Abstract
Alcohol abuse results in an increased incidence of pulmonary infection, in part attributable to impaired mucociliary clearance. Analysis of motility in mammalian airway cilia has revealed that alcohol impacts the ciliary dynein motors by a mechanism involving altered axonemal protein phosphorylation. Given the highly conserved nature of cilia, it is likely that the mechanisms for alcohol-induced ciliary dysfunction (AICD) are conserved. Thus we utilized the experimental advantages offered by the model organism, Chlamydomonas, to determine the precise effects of alcohol on ciliary dynein activity and identify axonemal phosphoproteins that are altered by alcohol exposure. Analysis of live cells or reactivated cell models showed that alcohol significantly inhibits ciliary motility in Chlamydomonas via a mechanism that is part of the axonemal structure. Taking advantage of informative mutant cells, we found that alcohol impacts the activity of the outer dynein arm. Consistent with this finding, alcohol exposure results in a significant reduction in ciliary beat frequency, a parameter of ciliary movement that requires normal outer dynein arm function. Using mutants that lack specific heavy-chain motor domains, we have determined that alcohol impacts the β- and γ-heavy chains of the outer dynein arm. Furthermore, using a phospho-threonine-specific antibody, we determined that the phosphorylation state of DCC1 of the outer dynein arm-docking complex is altered in the presence of alcohol, and its phosphorylation correlates with AICD. These results demonstrate that alcohol targets specific outer dynein arm components and suggest that DCC1 is part of an alcohol-sensitive mechanism that controls outer dynein arm activity.
Keywords: cilia, alcohol, ethanol, dynein
mucociliary clearance (MCC) is the primary defense mechanism for the lungs against inhaled particles and pathogens, and MCC is impaired in heavy drinkers. Previous studies have demonstrated that alcohol alters the movement of airway cilia, which are critical organelles that mediate MCC in the upper airway (43). This alcohol-induced ciliary dysfunction (AICD) is mediated by impairment of nitric oxide (NO) and cyclic nucleotide-dependent protein kinase signaling pathways in the ciliated airway epithelium (39–41).
The airway is exposed to alcohol via exhalation of volatized alcohol that is off-gassed from the bronchial circulation. Following brief alcohol exposure, the naïve airway epithelium rapidly and transiently increases ciliary beat frequency (CBF). In contrast, following prolonged alcohol exposure, airway cilia become desensitized to further stimulation. Mechanistic studies demonstrated that brief alcohol exposure activates the endothelial isoform of NO synthase (eNOS) to produce NO, which dually and sequentially activates PKA and PKG signaling pathways in intact cells and isolated airway axonemes (39–41). Thus alcohol has a direct effect on the ciliary responsiveness, verifying a key mechanism by which alcohol impairs MCC, as seen with prolonged alcohol consumption. Whereas the upstream elements of this cilia activation pathway are well defined, the downstream targets of the PKA/PKG signaling pathways remain unidentified. It is also unknown whether these same pathways are subsequently downregulated during prolonged alcohol consumption.
To identify downstream ciliary proteins targeted by alcohol, we utilized the model organism, Chlamydomonas reinhardtii, which provides a versatile experimental system for the study of cilia and regulatory pathways that control motility (10, 32, 50, 52). Studies in Chlamydomonas have directly led to the discovery of ciliary genes that cause a wide range of human diseases called ciliopathies (1, 7, 29, 30, 33, 37, 49). For example, our understanding of the molecular basis of primary ciliary dyskinesia comes from the discovery of Chlamydomonas genes encoding components of the outer dynein arms (ODAs), radial spokes, nexin-dynein regulatory complex (N-DRC), and central pair (6, 9, 13, 20, 28, 51). Our knowledge of how these axonemal structures contribute to motility is grounded in the experimental advantages offered in Chlamydomonas, including genetic, biochemical, and functional assays not available in mammalian systems.
Ciliary motility is generated by the microtubule motor, dynein. The ODA produces most of the power for movement and contributes to normal CBF; the inner dynein arm motors (IDA), of which there are several subtypes designated dynein a–g, contribute to normal ciliary waveform patterns. Genetic, pharmacological, and functional assays for dynein-driven microtubule sliding have revealed a role for the central pair, radial spokes, N-DRC, and kinases (PKA) and phosphatases (PP1, PP2A) in control of dynein activity (10, 50, 52). The ODA is anchored to the axoneme via a heterotrimeric complex called the ODA-docking complex (ODA-DC) (4, 25, 45, 46). This complex contains two coiled-coil proteins, DCC1 and DCC2, plus a redox-sensitive calcium-binding subunit, DLE3 (5).
In the current study, we demonstrate that alcohol exposure results in altered CBF attributable to direct effects on the ODA motor. Both the β- and γ-heavy-chain (HC) motor subunits are targets for AICD in Chlamydomonas. Furthermore, we identified DCC1, a component of the ODA-DC, as an alcohol-sensitive phosphoprotein. Phosphorylation of DCC1 is altered during alcohol exposure, and DCC1 phosphorylation correlates with AICD. Thus our current study, using Chlamydomonas, has revealed direct targets of alcohol and revealed a mechanism underlying the toxic effect of alcohol on ciliary motility.
MATERIALS AND METHODS
Strains and culture conditions.
The Chlamydomonas strains used in this study were as follows: wild-type (WT) (CC125), ida1, ida3, ida4, pf14, pf18, pf3, oda2, oda3 (CC2232), oda11 (CC2673), oda4-s7 (CC2999), sup-pf1 (CC1397), oda2-t. All strains with “CC” designations were obtained from the Chlamydomonas Center (St. Paul, MN). The oda2-t strain was a gift from Stephen M. King. All other strains were from the laboratory of Winfield S. Sale. All strains were grown in L medium for motility analysis with aeration on a 14-h:10-h light/dark cycle.
Flagella and axoneme extraction.
