Abstract
Increased endothelial cell (EC) permeability and vascular inflammation along with alveolar epithelial damage are key features of acute lung injury (ALI). Products of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine oxidation (OxPAPC) showed protective effects against inflammatory signaling and vascular EC barrier dysfunction induced by gram-negative bacterial wall lipopolysaccharide (LPS). We explored the more general protective effects of OxPAPC and investigated whether delayed posttreatment with OxPAPC boosts the recovery of lung inflammatory injury and EC barrier dysfunction triggered by intratracheal injection of heat-killed gram-positive Staphylococcus aureus (HKSA) bacteria. HKSA-induced pulmonary EC permeability, activation of p38 MAP kinase and NF-κB inflammatory cascades, secretion of IL-8 and soluble ICAM1, fibronectin deposition, and expression of adhesion molecules ICAM1 and VCAM1 by activated EC were significantly attenuated by cotreatment as well as posttreatment with OxPAPC up to 16 h after HKSA addition. Remarkably, posttreatment with OxPAPC up to 24 h post-HKSA challenge dramatically accelerated lung recovery by restoring lung barrier properties monitored by Evans blue extravasation and protein content in bronchoalveolar lavage (BAL) fluid and reducing inflammation reflected by decreased MIP-1, KC, TNF-α, IL-13 levels and neutrophil count in BAL samples. These studies demonstrate potent in vivo and in vitro protective effects of posttreatment with anti-inflammatory oxidized phospholipids in the model of ALI caused by HKSA. These results warrant further investigations into the potential use of OxPAPC compounds combined with antibiotic therapies as a treatment of sepsis and ALI induced by gram-positive bacterial pathogens.
Keywords: oxidized phospholipids, cytoskeleton, pulmonary endothelium, inflammation, vascular leak
sepsis is the 10th leading cause of death in the United States (33) and bacterial inflammation is a major contributor to the development of acute respiratory distress syndrome (ARDS). About 50% of all cases of sepsis are caused by gram-positive bacteria (38). In pneumonia caused by Staphylococcus aureus, the intense inflammatory response that is triggered by this infection can lead to rapid development of lung injury and ARDS (23, 28). Although antibiotics are used to ameliorate bacterial dissemination, even inactivated or killed bacteria continue acting as a “danger signal” and activate an inflammatory response often leading to a cytokine storm and severe lung injury.
Gram-positive bacterial pathogens stimulate inflammatory responses via the activation of Toll-like receptors (TLRs) (26, 45), leading to acute pulmonary inflammation characterized by the influx of neutrophils and increased cytokine levels in lung parenchyma and bronchoalveolar lavage (BAL) fluid (29). TLR2 is considered a major receptor involved in the host defense against gram-positive bacteria, although it also recognizes lipoproteins from other bacterial species, and other TLRs cooperate with TLR2 in activation of an inflammatory signaling (35, 41).
TLR-induced inflammatory events in endothelial cells are mediated by phosphorylation/activation of mitogen-activated protein kinases (MAPK) p42/p44, JNK1/2, p38, and the nuclear factor-κB (NF-κB) pathway (1). As a result, TLR2-induced activation of inflammatory signaling promotes the transient elevation of monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, IL-6, and tumor necrosis factor-α (TNF-α) in vivo and in vitro (30, 48) and also leads to the activation and recruitment of neutrophils and macrophages into the lungs (48).
Previous studies have described potent barrier-protective effects of specific products of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine oxidation (OxPAPC) (3, 13) on the vascular endothelium and demonstrated a key role of Rac1 and Rap1 GTPases in OxPAPC-induced endothelial cell (EC) barrier enhancement (3, 6, 9, 17). Because of direct barrier enhancing effects on lung endothelial monolayers, OxPAPC is capable of suppressing the lung vascular leak induced by edemagenic and inflammatory molecules (thrombin, LPS) (8, 37) or excessive lung distension during mechanical ventilation (36). Concurrent administration of OxPAPC has been also shown to antagonize interaction of TLR4 and TLR9 ligands, LPS, and CpG DNA with their cognate receptors. As a result, OxPAPC inhibited activation of MAPK and NF-κB signaling and inflammatory lung injury (18, 31, 37). The present model of OxPAPC anti-inflammatory effects assumes competitive interaction of OxPAPC with TLR receptors, which prevents receptor interaction with proinflammatory ligands, i.e., LPS. However, effects of OxPAPC posttreatment on modulation of ongoing inflammation and stimulation of organ recovery after initiation of inflammation remain to be explored.
