Abstract
This study aimed to examine the effects of sex (males vs. females) and sex hormones (menstrual cycle phases in women) on sympathetic responsiveness to severe chemoreflex activation in young, healthy individuals. Muscle sympathetic nerve activity (MSNA) was measured at baseline and during rebreathing followed by a maximal end-inspiratory apnea. In women, baseline MSNA was greater in the midluteal (ML) than early-follicular (EF) phase of the menstrual cycle. Baseline MSNA burst incidence was greater in men than women, while burst frequency and total MSNA were similar between men and women only in the ML phase. Chemoreflex activation evoked graded increases in MSNA burst frequency, amplitude, and total activity in all participants. In women, this sympathoexcitation was greater in the EF than ML phase. The sympathoexcitatory response to chemoreflex stimulation of the EF phase in women was also greater than in men. Nonetheless, changes in total peripheral resistance were similar between sexes and menstrual cycle phases. This indicates that neurovascular transduction was attenuated during the EF phase during chemoreflex activation, thereby offsetting the exaggerated sympathoexcitation. Chemoreflex-induced increases in mean arterial pressure were similar across sexes and menstrual cycle phases. During acute chemoreflex stimulation, reduced neurovascular transduction could provide a mechanism by which apnea-associated morbidity might be attenuated in women relative to men.
Keywords: muscle sympathetic nerve activity, hypoxia, hypercapnia, menstrual cycle, sex differences
chemoreflex activation, induced by hypoxia and/or hypercapnia, is a potent sympathoexcitatory stimulus that can elicit long-lasting elevations in muscle sympathetic nerve activity (MSNA) (31, 40). Sympathetic responses to chemoreflex activation have been studied frequently, because repeated activation of the chemoreflex is thought to contribute to the chronic elevations in baseline MSNA that are observed in patients with sleep apnea syndrome (3, 15). Indeed, heightened levels of baseline MSNA have been linked to the development of hypertension (51), and sleep apnea syndrome has been acknowledged as an independent risk factor for hypertension (16).
Interestingly, little is known regarding the effect of sex and sex hormones on sympathetic activation during chemoreflex stimulation, despite observations that the incidence of sleep apnea syndrome and hypertension is lower in premenopausal women than in age-matched men (38, 39, 53). Furthermore, the association between sleep apnea syndrome and hypertension appears to be weaker in women than men (35). These observations raise the possibility that chemoreflex-induced sympathoexcitation is attenuated in women relative to men. To this end, information regarding the fundamental sex-based patterning of sympathetic responses to chemoreflex activation is needed. Only one previous study has compared MSNA responses to chemoreflex activation between the sexes (18). This study (18) in young healthy individuals showed that absolute sympathoexcitatory responses to hypoxic breathing were similar between the sexes. However, peak MSNA activation occurred earlier in the hypoxic period in the women than the men, indicating that MSNA responses to chemoreflex stimulation were temporally potentiated in the women. Given the prominent role of the sympathetic nervous system in development of cardiovascular disease (1, 21, 26), an improved understanding of the extent to which chemoreflex-dependent sympathetic activation is affected by sex could help suggest mechanisms by which sleep apnea syndrome might be associated with sex-dependent morbidity.
Within the context of considering factors that may contribute to the development of hypertension, it is important to consider the extent to which neuronal activation is associated with alterations to hemodynamic outcomes. Recent data indicate that neurovascular transduction differs between men and women (12). That is, while a positive relationship exists between baseline MSNA and total peripheral resistance (TPR) in young men (7), no such association has been observed in young women (14). As a result, male-female differences in baseline MSNA are not coupled to differences in baseline TPR (14, 42). The previous comparison of MSNA responses to hypoxia did not include an indication of concurrent vascular resistance (18). Moreover, Patel et al. (37) recently demonstrated reduced forearm vasoconstriction in young healthy women during apnea-induced chemoreflex activation compared with young healthy men, but they did not study MSNA. Therefore, it remains unclear whether sex affects the MSNA response to apnea or whether sex differences in MSNA are coupled to hemodynamic outcomes.
Recent investigations into sympathetic regulation in women have indicated that the menstrual cycle exerts an influence over the control of MSNA: generally, a relative sympathoexcitation has been observed during the high-hormone [midluteal (ML)] phase relative to the low-hormone [early-follicular (EF)] phase of the menstrual cycle. This pattern has been observed at baseline (29, 30, 36) and in response to baroreceptor unloading (6, 10) but has not been examined during chemoreflex activation. Recently, our laboratory examined MSNA responses to chemoreflex activation in regular users of hormonal contraceptives and observed that the low-hormone phase of hormonal contraceptive use was associated with a greater sympathoexcitatory response to severe chemoreflex stimulation than the high-hormone phase (49). When generalized to pertain to changes in endogenous sex hormones that occur across the menstrual cycle, these data suggest that low levels of circulating hormones (i.e., in the EF phase) may be associated with greater chemoreflex-induced increases in MSNA than higher hormone levels (i.e., in the ML phase).
The purpose of this study was to determine whether sympathetic and hemodynamic responses to chemoreflex stimulation differ between young, healthy men and women and between the EF and the ML phase of the menstrual cycle in women. We hypothesized that sympathetic responses to a hypoxic-hypercapnic apnea would be altered by one's sex and the changes in hormone levels that occur across the menstrual cycle. Specifically, the study tested the hypotheses that 1) MSNA responses would be greater in women than men and 2) in women, MSNA responses would be greater in the EF than ML phase of the menstrual cycle. We also examined whether altered MSNA responses with menstrual phase would translate into modified neurovascular outcomes.
