Abstract
In young healthy men, passive leg movement (PLM) elicits a robust nitric oxide (NO)-dependent increase in leg blood flow (LBF), thus providing a novel approach to assess NO-mediated vascular function. While the magnitude of the LBF response to PLM is markedly reduced with age, the role of NO in this attenuated response in the elderly is unknown. Therefore, this study sought to determine the contribution of NO in the PLM-induced LBF with age. Fourteen male subjects (7 young, 24 ± 1 yr; and 7 old, 75 ± 3 yr) underwent PLM with and without NO synthase (NOS) inhibition achieved by intra-arterial infusion of NG-monomethyl-l-arginine (l-NMMA). LBF was determined second-by-second by Doppler ultrasound, and central hemodynamics were measured by finger photoplethysmography. NOS inhibition blunted the PLM-induced peak increase in LBF in the young (control: 668 ± 106; l-NMMA: 431 ± 95 Δml/min; P = 0.03) but had no effect in the old (control: 266 ± 98; l-NMMA: 251 ± 92 Δml/min; P = 0.59). Likewise, the magnitude of the reduction in the overall (i.e., area under the curve) PLM-induced LBF response to NOS inhibition was less in the old (LBF: −31 ± 18 ml) than the young (LBF: −129 ± 21 ml; P < 0.01). These findings suggest that the age-associated reduction in PLM-induced LBF in the elderly is primarily due to a reduced contribution to vasodilation from NO and therefore support the use of PLM as a novel approach to assess NO-mediated vascular function across the lifespan.
Keywords: aging, leg blood flow, nitric oxide, endothelial function, flow-mediated dilation
passive leg movement (PLM) evokes a robust leg blood flow (LBF) response (34, 56, 61), the magnitude of which is markedly reduced by aging (26, 35). Importantly, aging is also characterized by impaired NO-mediated vascular function (20, 45, 51), suggesting that the magnitude of the PLM-induced LBF response may be directly associated with impaired vascular function. Interestingly, in young healthy men, utilizing NO synthase (NOS) blockade, it has been documented that nearly 80% of the PLM-induced LBF response is NO dependent (55). This high reliance on NO coupled with a relatively simple methodology supports the use of the PLM paradigm as a novel approach for the in vivo assessment of NO bioavailability and endothelial function. However, the contribution of NO to PLM-induced LBF in populations other than the healthy young, most notably the elderly, is not yet known, thus limiting the implications of this assessment across the lifespan.
The assessment of endothelial function is of significance in both clinical and research settings as endothelial dysfunction precedes overt cardiovascular disease including hypertension, atherosclerosis, and coronary artery disease (1, 9, 17, 53). In the research arena, endothelial function is commonly assessed by flow-mediated dilation (FMD) following circulatory occlusion (10). However, recent studies have questioned the NO-dependent nature of this technique (42–44, 57, 63). Given the recognized vasoprotective and cardioprotective properties of NO, the growing uncertainty regarding the role of NO in the traditional FMD technique, and its failure to be embraced clinically, there is reason to search for a novel approach to assess NO-dependent endothelial function.
Therefore, this investigation sought to determine the mechanism responsible for the recognized impairment in PLM-induced LBF with age (26, 35) by assessing the role of NO in the peripheral hemodynamic response to this stimulus. Specifically, we hypothesized that due to the age-associated reduction in NO bioavailability, aging would be associated with a limited contribution of NO to the PLM-induced increase in LBF. Documentation of the capacity for PLM to discern changes in NO bioavailability across the lifespan will help to solidify the practical usefulness of PLM as a novel and clinically relevant approach to assess NO-mediated endothelial function.
METHODS
Subjects
Seven young healthy men (24 ± 1 yr of age) and seven older healthy men (75 ± 3 yr of age) volunteered to participate in this research study. Subjects were normally active, not taking any prescription medications, and free from overt cardiovascular disease. Protocol approval and written informed consent were obtained according to the University of Utah and Veterans Affairs Salt Lake City Institutional Review Boards, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place at the Utah Vascular Research Laboratory located in the Salt Lake City Veteran Affairs Medical Center's Geriatric Research, Education, and Clinical Center.
