Abstract
Brain-derived neurotrophic factor (BDNF) expression increases in the paraventricular nucleus of the hypothalamus (PVN) in response to hypertensive stimuli including stress and hyperosmolarity. However, it is unclear whether BDNF in the PVN contributes to increases in blood pressure (BP). We tested the hypothesis that increased BDNF levels within the PVN would elevate baseline BP and heart rate (HR) and cardiovascular stress responses by altering central angiotensin signaling. BP was recorded using radiotelemetry in male Sprague-Dawley rats after bilateral PVN injections of adeno-associated viral vectors expressing green fluorescent protein (GFP) or myc epitope-tagged BDNF fusion protein. Cardiovascular responses to acute stress were evaluated 3 to 4 wk after injections. Additional GFP and BDNF-treated animals were equipped with osmotic pumps for intracerebroventricular infusion of saline or the angiotensin type-1 receptor (AT1R) inhibitor losartan (15 μg·0.5 μl−1·h−1). BDNF treatment significantly increased baseline BP (121 ± 3 mmHg vs. 99 ± 2 mmHg in GFP), HR (394 ± 9 beats/min vs. 314 ± 4 beats/min in GFP), and sympathetic tone indicated by HR- and BP-variability analysis and adrenomedullary tyrosine hydroxylase protein expression. In contrast, body weight and BP elevations to acute stressors decreased. BDNF upregulated AT1R mRNA by ∼80% and downregulated Mas receptor mRNA by ∼50% in the PVN, and losartan infusion partially inhibited weight loss and increases in BP and HR in BDNF-treated animals without any effect in GFP rats. Our results demonstrate that BDNF overexpression in the PVN results in sympathoexcitation, BP and HR elevations, and weight loss that are mediated, at least in part, by modulating angiotensin signaling in the PVN.
Keywords: brain-derived neurotrophic factor, blood pressure, body weight, angiotensin, stress
brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family and has a key role in regulating neuronal development and survival (21). In addition, increasing evidence indicates that neuronal activity-dependent production and release of BDNF provokes both short-term and long-term changes in synaptic function mediating neuronal plasticity (41), and indications of BDNF-induced intracellular Ca2+ elevations in postsynaptic neurons (2) suggests that BDNF may also act as a neurotransmitter. Owing to the combination of these properties, BDNF is capable of modulating complex regulatory pathways in the central nervous system. For instance, BDNF is involved in several homeostatic control mechanisms in the hypothalamus including activation of the hypothalamic-pituitary-adrenal (HPA) axis (18, 22) and regulation of food intake and body weight (26, 40, 42, 51). In addition, BDNF mRNA expression within the paraventricular nucleus of hypothalamus (PVN) is reported to increase in response to stimuli that are associated with elevations of blood pressure such as stress (18, 20, 49, 50), hyperosmolarity (1), and repeated amphetamine administration (33). Although BDNF has been shown to exert hypertensive actions in the brainstem (11, 54), it remains to be investigated whether BDNF-mediated mechanisms within the PVN influence regulation of the sympathetic nervous system and blood pressure.
Parvocellular neurons within the PVN play a key role in activating preganglionic sympathetic neurons in the intermediolateral column of the spinal cord via either direct projections or descending pathways including a synapse in the rostral ventrolateral medulla (RVLM) (13). These PVN neurons receive stimulatory and inhibitory input from higher centers in the brain, from medullary nuclei, and from peripheral sensors (13). Importantly, ANG II-induced activation of angiotensin type-1 receptors (AT1R) in the PVN is implicated in sympathoexcitation associated with stress (7, 14, 43) and hyperosmolarity (10). Since BDNF is elevated by these same stimuli in the PVN, we postulated that BDNF could activate ANG II-AT1R signaling to increase sympathetic tone. In addition, BDNF has been shown to regulate synaptic transmission and activity-dependent plasticity in the hippocampus (36) leading to stress-induced changes in learning and memory, but it is unknown whether long-term elevations in BDNF, such that may be caused by chronic stress (20, 50), could affect the amplitude and duration of cardiovascular stress responses.
Therefore, we tested the hypothesis that increased expression of BDNF in the PVN elevates blood pressure, heart rate, and sympathetic tone and enhances cardiovascular responses to acute stressors. Furthermore, we hypothesized that pressor, tachycardic, and sympathoexcitatory actions of BDNF are mediated by AT1R signaling. Clarifying these BDNF-mediated regulatory pathways within the PVN is important to better understand the central mechanisms leading to blood pressure elevations induced by stress and changes in plasma osmolarity. Our approach of vector-mediated upregulation of BDNF in the PVN enabled us to examine the contribution of BDNF to neural control of blood pressure selectively in the PVN without interference from other regulatory mechanisms activated by stress or hyperosmolarity. In addition, due to its effects on food intake and body weight regulation, increasing hypothalamic BDNF levels using gene therapy or other therapeutic approaches have recently emerged as potential treatments for obesity and diabetes (8, 37). However, if our hypothesis that BDNF exerts sympathoexcitatory and hypertensive effects within the PVN proves to be correct, such therapeutical approaches could induce unwanted cardiovascular side effects that need to be characterized.
METHODS
All animal housing, handling, and surgical and experimental procedures were conducted within an Association for the Assessment and Accreditation of Laboratory Care International-accredited animal care facility at the University of Florida, in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Florida approved all animal housing, handling, and surgical and experimental procedures. Male Sprague-Dawley rats were obtained from Charles River (Wilmington, MA) at 7 wk of age. Rats were housed individually with a 12-h:12-h light/dark cycle, and standard rat chow and water were provided ad libitum.
Experimental Design
Experiment 1.
The aim of these experiments was to characterize the effects of BDNF on body weight, blood pressure, heart rate, indexes of autonomic function, and cardiovascular responses to acute stressors. Radiotelemetric transmitters were implanted at 8 to 9 wk of age. Following a 2-wk post-operative recovery period, baseline parameters were recorded for a week. Then, adeno-associated viral vector serotype 2 (AAV2)-green fluorescent protein (GFP) (n = 8) or AAV2-myc epitope-tagged BDNF fusion protein (AAV2-BDNFmyc; n = 9) were injected bilaterally into the PVN. Blood pressure was recorded for 4 wk after injections, and rats were subject to an acute water stress procedure at the end of week 3 and to an acute restraint stress procedure at the end of week 4. Animals were euthanized after restraint stress by transcardiac perfusion under deep isoflurane anesthesia, and accuracy of vector injections was verified with immunohistochemistry.
Experiment 2.
The goal of these experiments was to study the effects of BDNF on the expression of renin-angiotensin system components in the PVN and tyrosine hydroxylase in the adrenal medulla, as a marker of sympathetic activity (3). AAV2-GFP (n = 7) or AAV2-BDNFmyc (n = 7) viral vectors were injected bilaterally into the PVN at 11 to 12 wk of age. Four weeks later, animals were euthanized and the brain and adrenals were removed and snap-frozen in liquid nitrogen. The region of the PVN was isolated from a 2-mm-thick brain section using a 1-mm diameter punch tool. The isolated PVN and adrenal medulla were processed for quantitative RT-PCR and Western blot analysis, respectively.
Experiment 3.
These experiments were designed to determine whether effects of BDNF on blood pressure, heart rate, and body weight are mediated by AT1Rs. Animals were divided into four groups: GFP + saline (n = 6), GFP + losartan (n = 5), BDNF + saline (n = 8), and BDNF + losartan (n = 7). Radiotelemetric transmitters were implanted at 8 to 9 wk of age. Following a 2-wk post-operative recovery period, baseline parameters were recorded for a week. Then, AAV2-GFP or AAV2-BDNFmyc was injected bilaterally into the PVN and an icv cannula connected to an osmotic minipump was introduced into the right lateral ventricle. Saline or losartan (15 μg·0.5 μl−1·h−1 icv) was infused for 2 wk, pumps were then replaced with new pumps containing the same solutions, and the infusion continued for 2 more wk. Blood pressure was recorded for the last 3 days of each 2-wk period, and changes in radiotelemetric parameters from baseline values were calculated. At the end of the experiment, animals were euthanized by transcardiac perfusion under deep isoflurane anesthesia, and accuracy of vector injections was verified with immunohistochemistry.
