Abstract
Transient receptor potential A1 (TRPA1) is a newly defined cationic ion channel, which selectively expresses in primary sensory afferent nerve, and is essential in mediating inflammatory nociception. Our previous study demonstrated that TRPA1 plays an important role in tissue mast cell activation-induced increase in the excitability of esophageal vagal nodose C fibers. The present study aims to determine whether prolonged antigen exposure in vivo sensitizes TRPA1 in a guinea pig model of eosinophilic esophagitis (EoE). Antigen challenge-induced responses in esophageal mucosa were first assessed by histological stains and Ussing chamber studies. TRPA1 function in vagal sensory neurons was then studied by calcium imaging and by whole cell patch-clamp recordings in 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled esophageal vagal nodose and jugular neurons. Extracellular single-unit recordings were performed in vagal nodose and jugular C-fiber neuron subtypes using ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus. Antigen challenge significantly increased infiltrations of eosinophils and mast cells in the esophagus. TRPA1 agonist allyl isothiocyanate (AITC)-induced calcium influx in nodose and jugular neurons was significantly increased, and current densities in esophageal DiI-labeled nodose and jugular neurons were also significantly increased in antigen-challenged animals. Prolonged antigen challenge decreased esophageal epithelial barrier resistance, which allowed intraesophageal-infused AITC-activating nodose and jugular C fibers at their nerve endings. Collectively, these results demonstrated that prolonged antigen challenge sensitized TRPA1 in esophageal sensory neurons and afferent C fibers. This novel finding will help us to better understand the molecular mechanism underlying esophageal sensory and motor dysfunctions in EoE.
Keywords: eosinophilic esophagitis, nodose, jugular, dysphagia, heartburn
eosinophilic esophagitis (EoE) is characterized by increased infiltrations of eosinophils and mast cells in the esophagus. Patients with EoE often present with symptoms that are considered to relate to esophageal dysfunctions, such as dysphagia, food impaction, and esophageal pain or heartburn (3). Altered function of the esophageal sphincter and swallowing reflexes, as well as painful swallowing, can be a result of dysregulation of neuronal activity in the esophagus. This is in keeping with the altered sensory and autonomic nerve function that typifies the allergic inflammatory response in all tissues (21). Little progress has been made in our understanding of mechanisms by which immune processes and tissue remodeling lead to alteration of sensory nerve function and their contribution to the symptoms of allergic esophageal disorders.
Vagal afferents in the esophagus, not only participate in maintaining esophageal physiological functions, but also play the important role in sensing potential tissue damage from noxious stimuli. Sensory transductions in esophageal vagal afferents in response to different stimuli require specific ion channels and receptors. Inflammatory mediators released in the tissue can activate and/or sensitize those ion channels and receptors in sensory nerve terminals, which leads to heightened sensitivity to stimuli that normally evoke no or mild painful sensation, so-called inflammatory hyperalgesia (14). Transient receptor potential A1 (TRPA1) is a newly identified nonselective cationic ion channel, which is selectively expressed in sensory neurons and nociceptive afferent C fibers. TRPA1 acts as a sensor for chemical irritants and inflammatory mediators in addition to modulating inflammatory nociception (5, 11, 14). Whether allergic inflammation in the esophagus sensitizes TRPA1 in esophageal afferents has yet to be determined.
Our previous studies have demonstrated that TRPA1 is selectively expressed in transient receptor potential vanilloid (TRPV1)-positive small- and medium-sized esophageal nodose and jugular neurons and plays crucial roles in regulating bradykinin- and mast cell activation-induced hyperexcitabilities in esophageal vagal nociceptive C-fiber subtypes (23, 26). Our newly published studies demonstrated that repeated antigen challenge in vivo in antigen-sensitized guinea pig led to allergic inflammation in the esophagus and increased acid responsiveness via sensitization of TRPV1 in esophageal vagal nodose and jugular neurons (10, 29). Using these established models and methods, in the present study, we tested the hypothesis that prolonged antigen challenge induces allergic response in the esophagus and sensitizes TRPA1 in esophageal vagal nociceptive afferent subtypes. Our results supported the conclusion that repeated antigen challenge in vivo for 2 wk increased the infiltrations of both eosinophils and mast cells in the esophagus, decreased epithelial resistance, and sensitized TRPA1 in esophageal nodose and jugular neuron and C-fiber subtypes.
MATERIALS AND METHODS
Antigen sensitization and antigen challenge.
The experiments were started with 4-wk-old male guinea pigs (Hilltop, Scottsdale, PA) weighing ∼150–200 g. All animals were kept in pathogen-free conditions and handled under approved protocols of the Johns Hopkins University Animal Care and Use Committee (ACUC No. GP12M417). Antigen sensitization and challenge were performed as previously described (10, 24, 27). Briefly, guinea pigs were sensitized by three intraperitoneal injections of 10 mg/kg ovalbumin (OVA) in saline every 48 h. Three weeks after the last injection, guinea pigs were challenged for 30 s each morning with aerosolized 0.1% OVA for 2 wk. This group (OVA sensitization plus OVA challenge) was expressed as OVA (2w). The weight of OVA (2w) guinea pigs averaged 563 ± 19 g, and naïve animals with the same age (11 ± 1 wk) and weight (578 ± 15 g) were used as controls.