This study investigated the protective effects of OxPAPC against lung inflammation and EC barrier dysfunction caused by gram-positive pathogens, the heat-killed S. aureus particles (HKSA). This study used pulmonary EC culture and a mouse model of HKSA-induced lung injury to test therapeutic effects of OxPAPC posttreatment (2–24 h) on modulation of HKSA-induced pulmonary EC barrier dysfunction and inflammation in vitro and parameters of lung injury in vivo. We evaluated anti-inflammatory and barrier-protective effects of OxPAPC posttreatment as a potential therapeutic strategy to mitigate lung injury and promote recovery in the model of lung injury caused by gram-positive pathogens.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (Allendale, NJ). Cells were maintained in a complete culture medium according to the manufacturer's recommendations and used for experiments at passages 5–8. Phospho-p38, IκBα, and p65-NF-κB and phosphoS536-p65-NF-κB antibodies were obtained from Cell Signaling (Beverly, MA); VE-cadherin, fibronectin, ICAM1, and VCAM1 from Santa Cruz Biotechnology (Santa Cruz, CA). HKSA was obtained from InvivoGen (San Diego, CA). Nonoxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC) and dimirystoyl-sn-glycero-3-phosphorylcholine (DMPC) were obtained from Avanti Polar Lipids (Alabaster, AL). OxPAPC was obtained by exposure of dry lipid to air as previously (3, 46). The extent of oxidation was measured by positive ion electrospray mass spectrometry (ESI-MS) described elsewhere (46). OxPAPC dissolved in chloroform was stored at −70°C and used within 2 wk after mass spectrometry testing. Reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Assessment of endothelial permeability.
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as previously described (5, 7). Express micromolecule permeability testing assay (XPerT) was recently developed by our group (21) and currently available from Millipore (Vascular Permeability Imaging Assay, catalog no. 17-10398). This assay is based on the high-affinity binding of avidin-conjugated FITC-labeled tracer to the biotinylated extracellular matrix proteins immobilized on the bottom of culture dishes after the EC barrier is compromised by treatment with a barrier-disruptive agonist. XPerT permeability assays were performed in 96-well plates. Visualization of EC monolayer permeability was performed in HPAEC plated on glass coverslips coated with biotinylated gelatin followed by agonist stimulation, incubation with FITC-avidin tracer, fluorescence microscopy, and imaging analysis as previously described (21, 42).
Fractionation and immunoblotting.
Confluent HPAEC were stimulated with HKSA and the nuclear fraction was isolated using the S-PEK kit (EMD Chemicals, Gibbstown, NJ). Immunoblotting detection of proteins of interest was performed as described previously (3). Protein extracts from mouse lungs or EC were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and the membranes were incubated with specific antibodies of interest. Immunoreactive proteins were detected with the enhanced chemiluminescence detection system according to the manufacturer's protocol (Amersham, Little Chalfont, UK).
Immunofluorescence.
Endothelial monolayers plated on glass coverslips were subjected to immunofluorescence staining with the appropriate antibody, as described previously (11). Texas red phalloidin was used to visualize F-actin. After immunostaining, slides were analyzed with a Nikon video imaging system (Nikon Instech, Tokyo, Japan). Images were processed with Image J software (National Institutes of Health, Bethesda, MD) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software (11). Quantitative analysis of VE-cadherin-positive adherens junctions was performed by measurements of junctional VE-cadherin immunoreactivity normalized to square area in control and stimulated cells as previously reported (15). Quantitative analysis of paracellular gap formation and F-actin cytoskeleton was performed as previously described (4, 7, 12). At least 20 microscopic fields for each experimental condition were analyzed. The 16-bit images were analyzed by use of MetaVue 4.6 software (Universal Imaging, Downington, PA). Paracellular gaps were manually marked out, and images were differentially segmented between gaps and cells based on image grayscale levels. The gap formation was expressed as a ratio of the gap area to the area of the whole image. The values were statistically processed by use of Sigma Plot 7.1 (SPSS Science, Chicago, IL) software. For analysis of stress fiber formation, F-actin immunoreactivity were captured based on image grayscale levels, and immunofluorescence signal intensity was measured and expressed in arbitrary units per microscopic field. The values were statistically processed with Sigma Plot 7.1 (SPSS Science, Chicago, IL) software.
Cytokine analysis.
The concentrations of KC, MIP-1, TNF-α, and IL-13 in mouse BAL fluid samples were measured by using a Mouse Cytokine Multiplex Panel according to the manufacturer's protocol (Millipore, Billerica, MA). For IL-8 and soluble ICAM1 (sICAM1) measurements in preconditioned medium of human pulmonary EC cultures, supernatants from treated EC were collected and centrifuged to remove debris. IL-8 and sICAM1 levels were determined by ELISA (R&D Systems, Minneapolis, MN) following the manufacturer's protocol.
