Abstract
Myeloid translocation genes (MTGs) are transcriptional corepressors implicated in development, malignancy, differentiation, and stem cell function. While MTG16 loss renders mice sensitive to chemical colitis, the role of MTG16 in the small intestine is unknown. Histological examination revealed that Mtg16−/− mice have increased enterocyte proliferation and goblet cell deficiency. After exposure to radiation, Mtg16−/− mice exhibited increased crypt viability and decreased apoptosis compared with wild-type (WT) mice. Flow cytometric and immunofluorescence analysis of intestinal epithelial cells for phospho-histone H2A.X also indicated decreased DNA damage and apoptosis in Mtg16−/− intestines. To determine if Mtg16 deletion affected epithelial cells in a cell-autonomous fashion, intestinal crypts were isolated from Mtg16−/− mice. Mtg16−/− and WT intestinal crypts showed similar enterosphere forming efficiencies when cultured in the presence of EGF, Noggin, and R-spondin. However, when Mtg16−/− crypts were cultured in the presence of Wnt3a, they demonstrated higher enterosphere forming efficiencies and delayed progression to mature enteroids. Mtg16−/− intestinal crypts isolated from irradiated mice exhibited increased survival compared with WT intestinal crypts. Interestingly, Mtg16 expression was reduced in a stem cell-enriched population at the time of crypt regeneration. This is consistent with MTG16 negatively regulating regeneration in vivo. Taken together, our data demonstrate that MTG16 loss promotes radioresistance and impacts intestinal stem cell function, possibly due to shifting cellular response away from DNA damage-induced apoptosis and towards DNA repair after injury.
Keywords: intestinal crypt regeneration, enteroid, radiation enteritis, MTG16, transcriptional corepressors
radiation enteritis is a pathological condition in which the small intestine is injured following exposure to ionizing radiation (21). Risk factors associated with radiation enteritis include mutations in DNA repair genes such as BRCA1 and BRCA2 (13, 35) or in DNA damage response genes such as Tp53 (32) and B-cell lymphoma 6 protein (BCL6) (30). Sensitivity to intestinal radiation-induced injury may be further influenced by changes in cell cycle kinetics, synchronization of replicating cell populations, or inhibition of effective DNA repair (6, 42). Typically, DNA repair of radiation-induced DNA double-strand breaks depends on the activation of DNA damage response programs that induce phosphorylation of histone H2A.X and activation of a number of mediators that phosphorylate p53. Together, the actions of these proteins ultimately lead to DNA repair or apoptosis if DNA repair is insufficient (44).
Myeloid translocation genes (MTGs) were discovered in acute myeloid leukemia (33). The MTGs, MTG8, MTGR1, and MTG16, serve as scaffold proteins and facilitate the formation of transcriptional repression complexes containing histone deacetylases (HDACs), nuclear receptor corepressor 1 (NcoR), and mammalian switch-independent 3A (mSin3A) (3, 11, 28, 46). Because MTGs are unable to bind DNA directly, association with transcription factors such as BCL6, promyelocytic leukemia zinc finger (PLZF), and T-cell factor 4 (TCF4) dictate target specificity (34). We have recently shown that MTGs compete with β-catenin for TCF4 occupancy and MTG binding attenuates TCF4-mediated transcriptional activation (34). Given that TCF4 is critical for stem cell renewal in the adult intestine (14), MTGs may regulate key stem cell signaling pathways necessary for homeostasis and injury repair (11, 34). In support of this concept, Mtg16−/− mice have stress-induced hematopoietic stem cell defects (10), as well as abnormal crypt regeneration in the colon after injury-induced inflammation (47). However, the effect of Mtg16 deletion on small intestine injury responses has yet to be determined.
Given that MTG16 impacts colonic responses to chemically induced colitis, we hypothesized that MTG16 may alter radiation-induced small intestinal regenerative responses. In the present study, we link MTG16 to epithelial regeneration after radiation-induced injury. At baseline, Mtg16−/− mice exhibited decreased goblet cell numbers and higher proliferation. Furthermore, after 12 Gy whole body radiation, Mtg16−/− mice showed protection from radiation-induced DNA damage and p53 activation. Ex vivo culturing of Mtg16−/− enteroids revealed increased Wnt responsiveness and delayed maturation. Complementary to in vivo findings, Mtg16−/− enteroids were more radioresistant than WT counterparts, indicating an epithelial cell-autonomous role for Mtg16 in radiation-induced epithelial responses. Lastly, examination of a postirradiation gene expression array dataset indicated that during the proliferative recovery phase Mtg16 expression was reduced in stem cell populations.
MATERIALS AND METHODS
Mouse Models
WT (C57BL/6 background) mice were obtained from the Jackson Laboratories. Mtg16−/− mice were obtained from S. W. Hiebert (Vanderbilt University) and have been described in detail (10). All experiments were performed with 8- to 12-wk-old WT and Mtg16−/− male and female mice on C57BL/6 background. All in vivo experimental procedures were performed under guidelines approved by the Vanderbilt Institutional Animal Care and Use Committee.
Gamma Irradiation
WT and Mtg16−/− mice were placed in a plexiglass-partitioning device and onto a turntable delivery platform, ensuring uniform radiation dosing of all mice. Mice received 12 Gy whole body radiation from a Mark I 137Cs source delivered at 1.58 Gy/min. To assess early injury responses, mice were killed 4 h after irradiation, a time known in WT mice to be associated with maximal induction of p53-mediated apoptosis (25).