Experimental Protocol
Before the experimental day all subjects reported to the laboratory for a familiarization trial. During this session, PLM and Doppler ultrasound imaging of the femoral artery were performed to both familiarize the subjects and ensure that acceptable images could be obtained with and without movement. Upon arrival at the laboratory on the experimental day, body mass and height were recorded and the right femoral artery was catheterized (18-gauge central venous catheter; Arrow International, Reading, PA) using the Seldinger technique. Following a 30-min rest period, subjects were positioned in the supine position. Due to the lasting effects of NG-monomethyl-l-arginine (l-NMMA), the control trials were always performed before l-NMMA infusion. Trials were separated by at least 30 min to ensure blood flow and central hemodynamics returned to baseline values. Before the commencement of PLM, stable baseline central and peripheral hemodynamic measures were documented. An automated occlusion cuff (Hokansen, Bellevue, WA) was placed just below the knee of the passively moved leg and inflated to 250 mmHg during both control and l-NMMA trials to ensure that the infusate did not perfuse the lower portion of the limb. Single leg PLM was performed by a member of the research team who moved the leg through a 90° range of motion at 1 Hz. The starting position of the leg was the full extension at the knee (i.e., 180°), and therefore, the first movement served to passively flex the knee from 180 to 90°. Real-time knee joint angle feedback was provided to the researcher by digital display. Before the start and throughout the protocol, subjects were encouraged to remain passive and resist the urge to assist with leg movement. To avoid the startle reflex and active resistance to the PLM, subjects were made aware that PLM would take place in ∼1 min, but to minimize the chance of an anticipatory response, they were not informed of exactly when the movement would begin. When commenced, PLM was performed for 2 min at 1 Hz. We previously reported no discernable difference in the time course and magnitude of the PLM-induced LBF response when performed on separate days (coefficient of variation = 5%), indicating the peripheral hemodynamic response to PLM is reproducible (55).
l-NMMA infusion.
Thigh volume was determined anthropometrically (31) and then used for the calculation of drug dosing. l-NMMA (Bachem, Bubendorf, Switzerland) was diluted from 250 mg lypholyzed powder in normal saline to a concentration of 5 mg/ml. l-NMMA was infused at a priming dose of 0.48 mg/dl thigh volume for 5 min before PLM. During the minute before PLM, l-NMMA was infused at a maintenance dose of 0.24 mg/dl thigh volume and this infusion rate was maintained for the duration of PLM. During control trials, normal saline was infused intra-arterially at the same rate and duration as l-NMMA.
Measurements
Femoral blood flow.
Measurements of femoral arterial blood velocity and vessel diameter were performed in the passively moved and the nonmoved legs distal to the inguinal ligament and proximal to the deep and superficial femoral bifurcation with Logic 7 and Logic e Doppler ultrasound systems (General Electric Medical Systems, Milwaukee, WI). The ultrasound systems were equipped with a linear transducer operating at an imaging frequency of 10 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel. Arterial diameter was measured, and mean velocity (Vmean; angle corrected and intensity-weighted area under the curve) was automatically calculated (Logic 7 and Logic e). with the use of femoral arterial diameter and Vmean, LBF was calculated as LBF = Vmean·π (vessel diameter/2)2·60, where LBF is in milliliters per minute. Leg vascular conductance (LVC) was calculated as LVC = LBF/mean arterial pressure (MAP).
Central hemodynamic variables.
Heart rate (HR), stroke volume (SV), and cardiac output (CO) were determined with a finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was calculated from beat-by-beat pressure waveforms assessed by photoplethysmography using the Modelflow method (Beatscope version 1.1; Finapres Medical Systems), which, in combination with HR, has been documented to accurately estimate CO during a variety of experimental protocols (3, 18, 19, 50, 58). Intravascular systolic and diastolic arterial pressures were measured with an in-line pressure transducer (Baxter, Deerfield, IL) placed at the level of the catheter. MAP was calculated as diastolic + 1/3(systolic − diastolic).
Data acquisition.
Throughout each protocol, HR, SV, CO, MAP, and ECG signals underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) using a data acquisition system (AcqKnowledge; Biopac Systems, Goleta, CA).
Data and Statistical Analysis
The data acquisition software (AcqKnowledge; Biopac Systems) allowed second-by-second analyses of HR, SV, CO, and MAP. The second-by-second blood velocities were analyzed on the ultrasound systems (GE Logic 7 Logic e) for the first 60 s of movement, and 12-s averages were assessed from 60 to 120 s of PLM. All analyses were performed using a 5-s moving average. Baseline was analyzed using the 60 s before initiation of PLM. A 2 × 2 repeated-measures ANOVA was used to determine significant differences in the absolute change from baseline to peak for HR, SV, CO, MAP, LBF, LVC, and area under the curve (AUC) for LBF, LVC, and MAP. Cumulative AUC was calculated as the summed second-by-second response during the first 60 s of PLM after normalizing for baseline differences. Post hoc comparisons were made using Fischer's least significant difference when appropriate. Subject characteristics and blood parameters were compared by independent sample t-tests.
The rate of increase in the hyperemic response from baseline to peak was determined a posteriori by linear regression using an iterative process minimizing the sum of squared residuals between the fitted function and the observed values. To avoid the inclusion of nonessential points in the regression, the data were initially fit up to the peak of the hyperemic response and then decreased iteratively by 1 s. The best fitting window was then established using the following three indexes: 1) the flat profile of the residual plot being no longer sustained as determined by visual inspection, 2) the demonstration of a local “threshold” in the χ2-squared values, and 3) a significant correlation (range: r = 0.89 ± 0.05 to 0.98 ± 0.01) between the actual data and the predicted values as determined by linear regression (46). A 2 × 2 repeated-measures ANOVA was used to test differences in the rate of increase of the hyperemic response and post hoc analysis was performed with Fischer's least significant difference when appropriate. Significance was set at an α-level of 0.05, and data are presented as means ± SE.
