Abstract
Sensory transduction in esophageal afferents requires specific ion channels and receptors. TRPM8 is a new member of the transient receptor potential (TRP) channel family and participates in cold- and menthol-induced sensory transduction, but its role in visceral sensory transduction is still less clear. This study aims to determine TRPM8 function and expression in esophageal vagal afferent subtypes. TRPM8 agonist WS-12-induced responses were first determined in nodose and jugular neurons by calcium imaging and then investigated by whole cell patch-clamp recordings in Dil-labeled esophageal nodose and jugular neurons. Extracellular single-unit recordings were performed in nodose and jugular C fiber neurons using ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus. TRPM8 mRNA expression was determined by single neuron RT-PCR in Dil-labeled esophageal nodose and jugular neurons. The TRPM8 agonist WS-12 elicited calcium influx in a subpopulation of jugular but not nodose neurons. WS-12 activated outwardly rectifying currents in esophageal Dil-labeled jugular but not nodose neurons in a dose-dependent manner, which could be inhibited by the TRPM8 inhibitor AMTB. WS-12 selectively evoked action potential discharges in esophageal jugular but not nodose C fibers. Consistently, TRPM8 transcripts were highly expressed in esophageal Dil-labeled TRPV1-positive jugular neurons. In summary, the present study demonstrated a preferential expression and function of TRPM8 in esophageal vagal jugular but not nodose neurons and C fiber subtypes. This provides a distinctive role of TRPM8 in esophageal sensory transduction and may lead to a better understanding of the mechanisms of esophageal sensation and nociception.
Keywords: C fiber, esophagus, menthol, TRPM8, vagal
esophageal sensory transduction is initiated by the stimuli in the primary sensory afferent nerve endings in the wall of the esophagus and transmitted through both spinal and vagal pathways to the central nervous system. Those subtypes of sensory nerves, which are able to discriminate noxious and innoxious stimuli, are defined as nociceptors. In the esophagus, noxious mechanical (distension), chemical (acid reflux), and thermal (hot or cold) stimuli all are able to activate esophageal nociceptors and induce painful sensation such as heartburn or esophageal-related chest pain (17). At present, the cellular and molecular mechanisms underlying esophageal sensation and nociception are still less clear.
Vagal afferents in the esophagus not only participate in maintaining esophageal physiological functions but also play the important role in sensing potential tissue damage from noxious stimuli (4, 5). They are derived from two distinct embryonic tissues: cranial placodes and neural crest. Placode-derived sensory nerves innervating the esophagus have their neuronal cell bodies in nodose ganglion while neurocrest-derived sensory neurons are located in jugular (supranodose) ganglion. These two vagal ganglia have different neurotrophin regulation during development and play distinctive roles in esophageal sensory transduction (1, 7, 21). Our previous studies have characterized three subtypes of esophageal vagal afferent nerve fibers in guinea pig and demonstrated that in addition to the classical low-threshold mechanosensitive vagal nodose Aδ-fibers (“tension receptors”), there are at least two high-threshold nociceptive vagal afferent subtypes in the esophagus, namely vagal nodose C fibers and jugular C fibers (25).
These two vagal C fiber subtypes are able to discriminate noxious esophageal distension and respond strongly to nociceptor-selective stimuli such as agonists of TRPV1 and TRPA1 (3, 9, 23, 25). Like TRPV1 and TRPA1, transient receptor potential melastatin 8 (TRPM8) is a nonselective cationic channel of the transient receptor potential (TRP) superfamily (10). TRPM8 is expressed selectively in a subpopulation of primary sensory neurons and can be activated by cold temperature (6, 15). In addition, TRPM8 is activated by chemicals that provide a sensation of cooling such as menthol and icilin (2, 18, 22). Clinical observations indicate that both cold and menthol can induce abnormal esophageal sensations. Drinking cold water, a stimulus for TRPM8 (and maybe TRPA1), can trigger esophageal-related chest pain, which is unlikely to involve esophageal smooth muscle spasm (16). Menthol (and peppermint oil) usually worsens heartburn symptoms, but it also has been added in many antacid formulations to soothe heartburn. These suggest that cold and menthol might regulate esophageal afferent function via TRPM8 and possibly affect esophageal sensory transduction and nociception. TRPM8 expression and function have been well-defined in dorsal root ganglion (DRG) and trigeminal ganglion (TG) (8, 13, 27), but relatively little is known about the involvement of TRPM8 in the function of vagal sensory neurons. Moreover, virtually nothing is known about the role of TRPM8 in esophageal sensory transduction.
TRPM8 expression in vagal sensory neuron was first determined by an elegant study using single-cell RT-PCR in Dil retrograde-labeled rat vagal nodose neurons from the upper gut (26). Using the same approach, another group also detected TRPM8 transcript in rat nodose neurons, and its expression was largely affected by tissue disruption (28). In mice and rats the nodose and jugular neurons often fuse into a single ganglion, making it difficult to differentiate between the placodal vs. neural crest-derived nociceptors. In guinea pigs the vagal nodose and jugular ganglia are easily identified as separate ganglia. In the present study, we took advantage of such distinctive anatomic and physiological features in guinea pig vagal nodose and jugular ganglia to evaluate and characterize the relative contribution of TRPM8 in regulating the activity of nodose vs. jugular C fiber neurons. Our results lead to the conclusion that in healthy animals, TRPM8 plays little role in nodose C fibers innervating the esophagus but may play a major role in sensory transduction in jugular C fibers.