Axonemes were isolated as described with slight modifications (53). In some preparations, the cells were not washed with 10 mM Hepes, pH 7.4. Cells were grown in liquid L media or on solid L media plates and flagellated for 2–4 h in liquid L media. Deflagellation was induced by dibucaine, and the flagella was collected, then resuspended in HMDEKP-1% NP-40 buffer [30 mM Hepes pH 7.4, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, 25 mM KCl, 1 mM PMSF, protease inhibitors (Roche, Indianapolis, IN), phosphatase inhibitors (Roche), and 1% Calbiochem NP-40 alternative (EMD Millipore, Billerica, MA)]. Demembranation proceeded for 15–30 min on ice, and then axonemes were collected by centrifugation. Isolated axonemes were treated with 1 mM ATP for 2 min, 1 mM ATP plus 100 mM ethanol for 2 min, or 10 U of calf intestinal phosphatase for 30 min. For immunoprecipitations, axonemes were extracted with high-salt buffer (HMDEKP buffer + 0.6 M NaCl), and the high-salt extract (HSE) was collected by centrifugation. Samples were then fixed for SDS-PAGE by the addition of 5× sample buffer. For immunoblots, axonemes and HSE were loaded in a 1:1 stoichiometry.
Phos-Tag SDS-PAGE and immunoblots.
Axonemal proteins were separated on standard SDS-PAGE gels or SDS-PAGE gels supplemented with 0.1 mM Phos-Tag acrylamide (Wako Chemicals USA, Richmond, VA) and 0.1 mM MnCl2 and then transferred to nitrocellulose (Bio-Rad, Hercules, CA). The blots were probed with the following primary antibodies: anti-phosphothreonine (pThr) 1:100 and anti-DCC1 1:2,000 (gift from Dr. Ken-ichi Wakabayashi).
Immunoprecipitation.
Axonemal HSEs were collected and diluted to a final salt concentration of 100 mM NaCl with HMDEP buffer (30 mM Hepes pH 7.4, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, 1 mM PMSF, and protease and phosphatase inhibitors). Magnetic beads (EMD Millipore) were washed three times and incubated in HMDEKP buffer with 5% Tween 20 at room temperature for 5 h. The diluted HSE was divided into two tubes: controls included 20 μl of HMDEP buffer, 10 μl magnetic beads, and 0.1% Tween 20; immunoprecipitations included 20 μl of the anti-phospho threonine antibody, 10 μl magnetic beads, and 0.1% Tween 20. Both samples were incubated at room temperature for 1 h on a rotisserie shaker. The beads were recovered and washed three times with HMDEKP-0.1% Tween, and the beads and supernatants were fixed for SDS-PAGE by addition of 5× sample buffer. Protein samples were separated in 7% acrylamide gels, transferred to nitrocellulose, and blotted with antibodies against phosphorylated threonine (Cell Signaling, Danvers, MA) or DCC1 (Ken-ichi Wakabayashi, Tokyo Institute of Technology, Japan). Antibody reactivity was visualized with horseradish peroxidase-conjugated anti-rabbit IgG (Bio-Rad) and chemiluminescence (GE Healthcare, Pittsburgh, PA). For protein-loading controls, the same protein samples were stained with Coomassie Brilliant Blue. For mass spectrometry, gels were stained with sypro ruby (Molecular Probes, Life Technologies, Grand Island, NY).
Analysis of swimming speed.
Cells were grown in L or Tris-acetate-phosphate (TAP) media (19) for 1–2 days, and cell counts were measured. Motility assays were performed with cell densities of ∼1.25–9.00 × 106 cells/ml. The cells were divided into two groups: one was diluted with an equal volume of L media; the second was diluted with an equal volume of L media with either 20 mM or 100 mM or 200 mM ethanol (alcohol). The final alcohol concentrations were 10 mM, 50 mM, and 100 mM, respectively. Cells were incubated for 10 min, and then cell movement was captured with an ANDOR Zyla cMOS high-speed camera (South Windsor, CT). The movement of individual cells was tracked with MetaMorph Basic to determine the distance each cell traveled during a given time period. Note, we observed a difference in the level of AICD induced in cells grown in TAP vs. L media. Cells grown in TAP showed reduced AICD compared with cells grown in L media. Thus the data presented here are generated from cells grown in L media. The data are represented as the mean swimming speed for each strain, which is derived from the average of at least three independent cultures of ∼60 cells each. Error bars represent the standard deviation of each data set. Statistical significance was determined by the t-test method calculated using Microsoft Excel software.
Analysis of CBF.
Chlamydomonas cells were grown in L or TAP media, and CBF was measured using the Sisson-Ammons Video Analysis system (42). Cells were cultured with or without alcohol (10 mM), and CBF measurements were recorded from cells that were adhered to the glass slide by the cell body. Measurements were taken in 1-min increments up to a total of 15 min.
Mass spectrometry.
Immunoprecipitations from WT or oda3 HSEs were separated on 7% polyacrylamide gels and stained with sypro ruby (Molecular Probes, Life Technologies). The region of the gels corresponding to p100 was excised, and the protein content was determined by tandem mass spectrometry at the University of Alabama Mass Spectrometry and Proteomics Facility. Peptides found in both the WT and oda3 samples were omitted, as they were considered background because of nonspecific binding. Peptides found in the WT sample were examined, and any peptides corresponding to predicted proteins above 150 kDa were omitted because they were considered to be proteolytic fragments from proteins of a higher molecular weight and unrelated to p100.
RESULTS
Alcohol exposure reduces swimming speed in Chlamydomonas by altering the ODA.
Chlamydomonas is the premiere system for discovery of genes important for ciliary function. Here, we sought to test the usefulness of this organism to determine the mechanisms underlying AICD. Forward swimming is generated by the coordinated action of the ciliary dynein motors. Thus measuring swimming speed is a rapid and simple way to assess ciliary function. We exposed WT Chlamydomonas cells (CC125) to biologically relevant concentrations of alcohol (22, 38, 47) and measured the swimming velocity. Alcohol exposure caused a dose-dependent decrease in forward swimming speed (Fig. 1A), demonstrating that alcohol slows ciliary motility and causes AICD in Chlamydomonas. Thus we can utilize the experimental advantages of this system to address the mechanisms underlying AICD.