Surgical Procedures
All surgeries were performed using aseptic technique under continuous isoflurane anesthesia (5% induction, 2% to 3% maintenance) delivered in oxygen. Depth of anesthesia was assured by lack of a reflex response to pinch of the hind paw. Carprofen (5 mg·kg−1·day−1 sc) was used for postsurgical analgesia administered at the beginning of surgery and for 2 days after surgery.
Radiotelemetric transducers (model PA-C40; Data Sciences International, St. Paul, MN) were implanted into the descending aorta via a midline abdominal incision. The aorta was isolated and briefly occluded, and the tip of the catheter was inserted using a 21-gauge needle. Surgical glue (3M Vetbond Tissue Adhesive) and a nitrocellulose patch were applied to secure the catheter in place. The transducer was sutured to the abdominal muscle, and the incision closed in layers.
For the injections of viral vectors, rats were placed in a stereotaxic frame, and viral vector stock solutions were injected bilaterally into the PVN using pipettes pulled from thin walled borosilicate glass capillary tubes (OD, 1 mm; ID, 0.58 mm; tip diameter, ∼25 μm) at the following stereotactic coordinates: 1.8 mm posterior from bregma, 0.3–0.35 mm lateral from midline, and 7.8 mm ventral from the dura mater. Virus stocks (1012 viral particles/ml; 200 nl/side) were injected over 5 min using a pneumatic pico pump (World Precision Instruments, Sarasota, FL). The pipette was left in place for an additional 3 min before being withdrawn.
For central inhibition of AT1Rs, an infusion/guide combo cannula (Plastics One, Roanoke, VA) was inserted into the right lateral ventricle (coordinates: 1.3 mm lateral, 0.8 mm posterior from the bregma; the infusion cannula and the guide cannula were 4 mm and 3 mm long, respectively). The infusion cannula was connected to an osmotic minipump (Durect, Cupertino, CA), while the guide cannula was closed with a dummy cannula of the same length. After 2 wk of infusion of saline or losartan (15 μg·0.5 μl−1·h−1), pumps were replaced with new pumps containing the same solutions via a 1-cm skin incision. AT1R inhibition by losartan was verified at the end of the infusion periods by intracerebroventricular injections of ANG II (20 ng·2 μl−1·2 min−1) via the intracerebroventricular guide cannula using a 28-gauge stainless steel cannula reaching 4 mm below the bone surface.
Viral Vectors and Gene Transfer into the PVN
AAV2 to elicit the expression of enhanced GFP and BDNFmyc derived from rat bdnf were constructed and packaged by Vector Biolabs (Philadelphia, PA). The expression of GFP and BDNFmyc was driven by a chicken-β-actin promoter with human cytomegalovirus enhancer, and a woodchuck post-transcriptional regulatory element, which enhanced the expression of transgenes, was present downstream of GFP and BDNFmyc. The BDNFmyc plasmid was a generous gift from Dr. Ronald Klein (LSU Health Sciences Center Shreveport) and was used previously to protect retinal ganglion cells in a rat glaucoma model (31). In addition, full efficacy of BDNFmyc expression driven by the rat neuron-specific enolase promoter was confirmed previously both in vitro and in vivo (24).
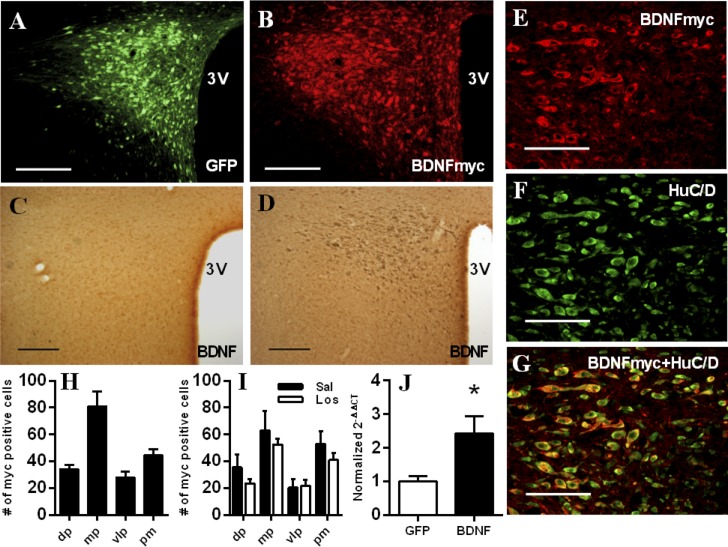
PVN injections of AAV2-GFP and AAV2-BDNFmyc resulted in marked expressions of GFP and BDNFmyc in the PVN as confirmed by fluorescent imaging and an antibody against the myc tag in experiments 1 and 3 (Fig. 1, A and B). In addition, BDNF overexpression in the PVN was confirmed with immunohistochemistry and an anti-BDNF primary antibody (Fig. 1, C and D), and by real-time RT-PCR in experiment 2 (Fig. 1J). Using confocal microscopy and double labeling with antibodies against the myc tag and HuC/HuD neuronal markers (Fig. 1, E–G), we have also verified that 97 ± 1% (n = 4) of myc positive cells were also positive for HuC/HuD neuronal markers in the PVN indicating that BDNFmyc was predominantly expressed in neurons. Only animals with bilateral GFP or BDNF expression in the PVN were included in the study, and there were no significant differences between numbers of myc positive cells in any subnuclei of the PVN from the three animal groups that received AAV2-BDNFmyc injections (experiment 1; experiment 3-saline and losartan infused; Fig. 1, H and I).
Fig. 1.
Verification of green fluorescent protein (GFP) and myc epitope-tagged brain-derived neurotrophic factor (BDNF) fusion protein (BDNFmyc) expression in the paraventricular nucleus of the hypothalamus (PVN). A and B: representative fluorescence photomicrographs showing PVN expression of GFP and BDNFmyc detected with an anti-c-Myc antibody in coronal sections ∼1.8 mm posterior from bregma; scale bars = 200 μm. C and D: representative immunohistochemistry images showing PVN expression of BDNF detected with an anti-BDNF antibody in adeno-associated viral vector serotype 2 (AAV2)-GFP-injected and in AAV2-BDNFmyc-injected animals in coronal sections ∼1.8 mm posterior from bregma; scale bars = 200 μm. E–G: representative confocal images from the PVN of AAV2-BDNFmyc-injected rats in coronal sections ∼1.8 mm posterior from bregma showing immunoreactivity for the myc tag, immunoreactivity for HuC/D neuronal markers, and overlap of BDNFmyc and HuC/D expressions demonstrating that BDNFmyc is expressed predominantly in neurons; scale bars = 100 μm. H and I: number of myc-positive cells detected in subnuclei of the PVN in coronal sections ∼1.8 mm posterior from bregma in AAV2-BDNFmyc-injected rats from experiment 1 and in AAV2-BDNFmyc-injected rats infused with saline (Sal) or losartan (Los) in experiment 3. J: expression of BDNF mRNA assessed with real-time RT-PCR in the PVN of AAV2-GFP- (n = 7) and AAV2-BDNFmyc-injected (n = 6) rats. 3V, third ventricle; dp, dorsal parvocellular subnucleus; mp, medial parvocellular subnucleus; vlp, ventrolateral parvocellular subnucleus; pm, posterior magnocellular subnucleus. Results are represented as means ± SE. *P < 0.05 GFP vs. BDNF.