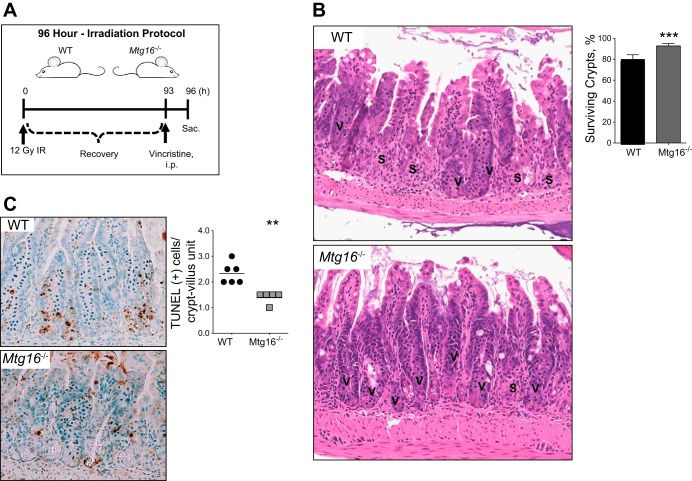
To assess regenerative response, WT and Mtg16−/− mice were dosed with 12 Gy irradiation as described above. Ninety-three hours after irradiation, mice were injected with 0.02 mg/kg of vincristine sulfate (Sigma-Aldrich, St. Louis, MO) to arrest cells in metaphase, facilitating identification of crypt cells entering mitosis over the 3-h period between administration and tissue harvest (1, 36). Mice were euthanized 3 h later (see Fig. 4A) at the 96-h time point. The 96-h postirradiation time point was chosen as it is a time point of crypt regeneration (27, 39, 45).
Fig. 4.
Mtg16−/− mice are protected from radiation-induced injury. A: schematic diagram of 96-h irradiation protocol. B: representative H&E demonstrating small intestinal crypt survival in WT and Mtg16−/− mice. The letter “V” denotes viable crypts and the letter “S” denotes sterile crypts. Mtg16−/− (n = 8) mice have a higher percentage of surviving crypts than WT (n = 6; ***P = 0.0002). Crypts were considered viable if 3 or more mitotic bodies were observed per crypt. Forty sequential, well-aligned crypts in the distal one-third of the small intestine were counted per data point. The percent of surviving crypts was calculated using the following equation: (# of viable crypts/total # of crypts counted) × 100. C: terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining demonstrated a reduction in the number of TUNEL+ cells in Mtg16−/− (n = 6) vs. WT (n = 5; **P = 0.001). All images were captured at ×10 magnification.
Immunohistochemistry and Immunofluorescence
Baseline characterization.
Following death, small intestines were removed, rinsed with PBS, and Swiss-rolled for histological examination. The tissues were fixed in 10% formalin overnight and transferred to 70% ethanol. Tissues were submitted to the Vanderbilt University Translational Pathology Shared Resource (TPSR) core for processing and paraffin embedding. Five-micrometer sections were cut for histology. The distal one-third of small intestinal sections from WT and Mtg16−/− mice was evaluated for crypt morphology, crypt depth, villus height, and biomarkers of proliferation and secretory lineages. Goblet cells were identified by periodic acid-Schiff (PAS) staining. Enteroendocrine cells were assessed by chromogranin A (CgA) staining using anti-CgA (ImmunoStar, Hudson, WI) at 1:1,000 dilution. Paneth cells were identified using anti-lysozyme antibody (Dako, Carpentaria, CA) at 1:500 dilution. Proliferation was measured using anti-phospho-histone H3 (pH3) Ser10 antibody (Millipore/Upstate, Bedford, MA) that labels cells in the mitotic (M) phase of the cell cycle at 1:150 dilution. Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) was used for secondary antibody and visualization.
Four hours postirradiation analyses.
Small intestines were harvested 4 h postirradiation and ∼3- to 4-cm segments of the distal small intestine were excised and further dissected before snap freezing in liquid nitrogen for use in subsequent flow cytometric analysis (8, 9). The remaining section of the distal small intestine was Swiss-rolled, fixed, and submitted to the Vanderbilt TPSR core for processing and sectioning. For phospho-histone H2A.X immunofluorescence, antigen retrieval was performed by using 500 ml of 1 M sodium citrate buffer (pH 6.0). Slides were placed in a pressure cooker and heated for 15 min on high pressure. Slides were then rinsed with deionized H2O to remove excess citrate buffer. Tissue sections were permeabilized by adding 50 μl of 0.1% Tween 20 to each section and allowed to incubate for 30 min in a covered chamber. Slides were washed twice to remove permeabilization buffer. Tissue sections were blocked in 5% goat serum in TBS. Anti-phospho-histone H2A.X (Ser139) from Millipore (EMD Millipore, Billerica, MA) was used at 1:100 dilution and slides incubated overnight at 4°C. Isotype-matched antibodies were included as negative controls. Sections were then washed in 1× PBS and incubated for 1 h at room temperature in Alexa Fluor 488 goat anti-mouse-IgG (Invitrogen, Grand Island, NY) at 1:100 dilution. Slides were counterstained and mounted with ProLong Gold Antifade with DAPI (Invitrogen).
Ninety-six hours postirradiation analyses.
At 96 h postirradiation, small intestines were harvested and Swiss-rolled as described above. Crypt regeneration was assessed by examination of hematoxylin and eosin-stained sections for the number of mitotic figures present per crypt in the distal one-third of the small intestine.
