Abstract
Mitochondria are in close proximity to the redox-sensitive sarcoplasmic reticulum (SR) Ca2+ release [ryanodine receptors (RyRs)] and uptake [Ca2+-ATPase (SERCA)] channels. Thus mitochondria-derived reactive oxygen species (mdROS) could play a crucial role in modulating Ca2+ cycling in the cardiomyocytes. However, whether mdROS-mediated Ca2+ dysregulation translates to abnormal electrical activities under pathological conditions, and if yes what are the underlying ionic mechanisms, have not been fully elucidated. We hypothesize that pathological mdROS induce Ca2+ elevation by modulating SR Ca2+ handling, which activates other Ca2+ channels and further exacerbates Ca2+ dysregulation, leading to abnormal action potential (AP). We also propose that the morphologies of elicited AP abnormality rely on the time of mdROS induction, interaction between mitochondria and SR, and intensity of mitochondrial oxidative stress. To test the hypotheses, we developed a multiscale guinea pig cardiomyocyte model that incorporates excitation-contraction coupling, local Ca2+ control, mitochondrial energetics, and ROS-induced ROS release. This model, for the first time, includes mitochondria-SR microdomain and modulations of mdROS on RyR and SERCA activities. Simulations show that mdROS bursts increase cytosolic Ca2+ by stimulating RyRs and inhibiting SERCA, which activates the Na+/Ca2+ exchanger, Ca2+-sensitive nonspecific cationic channels, and Ca2+-induced Ca2+ release, eliciting abnormal AP. The morphologies of AP abnormality are largely influenced by the time interval among mdROS burst induction and AP firing, dosage and diffusion of mdROS, and SR-mitochondria distance. This study defines the role of mdROS in Ca2+ overload-mediated cardiac arrhythmogenesis and underscores the importance of considering mitochondrial targets in designing new antiarrhythmic therapies.
Keywords: reactive oxygen species, ryanodine receptors, ROS-induced Ca2+ release, arrhythmias, mitochondria, computational modeling
cardiovascular diseases kill nearly 400,000 Americans each year (41), and many of these deaths are the result of ventricular arrhythmias leading to sudden cardiac arrest. Although arrhythmias are multifactorial and involve a sophisticated interplay among various subcellular systems such as electrophysiology, force generation, and energy metabolism, a defective intracellular Ca2+ cycling has been implicated to play a crucial role in arrhythmogenesis (10, 14, 51). However, in spite of extensive efforts, the detailed molecular mechanisms underlying Ca2+ dysregulation and Ca2+-mediated arrhythmias are not completely understood, hindering the development of effective therapeutic strategies.
As an important universal signaling ion, cardiac Ca2+ is precisely regulated by a complicated system comprising of multiple sarcolemmal and intracellular ion channels and transporters that activate and inactivate in a coordinated fashion during the cardiac cycle (6). Of those channels, the sarcoplasmic reticulum (SR) Ca2+ release channels, also known as ryanodine receptors (RyRs), are the primary Ca2+ release sites. Structural studies have shown that tetrameric RyRs have 89 cysteine residues, of which ∼21 are susceptible to oxidation by free radicals (61). Oxidation of RyR cysteine thiol residues forms disulfide bonds, leading to reversible activation of channel activity. Gen et al. (25) have shown that extracellular H2O2 activates SR Ca2+ release and causes Ca2+ overload in rat ventricular myocytes. Similarly, exogenous H2O2 can directly modify the gating of sheep cardiac RyRs and increase the channel open probability (21). In addition to RyRs, reactive oxygen species (ROS) also affect SR Ca2+ uptake proteins such as SR Ca2+-ATPase (SERCA). It has been shown that H2O2 and superoxide (a.k.a. O2·−) inhibit SERCA activity by directly oxidizing its thiol groups (42, 68) or indirectly interfering with its ATP binding sites (60). Although the effects of exogenous ROS on SR Ca2+ handling proteins are evident (4, 29, 59), study of how endogenous ROS influence Ca2+ homeostasis has begun only recently (49, 62, 63, 66), partially due to the difficulty of inducing controllable endogenous ROS production in the cell in the experimental setting.
There is convincing evidence that mitochondria, the major sites of intracellular ROS production, and SR are in close proximity and physically tethered via mitofusin proteins (12, 19). This unique mitochondria-SR tethering forms a close coupling of Ca2+ signaling between SR release sites and nearby mitochondria, facilitating rapid mitochondrial Ca2+ uptake that stimulates oxidative phosphorylation (11). This coupling is essential for matching energy supply with demand in response to increased workload (39, 52). We speculate that the mitochondria-SR tethering is also involved in the interorganellar redox signaling, allowing dynamic modulation of SR Ca2+ handling by mitochondria-derived ROS (mdROS). In supporting this notion, recent studies have shown that mitochondrial depolarization and associated ROS-induced ROS release (RIRR) are closely correlated to enhanced Ca2+ sparks in resting guinea pig cardiomyocytes (62, 66). In addition, others have reported that mdROS cause significant changes in global Ca2+ transients and regional Ca2+ sparks in smooth muscle cells (13), skeletal muscles (46), and cerebral arteries (58). Given these advancements, how mdROS may influence Ca2+ cycling and electrical activity in paced cardiomyocytes has not been fully elucidated. We hypothesize that the pathological mdROS can diffuse to the proximal SR and dynamically alter SR Ca2+ handling (e.g., RyRs and SERCA), disrupting Ca2+ cycling and causing erratic action potential (AP) generation.
To test the hypothesis, we developed a novel guinea pig cardiomyocyte model that integrates exciation-contraction coupling, Ca2+ handling, and mitochondrial energetics, as well as the modulations of RyRs/SERCA by the surrounding mdROS. The simulations show that mdROS bursts rapidly induce cytosolic Ca2+ ([Ca2+]i) overload by stimulating RyRs and inhibiting SERCA. The elevating [Ca2+]i activates the Na+/Ca2+ exchanger (NCX) and Ca2+-sensitive nonspecific cationic channels (nsCa) that underlie transient inward currents (Iti), further exacerbating Ca2+ dysregulation and leading to APs triggered by the abnormal automaticity.
METHODS
Model Development
In this study, a multiscale guinea pig cardiomyocyte model was developed to examine the effect of mitochondrial oxidative stress on Ca2+ regulation and cellular electrophysiological behaviors. This model was based on our recently published exciation-contraction coupling, mitochondrial energetics and ROS-induced ROS release (ECME-RIRR) model (37, 67) and incorporated new model components including local control Ca2+-induced Ca2+-release (CICR), mitochondria-SR microdomain (MSM), and mdROS modulations of RyRs and SERCA. In a recent study we showed that the mdROS-mediated Ca2+ sparks can be suppressed by TMPyP (a O2·− dismutase mimetic) or 4′-Cl diazepam (the peripheral benzodiazepine receptor ligand that blocks mitochondrial O2·− release) (66), suggesting that mitochondria-derived O2·− (mdO2·−) plays a major role in the dynamic modulation of SR Ca2+ release during RIRR. Thus only the direct effect of mdO2·− on SR Ca2+ handling proteins was considered in the present model development. For simplification, we assumed that all mitochondria in the cardiomyocyte depolarize/oscillate synchronously (1). It is worth noting that such a synchronization may only occur in pathological, rather than physiological, conditions (2). Consequently, the mitochondria were lumped as a giant mitochondrion and mitochondria-SR subspaces were modeled as a single compartment. The schemes of the new whole cell model and the MSM model are shown in Supporting Information Fig. S1 and Fig. S2, respectively (see endnote).
mdO2·− diffusion.
Studies have shown that under pathophysiological conditions, mitochondrial O2·− production increases significantly and upon reaching a threshold triggers the opening of the inner membrane anionic channels (1, 3, 65) and/or mitochondrial permeability transition pore, leading to RIRR (69). We hypothesize that the pathological mdO2·− could diffuse from the perimitochondrial space to the proximal SR, dynamically modulating the redox-sensitive Ca2+ channels. Assuming that MSM is homogeneous, the concentration profiles of mdO2·− in the microdomain ([O2·−]MSM) can be described by the Fick's second law (3):
| (1) |
where DO2·− is O2·− diffusion coefficient, x is the distance from the mitochondrion, vcyto_MSM is cytosol and MSM effective volume ratio, and f([O2·−]MSM(x, t)) is O2·− scavenging rate.