In vivo model of acute lung injury.
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6J mice, 8–10 wk old, with an average weight 20–25 g (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). HKSA (2 × 108 bacterial cells/mouse) or sterile saline solution was injected intratracheally in a small volume (20–30 μl) using a 20-gauge catheter (Penn-Century, Philadelphia, PA). Five hours after intratracheal HKSA instillation, OxPAPC (1.5 mg/kg), DMPC (1.5 mg/kg), or sterile saline solution was administrated by retro-orbital injection or intravenous injection in the external jugular vein. At 24 h, animals were euthanized by exsanguination under anesthesia. Measurements of cell count and protein concentration in BAL and determination of myeloperoxidase activity in lung tissue homogenates were performed as previously described (8, 22). Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 h before termination of ventilation to assess vascular leak as described previously (34, 36). Histological assessment of lung injury was performed as descried elsewhere (22, 36).
Statistical analysis.
Results are expressed as means ± SD of three to six independent experiments. Stimulated samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way ANOVA, followed by the post hoc Tukey test, were used. P < 0.05 was considered statistically significant.
RESULTS
HKSA induces barrier disruption in pulmonary endothelium.
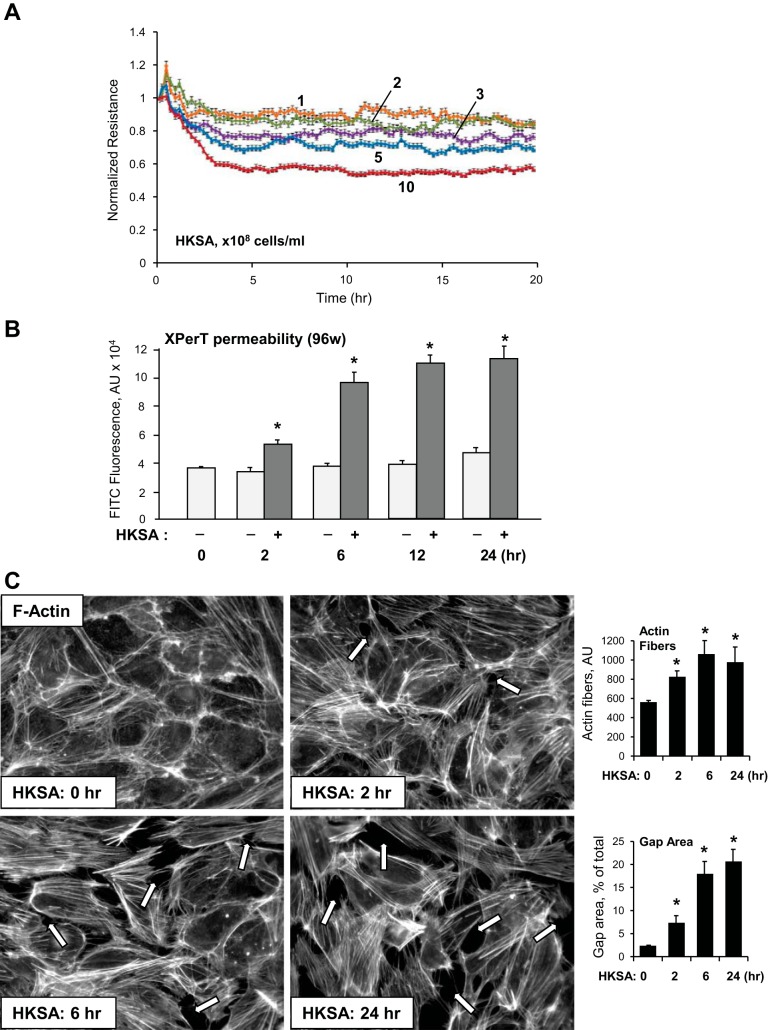
Dose-dependent (1–10 × 108 bacterial cells/ml) effects of HKSA on pulmonary EC permeability were assessed by measuring TER. All HKSA concentrations significantly reduced TER within 4–5 h, which remained decreased up to 20 h following treatment (Fig. 1A).
Fig. 1.