Flow Cytometric Analysis of Epithelial Cell Isolates
Flow cytometric analysis of epithelial cell isolates including both crypt and villus populations was performed as previously described (9). Frozen tissue segments were thawed in calcium and magnesium free 1× Dulbecco's phosphate-buffered saline (DPBS). DPBS was decanted and samples were resuspended in cold Hanks' balanced salt solution (HBSS) containing 3 mM ethylenediaminetetraacetic acid (EDTA) and dithiothreitol (DTT) for 1 h with gentle shaking every 15 min. HBSS/EDTA/DTT solution was decanted and epithelial cells from crypts, and villi were resuspended three times in 25 ml of 1× DPBS. After each resuspension, conical tubes were shaken vigorously for a minimum of 30 s. The cell suspension was passed through a 70-μm cell strainer to remove clumps. Epithelial cells were pelleted, centrifuged at 1,500 rpm for 10 min, and resuspended in 1 ml of 1× DPBS. Cells were manually counted using a hemocytometer. Cells (1 × 106) were resuspended in 1 ml of 37% formaldehyde (Ted Pella, Redding, CA) diluted to a final concentration of 4% and incubated at room temperature for 10 min. Epithelial cell isolates were stained according to manufacturer's instructions for expression of the following antibodies: biotinylated E-cadherin (Abcam, Cambridge, MA) and streptavidin-peridinin chlorophyll protein (PerCP)-Cy5.5 tagged antibody (BD Bioscience, San Jose, CA) were used to identify epithelial cells. Phospho-histone H2A.X (Ser139) PE conjugated antibody (Cell Signaling, Danvers, MA) was used to identify DNA damage in epithelial cells. p53 antibody conjugated to Alexa Fluor 488 (Cell Signaling) was used as a marker to detect p53 induction since this is a critical mediator of radiation-induced apoptosis or DNA repair. All cells were analyzed by flow cytometry on a Becton Dickinson LSR II and first gated for E-cadherin expression. At least 10,000 events were collected. The percentage of epithelial cells positive for phospho-histone H2A.X or p53 was calculated using FlowJo software (TreeStar, Ashland, OR).
Apoptosis Assays
Apoptosis in epithelial cell isolates was quantified using the Cell Death Detection ELISAPLUS kit (Roche Applied Sciences, Indianapolis, IN) following the manufacturer's protocol. Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining on tissue sections was conducted with ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (EMD Millipore, Billerica, MA) according to the manufacturer's protocol. Control stains were obtained by omitting the terminal deoxynucleotide transferase (TdT) enzyme. Crypt apoptotic indexes were generated by averaging the number of apoptotic cells in 40 sequential, well-aligned crypts per mouse in the distal one-third of the small intestine. This is presented as the mean number of TUNEL+ cells per crypt in each animal.
Enteroid Cultures
The crypt-enteroid culture method was modified from Sato et al. (29, 40). Briefly, mouse proximal small intestine (∼10 cm) was excised, opened longitudinally, and washed with ice-cold 1× DPBS. The intestine was cut into small pieces and incubated in ice-cold 1× DPBS containing 1 mM EDTA on a rocking platform for 30 min. After being rinsed once with ice-cold PBS to remove EDTA, the intestinal fragments were resuspended three times by gentle shaking in 5 ml of ice-cold 1× DPBS. After each resuspension, the supernatant was collected and passed through a 70-μm cell strainer (Fisher Scientific) to remove villus fragments. The cell strainer was cleared using 5 ml of dissociation buffer. Four-hundred crypts were resuspended in Matrigel (BD Bioscience) containing growth factors all obtained from R&D Systems (Minneapolis, MN): 50 ng/ml EGF, 100 ng/ml Noggin, and 500 ng/ml R-spondin (ENR media) or 100 ng/ml Wnt3A + ENR (WENR media). Neither media nor growth factors were replaced throughout the course of the experiment. Plating efficiencies were calculated by dividing the total number of enterospheres formed by the original number of crypts plated at day 0 and multiplying by 100. Enterospheres were visualized and counted at 24, 48, and 72 h after plating. Experiments were performed in duplicate and repeated three times.
Statistical Analysis
Statistical analysis and graphs were generated using GraphPad Prism 6.0. All data are represented as the standard deviation, unless stated otherwise. Student t-test was performed to compare two groups. One-way ANOVA with Tukey's multiple comparison tests was performed to compare more than two groups. P < 0.05 was considered statistically significant.
RESULTS
MTG16 Regulates Crypt Proliferation and Goblet Cell Numbers In Vivo
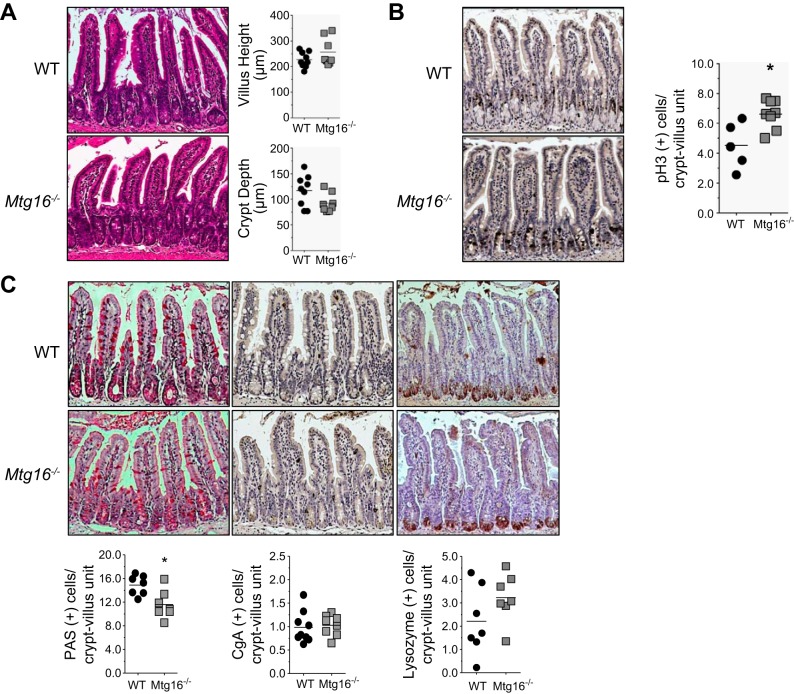
Previous studies have demonstrated that MTGs regulate lineage specification and proliferation in a number of tissues [hematopoiesis (10, 26); colon (47)]. In the small intestine, Mtgr1 knockout mice exhibit defects in secretory lineage allocation (2), while loss of Mtg16 has been reported to promote colonocyte proliferation and exacerbate colonic response to injury (47). The role of MTG16 in small intestinal biology is unknown. To define whether MTG16 deletion alters morphology, proliferation, or secretory cell lineage allocation in the small intestine, we performed a histological characterization of Mtg16−/− mice. Mtg16−/− mice had normal crypt architecture with both villus height and crypt depth being comparable to WT mice (Fig. 1A). In contrast, pH3+ cells per crypt-villus unit were increased in Mtg16−/− intestine (Fig. 1B), indicating increased enterocyte proliferation. There were no significant differences in the numbers of enteroendocrine or Paneth cells (Fig. 1C). However, PAS-labeled goblet cells per crypt-villus unit were significantly reduced in Mtg16−/− mice (Fig. 1C). Thus MTG16 regulates proliferation in the small intestinal crypts and is required for efficient goblet cell production.