METHODS
Male Hartley guinea pigs (150–200 g) were purchased from Hilltop Laboratory Animals (Scottsdale, PA). All experiments were approved by the Johns Hopkins University Animal Care and Use Committee.
Calcium Imaging in Dissociated Nodose and Jugular Neurons
Nodose and jugular neurons were prepared as described previously (9). Briefly, nodose and jugular ganglia from guinea pigs were first dissected and collected in an ice-cold Krebs bicarbonate solution (118 mM NaCl, 1.0 mM NaH2PO4, 25.0 mM NaHCO3, 5.4 mM KCl, 1.9 mM CaCl2, 1.2 mM MgSO4, and 11.1 mM dextrose, pH 7.4, gassed with 95% O2-5% CO2) and then treated with enzyme (2 mg/ml collagenase and 2 mg/ml dispase in Ca2+/Mg2+-free HBSS buffer) at 37°C for 2 h. During the incubation, neurons were dissociated by mild trituration and then harvested by centrifugation. After resuspending in fresh L15 media, these neurons were transferred onto coverslips (Warner Instrument) precoated with poly-d-lysine (0.1 mg/ml) and laminin (5 μg/ml), allowed to adhere for 2 h in an incubator at 37°C. Then, the cells were washed with fresh media and cultured in fresh L15 media overnight in the incubator at 37°C and used within 24 h.
Calcium imaging studies were performed as described previously (9). Briefly, cultured vagal sensory neurons were loaded with 2 mM fura-2-AM and 0.05% Pluronic F-127 dissolved in normal extracellular solution (ECS, in mM: 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH) in a dark environment at 37°C for 45 min. After washing three times with ECS, these neurons were allowed to deesterify for at least 30 min before use. Fluorescence changes were measured with a Zeiss Upright Scope equipped with PTI-RatioMaster. Chemicals were applied with a custom-built perfusion system. At the end of each experiment, a 50 mM KCl buffer (95 mM NaCl, 50 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.4 with NaOH) was applied to distinguish excitable cells. Only KCl-responsive cells were considered to be excitable cells and used for analysis.
Patch-Clamp Recording in Dil-Labeled Esophageal Nodose and Jugular Neurons
Retrograde labeling of nodose and jugular neurons from the esophagus with DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) (Molecular Probe, Eugene, OR) were performed in four guinea pigs according to our previously described method (9). Briefly, under ketamine (50 mg/kg) and xylazine (5 mg/kg) anesthesia, the cervical esophagus was surgically exposed, DiI solution (1–2 μl, 1% in dimethyl sulfoxide and normal saline mixture) was injected in the wall of the esophagus at 50–60 mm above the gastric-esophageal junction (the injection site was confirmed by the time to dissect the ganglia). Each esophagus received two to three injections. Postoperatively, animals were carefully monitored, and if necessary treated for pain, until totally recovery. After 2 wk, both nodose and jugular ganglia (2 of each per animal) were collected and disassociated (see above) for whole cell patch-clamp recordings or single-neuron RT-PCR (see below).
Whole cell patch-clamp recordings in Dil-labeled esophageal nodose and jugular neurons were performed according to our previously described methods (9). Briefly, borosilicate glass (WPI, Sarasota, FL) electrodes were 2–3 MΩ when filled with the pipette solution (in mM): 140 CsCl, 1 MgCl2, 5 MgATP, 2 EGTA, 10 HEPES (pH 7.2 with CsOH). Whole cell patch-clamp was performed using an Axopatch 200B patch-clamp amplifier and Axograph software (Axon Instruments, Foster City, CA). Currents were typically digitized at 10 kHz and filtered at 2 kHz. The whole cell currents were recorded using voltage ramp from −100 mV to 100 mV in 100-ms duration while cells were patched with a holding potential of 0 mV.
Extracellular Single-Unit Recording Ex Vivo in Esophageal-Vagal Preparation
Extracellular single-unit recordings from nodose or jugular neurons were performed in ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus according to our previous studies (23, 25). Briefly, guinea pigs were killed by CO2 inhalation and exsanguination, and the esophagus and trachea with intact bilateral extrinsic vagal innervation (including jugular and nodose ganglia) were dissected. The tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate buffer. The two compartments were separately superfused with KBS (pH 7.4, 35°C, 4–6 ml/min). Polyethylene tubing was inserted 3–5 mm into the cranial and caudal esophagus and secured for esophageal distension. Isobaric (constant pressure) distension of the esophagus was achieved by increasing intraluminal esophageal pressure to 10, 30, and 60 mmHg. The pressure was generated by a calibrated device utilizing fluid (KBS) columns.
Extracellular recordings were performed using an aluminosilicate glass microelectrode (pulled with a Flaming-Brown micropipette puller, Sutter Instrument, Novato, CA) and filled with 3 M sodium chloride (electrode resistance 2 MΩ). The electrode was placed into an electrode holder connected directly to the headstage (A-M Systems, Everett, WA). A return electrode of silver-silver chloride wire and earthed silver-silver chloride pellet was placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut-off, 0.3 kHz; high cut-off, 1 kHz), and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR) and a model TA240 chart recorder (Gould, Cleveland, OH). The data were stored and analyzed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD) and further processed using spreadsheet software (Microsoft Excel 2007).
