Abstract
The consumption of flavan-3-ol-containing foods, including (−)-epicatechin (EC), has been linked to lower incidence of cardiovascular disease and stroke. We previously demonstrated nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) -dependent EC efficacy in reducing stroke-induced deficits in 2-mo-old mice; yet stroke is primarily a disease of the elderly. Because neuroinflammation, oxidative stress, and vascular dysfunction are hallmarks of aging, we tested whether Nrf2 mediates EC efficacy in aging mice through modulation of glial responses and blood brain barrier permeability. First, we compared anastomosis in naïve wild-type and C57BL/6 Nrf2−/− mice to identify potential differences in cerebrovascular architecture. Data showed no significant differences in the number of anastomoses or mean intersection points, indicating similar gross vascular physiology. To assess efficacy and mechanisms of protection, wild-type or Nrf2−/− mice were administered the minimum effective EC dose established in our previous studies before the permanent distal middle cerebral artery occlusion. Similar to previous results with young mice, 12-mo-old wild types also showed significant reductions in infarct volume (41.01 ± 29.57%) and improved performance in removing adhesive tape relative to vehicle-treated controls, whereas a trend toward protection was observed in Nrf2−/−. However, EC did not reduce immunoreactivity for the microglia/macrophage marker anti-ionized calcium-binding adapter molecule 1, suggesting that dampened activation/recruitment did not account for EC protection. Furthermore, there were no differences in mouse IgG extravasation or spontaneous hemorrhage between EC-treated groups. These data demonstrate that EC protection occurs independent of microglia/macrophage modulation or blood brain barrier preservation, suggesting that the glial cell responses in young mice are compensatory to another, and potentially novel, protective mechanism.
Keywords: behavior, cerebrovascular, central nervous system, inflammation, stroke
the only viable treatment for ischemic stroke, aside from supportive care, is thrombolytic therapy, which must be initiated within 4.5 h of stroke onset (1, 21, 32). In the aging population, however, health risks due to preexisting medical conditions often prohibit this therapeutic option. Because high blood pressure, high cholesterol, diabetes, and other factors limit clinical treatment options in a majority of patients, the ability to mitigate stroke injury through prophylactic neuroprotective “priming” is currently an area of great interest. Complementary and alternative medicinal (CAM) therapy represents a distinct approach whereby the consumption of natural compounds induces protective pathways that enable the brain to mitigate injury when an ischemic event arises. The success of such approaches would therefore benefit patients by eliciting a neuroprotective response, regardless of whether or not they are eligible for clinical treatment after stroke onset.
Our laboratory and others have been investigating the potential of CAM therapies, particularly (−)-epicatechin (EC) and resveratrol, in protective priming against ischemic stroke injury. These natural bioactive compounds are enriched in many food sources, thus representing a safe, noninvasive therapeutic option to bolster endogenous protection against vascular injury and stroke. Indeed, studies have documented improvements in vascular function (13, 52, 56, 60), reduced the risk for cardiovascular disease and stroke (18, 19, 28, 45), and heightened cognition following neurodegenerative conditions (35, 36).
EC and other flavan-3-ols are extensively metabolized in the small intestine and in the liver in humans and rodents following oral consumption (4, 37), which has generated questions as to whether the parent compound or bioactive metabolites are responsible for its beneficial effects. We and others have hypothesized an alternative mechanism of protection involving activation of the nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2). This transcriptional activator regulates cell defense/survival in multiple organ systems throughout the body (25). In response to injury and oxidative and xenobiotic stress, Nrf2 regulates the production of cytoprotective proteins that protect cells from the deleterious microenvironment (31, 64) which persists in various pathological states (39, 40), including stroke (26, 68). Interestingly, the beneficial effects of EC and resveratrol in protecting against vascular dysfunction and cerebral ischemia have been linked to Nrf2 induction (28, 29, 45, 62, 63).
Our laboratory has previously shown that EC effectively reduced infarct volume and basic neurological deficits when measured 72 h after transient brain ischemia (45). Additionally, we have recently documented Nrf2-dependent improvements in anatomical outcomes and complex sensorimotor function in young mice after permanent cerebral ischemia (28). However, no studies have evaluated the prophylactic efficacy of EC in aging mice subjected to experimental stroke. Because the aging brain exhibits heightened inflammation and vascular vulnerability, we subjected 12-mo-old mice to permanent cerebral ischemia to ask the question of whether EC can protect the aging brain from permanent focal ischemia and, if so, whether the benefits of EC are associated with glial cell reactivity and cerebrovascular preservation.
MATERIALS AND METHODS
Animal care.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida, in accordance with the Guide for the Care and Use of Laboratory Animals and the guidelines laid down by the National Institutes of Health regarding the care and use of animals for experimental procedures. For all experiments, male wild-type (WT) and Nrf2 knockout (Nrf2−/−) C57BL/6 mice were housed in a climate-controlled facility (23 ± 1°C) on a 12-h:12-h reversed light-dark cycle and were provided food and water ad libitum. Mice (4 mo old) were used for anastomosis and hemorrhage evaluations, and 12-mo-old (aging) mice were used for all outcomes measured.
EC treatment and permanent distal middle cerebral artery occlusion.
WT or Nrf2−/− mice were randomized and administered either methylcellulose vehicle (WT = 8; Nrf2 = 9) or 15 mg/kg of freshly prepared EC (WT = 7; Nrf2 = 9) (Sigma Aldrich, St. Louis, MO) by gavage 90 min before permanent distal middle cerebral artery (MCA) occlusion (pdMCAO), as previously described (28, 45). The pdMCAO procedure was performed under halothane anesthesia (4% induction and 2% maintenance, 1.5/3.0% oxygen/air balance). All possible measures were taken to minimize pain and discomfort, and mice showed no overt signs of stress. Halothane was used because isoflurane has been shown to induce Nrf2-responsive protein expression (42), which would potentially confound the interpretation of results. Complete occlusion was confirmed by severance of the vessel. Because 15 mg/kg was determined to be the minimum effective dose in previous studies using young, healthy mice (28), the same dose was used in the present study for comparative purposes.
