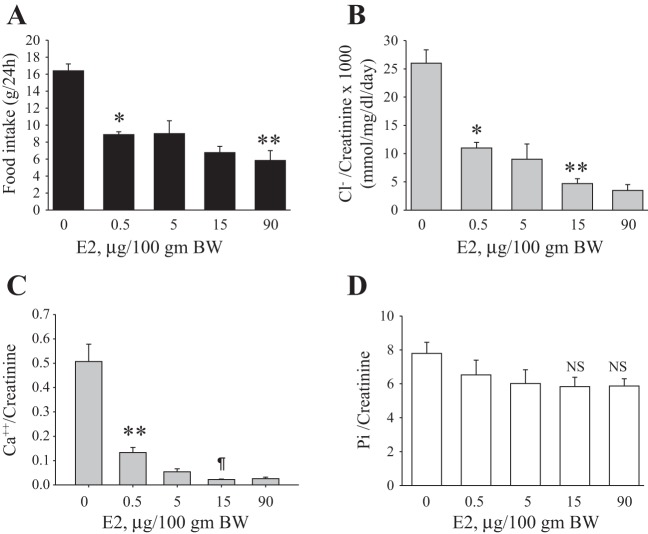
In our previous study, we demonstrated that a single dose of estrogen 15 μg/100 g body wt administered once daily for 3 days to OVX rats caused a significant downregulation of NaPi-IIa and phosphaturia (23). In the present work, we examined these effects in a dose-response study as described in methods. The results shown in Fig. 1A indicate that estrogen treatment is associated with a significant reduction in food intake at a lower dose of 0.5 μg/100 g body weight (P < 0.001 vs. vehicle; Fig. 1A) and further decreased at 15 and 90 μg/100 g body wt (P < 0.0001 vs. vehicle, Fig. 1A). A parallel dose-dependent decrease in chloride/creatinine (Fig. 1B) and calcium/creatinine (Fig. 1C) excretion was also observed in response to estrogen. However, Pi/creatinine excretion remained unchanged in response to all doses of estrogen (Fig. 1D) despite a reduction Pi intake. Lastly, estrogen decreased serum Pi concentration significantly (P < 0.05, Table 2) but did not change the circulating levels of calcium (Table 1).
Fig. 1.
Dose-dependent effects of estrogen on food intake, chloride, and phosphate (Pi) excretion. Ovariectomized (OVX) rats were placed in metabolic cages and injected daily with vehicle (0) or indicated doses of estrogen for 3 days. Food intake (A), chloride/creatinine excretion (B), calcium/creatinine excretion (C), and phosphate/creatinine excretion (D). These parameters were measured on the third day of treatment. *P < 0.001, **P < 0.0001 vs. vehicle (0). ¶P < 0.04 vs. E2 (0.5 μg/100 g body wt). n = 4 rats in each group. NS, not significant vs. vehicle (0).
Table 2.
Expression levels of NaPi-IIc, Pit2, and Pit1 in the kidney cortex
| mRNA |
Protein |
|||
|---|---|---|---|---|
| Vehicle | EST | Vehicle | EST | |
| NaPi-IIc | 100 ± 3.0 | 92 ± 4.0 | 100 ± 13 | 97 ± 10 |
| Pit2 | 100 ± 4.5 | 106 ± 4.0 | 100 ± 6.0 | 100 ± 10 |
| Pit1 | 100 ± 5.0 | 98 ± 5.0 | ND | ND |
Data are expressed as means ± SE; n = 4 rats in each group. Ovariectomized (OVX) rats were treated with estrogen (EST; 15 μg/100 g body weight) or vehicle for 3 days. The mRNA and protein expression of Pi transporters were examined with Northern hybridization and immunoblotting, respectively.
Table 1.
Serum levels of inorganic phosphate and calcium
| Vehicle-PF | PPT, 150 μg/100 g body wt | Vehicle | DPN, 150 μg/100 g body wt | PPT + DPN | Estrogen, 15 μg/100 g body wt | |
|---|---|---|---|---|---|---|
| Pi, mM | 3.60 ± 0.22 | 3.31 ± 0.39 | 3.56 ± 0.32 | 3.90 ± 0.42 | 2.31 ± 0.09* | 2.63 ± 0.08* |
| Ca2+, mg/dl | 7.72 ± 1.93 | 9.46 ± 1.28 | 10.43 ± 0.85 | 13.0 ± 1.42 | 10.40 ± 0.80 | 10.32 ± 0.43 |
Data are expressed as means ± SE. 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) is compared to pair-fed vehicle (vehicle-PF) Vehicle group combines vehicles for 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) and PPT+DPN and estrogen pooled in one vehicle group. Pi, inorganic phosphate.
P < 0.05 compared with vehicle; n = 4 to 7 rats in each group.
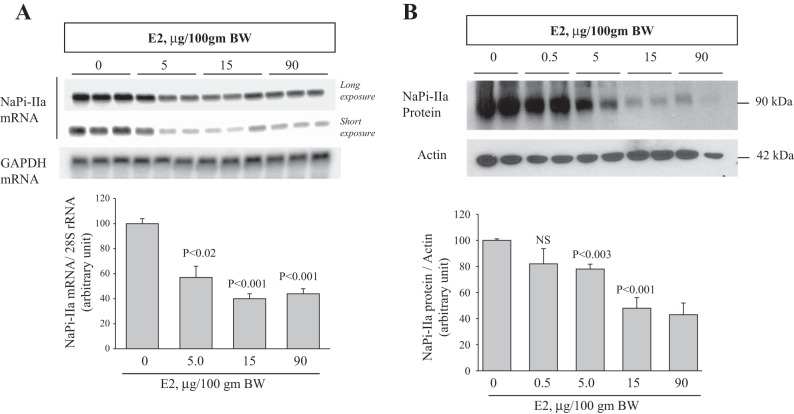
The expression of NaPi-IIa was then examined in the kidney cortex of vehicle or estrogen-treated OVX rats. The results shown in Fig. 2A indicate that estrogen caused a significant decrease in the mRNA expression of NaPi-IIa at 5 μg/100 g body wt (−45%, P < 0.02; Fig. 2A) and further decreased by 63% (P < 0.001) and 52% (P < 0.001) at 15 and 90 μg/100 g body wt, respectively, compared with vehicle (Fig. 2A). Data depicted in Fig. 2B indicate that NaPi-IIa protein abundance is significantly reduced by ∼22% (P < 0.003) at 5 μg/100 g body wt by ∼52% at 15 and 90 μg/100 g body wt (P < 0.001) of estrogen, compared with vehicle (Fig. 2B).