RESULTS
Young and old subjects were well-matched for stature, thigh volume, MAP, and blood characteristics (Table 1).
Table 1.
Subject stature and blood characteristics
| Young | Old | |
|---|---|---|
| Age, yr | 24 ± 1 | 75 ± 3* |
| Height, cm | 177 ± 3 | 177 ± 3 |
| Weight, kg | 75 ± 4 | 78 ± 5 |
| Body mass index, kg/m2 | 24 ± 1 | 25 ± 2 |
| Thigh volume, dl | 74 ± 4 | 63 ± 4 |
| Mean arterial pressure, mmHg | 95 ± 3 | 98 ± 4 |
| Glucose, mg/dl | 68 ± 4 | 75 ± 4 |
| Cholesterol, mg/dl | 172 ± 14 | 178 ± 13 |
| Triglycerides, mg/dl | 89 ± 15 | 102 ± 17 |
| HDL, mg/dl | 49 ± 3 | 50 ± 4 |
| LDL, mg/dl | 107 ± 12 | 117 ± 9 |
Values are means ± SE.
P < 0.05, significant difference between young and old.
Baseline Effects of l-NMMA
Baseline hemodynamics with and without l-NMMA are presented in Table 2. Baseline LBF was ∼30% lower in the old compared with the young; however, this difference did not reach statistical significance (P = 0.19). NOS inhibition, achieved by the intra-arterial infusion of l-NMMA, decreased baseline LBF in the young (P < 0.01) and the old (P = 0.02); however, the magnitude of this reduction was not different between groups (P = 0.21). l-NMMA reduced baseline HR (P = 0.03) and CO (P = 0.024) in the young but had no effect in the old. SV and MAP were not different between groups and were not altered by l-NMMA (Table 2).
Table 2.
Baseline hemodynamics with and without l-NMMA
| Young |
Old |
|||
|---|---|---|---|---|
| Control | l-NMMA | Control | l-NMMA | |
| Leg blood flow, ml/min | 310 ± 27 | 161 ± 16† | 217 ± 62 | 121 ± 50† |
| Leg vascular conductance, ml·min−1·mmHg−1 | 3.3 ± 0.3 | 1.7 ± 0.1† | 2.3 ± 0.6 | 1.3 ± 0.5† |
| Heart rate, beats/min | 54 ± 2 | 50 ± 2† | 59 ± 4 | 61 ± 6 |
| Stroke volume, ml/beat | 98 ± 6 | 96 ± 8 | 93 ± 19 | 79 ± 15 |
| Cardiac output, l/min | 5.5 ± 0.4 | 4.7 ± 0.3† | 5.2 ± 1.0 | 4.1 ± 0.8 |
| Mean arterial pressure, mmHg | 95 ± 3 | 96 ± 3 | 98 ± 4 | 98 ± 5 |
Values are means ± SE.
P < 0.05, significant difference between control and NG-monomethyl-l-arginine (l-NMMA).
Peripheral Hemodynamics During PLM
In both groups and trials, LBF increased immediately at the onset of PLM (Fig. 1A). To take into account the l-NMMA-induced reduction in baseline LBF, the peak change in LBF was calculated (Fig. 1B). The peak change in LBF during control PLM was 2.5-fold greater in the young (668 ± 106 ml/min) than the old (266 ± 98 ml/min; P = 0.016). NOS inhibition evoked a significant reduction in peak LBF in the young (Δ −237 ± 83 ml/min; P = 0.03) but not the old (Δ −15 ± 26 ml/min; P = 0.59). Similarly, l-NMMA reduced the overall LBF response (as measured by LBF AUC) in the young (−129 ± 21 ml; P < 0.01) but not the old (−31 ± 18 ml, P = 0.13; Fig. 1C). PLM-induced changes in LVC (second-by-second data not illustrated), an indicator of leg vasodilation, mirrored the changes in LBF, with a threefold greater peak change in LVC in the young (7.7 ± 1.1 ml·min−1·mmHg−1) compared with the old (2.5 ± 0.9 ml·min−1·mmHg−1; P < 0.01). Similar to LBF, NOS inhibition significantly attenuated the peak change in LVC in young (Δ −3.3 ± 1.0 ml·min−1·mmHg−1; P = 0.02) but not the old (Δ −0.2 ± 0.4 ml·min−1·mmHg−1; P = 0.62). The overall vasodilatory response to PLM (as measured by LVC AUC) was reduced with age (P < 0.05) and by l-NMMA in both the young (P < 0.01) and the old (P < 0.05; Fig. 2A); however, the magnitude of the l-NMMA-induced reduction in LVC AUC was far greater in the young (P = 0.02; Fig. 2B).
Fig. 1.