Immunohistochemistry
Animals were anesthetized with 5% isoflurane and were perfused with 400 ml of cold PBS followed by 400 ml of cold 4% paraformaldehyde in PBS. Brains were postfixed for 2 h in 4% paraformaldehyde, then equilibrated in 30% sucrose solution at 4°C. Coronal sections (40 μm) were cut on a microtome (Thermo Scientific - Microm HM 450) and mounted on Fischer Superfost Plus slides. BDNFmyc and HuC/HuD neuronal markers were detected using the following primary antibodies: anti-c-Myc (Santa Cruz-9E10, 1:200, overnight incubation at 4°C to detect BDNFmyc), anti-HuC/HuD (Invitrogen-A21271, 1:200, overnight incubation at 4°C to detect human neuronal-specific protein). Secondary antibodies were anti-rabbit AF488, anti-mouse AF546 (Invitrogen, 1:200, 2-h incubation at room temperature). Immunofluorescence and GFP were detected with either a fluorescent microscope (Olympus BX41 with Olympus DP71 digital camera) or a confocal microscope (Zeiss LSM 510 META). BDNF expression in the PVN was also detected using an anti-BDNF primary antibody (Promega G1641, 1:25, 48-h incubation at 4°C) following antigen retrieval using a target retrieval solution (Dako S1699) and quenching endogenous peroxidase activity with 0.3% H2O2 in PBS (10 min at room temperature). Bound primary antibodies were visualized using the Polink-2 HRP Plus Chicken IgY DAB Detection System (D84-6; GBI Labs) and an Olympus BX50 light microscope.
Western Blot
Adrenal medullae were isolated from six GFP and seven BDNF rats from experiment 2 and homogenized in modified RIPA buffer of 150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA, 0.5% deoxycholate, 1% Nonidet P-40; 10% glycerol mixed with a protease inhibitor cocktail (Sigma-P2714) according to manufacturer's instructions. The homogenates were centrifuged at 14,000 g for 30 min, and the protein extract was separated by electrophoresis (100V) on a 10% SDS polyacrylamide and transferred electrophoretically (100V) to a nitrocellulose membrane. The membranes were blocked in Tris-borate-saline Tween-20 (TBST-20) buffer containing 3% nonfat dry milk. The membrane was then incubated overnight with purified rabbit anti-tyrosine hydroxylase antibody (1:2,000; Pel-Freez Biologicals, Rogers, AR) or mouse anti-β-actin antibody (Cell Signaling-3700). The membrane was washed several times with TBST-20 buffer and then incubated with horseradish peroxidase (HRP)-coupled anti-rabbit or anti-mouse IgG secondary antibodies (711-035-152 or 715-035-150; Jackson ImmunoResearch) for 1 h at room temperature. The membrane was washed with TBST-20 buffer and then developed using Clarity Western ECL Substrate (170-5061; Bio-Rad) and a Chemidoc gel imaging system (Bio-Rad).
Real-time RT-PCR
Expression of BDNF, AT1R, ANG II-type-2 receptor (AT2R), Mas receptor (Mas), (pro)renin receptor (PRR), angiotensinogen, angiotensin converting enzyme (ACE), ACE2, and 18s RNA were analyzed using quantitative real-time RT-PCR using specific oligonucleotide primers and TaqMan probes (Applied Biosystems, Foster City, CA). Total RNA was extracted from ∼2 mm3 brain punches containing the PVN (the 2 sides were combined from each animal) using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA samples were treated with an RNase-free DNase Set (Qiagen) to remove genomic DNA and were used to create cDNA with a high capacity cDNA archive kit (Applied Biosystems) according to manufacturer's instructions and stored at −20°C. All quantitative RT-PCR reactions were run in triplicate in 8-well optical grade strips in a Prism 7000 Sequence Detection System (Applied Biosystems) and quantified with the cycle threshold method. 18S mRNA expression and all other mRNA sequences were determined by quantitative RT-PCR using primers and FAM Taqman probes. Control reactions containing no template were run for each plate. One upper outlier was eliminated from BDNF and AT1R datasets from the BDNF group using Grubbs′ method with α = 0.05.
Acute Stress Procedures
Acute stress procedures were performed between 8 am and 12 pm and were started after obtaining baseline blood pressure and heart rate recordings for a minimum of 30 min. For water stress, rats were placed in standard rat cages filled with 1 cm deep water (room temperature, ∼25°C) for 15 min. Restraint stress was performed by placing animals in cylindrical plastic restrainers for 60 min. After animals were returned to their home cages, blood pressure and heart rate were recorded for an additional 30-min (water stress) or 60-min (restraint stress) recovery period. Blood pressure and heart rate data were exported with 1-min moving average from continuously recorded blood pressure data, and baseline values were calculated by averaging the baseline period after physical activity-related peaks from blood pressure and heart rate datasets were removed. Average blood pressure and heart rate changes from baseline (during stress and recovery periods), as well as amplitude and time delay of peak responses, were calculated. Two of the BDNF-treated rats had to be excluded from the analysis of restraint stress-induced responses since we could not establish steady baseline blood pressure before restraint stress due to increased spontaneous physical activity.
Analysis of Radiotelemetry Data
Blood pressure and heart rate radiotelemetry data were analyzed using Dataquest A.R.T. Analysis software (Data Sciences International). Data were recorded every 10 min for 15 s, and data collected between 8 am and 4 pm were averaged to calculate daytime values, whereas data collected between 8 pm and 4 am were averaged to calculate nighttime values for each animal. The telemetry system also assessed spontaneous physical activity of the animals based on variation in signal strength received from the transmitter and was expressed in arbitrary units.
Heart rate- and blood pressure-variability data analysis was performed using the freely available HemoLab software (http://www.haraldstauss.com/HemoLab/HemoLab.html). Blood pressure was recorded continuously at 500 Hz sampling rate for 3 h between 9 am and 12 pm 18–21 days after vector injections before animals were subjected to acute stress. Sampling rate of the datasets was increased to 1,500 Hz by using spline interpolation. Blood pressure datasets were visually inspected and three 5- to 10-min-long artifact-free segments per animal were extracted. Beat-by-beat pulse interval and systolic blood pressure time series were spline interpolated to an equidistant sampling rate of 15 Hz from the 1,500 Hz datasets. Power spectra were computed by the fast Fourier transform using the full length of each time series with 50% overlapping segments of 2,048 data points. Spectral powers of pulse intervals in the low frequency range (HRV-LF; 0.2–0.6 Hz) and high frequency range (HRV-HF; 1.0–3.0 Hz) and low frequency (0.2–0.6 Hz) spectral power of systolic blood pressure (BPV-LF) were calculated as the area under the curve of the respective power spectra (5). Parameters obtained from the three datasets were averaged for each animal.
Statistical Analysis
Data were analyzed by Student's t-test, one-way ANOVA, or two-way repeated-measures ANOVA, and, if the main effect was significant, Tukey's post hoc test was applied to determine individual differences between means. Results are expressed as means ± SE, and a value of P < 0.05 was considered significant.