Fig. 2.
Dose-response effect of estrogen on NaPi-IIa expression in the kidney. A, top: representative Northern blots showing long and short exposure of NaPi-IIa mRNA (light bands) and GAPDH mRNA (dark band) in the renal cortex of rats injected daily for 3 days with indicated doses of estrogen vs. vehicle (0). GAPDH was used as a constitutive gene for the control of the equity of RNA loading into Northern gels. Bottom: corresponding densitometric analysis showing the mean of NaPi-IIa mRNA-to-GAPDH mRNA ratio (n = 4 rat in each group). B, top: representative immunoblots showing the abundance of NaPi-IIa and actin proteins in membrane fractions isolated from renal cortex of vehicle- or estrogen-treated rats. B, bottom: average densitometric analysis of NaPi-IIa/actin bands (n = 4 rat in each group). Each lane was loaded with 30 μg of total RNA or 40 μg of membrane proteins from a different rat. n = 4 rats in each group.
Specificity of the Effects of Estrogen on Pi Transporters in the Kidney
As described above, several transporters, including NaPi-IIa, NaPi-IIc, and Pit2, are involved in Pi reabsorption in the proximal tubule. The results shown in Table 2 indicate that the mRNA expression levels and protein abundance of NaPi-IIc and Pit2 in the kidney cortex of OVX rats were not altered in response to 3 days of estrogen treatment (15 μg/100 g body wt), compared with vehicle (P > 0.05; Table 2). In addition, the mRNA expression level of Pit1 was also not affected by estrogen (Table 2).
Expression of ER Isoforms in Whole Cortex and Cortical Tubule Suspensions of Female Rat Kidney
The RT-PCR data depicted in Fig. 3 indicate the presence of mRNA of both ERα and ERβ in the kidney cortex of ovariectomized (OVX) rats (Fig. 3A). Both receptors are also detected in a preparation of cortical tubule suspension harvested from cortex of OVX rat kidneys using collagenase digestion, as described in methods. The cortical suspension is enriched with PTS, as indicated by increased mRNA expression levels of SGLT1 and SNAT3 used as specific markers of proximal tubule cells (Fig. 3B). As expected, distal nephron segments are less abundant in PTS, as indicated by lower expression levels of calcium transporters ECaC1 and NCX (Fig. 3C), which are considered the specific makers of distal tubule segments.
Effect of Long-Term Estrogen Treatment on NaPi-IIa Expression in Cortical Tubular Suspensions In Vitro
Viability of proximal tubule suspensions.
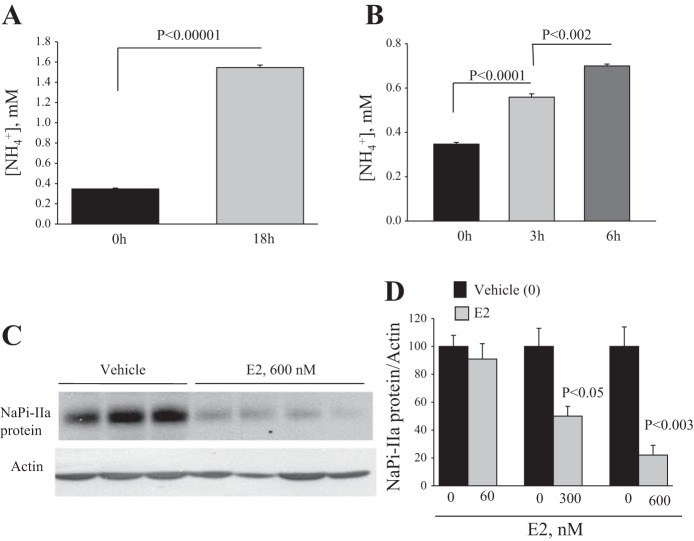
PTS incubated in serum- and phenol red-free DMEMF/12 for 24 h are viable, as indicated by the absence of dead cells, as determined by Trypan blue exclusion method. Further, PTS are metabolically highly active, as indicated by their ammoniagenic capacity assessed by measuring NH4+ production and accumulation in cultured media. Indeed, the results depicted in Fig. 4 indicate that NH4+ production increased from 0.35 ± 0.008 mM at time 0 to 1.55 ± 0.026 mM (P < 0.00001; Fig. 4A) after an 18-h incubation period. Time 0 is defined here as [NH4+] in the medium before the addition of PTS. After 18 h, PTS were washed by centrifugation and resuspended in fresh serum-free medium and assayed again for NH4+ production after 3- and 6-h incubation periods. The results indicate an increase in NH4+ production by PTS from 0.33 ± 0.008 mM at time zero to 0.56 ± 0.015 mM (P < 0.0001 vs. time zero; Fig. 4B) and to 0.70 ± 0.008 mM (P < 0.002 vs. 3 h; Fig. 4B) after 3- and 6-h incubation periods, respectively. These data clearly demonstrate that PTS incubated for 24 h in a serum-free medium are viable, and highly ammoniagenic, under baseline and unstimulated condition.
Fig. 4.
Long-term effect of estrogen on NaPi-IIa protein expression in proximal tubular suspension in vitro. PTS was prepared and incubated in phenol red- and serum-free medium on a shaker in cell culture incubator, as described in methods. Equal parts of PTS were distributed in 100-mm culture dishes and incubated for 18 h under normal conditions. A: after 18 h, NH4+ produced by PTS was measured in the medium and compared with its level in the medium at time 0 (0 h), before the addition of PTS (n = 4 dishes in each). B: after 18 h, PTS were washed by centrifugation and resuspended in the same medium for up to 6 h. NH4+ was measured in the media after 3 and 6 h and compared with its level at time 0 (n = 4 dishes in each group). C and D: PTS were treated with vehicle (0.016% ethanol) or 60, 300, or 600 nM of E2 and incubated for 24 h. C: representative immunoblots showing the abundance of NaPi-IIa and actin proteins in membrane fractions isolated from PTS treated with vehicle or 600 nM E2. D: average densitometric analysis of NaPi-IIa/actin bands for each E2 dose vs. vehicle. Each lane was loaded with 40-μg membrane proteins from a different 100-mm culture dish. n = 4 or 5 dishes for each group in two separate experiments. P < 0.05 and P < 0.003 vs. no estrogen (vehicle).
Chronic treatment of PTS by estrogen.