The numerical simulation of Eq. 1 was performed with a finite difference method, whereby the spatial component at x was approximated by the following expression (Supporting Information Fig. S3A):
| (2) |
where Δx is the spatial step size. To reduce computation time, the MSM was discretized as two subcompartments, with one (MSM_SR) adjacent to the peri-SR space and another (MSM_mito) adjacent to the perimitochondrial space (Supporting Information Fig. S3B). Assuming nonflux boundary conditions (i.e., = 0 and = 0, where X is SR-mitochondrion distance), the concentration of mdO2·− in the peri-SR space ([O2·−]SR), and perimitochondrial space ([O2·−]p_mito) can be approximated to equal [O2·−]MSM_SR and [O2·−]MSM_mito, respectively. Consequently, the concentration profile of [O2·−]SR can be described by the following equation (the detailed derivation of the equation can be found in Supporting Information S1):
| (3) |
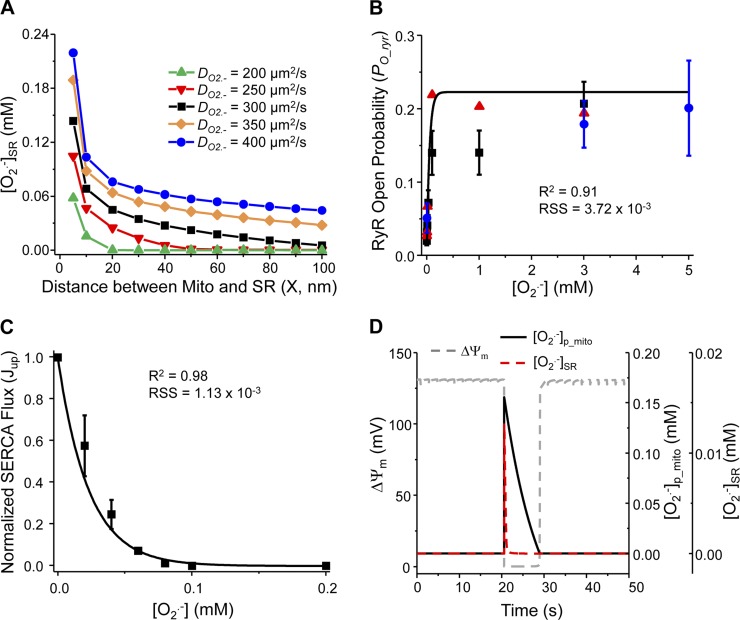
The values of newly added model parameters (e.g., DO2·− and X) were taken from the literature and listed in the Supporting Information (Table S7.16). The effect of diffusion coefficient on mdO2·− concentration in the peri-SR space is plotted in Fig. 1A, which shows that the larger the diffusion coefficient, the higher the SR mdO2·− concentration.
Fig. 1.
Dynamics of mitochondria-derived O2·− (mdO2·−) and its effect on sarcoplasmic reticulum (SR) Ca2+ handling channels. A: model simulated concentration of mdO2·− in the per-SR space ([O2·−]SR) with different diffusion coefficient, DO2·−. B: stimulatory effect of O2·− on ryanodine receptor (RyR) open probability (PO_ryr). Line is the least square curve fit and dots are experimental data [circles from Boraso and Williams (8); triangles and squares from Oba et al. (44, 45)]. C: inhibitory effect of O2·− on SR Ca2+-ATPase (SERCA) Ca2+ uptake (Jup). Line is the least square curve fit and square dots are experimental data taken from Ref. 60. D: model simulated mitochondrial depolarization and associated O2·− burst triggered by increasing the shunt (from 2% to 10%), which shows that [O2·−]p_mito and [O2·−]SR bursts occurred concurrently with mitochondrial depolarization. RSS, residual sum of squares; ΔΨm, membrane potential.
Local Ca2+ control.
To account for the local interaction of L-type Ca2+ channels (LCCs) and RyRs in the dyadic microdomain that controls CICR, we incorporated the coupled 40-state LCC-RyR model developed by Gauthier et al. (24) into the ECME-RIRR model (67) (see Supporting Information S2 for details). Since in the ECME-RIRR model the SR is separated into two compartments (i.e., NSR and JSR) that is different from the Gauthier et al. model, several model equations and parameters were modified (see Supporting Information S3 for details) to better fit the simulated current-voltage (I– V) relationship and L-type Ca2+ current (ICaL) trace during steady-state AP with experimental data (Supporting Information Fig. S4). It is worthy to note that the values of the peak current of ICaL were different among experiments, which might be caused by the variance of channel density (24). In our model, the number of LCC [i.e., the number of Ca2+ release unit (NCaRU)] was set to 300,000, which is within the range of experimental measurements [from ∼276,000 (7) to ∼500,000 (27)]. Other model parameters were the same as those used in the previous model (24). The modified ECME-RIRR model showed a ∼37% decrease of SR Ca2+ content during a normal AP cycle, which is comparable to that reported in the previous studies (∼35%) (5, 24).
ROS and RyR Ca2+ release.
Experimental studies have shown that ROS exponentially increase RyR open probability (PO_ryr), with the enhancement effect determined by both the ROS concentration and the state of the channel: when PO_ryr is low, the enhancing effect of ROS is dramatic, but when PO_ryr is already high, the stimulatory effect of ROS is much less significant. Consequently, the RyR open probability in the presence of ROS (PO_ryr_ROS) can be described by an exponential equation, which is a function of [O2·−]SR and PO_ryr:
| (4) |
where cryr is ROS enhancement coefficient and kryr is effective factor. The values of these parameters (0.20 and 19.55 mM−1, respectively) were obtained by the least-square curve fitting using experimental data from the literature (8, 44, 45) (Fig. 1B). The enhancement of ROS on PO_ryr was incorporated into the RyR Ca2+ release formula (Supporting Information S4).
ROS and SERCA Ca2+ uptake.
Unlike RyRs, SERCA Ca2+ uptake (Jup; Supporting Information Eq. S6.E47) is exponentially inhibited by ROS and the effect can be described by:
| (5) |
where cSERCA (= 1.02) is the inhibition coefficient and kSERCA (= 43.67 mM−1) is the effective factor of ROS inhibition. These values were obtained using the least-square curve fitting method. The experimental data used for parameter optimization is from Refs. 36, 60, and the fitting result is shown in Fig. 1C. Equation 5 (Jup_ROS) was then added back to ECME-RIRR model to replace Jup in Eq. S6.E47 (Supporting Information).
Simulation Protocol
The model formulations and parameters of other processes (e.g., ion currents and metabolic reactions) were the same as those in the ECME-RIRR model (67) and CICR model (24) unless otherwise indicated (see Supporting Information S6 and S7). The whole cell model was coded in C++ (Visual Studio; Microsoft, Redmond, WA). The nonlinear ordinary differential equations were integrated numerically with CVODE as described previously (67).
Before examining the effect of mdO2·− on SR Ca2+ handling and cellular electrophysiology, the behavior of a paced (0.5 Hz) cardiomyocyte was simulated under physiological conditions (i.e., mdO2·− production was at physiological level). The obtained steady-state values were then fed into the model as initial conditions (Supporting Information Table S7.17) for all runs in the subsequent simulations. Mitochondrial depolarization (and associated mdO2·− burst) was induced by increasing the fraction of O2·− production from the electron transport chain (a.k.a. shunt) from 2 to 10% as described previously (67). To systematically examine the effect of mdO2·− on Ca2+ handling and AP generation, the mdO2·− burst was induced at different time during the AP cycle (e.g., phase 2 or 4). In some subset simulations, the extent of mdO2·− production or the distance between mitochondria and SR was altered to examine its effect on the mdROS-mediated Ca2+ transient and AP alterations. APD90 was defined as the interval between the time of the maximum upstroke velocity of the AP, [dV/dt]max, and 90% repolarization.