Effects of heat-killed gram-positive Staphylococcus aureus (HKSA) on pulmonary endothelial cell (EC) permeability and F-actin remodeling. A: EC grown on microelectrodes to confluence were treated with HKSA (1 × 108, 2 × 108, 3 × 108, 5 × 108, or 10 × 108 bacterial particles/ml) and used for measurements of transendothelial electrical resistance (TER). B: EC grown in 96-well plates with immobilized biotinylated gelatin were treated with vehicle of HKSA (5 × 108 particles/ml) for indicated time periods followed by addition of FITC-avidin tracer in the last 5 min of incubation. Unbound FITC-avidin was removed, and FITC fluorescence was measured. Data are expressed as means ± SD of 4 independent experiments; *P < 0.05. XPerT, express micromolecule permeability testing assay. C: EC monolayers were treated with HKSA (5 × 108 particles/ml), and cytoskeletal remodeling was assessed by immunofluorescence staining for F-actin with Texas red phalloidin at 2, 6, or 24 h of HKSA stimulation. Paracellular gaps are shown by arrows. Bar graphs depict quantitative analysis of stress fibers and gap area in EC monolayers after incubation with HKSA. Data are expressed as means ± SD of 3 independent experiments; *P < 0.05.
The effect of HKSA on lung EC monolayer permeability associated with septic inflammation was further analyzed with an express assay testing EC monolayer permeability for macromolecules, which was described in our recent study (21). In agreement with TER measurements, HKSA gradually increased EC monolayer permeability for FITC-labeled avidin during a 2- to 24-h period (Fig. 1B). Analysis of cytoskeletal remodeling in HKSA-challenged cells showed the disappearance of the cortical F-actin rim and formation of actin stress fibers and paracellular gaps, which reflect compromised EC monolayer integrity (Fig. 1C).
HKSA induces inflammatory activation of pulmonary endothelium.
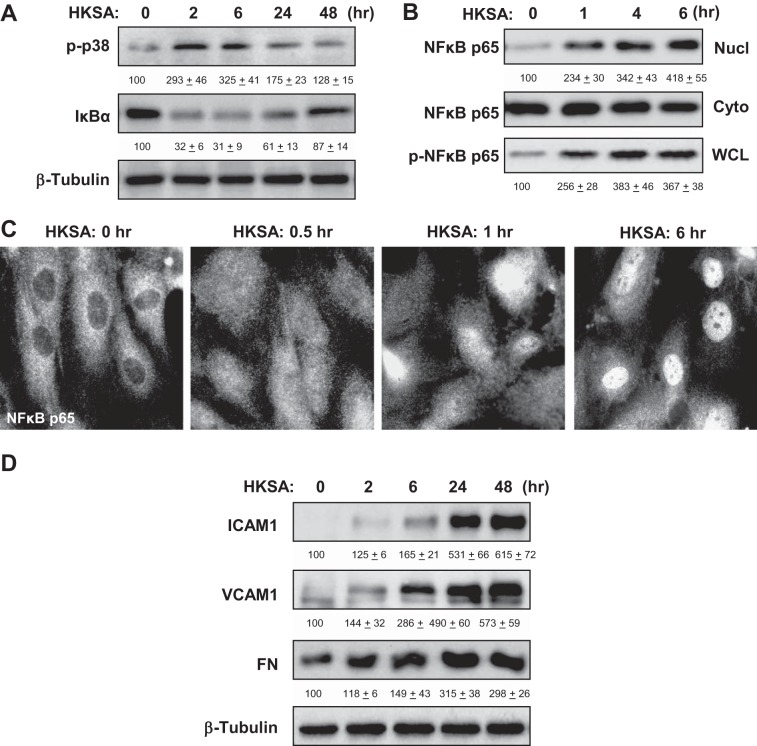
Activation of intracellular signaling by HKSA was evaluated by the analysis of phosphorylation/activation status of signaling proteins involved in the stress response and inflammatory cascades. HKSA induced rapid time-dependent phosphorylation of stress-activated p38 MAP kinase. Activation of inflammatory signaling stimulates IκBα phosphorylation by IκB kinase, subsequent degradation by the proteasome and IκBα degradation leading to NF-κB release and translocation to the nucleus, where it triggers the transcription of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 (20). HKSA caused increased phosphorylation of p38 stress MAP kinase and degradation of IκBα, an inhibitory subunit of the NF-κB complex (Fig. 2A), resulting in the activation of NF-κB-dependent transcription.
Fig. 2.