PTS were freshly isolated and incubated in serum- and phenol red-free DMEMF/12 in the presence of 60, 300, and 600 nM estrogen or its vehicle for 24 h. Membrane proteins were then extracted from these suspensions and used for immunoblotting studies. The data depicted in Fig. 4 show a significant reduction in NaPi-IIa protein abundance in PTS in response to 300 nM (−50%; P < 0.05) and 600 nM (−80%; P < 0.003) of E2, compared with vehicle (Fig. 4, C and D). Estrogen did not alter NaPi-IIa protein abundance in PTS at 60 nM, compared with vehicle (P > 0.05; Fig. 4D).
Effects of specific activation of ERα on food intake, Pi excretion, and the expression of NaPi-IIa
The results shown in Fig. 5 indicate that specific activation of ERα using 150 or 500 μg/100 g body wt of the ERα-specific agonist PPT resulted in a significant reduction in food intake (Fig. 5, A and B), which correlated with a significant reduction in urinary Pi/creatinine excretion (Fig. 5C), compared with baseline levels. Both of these effects are comparable to those obtained in pair-fed and vehicle-treated animals. Note that food intake and Pi/creatinine excretion at baseline or after PPT treatment were not significantly different between the three groups. Lastly, PPT did not alter the protein abundance of NaPi-IIa in the kidney cortex at either dose used (P > 0.05, Fig. 6, A and B), compared with the pair-fed vehicle group (vehicle-PF). PPT did not alter the serum levels of Pi or calcium compared with vehicle-PF group (Table 1).
Fig. 5.
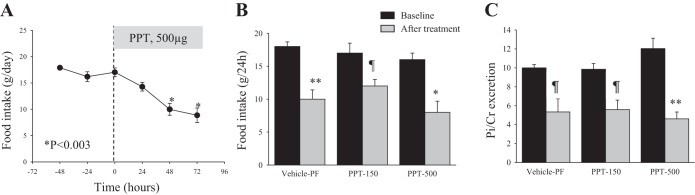
Effects of ERα agonist [4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol, PPT] on food intake and Pi excretion. OVX rats were housed in metabolic cages, and after 3 days of adjustment (baseline), they were injected daily with PPT (150 or 500 μg/100 g body wt) or vehicle for 3 days. Vehicle group was pair-fed with PPT-treated animals. A: time course of food intake before (baseline) and after 500 μg/100 g body wt PPT injection. B and C: average of 3 days of food intake and Pi excretion/creatinine at baseline (black bars) and 3 days after treatment (gray bars). n = 4 rats in each group. *P < 0.003. **P < 0.002. ¶P < 0.05 vs. average baseline.
Fig. 6.
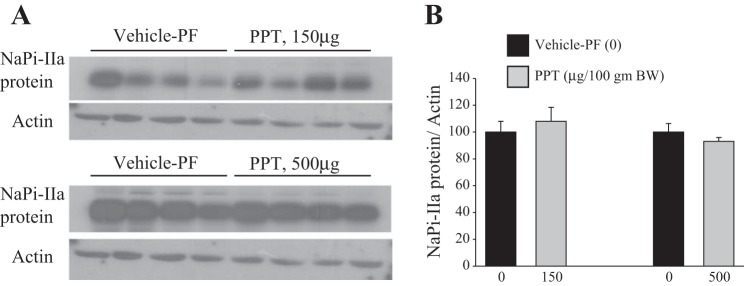
Effect of ERα agonist (PPT) on NaPi-IIa protein expression in the kidney cortex. Total membrane proteins were isolated from vehicle and PPT-treated OVX rats and used for immunoblotting experiments. A: immunoblots of NaPi-IIa and actin proteins in the cortex of rats treated with PPT at 150 μg/100 g body wt (top) or 500 μg/100 g body wt (bottom) vs. pair-fed vehicle. B: corresponding densitometric analysis of NaPi-IIa/actin bands for each dose of PPT vs. vehicle-PF (0). Each lane was loaded with 40 μg of proteins harvested from a different rat kidney cortex. The PPT experiment at 150 μg/100 g body wt was repeated twice (n = 8 rats in each group). n = 4 rats in each group were used for the experiment of PPT at 500 μg/100 g body wt.
Effects of Specific Activation of ERβ on Food Intake, Pi Excretion, and the Expression of NaPi-IIa
The data depicted in Fig. 7 indicate that specific activation of ERβ using 150 μg/100 g body wt of DPN did not affect food intake (Fig. 7, A and B), but caused a decrease, albeit not significant, in Pi/creatinine excretion (Fig. 7C), compared with vehicle-treated animals. A higher dose of DPN (500 μg/100 g body wt) also did not alter food intake but significantly decreased Pi/creatinine excretion by 40% (P < 0.001; Fig. 7C), compared with vehicle-treated animals. Lastly, Fig. 8 shows that the protein abundance of NaPi-IIa was not altered in response to DPN at the lower dose (150 μg/100 g body wt), but was rather increased (+40%, P < 0.02) at the higher dose (500 μg/100 g body wt), compared with the vehicle-treated group (Fig. 8, A and B). DPN did not alter the serum levels of Pi or calcium compared with vehicle group (Table 1).
Fig. 7.
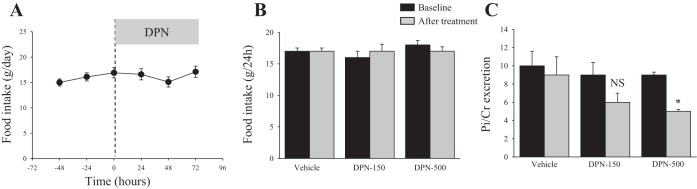
Effects of ERβ agonist [2,3-bis(4-hydroxyphenyl)-propionitrile, DPN] on food intake and Pi excretion. OVX rats were housed in metabolic cages and after 3 days of adjustment (baseline), they were injected daily with DPN (150 or 500 μg/100 g body wt) or vehicle for 3 days. A: time course of food intake before and after 150 μg/100 g body wt DPN injection. B: average of 3 days of food intake before (baseline, dark bars) or food consumed on the third day after vehicle or DPN injection (gray bars). C: average of 3 days Pi excretion/creatinine excretion before (baseline, dark bars) and Pi excretion/creatinine excretion of the third day after vehicle or DPN injection (gray bars). n = 4 rats in each group. *P < 0.001 vs. baseline of DPN group.
Fig. 8.