Behavioral testing.
Because of the relatively mild functional deficits produced by pdMCAO cortical injury, mice were tested for performance on the adhesive removal test and were subjected to a DigiGait (Mouse Specifics, Framingham, MA) gait analysis evaluation.
Adhesive removal test.
The adhesive removal task requires forelimb strength, sensation, and complex sensorimotor coordination. Impaired animals mimic clinical pathology in which stroke patients often report weakness and/or loss of function in one or more extremities. For testing, adhesive tape was placed on the planar surface of the forepaw and latency to remove was recorded. Mice were trained for 3 days, tested for baseline performance before stroke, and posttested 1 day after stroke.
DigiGait.
For motor sensory testing using the DigiGait testing, mice were placed on a treadmill, which was set at 20 rpm and activated. Mice were then recorded while running for 3 to 5 s on the treadmill. Paw print placement for each trial was recorded by the DigiGait tracking system. The interfaced software evaluated various parameters associated with swing and stride. Mice were tested once before stroke and again at 1 day poststroke. For analysis, each parameter was first expressed as a ratio of the opposing side (left/right), and those ratios were expressed relative to baseline gait values.
Histology and immunohistochemistry.
Lesion volume and total immunoreactivity were calculated from 16 brain sections collected at even intervals throughout the expanse of the cortical infarct. At 7 days poststroke, mice were perfused, brains were fixed and cryosectioned, and sections were stained with Cresyl violet for lesion volume quantification. Immunohistochemistry was performed to evaluate reactive gliosis by using anti-ionized calcium-binding adapter molecule 1 (Iba1) (1: 1,000; Wako, Richmond, VA) for microglia/macrophages. This antibody is listed and cited in the Journal of Comparative Neurology database. Primary antibody was omitted in control experiments to validate antibody selectivity. Blood brain barrier permeability was assessed using mouse IgG secondary antibody (peroxidase-labeled mouse IgG, 1: 300) (30). For gliosis, biotinylated secondary antibodies were complexed using an elite ABC kit (Vector) and visualized using diaminobenzidine with nickel enhancement (Vector). Images were acquired using an Aperio ScanScope XT, and quantifications were performed using ImageScope software (Aperio, Vista, CA). The ImageScope algorithm “positive pixel count” was used to quantify pixel numbers corresponding to weak positive, positive, and strong positive signal based on preset criteria. Because of the abundance of quiescent microglia/macrophages and resting astrocytes, strong positive pixels were used to identify staining that reflected activated glial cells. The positive pixel counts were expressed as a ratio of the total cortical area measured, and ipsilateral calculations were then expressed relative to the contralateral hemisphere for each section to control for potential variations in antibody penetrance or tissue processing.
Anastomosis.
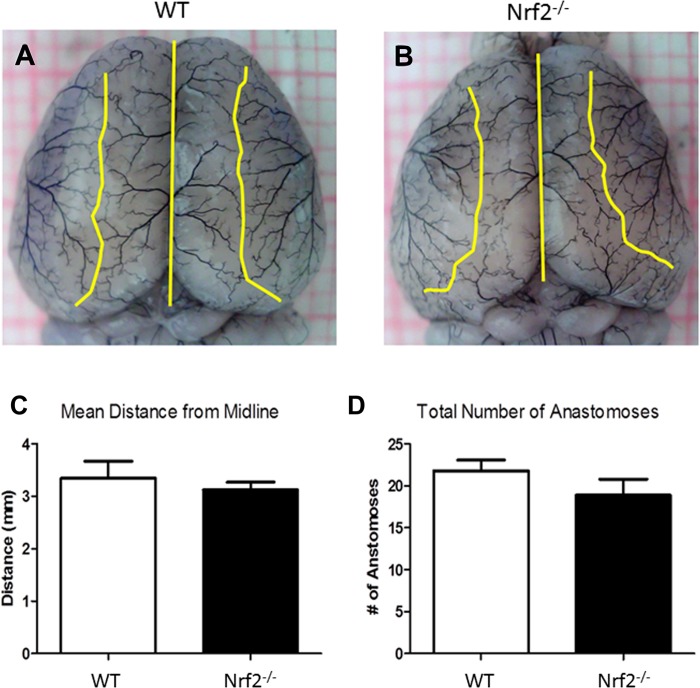
To examine potential variations in the cerebral vasculature of WT and Nrf2−/− mice, animals were deeply anesthetized with isoflurane and perfused with PBS followed by 1 ml of black latex paint. Flow rates of both PBS and latex paint did not exceed 1 ml/min. Brains were harvested immediately following perfusion and post-fixed in 10% paraformaldehyde for 24 h at 4°C before imaging of the dorsal surface. Images were obtained using a desktop endoscope/microscope (Oasis Scientific, Greenville, SC). For analysis, the distal branches of the anterior cerebral artery (ACA) and the MCA were traced to the point of anastomosis, which was defined as the narrowest point along the vessel connecting the ACA and MCA (or the midpoint between the nearest branching points in cases of no connecting point). All anastomoses points were then connected to form a line (Fig. 1), and the distance from the midline was determined by measuring the distance from the midline to the anastomosis line. Measurements were taken at 1-mm intervals from the frontal pole to the end of the dorsal surface.
Fig. 1.
nuclear transcription factor erythroid 2p45-related factor-2 knockout (Nrf2−/−) and wild-type (WT) mice show similar cerebrovascular architecture. Anastomosis was assessed to determine potential variations in the cerebral vasculature of WT and Nrf2−/− mice. A and B: representative images from C57BL/6 WT and Nrf2−/− mice show line tracings connecting anastomosis points. C and D: no significant differences were found in the mean intersection distance from the midline or the total number of anastomoses, indicating that cerebral vascular physiology is similar. Data are expressed as mean ± SD.
Hemorrhage frequency.