RESULTS
Model Validation
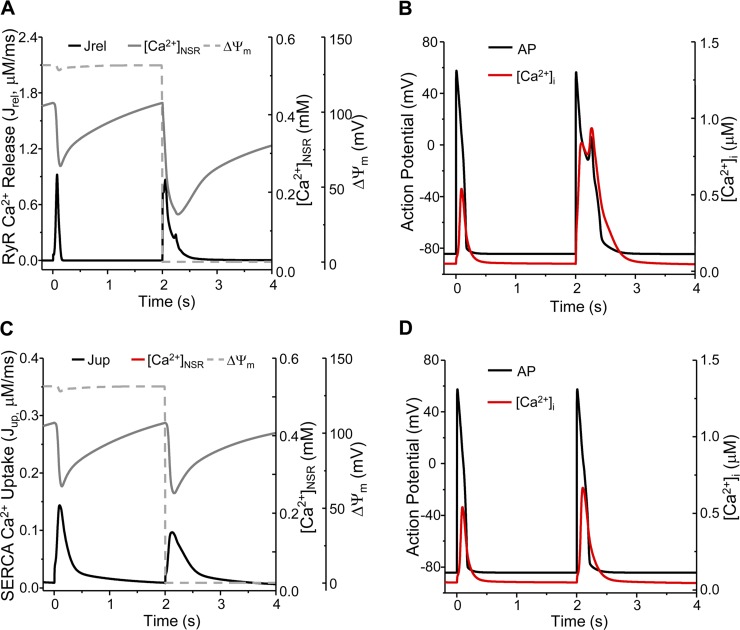
After parameters were optimized, the new/modified model modules were incorporated into the ECME-RIRR model. We first simulated the dynamics of mitochondrial energetics under stress conditions. Increasing shunt from 2 to 10% triggered sustained mitochondrial oscillations including membrane potential (ΔΨm) and mdO2·− production (Supporting Information Fig. S5), as well as NADH oxidation and reduced glutathione depletion (data not shown) in a paced cardiomyocyte (0.5 Hz). These simulations were consistent with our previous experimental and computational studies (1, 17, 65, 67), suggesting that the addition of new model components (e.g., mdO2·− modulation of RyRs and local Ca2+ control) did not influence the dynamics of the existing model subsystems. Simulations also showed that [O2·−]p_mito and [O2·−]SR bursts occurred concurrently with ΔΨm depolarization during each oscillation cycle (see Fig. 1D for enlargement). Thus, for better visualization, ΔΨm depolarization was plotted to represent the O2·− burst in some figures.
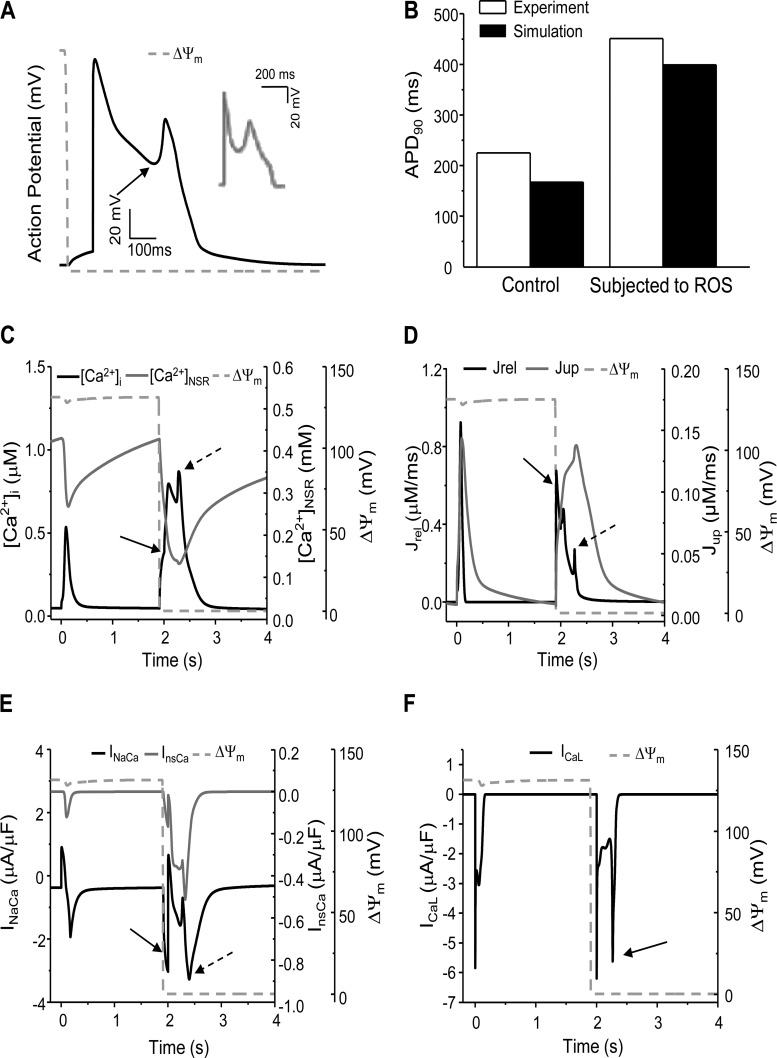
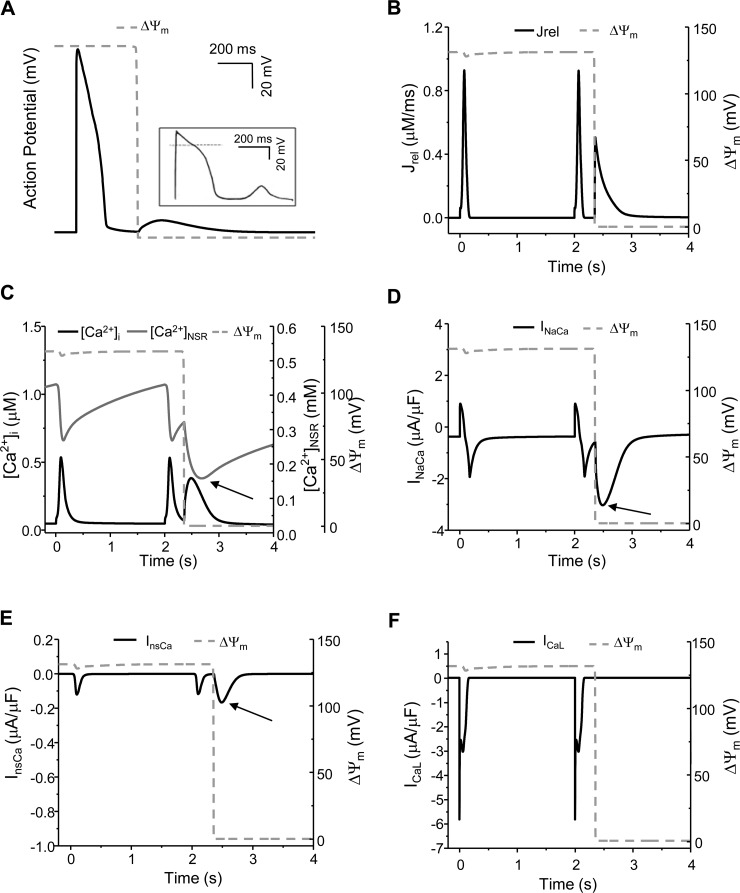
The model was further validated by simulating mdROS-mediated abnormal APs and comparing the results with experimental data. Due to the lack of experimental studies on the direct effect of mdROS on APs in guinea pig cardiomyocytes, the comparisons were made between model simulations and data obtained from isolated rabbit cardiomyocytes exposed to external oxidative stress. A mdO2·− burst induced before AP firing led to an early afterdepolarization (EAD; Fig. 2A) similar to that observed in a cardiomyocyte subject to H2O2 perfusion (Fig. 2A, inset) (59), including the ∼2.5-fold APD90 prolongation (Fig. 2B). The mdO2·− burst also caused significant increase in Ca2+ transient (1.63-fold higher than that of normal AP cycles) and reduction in SR Ca2+ storage (∼69%; Fig. 2C). The mdO2·− burst-induced [Ca2+]i elevation was comparable to that reported in a recent experimental study (57). In addition, a mdO2·− burst induced during phase 4 of the AP cycle elicited a delayed afterdepolarization (DADs) that was also observed in the H2O2 perfusion experiments (Fig. 3A, inset) (59). These validations, although semiquantitatively, demonstrated the utility and capability of our model in examining the interaction between mitochondrial energetics and cellular electrophysiology.
Fig. 2.