RESULTS
Effect of Increased BDNF Expression on Telemetry Parameters and Body Weight
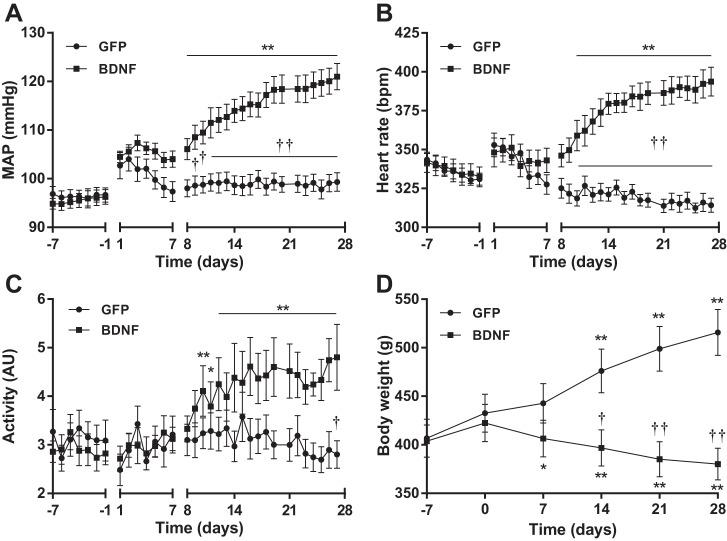
Baseline diastolic, systolic and mean arterial blood pressure (MAP) as well as heart rate, pulse pressure, and physical activity were similar in GFP and BDNF rats before vector injections both during day- and nighttime in experiment 1 (Table 1). MAP increased transiently after vector injections in both groups, but MAP returned to pre-injection levels in GFP animals, whereas in BDNF rats, MAP was significantly elevated compared with baseline 8 days after injections, and became significantly different from GFP rats 9 days after injections (Fig. 2A). Changes in heart rate were similar to MAP, and in the BDNF group, heart rate became significantly elevated compared with baseline and higher compared with GFP rats 10 days after injections, whereas GFP rats demonstrated a decline in heart rate over time (Fig. 2B). In parallel with increases in MAP and heart rate, nighttime physical activity also became significantly elevated compared with baseline in BDNF rats 10 days after vector injections and different from GFP animals on day 27 (Fig. 2C). Diastolic, systolic, and pulse pressure increased similarly in response to BDNF overexpression (Table 1).
Table 1.
Radiotelemetry parameters recorded on the day before vector injections (day −1) and on the day before restraint stress (day 27) in experiment 1
| Time, day | MAP, mmHg | HR, beats/min | DIA, mmHg | SYS, mmHg | PP, mmHg | ACT, AU | |
|---|---|---|---|---|---|---|---|
| GFP-D | −1 | 96.7 ± 1.5 | 331 ± 4 | 79.4 ± 1.2 | 117.8 ± 1.7 | 38.4 ± 0.8 | 0.7 ± 0.2 |
| BDNF-D | −1 | 96.2 ± 1.7 | 332 ± 6 | 77.8 ± 1.6 | 116.7 ± 2.4 | 38.1 ± 1.1 | 1.0 ± 0.1 |
| GFP-D | 27 | 99.3 ± 1.9 | 314 ± 4 | 82.2 ± 1.7 | 121.1 ± 2.2 | 39.0 ± 0.6 | 1.0 ± 0.3 |
| BDNF-D | 27 | 121.8 ± 2.9** | 394 ± 9** | 98.7 ± 2.3** | 150.7 ± 3.2** | 48.6 ± 0.6** | 1.9 ± 0.4 |
| GFP-N | −1 | 102.1 ± 1.8 | 394 ± 3 | 85.1 ± 1.1 | 123.1 ± 2.0 | 38.0 ± 0.6 | 3.1 ± 0.4 |
| BDNF-N | −1 | 99.3 ± 1.7 | 390 ± 8 | 82.6 ± 1.3 | 120.8 ± 2.3 | 37.4 ± 1.4 | 2.8 ± 0.3 |
| GFP-N | 27 | 104.3 ± 1.8 | 372 ± 5 | 87.1 ± 1.5 | 126.3 ± 2.2 | 39.3 ± 0.8 | 2.8 ± 0.3 |
| BDNF-N | 27 | 123.4 ± 2.1** | 447 ± 8** | 100.3 ± 1.9** | 155.1 ± 3.0** | 51.3 ± 1.2** | 4.6 ± 0.4* |
Values are means ± SE. GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor; D, daytime; N, nighttime; MAP, mean arterial blood pressure; HR, heart rate; DIA, diastolic blood pressure; SYS, systolic blood pressure; PP, pulse pressure; ACT, physical activity; AU, arbitrary unit.
P < 0.05,
P < 0.01 BDNF vs. GFP.
Fig. 2.
Change in daytime mean arterial blood pressure (MAP; A), daytime heart rate (B), nighttime physical activity (C), and body weight (D) of AAV2-GFP (n = 8) and AAV2-BDNFmyc-injected rats (n = 9) from experiment 1. MAP and heart rate were recorded for 1 wk to assess baseline values (day −7 to day −1), and viral vectors were injected on day 0 (recording during the first postoperative week was done in only 6 rats per group, since some transmitters were turned off to save battery; statistical analysis did not include this period). Values were omitted from day 0 (vector injections), day 21 (water stress), and day 28 (restraint stress). The decline in heart rate with time in the GFP group was statistically significant (P < 0.05), but it is not marked on the figure for clarity. Results are represented as means ± SE. *P < 0.05, **P < 0.01 compared with pre-injection values; †P < 0.05, ††P < 0.01 BDNF vs. GFP. AU, arbitrary units; bpm, beats/min.
Body weights of animals in the GFP and BDNF groups were similar at the time of telemetry transmitter implantation (GFP, 286 ± 19 g; BDNF, 285 ± 14 g) and at the time of vector injections (GFP, 433 ± 19 g; BDNF, 422 ± 19 g). However, increased BDNF expression in the PVN significantly reduced body weight, and by the end of the experiment, body weight increased by 83 ± 8 g in the GFP group but decreased by 42 ± 9 g in the BDNF group (P < 0.01; Fig. 2D) compared with pre-injection values.
Indexes of Autonomic Function
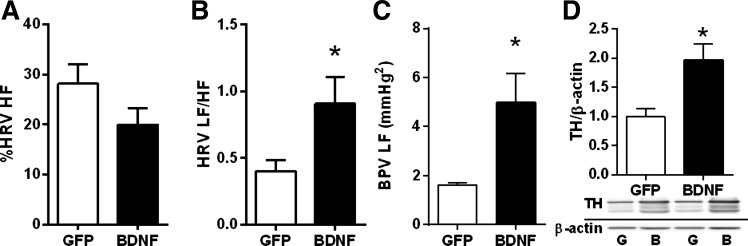
HRV LF-to-HF power ratio and the LF power of BPV increased significantly in BDNF rats compared with GFP rats in experiment 1, indicating marked elevations in cardiac and vascular sympathetic tone, but the normalized HRV HF power, an index of cardiac parasympathetic tone, was unaffected by BDNF (Fig. 3, A–C). In addition, adrenomedullary expression of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis, was also markedly elevated in the adrenal medulla of BDNF- versus GFP-treated animals from experiment 2, further supporting an increase in sympathetic activity (Fig. 3D).
Fig. 3.
Heart rate variability (HRV)-high frequency (HF) power normalized to total HRV power, an index of cardiac parasympathetic activity (A); heart rate variability-low frequency (LF) to high frequency (HF) power ratio, an index of cardiac sympathetic activity (B); blood pressure variability-LF power, an index of vascular sympathetic tone (C), from AAV2-GFP (n = 8) and AAV2-BDNFmyc-injected (n = 9) animals from experiment 1 and ratio of tyrosine hydroxylase (TH) and β-actin protein expressions in the adrenal medulla were assessed with Western blot (D) from AAV2-GFP (n = 6) and AAV2-BDNFmyc-injected (n = 7) animals from experiment 2. Results are represented as means ± SE. *P < 0.05 BDNF vs. GFP.