For evaluations of rebleeding following stroke, Cresyl violet-stained sections collected throughout the entire cortical infarct were evaluated for intraparenchymal blood. Because of the nature of the occlusion at the cortical surface, traces of surface blood were discounted from the analysis. Only sections showing intraparenchymal blood within or lateral to the cortical infarct were counted, and hemorrhage was recorded as “absent” or “present” for each animal. Hemorrhage frequency was then expressed as the number of mice showing intraparenchymal blood within each experimental group.
Statistical analyses.
All analyses were performed blinded such that experimenters performing surgery, behavioral testing, anatomical measures, and data analysis were unaware of the genotypes and treatments. Power analysis was completed based on previous data using the same model. Data are expressed as means ± SE, except in the case of anastomosis, where mean ± SD was used. Statistical significance was set at P < 0.05. Bartlett's tests showed no significant differences in group variances; therefore, data were evaluated using parametric statistics. Data from experiments evaluating a single factor between two groups were analyzed by two-tailed, unpaired Student's t-test. For experiments with greater than two groups, significance was determined using one-way ANOVA. Where main effects occurred, Bonferroni post hoc analyses were then used to evaluate multiple group comparisons. For behavioral experiments, the use of a two-by-two factorial design [treatment (vehicle or EC) and time (pretest or posttest)] required analysis by two-way ANOVA, followed by Bonferroni post hoc analyses. The nonparametric Kruskal-Wallis test was used to evaluate hemorrhage frequency. All statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA) data analysis software.
A power analysis was performed based on previous experiments using the same pdMCAO model and C57BL/6 Nrf2−/− mouse strain. The analysis showed that a sample size of 8 was needed to achieve significant difference [statistical power = 0.80, α = 0.05 (2 sided)].
RESULTS
Cerebrovascular architecture is similar in WT and Nrf2−/− mice.
Anastomosis was evaluated in naïve WT and Nrf2−/− mice to determine whether variations in the cerebral arterial vasculature might account for differences in severity of stroke injury or treatment efficacy. Mice were perfused with black latex paint and the cerebral vasculature was analyzed (Fig. 1) to determine the total number of ACA/MCA intersection points, as well as the mean distances of anastomoses from the dorsal midline. The method for identifying intersection points and line tracing is illustrated in representative macrographs from WT and Nrf2−/− mice (Fig. 1, A and B). Quantification showed no significant differences in the mean intersection distance from the midline (Fig. 1C) or the total number of anastomoses (Fig. 1D) between WT and Nrf2−/− mice.
EC improves anatomical and functional outcomes in aging mice.
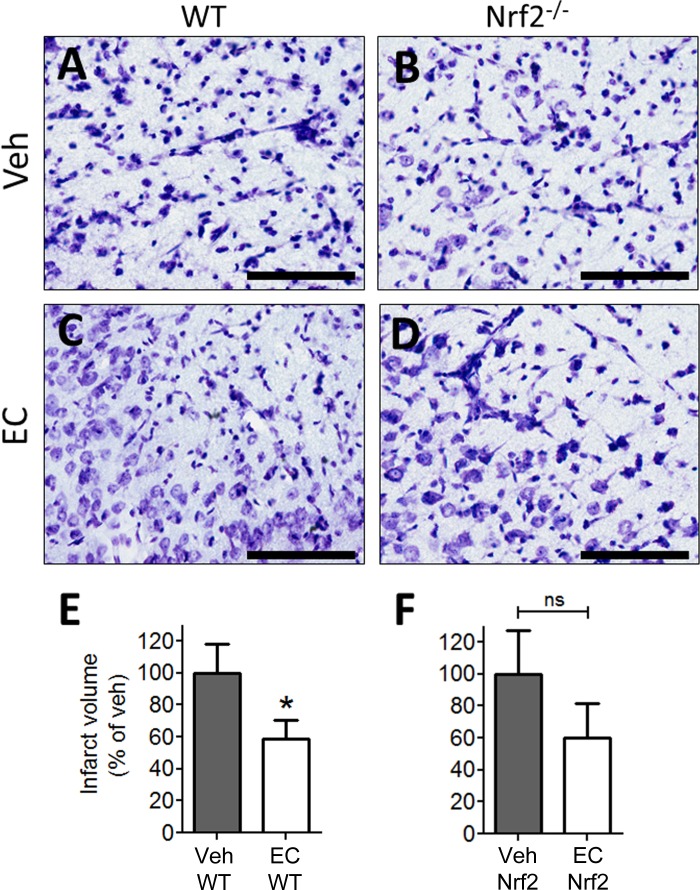
We previously documented the efficacy of EC in young, healthy animals such that a single oral dose of 15 mg/kg 90 min before pdMCAO reduced infarct volume and functional recovery in WT but not in Nrf2−/− mice (28). Therefore, the present study was conducted to determine whether the same prophylactic dose of EC also elicits protection in the aging brains of 12-mo-old mice. Cresyl violet staining revealed expanses of pyknotic nuclei and cellular degeneration in vehicle-treated mice (Fig. 2, A and B), whereas the cortical infarction zones of EC-treated mice showed larger numbers of viable cells and less tissue degradation (Fig. 2, C and D). Quantification showed that EC significantly reduced infarct volume in WT mice relative to vehicle-treated controls (P = 0.04), consistent with our previous study in young mice. Of note, EC treatment in 12-mo-old Nrf2−/− mice show a trend toward reductions in infarct volume relative to vehicle-treated Nrf2−/− group; however, this effect did not reach a significant level (P = 0.12).
Fig. 2.
(−)-Epicatechin (EC) pretreatment attenuates infarct volume in the aging brain. Representative photomicrographs of Cresyl violet-stained sections from vehicle- and EC-treated WT and Nrf2−/− mice. A and B: tissue voids and regions of degenerating morphology were indistinguishable in vehicle (Veh)-treated WT (N = 8) and Nrf2−/− (N = 9) mice. C and D: sections from EC-treated mice (WT = 7; Nrf2 = 9) displayed more preserved morphology in general, although regions of pyknotic nuclei and cell death were also present. E and F: quantification of infarct volume showed a significant reduction in EC-treated WT mice relative to vehicle controls. EC treatment in Nrf2−/− exhibits a trend toward a decrease in infarction volume; however, this trend did not reach a significant value (P = 0.12). Data are expressed as means ± SE. *P < 0.05; NS, not significant. Scale bars = 200 μm.