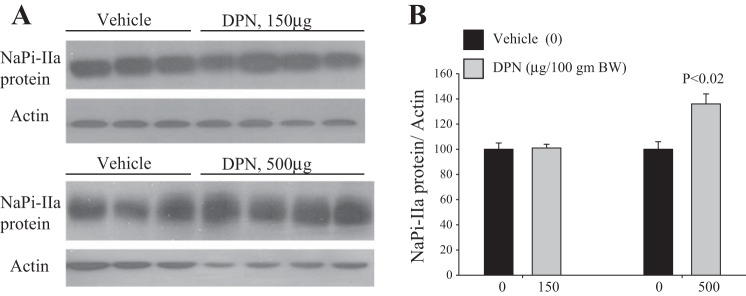
Effect of ERβ agonist (DPN) on NaPi-IIa expression in the kidney cortex. Total membrane proteins were isolated from vehicle and DPN-treated OVX rats and used for immunoblotting experiments. A: immunoblots of NaPi-IIa and actin proteins in the cortex of rats treated with DPN at 150 μg/100 g body wt (top) or 500 μg/100 g body wt (bottom) vs. vehicle. B: corresponding densitometric analysis of NaPi-IIa/actin bands for each dose of DPN vs. vehicle (0). Each lane was loaded with 40 μg proteins harvested from a kidney cortex of a different rat. The DPN experiment at 150 μg/100 g body wt was repeated twice (n = 8 rats in each group). n = 4 rats in each group were used for the experiment of DPN at 500 μg/100 g body wt. P < 0.02 vs. vehicle (0).
Effects of Simultaneous Activation of ERα and ERβ on Food Intake, Pi Excretion and the Expression of NaPi-IIa
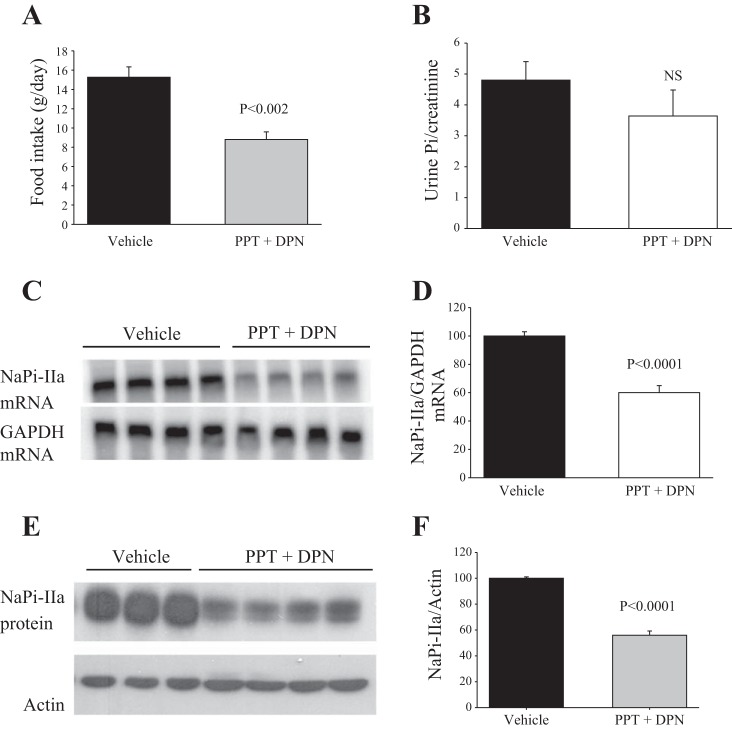
The results shown in Fig. 10 indicate that simultaneous activation of ERα and ERβ using daily injections of a cocktail containing 150 μg/100 g body wt of each of PPT and DPN (PPT + DPN) for 3 days resulted in a 42% reduction in food intake (P < 0.001; Fig. 9A) but did not decrease Pi/creatinine excretion (P > 0.05; Fig. 9B). The results further indicate that the mRNA expression level of NaPi-IIa is significantly reduced (−40%) in PPT + DPN vs. vehicle-treated rats (P < 0.0001, Fig. 9, C and D). Unlike PPT or DPN alone, combined treatment with PPT + DPN significantly reduced the protein abundance of NaPi-IIa (−44%; P < 0.001), compared with the vehicle-treated group (Fig. 9, E and F). Lastly, PPT + DPN treatment caused a significant reduction in serum Pi concentration (P < 0.05; Table 1), but did not alter the serum levels of calcium (Table 1).
Fig. 10.
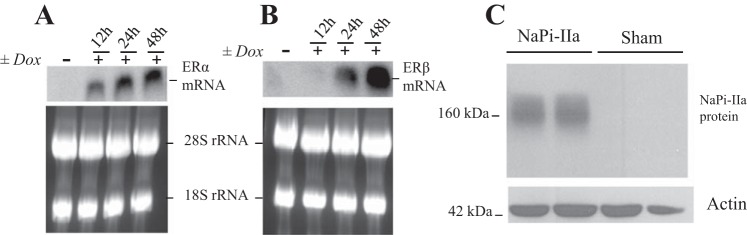
Expression of ERα and ERβ mRNA and NaPi-IIa protein in U20S cells. Northern hybridization of ERα (A), ERβ (B), and ribosomal 28S-18S RNA in U20S cells treated with doxycycline or vehicle for indicated time. Each lane was loaded with 30 μg of total RNA isolated from three culture dishes (100 mm) pooled together in one sample. C: immunoblot showing NaPi-IIa protein (∼160 kDa) and actin bands in lysates harvested from U20S cells transfected with rat NaPi-IIa expression construct or the empty vector (Sham). Each lane was loaded with 30 μg of proteins harvested from different culture dishes (60 mm) in each group.
Fig. 9.
Effects of combined ERα and ERβ agonists (PPT + DPN) on food intake, Pi excretion, and NaPi-IIa expression. OVX rats were placed in metabolic cages and injected with PPT + DPN (150 μg/100 g body wt each) or vehicle. Averages of food intake (A) and Pi/creatinine excretion (B) of the last day of treatment with Vehicle or PPT + DPN. C: Northern hybridization of NaPi-IIa and GAPDH mRNA. E: immunoblots of NaPi-IIa and actin proteins in the cortex of rats treated with PPT + DPN vs. vehicle. Corresponding densitometric analysis of NaPi-IIa mRNA/GAPDH mRNA (D) and NaPi-IIa protein/actin (F). Each lane was loaded with 30 μg of total RNA or 40 μg proteins harvested from a different rat. n = 3 or 4 rats in each group. NS, not significant.