Model-simulated mdO2·− burst-induced early afterdepolarization (EAD) in a “beating” (0.5 Hz) cardiomyocyte. A: mitochondria-derived reactive oxygen species (mdROS) burst-elicited EAD (inset: EAD observed in a rabbit cardiomyocyte subjected to ROS perfusion; Ref. 59). B: comparison of APD90 between model simulation (guinea pig) and experimental data (rabbit) under control and oxidative stress conditions {where APD90 is the interval between the time of the maximum upstroke velocity of the action potential (AP), [dV/dt]max, and 90% repolarization}. C– F: model simulated intracellular Ca2+ concentration ([Ca2+]i) and SR Ca2+ concentration ([Ca2+]SR) (C), RyR Ca2+ release (Jrel), and SERCA Ca2+ uptake (Jup) (D); Na+/Ca2+ exchanger current (INaCa) and Ca2+-sensitive nonspecific cationic current (InsCa) (E); and ICaL (F) before and after a mdROS burst. The solid arrow in A represents the AP reversal; in C, D, and E, the [Ca2+]i elevation, SR Ca2+ release, and activations of INaCa and InsCa caused by the mdO2·− burst, respectively; in F, the L-type Ca2+ current (ICaL) reactivation. The dashed arrow in C, D, and E represents the second [Ca2+]i elevation, SR Ca2+ release, and activations of INaCa and InsCa caused by ICaL reactivation, respectively. The dashed lines represent ΔΨm and its depolarization represents the acute mdO2·− burst.
Fig. 3.
Model-simulated mdO2·− burst-induced delayed afterdepolarizations (DAD) in a “beating” (0.5 Hz) cardiomyocyte. A: mdROS burst-induced DAD (inset: DAD observed in a cardiomyocyte perfused by external H2O2; Ref. 59). B– F: model simulated SR Ca2+ release (Jrel) (B), [Ca2+]i and [Ca2+]SR (C), INaCa (D), InsCa (E), and ICaL (F) before and after the induction of a mdROS burst. The dash lines represent ΔΨm and its depolarization represents the acute mdO2·− burst. The arrow in C indicates the mdO2·− burst-induced SR Ca2+ depletion and in D and E represents activation of INaCa and InsCa.
Effect of mdO2·− on Ca2+ Handling Channels
We next examined the ionic mechanisms underlying the mdO2·−-induced EAD or DAD described above. When a mdO2·− burst was induced before AP firing, the activated Jrel (Fig. 2D, black line, solid arrow) and inhibited Jup (Fig. 2D, gray line) caused not only [Ca2+]i elevation but also [Ca2+]SR reduction (Fig. 2C, solid arrow). The accumulated cytosolic Ca2+ subsequently enhanced the Na+/Ca2+ exchanger current (INaCa) in the forward mode (Fig. 2E, black curve, solid arrow) and the Ca2+-sensitive nonspecific cationic current (InsCa) (Fig. 2E, gray curve), eliciting Iti that caused the reduction of AP repolarization reserve (Fig. 2A, arrow). Intriguingly, the transient reversal of AP repolarization reactivated the ICaL (Fig. 2F, arrow), which triggered a second CICR (Fig. 2D, dashed arrow), causing a further [Ca2+]i elevation and [Ca2+]SR depletion (Fig. 2C, dashed arrow). The resultant Ca2+ overload caused further INCX and InsCa activation (Fig. 2E, dashed arrow), which eventually elicited an EAD (Fig. 2A).
In the case of DAD (Fig. 3A, inset), the mdO2·−-activated Jrel (Fig. 3B) evoked an extra Ca2+ transient, which was slightly smaller than that of normal AP cycles (Fig. 3C). The enhanced RyR Ca2+ release also caused a significant SR Ca2+ depletion (Fig. 3C, arrow). Consequently, both INaCa and InsCa were enhanced (Fig. 3, D and E, arrow), which induced Iti that underlay the DAD. However, ICaL was not reactivated since the membrane potential was low (Fig. 3F).
Effect of Timing of mdROS Burst Induction on AP Morphology
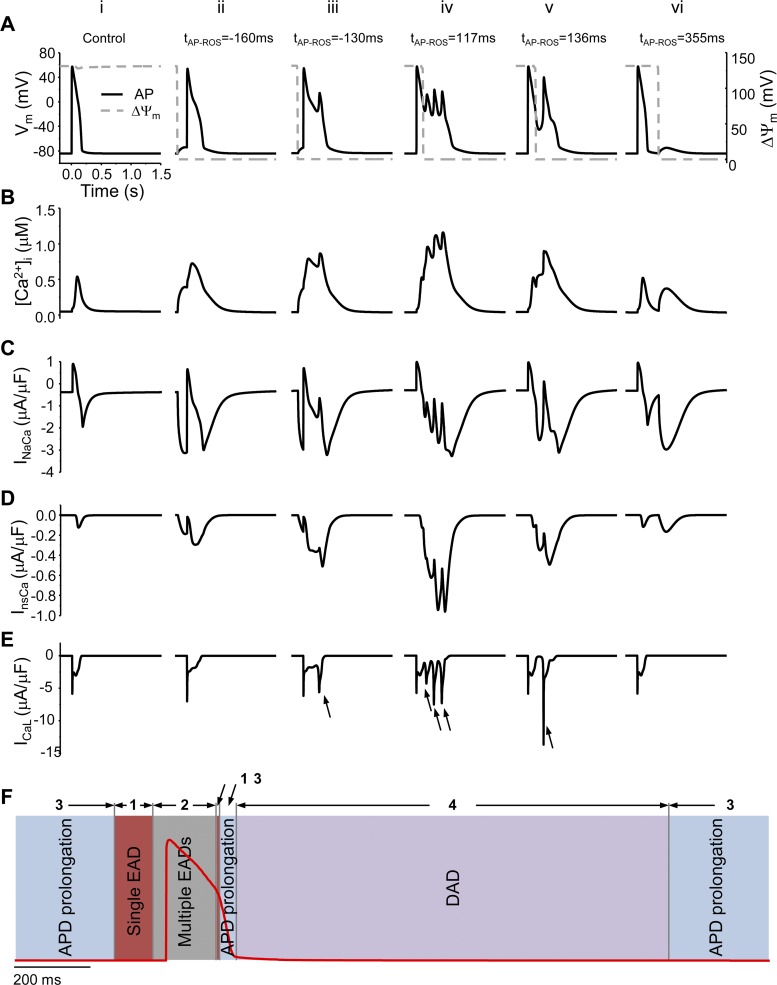
Simulations shown in Figs. 2 and 3 suggest that the pattern of mdO2·−-induced AP abnormality is dependent on the timing of mdO2·− burst induction during a AP cycle or the time interval between the firing of AP and the burst of mdO2·− (tAP-mdROS). To better understand the ionic mechanisms underlying the mdO2·−-induced AP abnormality, we analyzed the correlation between tAP-mdROS and the morphology of mdO2·−-mediated AP.
Figure 4Ai shows the control AP (i.e., mdO2·− production was at physiological level). The mdO2·− burst induced before AP firing (e.g., tAP-mdROS = −160 ms) caused APD90 prolongation (from 167 to 274 ms) (Fig. 4Aii). A mdO2·− burst began to elicit EADs when tAP-mdROS gradually increased. The mdROS burst induced at tAP-mdROS = −130 ms caused a single EAD (Fig. 4Aiii), and a delayed mdO2·− burst (e.g., tAP-mdROS = 117 ms) elicited multiple EADs (Fig. 4Aiv). The multiple EADs degraded into a single EAD (Fig. 4Av) and then APD prolongation (data not shown) when the occurrence of mdO2·− burst was further postponed to phase 3 of AP. Finally, when mdO2·− burst was induced after the AP was fully repolarized (e.g., tAP-mdROS = 355 ms), a DAD (Fig. 4Avi) ensued.
Fig. 4.
Effect of tAP-mdROS (the time interval between mdO2·− burst induction and AP firing) on the mdO2·−-induced [Ca2+]i and AP abnormality. i: control; ii: tAP-mdROS = −160 ms; iii: tAP-mdROS = −130 ms; iv: tAP-mdROS = 117 ms; v: tAP-mdROS = 136 ms; and 6) tAP-mdROS = 355 ms. A– E: AP, [Ca2+]i, INaCa, InsCa, and ICaL, respectively. The arrows in Eiii-Ev represent ICaL reactivation(s). F: summary of the effect of tAP-mdROS on mdO2·− burst-induced AP abnormality: a mdO2·− burst induced in zone 1 (−135 to −37 and 136 to 140 ms) elicited a single EAD, in zone 2 (−36 to 135 ms) elicited multiple EADs, in zone 3 (147 to 179 ms and −680 to −136 ms) elicited APD prolongation, and in zone 4 (the remaining time window) elicited a DAD. The dash lines represent ΔΨm and its depolarization represents the acute mdO2·− burst.