METHODS
Participants.
Eighteen healthy nonsmoking subjects [9 women (24 ± 3 yr, 166 ± 6 cm, 64 ± 9 kg) and 9 men (26 ± 2 yr, 179 ± 4 cm, 82 ± 11 kg)] were tested after providing written, informed consent. Participants were physically active, engaging in both endurance and resistance exercise on a regular basis [mean ± SD: 4 ± 2 bouts/wk, 81 ± 43 min/bout (women); 5 ± 2 bouts/wk, 66 ± 25 min/bout (men)]. The majority of the participants were Caucasian (7 of 9 women, 8 of 9 men). All women reported regular menstrual cycles of ∼28 days, and none had used hormonal contraceptives within the last 6 mo. Test protocols were approved by the Health Sciences Research Ethics Board at the University of Western Ontario and conformed to the standards set by the latest revision of the Declaration of Helsinki.
Experimental design.
Women were tested in random order across the EF (days 1–4 after onset of menstruation) and ML (days 20–24) phases of the menstrual cycle. Men were tested once. Intravenous blood samples were taken to assess circulating sex hormone levels and to confirm the target menstrual cycle phases in the women (Table 1). Before testing, all participants attended a familiarization session, during which they became accustomed to the experimental protocols and the noninvasive aspects of data acquisition. On test dates, participants arrived at the laboratory having fasted for 3 h and having abstained from caffeine, alcohol, and exercise for 12 h.
Table 1.
Baseline subject characteristics
| Women |
|||
|---|---|---|---|
| EF | ML | Men | |
| 17β-Estradiol, pmol/l | 151 ± 50 | 638 ± 175* | 161 ± 64† |
| Progesterone, nmol/l | 1.2 ± 0.5 | 35.8 ± 9.3* | 1.5 ± 0.3† |
| Testosterone, nmol/l | 1.0 ± 0.2 | 1.0 ± 0.4 | 19.6 ± 6.5*† |
| MAP, mmHg | 86 ± 6 | 84 ± 6 | 92 ± 8† |
| SBP, mmHg | 114 ± 13 | 112 ± 11 | 127 ± 9*† |
| DBP, mmHg | 67 ± 9 | 67 ± 4 | 73 ± 7 |
| HR, beats/min | 61 ± 7 | 64 ± 7 | 54 ± 7*† |
| Q̇, l/min | 3.7 ± 0.7 | 4.1 ± 0.9 | 4.2 ± 9 |
| Q̇i, l·min−1·m−2 | 2.2 ± 0.4 | 2.3 ± 0.4 | 2.1 ± 0.4 |
| TPR, mmHg·l−1·min−1 | 24 ± 7 | 22 ± 7 | 23 ± 5 |
| TPRi, mmHg·l−1·min−1·m−2 | 41 ± 10 | 37 ± 10 | 46 ± 10 |
| MSNA burst frequency, bursts/min | 10 ± 5 | 14 ± 7* | 18 ± 5* |
| MSNA burst incidence, bursts/100 heartbeats | 16 ± 8 | 21 ± 8* | 34 ± 12*† |
| Total MSNA, arbitrary units | 466 ± 203 | 714 ± 317* | 854 ± 239* |
Values are means ± SD. EF, early-follicular phase; ML, midluteal phase; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Q̇, cardiac output; Q̇i, cardiac index; TPR, total peripheral resistance; TPRi, TPR index; MSNA, muscle sympathetic nerve activity.
P < 0.05 vs. EF;
P < 0.05 vs. ML.
End-inspiratory apnea protocol.
Participants were supine and breathed through a mouthpiece (series 9060, Hans Rudolph, Kansas City, MO) attached to a three-way valve that opened to room air or to a Y connector (VacuMed, Ventura, CA) leading to two 3-liter rebreathing bags. A noseclip prevented nasal breathing (series 9015, Hans Rudolph), and a pulse oximetry ear clip (Dura-Y D-YSE, Covidien-Nellcor, Boulder, CO) connected to a pulse oximeter (OxiMax N-560, Covidien-Nellcor) was used to monitor blood oxygen saturation. Gases were analyzed online using an infrared carbon dioxide sensor and an optical oxygen detector fed from a damped micro-vacuum sampling pump (ML206 gas analyzer, ADInstruments, Colorado Springs, CO). After 5 min of baseline recording, participants commenced rebreathing, which continued until an end-tidal Po2 of 65 Torr was achieved. Immediately thereafter, participants commenced a voluntary maximal end-inspiratory apnea. After the apnea, participants took two breaths from the rebreathing bags for quantification of peak hypoxia and hypercapnia.
Measurements.
Sympathetic nerve activity was assessed using the microneurographic technique (50). A tungsten recording electrode (200 μm diameter, 35 mm long) with an uninsulated 1- to 5-μm tip was inserted transcutaneously into the peroneal nerve, and a reference electrode was inserted subcutaneously 1–3 cm from the recording site. Adequate MSNA recording sites produced pulse-synchronous bursts of activity that increased in frequency during apnea maneuvers and were unaffected by arousal to a loud noise (9). The MSNA signal was amplified 75,000 times, band-pass-filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s; model 662C-3, Department of Bioengineering, University of Iowa, Iowa City, IA).