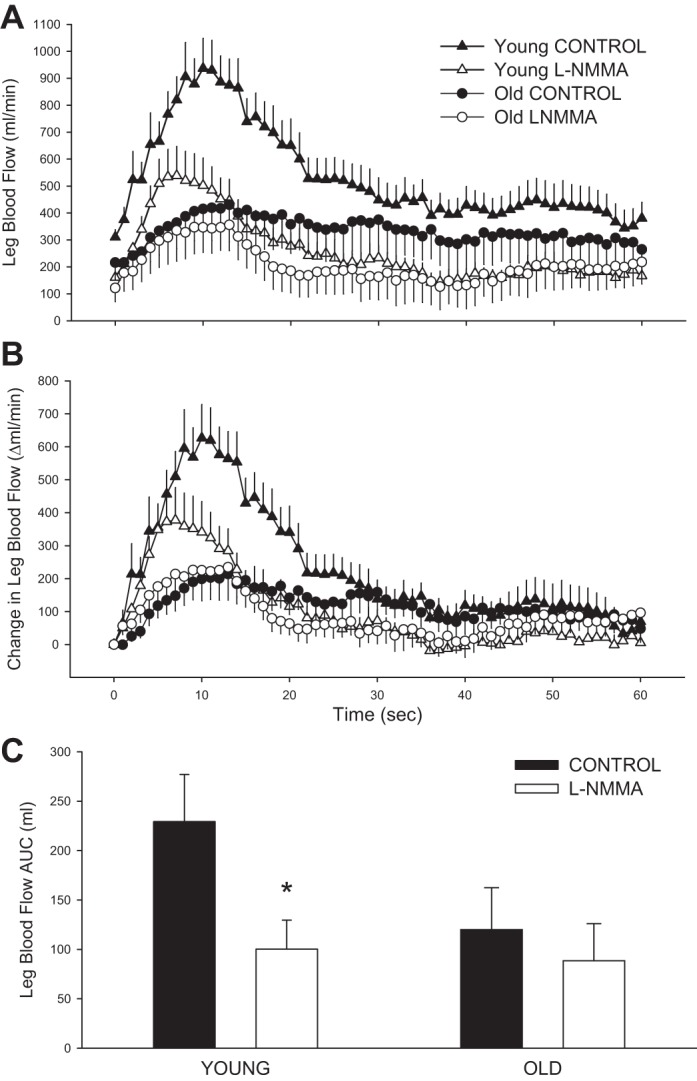
Passive limb movement (PLM)-induced hyperemia in young and old with and without intra-arterial NG-monomethyl-l-arginine (l-NMMA) infusion. A: absolute leg blood flow (LBF; ml/min). B: change in LBF, normalized for the resting reduction in LBF due to l-NMMA. C: LBF area under the curve (AUC) calculated as the summed second-by-second response during the first 60 s of movement. Values are means ± SE. To increase clarity of the data presented, only the first 60 s of PLM are displayed. One minute of baseline data was collected before PLM occurred. *P < 0.05, significant difference between control and l-NMMA.
Fig. 2.
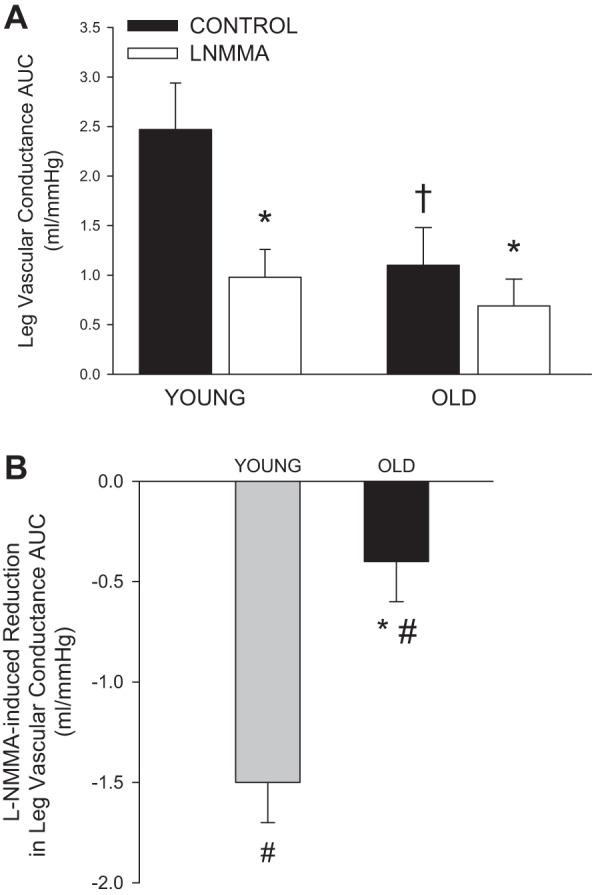
PLM-induced vasodilatory response in young and old with and without intra-arterial l-NMMA infusion. A: leg vascular conductance (LVC) AUC calculated as the summed second-by-second response during the first 60 s of movement. B: l-NMMA-induced reduction in LVC AUC. Values are means ± SE. *P < 0.05, significant difference between control and l-NMMA. †P < 0.05, significant difference between young and old. #P < 0.05, significant reduction due to l-NMMA.
The rate of the initial (first 7–10 s) increase in the LBF response to PLM in the control trial was nearly three times faster in the young (74 ± 11 ml·min−1·s) than the old (26 ± 6 ml·min−1·s; P = 0.004). l-NMMA, however, had no impact on the rate of the LBF response in either group (young: 65 ± 15 ml·min−1·s, P = 0.63; old: 35 ± 12 ml·min−1·s, P = 0.53).