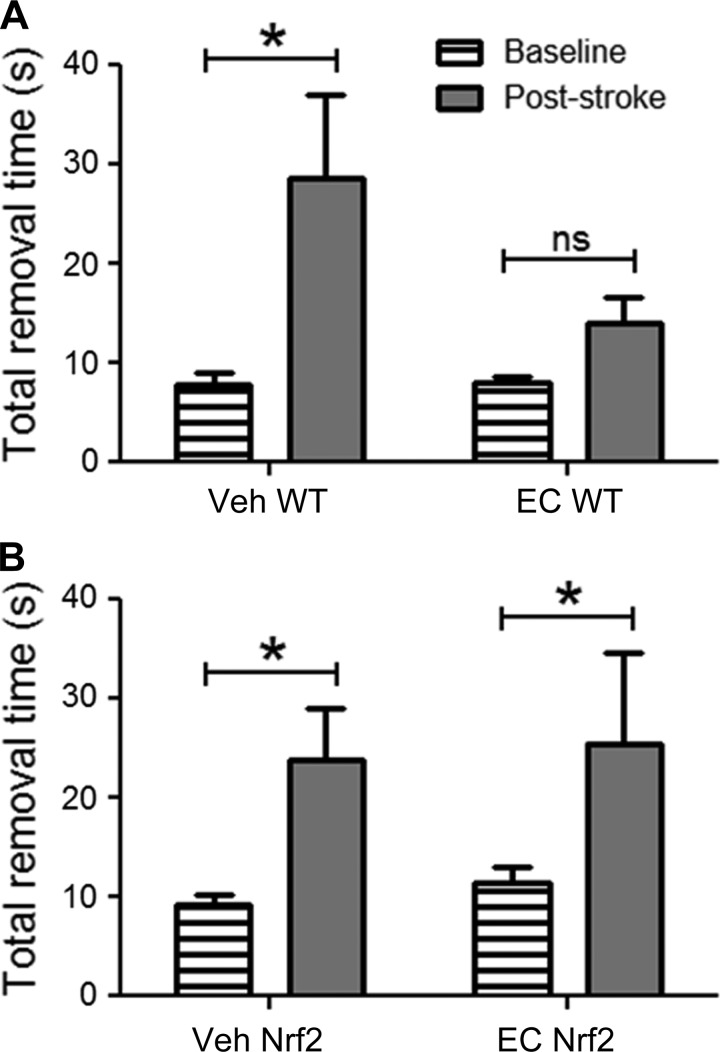
In addition to infarct volume, mice were evaluated for performance on the adhesive removal test, which demonstrates sensitivity in detecting forelimb sensorimotor deficits. The results from WT mice (Fig. 3A) showed a main effect of time, with vehicle-treated mice showing significant elevations in removal time relative to presurgical baseline scores (P = 0.001). In contrast, there was no significant difference between pre- and poststroke removal time in EC-treated mice. Furthermore, results from Nrf2−/− mice (Fig. 3B) showed a main effect of time, and subsequent analyses showed significant elevations in both vehicle-treated (P = 0.002) and EC-treated (P = 0.03) mice relative to their respective baseline scores.
Fig. 3.
EC pretreatment promotes functional recovery following stroke. A: Vehicle-treated WT mice subjected to permanent distal middle cerebral artery occlusion showed significant elevations in latency to remove adhesive tape relative to presurgical baseline scores, whereas there was no significant difference in removal time for EC-treated WT mice. B: latency to remove adhesive tape was significantly elevated in Nrf2−/− mice after treatment with either vehicle or EC relative to presurgical baseline scores, and there was no significant difference in poststroke removal time between treatments. Data are expressed as means ± SE. *P < 0.05.
Gait analysis was also evaluated on these mice to determine whether milder deficits could be captured, as the DigiGait system was designed to identify impairments with high sensitivity. Although WT mice showed clear forelimb deficits in removing adhesive tape, no major gait deficits were detected upon analyzing various parameters designed to reflect asymmetries associated with stance/stride length, frequency, etc. (data not shown).
EC does not alter microglia/macrophage activation 7 days after stroke.