Baseline blood pressures were assessed using manual sphygmomanometry. The average of three blood pressure values was used to calibrate beat-to-beat blood pressures obtained through photoplethysmographic methods (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Baseline cardiac output (Q̇) was calculated from Doppler-derived measures of stroke volume velocity obtained in the ascending aorta using a 2.0-MHz pulsed wave probe (model CFM750, Vingmed). Aortic diameters were obtained from two-dimensional B-mode echo-Doppler images (2.5-MHz probe, Vivid 7 Dimension system, GE Healthcare, Baie d'Urfe, PQ, Canada). Because placement of the rebreathing apparatus prevented measurement of stroke volume velocity, the change in Q̇ during rebreathing and apnea maneuvers was assessed using the Modelflow algorithm in the Finometer. Heart rate (HR) was measured using a standard three-lead electrocardiogram. All data were analyzed offline (PowerLab/16SP with LabChart 7, ADInstruments).
Data analysis.
Rebreathing data were averaged across the final period of rebreathing (75–65 Torr end-tidal Po2). Because end-inspiratory apnea is associated with biphasic sympathetic and hemodynamic responses, apnea data were divided into two halves: APN-P1 and APN-P2.
The brachial blood pressure waveform was analyzed to determine mean arterial (MAP), systolic, and diastolic blood pressures. TPR was calculated as MAP/Q̇. Body surface area was estimated using the Mosteller formula (24, 32) to calculate cardiac index (Q̇i) and TPR index (TPRi).
Sympathetic outflow was quantified as burst frequency (bursts/min and bursts/100 heartbeats), burst size (amplitude), and total sympathetic activity (frequency × amplitude). The amplitude component was quantified because of its importance in distinguishing between conditions that have similar burst frequencies (47) and because burst amplitude is regulated in a manner distinct from burst frequency (22, 27), which is reflective of axonal recruitment (41, 45). After raw neurogram voltages were compared between baseline and postapnea periods to ensure that that sympathetic nerve sites had not shifted during the rebreathing or apnea, all bursts were normalized to the largest burst observed at baseline in each recording, which was assigned a value of 100.
To quantify the extent to which the MSNA signal was associated with a change in vascular function between menstrual cycle phases and sexes, neurovascular transduction was calculated as the quotient of TPRi and total MSNA. These comparisons were made at baseline (absolute TPRi/total MSNA) and during APN-P2 (ΔTPRi/Δtotal MSNA).
Statistical analyses.
Baseline comparisons of sex hormones, hemodynamics, and MSNA between sexes and menstrual cycle phases were assessed using paired (EF vs. ML) and unpaired (men vs. EF and ML) two-tailed t-tests. The effects of menstrual phase on hemodynamic and sympathetic responses to chemoreflex stimulation were analyzed using two-way repeated-measures ANOVAs. The effect of sex was examined using two-way split-plot ANOVAs, which separately compared men with EF and men with ML. In the comparisons of neurovascular transduction, values were compared between groups using two-tailed, Bonferroni-corrected t-tests. Alpha was set at 0.05 for all comparisons.
RESULTS
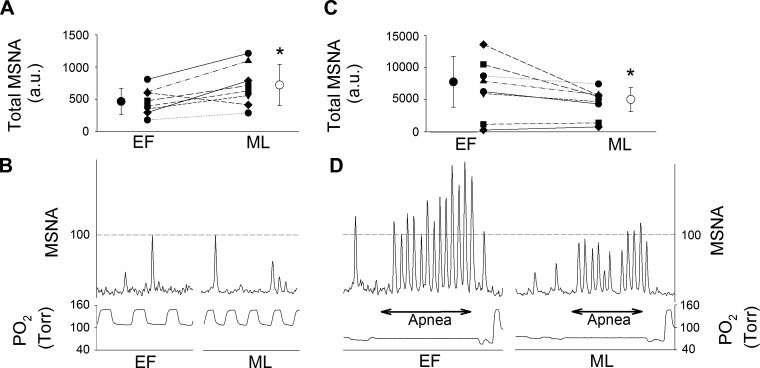
Baseline MSNA burst frequency, burst incidence, and total MSNA were greater in the ML than the EF phase of the menstrual cycle (Table 1). Individual patterns of change in baseline MSNA from the EF to the ML phase are presented in Fig. 1. We observed an increase in baseline MSNA from the EF to the ML phase in the majority of women (Fig. 1A). Total MSNA and burst frequency were greater in men than in women in the EF phase but similar between men and women in the ML phase. Burst incidence was greater in men than women, regardless of menstrual cycle phase.
Fig. 1.
Individual and sample patterns of total muscle sympathetic nerve activity (MSNA) during early-follicular (EF) and midluteal (ML) phases of the menstrual cycle. A: individual data from all women at baseline. ●, Mean ± SD for EF phase; ○, mean ± SD for ML phase. *P < 0.05 vs. EF. B: traces of 15 s of baseline recordings from a representative subject during EF and ML phases. au, Arbitrary units. C: individual data from all women during the 2nd half of apnea (APN-P2). ●, Mean ± SD for EF phase; ○, mean ± SD for ML phase. *P < 0.05 vs. EF. D: traces of end-inspiratory apnea performed during EF and ML phases.