The dynamics of [Ca2+]i and Ca2+ currents were then analyzed to explore the ionic mechanism accounting for the variance of the mdO2·− burst-induced APs. When tAP-mdROS = −160 ms (Fig. 4Aii), a mdO2·− burst caused a moderate [Ca2+]i increase (Fig. 4Bii), which activated INaCa (Fig. 4Cii) in the forward mode and enhanced InsCa (Fig. 4Dii). The resultant Iti caused a small AP depolarization and prolongation of APD90 (Fig. 4Aii) and ICaL (Fig. 4Eii) but could not reverse AP repolarization.
When tAP-mdROS = −130 ms, the mdO2·− burst gradually raised [Ca2+]i (Fig. 4Biii), which augmented INaCa and InsCa (Fig. 4, Ciii and Diii) and evoked Iti, which reduced AP repolarization reserve, reactivated ICaL (Fig. 4Eiii), and elicited an EAD (Fig. 4Aiii). The reactivated ICaL subsequently triggered CICR and led to further increases of [Ca2+]i and augmentation of Iti. Further postponing the induction of mdROS (e.g., tAP-mdROS = 117 ms; Fig. 4Aiv) led to a larger Ca2+ transient, which enhanced INaCa and InsCa (Fig. 4, Biv-Div). The resultant large Iti activated ICaL repetitively (Fig. 4Eiv), generating an EAD with multiple phases (Fig. 4Aiv).
When the mdROS was induced in phase 3 (e.g., tAP-mdROS = 136 ms) of the AP cycle, the mdO2·− burst-induced Ca2+ elevation (Fig. 4Bv) and INaCa and InsCa enhancements (Fig. 4, Cv and Dv) could only trigger a single EAD (Fig. 4Av). As the cell membrane voltage was low when ICaL was reactivated, the amplitude of reactivated ICaL was larger (Fig. 4Ev, arrow) than that evoked in the previous cases (Fig. 4, Eiii and Eiv). This is consistent with the experimental result showing that a more repolarized AP produced larger EAD amplitude (59). Further delay of the mdROS burst in phase 3 diminished mdO2·− burst-induced Ca2+ elevation and induced APD prolongation (data not shown).
When the mdO2·− burst was induced in phase 4 of the AP cycle (Fig. 4Avi), the mdO2·− burst-induced SR Ca2+ release generated a second Ca2+ transient (Fig. 4Bvi). In this case, the gradual AP depolarization induced by INaCa and InsCa activations (Fig. 4, Cvi and Dvi) slightly depolarized the membrane potential and evoked a DAD (Fig. 4Avi). However, ICaL was not activated (Fig. 4Evi). The relationship between tAP-mdROS and mdO2·− burst-induced AP abnormality is summarized in Fig. 4F, with zones 1–4 representing single EAD, multiple EADs, APD prolongation, and DADs, respectively.
Effect of mdO2·−-Mediated RyR Activation or SERCA Inhibition Alone on Ca2+ Cycling and AP
We next examined how mdO2·− could affect Ca2+ transients and AP via modulating solely the activity of RyRs or SERCA. A mdO2·− burst induced during AP firing, when modeled to activate RyR Ca2+ release solely, caused a substantial [Ca2+]SR reduction (∼65%) (Fig. 5A) and a significant [Ca2+]i elevation (∼1.7-fold in amplitude and ∼2.3-fold in duration) (Fig. 5B). The elevated Ca2+, as we expected, caused an EAD (Fig. 5B) similarly to that shown in Fig. 2A in which the mdO2·− burst affected both RyRs and SERCA. The mdO2·− burst-induced RyR activation itself, however, could not elicit multiple EADs regardless the timing of mdROS burst induction during the AP cycle (data not shown).
Fig. 5.
Model simulated effect of mdO2·− burst-mediated RyR activation or SERCA inhibition alone on SR Ca2+ content (A and C) and AP/[Ca2+]i morphology (B and D). In simulations shown in A and B, mdO2·− burst had no effect on SERCA activity, and in C and D mdO2·− burst had no effect on RyR activity. The dash line is ΔΨm and its depolarization represents the acute mdO2·− burst.
The effects of mdO2·− burst on Ca2+ transients and AP were much less evident when it was modeled to modulate SERCA only. Particularly, the mdO2·− burst-inhibited SERCA Ca2+ uptake (Jup, from ∼0.144 to ∼0.097 mM/s) led to a small [Ca2+]SR reduction (Fig. 5C) and a modest [Ca2+]i increase (Fig. 5D). In this case, the mdO2·− burst could only cause APD prolongation (from 167 to 207 ms in Fig. 5D), implying that the mdO2·−-mediated SERCA inhibition had a smaller effect on AP than mdO2·−-mediated RyR activation in our model.
Effect of Shunt or Mitochondria-SR Distance on AP Morphology
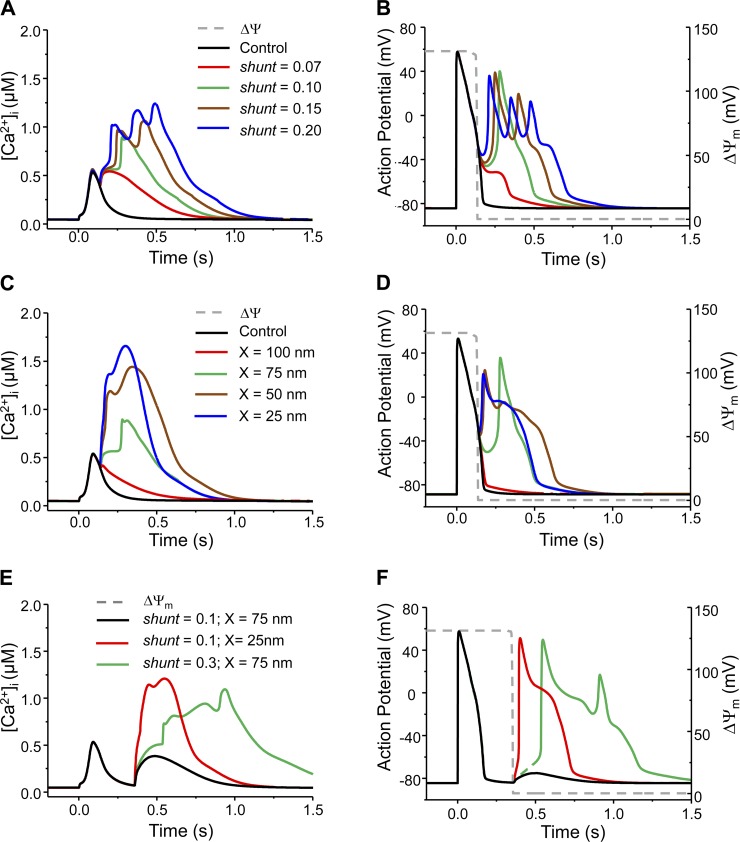
In addition to mdO2·− induction timing, the pattern of mdROS-mediated abnormal AP was also affected by the concentration of [O2·−]SR, which was determined by mdO2·− production rate (shunt) and mitochondria-SR distance (X) (or diffusion coefficient). Specifically, increasing shunt enhanced mdO2·− burst-induced Ca2+ elevation (Fig. 6A), promoting earlier occurrence of EAD, and potentially shifting APD prolongation to EAD and single EAD to multiple EADs (Fig. 6B). Reducing X had the similar effect: the shorter the distance, the stronger the effects of mdO2·− on Ca2+ and AP (Fig. 6, C and D). It is worth noting that the Ca2+ transient became “nonphysiologically” large when O2·− production was very high (e.g., shunt = 20%) or mitochondria-SR distance was very short (e.g., X = 25 nm) (Fig. 6, A and C). Under those extreme conditions, mdO2·− caused dramatic SR Ca2+ depletions compared with those under normal conditions (76–85 vs. ∼37%). In the case when mdO2·− burst was induced in phase 4 of the AP cycle, increasing shunt (from 10 to 30%) or reducing X (from 75 to 25 nm) significantly exaggerated Ca2+ elevation (Fig. 6E). The resultant large Iti depolarized the membrane potential to the threshold for INa activation, leading to triggered activities (Fig. 6F). Increasing mdO2·− diffusion coefficient (DO2·−) similarly enhanced the effects of mdO2·− burst on Ca2+ transients and APs (Supporting Information Fig. S6).