Respective Roles of ERα and ERβ in NaPi-IIa Regulation by Estrogen Using an In Vitro Expression System
Rationale for using bone cells (U20S) for the following studies.
The above results (Fig. 9) clearly suggest that the downregulation of NaPi-IIa by estrogen likely requires the activation of both receptor isoforms ERα and ERβ. Despite several attempts, we could not find specific and sensitive antibodies against rat ERα and ERβ to further dissect the mechanism(s) by which estrogen downregulates NaPi-IIa in rat kidney. Most of the commercially available antibodies were nonspecific, as shown by others (53). Moreover, the existing proximal tubule cells from rat (NRK), opossum (OK), and HEK (human) either do not express both ER isoforms or express ERα at a very low level by RT-PCR (data not shown). To overcome these difficulties and determine the mechanism by which both ER isoforms mediate the downregulation of NaPi-IIa, we used a human bone cell line (U20S), which stably expresses either ERα alone, ERβ alone, or both ERα and ERβ under doxycycline control (37). Most importantly, ERα and ERβ have been shown to form a functional heterodimer complex, which mediates the regulation of several target genes by estrogen in these cells (37). These cells were transiently transfected with rat kidney full-length NaPi-IIa construct and used for the following experiments.
Effects of estrogen on rat NaPi-IIa expression in U20S cells.
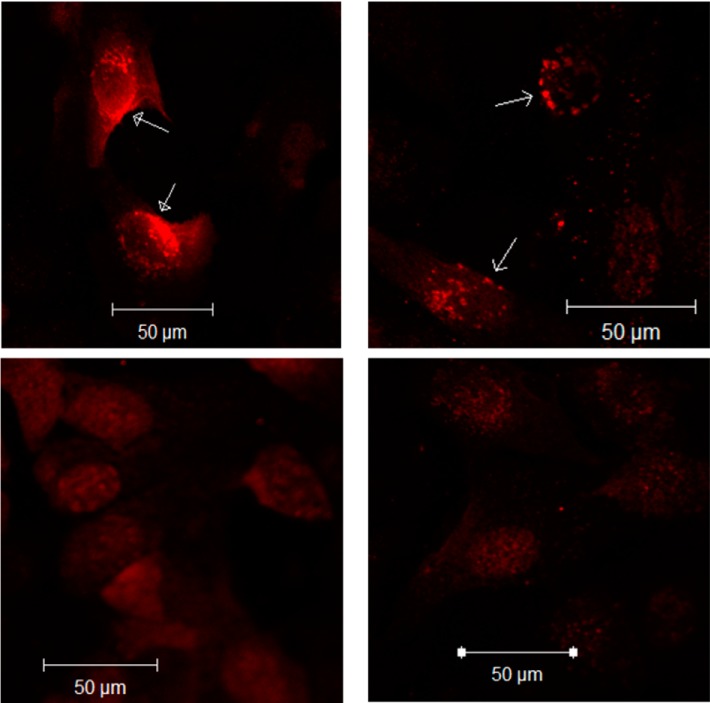
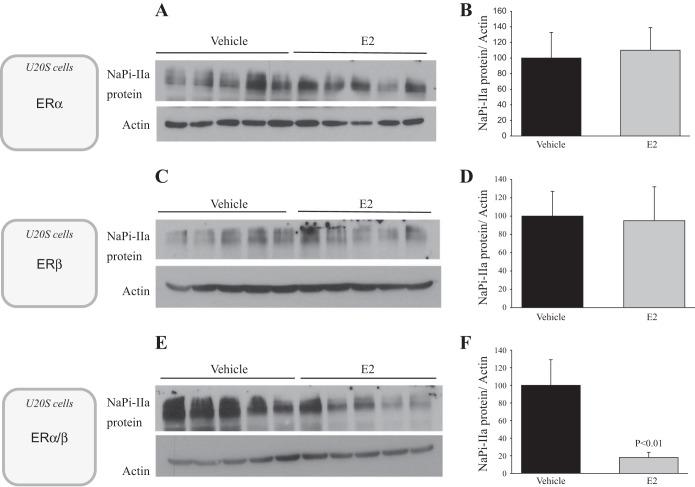
First, Northern blotting experiment demonstrated that doxycycline treatment induced the expression of both ERα and ERβ in U20S cells (Fig. 10, A and B). The induction of ERα precedes that of ERβ, but their mRNA expression levels is significantly higher after 48 h of doxycycline treatment (Fig. 10, A and B). Second, immunoblotting experiment shows that NaPi-IIa protein is expressed only in cells transfected with the rat NaPi-IIa expression plasmid (Fig. 10C). NaPi-IIa protein in these cells is expressed as a ∼160-kDa band, as previously described by other investigators in rat and mouse bone cells (35). Third, immunofluorescence studies depicted in Fig. 11 shows that rat NaPi-IIa is expressed in the plasma membrane of transfected and vehicle-treated U20S cells expressing both ERα and ERβ (Fig. 11, top) and that the abundance of its expression is significantly reduced in the presence of 100 nM estrogen (Fig. 11, bottom). Finally, immunoblotting experiments of U20S cells incubated with 100 nM estrogen or vehicle for 24 h show that NaPi-IIa protein abundance was not altered by E2 in U20S cells expressing ERα alone (P > 0.05; Fig. 12, A and B) or ERβ alone (P > 0.05; Fig. 12, C and D). However, estrogen caused a sharp decrease in the abundance of NaPi-IIa protein (−80%; P < 0.01) in cells expressing both ERα and ERβ (Fig. 12, E and F), compared with the vehicle-treated group.
Fig. 11.
Immunofluorescence of rat NaPi-IIa in U20S cells. U20S cells were grown to confluence and incubated in serum- and phenol red-free medium containing doxycycline. Cells were transfected with rat NaPi-IIa full-length construct and then treated with vehicle (top) or 100 nM E2 (bottom) for 24 h. The immunofluorescence was then revealed by confocal microscopy at original magnification of ×20 with the same settings (gain, brightness, zoom) between groups.
Fig. 12.
Effects of estrogen on NaPi-IIa protein abundance in U20S cells expressing ERα, ERβ, or ERα and ERβ and transfected with rat NaPi-IIa construct. U20S cells were pretreated with doxycycline and transfected with rat NaPi-IIa full-length construct and then treated with E2 (100 nM) or vehicle for 24 h. The Immunoblots show the abundance of NaPi-IIa and actin proteins (A, C, E) and the corresponding densitometric analysis of NaPi-IIa/actin (B, D, F) in cells expressing ERα alone (A, B) or ERβ alone (C, D) or both ERα and ERβ (E, F). Each lane is loaded with 30 μg of protein harvested from a different cell culture dish. Five different dishes were used for each group and in each experiment.