Histological assessments.
For hematoxylin and eosin (H and E) staining, the esophagus was fixed in 10% formalin solution (Sigma-Aldrich, St. Louis, MO) for 24 h, embedded in paraffin blocks, cut into 6-μm cross-sections, and placed on slides. The slides were dewaxed with fresh xylenes and a descending ethanol series (100%, 95%, 70%), stained in diluted hematoxylin for 2 min, destained in running tap water for 5 s, and then counterstained in eosin solution for 10 s. Finally, the slides were dehydrated through ascending ethanol series (70%, 95%, 100%) into xylene and mounted with coverslips. For Giemsa staining, the esophagus was fixed in 4% paraformaldehyde in phosphate buffer pH 7.4 for 24 h, embedded in optimal cutting temperature compound (OCT; Sakura Finetek, Torrance, CA) at −20°C, cut into 12-μm cross-sections, mounted on lysine-coated slides (Fisher, Waltham, MA), and then allowed to air dry for 30 min at room temperature before being stained. Slides were rinsed with deionized water for 1 min, stained with diluted Giemsa buffer for 30 min, rinsed in deionized water for 1 min, then differentiated with 0.5% aqueous acetic acid for 1 min. Slides were dehydrated through ethanol series, cleared in xylene, and mounted with coverslips. For Toluidine blue staining, the esophagus was fixed in Carnoy's solution (60% ethanol, 30% chloroform, 10% glacial acetic acid) for 24 h, cut into 12-μm cross-sections from frozen OCT-embedded blocks, mounted on lysine-coated slides, and air dried for 30 min at room temperature before being stained. Slides were rinsed by deionized water for 1 min, stained with Toluidine blue (1% in 0.1 N HCl) for 1 min, rinsed in deionized water for 1 min, then dehydrated through ethanol series, cleared in xylene, and mounted with coverslips.
All histological slides were analyzed by a researcher blinded to the identities of the samples. The inflammation grade of the esophagus was evaluated under H and E stain according to our previous reported method (29), including the assessments of active inflammation (neutrophil infiltration in the epithelium), the length of vascular papillae, basal-zone hyperplasia, and the number of intraepithelial eosinophils. The total numbers of eosinophils and mast cells were counted per cross-section in Giemsa- and Toluidine blue-stained slides by the aid of an Olympus light microscope (Olympus, Tokyo, Japan) at ×400 high-power field.
Ussing chamber studies.
The Ussing chamber experiments were performed as described previously (9). Briefly, freshly isolated esophagi from either naïve or OVA (2w) animals were cut into four 1-cm segments, split longitudinally, and mounted on the Ussing chamber (Physiologic Instruments, San Diego, CA). Segments of tissues were set as a flat sheet between two Lucite-modified slides, and 0.036 cm2 of tissue was exposed to 10 ml of Krebs bicarbonate buffer (composed of 118 mM NaCl, 5.4 mM KCl, 1.0 mM NaH2PO4, 1.2 mM MgSO4, 1.9 mM CaCl2, 25.0 mM NaHCO3, and 11.1 mM dextrose, gassed with 95% O2-5% CO2) at 37°C. The transepithelial potential difference was detected by two paired electrodes, which contained 4% agar in 3 mol/l KCl. The electrodes were connected to a current-clamp amplifier. The potential difference between electrodes was compensated before tissue was mounted into the chamber. After equilibrium was adjusted, potential difference was continuously monitored under open circuit; voltage of each chamber was recorded for 30 min every 20 s, and the average transepithelial resistance (TER) was calculated by Ohm's law. The esophagus was cut into four segments and recorded in four chambers, and then averaged TER was calculated for each animal.
Calcium imaging.
TRPA1 agonist allyl isothiocyanate (AITC)-induced calcium influx responses were studied using calcium imagings in dissociated nodose and jugular neurons, as described previously (10). Briefly, cultured vagal sensory neurons were loaded with 2 mM fura 2-AM and 0.05% Pluronic F-127 dissolved in normal extracellular solution (ECS, in mM: 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH) in a dark environment at 37°C for 45 min. After being washed three times with ECS, these neurons were allowed to deesterify for at least 30 min before use. Fluorescence changes were measured with a Zeiss Upright Scope equipped with PTI-RatioMaster. Chemicals were applied with a custom-built perfuse system. At the end of each experiment, a 50 mM KCl buffer (95 mM NaCl, 50 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES and 10 mM glucose, adjusted to pH 7.4 with NaOH) was applied to distinguish excitable cells. Only KCl-responsive cells were considered to be excitable cells and used for analysis.
Retrograde labeling and patch-clamp recording.