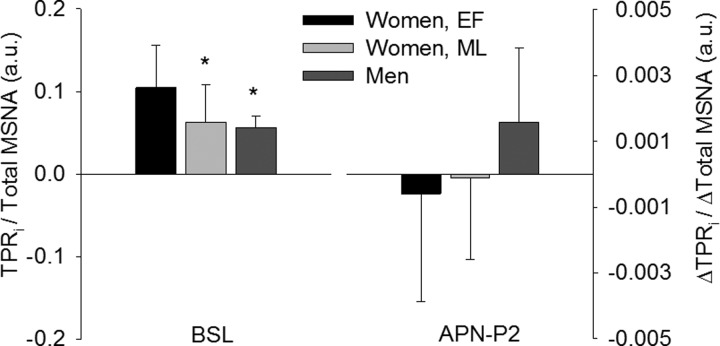
Baseline MAP and systolic blood pressure were higher in men than women, regardless of menstrual cycle phase. Conversely, HR was lower in men than women. However, Q̇ and TPR were similar between the sexes, in absolute terms and when normalized to body size. As such, baseline neurovascular transduction was greatest in women in the EF phase of the menstrual cycle (Fig. 2). Baseline neurovascular transduction was similar between women in the ML phase and men (P = 0.2).
Fig. 2.
Neurovascular transduction. Total peripheral resistance index (TPRi) normalized to total MSNA at baseline was higher in women in the EF than ML phase of the menstrual cycle and men (*P < 0.05 vs. EF). During chemoreflex activation, relative neurovascular transduction tended to be higher in men than in women in the EF (P = 0.06) and ML (P = 0.09) phases of the menstrual cycle, although the difference was not statistically significant. Values are means ± SD. BSL, baseline.
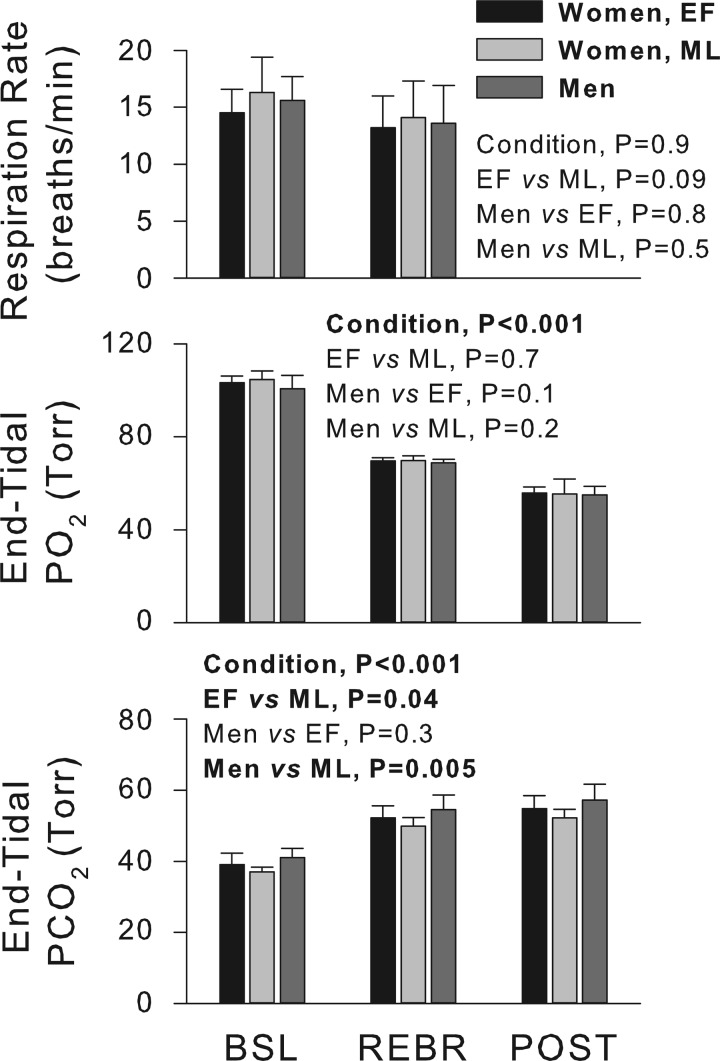
Respiration rates were similar between menstrual cycle phases and between sexes and were not significantly changed from baseline to rebreathing (Fig. 3). Significant reductions in end-tidal Po2 and elevations in end-tidal Pco2 were elicited by rebreathing and apnea (P < 0.001). End-tidal Po2 fell to similar levels in men and in women during the EF and ML phases, while end-tidal Pco2 levels were slightly, yet significantly, lower in the ML than EF phase and in men (Fig. 3). Apneas were maintained for similar durations in the EF (22 ± 4) and ML (22 ± 5) phases and in men (26 ± 4 s; all comparisons not significant).
Fig. 3.
Respiratory characteristics. Respiration rates and end-tidal Po2 values were similar between all groups. End-tidal Pco2 values were higher in men than in women in the ML phase of the menstrual cycle and higher during the EF than the ML phase. Values are means ± SD. REBR, rebreathing; POST, postapnea.