The recording electrode was micromanipulated into the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when esophageal distension (with a rapid increase in intraluminal pressure to 60 mmHg for 5 s) evoked action potential discharge. The serosal surface of the esophagus was then searched with a stimulating electrode (pulse duration 1.5 ms, frequency 1 Hz) applied to the tissue. A mechanosensitive receptive field was located when the electronic stimulus evoked discharge of action potentials with waveforms identical to the action potentials evoked by distension. Conduction time was measured as the time between the stimulation pulse and the action potential (visualized by oscilloscope). Conduction velocity was calculated by dividing the length of the approximated nerve pathway by conduction time. Isobaric esophageal distension for 20 s with an intraluminal pressure of 10, 30, and 60 mmHg separated by at least 60 s was used to determine the distension pressure-nerve activity relationship of an esophageal afferent fiber. To assess the reproducibility of distension-evoked activation, this distension protocol was repeated after at least 5 min. The distension-evoked response was quantified as the peak frequency of action potentials discharged during the 20 s of distension from which the spontaneous activity (if present) was subtracted. The peak frequency (Hz) was defined as the maximal frequency of action potential discharge.
After recording the baseline spontaneous activity and mechanical excitability (esophageal distension under the pressure of 10, 30, and 60 mmHg) of esophageal vagal C fiber, the TRPM8 agonist WS-12 (1 μM) was perfused to the serosal surface of the esophagus for 30 min. The action potential discharges of esophageal nodose or jugular C fibers induced by WS-12 were monitored continuously for 30 min and analyzed in 1-s bins (yielding the number of action potentials in each second, Hz). The esophageal distension-evoked responses of these fibers were also detected at the end of agonist perfusion. Then WS-12 was washed out with fresh KBS (pH = 7.4) for 30 min, and AITC- and capsaicin-evoked action potential discharges were recorded (30-min washing with fresh KBS between each chemicals).
Single-Cell RT-PCR in Dil-Labeled Nodose and Jugular Neurons
Single-cell RT-PCR studies were performed on individual neurons as described previously (21). The sensory ganglia were dissected, incubated in the enzyme buffer (2 mg/ml collagenase type 1A and 2 mg/ml dispase II in Ca2+-, Mg2+-free Hanks balanced salt solution) for 3 × 15 min at 37°C. Neurons were dissociated by trituration with three glass Pasteur pipettes of decreasing tip pore size between and after incubations, washed by centrifugation (3 times at 1,000 g for 2 min) and suspended in L-15 medium containing 10% fetal bovine serum (L-15/FBS). The cell suspension was transferred onto poly-d-lysine/laminin-coated coverslips. After the suspended neurons had adhered to the coverslips for 2 h, the neuron-attached coverslips were flooded with L-15/FBS and used within 8 h. Coverslips with dissociated neurons were perfused with PBS, and the DiI-labeled neurons identified under fluorescent microscope (rhodamine filter) were individually harvested into a glass pipette (tip 50–150 μm) pulled with a micropipette puller (P-87, Sutter) by applying negative pressure. The pipette tip containing the cell was broken into a PCR tube containing RNase Inhibitor (1 μl, RNAseOUT, 2 U/l, Invitrogen), immediately frozen, and stored at −80°C. Only the neurons free of debris or attached cells were collected. From one coverslip, one to five cells were collected. A sample of the bath solution was collected from some coverslips for no-template experiments (bath control).
First-strand cDNA was synthesized from single neurons by using the Super-Script III CellsDirect cDNA Synthesis System (Life Technologies) according to the manufacturer's recommendations. Samples were defrosted, lysed (10 min, 75°C), and treated with DNase I. Then, poly(dT) and random hexamer primers (Roche Applied Bioscience) were added. The samples were reverse transcribed by adding SuperscriptIII RT for cDNA synthesis. Two microliters of each sample (cDNA, RNA control, or bath control, respectively) was used for PCR amplification by the HotStar Taq Polymerase Kit (Qiagen) according to the manufacturer's recommendations in a final volume of 20 μl. After an initial activation step at 95°C for 15 min, cDNAs were amplified with custom-synthesized primers (Life Technologies) by 50 cycles of denaturation at 94°C for 30 s, with annealing at 60°C for 30 s and extension at 72°C for 1 min followed by a final extension at 72°C for 10 min. Products were visualized in ethidium bromide-stained 1.5% agarose gels with a 50- or 100-bp DNA ladder. The figures were constructed by using Microsoft PowerPoint and Apple Preview.
The primers were designed by using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/): TRPV1 (sequence NM_001172652.1), forward primer (start 992) CCAACAAGAAGGGGTTCACA, reverse primer (start 1159) ACAGGTCATAGAGCGAGGAG, predicted product 168 bp, predicted genomic product >1,000 bp; TRPM8 (sequence NM_001173090.1), forward primer (start 3031) ATCCCCTTCCCCTTTGTG, reverse primer (start 3156) GGTCTCGTTGTCCTCATTTTTG, predicted product 126 bp, predicted genomic product >1,000 bp. The primers are intron-spanning, and no genomic product can be amplified because its predicted size >1,000 bp is not achievable with the extension time of 30 s used for PCR, thus preventing false-positive results.