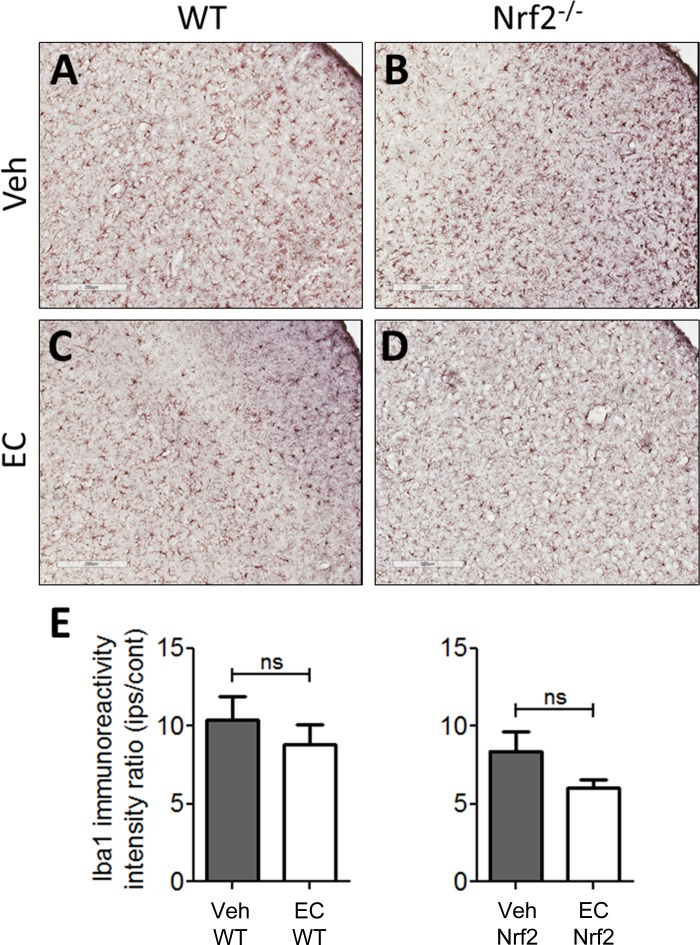
Our previous report in young, healthy mice documented reductions in microglia/macrophage activation and recruitment following EC treatment (28). To determine whether the protective effects of EC in aging mice are associated with alterations in gliosis, sections were subjected to immunohistochemical staining for the microglia/macrophage marker Iba1 (Fig. 4). In sections from vehicle-treated mice, Iba1-positive cells displayed partially to fully activated morphology and were distributed abundantly throughout the cortical infarction zone. Furthermore, there was no observable distinction between the quantity or relative activation state when comparing WT and Nrf2−/− mice (Fig. 4, A and B). Similarly, sections from EC-treated WT and Nrf2−/− mice contained regions of abundant Iba1-positive cells throughout the cortical infarct, although EC-treated Nrf2−/− mice appeared to show less microglia/macrophage recruitment (Fig. 4D) relative to EC-treated WT mice (Fig. 4C). Quantification (Fig. 4E) revealed no significant differences in Iba1 immunoreactivity across treatment (P = 0.17). Astrogliosis was also estimated by quantifying glial fibrillary acidic protein immunoreactivity to determine whether an astrocytic response might be associated with EC protection, and there were no significant differences across treatments or genotypes (data not shown).
Fig. 4.
EC does not alter microglia/macrophage activation in aging mice. Representative micrographs show immunohistochemical anti-ionized calcium-binding adapter molecule 1 (Iba-1) staining in sections from WT and Nrf2−/− mice treated with vehicle or EC. A and B: sections from vehicle-treated WT and Nrf2−/− mice showed densely distributed cell populations, including large numbers of hypertrophic cells with thick processes that are characteristic of the activated phenotype throughout the ischemic cortex. There was no apparent difference in the number or expanse of activated cells between genotypes. C and D: cells with activated morphology were numerous throughout the ischemic regions of mice treated with EC, and these cells resembled those in vehicle-treated mice. Treatment with EC appeared to reduce the number of fully activated cells. E: quantification revealed no significant differences in Iba1 immunoreactivity between vehicle- and EC-treated WT or Nrf2−/− mice. IPS/Cont, ratio of ipsilateral to contralateral immunoreactivity. Data are expressed as means ± SE. Scale bars = 200 μm.
EC does not alter gross vascular permeability or hemorrhage frequency.
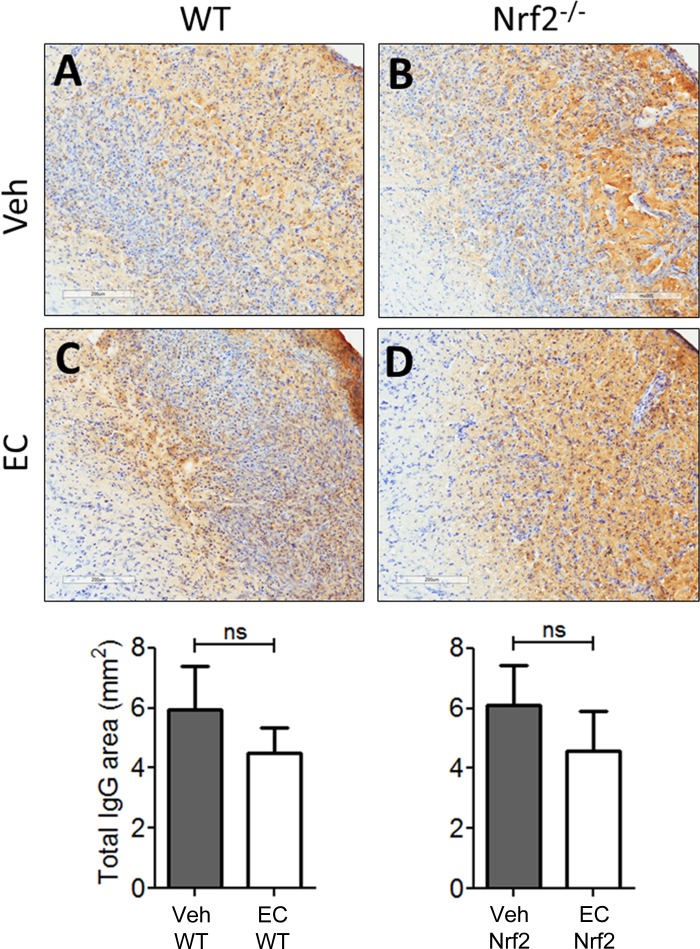
Because the protective effects of EC were not associated with alterations in gliosis 7 days after pdMCAO, we sought to determine whether protection might occur through preservation of cerebrovascular integrity. Immunohistochemistry for mouse IgG was performed on sections from WT and Nrf2−/− mice treated with vehicle or EC (Fig. 5). Consistent with the microglia/macrophage profile, animals treated with vehicle (Fig. 5, A and B) showed intense IgG staining throughout the cortical infarction zone that resembled sections from EC-treated mice (Fig. 5, C and D) within each genotype. Quantification of the area occupied by IgG showed no significant differences across treatments (P = 0.27) or genotypes (P = 0.92).
Fig. 5.
EC does not alter gross vascular permeability or hemorrhage frequency. Representative micrographs show mouse IgG immunohistochemical staining in sections counterstained with Cresyl violet. A and B: sections from vehicle-treated WT and Nrf2−/− mice showed expansive regions of mouse IgG staining that is indicative of blood brain barrier permeability, with no apparent difference in the expanse of parenchymal IgG immunoreactivity between genotypes. C and D: IgG immunoreactivity was abundant throughout the ischemic cortex in sections from EC-treated mice and appeared more prominent in Nrf2−/− mice relative to WTs. Bottom: quantification revealed no apparent difference in IgG immunoreactivity between vehicle- and EC-treated WT (P = 0.28) or Nrf2−/− mice (P = 0.92). Data are expressed as means ± SE. ns, not significant. Scale bars = 200 μm.