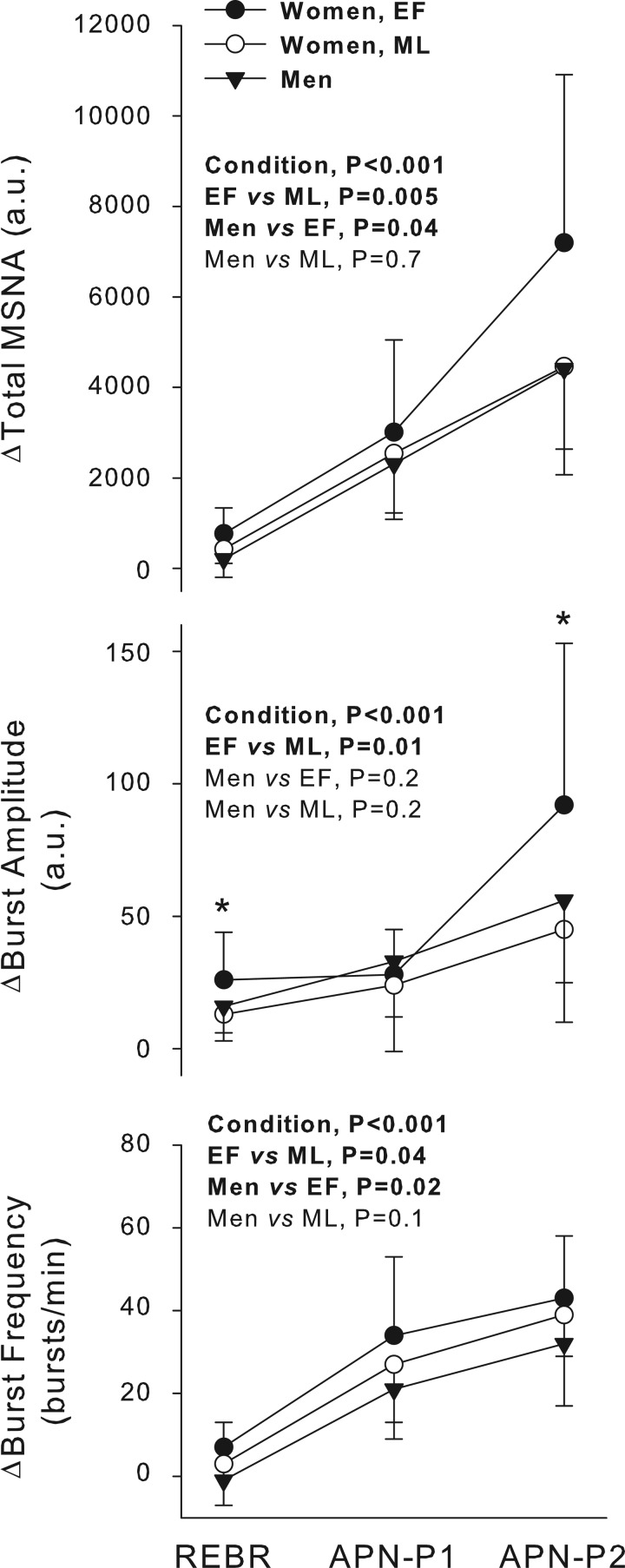
In one female participant, the rebreathing and apnea periods were associated with activation of motor units, which obscured the MSNA signal. Thus this participant was not included in the analyses of sympathetic responses to chemoreflex stimulation. Progressive increases in total MSNA occurred between rebreathing, APN-P1, and APN-P2 through elevations in MSNA burst frequency and amplitude (Fig. 4). The relative increases in total MSNA and burst frequency were greater in women in the EF phase than both the ML phase and men. Absolute levels of total MSNA achieved during APN-P2 were also greater during the EF than ML phase, a pattern that was observed in most women (Fig. 1C). Relative increases in MSNA burst amplitude were greater during the EF than ML phase, and a statistical interaction between chemoreflex condition and menstrual cycle phase (P = 0.05) indicated that burst amplitude was greater in the EF than ML phase specifically during rebreathing (P = 0.01) and APN-P2 (P = 0.02; Fig. 4). No significant differences in burst amplitude were observed between the sexes.
Fig. 4.
Relative sympathoexcitation during chemoreflex stimulation. Total MSNA and burst frequency were increased to the greatest extent in women in the EF phase. Increase in burst amplitude was greater in the EF than ML phase but was not different between sexes. In women, a statistical interaction between menstrual cycle phase and chemoreflex condition was observed in the burst amplitude response: *P < 0.05, EF vs. ML. Values are means ± SD. APN-P1, 1st half of apnea.
Because of the significant reduction in end-tidal Pco2 in women in the ML phase of the menstrual cycle relative to the EF phase and relative to men, a post hoc subanalysis was conducted in which all sympathetic responses were expressed relative to end-tidal Pco2. However, no statistical outcomes were affected by this analysis, indicating that end-tidal Pco2 was not the primary driver of differences in sympathetic responses between the sexes or between menstrual cycle phases.