Fig. 1.
Myeloid translocation gene 16 (MTG16) regulates epithelial progenitor cell lineage allocation and proliferation. Small intestines were isolated and Swiss-rolled. A: representative hematoxylin and eosin (H&E) demonstrating normal crypt morphology in wild-type (WT) and Mtg16−/− small intestine. Measurement of villus height and crypt depth of 100 crypts from the distal small intestine demonstrating no differences between Mtg16−/− and WT mice (n = 17 mice). B: pH3+ cells demonstrate increased proliferation in Mtg16−/− small intestine (n = 5) compared with WT (n = 8; *P = 0.01). C, left: periodic acid-Schiff (PAS) stain demonstrated reduced number of goblet cells in Mtg16−/− small intestine compared with WT (n = 7 each; *P < 0.05). C, middle: chromogranin A (CgA) staining demonstrated no difference in the number of enteroendocrine cells between Mtg16−/− (n = 8) and WT (n = 9; P = 0.75). C, right: lysozyme staining demonstrated no differences in number of Paneth cells between WT and Mtg16−/− mice (n = 7 in each group; P = 0.16). All images were captured at ×10 magnification.
MTG16 Is Critical for Radiation-Induced DNA Damage Response
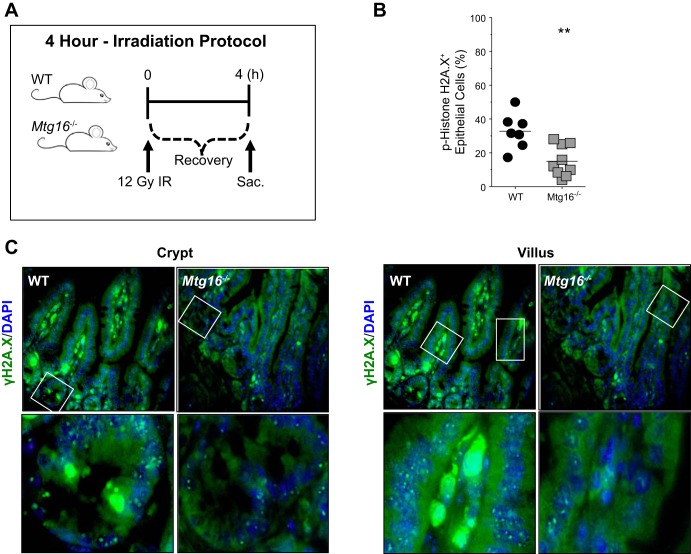
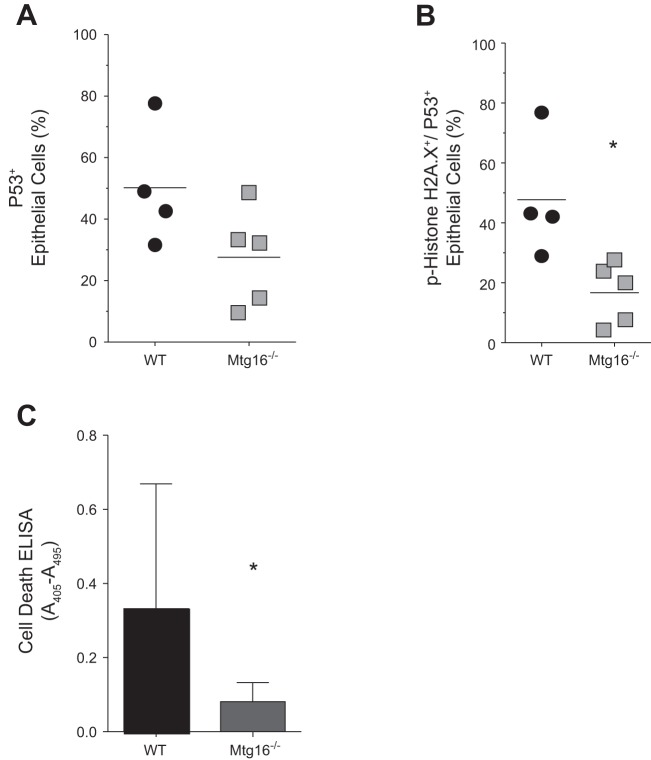
To assess intestinal injury responses, WT and Mtg16−/− mice were exposed to 12 Gy of ionizing radiation. DNA damage and apoptosis were quantified 4 h postirradiation (Fig. 2A), the peak time for detection of p53-induced apoptosis in the small intestine in response to irradiation (25). Flow cytometric analysis for phospho-histone H2A.X on epithelial isolates from irradiated WT and Mtg16−/− mice revealed significantly decreased levels in Mtg16−/− intestine compared with WT (Fig. 2B). This was confirmed by immunofluorescence staining of WT and Mtg16−/− intestine for phospho-histone H2A.X (Fig. 2C). In addition, while analysis of p53-positive epithelial cells by flow cytometry revealed no significant differences between the cohorts (Fig. 3A), analysis of phospho-histone H2A.X/p53 double-positive epithelial cells indicated a significant reduction in the percentage of double-positive cells in epithelial isolates from Mtg16−/− mice at 4 h postirradiation (Fig. 3B). Furthermore, apoptosis assessed by cell death ELISA was also reduced in Mtg16−/− intestine (Fig. 3C). Taken together, these data suggest that Mtg16 deletion protects from injury via decreased triggering of apoptosis after radiation-induced injury.