Acute Water Stress
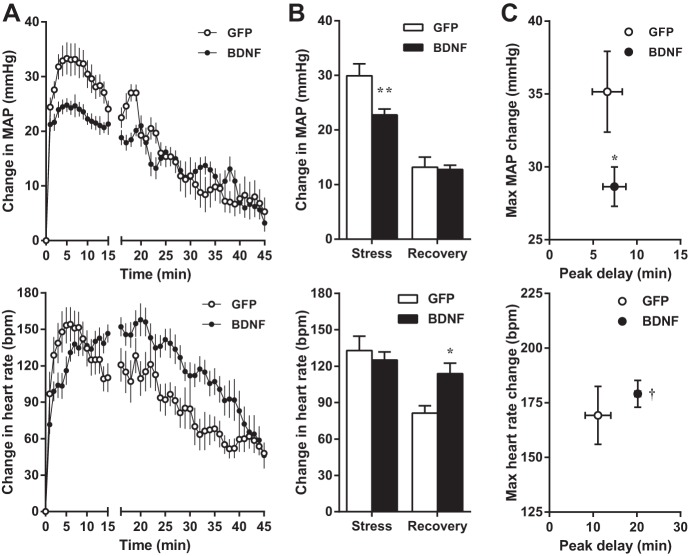
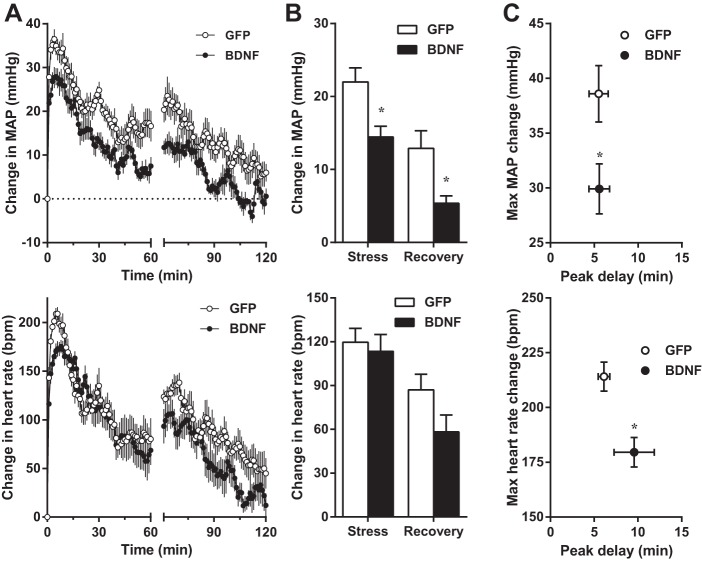
Water stress-induced increases in MAP averaged for the duration of stress were significantly reduced in BDNF rats compared with GFP, whereas the average change in MAP during the recovery phase was similar in the two groups (Fig. 4). Peak MAP increases were also significantly reduced in BDNF rats, whereas the delay of peak response from the onset of stress was unaffected by BDNF. In contrast, the average heart rate increase during stress and the amplitude of peak heart rate responses were similar in GFP and BDNF rats, but the peak was significantly delayed in the BDNF group. As a result, the average increase in heart rate during recovery was significantly higher in BDNF versus GFP rats. Physical activity during stress and recovery was not different between the two groups (not shown).
Fig. 4.
A–C: changes in MAP (top) and heart rate (bottom) from pre-stress baseline values during acute water stress in GFP (n = 8) and BDNF (n = 9) rats from experiment 1. Baseline MAP and heart rate were 98.6 ± 1.3 mmHg and 307 ± 7 beats/min (bpm) in GFP and 116.0 ± 3.1 and 365 ± 7 beats/min in BDNF rats. A: MAP and heart rate traces obtained with 1-min moving average during stress (15 min) and post-stress recovery (30 min). B: MAP and heart rate increases averaged during stress and recovery. C: amplitude and delay of peak MAP and heart rate responses. Statistical analysis was done on average and peak MAP and heart rate values. Results are represented as means ± SE. *P < 0.05, **P < 0.01 amplitude of change in BDNF vs. GFP; †P < 0.01 time delay of peak in BDNF vs. GFP.
Acute Restraint Stress
Restraint stress-induced average and peak increases in MAP were significantly reduced in BDNF versus GFP rats both during stress and recovery, and the amplitude of peak MAP increase during stress was also markedly diminished in BDNF rats (Fig. 5). The timing of the peak MAP response was unaffected by BDNF treatment, and average changes in heart rate during stress and recovery were also similar in GFP and BDNF animals. However, the amplitude of peak heart rate elevation was significantly reduced in BDNF than in GFP-treated rats, while the timing of the peak was unaffected. Physical activity during recovery was not different between the two groups (not shown).
Fig. 5.
A–C: changes in MAP (top) and heart rate (bottom) from pre-stress baseline values during acute restraint stress in GFP (n = 8) and BDNF (n = 7) rats from experiment 1. Baseline MAP and heart rate were 97.2 ± 1.9 mmHg and 306 ± 5 beats/min in GFP and 119.6 ± 4.0 and 369 ± 11 beats/min in BDNF rats. A: MAP and heart rate traces obtained with 1-min moving average during stress (60 min) and post-stress recovery (60 min). B: MAP and heart rate increases averaged during stress and recovery. C: amplitude and delay of peak MAP and heart rate responses. Statistical analysis was done on average and peak MAP and heart rate values. Results are represented as means ± SE. *P < 0.05, amplitude of change in BDNF vs. GFP.
Expression of Renin-angiotensin System Components in the PVN
Real-time RT-PCR analysis on PVN tissue samples from experiment 2 indicated that increased BDNF expression markedly elevated AT1R mRNA and significantly reduced Mas mRNA in the PVN (Fig. 6). In contrast, mRNA expressions of angiotensinogen, ACE, ACE2, AT2R, and PRR were unaffected by BDNF [2−ΔΔCt values compared with 18s RNA in the BDNF group were 0.79 ± 0.10 (angiotensinogen); 1.00 ± 0.11 (ACE); 0.84 ± 0.11 (ACE2); 0.75 ± 0.09 (AT2R); 1.00 ± 0.11 (PRR) relative to the GFP group].
Fig. 6.
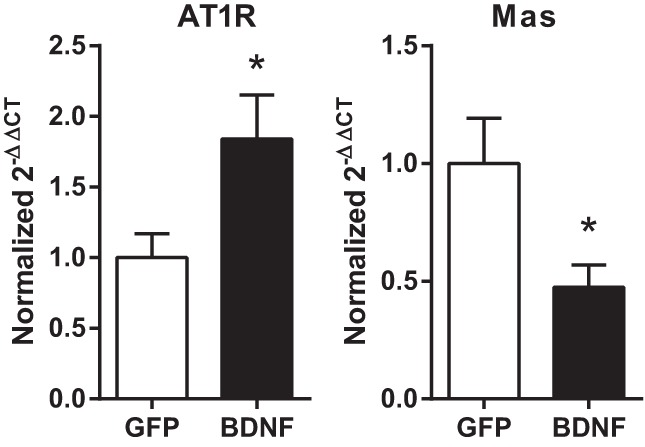
Expressions of angiotensin type-1 receptor (AT1R) and Mas receptor (Mas) mRNA compared with 18s RNA levels using the 2−ΔΔCt method in GFP (n = 7) and BDNF (n = 6 for AT1R and n = 7 for Mas) animals from experiment 2. Results are represented as means ± SE normalized to the GFP group. *P < 0.05 GFP vs. BDNF.
Effects of Central AT1R Inhibition
Telemetry parameters averaged for the week before vector injections were similar in all four groups in experiment 3 (Table 2). AT1R inhibition by losartan was verified at the end of the infusion periods by intracerebroventricular injections of ANG II (20 ng·2 μl−1·2 min−1). Average increases in blood pressure during 30 min following ANG II injection were 16.3 ± 3.4 mmHg (GFP + saline) vs. 2.8 ± 2.1 mmHg (GFP + losartan), and 14.5 ± 2.8 mmHg (BDNF + saline) vs. 3.1 ± 1.7 mmHg (BDNF + losartan). The effect of losartan infusion on ANG II-induced blood pressure elevations was significant (P < 0.01).
Table 2.