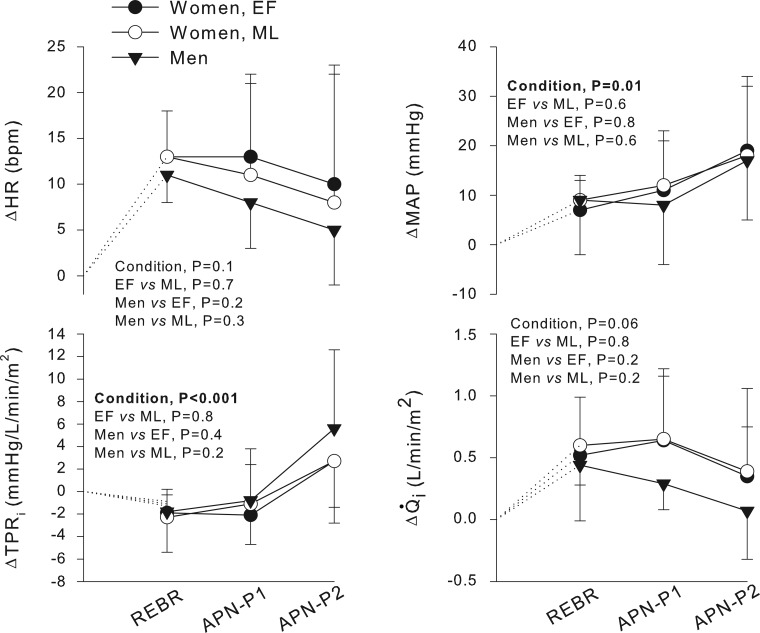
Relative changes in hemodynamic outcomes during chemoreflex stimulation are presented in Fig. 5. In all participants, chemoreflex activation was associated with graded increases in TPRi and MAP, but not HR or Q̇i. None of the hemodynamic outcomes differed between menstrual cycle phases or between the sexes. Nevertheless, relative neurovascular transduction, as assessed during peak chemoreflex stress (i.e., APN-P2), was not significantly different between menstrual cycle phases (P = 0.1) or between men and women in the EF (P = 0.06) or ML (P = 0.09) phase (Fig. 2).
Fig. 5.
Relative changes in hemodynamics during chemoreflex stimulation. Significant changes in mean arterial pressure (MAP) and total peripheral resistance index (TPRi) occurred across chemoreflex stimuli. Relative changes in MAP and TPRi, as well as cardiac index (Q̇i) and heart rate (HR), were not significantly different between sexes or menstrual cycle phases. Values are means ± SD.
DISCUSSION
This study aimed to establish the impact of sex and sex hormone levels on sympathetic responses to acute chemoreflex activation. When compared with those of women in the EF phase of the menstrual cycle, baseline MSNA burst frequency and total MSNA (but not burst incidence) were greater in men and also in women during the ML phase of the menstrual cycle. During chemoreflex activation, reflex increases in MSNA were larger in women in the EF than ML phase (all MSNA characteristics). The reflex increases in MSNA burst frequency and total MSNA in women in the EF phase were also greater than those observed in men. However, baseline and chemoreflex-driven hemodynamic outcomes were similar across menstrual cycle phases and between the sexes, suggesting a relative lack of neurovascular transduction during elevated MSNA in the women. Together, these data support a direct effect of sex hormones on baseline MSNA and on sympathetic reflex responses to chemoreflex stimulation. These data also confirm previous findings (14) that neurovascular transduction appears to be damped in women compared with men.
The first direct comparisons of MSNA between men and women demonstrated greater resting MSNA burst frequency in men than women (34). Several subsequent studies reproduced this finding (14, 19, 20, 28, 34, 42, 52), albeit inconsistently (10, 11, 18, 23, 33). This inconsistency may be attributable to menstrual cycle phase effects on MSNA, as only a few previous sex-based studies have accounted for menstrual cycle phase in the female participants (14, 23, 52). The consideration of menstrual cycle phase has been deemed important following reports of elevations in baseline MSNA during the ML relative to the EF phase of the menstrual cycle (4, 29, 30, 36). While it should be noted that associations between menstrual cycle phase and baseline MSNA have not been observed universally (5, 6, 10, 17, 20), collectively the data indicate that the menstrual cycle, and specifically the changes in sex hormone levels that occur along the menstrual cycle (4), are associated with changes in the regulation of baseline MSNA. In the present group of participants, the net result of these effects was the exaggeration of baseline MSNA burst frequency and total MSNA in men relative to women in the EF, but not the ML, phase of the menstrual cycle. Additionally, our data suggest that the menstrual cycle effect on baseline MSNA is manifest in the burst frequency, but not the burst amplitude, component of MSNA.
In the present study we also compared sex and menstrual cycle effects on MSNA regulation during a potent chemoreflex stimulus. In coupling the sympathoexcitatory effect of combined hypoxia-hypercapnia (31) with the elimination of the sympathoinhibitory effect of the thoracic afferent nerves through the use of apnea (43, 44), the stimulus was designed to elicit a strong sympathoexcitatory response. During chemoreflex activation, increases in total MSNA and MSNA burst frequency were greatest in women, particularly during the EF phase of the menstrual cycle. As such, these data support the findings of Jones et al. (18), who reported an amplified hypoxia-driven total MSNA response in women relative to men. However, Jones et al. did not systematically study the effect of menstrual cycle on sympathetic responses to chemoreflex stimulation. Our current observations suggest that chemoreflex responses are not regulated equally across the menstrual cycle. To the best of our knowledge, ours is the first study to examine sympathetic responses to chemoreflex stimulation across the menstrual cycle. However, in a similar study of chemoreflex activation in regular users of hormonal contraceptives (49), we found a greater total MSNA response to hypoxic-hypercapnic apnea associated with the low- than the high-hormone phase of hormonal contraceptive use. The similarity between the patterns of sympathetic activation across phases of hormonal contraceptive use (49) and the phases of the menstrual cycle implies that the changes in endogenous hormones that occur across the menstrual cycle exert an influence over the regulation of MSNA during chemoreflex activation similar to the changes in the exogenous hormones contained within the hormonal contraceptives.