Chemicals
All chemicals used in the experiments were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Collagenase/dispase was purchased from Roche Applied Science (Indianapolis, IN). Fetal bovine serum, HBSS, and Pluronic(R) F-127 were purchased from Life Technologies (Grand Island, NY). The stock solution of capsaicin (10 mM) was prepared in ethanol; those of collagenase/dispase(2 mg/ml), laminin (5 μg/ml) were in sterile Ca2+/Mg2+-free Hanks Balanced Salt Solution (HBSS); fura-2-AM (2M) was prepared in acetone and poly-l-lysine (1 mg/ml) was diluted in sterile water. WS-12 and AMTB (both from TOCRIS Bioscience, Bristol, UK) were diluted in dimethyl sulfoxide (DMSO). All the stock solutions were separated into small aliquots and stored in −20°C, and working solution were prepared 1–2 days before use.
Data Analysis
In extracellular study, we only analyzed the results from capsaicin- or AITC-responsive C fibers, which were confirmed by the end of each recording to indicate that the nerve terminals were exposed to chemical perfusion. The agonist-evoked nerve response was quantified as the peak frequency of the action potential discharge within a 5-min period, and averaged from 6 recording periods for a total of 30 min. The peak frequency (Hz) of the action potential discharges are presented as means ± SE and compared by paired t-test or one-way ANOVA. For all experiments, significance was defined as P < 0.05.
In calcium imaging studies, neurons were defined as “responders” to a given compound if the mean response was greater than the mean baseline plus 2 × the SD using unpaired t-test. Patch-clamp data were analyzed with Sigmaplot 11.0 (SPSS). Dose-response curves for the agonist were fitted with a modified Hill equation. All data are presented as means ± SE. Statistical comparisons were made with unpaired Student's t-test and Wilcoxon rank-sum test, and differences were considered significant at P < 0.05.
RESULTS
TRPM8 Selectively Expressed in Dil-Labeled TRPV1-Positive Jugular but Not Nodose Neurons
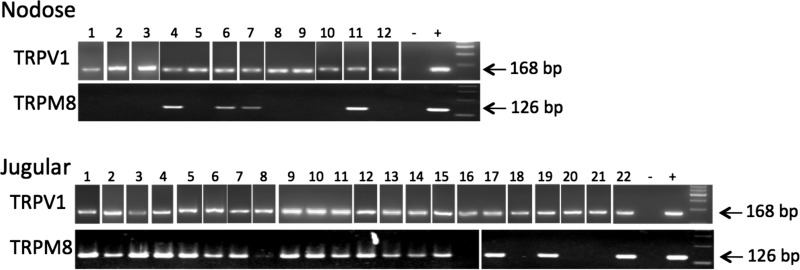
We first evaluated the TRPM8 mRNA expression in the nodose and jugular neurons retrogradely labeled from the esophagus by using single-cell RT-PCR. The neurons projecting the capsaicin-sensitive fibers (putative C fiber neurons) were identified by the expression of the capsaicin receptor TRPV1. The expression of TRPM8 was evaluated in these TRPV1-positive esophageal neurons. We found that TRPM8 was expressed in the vast majority (18/22) of jugular esophageal TRPV1-positive neurons. In contrast, only a minority (4/12) of the nodose TRPV1-positive esophageal C fiber neurons expressed TRPM8 (P < 0.05, Fisher exact test compared with jugular) (Fig. 1).
Fig. 1.
Transient receptor potential melastatin 8 (TRPM8) mRNA expression in Dil-labeled esophageal nodose and jugular neurons using single-neuron RT-PCR method. TRPM8 was expressed in the majority (18/22) of jugular esophageal TRPV1-positive neurons. In contrast, only a minority (4/12) of the nodose TRPV1-positive esophageal C fiber neurons expressed TRPM8 (P < 0.05, Fisher exact test compared with jugular).