LBF in the nonmoved leg exhibited a slight increase following the onset of PLM and then fluctuated around the baseline for the remaining portion of PLM in the young and old in the control trial and the old l-NMMA trial (Fig. 3). In contrast, during the l-NMMA trial in the young, LBF in the nonmoved leg exhibited a clear transient increase during the first minute of PLM (Fig. 3).
Fig. 3.
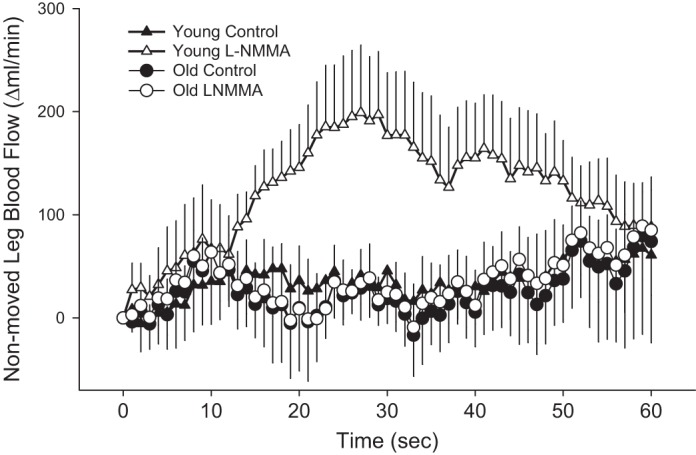
Change in LBF in the nonmoved limb during PLM in young and old with and without intra-arterial l-NMMA infusion. Significant main effect of interaction for the young (trial × time). One minute of baseline data was collected before PLM. To increase clarity of the data presented, only the first 60 s of PLM are displayed. Values are means ± SE.
Central Hemodynamics During PLM
PLM evoked significant increase in HR, SV, and CO in both groups and across all trials (Table 3). Peak changes in HR and CO were not different between groups or conditions. The change in SV was greater in the young during the control trial compared with the l-NMMA trial while there was no effect of l-NMMA on SV in the old. Due to the dynamic changes in MAP during PLM (Fig. 4A), the overall MAP response, as determined by AUC during the first minute of PLM, was compared between groups and trials. Young subjects exhibited an overall decrease in MAP during the control trial, whereas a net increase was evident with l-NMMA infusion (Fig. 4B). In contrast, MAP in the old increased during PLM, and when expressed as AUC, the overall MAP response also revealed a net increase that was similar between the control and l-NMMA trials (Fig. 4D).
Table 3.
Peak changes in central hemodynamics during passive limb movement
| Young |
Old |
|||
|---|---|---|---|---|
| Control | l-NMMA | Control | l-NMMA | |
| Heart rate, beats/min | 10 ± 3 | 10 ± 3 | 7 ± 2 | 7 ± 3 |
| Stroke volume, ml/beat | 7 ± 2 | 3 ± 1† | 10 ± 5 | 6 ± 2 |
| Cardiac output, l/min | 0.9 ± 0.2 | 1.1 ± 0.3 | 1.4 ± 0.6 | 0.7 ± 0.4 |
Values are means ± SE.
P < 0.05, significant difference between control and l-NMMA.
Fig. 4.
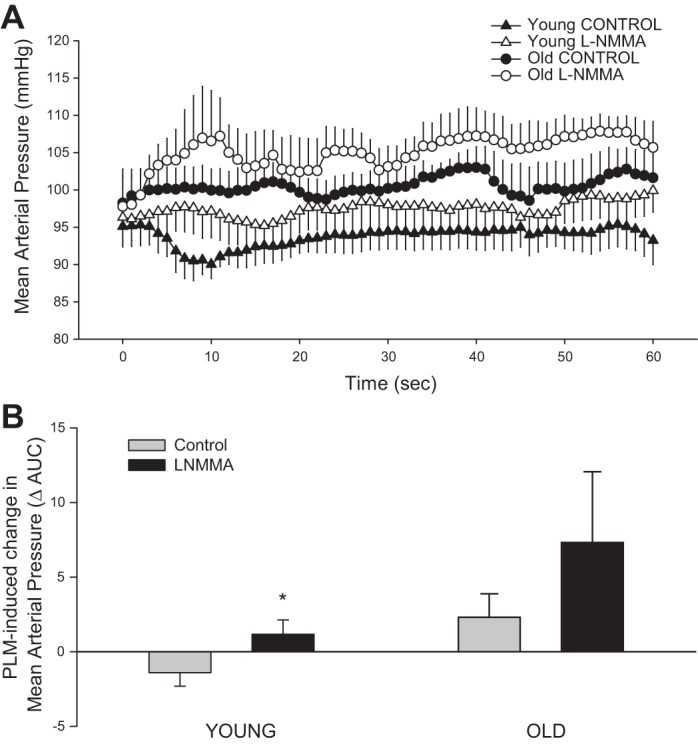
PLM-induced changes in mean arterial pressure in young and old with and without intra-arterial l-NMMA infusion. Absolute mean arterial pressure (MAP; mmHg; A) and MAP AUC (B) calculated as the summed second-by-second response during the first 60 s of movement. Values are means ± SE. *P < 0.05, significant difference between control and l-NMMA.