Baseline radiotelemetry parameters averaged for the week before vector injections in experiment 3
| MAP, mmHg | HR, beats/min | DIA, mmHg | SYS, mmHg | PP, mmHg | ACT, AU | |
|---|---|---|---|---|---|---|
| GFP + Sal-D | 96.1 ± 2.2 | 355 ± 12 | 79.4 ± 2.0 | 115.9 ± 2.3 | 36.6 ± 2.0 | 0.9 ± 0.2 |
| GFP + Sal-N | 100.1 ± 2.4 | 409 ± 10 | 83.9 ± 2.4 | 119.6 ± 2.5 | 35.6 ± 2.2 | 2.8 ± 0.3 |
| GFP + Los-D | 95.4 ± 1.8 | 365 ± 10 | 77.5 ± 2.0 | 116.2 ± 1.5 | 38.7 ± 1.1 | 1.2 ± 0.3 |
| GFP + Los-N | 99.7 ± 1.6 | 413 ± 9 | 82.6 ± 2.0 | 119.8 ± 1.1 | 37.2 ± 1.3 | 3.3 ± 0.6 |
| BDNF + Sal-D | 98.2 ± 1.2 | 350 ± 11 | 79.3 ± 1.2 | 120.7 ± 1.6 | 41.4 ± 1.3 | 0.9 ± 0.1 |
| BDNF + Sal-N | 101.9 ± 1.7 | 401 ± 12 | 83.5 ± 1.7 | 124.5 ± 2.0 | 41.1 ± 1.3 | 2.6 ± 0.1 |
| BDNF + Los-D | 99.8 ± 1.6 | 367 ± 7 | 82.4 ± 1.0 | 120.5 ± 2.4 | 38.0 ± 2.0 | 1.5 ± 0.4 |
| BDNF + Los-N | 104.7 ± 1.4 | 416 ± 7 | 87.5 ± 0.8 | 125.2 ± 2.3 | 37.7 ± 1.9 | 3.4 ± 0.6 |
Values are means ± SE. Sal, saline-infused; Los, losartan-infused.
Daytime MAP increased markedly in BDNF + saline animals. However, central infusion of losartan significantly attenuated, but did not completely abolish BDNF-induced elevations of MAP both at weeks 2 and 4 after vector injections with no effect in GFP animals (Fig. 7A). Similarly, daytime heart rate increased significantly in the BDNF + saline group, but losartan infusion diminished the tachycardic effect of BDNF (Fig. 7B). At week 2, there was no significant difference between BDNF + losartan rats compared with GFP rats, while at week 4, BDNF + losartan rats had significantly lower heart rate than BDNF + saline rats, but significantly higher heart rate than GFP rats. Nighttime MAP and heart rate values were affected similarly by vector and losartan treatments (data not shown).
Fig. 7.
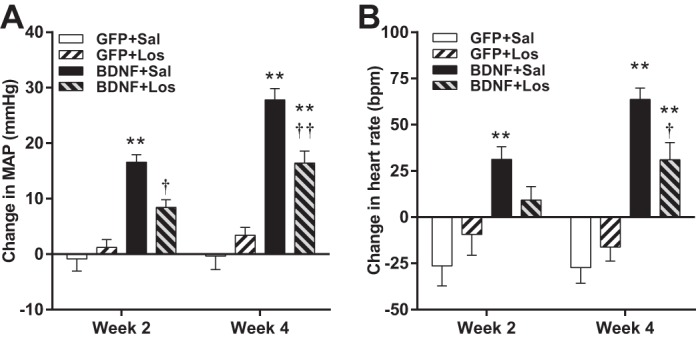
Changes in daytime MAP (A) and daytime heart rate (B) in AAV2-GFP-injected rats receiving intracerebroventricular infusions of saline (Sal; n = 6) or losartan (Los; n = 5) and AAV2-BDNFmyc-injected rats receiving intracerebroventricular infusions of saline (n = 8) or losartan (n = 7). Parameters were averaged for the last 3 days of the first and second 2-wk infusion periods. Results are represented as means ± SE. **P < 0.01 GFP vs. BDNF with same infusion treatment; †P < 0.05, ††P < 0.01 losartan vs. saline with same vector treatment.
Physical activity during the active nighttime period was significantly elevated in BDNF + saline rats both at weeks 2 and 4 compared with GFP + saline (Fig. 8A), and losartan had no effect on activity in either GFP or BDNF animals. Body weight decreased significantly in BDNF + saline rats compared with GFP + saline animals both at weeks 2 and 4 (Fig. 8B), and although central losartan infusion prevented the BDNF-induced weight loss, body weight in the BDNF + losartan group was still significantly lower than in GFP rats both at weeks 2 and 4. Losartan had no effect on the body weight of GFP rats.
Fig. 8.
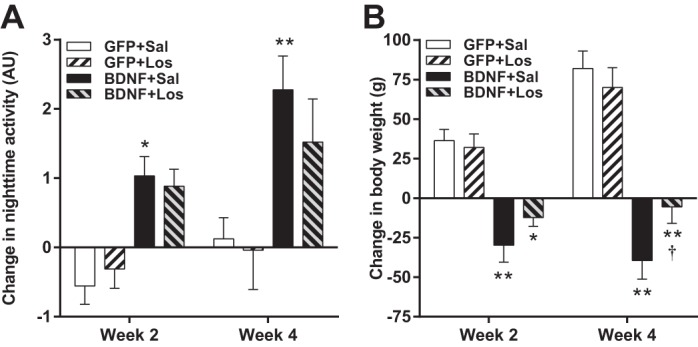
Changes in nighttime physical activity (A) and body weight (B) in AAV2-GFP-injected rats receiving intracerebroventricular infusions of saline (Sal; n = 6) or losartan (Los; n = 5) and AAV2-BDNFmyc-injected rats receiving intracerebroventricular infusions of saline (n = 8) or losartan (n = 7). Parameters were averaged for the last 3 days of the first and second 2-wk infusion periods. Results are represented as means ± SE. *P < 0.05, **P < 0.01 GFP vs. BDNF with same infusion treatment; †P < 0.05 losartan vs. saline with same vector treatment. AU, arbitrary units.
DISCUSSION
Our study demonstrates for the first time that long-term elevation of BDNF in the PVN significantly increases blood pressure, heart rate, and indexes of sympathetic activity, while lowering body weight. In addition, mRNA expression of AT1R increases, while expression of Mas decreases in response to elevated PVN levels of BDNF, and central inhibition of AT1Rs diminishes the BDNF-induced increases in blood pressure and heart rate and decrease in body weight. Collectively, these data suggest a novel role for BDNF in cardiovascular and metabolic control by regulating the balance between two opposing axes of angiotensin signaling mediated by ANG II/AT1R and angiotensin (1–7)/Mas, respectively.
The Role of BDNF in Neuronal Control of Blood Pressure and Heart Rate
Previous reports have indicated that BDNF is involved in blood pressure regulation in brainstem nuclei. For example, the NTS receives BDNF input from arterial baroreceptor afferents and BDNF may be involved in modulation of baroreflex function by mediating frequency-dependent depression of postsynaptic responses in NTS neurons (30). In addition, BDNF mRNA is strongly expressed in A2 noradrenergic neurons (9), and inhibition of BDNF TrkB receptors in the NTS decreases, whereas microinjection of BDNF increases blood pressure, heart rate, and lumbar sympathetic nerve activity in anesthetized rats (11). A1/C1 catecholaminergic neurons in the RVLM are also positive for BDNF mRNA (9), and BDNF-positive nerve terminals are also found in the vicinity of spinal-projecting sympathoexcitatory neurons (54). Furthermore, BDNF microinjections into the RVLM of anesthetized rats elicit rapid increases in blood pressure without elevating heart rate (54). Thus, both in the NTS and RVLM, BDNF has been shown to exert hypertensive effects. However, the role of BDNF in blood pressure regulation has not been previously investigated in the PVN. The potential significance of such BDNF-mediated mechanisms is that BDNF mRNA and protein levels increase in the PVN in response to stressors, hyperosmolarity, and repeated amphetamine administration (1, 18, 20, 33, 49, 50), and our findings suggest that blood pressure elevations to these stimuli may be mediated by BDNF-induced sympathoexcitation.