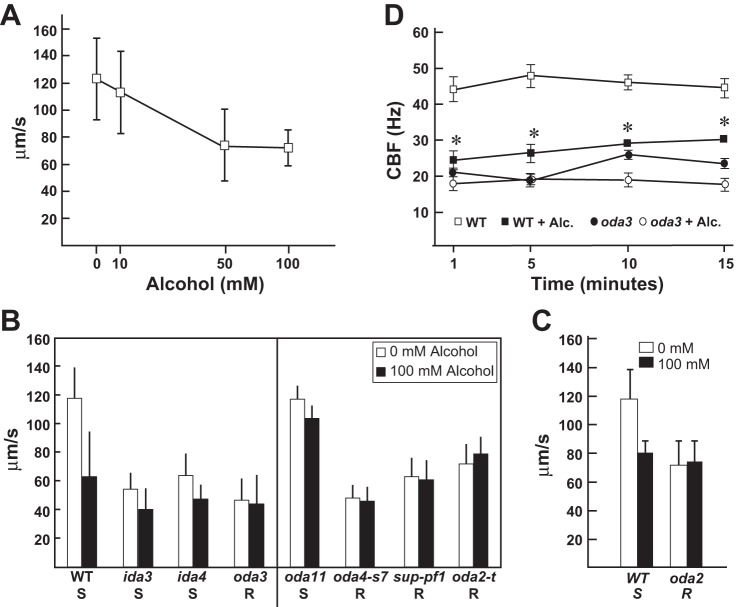
Fig. 1.
Alcohol treatment reduces ciliary beat frequency (CBF) and alters outer dynein arm (ODA) function via the β- and γ-heavy chain (HC). A: wild-type (WT) cells were treated with increasing levels of alcohol. Swimming speeds were measured and show a dose-dependent decrease in response to increasing alcohol concentration. B: motility analyses of WT cells and mutants strains defective in the inner dynein arm (IDA) and ODA motors (left) show that alcohol significantly reduces swimming velocities in WT cells and in mutant strains defective in the IDA motors [dyneins f (ida3) and dyneins a, b, and c (ida4)]. In contrast, the alcohol-induced decrease in swimming speed is not observed in oda3 (lacking the ODA). Motility analyses of oda HC mutants (right) show that alcohol reduces forward swimming speeds in oda11 (lacking the α-HC). In contrast, alcohol shows no ciliary-slowing effect in oda4-s7 (lacks β-HC motor domain), sup-pf1 [lacking 7 amino acids in the coiled-coil 1 (CC1) region of the dynein motor stalk domain], and oda2-t (lacks γ-HC motor domain). Thus WT, ida mutants, and oda11 are susceptible (S) to alcohol-induced ciliary dysfunction (AICD), whereas oda3 and the β- and γ-HC mutants are alcohol resistant (R). C: AICD is recapitulated in WT cell models, where the ciliary membrane is removed. Thus AICD is mediated by mechanisms intrinsic to the ciliary axoneme. Importantly, oda2 cell models are resistant to AICD and confirm that AICD targets the ODA motors. D: in the presence of 10 mM alcohol, CBF is significantly reduced in WT cells over the course of 15 min (*P ≤ 0.0001). CBF is not reduced by alcohol in a mutant that lacks the ODA (oda3). Measurements were performed using Sisson-Ammons Video Analysis (42).
Because alcohol slows forward swimming speed, we asked whether alcohol targets specific classes of ciliary dyneins by analyzing swimming speed in Chlamydomonas mutant strains that are defective in assembly of the ODA or IDA motors (Fig. 1B, left). Alcohol reduces swimming velocities in WT cells (P < 0.0001), ida3 (P < 0.0001; defective in assembly of dynein f, aka I1 dynein), and ida4 (P < 0.0001; defective in assembly of the single-headed inner arms, dyneins a, c, and d). Thus these strains are considered alcohol susceptible (S). In contrast, oda3 cells (defective in assembly of the ODA) show no reduction in swimming velocities in response to alcohol and are considered alcohol resistant (R) (Fig. 1B, left; P = 0.001). This result demonstrates that alcohol targets the ODA.
Alcohol targets the ODA β- and γ-HCs in the ciliary axoneme.
Analysis of swimming speed in cell models (where the ciliary membrane is removed by detergent leaving the cell body with 2 attached axonemes) reactivated with ATP recapitulates the whole cell AICD observed in WT cells (Fig. 1C; P ≤ 0.0001) and demonstrates that the effect of alcohol is on the ciliary axoneme. Consistent with the whole cell motility analyses (Fig. 1B), we find that oda-mutant cells (oda2) are AICD resistant (Fig. 1C; P = 0.7), confirming that AICD targets the ODA motors.
In Chlamydomonas, the ODA has three dynein HC subunits (α, β, and γ). To further understand how alcohol impacts ODA function, we took advantage of unique motor mutants available in Chlamydomonas that affect specific ODA HCs (Fig. 1B; right). The oda11 mutant fails to assemble the α-HC (35); oda4-s7 fails to assemble the motor domain of the β-HC (36); sup-pf1 has a small deletion in the β-HC motor domain (21, 31), whereas oda2-t fails to assemble the motor domain of the γ-HC (26). The oda11 strain swims at speeds comparable to our WT strain (Fig. 1B; P = 0.8 WT vs. oda11 no alcohol). Like WT, oda11 shows reduced swimming velocities upon alcohol exposure (P < 0.0001; oda11 vs. oda11 + alcohol); however, the reduction in swimming speed is not as pronounced as that observed in WT cells. The β-HC mutants, oda4-s7 and sup-pf1, are resistant to the ciliary-slowing effects of alcohol, showing no overall reduction in swimming speed upon alcohol exposure (P = 0.01 and 0.02, respectively). In the absence of alcohol, the oda4-s7 strain swims comparably to the oda3 strain, which completely lacks the ODA (P = 0.07); thus it is not surprising that oda4-s7 is resistant to the effects of alcohol. In contrast, untreated sup-pf1 swims significantly faster than untreated oda3 (P < 0.0001), yet sup-pf1 still shows alcohol resistance. Similarly, the γ-HC mutant, oda2-t, swims significantly faster than oda3 in the absence of alcohol (P > 0.0001), yet it also shows resistance to the ciliary-slowing effects of alcohol. Interestingly, we find that the oda2-t strain swims faster in alcohol (P > 0.0001). These results demonstrate that alcohol has a profound impact on the β- and γ-HC of the ODA while having a more modest effect on the α-HC in causing AICD.