A potential discrepancy in these data appears to exist wherein the high-hormone phase of the menstrual cycle produced higher baseline MSNA but smaller reflex chemoreflex sympathetic activation. While such changes may be due to a ceiling effect whereby higher baseline activity limits the reflexive increase in MSNA, differences in the pattern of increase deserve consideration. Specifically, the menstrual cycle-based difference in baseline MSNA was due to a change in the burst frequency component of MSNA. In contrast, the difference in MSNA during chemoreflex activation was due largely to changes in the burst amplitude component of MSNA. Within the context of the hypothesis that baroreceptor feedback gates MSNA burst frequency, rather than burst size (22, 25), the current data might indicate how sex hormones affect the central regulation of sympathetic outflow. Specifically, the data suggest that an elevated sex hormone milieu augments burst frequency and inhibits burst amplitude. Therefore, it may be that large reflexive increases in burst amplitude are achieved only in low-hormone phases. Stated differently, the absolute levels of sympathetic outflow in women at baseline and the reflexive increases in MSNA during chemoreflex activation are dependent on the phase of the menstrual cycle within which the measurements are obtained. These findings replicate our earlier observation that regulation of the burst amplitude component of MSNA varied across hormone phases in women taking hormonal contraceptives (49). In that earlier study, the low-hormone EF phase of the menstrual cycle was associated with a larger increase in MSNA burst amplitude during APN-P2. In combination, these data support the hypothesis of independent control of the frequency and strength (i.e., burst amplitude) components of MSNA through central integration sites (25).
A caveat to the data presented here is that the differences in sympathetic activation patterns between men and women and between menstrual cycle phases were not coupled with similar changes in peripheral resistance or MAP. A lack of congruity between MSNA and peripheral resistance has been reported previously in young women (14). It has been suggested that this occurs through buffering of the sympathetic signal through the activation of vasodilatory β-adrenoreceptors (13). Also, vascular production of nitric oxide is increased by elevations in circulating estradiol (46), which could act to increase vasodilation and, thereby, limit MSNA-induced vasoconstriction during the ML phase of the menstrual cycle. Although we did not measure these vascular factors in the present study, these mechanisms might contribute to a buffering of sympathetic activity at baseline, accounting for the lack of difference in TPR between the EF and the ML phase. However, we also observed similar levels of TPR during chemoreflex activation in men and in women across both menstrual cycle phases in the face of a greater sympathoexcitatory response during the EF phase. In the EF phase, circulating estradiol levels are low and, as such, cannot account for the lack of transduction of sympathetic nerve activity into TPR and MAP in this phase of the menstrual cycle. Further research is required to determine the mechanism by which neurovascular transduction may be damped in the EF relative to the ML phase, although these results do indicate that elevated concentrations of circulating estradiol are not necessary for this effect.
Limitations.
Menstrual cycle phases were selected on the basis of the desire to test the nadir of circulating sex hormone levels (i.e., in the EF phase) against a prolonged increase in circulating 17β-estradiol and progesterone (i.e., in the ML phase). In light of data that indicate a sympathoexcitatory role for progesterone and a sympathoinhibitory role for 17β-estradiol (4, 8), different experimental approaches may help differentiate between the independent effects of these hormones on MSNA responses to chemoreflex activation. Such approaches could include consideration of the late-follicular phase, which is associated with elevated 17β-estradiol but low progesterone, or the use of gonadotropin-releasing hormone antagonist (2, 8).
Implications.
These data, collected in young, healthy men and women, may provide new insight into the mechanisms at work in the clinical population of individuals with sleep apnea syndrome. This condition is more prevalent in men than women (38, 39, 53) and may be more strongly associated with hypertension in men than women (35). Indeed, the increases in end-tidal Pco2 elicited by the acute chemoreflex stimulus utilized in this study approximate the hypercapnic stress of a single sleep apnea episode (48). Therefore, the reflex increases in MSNA observed in the current study may also replicate those that occur in a single bout of sleep apnea. However, sleep apnea syndrome is a chronic condition associated with several apneic events each night, qualities that are likely to alter the profile of sympathetic and hemodynamic responses to apnea over time. In the context of our present findings, however, it may be that the blunting of neurovascular transduction in women during states of relative sympathoexcitation may contribute to a reduced incidence of hypertension following the development of sleep apnea syndrome relative to men. Furthermore, our finding of a sex hormone effect on the sympathetic responses to chemoreflex activation has implications for postmenopausal women, in whom sleep apnea syndrome appears to be far more prevalent than in premenopausal women (38). Subsequently, the transduction of chemoreflex-induced sympathoexcitation would be expected to increase in postmenopausal women, as has been previously observed under baseline conditions (13), predisposing postmenopausal women with sleep apnea syndrome to hypertension. Therefore, the current data provide the rationale to study sympathetic responses to a similar acute chemoreflex stimulus in adults with sleep apnea syndrome, including pre- and postmenopausal women, to determine the extent to which MSNA responses are altered in these groups.
Summary.