Sympathetic nerve activity is regulated differentially depending on the target organ (34, 35, 48). In this study, we have analyzed the effect of elevated BDNF within the PVN on HRV LF/HF ratio as an index of cardiac sympathetic tone and BPV-LF power as an index of vascular sympathetic tone. In addition, sympathetic tone to the adrenal medulla was assessed by measuring adrenomedullary expression of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis, based on previous findings that expression of this enzyme is regulated by preganglionic adrenal nerve activity (3). We found that BDNF overexpression in the PVN significantly increased all three of these indexes of sympathetic activity, indicating a broad sympathoexcitatory effect; however, further studies using nerve activity recordings of multiple sympathetic nerves are required to establish whether BDNF affects sympathetic tone to various target organs differentially.
Potential Mechanisms Mediating the Sympathoexcitatory, Pressor, and Tachycardic Effects of BDNF
Activation of AT1R by ANG II is one of the major mechanisms responsible for stimulation of PVN sympathoexcitatory neurons (13) and has been shown to be involved in the mediation of stress- and hyperosmolality-induced pressor responses (7, 10, 14). Considering that BDNF is also increased in the PVN by these stimuli (1, 18, 20, 50), we hypothesized that elevated BDNF levels may activate the ANG II/AT1R pathway in the PVN. Our results demonstrate that BDNF overexpression had no effect on angiotensinogen, ACE, or ACE2 mRNA levels in the PVN. However, it markedly increased AT1R mRNA expression and reduced Mas mRNA. These changes may result in a significant imbalance between the hypertensive ANG II/AT1R axis and the hypotensive angiotensin (1–7)/Mas axis (16, 56, 58), which is further supported by our findings that central infusion of losartan blocked, at least partially, the BDNF-induced increases in MAP and heart rate. A BDNF-mediated shift between these two opposing arms of angiotensin signaling could have major implications in stress-related hypertension, since repeated stress increases both BDNF and AT1R mRNA expression in the PVN, and AT1R-mediated mechanisms contribute to increased sympathetic and HPA-axis activity, cardiovascular responses, and even development of gastric ulcers (7, 20, 43). Likewise, in obesity, high circulating leptin levels lead to increased central BDNF concentration (55), which could be responsible for upregulation of AT1R in the hypothalamus of high-fat diet-fed obese rats (17) contributing to obesity-related sympathoexcitation and blood pressure elevation.
Inhibition of AT1Rs did not completely abolish BDNF-induced increases in blood pressure and heart rate, which could partially be explained by additional contribution of reduced Mas signaling that was unaffected by losartan treatment, and other mechanisms independent of angiotensin signaling may be involved as well. For example, BDNF enhances NMDA receptor-mediated synaptic transmission (27, 28), potentially activating pre-sympathetic neurons in the PVN that receive glutamatergic input (13, 38). BDNF has also been shown to affect morphological features of neurons including soma size and dendritic arborization, as well as synaptic strength (53). Therefore, chronically elevated BDNF concentration in the PVN, like that achieved in our experimental model or induced by chronic stress (20, 50), could affect the structure and synaptic function of neurons within the PVN, causing long-term adaptive changes in the sympathoexcitatory neuronal circuitry (47). BDNF is also involved in the regulation of the HPA axis by stimulating the expression of corticotrophin releasing factor (CRF) in the PVN leading to elevations in plasma corticosterone levels (18). Long-term elevations in plasma glucocorticoid levels in turn could result in increased blood pressure both by peripheral actions potentiating vasoconstrictor mechanisms (52) and by central actions in the NTS to diminish baroreflex regulation of heart rate and renal sympathetic nerve activity (44–46). Interestingly, BDNF-induced activation of the HPA axis may also be partially mediated by AT1R upregulation in the PVN, since it has been demonstrated that ANG II stimulates CRF production and release in the PVN via AT1R (4), and AT1R/CRF signaling has been implicated in food intake and body weight regulation as well (57). However, we cannot exclude the possibility that the BDNF-induced CRF production and HPA axis activation are mediated by multiple mechanisms and not solely by AT1R. A limitation of our study was that losartan was infused intracerebroventricularly; therefore, it is also possible that reductions in BDNF-induced blood pressure, heart rate and body weight changes were a result of AT1R inhibition in other cardiovascular nuclei besides the PVN such as the RVLM, NTS, area postrema, or the subfornical organ. Changes in AT1R signaling in these brain regions as well as the potential contribution of AT1R independent mechanisms to BDNF-induced pressor and tachycardic responses will have to be investigated in future studies.
Effect of Long-term BDNF Elevations in the PVN on Acute Stress Responses
BDNF can potentially modulate stress responses in the PVN by several mechanisms including influencing neuronal plasticity, neurite outgrowth (36), stimulation of CRF synthesis (18), and, as we have shown in this study, central angiotensin signaling. Here, we tested whether long-term elevation of BDNF in the PVN would affect the amplitude of cardiovascular responses to acute stressors, and contrary with our expectations, we found that the amplitude of pressor responses was slightly reduced in BDNF animals. However, it must be emphasized that due to the significantly higher baseline blood pressure in BDNF rats, absolute blood pressure was still considerably higher in the BDNF group during stress. Therefore, the attenuated increase in blood pressure could simply be a result of higher baseline blood pressure and a ceiling effect. Alternatively, due to elevated baseline BDNF concentration in the PVN of BDNF-treated rats, the stress-induced rise in BDNF may be proportionally smaller than in control animals, and assuming that BDNF levels in the PVN acutely correlate with blood pressure, this could lead to the relatively smaller increases but higher absolute blood pressure levels observed during stress. Finally, it is also possible that long-term elevations in BDNF induce neuronal plasticity and adaptive mechanisms in the PVN that result in reduced responses to stress. Such mechanisms could be responsible for the observed decline in cardiovascular responses when animals are repeatedly subjected to the same type of stressor (12). Interestingly, BDNF treatment had a somewhat different effect on heart rate responses that also depended on the type of stressor. During water stress, the main effect of BDNF was to delay peak heart rate elevations, while during restraint stress, the amplitude declined. Overall, results from our acute stress experiments demonstrated that BDNF upregulation in the PVN does not augment stress-induced cardiovascular responses, but whether BDNF induces adaptive mechanisms that result in reduced or delayed responses to stressors will need to be further investigated.
The Impact of Increased BDNF Expression in the PVN on Body Weight
The role of BDNF in body weight and food intake regulation has been demonstrated by numerous studies (26). Rats infused intracerebroventricularly with BDNF demonstrate significant reductions in food intake and body weight (40), whereas heterozygous BDNF+/− mice exhibit chronic hyperphagia and obesity (23, 29). BDNF has also been identified as a downstream mediator in hypothalamic leptin signaling pathways, since leptin-induced activation of proopio-melanocortin containing neurons in the arcuate nucleus led to melanocortin-4 receptor-mediated upregulation of BDNF in the ventromedial hypothalamus (25, 55). In addition, BDNF also exerts anorexic and metabolic effects in the PVN as indicated by a study from Toriya et al. (51), in which chronic BDNF infusion into the PVN decreased body weight and food intake in rats by upregulating CRF and activating CRF type-2 receptors. Our study verified that elevated BDNF in the PVN results in weight loss and also demonstrated for the first time that these effects can be maintained long term, since for the 4-wk duration of our experiments, body weight in the BDNF group showed a steady decline without any indication of developing resistance toward BDNF effects. Finally, we found that physical activity during the active nighttime period was also elevated by BDNF potentially contributing to BDNF-induced weight loss.