Fig. 6.
Model simulated effect of mdO2·− production rate (shunt) or mitochondrion-SR distance (X) on the mdO2·−-mediated Ca2+ transient and AP alteration. A and B: increasing shunt caused higher Ca2+ elevation and converted normal AP to APD prolongation, single EAD and eventually multiple EADs. In these simulations, X = 75 nm. C and D: decreasing X had a similar effect on Ca2+ elevation and AP as increasing shunt. In these simulations, shunt = 0.10. E and F: effects of shunt and/or X on the phase 4 mdO2·− burst-induced Ca2+ elevation and AP abnormality. Increasing shunt from 10 to 30% or reducing X from 75 to 25 nm caused extraordinary increase of [Ca2+]i and translated DAD to triggered activity. The dash gray lines represent ΔΨm and its depolarization represents the acute mdO2·− burst.
Effect of Blocking Ca2+ Handling Channel on mdROS-Induced EADs
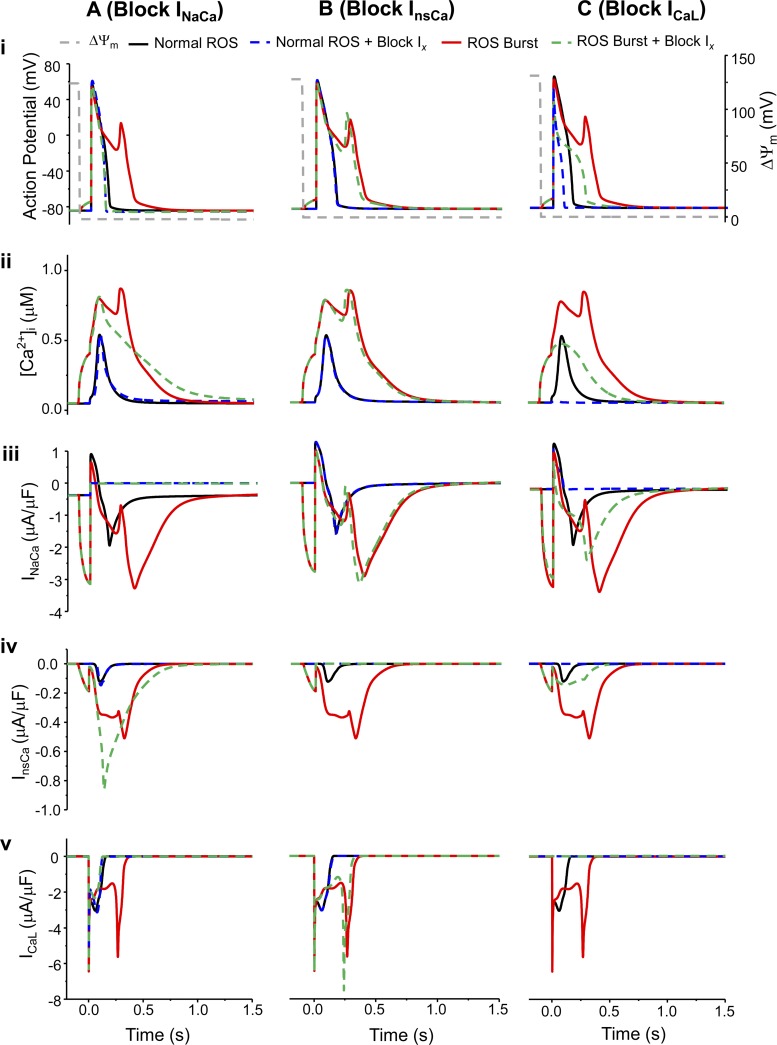
Finally, we examined the effect of blocking individual Ca2+ handling channels on the mdROS-induced AP abnormality. Under normal mdO2·− production conditions, blocking INaCa had minimal effects on both AP and [Ca2+]i (Fig. 7, Ai and Aii). In the presence of pathological mdO2·− bursts, the blockade of INaCa (Fig. 7Aiii) inhibited Iti in spite of enhanced InsCa activation (Fig. 7Aiv), which suppressed ICaL reactivation (Fig. 7Av). The lack of the subsequent CICR lowered Ca2+ elevation (Fig. 7Aii) and abolished the EAD (Fig. 7Ai). However, since the Ca2+ extrusion via INaCa was blocked, the duration of Ca2+ transient was prolonged (Fig. 7Aii).
Fig. 7.
Model simulated effects of blocking INaCa (A), InsCa (B), or ICaL (C) on AP (i), Ca2+ transient (ii), INaCa (iii), InsCa (iv), and ICaL (v) under control and mdO2·− burst conditions. In the legend, Ix represents INaCa (Ai-Av), InsCa (Bi-Bv), or ICaL (Ci-Cv), respectively. The dash gray lines represent ΔΨm and its depolarization represents the acute mdO2·− burst.
Blocking InsCa did not significantly affect AP (Fig. 7Bi) and Ca2+ transient (Fig. 7Bii) no matter whether there was a pathological mdO2·− burst. Particularly, blocking InsCa slightly shortened the APD (Fig. 7Bi) and shifted the second Ca2+ transient to the left by ∼10 ms (Fig. 7Bii). However, blocking InsCa slightly reduced Iti (Fig. 7Biv), shifted the ICaL reactivation to a more polarized AP, and therefore increased the amplitude of the reactivated ICaL (Fig. 7Bv).
Experimental studies have demonstrated that blocking LCC eliminates the oxidative stress-induced EADs and causes remarkable APD prolongation (59). Our simulations showed the similar results: the mdO2·−-induced EAD was suppressed but APD remained prolonged when ICaL was blocked (Fig. 7Ci, dashed green curve). The prolonged APD was mainly caused by the mdO2·−-induced RyR activation and SERCA inhibition, which resulted in a relatively small but prolonged [Ca2+]i elevation (Fig. 7Cii). However, blockade of ICaL (Fig. 7Cv) hindered the subsequent CICR and prevented the further Ca2+ elevation. Consequently, INaCa and InsCa enhancements were suppressed (Fig. 7, Ciii and Civ) and the Iti was too small to elicit an EAD. The outcomes of ICaL blockade in the absence of mdROS bursts were shortened APD and abolished AP plateau (Fig. 7Ci) as well as diminished Ca2+ transient (Fig. 7Cii), which were consistent with previous studies (16, 23).
DISCUSSION
In this study, a novel multiscale guinea pig cardiomyocyte model was developed, which incorporates mitochondrial energetics, excitation-contraction coupling, local Ca2+ control, and RIRR, as well as mdROS-mediated modulations of RyR and SERCA activities. This model, for the first time, provides a computational framework to quantitatively examine how mdROS may influence cardiomyocyte Ca2+ regulation and electrophysiological behaviors under oxidative stress. Our main findings are 1) mdO2·−-induced AP abnormality involves alterations of multiple Ca2+ handling channels in a coordinated fashion, including RyRs, SERCA, InsCa, INaCa, and ICaL; 2) the variance of mdO2·− burst-induced AP abnormality largely depends on the time interval between its induction and AP firing; and 3) the mdO2·−-induced AP abnormality is also influenced by mdO2·− dosage and/or mitochondria-SR distance.
It has been shown that the excessive O2·− produced by a stressed mitochondrion can diffuse to its immediate neighbors and activate their inner membrane anion channels, triggering RIRR in a self-amplifying mode (70). The present work suggests that if mdO2·− could travel to the proximal SR, they may activate the redox-sensitive RyRs, triggering ROS-induced Ca2+ release (RICR). This RICR alone, or together with inhibition of SERCA-mediated SR Ca2+ uptake, evoked erratic APs similar to those observed in cardiomyocytes exposed to H2O2 perfusion (59). Importantly, we showed that the mdO2·−-mediated SERCA inhibition had smaller effects on Ca2+ cycling and AP generation than the mdO2·−-mediated RyR activation, implying that enhanced RyR Ca2+ release might play a major role in the mdROS-induced cardiac arrhythmias. It is worth noting that in our simulations the inhibitory effect of mdO2·− on SERCA is transient. Sustained SERCA inhibition, such as that induced by continuous H2O2 perfusion, would have a much more prominent effect on Ca2+ handling. Intriguingly, while the proarrhythmic effect of mdO2·− burst-induced SERCA inhibition alone was minor, it significantly exacerbated the effect of RyR activation on AP. Particularly, concurrent SERCA inhibition shifted the RyR activation-induced APD prolongation to a single EAD and the single EAD to multiple EADs. These model simulations endorse the antiarrhythmic effect of increasing SERCA2a gene expression in heart failure treatment (18, 20).