DISCUSSION
Previously, we (55) and others (37) have reported that NO plays an essential role in the magnitude and duration of the PLM-induced increase in LBF, suggesting that such a paradigm may provide a novel assessment of NO-mediated vascular function and cardiovascular disease risk. The current study is the first to directly examine the contribution of NO to PLM-induced LBF with age. The PLM-induced increase in peak LBF and the contribution of NO to this response were severely attenuated with age, as NOS inhibition diminished the LBF response in the young but had no effect in the old. Moreover, the overall LBF response (i.e., AUC) was significantly blunted following NOS inhibition in the young but not the old. These findings reveal that aging reduces the role of NO in PLM-induced LBF and provide further support for PLM as a novel and clinically relevant approach to assess NO-mediated vascular function across the lifespan.
PLM as a Novel Method for the Assessment of NO-Mediated Vascular Function
The assessment of NO-mediated vascular function is important from both clinical and research perspectives as reductions in NO bioavailability and endothelial function precede overt cardiovascular disease (1, 17, 25, 53). Indeed, the prognostic and diagnostic importance of accurately and reliably assessing NO-mediated vascular function has led to the development and sustained interest in the occlusion-based FMD (10). This traditional FMD technique, as first described by Celermajer et al. (10), has been widely used as a noninvasive approach to determine endothelial derived NO bioavailability. However, recent evidence has challenged the usefulness of FMD in determining NO-dependent vascular function (42–44, 57, 63). This uncertainty and the failure of FMD to become adopted clinically prompted interest in determining whether an alternative method, PLM, can serve as a functional bioassay for endothelium-derived NO bioavailability and assess NO-dependent vascular function in humans.
This study provides novel evidence that aging greatly diminishes the contribution of NO to PLM-induced hyperemia. Confirming our previous findings (55), NOS inhibition reduced the peak change and overall LBF in the young, while having no effect in the old (Fig. 1). Interestingly, the significant reduction in overall LVC (Fig. 2A) and not LBF (Fig. 1C) in the older adults is likely due to the slight elevation in MAP during PLM in the NOS-inhibited trial in the old. Thus normalizing the LBF response for this minor elevation in MAP revealed that the overall vasodilatory response to PLM in the old is at least partially NO mediated, albeit at a level much less than that reported in the young (Fig. 2B). Overall, these findings imply that the attenuated PLM-induced increases in LBF and vasodilation associated with aging are largely due to a reduced contribution of NO.
Reductions in vascular function with advancing age are well established. Indeed, both intra-arterial infusion of acetylcholine and occlusion-based FMDs have predominantly revealed impaired endothelial function with aging (5, 11, 20, 24, 38, 39). Compared with these conventional methods of assessing NO-dependent endothelial function, PLM may be a superior alternative. First, unlike the intra-arterial acetylcholine infusion approach, PLM is noninvasive, significantly reducing the risks associated with the technique. Mortensen et al. (37) recently reported a strong correlation between acetylcholine infusion and PLM induced-LBF, thus providing additional support in favor of the NO dependency of the PLM-induced hyperemic response. Second, acute tissue ischemia, which is required to provide the shear stimulus for vasodilation in the traditional FMD, is uncomfortable, and may be contraindicated in some individuals, is not a component of PLM. Third, compared with cuff-occlusion FMD, PLM does not require the precise sequential measurement of conduit artery diameter as the common femoral artery does not dilate during passive movement or exercise, greatly reducing the technical requirements in terms of both sonography and analysis (29). Finally, our recent observations, along with the current study, reveal that the PLM-induced increase in LBF is primarily NO dependent (55), a contention that has recently been challenged with traditional FMD (43, 63). Establishing PLM as a biomarker of NO-dependent vascular function will undoubtedly require additional investigation and validation; however, recent investigations by our group indicate that in addition to aging (26, 35), conditions commonly associated with vascular dysfunction including spinal cord injury (60) and heart transplantation (30) exhibit reduced vasodilation during PLM. Taken together, these findings suggest that PLM is capable of determining impairments in NO-dependent vascular function in clinically relevant patient groups.
In terms of the mechanisms responsible for the reduced NO-mediated LBF response to PLM with age, upstream factors, including a reduction in eNOS protein expression, have been suggested. However, Donato et al. (22) reported no difference in endothelial cell eNOS protein expression between young and old men, despite impaired endothelial function, suggesting that the enzymatic machinery for NO production is present but may simply not be functioning optimally. Alternatively, downstream factors such as elevated oxidative stress and the concomitant reduction in NO bioavailability by the inactivation of NO to peroxynitrite (8, 13, 27) may add to the diminished role of NO with age. In agreement with this hypothesis our group recently reported that acute antioxidant treatment resulted in improved blood antioxidant status, reduced oxidative stress, and improved vascular function in older healthy individuals (62). Additionally, intra-arterial infusion of ascorbic acid markedly improved endothelium-dependent vasodilation during prolonged handgrip exercise (16, 32) and during coinfusion of acetylcholine (52), suggesting a causative relationship between oxidative stress and impaired endothelium-dependent vascular function with age. Therefore, further research is warranted to determine if the impaired age-associated hyperemic response to PLM can be augmented by either acute or chronic antioxidant supplementation.