TRPM8 Agonist WS-12 Evoked Calcium Influx in Jugular but Not Nodose Neurons
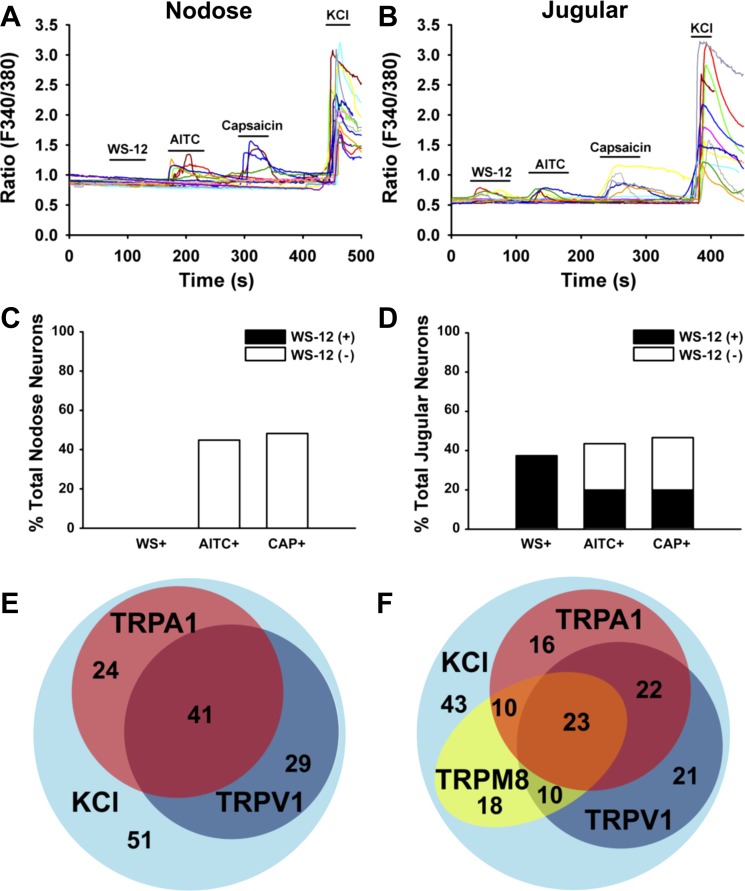
WS-12 is a chemical analog of menthol that stimulates TRPM8 more selectively and more potently than menthol (12, 20). We performed calcium imaging assay in the nodose and jugular neurons from naive guinea pigs (N = 7) to determine their responses to WS-12. As shown in Fig. 2, the population of WS-12-sensitive vagal neurons was limited only to those isolated from the jugular ganglia. We found that ∼40% (61/163) of jugular neurons were stimulated by WS-12. Nodose neurons were categorically unresponsive to WS-12 (0 of 135 nodose neurons responding) (Fig. 2). By contrast the percentage of neurons responding to capsaicin or AITC was similar between those isolated from nodose and jugular ganglia. We found that 44.8 ± 5.7% (65 of 145) of nodose neurons were excited by 100 μM AITC compared with that of 43.6 ± 4.3% (71 of 163) in jugular neurons (Fig. 2, A–D). Perfusion with capsaicin (100 nM) activated 48.3 ± 4.1% (70 of 145) of nodose neurons compared with that of 46.6 ± 4.9% (76 of 163) in jugular neurons (Fig. 2, A–D). The Venn diagram in Fig. 2, E and F, showed the overlaps among WS-12-, AITC-, and capsaicin-responsive neurons in jugular and nodose ganglia.
Fig. 2.
TRPM8 agonist WS-12 evoked calcium influx in vagal jugular but not nodose neurons. A: representative traces of calcium influxes in nodose neurons (left) and jugular neurons (right) evoked by TRP channel agonists (WS-12: 1 μM; AITC: 100 μM; capsaicin: 100 nM) and KCl (50 mM). B: summary data of the responsive rates of nodose and jugular neurons to TRP channel agonists. C: Venn diagram showing responsive overlaps of TRPM8 with TRPA1 and TRPV1 in jugular (right), but not nodose (left) neurons.
TRPM8 Agonist WS-12 Induced Inward Current in Dil-Labeled Esophageal Jugular but Not Nodose Neurons
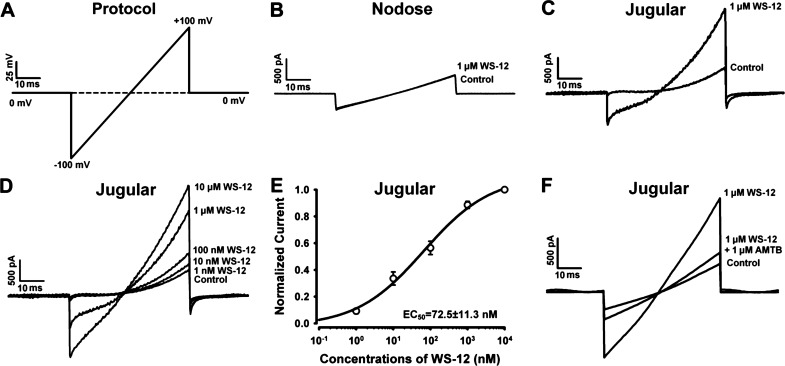
To further determine if TRPM8 function in vagal sensory neurons specifically innervated the esophagus, we dissociated DiI retrogradely labeled esophageal nodose and jugular neurons for whole cell patch-clamp recordings. WS-12 activated outwardly rectifying currents, with a reverse potential ∼0 mV in ∼40% of jugular neurons (7 of 17 DiI-labeled jugular neurons from 3 naive guinea pigs) (Fig. 3C). A concentration-response analysis revealed that WS-12 stimulated the current with an EC50 of 67.5 ± 10.6 nM (Fig. 3, D and E), which is consistent with the potency of WS-12 in activating TRPM8 in transfected HEK293 cells (20). The WS-12-evoked current was inhibited by AMTB, a selective inhibitor of TRPM8 (Fig. 3F). In contrast, WS-12 did not evoke a current in nodose neurons (0 of 13 DiI-labeled nodose neurons from 3 guinea pigs responded) (Fig. 3B).
Fig. 3.