Fig. 2.
MTG16 is required for proper response to radiation-induced DNA damage. A: schematic of 4-h irradiation (IR) protocol. B: detection of phospho-histone H2A.X by flow cytometry in epithelial cells isolated from WT (n = 7) and Mtg16−/− (n = 9) mice (**P = 0.003). C, top: representative immunofluorescent staining of WT and Mtg16−/− small intestine for phospho-γH2A.X with DAPI 4 h after 12 Gy irradiation (×10 magnification). The areas in white boxes are shown at higher magnification (×40 magnification) at bottom.
Fig. 3.
MTG16 is critical for p53-mediated apoptosis. A: flow cytometry detection of p53 in epithelial isolates from irradiated WT (n = 4) and Mtg16−/− (n = 5) mice. B: flow cytometry detection of phospho-histone H2A.X and p53 in epithelial isolates from irradiated WT (n = 4) and Mtg16−/− (n = 5) mice (*P = 0.02). C: apoptosis was measured by cell death ELISA (*P = 0.03; n = 10 in each group).
MTG16 Loss Promotes Crypt Regeneration
Since we observed decreases in DNA damage and apoptosis, we postulated that MTG16 would impact crypt regenerative dynamics in response to ionizing radiation (4, 25, 31, 36). Therefore, we exposed WT and Mtg16−/− mice to 12 Gy irradiation followed by a 93-h recovery period (Fig. 4A). Three hours before death, mice were injected with vincristine, a mitotic inhibitor, facilitating identification of regenerative crypts. At the 96-h time point, proliferation of stem cells leads to crypt regeneration (24, 31, 38, 39). Mtg16−/− mice had 20% increased crypt viability compared with WT mice (Fig. 4B) with a concurrent reduction in TUNEL-positive intestinal epithelial cells (Fig. 4C). Taken together, these data indicate that the absence of MTG16 protects the epithelium from radiation-induced apoptosis during the regenerative phase, as well.
MTG16 Impacts Stem Cell Growth, Maturation, and Wnt3A Response
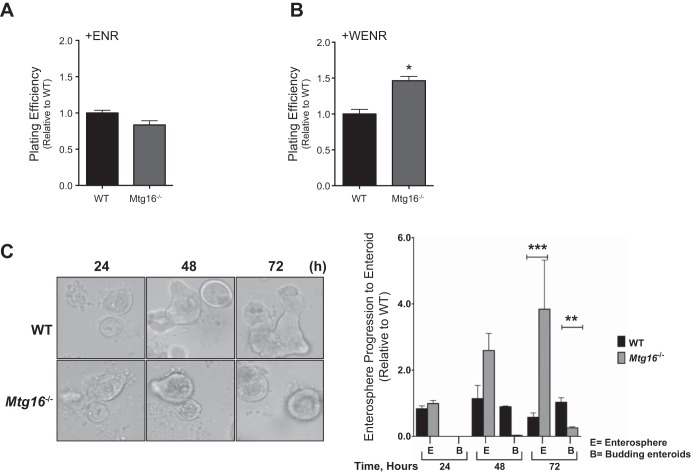
To investigate the mechanisms by which MTG16 might contribute to stem cell survival we examined growth patterns of three-dimensional enteroid cultures of crypts isolated from WT and Mtg16−/− mice. Specifically, we calculated the enterosphere forming efficiency. We considered enterospheres to be “spherical structures composed of several small intestinal epithelial cells that appear as a rounded-off epithelial cysts” when evaluated 24 h postplating (43). There were no differences in plating efficiencies between the two groups when cultured in Matrigel containing EGF, Noggin, and R-spondin (ENR) (Fig. 5A). Because MTG16 is a negative regulator of Wnt signaling, as it can competitively bind to TCF4 and oppose β-catenin-dependent transcriptional activation (34), we postulated that Mtg16−/− enteroids may be hyperresponsive to Wnt activation. Therefore, we added Wnt3A + ENR (WENR) to the Matrigel and plated freshly isolated crypts. In WENR-supplemented crypt cultures, there was a 50% higher plating efficiency of Mtg16−/− crypts (Fig. 5B). Interestingly, Mtg16−/− enteroids also showed reduced progression to budding enteroids compared with WT enteroids at 72 h postplating (Fig. 5C). These observations suggest that MTG16 affects stem cell growth and maturation in a Wnt-dependent manner, such that loss of Mtg16 promotes increases in Wnt responsiveness.
Fig. 5.
MTG16 regulates enteroid growth and Wnt3A response. Small intestinal crypts were isolated from WT or Mtg16−/− mice and plated at 400 crypts/well for all experiments. A: plating efficiency was calculated for crypts embedded in Matrigel containing EGF, Noggin, and R-spondin (ENR). There was no significant difference in plating efficiency between Mtg16−/− and WT intestinal crypts (P = 0.14). Plating efficiency was calculated using the following equation: (total # of crypts that formed enterospheres at 24 h/total # of crypts plated at 0 h). B: plating efficiency was calculated for crypts embedded in Matrigel containing Wnt3A + ENR (WENR). Mtg16−/− enteroids exhibited higher plating efficiency than WT enteroids (*P = 0.03) C, left: enteroid morphology at 24, 48, and 72 h (×10 magnification). C, right: Mtg16−/− enteroids exhibited delayed enterosphere progression to budding enteroids at 72 h (enterosphere: ***P < 0.0001; budding enteroids: **P < 0.001). E, enterosphere; B, budding enteroid. Experiments were performed in duplicate and repeated 3 times.