Alcohol exposure reduces CBF in Chlamydomonas.
Ciliary motility is generated by the combined action of the IDA and ODA motors, which control ciliary waveform and CBF, respectively. Any defect in these parameters of movement will result in decreased forward swimming speeds. In mammalian cilia, alcohol alters CBF; however, it is unclear what motors are specifically affected by alcohol. In Chlamydomonas, the ODA contributes to normal CBF, and oda-mutant cells are resistant to AICD (Fig. 1C). To further define the effect of alcohol on ciliary motility, the CBF of WT cells and mutant cells defective in assembly of the ODA (oda3) were measured in the absence and presence of alcohol (Fig. 1C). WT cells have an average CBF of 43.11 ± 1.03 Hz in the absence of alcohol, which is reduced upon alcohol exposure (Fig. 1C, P < 0.0001). This significant reduction was seen with alcohol concentrations of 1, 10, and 100 mM (data not shown). As expected, the oda3 mutant has greatly reduced CBFs of 20.74 ± 0.76 Hz compared with WT (P < 0.0001), which do not change significantly upon alcohol exposure (Fig. 1C). The time course of this inhibition shows significant reduction of CBF in WT cells over 15 min of alcohol exposure. This result strongly indicates that alcohol alters ODA function. Consistent with this idea, oda3 does not show any further reduction in CBF upon alcohol exposure. Taken together, these results provide the first direct evidence that alcohol targets specific ciliary dynein motors to slow ciliary motility. Because cilia are so highly conserved in structure and function, alcohol likely alters ODA function in mammalian cilia (27).
Alcohol alters ciliary protein phosphorylation.
In mammalian cilia, chronic alcohol exposure results in a decrease in cyclic nucleotide production (54). Inactivation of kinases leads to altered phosphorylation of ciliary proteins, yet the downstream targets altered by alcohol have not been identified. In Chlamydomonas, biochemical, genetic, and pharmacological studies have revealed phospho-regulatory pathways that involve the central pair, radial spoke, DRC, and conserved kinases and phosphatases that impinge on dynein motor function (10, 50, 52). Thus it is likely that alcohol alters conserved phosphorylation-based signaling pathways that control ODA function and CBF.
To determine the effects of alcohol exposure on axonemal phospho-signaling pathways, we used phospho-amino acid-specific antibodies to observe changes in phosphorylation of axonemal proteins in response to alcohol. We tested several commercially available antibodies and found one specific anti-phospho-threonine antibody that reacted strongly with a ∼100-kDa phosphoprotein (p100) in isolated axonemes (Fig. 2A). The p100 phosphorylation is greatly diminished when the axonemes are treated with phosphatase. When axonemes are treated with ATP, p100 becomes heavily phosphorylated, as evidenced by the increased smearing of the p100 band. However, in the presence of ATP and alcohol, this increased phosphorylation does not occur, indicating that alcohol may alter the activity of an axonemal-bound kinase or phosphatase. Alcohol treatment also induced the phosphorylation of several other axonemal proteins (Fig. 2A); however, the antibody reacted most consistently with p100, and thus we focused our studies on this phosphoprotein.
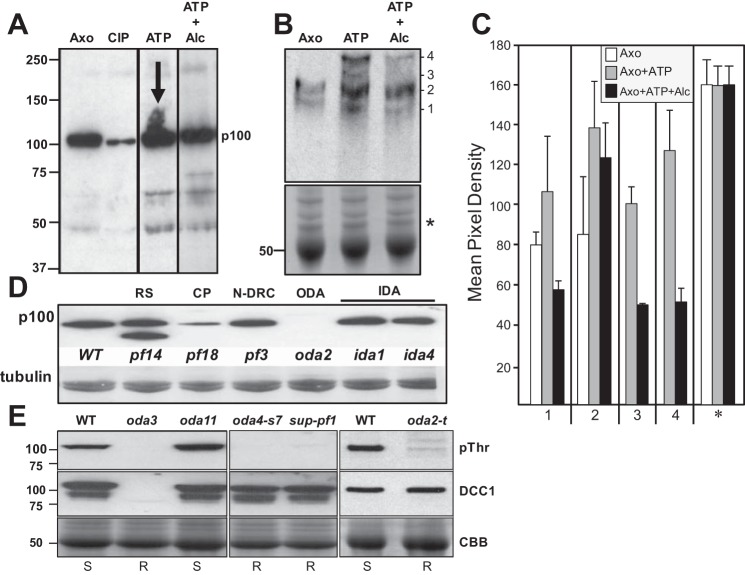
Fig. 2.