TRPM8 agonist WS-12 activated Dil-labeled esophageal jugular but not nodose neurons. A: the protocol for whole cell patch-clamp experiments of TRPM8 agonist (WS-12)-induced responses in esophageal nodose and jugular neurons. B and C: representative current traces showing that WS-12 (1 μM) activated esophageal jugular, but not nodose, neurons. D: representative current traces showing that WS-12 elicited currents in esophageal jugular neurons in a dose-dependent manner. E: the dose-dependent activation curve of WS-12 in esophageal jugular neurons (n = 7, N = 3). F: representative current traces showing that TRPM8 inhibitor AMTB prevented WS-12-induced activation effect in esophageal jugular neurons.
TRPM8 Agonist WS-12 Selectively Evoked Action Potential Discharges in Jugular but Not Nodose C Fiber Nerve Endings in the Esophagus
Extracellular single-unit recording of action potential discharges in response to WS-12 was performed in nodose or jugular neurons with intact nerve endings in the esophagus using our established ex vivo esophageal-vagal preparations. We evaluated the activity of one afferent C fiber (either nodose or jugular) per animal, so the total number of recorded C fibers (n) is equal to the number of animals (N) used in the experiments. In the present study, the average conduction velocity of nodose C fibers innervating the esophagus was 0.6 ± 0.04 m/s (n = 10), and that of jugular C fibers was 0.8 ± 0.2 m/s (n = 11).
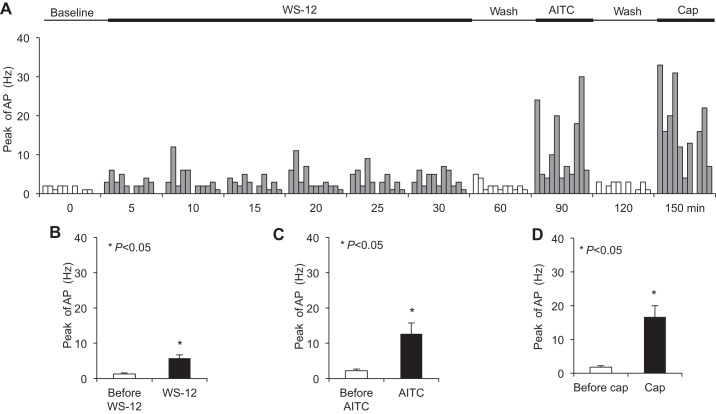
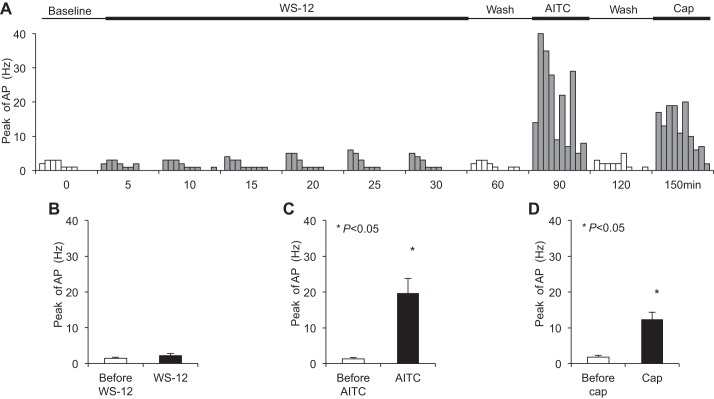
Perfusion with WS-12 (1 μM) for 30 min activated nearly every esophageal jugular C fiber studied (n = 10/11) (Fig. 4A). The peak discharge rate of action potentials averaged 5.80 ± 0.96 Hz after perfusion with WS-12 (compared with a baseline of 1.30 ± 0.26 Hz, P < 0.001, n = 10) (Fig. 4B). Following WS-12 perfusion for 30 min, the peak discharge rates evoked by esophageal distension were compared. They were 4.80 ± 2.20, 6.80 ± 2.13, and 8.71 ± 2.21 Hz at distension pressures of 10, 30, and 60 mmHg, respectively, which were not significantly changed compared with those before WS-12 perfusion (2.70 ± 0.40, 5.30 ± 0.72, and 9.00 ± 0.56 Hz, respectively, P > 0.05, n = 10). After washing with fresh KBS for 60 min, we tested repeat perfusion of WS-12-induced responses in 5 of 10 WS-12-responsive jugular C fibers. The peak discharge rate of action potentials averaged 6.6 ± 1.86 Hz after the first WS-12 perfusion (vs. baseline at 1.4 ± 0.4 Hz, P < 0.05, n = 5). The second WS-12 only activated 3 of those 5 jugular C fibers. The peak discharge rate of action potentials averaged 4.2 ± 1.5 Hz after the second WS-12 perfusion (vs. baseline at 2.0 ± 0.71 Hz, P = 0.063, n = 5). After washing out WS-12 for 60 min, TRPA1 agonist-induced responses were determined. AITC (300–400 μM) activated most of those WS-12-responsive C fibers (n = 9/10). The peak discharge rate of action potentials averaged 12.70 ± 3.02 Hz after AITC perfusion (compared with that before AITC at 2.20 ± 0.42 Hz, P = 0.006, n = 10) (Fig. 4C). At the end of each recording, after washing out of AITC for 30 min, the TRPV1 agonist capsaicin (1 μM) was perfused. Capsaicin also activated most of WS-12-responsive C fibers (n = 9/10). The peak discharge rates averaged 16.70 ± 3.31 Hz after capsaicin perfusion (compared with that before capsaicin at 1.80 ± 0.44 Hz, P = 0.001, n = 10) (Fig. 4D).