Retrograde labeling of nodose and jugular neurons from the esophagus with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probe, Eugene, OR) was performed in four guinea pigs according to our previously described method (10, 28). Briefly, animals were anaesthetized with ketamine (80 mg/kg) and xylazine (5 mg/kg) dissolved in PBS. Supplemental anesthesia was given as needed to abolish the hindpaw-pinch reflex. The cervical esophagus was surgically exposed, and DiI solution (1–2 μl, 1% in dimethyl sulphoxide and normal saline mixture) was injected in the wall of the esophagus at 50–60 mm above the gastric-esophageal junction (the injection site was confirmed by the time to dissect the ganglia). Each esophagus received two to three injections. The animals were sutured and allowed to recover for ∼2 wk for sufficient labeling of cell bodies in the vagal ganglia. Postoperatively, animals were carefully monitored on an hourly basis for several hours and twice daily thereafter and if necessary treated for pain until totally recovered. Any animal that displayed behaviors indicating excessive pain or infection was killed immediately by overdose of CO2. After 2 wk, both nodose and jugular ganglia (two of each per animal) were collected and disassociated (see above) for whole cell patch-clamp recordings.
Whole cell patch-clamp recordings in DiI-labeled esophageal nodose and jugular neurons were performed according to our previously described methods (10). Briefly, Borosilicate glass (WPI, Sarasota, FL) electrodes were 2–3 MΩ when filled with the pipette solution (in mM): 140 CsCl, 1 MgCl2, 5 MgATP, 2 EGTA, 10 HEPES (pH 7.2 with CsOH). Whole cell patch-clamp recordings were performed using an Axopatch 200B patch-clamp amplifier and Axograph software (Axon Instruments, Foster City, CA). Currents were typically digitized at 10 kHz and filtered at 2 kHz. The whole cell currents were recorded using a voltage ramp from −100 mV to 100 mV in 100-ms duration while cells were patched with a holding potential of 0 mV.
Extracellular single-unit recording ex vivo.
Extracellular single-unit recordings from nodose or jugular neurons were performed in ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus according to our previous studies (28, 29). Briefly, an aluminosilicate glass microelectrode (pulled with a Flaming-Brown micropipette puller; Sutter Instrument, Novato, CA) filled with 3 M sodium chloride (electrode resistance 2 MΩ) was placed into an electrode holder connected directly to the headstage (A-M Systems, Everett, WA). A return electrode of silver-silver chloride wire and earthed silver-silver chloride pellet was placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut off, 0.3 kHz; high cut off, 1 kHz), and the resultant activity was displayed on an oscilloscope (TDS 340; Tektronix, Beaverton, OR). The data were stored and analyzed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD).
The recording electrode was micromanipulated into the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when esophageal distension (with a rapid increase in intraluminal pressure to 60 mmHg for 5 s) evoked action-potential discharge. Conduction velocity was calculated by dividing the length of the approximated nerve pathway by conduction time. The peak frequency (Hz) was defined as the maximal frequency of action-potential discharge. On the basis of our previous studies, we selected distension-sensitive esophageal vagal C fibers for two considerations. First, mechanical distension-evoked action-potential discharges were easy to identify by distending the whole esophagus followed by electric stimulation to confirm the specific receptive field in the esophagus. Second, mechanical distension-evoked action-potential discharges were consistent and repeatable for more than 8 h. If we used chemicals to search the afferent fiber, most of the chemical-evoked action-potential discharges could be sensitized or desensitized by those chemicals themselves, making it difficult to compare the sensitization effect thereafter (26, 28).
After we recorded the baseline spontaneous activity and mechanical excitability (esophageal distension under the pressure of 10, 30, and 60 mmHg) of esophageal vagal C fiber, TRPA1 agonist AITC (380 μM) was infused into the lumen of the esophagus for 30 min. The action-potential discharges of esophageal nodose or jugular C fibers induced by AITC were monitored continuously for 30 min and analyzed both in 1-s bins (yielding the number of action potentials in each second, Hz) in 5-min intervals for 30 min. The esophageal distension-evoked responses of these fibers were also detected at the end of agonist perfusion.
Chemicals.
All chemicals used in the experiments were purchased from Sigma-Aldrich unless stated otherwise. Collagenase/dispase was purchased from Roche Applied Science (Indianapolis, IN). FBS, Hanks' balanced salt solution (HBSS), and Pluronic(R) F-127 were purchased from Life Technologies (Grand Island, NY). Collagenase/dispase (2 mg/ml) and laminin (5 μg/ml) were prepared in sterile Ca2+/Mg2+-free HBSS, and fura 2-AM (2 M) was prepared in acetone and poly-l-lysine (1 mg/ml) and diluted in sterile water. All the stock solutions were separated into small aliquots and stored in −20°C, and working solutions were prepared freshly on the day of use.
Data analysis.
Results from histological and Ussing chamber studies were expressed as means ± SE. Differences between the values were determined by Student's t-test or one-way ANOVA, and P < 0.05 was considered statistically significant.
In calcium-imaging studies, neurons were defined as “responders” to a given compound if the mean response was greater than the mean baseline plus 2 × the standard deviation using unpaired t-test. Patch-clamp data were analyzed with Sigmaplot 11.0 (SPSS, Chicago, IL). All data are presented as means ± SE. Statistical comparisons were made with unpaired Student's t-test and Wilcoxon rank-sum test, and differences were considered significant at P < 0.05.