MTG16 Modulates Intestinal Stem Cell Regenerative Response after Irradiation
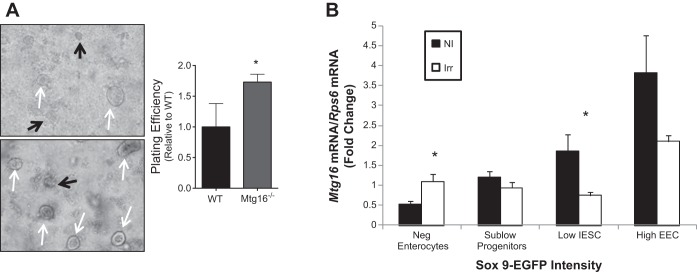
Because increased crypt regeneration was observed in vivo after irradiation of Mtg16−/− mice, we hypothesized that Mtg16−/− enteroid plating efficiency, a surrogate marker for stem cell survival or growth, would be similarly impacted. To test this, mice were dosed with 12 Gy radiation and crypts were isolated and plated in Matrigel containing ENR 4 h later. We observed a 70% increase plating efficiency in Mtg16−/− enteroids 24 h after plating (Fig. 6A).
Fig. 6.
MTG16 decreases stem cell regenerative response after radiation-induced injury. A: plating efficiency of intestinal crypts isolated from WT and Mtg16−/− mice dosed with 12 Gy irradiation. Higher plating efficiencies were observed in crypts isolated from Mtg16−/− mice compared with crypt isolation from WT mice (*P = 0.01; white arrows indicate live and black arrows indicate dead enterospheres, ×10 magnification). Experiments were performed in duplicate and repeated 3 times. B: quantitative PCR of Mtg16 expression in Sox9-EGFPNegative, Sox9-EGFPSublow, Sox9-EGFPLow, and Sox9-EGFPHigh cells from the intestines of both nonirradiated and irradiated mice. No significant differences were observed in Mtg16 mRNA levels across populations. Mtg16 mRNA expression is lower in intestinal epithelial stem cell (IESC) enriched Sox9-EGFPLow and higher in terminally differentiated Sox9-EGFPNegative cells 5 days postirradiation (IESCs: *P < 0.05; enterocytes: *P < 0.05; n = 5 per group). Black bars represent nonirradiated (NI) and white bars represents irradiated (Irr).
Given that specific gene expression programs are modified after intestinal injury and during regenerative phases, we sought to determine if Mtg16 was regulated in response to radiation-induced injury. Using a well-characterized Sox9 transgenic model (18, 24), we assessed Mtg16 RNA levels in different populations of Sox9-EGFP sorted cells. Prior studies have demonstrated that Sox9, a Wnt target gene, is a marker for self-renewing small intestinal epithelial stem cells (16–18). Studies using Sox9-EGFP reporter mice have demonstrated that FACS for different levels of Sox9-EGFP expression yields Sox9-EGFPNegative cells enriched for enterocyte markers, Sox9-EGFPSublow cells enriched for progenitors, Sox9-EGFPLow cells enriched for Lgr5+ and other intestinal stem cell markers, and Sox9-EGFPHigh cells enriched for enteroendocrine cells (24). In the present study, quantitative RT-PCR on these populations for Mtg16 indicated no significant difference in Mtg16 levels across populations; however, after irradiation, Mtg16 was specifically downregulated in the Sox9-EGFPLow stem cell-enriched compartment and increased in the Sox9-EGFPNegative cells (Fig. 6B). Thus these data suggest that MTG16 levels are regulated during the regenerative phase after radiation-induced injury.
DISCUSSION
The goal of the present study was to investigate the role of MTG16 in the small intestine by examining the effect of Mtg16 deletion on baseline mucosal homeostasis and response to injury after ionizing radiation. At baseline, Mtg16−/− mice demonstrate higher intestinal proliferation and altered lineage allocation. Mtg16−/− enteroids were hyperresponsive to Wnt activation and exhibited delayed progression to mature enteroids, suggesting altered stem cell activity in the Mtg16−/− intestine. After irradiation, Mtg16−/− mice were protected from DNA damage and had decreased p53 activation. Additionally, Mtg16−/− crypts isolated from mice at 4 h after 12 Gy radiation had increased plating efficiency, indicating an epithelial cell-autonomous role for MTG16 in protecting crypt stem cells. Lastly, examination of Mtg16 expression in isolated stem cells at the time of crypt regeneration demonstrated that Mtg16 expression was reduced in stem cell populations.
Despite an increase in proliferation in the Mtg16−/− mice, we did not observe a difference in gross morphology or morphometry. There are multiple factors that influence crypt morphometry, with apoptosis and proliferation each partially contributing to overall crypt depth or villus height. We suspect that the rather subtle, but significant, increase in proliferation may be insufficient to influence mucosal morphometry in the Mtg16−/− mice. Mtg16−/− mice exhibit hematopoietic lineage allocation defects with skewing of early myeloid progenitor cells toward granulocytic/macrophage lineages and a reduction in megakaryocyte-erythroid progenitor cells (10). We report that these lineage allocation differences are not limited to hematopoiesis, as Mtg16−/− small intestine also has decreased goblet cell numbers. While the functional role of goblet cells in radiation injury is unclear (5, 12, 22), it is possible that decreased goblet cells in Mtg16−/− mice might impact crypt viability after radiation injury.