Fig. 4.
TRPM8 agonist WS-12 activated esophageal jugular C fibers. A: summary of TRP channel agonists-induced responses in esophageal jugular C fibers. B: TRPM8 agonist WS-12 (1 μM, 30 min) evoked action potential discharges in esophageal jugular C fibers (*P < 0.05 vs. baseline, n = 10/11). C: TRPA1 agonist AITC evoked action potential discharges in those WS-12-responsive jugular C fibers (*P < 0.01 vs. control, n = 10). D: TRPV1 agonist capsaicin evoked action potential discharges in those WS-12-responsive jugular C fibers (*P < 0.01 vs. control, n = 10).
In contrast, perfusion of WS-12 (1 μM) for 30 min did not evoke action potential discharges in most of esophageal nodose C fibers (n = 9/10) (Fig. 5A). The peak discharge rate of action potentials averaged 2.2 ± 0.61 Hz after WS-12 perfusion (compared with that before WS-12 at 1.4 ± 0.4 Hz, P > 0.05, n = 10) (Fig. 5B). Following WS-12 perfusion for 30 min, the peak discharge rates evoked by esophageal distension were determined in these nodose C fibers. The peak discharge rates were 4.40 ± 1.73, 6.50 ± 2.58, and 7.80 ± 2.72 Hz at distension pressures of 10, 30, and 60 mmHg, respectively, which were not significantly different from those before WS-12 perfusion (4.00 ± 1.58, 7.00 ± 2.34, and 8.30 ± 2.50 Hz, respectively, P > 0.05, n = 10). After washing out WS-12 for 60 min, TRPA1 agonist-induced response was determined. AITC (300–400 μM) activated all these WS-12-nonresponsive esophageal nodose C fibers (n = 10). The peak discharge rate of action potentials averaged 19.7 ± 4.04 Hz by AITC perfusion (compared with that before AITC at 1.30 ± 0.37 Hz, P = 0.001, n = 10) (Fig. 5C). At the end of each recording, after washing out AITC for 30 min, TRPV1 agonist capsaicin (1 μM) was perfused. All these nodose C fibers could be activated by capsaicin. The peak discharge rates averaged 12.40 ± 1.98 Hz after capsaicin perfusion (compared with that before capsaicin at 1.80 ± 0.47 Hz, P < 0.001, n = 10) (Fig. 5D).
Fig. 5.
TRPM8 agonist WS-12 did not activate esophageal nodose C fibers. A: summary of TRP channel agonists-induced responses in esophageal nodose C fibers. B: TRPM8 agonist WS-12 (1 μM, 30 min) did not evoke action potential discharges in esophageal nodose C fibers (P > 0.05 vs. baseline, n = 9/10). C: TRPA1 agonist AITC evoked action potential discharges in those WS-12-responsive nodose C fibers (*P < 0.01 vs. baseline control, n = 10). D: TRPV1 agonist capsaicin evoked action potential discharges in those WS-12-responsive jugular C fibers (*P < 0.01 vs. baseline control, n = 10).
DISCUSSION
Vagal afferents innervated in the esophagus have two subtypes of C fibers. One type has its cell body situated in the nodose ganglion, the other in the jugular ganglion. Both C fiber subtypes fit the definition of nociceptors in that they can distinguish noxious from nonnoxious distension and are stimulated by various inflammatory mediators commonly associated with nociceptor activation (23–25). However, these two nerve subtypes have different embryological origins, are under different neurotrophic control, innervate distinct aspects of the esophagus, and, importantly, synapse with secondary neurons in distinct brain regions (5). For example, in viral tracing studies, jugular neurons have been suggested to have a more somatic central circuitry compared with the more visceral circuitry of the nodose fibers (14). This supports the idea that the jugular C fibers may transduce sensations and reflexes distinct from nodose C fibers. For these reasons it is critical to understand the activation profile of jugular vs. nodose C fiber nociceptors.
Many chemical stimuli activate both nodose and jugular C fibers in the esophagus; e.g., tissue distension and all substances that can gate either TRPV1 or TRPA1 (although nodose C fiber are more strongly activated by TRPA1 agonists than jugular C fibers) (3, 23). Chemical stimuli have been identified that selectively activate nodose but not jugular C fibers including ATP via P2X2/3 receptors (11, 25), 5-HT via 5-HT3 receptor (24), and adenosine via A2a receptor agonists (18). Until the present study, no stimuli has been identified that would stimulate jugular but not nodose C fibers. The evidence provided here indicates that stimuli that gate TRPM8 may selectively activate jugular and not nodose C fibers in the esophagus.
The conclusion that TRPM8 activators will stimulate jugular but not nodose esophageal C fibers is based on four lines of evidence. First, single-neuron RT-PCR revealed that TRPM8 mRNA was expressed in a large majority of esophageal-specific jugular C fiber neurons but only a minority of nodose neurons. Second, calcium image analysis revealed that a very selective TRPM8 agonist stimulated calcium influx in a majority of jugular neurons but in virtually no nodose neurons. Third, patch-clamp recording revealed that jugular neurons responded to the TRPM8 agonist with current properties consistent with TRPM8, and was sensitive to a TRPM8 inhibitor, whereas no such current was observed in nodose neurons. Last, the nerve endings in the esophageal wall of jugular C fibers but not nodose C fibers consistently responded with action potential discharge to TRPM8 activation.