In extracellular recording, TRPA1 agonist-evoked C-fiber response was quantified as peak frequency of action-potential discharges within a 5-min period and averaged from six recordings for a total of 30 min. The peak frequencies (Hz) of action-potential discharges were presented as means ± SE and compared by paired t-test or one-way ANOVA. For all experiments, significance was defined as P < 0.05.
RESULTS
Prolonged antigen challenge led to allergic inflammation in the esophagus.
Histological assessments in the esophagus were performed in naïve and antigen-challenged animals. Under H and E stain, OVA challenge did not induce gross tissue damages (such as ulcer or erosion) or change thicknesses of each layer in the esophagus (data not shown). However, the inflammation score was significantly increased (naïve vs. OVA 2w: 2.0 ± 0.47 vs. 4.33 ± 0.27, P < 0.05, n = 5 in each group) (Fig. 1A). Moreover, we found that OVA challenge for 2 wk significantly increased the infiltration of both eosinophils and mast cells in the esophagus. Increased mast cells were observed in both mucosal (from 8.2 ± 2.1 to 63.5 ± 4.5/cross-section, P < 0.05, n = 5 in naïve and n = 6 in OVA 2w) and muscle layers (from 14.0 ± 2.4 to 43.1 ± 4.5/cross-section, P < 0.05, n = 5 in naïve and n = 6 in OVA 2w). Increased eosinophils mainly occurred in the mucosa (from 2.6 ± 0.9 to 63.5 ± 20.0/cross-section, P < 0.05, n = 5 in naïve and n = 6 in OVA 2w) but only slightly in the muscle layer (from 0 to 5.2 ± 2.55/cross-section, P < 0.05, n = 5 in naïve and n = 6 in OVA 2w) (Fig. 1, B and C). These data demonstrated that OVA challenge for 2 wk led to the development of chronic allergic inflammation, which featured with predominant infiltrations of both mast cells and eosinophils in guinea pig esophagus.
Fig. 1.
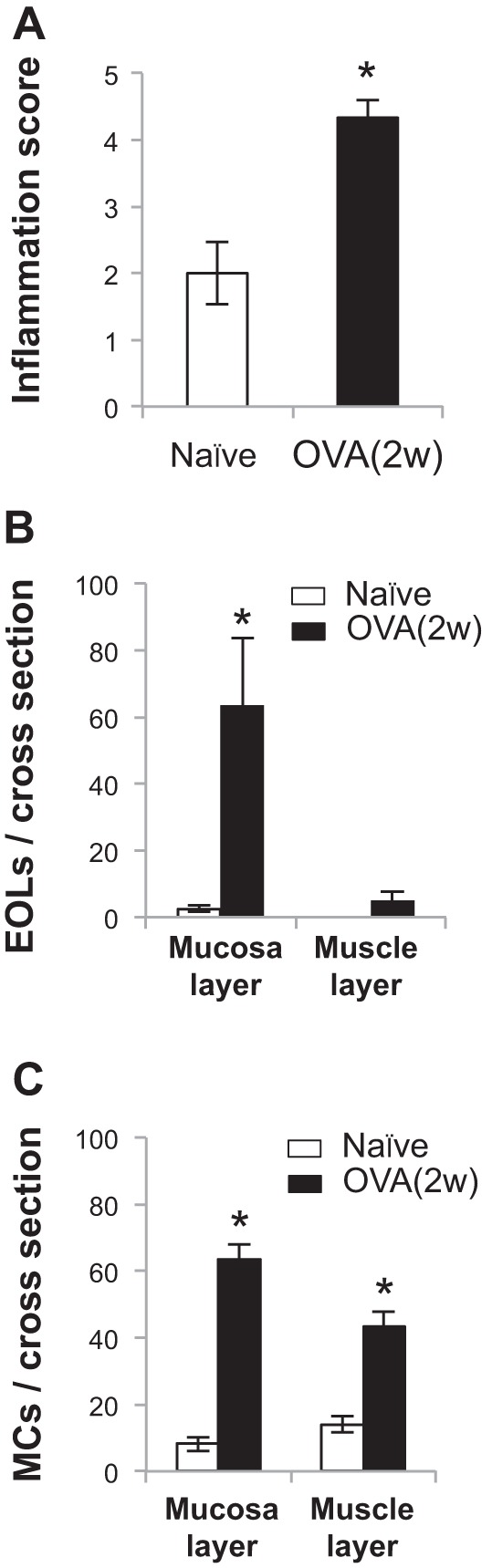
Histological assessments of the esophagus. A: with the use of hematoxylin and eosin stain, in guinea pigs, ovalbumin (OVA) challenge for 2 wk [OVA (2w)] did not induce gross tissue damage but increased the inflammation score in esophagus [naïve vs. OVA (2w): 2.0 ± 0.47 vs. 4.33 ± 0.27, P < 0.05, n = 5 in each group]. B: in Giemsa stain, OVA (2w) increases eosinophil (EOL) numbers per cross-section mainly in mucosa layer (naïve vs. OVA: 2.6 ± 0.9 vs. 63.5 ± 20.00, *P < 0.05) but slightly in muscle layer [naïve vs. OVA (2w): 0 vs. 5.2 ± 2.55, *P < 0.05]. C: in Toluidine blue stain, OVA (2w) increases mast cell (MC) numbers both in mucosa layer [naïve vs. OVA (2w): 8.2 ± 2.1 vs. 63.5 ± 4.5, *P < 0.05] and muscle layer [naïve vs. OVA (2w): 14.0 ± 2.4 vs. 43.1 ± 4.5, *P < 0.05] (for both stains, naïve group: n = 5, OVA: n = 6).