While we report higher crypt viability after radiation than what others have previously reported (36), we believe that differences in technique may account for this apparent discrepancy. With a Swiss-rolling technique and examination of 40 sequential, well-aligned crypts in the distal one-third of the small intestine at this time point and radiation dose, we reproducibly observed crypt viability in the 60–80% range in WT mice. We observed significantly higher crypt viability in the Mtg16−/− mice, suggesting either greater viability and survival postirradiation or faster crypt regeneration. Other methods of assessment, such as evaluating cross sections of the small intestine, often score lower indexes of viability (36). Given our experimental design, we may also have higher crypt viability indexes because regeneration occurs at 96 h postradiation, and it is possible that dead crypts may have already been cleared by this point and thus would not be factored into our assessment.
While decreased phosphorylation of H2A.X in the Mtg16−/− mice suggests accelerated repair and decreased DNA damage in this cohort, an alternative consideration is that MTG16 regulates the phosphorylation of H2A.X in response to irradiation and triggers activation of DNA repair machinery. Thus Mtg16 deletion may result in a failure to recognize damage after radiation due to compromised phosphorylation of H2A.X, and Mtg16−/− mice may suffer DNA damage and have defective initiation of repair mechanisms. Another important consideration for these studies is that while Mtg16−/− mice exhibit decreased apoptosis after radiation compared with WT mice, there is a possibility that intestinal cells that escape apoptosis may later undergo mitotic catastrophe, an indication of failed DNA repair. This phenomenon has been observed in p53−/− mice (20). We did not test this as Mtg16−/− mice do not survive, secondary to marrow failure, even at lower doses of radiation. This experiment may be performed when Mtg16 floxed mice become available.
After irradiation, and at time of crypt regeneration, Mtg16 expression is reduced in Sox9-EGFPLow stem cell-enriched compartments. Microarray analysis of Sox9-EGFPLow stem cell has shown that genes involved in differentiation, crypt repair, and radiation-induced apoptosis are repressed in Sox9-EGFPLow cells (24). Given this evidence, we postulate that lower MTG16 levels may permit activation of stem cell programs promoting epithelial reconstitution. We also observed that Mtg16 expression is increased in Sox9-EGFPNegative populations. As previously reported by van Landeghem et al. (24), Sox9-EGFPNegative cells are enriched for differentiated lineages. Thus, MTG16 might repress stem cell programs and allow differentiation to progress after injury in this population of cells.
The Wnt signaling pathway plays an important role in regulating intestinal epithelial stem cell function (15, 19, 37). We have previously shown that MTG16 competes with β-catenin for TCF4 occupancy and that the absence of MTG16 results in increased epithelial proliferation (11, 34). In support of enhanced TCF4 activity in response to Wnt, baseline characterization of Mtg16−/− crypts in the enteroid culture system showed increased plating efficiency and delayed maturation in the presence of Wnt3A. Furthermore, several lines of evidence support a role for WNT/β-catenin signaling in survival of stem/progenitor cell populations after radiation (23, 48). Ex vivo studies presented here show that enteroids isolated from irradiated Mtg16−/− mice have increased survival compared with WT enteroids. Together, these data suggest that MTG16 may be important in modifying survival programs in stem cell populations after radiation.
Our findings indicate that MTG16 is critical for multiple aspects of small intestinal homeostasis and response to injury. Specifically, MTG16 controls goblet cell allocation, controls enterocyte proliferation, and is important in radiation-induced injury responses. Importantly, because current treatment modalities are aimed at targeting the symptoms of radiation enteritis (21), this study offers promise in understanding the underlying molecular mechanisms that regulate responses to radiation therapy.
GRANTS
This study was supported by National Institutes of Health Grants R01-DK-099204 and K08-DK-080221 (to C. S. Williams), 1F30-DK-103498 and T32-GM-07347 (to V. K. Reddy), P50-CA-095103 (to M. K. Washington), P30-DK-058404 (to Vanderbilt Digestive Disease Research Center); American Cancer Society Research Scholar Grant 116552 (to C. S. Williams), and NIH Grant R01-DK-040247 (to P. K. Lund); Office of Medical Research, Department of Veterans Affairs Merit Review Grant 1I01BX001426 (to C. S. Williams). This publication was also supported in part by the National Cancer Institute Cancer Center Support Grant P30-CA-068485 and by Clinical and Translational Science Award UL1TR000445 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.V.P., V.K.R., A.M.W., M.K.W., E.H., S.W.H., K.S., R.C., P.K.L., and C.S.W. conception and design of research; S.V.P., V.K.R., M.K.M., A.M.W., E.H., A.T.M., K.S., R.C., and C.S.W. performed experiments; S.V.P., V.K.R., M.K.M., A.M.W., M.K.W., E.H., A.T.M., K.S., R.C., K.T.W., and C.S.W. analyzed data; S.V.P., V.K.R., A.M.W., K.S., R.C., K.T.W., P.K.L., and C.S.W. interpreted results of experiments; S.V.P., V.K.R., A.T.M., and C.S.W. prepared figures; S.V.P., V.K.R., and C.S.W. drafted manuscript; S.V.P., V.K.R., M.K.M., S.W.H., K.T.W., P.K.L., and C.S.W. edited and revised manuscript; S.V.P., V.K.R., M.K.M., and C.S.W. approved final version of manuscript.