Insight into the Initial PLM-Induced Hyperemic Response
Based on our previous investigation (55), and confirmed by the current findings, the LBF response to PLM in young healthy individuals involves two distinct phases: an initial rapid NO-independent component, in which LBF increases, followed by an NO-dependent component that consists of a continued rise to peak LBF that gradually decays toward baseline. Interestingly, the current study reveals that despite the initial onset of LBF apparently not being NO-dependent (Fig. 1B), aging is associated with a threefold slower rate of LBF increase with the onset of PLM (i.e., within the first 7–10 s; Fig. 1B). This suggests that additional, non-NO-mediated, mechanisms associated with vasodilation are impaired with age. It has been suggested that the immediate or rapid vascular response to muscle contraction, or changes in muscle length with PLM, occur following the first muscle “contraction,” without a measurable delay and without a contribution from NO (47, 48). The current finding of an age-associated reduction in PLM-induced LBF hyperemia agrees with previous reports (Kirby et al. 32; Crecelius et al. 16) utilizing a single brief muscle contraction (handgrip exercise), which also implied that endothelial function, per se, does not contribute to the age-associated reduction in blood flow following a single contraction. Additionally, endothelium denuded isolated rat feed arteries display a reduced, but not abolished, immediate vasodilation in response to mechanical compression (12), providing evidence that an intact endothelium is not essential for the initial response.
Although the exact mechanisms responsible for the initial LBF response to PLM are not known, the current findings and those of others indicate that local endothelium-dependent vasodilators (NO and prostaglandins) and neural mechanisms do not appear to be essential for the initial vasodilatory response (6, 7, 23, 47, 49). A smooth muscle-related mechanism may involve potassium (K+) flux resulting in smooth muscle hyperpolarization, as the inhibition of inwardly rectifying K+ channels has been demonstrated to result in a reduction in the immediate vasodilator response in animals (2, 28, 36) and humans (14, 15). Mechanosensitive ion channels present on the endothelium may respond to stretch- and shear-induced changes during PLM leading to vasodilation. Additionally, myogenic vasodilation may play a role as we have previously reported that an increase in perfusion pressure, evoked by an upright posture, augmented the initial onset of LBF at the start of PLM (26, 56).
Contribution of Central Hemodynamics to PLM-Induced Hyperemia
At the onset of passive movement, group III mechanosensitive skeletal muscle afferent fibers are stimulated eliciting a reflex-mediated cardio-acceleration and subsequently a central hemodynamic response (30, 34, 40, 54, 61). This central hemodynamic response (HR, SV, and CO) to PLM-induced hyperemia was confirmed by the current study; however, differences due to age and NOS inhibition were unremarkable (Fig. 4). Central factors may contribute to the differences in PLM-induced hyperemia; however, the contribution of the central hemodynamic response (< 30%) (54) is much less than that associated with peripheral factors (∼ 80%), indicating that the differences between individuals or groups are largely peripherally mediated.
Interestingly, the overall MAP response to PLM was affected by both NOS inhibition and age (Fig. 4). Notably, in the young subjects PLM resulted in a transient reduction in MAP corresponding temporally with the peak increase in LBF. Theoretically, this reduction in MAP should act to decrease LBF; however, PLM-induced vasodilation appears to outstrip the capacity of the CO increase to raise pressure. During the infusion of l-NMMA in the young, MAP exhibited a net increase due to the expected reduction in vasodilation or increased vascular tone. Importantly, this peripherally induced reduction in vascular conductance due to intra-arterial infusion of l-NMMA had a profound impact on systemic blood flow distribution as the control leg (nonmoved and noninfused leg) of the young subjects experienced a significant increase in LBF during PLM (Fig. 3). This increase in LBF in the nonmoved leg of the young is likely the direct hemodynamic consequence of eliminating the previously available vascular sink (the passively moved limb) for the increase in CO during PLM. Interestingly, the accompanying increase in MAP in the young was modest, likely due to the accommodation of the increased CO by the nonmoved leg, presumably by non-NOS-mediated mechanisms due to the systemic effects of circulating l-NMMA. In both control and NOS-inhibited trials, the old subjects displayed marked increases in MAP during PLM, providing further evidence of a reduced vasodilatory capacity with age. Again, perhaps, non-NOS mediated, because of the anticipated effect of circulating l-NMMA. Interestingly, in stark contrast to the young, blood flow distribution between the infused and noninfused legs of the old was not affected by the l-NMMA as the overall contribution of NO to PLM-induced LBF and vasodilation was greatly reduced in the old.