Antigen challenge increased the permeability of esophageal epithelium.
Esophageal epithelial barrier function was studied by the Ussing chamber method. Our result demonstrated that prolonged OVA challenge for 2 wk significantly decreased the TER in the esophagus (naïve vs. OVA 2w: 564.7 ± 63.4 vs. 356.0 ± 45.5 Ω/cm2, n = 10 in naïve and n = 9 in OVA 2w groups, P < 0.05) (Fig. 2). Decreased TER indicated a disruption of esophageal epithelial barrier function, which occurred after repeated OVA challenge for 2 wk.
Fig. 2.
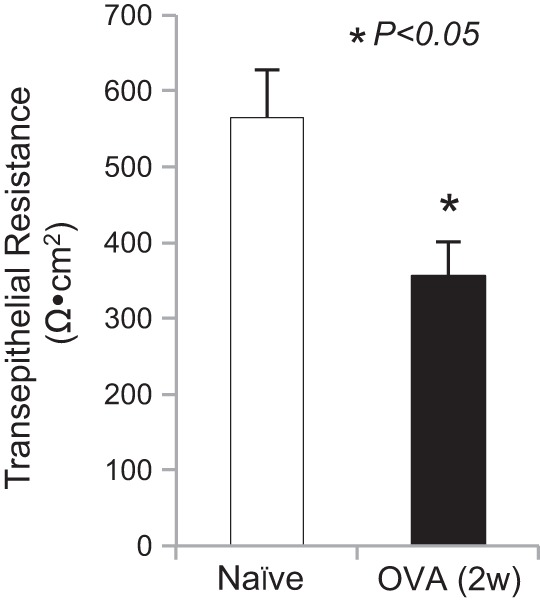
Antigen challenge decreased transepithelial resistance (TER) in the esophagus. Ussing chamber measurements of TERs in the esophagus were compared among naïve guinea pigs (n = 10) and OVA (2w) (n = 9). OVA inhalation significantly decreased TER in the esophagus after repeated antigen challenges for 2 wk [naïve vs. OVA (2w): 564.7 ± 63.4 vs. 356 ± 45.5 Ω/cm2, *P < 0.05].
Antigen challenge increased calcium influx induced by TRPA1 agonist AITC in nodose and jugular neurons.
With the use of calcium-imaging assay, the possible functional changes of TRPA1 after antigen challenge were compared in the nodose and jugular neurons between naïve (n = 7) and OVA-challenged guinea pigs (OVA 2w, n = 5) using AITC as a TRPA1 agonist. As shown in Fig. 3, significantly increased populations of AITC-responsive nodose and jugular neurons were observed in OVA-challenged guinea pigs compared with in naïve animals. About 66.7% (102/153) of nodose neurons can be activated by AITC (100 μM) in OVA-challenged animals, whereas the percentage was 43.5% (74/170) in naïve guinea pigs. Similarly, the percentage of AITC-responsive jugular neurons was increased from 38.9% (100/257) in the naïve group to 51.9% (83/160) in the OVA-challenged group (both P < 0.05) (Fig. 3).
Fig. 3.
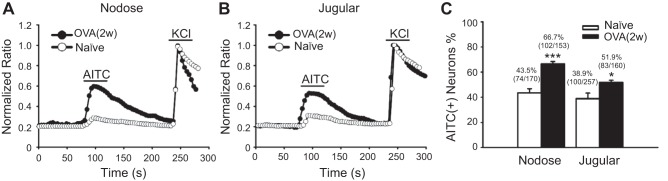
Antigen challenge increased allyl isothiocyanate (AITC)-responsive neurons in both nodose and jugular ganglia. A: representative traces of AITC (100 μM)-induced calcium influx in nodose neurons from naïve and OVA-challenged guinea pigs. B: representative traces of AITC-induced calcium influx in jugular neurons from naïve and OVA-challenged guinea pigs. C: summary of the percentages of AITC-responsive neurons in all KCl-responsive nodose and jugular neurons from naïve (n = 7 each) and OVA-challenged (n = 5 each) guinea pigs (*P < 0.05 and ***P < 0.001 were the levels of significance for naïve vs. OVA-challenged groups using two-tailed unpaired t-test).
Antigen challenge increased current density elicited by TRPA1 agonist AITC in esophageal DiI-labeled nodose and jugular neurons.