REFERENCES
- 1.Alferez D, Goodlad RA. To best measure cell proliferation in samples from the intestine. Cell Prolif 40: 231–240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann JM, Chyla BJ, Ellis TC, Martinez A, Moore AC, Franklin JL, McGhee L, Meyers S, Ohm JE, Luce KS, Ouelette AJ, Washington MK, Thompson MA, King D, Gautam S, Coffey RJ, Whitehead RH, Hiebert SW. Mtgr1 is a transcriptional corepressor that is required for maintenance of the secretory cell lineage in the small intestine. Mol Cell Biol 25: 9576–9585, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann JM, Nip J, Strom DK, Lutterbach B, Harada H, Lenny N, Downing JR, Meyers S, Hiebert SW. ETO, a target of t(8;21) in acute Leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through Its oligomerization domain. Mol Cell Biol 21: 6470–6483, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev 22: 1856–1864, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becciolini A, Fabbrica D. Quantitative changes in the goblet cells of the rat small intestine after irradiation. Acta Radiol Oncol 24: 291–299, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg 53, Suppl 1: 15S–21S, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, Delgado AG, Hill S, Casero AR, Bravo LE, Dominguez RL, Correa P, Polk DB, Washington MK, Rose KL, Schey KL, Morgan DR, Peek RM, Wilson KT. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 146: 1739–1751.e14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA Jr, Correa P, Gobert AP, Polk DB, Peek RM Jr, Wilson KT. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141: 1696–1708, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chyla BJ, Moreno-Miralles I, Steapleton AM, Thompson MA, Bhaskara S, Engel M, Hiebert SW. Deletion of Mtg16, a target of t(16;21), alters hematopoietic progenitor cell proliferation and lineage allocation. Mol Cell Biol 28: 6234–6247, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JN, McGhee L, Meyers S. The ETO (MTG8) gene family. Gene 303: 1–10, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen JM, Kooyman J, Visser WJ. The influence of 400 r x-irradiation on the number and the localization of mature and immature goblet cells and Paneth cells in intestinal crypt and villus. Cell Tissue Kinet 9: 65–75, 1976. [DOI] [PubMed] [Google Scholar]
- 13.Ernestos B, Nikolaos P, Koulis G, Eleni R, Konstantinos B, Alexandra G, Michael K. Increased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys 76: 1199–1205, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol 32: 1918–1927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27: 7551–7559, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 296: G1108–G1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298: G590–G600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Gudkov AV, Komarova AE. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 3: 117–129, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Harb AH, Abou Fadel C, Sharara AI. Radiation enteritis. Curr Gastroenterol Rep 16: 383, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kanter M, Akpolat M. Vitamin C protects against ionizing radiation damage to goblet cells of the ileum in rats. Acta Histochem 110: 481–490, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Kim KH, Lee J, Lee YA, Kim M, Lee SJ, Park K, Yang H, Jin J, Joo KM, Lee J, Nam DH. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest 92: 466–473, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibowitz BJ, Qiu W, Liu H, Cheng T, Zhang L, Yu J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol Cancer Res 9: 616–625, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg SR, Olsson A, Persson AM, Olsson I. The Leukemia-associated ETO homologues are differently expressed during hematopoietic differentiation. Exp Hematol 33: 189–198, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lund PK. Fixing the breaks in intestinal stem cells after radiation: a matter of DNA damage and death or DNA repair and regeneration. Gastroenterology 143: 1144–1147, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Lutterbach B, Westendorf JJ, Linggi B, Patten A, Moniwa M, Davie JR, Huynh KD, Bardwell VJ, Lavinsky RM, Rosenfeld MG, Glass C, Seto E, Hiebert SW. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol 18: 7176–7184, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 3: 217–240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margalit O, Amram H, Amariglio N, Simon AJ, Shaklai S, Granot G, Minsky N, Shimoni A, Harmelin A, Givol D, Shohat M, Oren M, Rechavi G. BCL6 is regulated by p53 through a response element frequently disrupted in B-cell non-Hodgkin lymphoma. Blood 107: 1599–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Martin K, Potten CS, Roberts AS, Kirkwood TB. Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J Cell Sci 111: 2297–2303, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Mazzatti DJ, Lee YJ, Helt CE, O'Reilly M, Keng PC. p53 modulates radiation sensitivity independent of p21 transcriptional activation. Am J Clin Oncol 28: 43–50, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi H, Kozu T, Shimizu K, Enomoto K, Maseki N, Kaneko Y, Kamada N, Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J 12: 2715–2721, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore AC, Amann JM, Williams CS, Tahinci E, Farmer TE, Martinez JA, Yang G, Luce KS, Lee E, Hiebert SW. Myeloid translocation gene family members associate with T-cell factors (TCFs) and influence TCF-dependent transcription. Mol Cell Biol 28: 977–987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieuwenhuis B, Van Assen-Bolt AJ, Van Waarde-Verhagen MA, Sijmons RH, Van der Hout AH, Bauch T, Streffer C, Kampinga HH. BRCA1 and BRCA2 heterozygosity and repair of X-ray-induced DNA damage. Int J Radiat Biol 78: 285–295, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Ottewell PD, Duckworth AC, Varro A, Dimaline R, Wang TC, Watson AJ, Dockray GJ, Pritchard DM. Gastrin increases murine intestinal crypt regeneration following injury. Gastroenterology 130: 1169–1180, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potten CS, Chadwick CA. Small intestinal growth regulatory factors extracted by simple diffusion from intact irradiated intestine and tested in vivo. Growth Factors 10: 63–75, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut 36: 864–873, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol 19: 185–198, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stelzner M, Helmrath M, Dunn JC, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH, Yu J. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 302: G1359–G1363, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulli G, Di Micco R, d'Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer 12: 709–720, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Hoshino T, Redner RL, Kajigaya SL. ETO, fusion partner in t (8;21) acute myeloid leukemia, represses transcription by interaction with the human. Proc Natl Acad Sci USA 95: 10860–10865, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams CS, Bradley AM, Chaturvedi R, Singh K, Piazuelo MB, Chen X, McDonough EM, Schwartz AD, Brown CT, Allaman MM, Coburn AL, Horst SN, Beaulieu DB, Choksi AY, Washington MK, Williams AD, Fisher AM, Zinkel SS, Peek RM, Wilson KT, Hiebert SW. MTG16 contributes to colonic epithelial integrity in experimental colitis. Gut 62: 1446–1455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodward AW, Chen MS, Behbod F, Alfaro MP, Buchholz AT, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA 104: 618–623, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]