The functional studies at the cell bodies and nerve endings showed a more categorical distinction in TRPM8 responses between jugular and nodose neurons compared with the gene expression data. Whereas very few if any nodose neurons responded to the TRPM8 agonist, ∼25% of the esophageal-specific nodose neurons expressed some TRPM8 mRNA. This is not a quantitative analysis, so it may be that some neurons express a small amount of mRNA that is insufficient to produce a meaningful density of functional channels.
These findings have potential clinical and experimental implications. Clinically it sets up the conditions whereby TRPM8 stimuli such as cold temperature and ingestion of menthol-related substances will lead to sensations and reflexes distinct from those stimuli that activate nodose C fibers. Experimentally the present result provides a tool to selectively activate jugular C fibers and not nodose C fibers. It will be of considerable interest to characterize the physiological consequences of this activation compared with selectively activating nodose but not jugular C fibers with stimuli such as the P2X3 agonist α,β-methylene ATP. The selective expression and function of TRPM8 in vagal afferent C fiber subtypes might also be helpful to further study the interaction of TRPV1 and TRPM8, relative contributions of TRPM8 and TRPA1 to cold, and integrated roles of these TRP channels in esophageal sensation and nociception.
In summary, the present study adds new knowledge on TRPM8 function and expression in vagal sensory neurons and afferents. Our data demonstrated that the TRPM8 agonist WS-12 selectively activated esophageal vagal jugular but not nodose nociceptive C fiber neurons. TRPM8 was highly expressed in esophageal jugular TRPV1-positive neurons. Such preferential function and expression of TRPM8 in esophageal vagal jugular neurons and C fibers provide a useful tool to further elucidate the cellular and molecular mechanisms of menthol- and cold-induced esophageal sensation and nociception.
GRANTS
This study was supported by National Institutes of Health Grant DK-087991 (S. Yu) and by Chinese NSF Grant 81200272 (to X. Yu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Y., Y.H., M.K., B.J.U., and S.Y. conception and design of research; X.Y., Y.H., F.R., and M.K. performed experiments; X.Y., Y.H., F.R., M.K., and S.Y. analyzed data; X.Y., Y.H., F.R., M.K., and S.Y. interpreted results of experiments; X.Y., Y.H., F.R., M.K., and S.Y. prepared figures; X.Y., Y.H., B.J.U., and S.Y. drafted manuscript; X.Y., Y.H., M.K., B.J.U., and S.Y. approved final version of manuscript; B.J.U. and S.Y. edited and revised manuscript.
REFERENCES
- 1.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol 232: 1–61, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Brozmanova M, Ru F, Surdenikova L, Mazurova L, Taylor-Clark T, Kollarik M. Preferential activation of the vagal nodose nociceptive subtype by TRPA1 agonists in the guinea pig esophagus. Neurogastroenterol Motil 23: e437–e445, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 10: 286–296, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev 60: 171–186, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 54: 371–378, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Farinas I, Cano-Jaimez M, Bellmunt E, Soriano M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res Bull 57: 809–816, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain 152: 1459–1468, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Liu Z, Yu X, Pasricha PJ, Undem BJ, Yu S. Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 307: G149–G157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 29: 355–384, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma S, GG, Ak VE, Jf D, HH. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci 21: 370–378, 2008. [PubMed] [Google Scholar]
- 13.Madrid R, Donovan-Rodríguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26: 12512–12525, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern AE, Davis-Poynter N, Yang SK, Simmons DG, Farrell MJ, Mazzone SB. Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Struct Funct. 2014August27 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 20002. [DOI] [PubMed] [Google Scholar]
- 16.Meyer GW, Castell DO. Human esophageal response during chest pain induced by swallowing cold liquids. JAMA 246: 2057–2059, 1981. [PubMed] [Google Scholar]
- 17.Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Hand Exp Pharmacol 194: 227–257, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ru F, Surdenikova L, Brozmanova M, Kollarik M. Adenosine-induced activation of esophageal nociceptors. Am J Physiol Gastrointest Liver Physiol 300: G485–G493, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherkheli MA, Vogt-Eisele AK, Bura D, Beltrán Márques LR, Gisselmann G, Hatt H. Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship. J Pharm Pharm Sci 13: 242–253, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Surdenikova L, Ru F, Nassenstein C, Tatar M, Kollarik M. The neural crest- and placodes-derived afferent innervation of the mouse esophagus. Neurogastroenterol Motil 24: e517–e525, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Teliban A, Bartsch F, Struck M, Baron R, Jänig W. Responses of intact and injured sural nerve fibers to cooling and menthol. J Neurophysiol 111: 2071–2083, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 296: G255–G265, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Ru F, Ouyang A, Kollarik M. 5-Hydroxytryptamine selectively activates the vagal nodose C-fibre subtype in the guinea-pig oesophagus. Neurogastroenterol Motil 20: 1042–1050, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563: 831–842, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 286: G983–G991, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat Cell Biol 14: 851–858, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]