To further investigate how TRPA1 function in sensory neurons specifically innervated the esophagus, nodose and jugular neurons were retrogradely labeled by DiI injections in the esophagus, and whole cell patch-clamp recordings in DiI-labeled neurons were performed 10–14 days thereafter. Perfusion with 100 μM AITC could elicit large currents in those labeled neurons from both naïve and OVA-challenged animals. In DiI-labeled nodose neurons, 100 μM AITC activated currents in 10/15 of the neurons from the naïve group with an average current density of 24.3 ± 5.4 pA/pF. Such response was significantly increased in OVA-challenged animals, by which 7/10 of nodose neurons responded to AITC with a significantly increased current density of 59.7 ± 4.7 pA/pF (Fig. 4A). Similarly, in DiI-labeled jugular neurons, 8/13 of the neurons from the naïve group were activated by 100 μM AITC (current density = 31.5 ± 5.3 pA/pF), whereas 8/11 of the neurons in the OVA-challenged group were activated by AITC with a significantly increased current density (65.8 ± 6.2 pA/pF) (Fig. 4B).
Fig. 4.
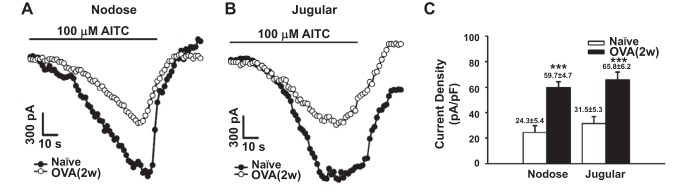
Antigen challenge increased AITC-elicited current densities in 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)-labeled esophageal nodose and jugular neurons. A: representative current traces of AITC (100 μM)-induced inward currents in nodose neurons from naïve (○) and OVA-challenged [●, OVA (2w)] guinea pigs. B: representative current traces of AITC (100 μM)-induced inward currents in jugular neurons from naïve guinea pigs (○) and OVA-challenged [●, OVA (2w)] guinea pigs. C: compared with naïve animals, OVA challenge significantly increased AITC-evoked current densities in DiI-labeled esophageal nodose (24.3 ± 5.4 vs. 59.7 ± 4.7 pA/pF, ***P < 0.001) and jugular (31.5 ± 5.3 vs. 65.8 ± 6.2 pA/pF, ***P < 0.001) neurons.
Antigen challenge increased action-potential discharges evoked by TRPA1 agonist AITC in esophageal nodose and jugular C fibers.
In extracellular recordings, the average conduction velocity of esophageal nodose C fibers was 0.55 ± 0.06 m/s in naïve (n = 8) and 0.74 ± 0.07 m/s in OVA-challenged (n = 8) animals. Those of jugular C fibers were 0.99 ± 0.1 m/s in naïve (n = 8) and 0.86 ± 0.09 m/s in antigen-challenged (n = 8) animals. In esophageal nodose C fibers, intraluminal infusion with AITC for 30 min did not evoke activation response in naïve animals. The peaks of action-potential discharges did not significantly increase over the baseline activity during the 30-min infusion with AITC (0.88 ± 0.3 vs. 1.13 ± 0.30 Hz, P > 0.05, n = 8). Allergen challenge significantly increased AITC-evoked activation responses. The peaks of action-potential discharges significantly increased over the baseline activity (0.75 ± 0.25 vs. 4.63 ± 1.03 Hz, P < 0.01, n = 8) during the 30-min infusion with AITC (Fig. 5, A and C). Similarly, in esophageal jugular C fibers, intraluminal infusion with AITC did not evoke activation response in naïve animals. The peaks of action-potential discharges did not significantly increase over the baseline activity during the 30-min infusion with AITC (1.25 ± 0.25 vs. 1.5 ± 0.33 Hz, P > 0.05, n = 8). Allergen challenge significantly increased AITC-evoked activation responses. The peaks of action-potential discharges significantly increased over the baseline activity (0.88 ± 0.25 vs. 2.5 ± 0.38 Hz, P < 0.01, n = 8) during the 30-min infusion with AITC (Fig. 5, B and D).
Fig. 5.