Another important finding is that mdO2·− burst can elicit various patterns of AP abnormality such as APD prolongation, single EAD, and multiple EADs and DAD, similar to those observed in experimental studies (59). Our analysis suggested that mdO2·− bursts elicit erratic APs by triggering the following events in a coordinated way: 1) the mdO2·− burst activates RyR Ca2+ release and inhibits SERCA Ca2+ uptake, resulting in a [Ca2+]i increase; 2) Ca2+ accumulation enhances INaCa in the forward mode and activates InsCa, evoking Iti; 3) Iti reduces AP repolarization reserve, reactivating ICaL that triggers CICR and causes further [Ca2+]i elevation; and 4) the resulting large [Ca2+]i increment augments INaCa and InsCa, eliciting larger Iti, which may repetitively reactivate ICaL. Depending on the timing (tAP-mdROS) of mdROS bursting, mdROS dosage, and mitochondria-SR distance, one or more of these events would not be activated, or be activated at different levels, resulting in various Ca2+ transient and AP morphologies (Fig. 4F). This paradigm is strengthened by the Ca2+ current inhibition studies, which showed that blocking INCX or ICaL significantly altered the pattern of mdO2·− burst-induced AP abnormality. These finding are highly significant, as under pathological conditions such as ischemia-reperfusion mitochondrial depolarization and associated ROS bursts may occur asynchronously in various cells, resulting in regional electrical heterogeneity that increases the propensity for arrhythmogenesis.
Some studies suggested that direct ROS activation of ICaL is a primary cause of oxidative stress-induced EADs (59), while others argued that the effects of ROS on ICaL are controversial (9, 30, 50, 55). Notably, we showed that ICaL were indirectly activated during mdROS bursts such as in the cases of single and multiple EADs (Fig. 4, Eiii and Eiv). Further analyses revealed that whether or not ICaL can be reactivated was largely determined by two factors: 1) the amplitude of mdO2·− burst-induced SR Ca2+ release, which must be large enough to enhance INaCa and InsCa so that the resultant Iti can reverse AP repolarization, and 2) the membrane potential when the reversal occurred, which needs to be in the range where ICaL can be activated. The latter factor also determined the amplitude of the reactivated ICaL, which was in agreement with previous experimental studies (59). Our simulations also suggested that when mdROS amplitude and duration are sufficiently large, ICaL can be reactivated repeatedly, producing multiple EADs. These types of substantial Ca2+ transients (and EADs) may be seen in real life in more extreme situations such as apoptotic conditions, when multiple aspects of the cellular homeostasis are changed significantly and irreversibly.
Transient inward currents (Iti) have been known to trigger EADs or DADs, eliciting Ca2+ overload-mediated arrhythmias. However, the contribution of InsCa to Iti is controversial and appears to vary with different cell types (26, 28, 31, 32, 34, 47). Ca2+-sensitive nonspecific cationic channels (nsCa) belong to the “transient receptor potential” protein family and are expressed in both excitable and nonexcitable mammalian cells (26). In human atrial myocytes InsCa was shown to contribute to the genesis of arrhythmias during Ca2+ overload (34). However, the situation in ventricular myocytes is more controversial. Several groups have shown that InsCa could be activated by extracellular oxidative stress in guinea pig ventricular myocytes (31, 32); but in rabbit ventricular myocytes, Pogwizd et al. (48) showed that the contribution of InsCa to Iti was insignificant in both control and failing cells. This may be due to the low expression of those cationic channels in ventricular myocardium (34). In our simulations, while blocking INaCa completely suppressed the mdROS burst-induced EAD (Fig. 7Ai), the blockade of InsCa had only minor effects on the morphologies of AP and Ca2+ transients. The results suggested that the contribution of InsCa to the mdROS burst-induced AP abnormality was smaller than that of INaCa, which is consistent with the observations of Pogwizd et al. (48). The differences in the contributions of these currents to Iti under stress conditions are also in agreement with their roles in shaping AP under physiological conditions. One limitation of our study is that InsCa might be activated directly by oxidative stress in guinea pig ventricular myocytes (31, 32) but this effect is not considered in the present model. Nevertheless, our simulations underscore the importance of targeting the appropriate Iti component in the treatment of oxidative stress-mediated cardiac arrhythmias.
It is well appreciated that mitochondrial dysfunction inhibits cell contraction. However, our simulations showed that the force of contraction was augmented during mitochondrial depolarization and ROS burst (Supporting Information Fig. S7). This paradox could be attributed to several factors: 1) Kohasi et al. (33) showed that enhanced ROS production could directly inhibit cardiomyocyte contraction; however, this effect is not included in the present model; and 2) it is known that ATP depletion directly inhibits cell contraction; however, the reduction of ATP during mitochondrial depolarization is very small in our simulations (∼3%) (Supporting Information Fig. S7). Under more stressed conditions such as higher pacing frequency or sustained mitochondrial dysfunction, the concentration of ATP may decline substantially, inhibiting SERCA and contraction (67).
Model Limitations and Future Directions
One of the major limitations is that the diffusion of mdROS in MSM cannot be validated by experimental study due to the lack of methods to measure ROS in such small domains. In addition, the present model does not consider the effects of mitochondrial-derived H2O2 or other oxidizing species. Our simplification is based on the experimental observations that the mdO2·− scavenger significantly suppressed enhanced SR Ca2+ release during RIRR. However, these results cannot completely exclude the effect of H2O2 as O2·− can be readily dismutated to generate H2O2. Whether it is O2·−, H2O2, or both that are responsible for the enhanced Ca2+ release deserves further investigations. Compared with O2·−, H2O2 is more stable and can diffuse further, thus affecting ion channels not only on the proximal SR membrane but also on the sarcolemmal membrane. In this context, various studies have shown that H2O2 can directly modulate ICaL, although the effect is controversial (or dependent on H2O2 concentration) (54–57). Furthermore, ROS can affect INa either directly (38) or indirectly by activating CaMKII (22, 53, 54). The ROS-mediated CaMKII activation can also enhance SERCA activity via phospholamban phosphorylation (40), counteracting the effect of ROS-induced SERCA inhibition. Other redox-sensitive ion channels include K+ channels (Kir and Kv) and the Na+/Ca2+ exchanger (NCX) (4, 15, 35, 43, 55, 68). It is expected that adding the effects of H2O2 on these sarcolemmal ion channels would exacerbate the influence of mdROS and lead to more complex AP morphologies as observed experimentally (59). Nonetheless, the lack of mdROS-induced ICaL, CaMKII, and/or INa modulations would not impact our main conclusions about the mechanisms underlying mdROS-induced Ca2+ dysregulation and abnormal APs. Indeed, the lack of mdROS-mediated membrane channel remodeling allowed us to focus on the crucial role of mdROS on SR Ca2+ handling and genesis of EADs and DADs under oxidative stress conditions. Nevertheless, the effects of mdROS on these proteins should be incorporated into the model when more experimental data become available.
Moreover, we assumed that all mitochondria in the cardiomyocyte depolarize and release ROS simultaneously so that we can focus on the ionic mechanisms underlying mdROS burst-induced alterations in Ca2+ cycling and cellular electrophysiology. In a real cardiomyocyte, the rate of ROS increase might be slower due to the heterogeneity of mitochondrial energetic state and network ultrastructure. The chronic, sustained mdROS increase may deplete SR Ca2+ loading if it spans over several beats or inhibits RyRs and ICaL activities if the dose is too high. Apparently, systematic experimental studies will be needed to gain a more complete understanding of how mdROS may exactly influence intracellular ion handling under certain pathological conditions. However, again these limitations are not expected to alter the mechanisms that underlie the mdROS-induced abnormal Ca2+ cycling and AP generation unraveled here.
Finally, a recent study showed that carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP)-induced mitochondrial depolarization increased the frequency and amplitude of Ca2+ waves and induced triggered activities, probably via the mitochondrial Ca2+ efflux mediated by the mitochondrial permeability pore (mPTP) (64). Since the present model did not incorporate mPTP, it cannot be used to examine this mechanism and study the interaction between the ROS-modulated and mPTP Ca2+ efflux-mediated cytosolic Ca2+ dynamics.