Experimental Considerations
The primary finding of this investigation is the novel determination that aging is associated with an attenuated contribution of NO to the PLM-induced increase in LBF. However, several experimental limitations should be recognized. First, the overall sample size (n = 14) is relatively small and may have contributed to our inability to detect age-associated differences in resting LBF. Several investigations (4, 21, 33, 41) report a significant 20–30% reduction in resting LBF, which is in agreement with the nonsignificant magnitude of reduction reported here (30%). Second, despite a numerically lower thigh volume in the old, there was not a detectable significant difference between groups, which, if actually altered by age, could influence the PLM-induced hyperemia (59). However, even after normalization for thigh volume the peak change in LBF during PLM remained attenuated in the older men compared with their young counterparts (young: 90 Δml·min−1·l thigh volume−1; old: 48 Δml·min−1·l thigh volume−1; P = 0.035), indicating that the smaller thigh volume was not the driving factor for age-related reduction in LBF during PLM. Third, unlike the subjects selected for this study, the proportion of older men (∼75 yr of age) in good health and not taking prescription medications in the general population is quite low (<20% based on recent statistics from the Centers for Disease Control). However, our careful matching of young and old subjects was required to determine the true impact of aging, independent of other confounding factors, on the contribution of NO to LBF during PLM across the lifespan. Finally, pharmacological inhibition of NOS with l-NMMA revealed a critical role of NO bioavailability in the PLM-induced hyperemic response in the young that was absent in the old; however, the contribution of factors upstream to NO release, for example, NOS phosphorylation and expression, remains to be elucidated. Indeed, future investigations are warranted to determine if directly augmenting NO bioavailability, with an NO donor, improves the LBF response to PLM in those with vascular dysfunction.
Conclusions
In young healthy men PLM evokes a robust increase in LBF, the magnitude of which is largely NO mediated. In contrast, the LBF response to PLM in the old is severely attenuated and the contribution of NO to this response in the old is markedly reduced as NOS inhibition failed to impact the peak and the overall hyperemic response in the old. Taken together, these findings indicate that PLM provides a novel noninvasive and relatively simple model capable of assessing NO-dependent changes in vascular function across the lifespan.
GRANTS
This study was supported by Veteran Affairs Rehabilitation Research and Development Grant 1IK2RX001215 CDA2 (to J. D. Trinity) and Merit Grants E1697R and E6910R (to R. S. Richardson); American Heart Association Grant 14SDG1850039 (to J. D. Trinity); and National Heart, Lung, Blood Institute Grant PO1-HL-091830 (to R. S. Richardson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.T., H.J.G., and R.S.R. conception and design of research; J.D.T., H.J.G., G.L., M.J.R., S.J.I., D.E.M., B.S.G., and A.D.B. performed experiments; J.D.T. and H.J.G. analyzed data; J.D.T., H.J.G., G.L., M.J.R., S.J.I., and R.S.R. interpreted results of experiments; J.D.T. and H.J.G. prepared figures; J.D.T. drafted manuscript; J.D.T., H.J.G., G.L., M.J.R., S.J.I., and R.S.R. edited and revised manuscript; J.D.T., H.J.G., G.L., M.J.R., S.J.I., D.E.M., B.S.G., A.D.B., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol 581: 841–852, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci (Lond) 106: 365–369, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 2014 May 12 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol 277: H1872–H1877, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Bult H, Herman AG, Matthys KE. Antiatherosclerotic activity of drugs in relation to nitric oxide function. Eur J Pharmacol 375: 157–176, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS. Reliable endothelial function testing: at our fingertips? Circulation 117: 2428–2430, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 48: 489–509, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol 590: 5349–5359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W, Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension 32: 9–15, 1998. [DOI] [PubMed] [Google Scholar]
- 18.de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth 95: 326–331, 2005. [DOI] [PubMed] [Google Scholar]
- 19.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009. [DOI] [PubMed] [Google Scholar]
- 20.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol 84: 754–758, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77–81, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol 557: 1013–1020, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Wray DW, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 32.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 34.McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohrman DE, Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res 35: 384–390, 1974. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993. [PubMed] [Google Scholar]
- 41.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 301: H1118–H1126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roig E, Cuppoletti A, Masotti M, Kianco R, Vallejos I, Sitges M, Ortiz J, Perez-Villa F. Assessment of peripheral endothelial-dependent vasodilatation within the first year after heart transplantation. J Heart Lung Transplant 28: 299–304, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol 537: 291–303, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Saunders NR, Tschakovsky ME. Evidence for a rapid vasodilatory contribution to immediate hyperemia in rest-to-mild and mild-to-moderate forearm exercise transitions in humans. J Appl Physiol 97: 1143–1151, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol 81: 1516–1521, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, A1537–1538, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barret-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 58.van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol 90: 131–137, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, Ives SJ, Yonnet G, Richardson R. Passive leg movement-induced hyperemia with a spinal cord lesion: Evidence of preserved vascular function. Acta Physiol (Oxf) 210: 429–439, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venturelli M, Amann MK, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive-limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 345–351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