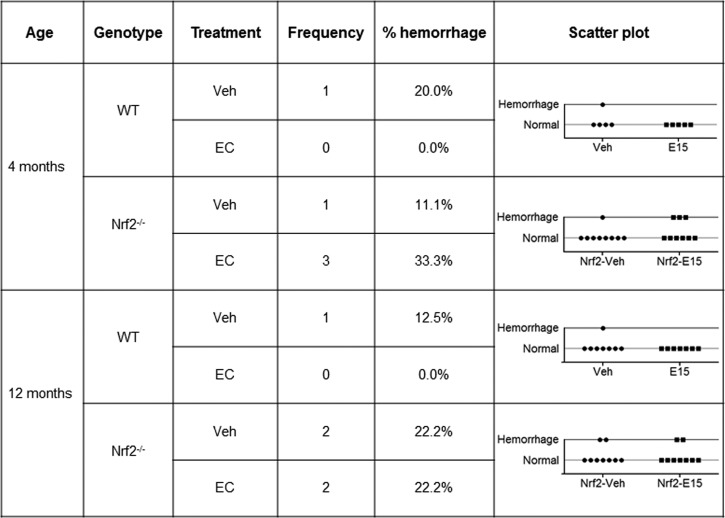
Despite the finding that there were no significant differences in IgG extravasation into the brain parenchyma, an additional comparison was performed to address whether EC protection could be explained by reductions in spontaneous bleeding after stroke. Because weakening of the cerebral vasculature is a hallmark of the aging process, Cresyl violet-stained sections from 4- and 12-mo-old WT and Nrf2−/− mice were examined to determine whether hemorrhage frequency differed in response to age, treatment, or genotype (Fig. 6). In the 4-mo-old WT mice (young mice group), there was only a single case of intraparenchymal bleeding in the vehicle group, with no events detected in EC-treated animals. In contrast, hemorrhage was detected in four cases with 4-mo-old Nrf2−/− mice, three of which belonged to the EC-treated group. A similar pattern was observed in 12-mo-old mice, with a single case of hemorrhage in a vehicle-treated WT mouse, and four cases in Nrf2−/− mice that were equally distributed across treatments. Despite a trend toward increased hemorrhage in Nrf2−/− mice, there was no significant differences between groups (P = 0.586).
Fig. 6.
Hemorrhage frequency is not altered by treatment, age, or genotype. Scatter plots show the numbers of young and aging WT and Nrf2−/− mice that displayed intraparenchymal bleeding following administration of vehicle or EC. Despite a trend toward increased hemorrhage for Nrf2−/− mice, analyses revealed no significant differences in intraparenchymal bleeding across treatment, age, or genotype (P = 0.59). E15, 15 mg/kg EC.
DISCUSSION
In this study, we tested the hypothesis that prophylactic EC administration would elicit protection from permanent focal ischemia in aging mice and that the benefits of EC would be associated with dampened glial cell reactivity and cerebrovascular preservation. Taken together, data here extend the benefits of prophylactic EC consumption to 12-mo-old mice, thus lending further support for flavan-3-ol use to protect against cerebrovascular disease and stroke. Similar to our previous reports (28, 45), a single prophylactic dose 90 min before pdMCAO effectively reduced infarct volume and reduced behavioral deficits in WT mice. The timing of EC treatment was selected based on peak plasma concentrations previously documented in rodents following oral administration (5, 49), which mirror the timing of stroke onset in the present study. The 15 mg/kg dose represents the minimum effective dose in diminishing microglia/macrophage activation and recruitment, decreasing infarct volume, and improving functional outcomes from our previous report using young mice (28). Consistent with previous data, EC was ineffective in improving histological and behavioral outcomes in aging Nrf2−/− mice, indicating that this transcriptional factor may also partially regulate protection in the aging phenotype.
Although the lack of functional recovery in EC-treated Nrf2−/− mice was pronounced, a trend toward reduced infarct volume in EC-treated Nrf2−/− mice was noted. Based on power analysis from previous experiments using 2-mo-old Nrf2−/− mice, the animal number used was sufficient to achieve adequate statistical power. However, the use of 12-mo-old mice in this study is an important difference that may reflect the need to adjust the animal number, which could not be further extended at this time. Given that these mice did not display an overt aged phenotype either in terms of behavior or anatomical measures, it is likely that the variance arose from deletion of the Nrf2 gene.
Previously, we have shown in young mice that pretreatment with 5, 10, and 15 mg/kg EC resulted in 54.5 ± 8.3, 40.1 ± 14.2\, and 50.4 ± 10.1% reductions in infarction volume, respectively. In the present study, use of 15 mg/kg EC extended its beneficial effect in aging mice and reduced infarct volume by 41.01 ± 29.57%. Because polyphenolic compounds have been shown to improve outcomes in models of ischemia (7, 27, 29, 50, 58, 59), the goal here was to evaluate possible effects on immune cell activation and cerebrovascular integrity. Interestingly, results did not support the hypothesized mechanisms. Although our previous study showed that the protective effects of EC in young mice were accompanied by reductions in microglia/macrophage activation and recruitment, there were no significant differences in these outcome measures in 12-mo-old mice, despite modest qualitative differences. The aging brain is generally considered to display a heightened glial response to insult (8, 34, 54, 65), which may account for the inability of EC to dampen microglia and macrophage activation. However, despite the fact that the aging process is widely assumed to include weakened vascular integrity (10, 16, 23, 24), spontaneous hemorrhage was not significantly altered in any of the experimental groups.