The goal of this investigation was to assess sympathetic responses to acute chemoreflex stimulation in young, healthy men and women. Consistent with previous research (18), increases in MSNA were greater in women than men, although this was observed only in the EF phase of the menstrual cycle in women. The change in sex hormones that occurs across the menstrual cycle was associated with a change in sympathetic responsiveness to acute chemoreflex activation. Specifically, the low-hormone EF phase was associated with a greater sympathoexcitation than the ML phase. Importantly, we did not observe a transduction of these effects into significant differences in hemodynamic outcomes between sexes or menstrual cycle phases.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant 217916-2013 (J. K. Shoemaker). C. W. Usselman was funded by the Ontario Graduate Scholarship Program and the Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship (CGS). T. I. Gimon and T. A. Luchyshyn were funded by a Natural Sciences and Engineering Research Council Undergraduate Student Research Award. C. A. Nielson, T. A. Luchyshyn, and N. S. Coverdale were funded by a CIHR Frederick Banting and Charles Best CGS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W.U. and J.K.S. developed the concept and designed the research; C.W.U., T.I.G., C.A.N., T.A.L., N.S.C., and J.K.S. performed the experiments; C.W.U., S.H.M.V.U., and J.K.S. analyzed the data; C.W.U., T.I.G., C.A.N., T.A.L., N.S.C., S.H.M.V.U., and J.K.S. interpreted the results of the experiments; C.W.U. prepared the figures; C.W.U. drafted the manuscript; C.W.U., T.I.G., C.A.N., T.A.L., N.S.C., S.H.M.V.U., and J.K.S. edited and revised the manuscript; C.W.U., T.I.G., C.A.N., T.A.L., N.S.C., S.H.M.V.U., and J.K.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Craig D. Steinback, Arlene Fleischhauer, and Jasna Twynstra for technical expertise and assistance. The authors also thank the research participants for their contribution to this study.
REFERENCES
- 1.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Brunt VE, Miner JA, Kaplan PF, Halliwill JR, Strycker LA, Minson CT. Short-term administration of progesterone and estradiol independently alter carotid-vasomotor, but not carotid-cardiac, baroreflex function in young women. Am J Physiol Heart Circ Physiol 305: H1041–H1049, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol 585: 635–641, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568: 315–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day DS, Gozansky WS, Bell C, Kohrt WM. Acute sex hormone suppression reduces skeletal muscle sympathetic nerve activity. Clin Auton Res 21: 339–345, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. [DOI] [PubMed] [Google Scholar]
- 10.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 29: 8–15, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl 6: S529–S531, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep apnea and hypertension. A population-based study. Ann Intern Med 120: 382–388, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PP, Davy KP, Seals DR. Influence of gender on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Clin Physiol 19: 153–160, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol Endocrinol Metab 270: E363–E366, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol Heart Circ Physiol 270: H350–H357, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmerly DS, Wong S, Menon R, Shoemaker JK. Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol Regul Integr Comp Physiol 292: R715–R722, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lam TK, Leung DT. More on simplified calculation of body-surface area. N Engl J Med 318: 1130, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Malpas SC. A new model for the generation of sympathetic nerve activity. Clin Exp Pharmacol Physiol 22: 11–15, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Malpas SC, Ninomiya I. The amplitude and periodicity of synchronized renal sympathetic nerve discharges in anesthetized cats: differential effect of baroreceptor activity. J Auton Nerv Syst 40: 189–198, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol 275: R1600–R1604, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol 302: H2560–H2566, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol 79: 205–213, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Park J, Middlekauff HR. Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol 297: H564–H568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel HM, Heffernan MJ, Ross AJ, Muller MD. Sex differences in forearm vasoconstrictor response to voluntary apnea. Am J Physiol Heart Circ Physiol 306: H309–H316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 149: 722–726, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal T, Oparil S. Hypertension in women. J Hum Hypertens 14: 691–704, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65: 1548–1552, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Salmanpour A, Frances MF, Goswami R, Shoemaker JK. Sympathetic neural recruitment patterns during the Valsalva maneuver. Conf Proc IEEE Eng Med Biol Soc 2011: 6951–6954, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67: 2101–2106, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Steinback CD, Breskovic T, Frances M, Dujic Z, Shoemaker JK. Ventilatory restraint of sympathetic activity during chemoreflex stress. Am J Physiol Regul Integr Comp Physiol 299: R1407–R1414, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Sverrisdottir YB, Rundqvist B, Johannsson G, Elam M. Sympathetic neural burst amplitude distribution: a more specific indicator of sympathoexcitation in human heart failure. Circulation 102: 2076–2081, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 85: 714–719, 1976. [DOI] [PubMed] [Google Scholar]
- 49.Usselman CW, Luchyshyn TA, Gimon TI, Nielson CA, Van Uum SH, Shoemaker JK. Hormone phase dependency of neural responses to chemoreflex-driven sympathoexcitation in young women using hormonal contraceptives. J Appl Physiol 115: 1415–1422, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y, Miyajima E, Tochikubo O, Matsukawa T, Ishii M. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension 13: 870–877, 1989. [DOI] [PubMed] [Google Scholar]
- 52.Yang H, Cooke WH, Reed KS, Carter JR. Sex differences in hemodynamic and sympathetic neural firing patterns during orthostatic challenge in humans. J Appl Physiol 112: 1744–1751, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993. [DOI] [PubMed] [Google Scholar]