Interestingly, results from our losartan experiments suggest that some actions of BDNF on energy homeostasis may also be mediated by changes in angiotensin signaling. Although BDNF + losartan-treated animals still had significantly lower body weight than GFP animals, inhibition of AT1Rs did abolish BDNF-induced weight loss resulting in no change in body weight over the 4-wk duration of the treatment. These findings correspond with recent studies indicating that central angiotensin signaling has a significant role in metabolic control and that upregulation of AT1Rs in the PVN reduces body weight gain in response to high-fat diet feeding (15, 19, 57). Therefore, leptin- and BDNF-induced elevation of AT1R expression in the PVN may be a compensatory mechanism in obesity to maintain normal body weight and adiposity. However, the finding that losartan did not completely eliminate BDNF-induced metabolic effects indicates that either downregulation of Mas or other mechanisms, such as CRF-dependent signaling, are also involved (32).
In conclusion, our findings indicate that BDNF acting within the PVN has a major impact on central regulation of sympathetic activity, blood pressure, heart rate, and body weight and suggest a novel mechanism by which BDNF regulates the balance between opposing arms of the brain renin angiotensin system in the PVN mediated by ANG II/AT1R and angiotensin (1–7)/Mas, respectively. Further investigation of downstream signaling mechanisms activated by BDNF is important for several reasons. First, brain and systemic levels of BDNF are influenced by numerous factors including stress (18, 20, 49, 50), plasma osmolality (1), exercise (39), and obesity (6), and BDNF-mediated mechanisms in the PVN could have a significant role in mediating both beneficial metabolic and adverse cardiovascular effects of these stimuli via different downstream mediators. Second, it has been suggested recently (6, 8) that enhancing BDNF signaling in the hypothalamus could be used to reduce body weight and treat obesity, but our results indicate that such interventions could have serious side effects and result in hypertension and increased risk of cardiovascular diseases. Third, inhibition of BDNF-induced sympathoexcitation and blood pressure elevation could be a novel therapeutic target to treat stress- or obesity-related hypertension; however, such treatment would need to be selective to avoid adverse metabolic effects such as increased food intake and weight gain.
GRANTS
This work was supported by American Heart Association Grant 11SDG7560022 (to B. Erdos), an American Federation of Aging Research Research Grant (to B. Erdos), and by National Heart, Blood, and Lung Institute Grants HL-76807 (to multiple principal investigators including D. A. Scheuer) and HL-93186 (to D. A. Scheuer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.E., I.B., L.F.H., and D.A.S. conception and design of research; B.E., I.B., and M.L.M. performed experiments; B.E., I.B., M.L.M., L.F.H., and D.A.S. analyzed data; B.E., I.B., L.F.H., and D.A.S. interpreted results of experiments; B.E. prepared figures; B.E. drafted manuscript; B.E., I.B., L.F.H., and D.A.S. edited and revised manuscript; B.E., I.B., M.L.M., L.F.H., and D.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Morgan E. Hernandez and Theresa L. Wellman for excellent technical assistance.
REFERENCES
- 1.Aliaga E, Arancibia S, Givalois L, Tapia-Arancibia L. Osmotic stress increases brain-derived neurotrophic factor messenger RNA expression in the hypothalamic supraoptic nucleus with differential regulation of its transcripts relation to arginine-vasopressin content. Neuroscience 112: 841–850, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Amaral MD, Pozzo-Miller L. BDNF induces calcium elevations associated with IBDNF, a nonselective cationic current mediated by TRPC channels. J Neurophysiol 98: 2476–2482, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science 224: 452–459, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Bali A, Jaggi AS. Angiotensin as stress mediator: role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol Res 76: 49–57, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Boughton CK, Murphy KG. Can neuropeptides treat obesity? A review of neuropeptides and their potential role in the treatment of obesity. Br J Pharmacol 170: 1333–1348, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busnardo C, Tavares RF, Correa FM. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience 270: 12–19, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med 15: 447–454, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castren E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience 64: 71–80, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281: R1844–R1853, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci 31: 12318–12329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti LH, Shannon MH, Murry JD, Printz MP. Repeated restraint stress-induced increase in baroreceptor reflex sensitivity: role of corticotropin-releasing factor. Neuropeptides 35: 71–81, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Davern PJ, Chen D, Head GA, Chavez CA, Walther T, Mayorov DN. Role of angiotensin II Type 1A receptors in cardiovascular reactivity and neuronal activation after aversive stress in mice. Hypertension 54: 1262–1268, 2009. [DOI] [PubMed] [Google Scholar]
- 15.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont AG, Brouwers S. Brain angiotensin peptides regulate sympathetic tone and blood pressure. J Hypertens 28: 1599–1610, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Erdos B, Broxson CS, Cudykier I, Basgut B, Whidden M, Landa T, Scarpace PJ, Tumer N. Effect of high-fat diet feeding on hypothalamic redox signaling and central blood pressure regulation. Hypertens Res 32: 983–988, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci 27: 280–295, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA 109: 1305–1310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19: 1290–1300, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein RL, Muir D, King MA, Peel AL, Zolotukhin S, Moller JC, Kruttgen A, Heymach JV Jr, Muzyczka N, Meyer EM. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience 90: 815–821, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 139: 1107–1115, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci 126–127: 30–38, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA 92: 8074–8077, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res 55: 20–27, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96: 15239–15244, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem 108: 450–464, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci 44: 4357–4365, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci 7: 271–280, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res 949: 218–227, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Miki K, Yoshimoto M. Sympathetic nerve activity during sleep, exercise, and mental stress. Auton Neurosci 174: 15–20, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Mueller PJ, Mischel NA, Scislo TJ. Differential activation of adrenal, renal, and lumbar sympathetic nerves following stimulation of the rostral ventrolateral medulla of the rat. Am J Physiol Regul Integr Comp Physiol 300: R1230–R1240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray PS, Holmes PV. An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int J Pept 2011: 654085, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10: 209–219, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr Neuropharmacol 9: 262–277, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyhuis TJ, Masini CV, Sasse SK, Day HE, Campeau S. Physical activity, but not environmental complexity, facilitates HPA axis response habituation to repeated audiogenic stress despite neurotrophin mRNA regulation in both conditions. Brain Res 1362: 68–77, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol 131: 229–238, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Rosas-Vargas H, Martinez-Ezquerro JD, Bienvenu T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch Med Res 42: 482–494, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Jezova M, Zhou J. Brain angiotensin II, an important stress hormone: regulatory sites and therapeutic opportunities. Ann N Y Acad Sci 1018: 76–84, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Scheuer DA, Bechtold AG. Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats. Am J Physiol Regul Integr Comp Physiol 282: R475–R483, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol 286: H458–H467, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Scheuer DA, Mifflin SW. Glucocorticoids modulate baroreflex control of renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 280: R1440–R1449, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 108: 560–565, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Scislo TJ, Augustyniak RA, O'Leary DS. Differential arterial baroreflex regulation of renal, lumbar, and adrenal sympathetic nerve activity in the rat. Am J Physiol Regul Integr Comp Physiol 275: R995–R1002, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Smith MA, Cizza G. Stress-induced changes in brain-derived neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol Aging 17: 859–864, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Smith MA, Makino S, Kim SY, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology 136: 3743–3750, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Toriya M, Maekawa F, Maejima Y, Onaka T, Fujiwara K, Nakagawa T, Nakata M, Yada T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 22: 987–995, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Ullian ME. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res 41: 55–64, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience 212: 1–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Zhou XF. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience 112: 967–975, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6: 736–742, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300: R804–R817, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto R, Akazawa H, Fujihara H, Ozasa Y, Yasuda N, Ito K, Kudo Y, Qin Y, Ueta Y, Komuro I. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J Biol Chem 286: 21458–21465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]