Alcohol affects the phosphorylation of axonemal proteins. A: isolated axonemes were treated with phosphatase or incubated with 1 mM ATP in the absence or presence of 100 mM alcohol. Samples were analyzed by Western blot using an antibody to detect phosphorylated threonine. A prominent 100-kDa axonemal phospho-protein is detected in untreated axonemes (Axo), which becomes dephosphorylated by phosphatase treatment (calf intestinal phosphatase, CIP). In the presence of ATP, this phospho-protein becomes heavily phosphorylated (ATP; note the increased smearing of the band marked by the arrow). In the presence of ATP and alcohol, this increased phosphorylation is not observed (ATP + Alc). Alcohol exposure results in altered phosphorylation of several additional proteins as well. B: to better separate phosphorylated proteins, axoneme samples were analyzed by the Phos-Tag SDS-PAGE method. Untreated axonemes show 2 distinct bands (1 and 2). In axonemes treated with ATP, bands 1 and 2 become more heavily phosphorylated, and 2 new bands are detected (3 and 4). In the presence of alcohol, all 4 bands are detected with decreased phosphorylation. Bottom: Coomassie Brilliant Blue (CBB)-stained gel demonstrating equivalent protein loading. C: densitometry of the bands detected by Western blot show the increase in phosphorylation with ATP and the corresponding decrease in phosphorylation in the presence of alcohol. Densitometry of the protein-stained gel (* in B) demonstrates equivalent loading of all samples. D: p100 is reduced or has less threonine phosphorylation in mutants that lack the central pair (CP) (pf18) and the ODA (oda2), whereas p100 phosphorylation is unaffected by loss of the radial spoke (RS) (pf14), nexin-dynein regulatory complex (N-DRC) (pf3), or IDAs (ida1, ida4). Tubulin is shown as a loading control. E: Western blots of isolated axonemes from WT and oda HC mutants. Phosphorylated p100 (pThr) is detected in WT and oda11 (lacking the α-HC) but is absent in oda3 (missing DCC1 and ODA), the β-HC mutants (oda4-s7 and sup-pf1), and the γ-HC mutant (oda2-t). DCC1 antibodies (DCC1) detect the presence of DCC1 in WT and the HC mutants but not oda3. CBB shows protein-stained tubulin bands for loading controls.
To further characterize the effect of alcohol on p100 phosphorylation, we separated isolated axonemal proteins by Phos-Tag SDS-PAGE (Fig. 2B). Phos-Tag is a molecule that specifically binds phosphorylated ions and, when incorporated into SDS-PAGE gels, will retard the migration of phosphorylated proteins to provide greater separation of phosphorylated vs. nonphosphorylated proteins (23, 24). Western blots of Phos-Tag gels probed for p100 show two distinct bands in untreated axonemes (Fig. 2B; bands 1 and 2). Four distinct bands are detected in axonemes treated with ATP and ATP plus alcohol, two corresponding to the bands detected in untreated axonemes (Fig. 2B; bands 1 and 2) and two new bands (Fig. 2B; bands 3 and 4). Densitometry of the bands demonstrates that bands 1 and 2 are increased in intensity in ATP-treated axonemes relative to untreated axonemes (Fig. 2C). In the presence of ATP and alcohol, all four bands show reduced intensity, demonstrating that alcohol diminishes the phosphorylation of p100 (Fig. 2C).
p100 phosphorylation correlates with AICD targeting the β- and γ-HCs.
To further understand whether p100 phosphorylation is linked to AICD, we analyzed p100 phosphorylation in our library of motility mutants defective in ciliary structures that contribute to motility (Fig. 2D). The p100 phosphoprotein is detected in WT axonemes, as well as axonemes from mutants missing the radial spokes (pf14), N-DRC (pf3), and various IDAs (ida1 and ida4). Notably, p100 phosphorylation is reduced in pf18, a mutant that lacks the central pair structure, and undetected in oda2, which lacks the ODA. This result is particularly interesting because genetic analyses have revealed that the ODA is part of a regulatory pathway that involves the central pair, and our current motility analyses have demonstrated that alcohol targets the ODA.
Consistent with our motility data (Fig. 1B), we find that p100 phosphorylation correlates with AICD (Fig. 2E). In WT and oda11 axonemes, p100 is phosphorylated, and these strains show AICD (reduced swimming speed in the presence of alcohol). In contrast, p100 phosphorylation is completely missing in the absence of the ODA and ODA-DC (oda3) as well as in the β- and γ-HC mutants (oda4-s7, sup-pf1–1, and oda2-t), all of which are alcohol resistant. These results demonstrate that p100 phosphorylation correlates with AICD in Chlamydomonas.
p100 is DCC1 of the ODA-DC.
Our biochemical analyses determined that p100 phosphorylation is altered by alcohol and also altered in the absence of the central pair and ODA structures. The relative mobility (Mr) of p100 is not consistent with known ODA subunits. However, it is consistent with the Mr of DCC1, a component of the ODA-DC-a heterotrimeric complex required for docking the ODA to its precise position in the axoneme structure (4, 25, 45, 46).
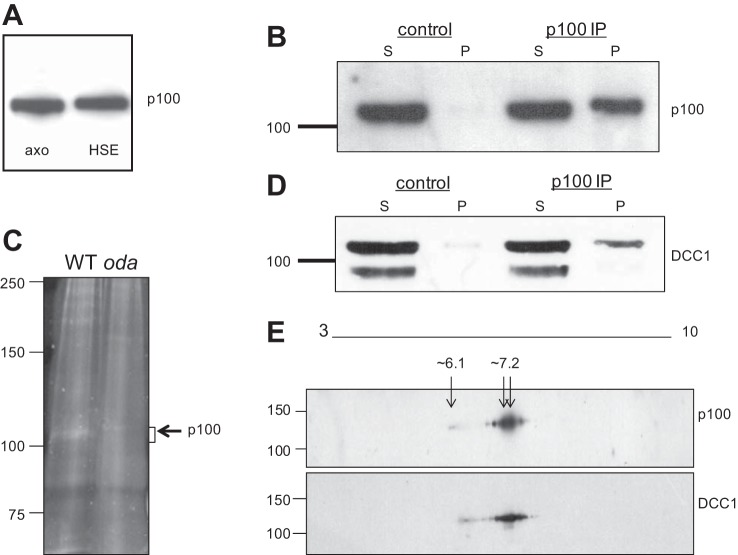
To determine whether p100 is DCC1, we performed immunoprecipitations followed by mass spectrometry of purified p100 protein. Isolated axonemes were extracted with high-salt buffers, conditions known to solubilize the ODA and ODA-DC from the axonemal microtubules. Western blots demonstrate that p100 is detected in the HSE (Fig. 3A), providing a source of soluble p100 that can be used for purification and identification. Western blots confirm that p100 is immunoprecipitated from HSEs with the anti-phosphothreonine antibody (Fig. 3B). The p100 band was excised from protein-stained gels (Fig. 3C) and analyzed by tandem mass spectrometry, which identified several proteins (Table 1). The protein with the most number of peptides with high confidence scores was DCC1, indicating that it is the most abundant protein in the sample. Moreover, the peptides identified covered the entire protein (data not shown).