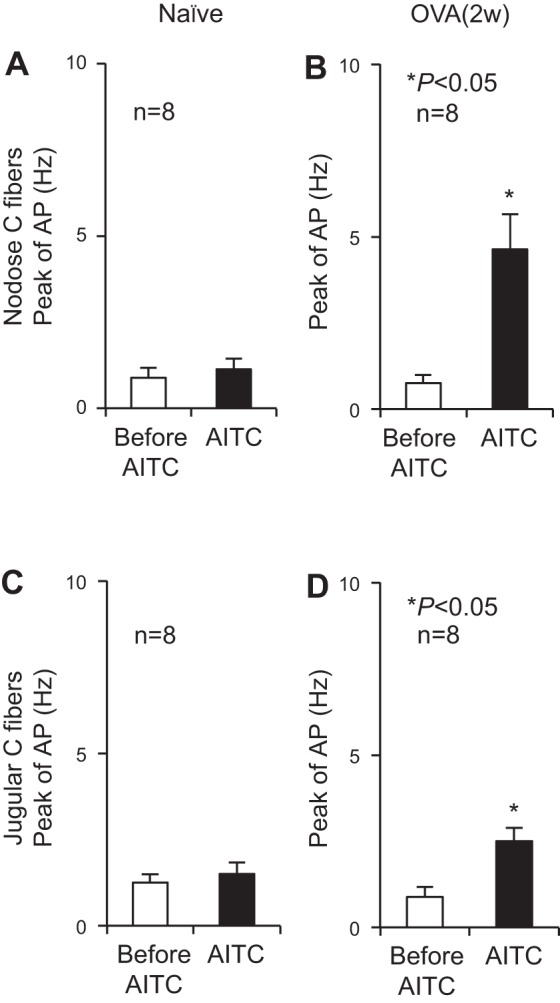
Intraesophageal infusion of AITC-evoked action-potential (AP) discharges in esophageal nodose and jugular C fibers after antigen challenge. A and B: in esophageal nodose C fibers, intraesophageal infusion of AITC for 30 min did not evoke activation responses in naïve animals but significantly increased AP discharges (from 0.75 ± 0.25 to 4.63 ± 1.03 Hz, *P < 0.01, n = 8) in OVA-challenged guinea pigs [OVA (2w)]. C and D: similarly, in esophageal jugular C fibers, intraesophageal infusion of AITC for 30 min did not evoke activation responses in naïve animals but significantly increased AP discharges (from 0.88 ± 0.25 to 2.5 ± 0.38 Hz, *P < 0.01, n = 8) in OVA-challenged guinea pigs [OVA (2w)].
DISCUSSION
EoE has emerged as an allergic disorder in the esophagus affecting both adult and pediatric populations for the last two decades. The diagnosis of EoE mainly depends on the symptoms of esophageal dysfunction and eosinophil count in esophageal mucosal biopsy. The clinical symptoms of EoE are varied among young children, adolescents, and adults, which may present as poor appetite/vomiting, heartburn, food impaction, and dysphagia (3, 7, 19). At present, how allergic inflammation leads to esophageal dysfunction is still less clear, and whether allergen exposure sensitizes esophageal sensory nerves is largely unknown. Allergy-related symptoms often are the result of alterations in the nervous system, depending on the organ and tissue in which the allergic reaction occurs, and could present as red itchy eyes, bronchoconstriction, and altered sensory and motor functions in the gastrointestinal tract (8, 21). With the use of antigen inhalation in antigen-sensitized guinea pigs, the present study added new knowledge and demonstrated that repeated allergen exposure increased mucosal infiltrations of eosinophils and mast cells, disrupted epithelial barrier function, and sensitized TRPA1 in vagal afferents in the esophagus. The present study exposed young animals to antigen repeatedly to induce allergic inflammation and sensory afferent dysfunction in the esophagus, which seems to apply to EoE in a young population.
Increased infiltration of eosinophils (and mast cells) in the esophagus has been considered a hallmark of EoE (2, 3, 4). The present study demonstrated that prolonged antigen challenge significantly increased infiltrations of eosinophils and mast cells in the esophagus. Increased eosinophils were mainly observed in mucosa, whereas mast cells were identified in both the mucosal and muscle layer of the esophagus. These features are consistent with clinical findings (2, 4) and in agreement with the results from mouse EoE models (15, 16, 17). Our data also revealed that prolonged allergen challenge did not induce severe structural changes (such as edema, erosion, and ulceration) in esophageal epithelium, as revealed by histological assessment, but significantly reduced epithelial barrier resistance. This is consistent with our newly published study that demonstrated that repeated antigen challenge for 3 days leads to decreased expression of tight-junction proteins and increased epithelial permeability in guinea pig esophagus (29), which is in agreement with a recent clinical observation that revealed a reduced expression of junction proteins in the esophagus in patients with EoE (1). In addition, the present study adds new knowledge on prolonged allergen challenge-induced sensory nerve dysfunction in the esophagus. Our results demonstrated that a brief allergen challenge every day for 2 wk sensitized TRPA1 in vagal sensory neurons and afferent C fibers in the esophagus.
TRPA1 is a nonselective cationic ion channel that selectively expressed in small- and medium-sized sensory neurons and afferent C fibers. It is well accepted that TRPA1 plays an essential role in chemical irritants and inflammatory mediator-induced inflammatory nociception (12). Our previous studies demonstrated that TRPA1 played a crucial role in acute mast cell activation-induced sensitization of esophageal vagal afferent C fibers (24). The present data extend to demonstrate that prolonged allergen challenge led to sensitization of TRPA1 in esophageal nociceptive afferents. This novel finding was demonstrated both at the neuronal cell body by patch-clamp recording in esophageal DiI-labeled nodose and jugular neurons and at the nerve terminals using extracellular single-unit recording in esophageal nodose and jugular C fibers. At present, the relative contributions of mast cell vs. eosinophils to such a sensitization effect in this EoE model are challenging to differentiate. The results from our previous studies support the notion that inflammatory mediators released from both mast cells and eosinophils in the tissue could contribute to such a sensitization effect. First, mast cell degranulation-released tryptase can sensitize TRPA1 in esophageal nodose C fibers via a protease-activated receptor 2-dependent mechanism (23). Second, synthetic cationic protein, which is similar to eosinophil cationic protein, is able to sensitize esophageal nodose C fiber (25). In addition, mediators released from nonimmune cells in inflamed tissue may also sensitize TRPA1. For example, our other study demonstrated that bradykinin was able to sensitize TRPA1 and induce hyperexcitabilities in esophageal nodose and jugular C fibers (26). It is noteworthy that intraesophageal AITC-activated esophageal vagal C fibers may require both disruption of epithelial barrier and sensitization of TRPA1 in esophageal nociceptive afferent, which allows AITC to reach to C-fiber nerve terminal and lowers the threshold of activation response to AITC in esophageal nociceptive C fibers. This is similar to our newly published study showing that repeated allergen challenge sensitized TRPV1 and disrupted the epithelial barrier, which led to intraesophageal acid-activating esophageal nodose C fiber (29). It is noteworthy that, in extracellular single-unit recording, each esophageal nodose C fiber in the present study was identified by esophageal distension at the beginning of the study. This might miss a subpopulation of distension-insensitive units, which may or may not display the similar response pattern to allergen challenge.