DISCUSSION
We have previously demonstrated that estrogen causes phosphaturia and hypophosphatemia in ovariectomized rats (23). This effect is mediated through the downregulation of the brush border sodium-dependent phosphate cotransporter NaPi-IIa at both the mRNA and protein levels (23). The present studies confirm the phosphaturic and hypophosphatemic effects of estrogen and its suppression of NaPi-IIa expression in a dose-dependent manner (Figs. 1 and 2). Increased electrolyte wasting despite decreased food intake is likely specific to Pi, as chloride excretion and calcium excretion correlated with reduction in food intake in response to estrogen (Fig. 1). Further, the present data clearly show that the phosphaturic effect of estrogen is specific to the downregulation of NaPi-IIa, as the expression of the other apical Pi-absorbing transporters in the proximal tubules (i.e., NaPi-IIc and Pit2) was not affected by estrogen (Table 2). We have used cortical total membrane fractions to examine the expression of NaPi-IIa and NaPi-IIc in these studies. Because both proteins are known to be downregulated by membrane retrieval and lysosomal degradation (reviewed in Ref. 9), a downregulation of NaPi-IIc would have been evident if targeted by estrogen in a chronic setting (i.e., 3-day treatment).
Our results demonstrate the presence of both estrogen receptor isoforms (ERα and ERβ) in the kidney cortex and in the proximal tubule suspension (Fig. 3). The level of expression of these receptors is identical between cortex and PTS preparations (Fig. 3), as the expression of ERα and ERβ in the whole cortex also includes the distal tubule where estrogen has been reported to upregulate calcium-absorbing transporters, at least, in mice kidney (60). These data confirm the presence of ERα and ERβ proteins in the proximal tubule of both mice and rat kidneys (29, 61). Estrogen receptors are ligand-regulated transcription factors that mediate the genomic effects of different estrogenic molecules, including 17β-estradiol (22). Our data (Fig. 3) are also in agreement with several ligand-binding studies, which reported the presence of estrogen receptor in both the cytosol and the nucleus of rat kidney cells (19, 25, 41). These receptors are expressed mainly in S1 and S2 segments of rat proximal tubule (19). All together, these results would suggest that estrogen likely acts directly through these receptors and downregulates NaPi-IIa in the proximal tubule cells. Our previous studies demonstrated that the downregulation of NaPi-IIa and phosphaturia by estrogen are also produced in parathyroidectomized rats, indicating that the estrogen effect on NaPi-IIa is independent of the circulating levels of PTH (23). In addition to PTH, FGF23 is a potent phosphaturic hormone, as it downregulates both NaPi-IIa and NaPi-IIc proteins in the proximal tubule (7, 34, 40, 52). Recent studies demonstrated that estrogen treatment increased FGF23 levels in rats (11). However, our studies clearly demonstrate that estrogen downregulates NaPi-IIa but does not alter NaPi-IIc or Pit2 expression levels (Table 2) in OVX rats. Further, the in vitro studies depicted in Figs. 4 and 11 clearly demonstrate that estrogen directly downregulates NaPi-IIa in proximal tubule suspensions harvested from OVX rat kidneys (Fig. 4), as well as in U20S cells expressing both ERα and ERβ and transfected with a rat NaPi-IIa expression construct (Figs. 11 and 12). Taken all together, these data indicate that FGF23 could not fully account for the downregulation of NaPi-IIa and subsequent phosphaturia in response to estrogen.
Altogether, this and previously published studies (6, 23) clearly define estrogen as a new phosphaturic hormone, which specifically targets NaPi-IIa in the rat kidney proximal tubule. This implies that estrogen is an important regulatory hormone of Pi metabolism. This new role of estrogen also exists in humans and is supported by abundant clinical data in the literature. Indeed, it has been shown that estrogen replacement therapy in postmenopausal women reduces bone resorption and prevents bone loss and osteoporosis (48). Such treatment is associated with a reduction in the circulating levels of inorganic phosphate in postmenopausal women (12, 55, 56). The phosphaturic effect of estrogen is not specific to female subjects, as a significant decrease in serum Pi levels was also reported in male patients with metastatic prostate cancer treated with estrogen (17). On the basis of our studies, it is likely that the hypophosphatemic effect of estrogen observed in these patients results from the downregulation of NaPi-IIa in the kidney with subsequent uncompensated phosphaturia.
Interestingly, the physiological significance of the phosphaturic effect of estrogen is not clear at this time. This effect appears to contradict the general view that this hormone is essential for bone health. Estrogen stimulates absorption of calcium from both the intestine and kidney and stimulates absorption of Pi from the intestine. Hence, it is possible that estrogen promotes bone mineralization, but also increases renal Pi wasting to offset the increase in its intestinal absorption and maintain the blood level of Pi at a very narrow physiologic range. With this coordinated action, estrogen will minimize Ca-PO4 precipitation in soft tissues and prevent vascular calcification and cardiovascular disease. This would suggest that women are more prone to vascular calcification in postmenopausal life in the absence of HRT. Indeed, studies have shown that there is an association between arterial calcification and osteoporosis in postmenopausal women (reviewed in Refs. 21 and 32). Subsequent studies demonstrated that postmenopausal women treated with estrogen exhibited a decrease in the levels of coronary artery calcification (36). However, the role of Pi excretion in this protective effect of estrogen remains to be demonstrated.