Fig. 3.
The p100 phosphoprotein is DCC1. A: isolated WT axonemes containing p100 (axo) were extracted with high-salt buffers, and the fractions were analyzed by Western blot using the anti-phosphothreonine antibody. p100 is solubilized from the axoneme with high salt and is detected in the high-salt extract (HSE). B: p100 is immunoprecipitated from HSEs with the anti-phosphothreonine antibody but not with control IgG. C: protein-stained gel showing a band at the expected size for p100 in WT immune complexes, which is absent in immune complexes from an ODA mutant, oda3. The p100 region in both WT and oda3 were excised and analyzed by tandem MS/MS. D: blots in B were stripped and reprobed with the DCC1 antibody, which shows a band for DCC1 in the immune pellet (p100 immunoprecipitation, IP) which comigrates with the p100 band. E: p100 and DCC1 comigrate on 2D gels. WT axonemes were separated on 2D gels followed by Western blotting with the anti-phosphothreonine and DCC1 antibodies. The spot patterns detected by both antibodies comigrate at the same molecular weight and pI range (∼6.1–7.2).
Table 1.
Proteins identified by mass spectrometry
| Protein | Number of Unique Peptides | MW | pI | Confidence Scores |
|---|---|---|---|---|
| DCC1 | 18 | 83 (Mr approximately 100 kDa) | 5.7 | 1.0 |
| Uncharacterized protein similar to FAP59 | 5 | 75 | 5.4 | 1.0 |
| FAP135 | 14 | 95 | 5.6 | 1.0 |
| FAP147 | 8 | 102 | 6.7 | 0.99 |
| RSP1 | 6 | 87 | 4.6 | 0.98 |
| FAP256 | 3 | 92 | 6.4 | 0.82 |
| FAP180 (AKT5) | 4 | 114 | 4.5 | 0.99 |
| FAP59 (CCDC39) | 2 | 105 | 5.9 | 0.99 |
| FAP70 | 2 | 111 | 5.3 | 0.98 |
| PKG | 2 | 115 | 6.4 | 0.71 |
| Uncharacterized protein | 1 | 115 | 5.5 | 0.99 |
MW, molecular weight; Mr, relative mobility.
To verify the mass spectrometry results, we probed our immunoprecipitations for DCC1 (Fig. 3D). Anti-DCC1 antibodies detect two bands in HSEs; the upper band corresponds to full-length DCC1, and the lower band is a proteolytic fragment. The full-length DCC1 is detected in the immune pellet, demonstrating that DCC1 is immunoprecipitated with the anti-phosphothreonine antibody. As further proof, we analyzed isolated axonemes by 2D Western blots (Fig. 3E). Both DCC1 and anti-phospho threonine antibodies detect comigrating spot patterns with a series of spots clustered around pI ∼7.2 with some weaker spots migrating at pI ∼6.1. Whereas the predicted pI of DCC1 is 5.7 (Table 1), the phosphorylation events likely shift the observed pI to a more basic position. Taken together, our data strongly support that p100 is DCC1 of the ODA-DC.
DISCUSSION
Here, we demonstrate the usefulness of Chlamydomonas for revealing the molecular targets involved in mediating AICD. Our results demonstrate that alcohol exposure of Chlamydomonas cells mimics the AICD observed in mammalian cilia; alcohol treatment reduces forward swimming speeds by reducing CBF. Whereas CBF is altered in mammalian cilia exposed to alcohol, it is unknown what ciliary dynein motors are perturbed by alcohol. Studies in Chlamydomonas have determined that the ODA functions in control of CBF. Our findings demonstrate that the presence of the ODA is required for AICD in Chlamydomonas and reveal for the first time a specific ciliary dynein motor that is affected by alcohol (Fig. 1). Moreover, AICD is likely mediated by a mechanism that targets the β- and γ-HC of the ODA (Fig. 2B) while having only a marginal effect on the α-HC. Of interest, the β- and γ-HC are conserved in mammalian ODAs, which lack an equivalent α-HC subunit. It is interesting that alcohol has such a profound and specific impact on the ODA motor and that alcohol does not affect all ciliary dynein motors universally. Notably, our results go beyond confirmation of the effects of alcohol on ciliary motility and CBF and reveal specific targets of alcohol in the ciliary axoneme.
In mammalian airways, brief alcohol exposure leads to upregulation of NO production and activation of downstream PKA/PKG signaling. It is likely that these signaling pathways are downregulated with chronic alcohol consumption. Consistent with this idea, chronic alcohol exposure results in the inactivation of PKA and downregulates ciliary beating in bovine bronchial epithelial cells (54). Here, we identify a specific axonemal protein, DCC1, with alcohol-sensitive phosphorylation (Fig. 2, A–C). DCC1 has been previously shown to be an axonemal phosphoprotein (2). Of the eight phosphosites identified, three are threonine (T351, T698, and T713). However, these studies did not analyze axonemes exposed to ATP. Thus we do not definitively know the identity of the sites that become phosphorylated upon ATP exposure, nor do we know the sites altered by alcohol exposure. A further understanding of how DCC1 functions in control of the ODA will require further studies involving identification and mutagenesis of the relevant DCC1 phosphosites.
Notably, baseline DCC1 phosphorylation (as detected with an anti-phospho threonine antibody) is absent in mutants lacking the entire ODA or in mutants with defects in the β- and γ-HC motor domains (Fig. 2, D and E). These findings are novel and indicate that DCC1 may function in a phospho-regulatory pathway that controls ODA activity by modulating specific ODA HCs (Fig. 4).
Fig. 4.