These data beg several questions as to why these outcomes were not altered by treatment, and a few important points may account for these observations. First, underlying differences in vascular permeability and gliosis may not have been captured because of the 7-day time point examined. Additional studies with multiple time points, encompassing both early and late phases of neurodegenerative events, are currently planned. It is also possible that 12-mo-old mice, while considered to be older relative to young mice, have not yet developed the “fully” aged neuropathology that renders them susceptible to heightened glial responses and vascular dysfunction. Importantly, blood brain barrier disruption has been documented in aged (24 mo old) mice (61), supporting the notion that 12-mo-old mice do not yet display the fully aged phenotype. This would explain the lack of associated alterations in IgG extravasation with EC efficacy, and it is an important point to consider when investigating these mechanisms of injury/protection. Therefore, future studies with aged mice (at least 1.5 years) are necessary to fully investigate this possibility, and indeed, several other studies have documented vascular abnormalities in aged rodents (14, 17, 38). Finally, the protective effects of EC may not be contingent on microglia/macrophage responses or blood brain barrier permeability per se. Instead, these outcome measures may simply reflect biological processes that occur in response to other signals within the ischemic microenvironment. Although the nature of such alterations was not uncovered here, these data fill a gap in the literature regarding the mechanisms of flavan-3-ol protection in mice that are transitioning between young and fully aged. Because the neural phenotype changes over time and appears to be species specific to some extent, repeated investigations using multiple ages and animal strains are necessary to validate experimental research (55).
Notably, several key pathways have been identified within the context of CAM therapies. For example, activation of the Nrf2 and pathways by polyphenols has been linked to neuroprotection from oxidative damage (6). In vitro, primary neuronal cultures treated with EC upregulated the expression of the Nrf2-responsive, cytoprotective proteins ferritin light chain, and biliverdin reductase, in addition to heme oxygenase 1. Although we have previously documented the involvement of these pathways in regulating the response to focal ischemia (46, 67), other signaling pathways may mediate the extended benefits observed here. For example, direct antioxidant effect of EC treatment on oxidative stress, as reported in several studies, cannot be ruled out (9, 41, 43), although we do not think it is likely simply on the basis of stoichiometry. It has been proposed that NDAPH oxidase activity augments with aging, thereby it participates in accentuating the oxidative stress and making aging brain more vulnerable to diseases (22, 66). On the other hand, it has been shown that EC inhibits NADPH oxidase in endothelial cells and thereby elevates the level of endothelial nitric oxide and induces vasodilation (53). Moreover, EC may have similar beneficial effects as reported for resveratrol and Gingko biloba, natural compounds that have been extensively studied by our laboratory and others because of its multiplicity of health benefits and pleiotropic actions (44, 46, 57). These natural compounds have been shown to elicit neuroprotection, in part, through downregulation of apoptotic enzymes (2, 20, 44, 48, 51, 57). Additionally, the neuroprotective effects of these natural compounds are also linked with the reductions in hypoxia-inducible factor-1α mRNA expression (11, 15).
With these emerging lines of evidence, future studies investigating the cell-specific, temporal alterations of these pathways may lead to identification of the key player(s) that mediate protection from delayed degenerative injury following brain ischemia. For example, it is possible that endothelial cell death and subsequent disruption of the neurovascular unit at early time points initiates a series of apoptotic signaling events that lead to delayed neurodegeneration, possibly through hypoxia-inducible factor 1α. Such events are known to occur after cerebral ischemia (3, 12, 33) and would fit with both the injury profile and the effects of EC observed in the present study. Notably, resveratrol has been shown to exert beneficial effects on endothelial cell function, which in turn promotes vascular function (60, 62, 63). Therefore, detailed studies investigating these signaling events throughout the neurodegenerative process will be essential in elucidating the mechanism of action that accounts for EC protection in both the young and aging brain.
Conclusion and potential limitations.
Although this study shows the efficacy of prophylactic EC in attenuating permanent focal ischemic brain damage in 12-mo-old mice, there are certain caveats associated with this study. To fully understand and determine the role of EC in regulating ischemic brain damage in aged populations (one of the most common and strong risks factor for stroke), a study on >26-mo old mice is needed. In our young animal studies, we have shown that the beneficial effect of EC is regulated partially through Nrf2 pathways; however, in the current study where we used 12-mo-old mice, the use of EC in Nrf2−/− shows a trend toward beneficial outcomes in Nrf2−/− mice. This suggests that perhaps with aging, the effect of Nrf2 is weakened and the primary beneficial effect of EC treatment in the aging brain is mediated through regulating the antioxidant defense system. Although WT and Nrf2−/− mice show similar vascular anastomosis system, the vascular protective effect of Nrf2 affecting stroke outcomes cannot be ruled out. To fully explore this avenue, studies with relative and absolute cerebral blood flow changes and vasomotor activity following EC treatment with or without stroke should be performed. To fully understand the role of EC treatment on gliosis on the final outcomes after stroke, the role of microglia should be tested in a time-dependent manner, and costaining of CD68 with Iba-1 could potentially provide further evidence. Nevertheless, it can be safely concluded from our previous studies, along with this current study, that consumption of EC protects the young and aging brain from ischemic brain damage. The exact mechanism(s) pertaining to the beneficial effect of EC is yet to be fully understood.