Lastly, we examined the respective roles of ER isoforms in estrogen-induced downregulation of NaPi-IIa and phosphaturia in OVX rats. Our results indicate that the specific activation of ERα with PPT caused a significant reduction in food intake (Fig. 5, A and B), as previously shown by others (57). However, unlike estrogen, the decrease in food intake by PPT correlates with a significant reduction in urinary Pi excretion in (Fig. 5C). PPT treatment did not alter NaPi-IIa protein abundance (Fig. 6, A and B), indicating that its activity is maintained during specific activation of ERα, which explains the absence of phosphaturia in PPT vs. pair-fed vehicle animals (Fig. 5C). When ERβ was specifically activated using DPN, food intake was not altered at any dose, whereas urinary Pi excretion was rather reduced at a higher dose (Fig. 7). The abundance of NaPi-IIa protein was unchanged (lower dose) and significantly increased at a higher dose of DPN (Fig. 8, A and B). The increase in NaPi-IIa protein abundance/activity could account for the reduction in urinary Pi excretion observed at a higher dose of DPN (Fig. 7C). However, when the animals were treated with a combination of PPT + DPN (activation of both ERα and ERβ, respectively), a significant reduction in food intake (Fig. 9A), phosphaturia (Fig. 9B), and significant downregulation of NaPi-IIa at both the mRNA (Fig. 9, C and D) and protein levels (Fig. 9, E and F) were observed. These effects of PPT + DPN reproduce the exact effects of estrogen on feeding, renal Pi handling, and NaPi-IIa expression. The effects of estrogen on NaPi-IIa expression and Pi handling are more pronounced than that of PPT + DPN, indicating that estrogen is also likely acting through other pathways, including membrane-bound receptor, such as GPR30, in addition to nuclear receptors ERα and ERβ. The in vivo findings were confirmed by in vitro experiments showing that estrogen decreased the expression of rat NaPi-IIa protein only in U20S cells expressing both ERα and ERβ (Figs. 11 and 12, E and F) but not in cells expressing either ERα alone (Fig. 12, A and B) or ERβ alone (Fig. 12, C and D). In various tissues and cells, including U20S cells, ERα and ERβ have been shown to function as a heterodimer complex and mediate the regulation of a set of genes and cell functions that are different from those regulated by ER homodimers (13, 37, 44, 47). All together, these data clearly demonstrate, for the first time, that the effects of estrogen on NaPi-IIa and Pi transport in the rat kidney proximal tubule require the activation of both ERα and ERβ; whereas its effect on food intake is mediated through the specific activation of ERα. Whether ERα and ERβ form a functional heterodimer in the rat kidney proximal tubule or activate synergistic pathways that downregulate NaPi-IIa remains to be determined when specific and sensitive antibodies for rat ERα and ERβ become available (53). In our previous studies (23), we reported that ICI182,780, an inhibitor of ERα (42, 62), partially inhibited the anorexic effect of estrogen but did not prevent the downregulation of NaPi-IIa by estrogen in OVX rats (23). In light of the present results, it is likely that the dose of ICI182,780 used in these studies was not high enough to completely block the anorexic effect of estrogen or to prevent its downregulation of NaPi-IIa in the kidney.
In conclusion, our data demonstrate that estrogen specifically downregulates NaPi-IIa but not NaPi-IIc or Pit2 in the kidney proximal tubule and causes phosphaturia in OVX rats. This effect is direct and independent of circulating factors, as shown by in vitro proximal tubular suspensions and U20S cells expressing rat kidney NaPi-IIa. The results further demonstrate that inhibition of food intake by estrogen is mediated solely by activation of ERα, whereas the downregulation of NaPi-IIa and phosphaturia are mediated through the activation of both ERα and ERβ.
GRANTS
These studies were supported by National Institutes of Health Grant DK-083582 to H. Amlal.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.B., R.W., S.S., R.F., M.L., J.R.H., and H.A. performed experiments; D.B., R.W., S.S., R.F., and H.A. analyzed data; D.B., R.W., S.S., R.F., and H.A. prepared figures; D.B., R.W., S.S., R.F., M.L., J.R.H., and H.A. edited and revised manuscript; D.B., R.W., S.S., R.F., M.L., J.R.H., and H.A. approved final version of manuscript; H.A. conception and design of research; H.A. interpreted results of experiments; H.A. drafted manuscript.
ACKNOWLEDGMENTS
Parts of these studies were published in abstract form and presented at the annual meeting of the American Society of Nephrology in Atlanta, GA, in November 2013.
REFERENCES
- 1.Abu Hossain S, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: F969–F978, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Adami S, Gatti D, Bertoldo F, Bertoldo F, Rossini M, Fratta-Pasini A, Zamberlan N, Facci E, Lo Cascio V. The effects of menopause and estrogen replacement therapy on the renal handling of calcium. Osteoporos Int 2: 180–185, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Amlal H, Habo K, Soleimani M. Potassium deprivation upregulates expression of renal basolateral Na+-HCO3− cotransporter (NBC-1). Am J Physiol Renal Physiol 279: F532–F543, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M. Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl− cotransporter of the rat medullary thick ascending limb. J Biol Chem 273: 33,681–33,691, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Attmane-Elakeb A, Sibella V, Moreau A, Vernimmen C, Feldmann G, Paillard M, Bichara M. Long-term shake suspension and membrane vesicles of medullary thick ascending limb. Kidney Int 53: 439–447, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Beers KW, Thompson MA, Chini EN, Chini EN, Dousa TP. β-estradiol inhibits Na+-Pi cotransport across renal brush border membranes from ovariectomized rats. Biochem Biophys Res Commun 221: 442–445, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 61: 91–104, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndt TJ, Knox FG. Renal regulation of phosphate excretion. In: The Kidney, Physiology and Pathophysiology, edited by Seldin DW and Giebisch G. New York: Raven, p. 2511–2532, 1992. [Google Scholar]
- 9.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch 458: 39–52, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Breusegem SY, Takahashi H, Giral-Arnal H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Miyamoto K, Barry NP, Levi M. Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol 297: F350–F361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrillo-López N, Román-García P, Rodríguez-Rebollar A, Fernández-Martín JL, Naves-Díaz M, Cannata-Andía JB. Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol 20: 2009–2017, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelo-Branco C, Martínez de Osaba MJ, Pons F, González-Merlo J. The effect of hormone replacement therapy on postmenopausal bone loss. Eur J Obstet Gynecol Reprod Biol 44: 131–136, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty S, Willett H, Biswas PK. Insight into estrogen receptor beta-beta and alpha-beta homo- and heterodimerization: A combined molecular dynamics and sequence analysis study. Biophys Chem 170: 42–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalumeau C, du Cheyron D, Defontaine N, Kellermann O, Paillard M, Poggioli J. NHE3 activity and trafficking depend on the state of actin organization in proximal tubule. Am J Physiol Renal Physiol 280: F283–F290, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citrin DL, Elson P, Kies MS, Lind R. Decreased serum phosphate levels after high-dose estrogens in metastatic prostate cancer. Possible implications. Am J Med 76: 787–793, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Danisi G, Murer H. Inorganic phosphate absorption in small intestine. In: Handbook of Physiology. The Gastrointestinal System: Intestinal Absorption and Secretion, edited by Field M and Frizzel RA. Bethesda, MD: Am Physiol Soc., 1991, p. 323–336. [Google Scholar]