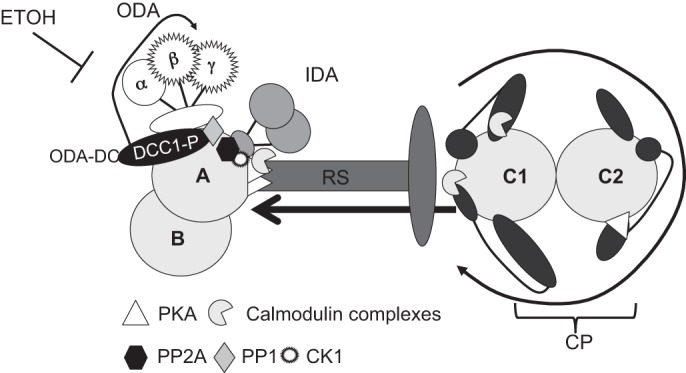
Model for regulation of ODA activity. The CP contains appendage structures (dark gray) that are asymmetrically oriented within the CP apparatus. The rotation of the CP (black round arrow) mechanically activates signaling pathways built into the CP and RS structures. The signal initiated in the CP is transmitted through the RS (arrow) to signaling molecules at the base of the RS and on the outer doublet microtubules. The predicted locations of signal transduction proteins [PKA, CK1, protein phosphatase 1 (PP1), PP2A, and calmodulin complexes] are shown. The ODA-docking complex (ODA-DC) at the base of the ODA contains phosphorylated DCC1. Upon activation of motility, DCC1 is further phosphorylated and may regulate the activity of the β-and γ-HCs. Alcohol may interfere in this pathway.
Consistent with these results, DCC1 phosphorylation is markedly reduced in the central pair mutant pf18 (Fig. 2D). Pharmacological and genetic studies have revealed a pathway for control of ciliary dynein function that involves the central pair and radial spoke complexes (21, 31). Rotation of the central pair apparatus leads to contact between the central pair appendages and the radial spoke head, which mechanically induces multiple signaling pathways built into the central pair, radial spokes, and outer doublet microtubules. These pathways include calcium-mediated signaling events involving calmodulin-containing complexes (8, 9, 44, 48) and cyclic-nucleotide-dependent signaling pathways involving PKA and CK1 (10, 15, 17, 50, 52). Axonemal phosphatases, such as PP1 and PP2A, also participate in the regulation of axonemal dynein activity (12, 18, 55). PKA is localized by A-kinase anchoring proteins positioned in the central pair and radial spokes (14) (Fig. 4). CK1 is likely positioned at the base of the dynein f inner arm motor along with PP2A (12, 17, 50). The location of PP1 is unknown.
Genetic analyses of suppressor mutations have revealed a pathway for regulation of the ODA that involves the central pair-radial spoke mechanism (3, 31, 34). The sup-pf1 mutation was identified in these suppressor screens as an extragenic suppressor that partially restores motility in the paralyzed central pair mutant, pf6 (21, 31). The sup-pf1–1 allele (used here) contains a mutation that deletes seven amino acids in a conserved region of the β-HC motor domain, downstream of the last nucleotide-binding site, which is predicted to form a small α-helical coiled coil (31). This region corresponds to CC1 of the coiled-coil stalk of the dynein microtubule-binding domain. Deletion of one heptad from CC1 abolishes microtubule binding of this domain in vitro (16). Our finding that sup-pf1 is resistant to AICD strengthens the idea that this region is critical for regulation of the β-HC motor subunit. It is interesting to speculate that alcohol may target this area, mediating its effects on dynein motor function by disrupting the microtubule binding function of this critical region of the dynein HCs.
GRANTS
This work was supported by grant NIAAA 5R01AA008769-22 (J. Sisson), NIAAA subcontract 34-5237-2020-016 (M. Wirschell), and NIAAA P50 AA013757 10 pilot project 00006748 (W. Sale).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.Y., W.S.S., J.H.S., and M.W. conception and design of research; F.Y., J.A.P., L.F., C.S., and M.W. performed experiments; F.Y., J.A.P., L.F., W.S.S., J.H.S., and M.W. analyzed data; F.Y., J.A.P., W.S.S., J.H.S., and M.W. interpreted results of experiments; J.A.P., L.F., J.H.S., and M.W. prepared figures; J.A.P., W.S.S., J.H.S., and M.W. edited and revised manuscript; W.S.S., J.H.S., and M.W. approved final version of manuscript; M.W. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ken-ichi Wakabayashi for the DCC1 antibody and Dr. Stephen M. King for the oda2-t strain. Special thanks go to Dr. George B. Witman for thoughtful scientific discussions on this manuscript.
REFERENCES
- 1.Afzelius BA. Cilia-related diseases. J Pathol 204: 470–477, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boesger J, Wagner V, Weisheit W, Mittag M. Analysis of flagellar phosphoproteins from Chlamydomonas reinhardtii. Eukaryot Cell 8: 922–932, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower R, Tritschler D, Vanderwaal K, Perrone CA, Mueller J, Fox L, Sale WS, Porter ME. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol Biol Cell 24: 1134–1152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DM, Inaba K, Pazour GJ, Takada S, Wakabayashi K, Wilkerson CG, Kamiya R, Witman GB. DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol Biol Cell 14: 3650–3663, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DM, Yagi T, Kamiya R, Witman GB. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J Biol Chem 278: 42652–42659, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, Parker KA, Ybot-Gonzalez P, Emes RD, Wilson SW, Wallis C, Johnson CA, Herrera RJ, Rutman A, Dixon M, Shoemark A, Bush A, Hogg C, Gardiner RM, Reish O, Greene ND, O'Callaghan C, Purton S, Chung EM, Mitchison HM. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet 84: 197–209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Angelo A, Franco B. The dynamic cilium in human diseases. Pathogenetics 2: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiPetrillo C, Smith E. Calcium regulation of ciliary motility analysis of axonemal calcium-binding proteins. Methods Cell Biol 92: 163–180, 2009. [DOI] [PubMed] [Google Scholar]
- 9.DiPetrillo CG, Smith EF. Pcdp1 is a central apparatus protein that binds Ca(2+)-calmodulin and regulates ciliary motility. J Cell Biol 189: 601–612, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elam CA, Sale WS, Wirschell M. The regulation of dynein-driven microtubule sliding in Chlamydomonas flagella by axonemal kinases and phosphatases. Methods Cell Biol 92: 133–151, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Elam CA, Wirschell M, Yamamoto R, Fox LA, York K, Kamiya R, Dutcher SK, Sale WS. An axonemal PP2A B-subunit is required for PP2A localization and flagellar motility. Cytoskeleton (Hoboken) 68: 363–372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier E, Duquesnoy P, Papon JF, Amselem S. Ciliary defects and genetics of primary ciliary dyskinesia. Paediatr Respir Rev 10: 51–54, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard AR, Diener DR, Rosenbaum JL, Sale WS. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP). J Cell Biol 153: 443–448, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillard AR, Fox LA, Rhea JM, Craige B, Sale WS. Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility. Mol Biol Cell 17: 2626–2635, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons IR, Garbarino JE, Tan CE, Reck-Peterson SL, Vale RD, Carter AP. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J Biol Chem 280: 23960–23965, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokhale A, Wirschell M, Sale WS. Regulation of dynein-driven microtubule sliding by the axonemal protein kinase CK1 in Chlamydomonas flagella. J Cell Biol 186: 817–824, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habermacher G, Sale WS. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci 109: 1899–1907, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Harris E. Chlamydomonas in the laboratory. In: The Chlamydomonas Sourcebook, edited by Harris E. Kidlington. Oxford, UK: Academic, 2009, p. 241–301. [Google Scholar]