GRANTS
This work was partially supported by National Center for Complementary and Alternative Medicine Grants R21AT005085 and R21AT005246 (to S. Doré).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.C.L. performed experiments; C.C.L., M.M., and A.S.A. analyzed data; C.C.L., A.S.A., and S.D. interpreted results of experiments; C.C.L., M.M., and A.S.A. prepared figures; C.C.L., M.M., and A.S.A. drafted manuscript; C.C.L., M.M., A.S.A., and S.D. edited and revised manuscript; S.D. conception and design of research; S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Matthew Mills and all other members of the Doré laboratory
REFERENCES
- 1.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38: 1655–1711, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal M, Kumar V, Kashyap MP, Khanna VK, Randhawa GS, Pant AB. Ischemic insult induced apoptotic changes in PC12 cells: protection by trans resveratrol. Eur J Pharmacol 666: 5–11, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal M, Kumar V, Singh AK, Kashyap MP, Khanna VK, Siddiqui MA, Pant AB. trans-Resveratrol protects ischemic PC12 cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem Neurosci 4: 285–294, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J Nutr 131: 2885–2891, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Baba S, Osakabe N, Natsume M, Terao J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic Biol Med 33: 142–148, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bhullar KS, Rupasinghe HP. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev 2013: 18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res 1019: 47–54, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Conde JR, Streit WJ. Effect of aging on the microglial response to peripheral nerve injury. Neurobiol Aging 27: 1451–1461, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cuevas E, Limon D, Perez-Severiano F, Diaz A, Ortega L, Zenteno E, Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25–35 in rats. Eur J Pharmacol 616: 122–127, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16: 209–221, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Doré S. Unique properties of polyphenol stilbenes in the brain: more than direct antioxidant actions; gene/protein regulatory activity. Neurosignals 14: 61–70, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med 17: 1391–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher ND, Sorond FA, Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol 47, Suppl 2: S210–S214, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gao P, Shen F, Gabriel RA, Law D, Yang E, Yang GY, Young WL, Su H. Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke 40: 3596–3600, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granado-Serrano AB, Martin MA, Haegeman G, Goya L, Bravo L, Ramos S. Epicatechin induces NF-kappaB, activator protein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) via phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 cells. Br J Nutr 103: 168–179, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res 2: 409–423, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke 43: 3052–3062, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Keen CL, Kelm M. Flavanols and cardiovascular disease prevention. Eur Heart J 31: 2583–2592, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Hollenberg NK, Fisher ND, McCullough ML. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J Am Soc Hypertens 3: 105–112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T, Huang T, Gao D, Gao D, Jiang X, Jiang X, Hu S, Hu S, Zhang L, Zhang L, Fei Z, Fei Z. Resveratrol inhibits oxygen-glucose deprivation-induced MMP-3 expression and cell apoptosis in primary cortical cells via the NF-κB pathway. Mol Med Rep 10: 1065–1071, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44: 870–947, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis 40: 185–192, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN. Brain microvasculature in aging. Neurobiol Aging 15: 765–766, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood-brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cereb Blood Flow Metab 31: 1874–1885, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci 24: 1101–1112, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res 77: 892–900, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Leonardo CC, Agrawal M, Singh N, Moore JR, Biswal S, Doré S. Oral administration of the flavanol (−)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur J Neurosci 38: 3659–3668, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonardo CC, Doré S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr Neurosci 14: 226–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonardo CC, Hall AA, Collier LA, Ajmo CT Jr, Willing AE, Pennypacker KR. Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD11b-expressing, isolectin-binding proinflammatory cells after middle cerebral artery occlusion. J Neurosci Res 88: 1213–1222, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol 173: 3467–3481, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Maiser SJ, Georgiadis AL, Suri MF, Vazquez G, Lakshminarayan K, Qureshi AI. Intravenous recombinant tissue plasminogen activator administered after 3 h following onset of ischaemic stroke: a metaanalysis. Int J Stroke 6: 25–32, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 301: 173–187, 2000. [DOI] [PubMed] [Google Scholar]
- 34.McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann NY Acad Sci 1035: 104–116, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol 75: 716–727, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oomen CA, Farkas E, Roman V, Van Der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009December9; 1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H. Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards. Free Radic Biol Med 52: 1403–1412, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol 113: 277–293, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98: 3410–3415, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruijters EJ, Weseler AR, Kicken C, Haenen GR, Bast A. The flavanol (−)-epicatechin and its metabolites protect against oxidative stress in primary endothelial cells via a direct antioxidant effect. Eur J Pharmacol 715: 147–153, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, Geiger KK, Pannen BH. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg 245: 931–942, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg N. K, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103: 1024–1029, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarzkopf TM, Koch KA, Klein J. Neurodegeneration after transient brain ischemia in aged mice: Beneficial effects of bilobalide. Brain Res 1529: 178–187, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab 30: 1951–1961, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 180: 248–255, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin JA, Lee H, Lim YK, Koh Y, Choi JH, Park EM. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J Neuroimmunol 227: 93–100, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Shrestha SP, Thompson JA, Wempe MF, Gu M, Agarwal R, Agarwal C. Glucuronidation and methylation of procyanidin dimers b2 and 3,3′′-di-o-galloyl-b2 and corresponding monomers epicatechin and 3-o-galloyl-epicatechin in mouse liver. Pharm Res 29: 856–865, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31: 135–147, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Singh N, Agrawal M, Doré S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem Neurosci 4: 1151–1162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorond FA, Hollenberg NK, Panych LP, Fisher ND. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultrasound Med 29: 1017–1022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steffen Y, Schewe T, Sies H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun 359: 828–833, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res 27: 685–691, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30: 2752–2758, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Sudano I, Flammer AJ, Roas S, Enseleit F, Ruschitzka F, Corti R, Noll G. Cocoa, blood pressure, and vascular function. Curr Hypertens Rep 14: 279–284, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Sun L, Zhuang W, Xu X, Yang J, Teng J, Zhang F. The effect of injection of EGb 761 into the lateral ventricle on hippocampal cell apoptosis and stem cell stimulation in situ of the ischemic/reperfusion rat model. Neurosci Lett 555: 123–128, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (−)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J 19: 258–260, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki M, Tabuchi M, Ikeda M, Umegaki K, Tomita T. Protective effects of green tea catechins on cerebral ischemic damage. Med Sci Monit 10: BR166–BR174, 2004. [PubMed] [Google Scholar]
- 60.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35: 238–245, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci 28: 1316–1328, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Woodfin A, Hu DE, Sarker M, Kurokawa T, Fraser P. Acute NADPH oxidase activation potentiates cerebrovascular permeability response to bradykinin in ischemia-reperfusion. Free Radic Biol Med 50: 518–524, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeynalov E, Shah ZA, Li RC, Doré S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis 35: 264–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett 393: 108–112, 2006. [DOI] [PubMed] [Google Scholar]