- 19.Davidoff M, Caffier H, Schiebler TH. Steroid hormone binding receptors in the rat kidney. Histochemistry 69: 39–48, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Dennis VW. Phosphate homeostasis. In: Handbook of Physiology: Renal Physiology, edited by Windhager EE. Bethesda, MD: Am Physiol Soc., sect 8, vol. II, 1992, p. 1785–1815. [Google Scholar]
- 21.Doherty TM, Uzui H, Fitzpatrick LA, Tripathi PV, Dunstan CR, Asotra K, Rajavashisth TB. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J 16: 577–582, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67: 335–376, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 73: 1141–1150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallazzini M, Attmane-Elakeb A, Mount DB, Hebert SC, Bichara M. Regulation by glucocorticoids and osmolality of expression of ROMK (Kir 1.1), the apical K channel of thick ascending limb. Am J Physiol Renal Physiol 284: F977–F986, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Hagenfeldt Y, Eriksson HA. The estrogen receptor in the rat kidney. Ontogeny, properties and effects of gonadectomy on its concentration. J Steroid Biochem 31: 49–56, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206: 13–22, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143: 4172–4177, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int 50: 1496–1505, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERα splice variants in the mouse. PLos One 8: e70926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karim Z, Attmane-Elakeb A, Sibella V, Bichara M. Acid pH increases the stability of BSC1/NKCC2 mRNA in the medullary thick ascending limb. J Am Soc Nephrol 14: 2229–2236, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Karim Z, Defontaine N, Paillard M, Poggioli J. Protein kinase C isoforms in rat kidney proximal tubule: acute effect of angiotensin II. Am J Physiol Cell Physiol 269: C134–C140, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 68: 271–276, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper GG, Enmark E, Pelto-Huikko M, Treuter E, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9: 650–660, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Lundquist P, Murer H, Biber J. Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate. Cell Physiol Biochem 19: 43–56, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML, WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med 356: 2591–2602, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 19: 1555–1568, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Monroe DG, Spelsberg T. General mechanisms of steroid receptors and receptor coregulator action. In: Advances in Molecular and Cell Biology, 34th ed., edited by Miller V and Hay M, Amsterdam: Elsevier B.V., 2004, p. 39–48. [Google Scholar]
- 39.Monroe DG, Spelsberg TC. Gonadal steroids and receptors. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 5th ed., edited by Favus MJ, Philadelphia: Lippincot, Williams and Wilkins, 2004, p. 32–38. [Google Scholar]
- 40.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Arch 447: 763–767, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Murono EP, Kirdani RY, Sandberg AA. Specific estradiol-17β binding component in adult rat kidney. J Steroid Biochem 11: 1347–1351, 1979. [DOI] [PubMed] [Google Scholar]
- 42.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96: 1858–1862, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patzer L, Hernando N, Ziegler U, Beck-Schimmer B, Biber J, Murer H. Ifosfamide metabolites CAA, 4-OH-Ifo and Ifo-mustard reduce apical phosphate transport by changing NaPi-IIa in OK cells. Kidney Int 70: 1725–1734, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Paulmurugan R, Tamrazi A, Massoud TF, Massoud TF, Katzenellenbogen JA, Gambhir SS. In vitro and in vivo molecular imaging of estrogen receptor α and β homo- and heterodimerization: exploration of new modes of receptor regulation. Mol Endocrinol 25: 2029–2040, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poggioli J, Lazar G, Houillier P, Gardin JP, Paillard M. Acute variations in extracellular pH modulate transduction pathways of PTH in rat proximal tubule. Am J Physiol Cell Physiol 263: C941–C947, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Poggioli J, Lazar G, Houillier P, Gardin JP, Achard JM, Paillard M. Effects of angiotensin II and nonpeptide receptor antagonists on transduction pathways in rat proximal tubule. Am J Physiol Cell Physiol 263: C750–C758, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Powell E, Xu W. Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proc Natl Acad Sci USA 105: 19012–19017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riggs BL, Jowsey J, Kelly PJ, Jones JD, Maher FT. Effect of sex hormones on bone in primary osteoporosis. J Clin Invest 48: 1065–1072, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87: 39–44, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293: R2194–R2201, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int 65: 1–14, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Silver J, Naveh-Many T. FGF23 and the parathyroid. Adv Exp Med Biol 728: 92–99, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ER β antisera label in ER β knockout and null mouse tissues. J Neurosci Methods 188: 226–234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43: 4934–4947, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Stock JL, Coderre JA, Mallette LE. Effects of a short course of estrogen on mineral metabolism in postmenopausal women. J Clin Endocrinol Metab 61: 595–600, 1985. [DOI] [PubMed] [Google Scholar]
- 56.Stock JL, Coderre JA, Posillico JT. Effects of estrogen on mineral metabolism in postmenopausal women as evaluated by multiple assays measuring parathyrin bioactivity. Clin Chem 35: 18–22, 1989. [PubMed] [Google Scholar]
- 57.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res 1268: 88–96, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Tremblay GB, Tremblay A, Copeland NG, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguère V. Cloning, chromosomal location and functional analysis of the murine estrogen receptor β. Mol Endocrinol 11: 353–365, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Uemura H, Irahara M, Yoneda N, Yasui T, Genjida K, Miyamoto KI, Aono T, Takeda E. Close correlation between estrogen treatment and renal phosphate reabsorption capacity. J Clin Endocrinol Metab 85: 1215–1219, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Van Abel M, Hoenderop JG, Dardenne O, St Arnaud R, Van Os CH, Van Leeuwen HJ, Bindels RJ. 1,25-dihydroxyvitamin D3-independent stimulatory effect of estrogen on the expression of ECaC1 in the kidney. J Am Soc Nephrol 13: 2102–2109, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med 2: 227–237, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Wijayaratne AL, McDonnell DP. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem 276: 35,684–35,692, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Uno JK, Inouye M, Inouye M, Xu L, Drees JB, Collins JF, Ghishan FK. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol 285: G1317–G1324, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Zajicek HK, Wang H, Puttaparthi K, Halaihel N, Markovich D, Shayman J, Béliveau R, Wilson P, Rogers T, Levi M. Glycosphingolipids modulate renal phosphate transport in potassium deficiency. Kidney Int 60: 694–704, 2001. [DOI] [PubMed] [Google Scholar]