In summary, the developed multiscale model is capable of simulating the acute effect of mdROS on Ca2+ cycling, providing a novel tool to examine how alterations in mitochondrial energetics can impact cardiomyocyte electrophysiology and electrical activity. The results highlight the role of mdROS in Ca2+ overload-mediated cardiac arrhythmogenesis and abnormal automaticity. They also underscore the importance of considering mitochondrial targets in designing new antiarrhythmic therapies in the context of sudden cardiac death.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R00-HL-095648 (to L. Zhou), R01-HL-101235, and R37-HL-054598 (to B. O'Rourke) and American Heart Association Grant GRNT8000059 (to S. M. Pogwizd).
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of one of the authors, which at the time of publication they indicate is http://www.uab.edu/medicine/cscb/images/paper_pdf/Li_et_al_Supporting_Information.pdf. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.L., D.S., and L.Z. performed experiments; Q.L., D.S., and L.Z. analyzed data; Q.L., B.O., S.M.P., and L.Z. interpreted results of experiments; Q.L. and L.Z. prepared figures; Q.L., D.S., and L.Z. drafted manuscript; B.O., S.M.P., and L.Z. edited and revised manuscript; L.Z. conception and design of research; L.Z. approved final version of manuscript.
REFERENCES
- 1.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aon MA, Cortassa S, O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol 641: 98–117, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci USA 101: 4447–4452, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrington PL, Meier CF Jr, Weglicki WB. Abnormal electrical activity induced by free radical generating systems in isolated cardiocytes. J Mol Cell Cardiol 20: 1163–1178, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 268: C1313–C1319, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM, Stiffel VM. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol Cell Physiol 264: C1587–C1593, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Boraso A, Williams S. Modification of the gating of the cardiac sarcoplasmic reticulum Ca2+-release channel by H2O2 and dithiothreitol. Am J Physiol Heart Circ Physiol 267: H1010–H1016, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Cerbai E, Ambrosio G, Porciatti F, Chiariello M, Giotti A, Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation 84: 1773–1782, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Chelu MG, Wehrens XH. Sarcoplasmic reticulum calcium leak and cardiac arrhythmias. Biochem Soc Trans 35: 952–956, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res 111: 863–875, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Liu Y, Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109: 1327–1331, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol 556: 755–771, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit Rev Clin Lab Sci 40: 337–375, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Coetzee WA, Opie LH. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: electrophysiological studies. J Mol Cell Cardiol 24: 651–663, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Corrias A, Giles W, Rodriguez B. Ionic mechanisms of electrophysiological properties and repolarization abnormalities in rabbit Purkinje fibers. Am J Physiol Heart Circ Physiol 300: H1806–H1813, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J 87: 2060–2073, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation 126: 2095–2104, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008. [DOI] [PubMed] [Google Scholar]
- 20.del Monte F, Hajjar RJ, Harding SE, Inesi G. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure response. Circ Res 88: e66-e67, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Eager KR, Roden LD, Dulhunty AF. Actions of sulfhydryl reagents on single ryanodine receptor Ca2+-release channels from sheep myocardium. Am J Physiol Cell Physiol 272: C1908–C1918, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink M, Noble D. Pharmacodynamic effects in the cardiovascular system: the modeller's view. Basic Clin Pharmacol Toxicol 106: 243–249, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier LD, Greenstein JL, Winslow RL. Toward an integrative computational model of the guinea pig cardiac myocyte. Front Physiol 3: 244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gen W, Tani M, Takeshita J, Ebihara Y, Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol 96: 623–629, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol 558: 75–83, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadley RW, Lederer WJ. Properties of L-type calcium channel gating current in isolated guinea pig ventricular myocytes. J Gen Physiol 98: 265–285, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X, Ferrier GR. Ionic mechanisms of transient inward current in the absence of Na+-Ca2+ exchange in rabbit cardiac Purkinje fibres. J Physiol 456: 19–38, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horackova M, Ponka P, Byczko Z. The antioxidant effects of a novel iron chelator salicylaldehyde isonicotinoyl hydrazone in the prevention of H(2)O(2) injury in adult cardiomyocytes. Cardiovasc Res 47: 529–536, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Hudasek K, Brown ST, Fearon IM. H2O2 regulates recombinant Ca2+ channel alpha1C subunits but does not mediate their sensitivity to acute hypoxia. Biochem Biophys Res Commun 318: 135–141, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Jabr RI, Cole WC. Alterations in electrical activity and membrane currents induced by intracellular oxygen-derived free radical stress in guinea pig ventricular myocytes. Circ Res 72: 1229–1244, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Jabr RI, Cole WC. Oxygen-derived free radical stress activates nonselective cation current in guinea pig ventricular myocytes. Role of sulfhydryl groups. Circ Res 76: 812–824, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Kohashi M, Kawahara K, Yamauchi Y. Carbachol-induced suppression of contraction rhythm in spontaneously beating cultured cardiac myocytes from neonatal rats. Biol Rhythm Res 34: 367–381, 2003. [Google Scholar]
- 34.Koster OF, Szigeti GP, Beuckelmann DJ. Characterization of a [Ca2+]i-dependent current in human atrial and ventricular cardiomyocytes in the absence of Na+ and K+. Cardiovasc Res 41: 175–187, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275: C1–C24, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Kukreja RC, Kearns AA, Zweier JL, Kuppusamy P, Hess ML. Singlet oxygen interaction with Ca2+-ATPase of cardiac sarcoplasmic reticulum. Circ Res 69: 1003–1014, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Pogwizd SM, Prabhu SD, Zhou L. Inhibiting Na+/K+ ATPase can impair mitochondrial energetics and induce abnormal Ca2+ cycling and automaticity in guinea pig cardiomyocytes. PLoS One 9: e93928, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Liu H, Dudley SC Jr. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res 107: 967–974, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res 73: 631–640, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics–2012 Update. Circulation 125: e2-e220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca(2+)-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med 22: 37–47, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Nakaya H, Takeda Y, Tohse N, Kanno M. Mechanism of the membrane depolarization induced by oxidative stress in guinea-pig ventricular cells. J Mol Cell Cardiol 24: 523–534, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am J Physiol Cell Physiol 271: C810–C818, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Oba T, Kurono C, Nakajima R, Takaishi T, Ishida K, Fuller GA, Klomkleaw W, Yamaguchi M. H2O2 activates ryanodine receptor but has little effect on recovery of releasable Ca2+ content after fatigue. J Appl Physiol 93: 1999–2008, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Plant DR, Lynch GS, Williams DA. Hydrogen peroxide increases depolarization-induced contraction of mechanically skinned slow twitch fibres from rat skeletal muscles. J Physiol 539: 883–891, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogwizd SM, Bers DM. Calcium cycling in heart failure: the arrhythmia connection. J Cardiovasc Electrophysiol 13: 88–91, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Tarr M, Valenzeno DP. Modification of cardiac ionic currents by photosensitizer-generated reactive oxygen species. J Mol Cell Cardiol 23: 639–649, 1991. [DOI] [PubMed] [Google Scholar]
- 51.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 87: 457–506, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem 276: 2586–2599, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner S, Rokita AG, Anderson ME, Maier LS. Redox regulation of sodium and calcium handling. Antioxid Redox Signal 18: 1063–1077, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 500: 631–642, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward CA, Moffat MP. Role of protein kinase C in mediating effects of hydrogen peroxide in guinea-pig ventricular myocytes. J Mol Cell Cardiol 27: 1089–1097, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104: 79–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ Res 80: 76–81, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279: 234–237, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res 77: 432–441, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie LH. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol 50: 128–136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Z, Gordan R, Wen H, Fefelova N, Zang WJ, Xie LH. Modulation of intracellular calcium waves and triggered activities by mitochondrial Ca flux in mouse cardiomyocytes. PLoS One 8: e80574, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol 6: e1000657, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L, Aon MA, Liu T, O'Rourke B. Dynamic modulation of Ca(2+) sparks by mitochondrial oscillations in isolated guinea pig cardiomyocytes under oxidative stress. J Mol Cell Cardiol 51: 632–639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L, Cortassa S, Wei AC, Aon MA, Winslow RL, O'Rourke B. Modeling cardiac action potential shortening driven by oxidative stress-induced mitochondrial oscillations in guinea pig cardiomyocytes. Biophys J 97: 1843–1852, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]