- 20.Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res 75: 158–164, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28: 115–124, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RA, Noll EC, Rodney WM. Survival after a serum ethanol concentration of 1 1/2%. Lancet 2: 1394, 1982. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc 4: 1513–1521, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita E, Kinoshita-Kikuta E, Ujihara H, Koike T. Mobility shift detection of phosphorylation on large proteins using a Phos-tag SDS-PAGE gel strengthened with agarose. Proteomics 9: 4098–4101, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Koutoulis A, Pazour GJ, Wilkerson CG, Inaba K, Sheng H, Takada S, Witman GB. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J Cell Biol 137: 1069–1080, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Takazaki H, Nakazawa Y, Sakato M, Yagi T, Yasunaga T, King SM, Kamiya R. Partially functional outer-arm dynein in a novel Chlamydomonas mutant expressing a truncated gamma heavy chain. Eukaryot Cell 7: 1136–1145, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Toole ET, Giddings TH Jr, Porter ME, Ostrowski LE. Computer-assisted image analysis of human cilia and Chlamydomonas flagella reveals both similarities and differences in axoneme structure. Cytoskeleton (Hoboken) 69: 577–590, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onoufriadis A, Shoemark A, Schmidts M, Patel M, Jimenez G, Liu H, Thomas B, Dixon M, Hirst RA, Rutman A, Burgoyne T, Williams C, Scully J, Bolard F, Lafitte JJ, Beales PL, Hogg C, Yang P, Chung EM, Emes RD, O'Callaghan C, Bouvagnet P, Mitchison HM. Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects. Hum Mol Genet 23: 3362–3374, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J. Cilia and ciliopathies: from Chlamydomonas and beyond. Sci China C Life Sci 51: 479–486, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Piatti G, De Santi MM, Brogi M, Castorina P, Ambrosetti U. Emerging ciliopathies: are respiratory cilia compromised in Usher syndrome? Am J Otolaryngol 35: 340–346, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Porter ME, Knott JA, Gardner LC, Mitchell DR, Dutcher SK. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J Cell Biol 126: 1495–1507, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol 151: F37–F42, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao Damerla R, Gabriel GC, Li Y, Klena NT, Liu X, Chen Y, Cui C, Pazour GJ, Lo CW. Role of cilia in structural birth defects: Insights from ciliopathy mutant mouse models. Birth Defects Res C Embryo Today 102: 115–125, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Rupp G, O'Toole E, Gardner LC, Mitchell BF, Porter ME. The sup-pf-2 mutations of Chlamydomonas alter the activity of the outer dynein arms by modification of the gamma-dynein heavy chain. J Cell Biol 135: 1853–1865, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakakibara H, Mitchell DR, Kamiya R. A Chlamydomonas outer arm dynein mutant missing the alpha heavy chain. J Cell Biol 113: 615–622, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakakibara H, Takada S, King SM, Witman GB, Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated beta heavy chain. J Cell Biol 122: 653–661, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol 85: 371–427, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Simet SM, Wyatt TA, DeVasure J, Yanov D, Allen-Gipson D, Sisson JH. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcohol Clin Exp Res 36: 432–442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 268: L596–L600, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res 23: 1528–1533, 1999. [PubMed] [Google Scholar]
- 41.Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res 33: 610–616, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211: 103–111, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Sisson JH, Stoner JA, Romberger DJ, Spurzem JR, Wyatt TA, Owens-Ream J, Mannino DM. Alcohol intake is associated with altered pulmonary function. Alcohol 36: 19–30, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell 13: 3303–3313, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takada S, Kamiya R. Functional reconstitution of Chlamydomonas outer dynein arms from alpha-beta and gamma subunits: requirement of a third factor. J Cell Biol 126: 737–745, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell 13: 1015–1029, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teplin LA, Abram KM, Michaels SK. Blood alcohol level among emergency room patients: a multivariate analysis. J Stud Alcohol 50: 441–447, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Wargo MJ, Dymek EE, Smith EF. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci 118: 4655–4665, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol 26: 1039–1056, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirschell M, Hendrickson T, Sale WS. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton 64: 569–579, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Wirschell M, Olbrich H, Werner C, Tritschler D, Bower R, Sale WS, Loges NT, Pennekamp P, Lindberg S, Stenram U, Carlen B, Horak E, Kohler G, Nurnberg P, Nurnberg G, Porter ME, Omran H. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet 45: 262–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wirschell M, Yamamoto R, Alford L, Gokhale A, Gaillard A, Sale WS. Regulation of ciliary motility: Conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch Biochem Biophys 510: 93–100, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol 134: 280–290, 1986. [DOI] [PubMed] [Google Scholar]
- 54.Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L575–L581, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci 113: 91–102, 2000. [DOI] [PubMed] [Google Scholar]