The consequence of sensitization of TRPA1 in esophageal nociceptive afferents by allergic inflammation, so far, has not been experimentally addressed. It may contribute to esophageal dysfunction in EoE for two reasons. First, TRPA1 can directly mediate inflammatory hyperalgesia (12), making it a strong candidate in mediating esophageal painful sensation under allergic inflammation condition. Second, sensitizing TRPA1 in nociceptive afferent may lead to neurogenic inflammation by release of neuropeptides such as substance P and calcitonin gene-related peptide in the esophagus, which are able to regulate both sensory nerve and smooth muscle functions (20). Such consequences have recently been reported in airway and skin that sensitization of TRPA1 by allergic inflammation contributed to airway hyperreactivity in asthma (6, 13) and to itch sensation in atopic dermatitis (22). It is of considerable interest to further investigate TRPA1 sensitization-induced esophageal sensory and motor dysfunctions in this animal model of EoE.
In summary, the present study for the first time demonstrated that prolonged allergen challenge sensitized TRPA1 in esophageal vagal sensory neurons and afferent C fibers. This adds new knowledge on allergic inflammatory-induced sensitization of esophageal afferents and will help us to better understand the molecular mechanism of esophageal sensory/motor dysfunction in EoE. Targeting on the key molecular downstream receptors of inflammatory mediators in esophageal nociceptor will add new treatment approaches to relieve esophageal dysfunction-related symptoms in patients with EoE.
GRANTS
This study was supported by NIH grant DK087991 (S. Yu) and supported by the Johns Hopkins Conte Digestive Disease Core Center for histological data process, calcium imaging, and Ussing chamber study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.L., X.F., C.-M.T., A.C.M., X.L., and S.Y. conception and design of research; Z.L., Y.H., X.Y., and J.X. performed experiments; Z.L., Y.H., X.Y., J.X., and S.Y. analyzed data; Z.L., Y.H., X.Y., J.X., C.-M.T., A.C.M., and S.Y. interpreted results of experiments; Z.L., Y.H., X.Y., J.X., and S.Y. prepared figures; Z.L., Y.H., X.Y., and S.Y. drafted manuscript; Z.L., Y.H., X.Y., P.J.P., and S.Y. edited and revised manuscript; Z.L., Y.H., X.Y., J.X., X.F., C.-M.T., A.C.M., X.L., and S.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Eric Zaccone (JHU) for the comments on the manuscript.
REFERENCES
- 1.Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, Brown KL, Hansen M, Butcher KF, Eidelwein AP, Noel RA, Rabon E, Posta A, Nakhoul NL. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clin Immunol 148: 265–278, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol 126: 140–149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aceves SS. Eosinophilic esophagitis. Immunol Allergy Clin North Am 35: 145–159, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol 126: 1198–1204, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106: 9099–9104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology 147: 1238–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 454: 445–454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoque KM, Woodward OM, van Rossum DB, Zachos NC, Chen L, Leung GP, Guggino WB, Guggino SE, Tse CM. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol 135: 43–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Liu Z, Yu X, Pasricha PJ, Undem BJ, Yu S. Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 307: G149–G157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 29: 355–384, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27: 3549–3563, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Hand Exp Pharmacol 194: 227–257, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 302: G1347–G1355, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107: 83–90, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 304: G1087–G1094, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Straumann A, Aceves SS, Blanchard C, Collins MH, Furuta GT, Hirano I, Schoepfer AM, Simon D, Simon HU. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy 67: 477–490, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104: 13519–13524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol 133: 1521–1534, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155: 285–295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 297: G34–G42, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 293: G850–G856, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Ouyang A. Effect of synthetic cationic protein on mechanoexcitability of vagal afferent nerve subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 301: G1052–G1058, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 296: G255–G265, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci 82: 324–330, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563: 831–842, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Liu Z, Heldsinger A, Owyang C, Yu S. Intraluminal acid activates esophageal nodose C fibers after mast cell activation. Am J Physiol Gastrointest Liver Physiol 306: G200–G207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