Effects of HKSA on inflammatory signaling. A: human pulmonary artery endothelial cells (HPAEC) were challenged with HKSA (5 × 108 bacterial particles/ml) for indicated periods of time. Phosphorylation of p38 MAP kinase and degradation of IκBα was detected by immunoblotting with corresponding antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. B: after EC treatment with HKSA, the content of NF-κB p65-subunit in nuclear (Nucl) and cytosolic (Cyto) fractions was performed using immunoblotting. The level of phosphorylated NF-κB p65 in the whole cell lysates (WCL) was monitored by Western blot with phospho-specific NF-κB p65 antibody. C: HKSA-induced nuclear translocation of NF-κB was visualized by immunofluorescence staining of EC culture with NF-κB antibody. D: time-dependent expression of ICAM1, VCAM1, and fibronectin (FN) caused by HKSA stimulation of pulmonary was monitored by immunoblotting. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. The results of Western blot quantitative densitometry are presented as means ± SD of 5 independent experiments.
The nuclear fractionation assay showed HKSA-induced phosphorylation of NF-κB p65-subunit at Ser536 and its translocation to the nuclear fraction (Fig. 2B). HKSA-induced nuclear localization of p65-NF-κB was further confirmed by immunofluorescence staining of control and HKSA-stimulated EC with NF-κB antibody (Fig. 2C).
Activation of inflammatory signaling led to increased expression of adhesion molecules ICAM1 and VCAM1 and increased EC production of fibronectin, the extracellular matrix protein involved in the propagation of the inflammatory response, which was observed at 4–48 h after HKSA challenge (Fig. 2D).
OxPAPC protects against HKSA-induced endothelial monolayer disruption.
Previously described barrier-protective effects of OxPAPC against EC barrier disruption caused by gram-negative pathogen LPS were mediated at least in part by direct inhibition of TLR4 receptor. Effects of OxPAPC in the model of Staphylococcus aureus-induced EC barrier dysfunction remained unclear and were investigated in the present study.
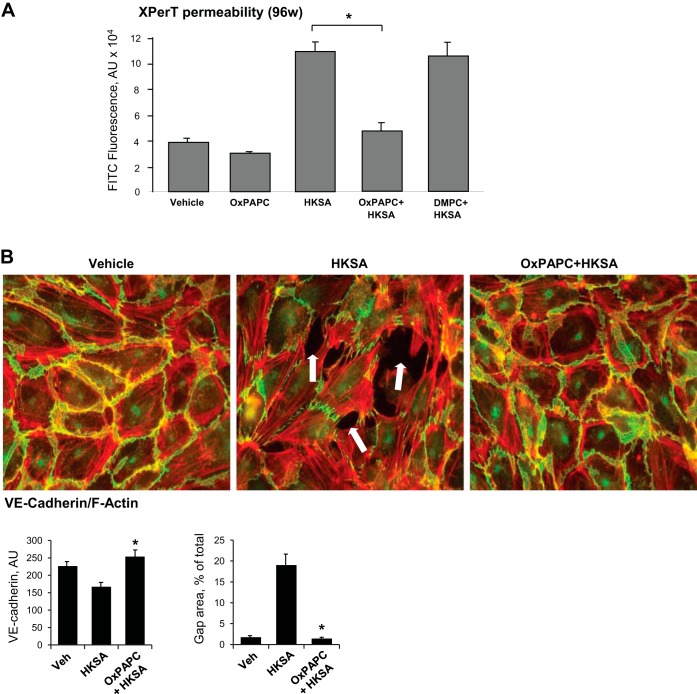
Effects of OxPAPC on EC permeability induced by HKSA were examined using the XPerT permeability assay described in methods. Quantitative analysis was first performed in 96-well plates with EC monolayers and the measurement of matrix-bound FITC-avidin fluorescence using a microplate fluorimeter reader. In these experiments, HPAEC were cotreated with OxPAPC and HKSA for 6 h. Control experiments were performed with oxidation-resistant phospholipid DMPC as a negative control. OxPAPC, but not DMPC, dramatically attenuated disruptive effects of HKSA (Fig. 3A).
Fig. 3.
Effects of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine oxidation (OxPAPC) cotreatment on HKSA-induced EC permeability, F-actin, and adherens junction remodeling. A: pulmonary EC grown in 96-well plates with immobilized biotinylated gelatin were treated with HKSA (5 × 108 particles/ml) with or without cotreatment with OxPAPC or DMPC (15 μg/ml), and permeability for FITC-avidin was evaluated by fluorimetric XPerT assay described in methods. Data are expressed as means ± SD of 6 independent experiments; *P < 0.05. B: endothelial monolayers were challenged with HKSA (5 × 108 particles/ml) with or without OxPAPC cotreatment and incubated for 6 h. Cytoskeletal remodeling was assessed by immunofluorescence staining for F-actin with Texas red phalloidin. VE-cadherin was detected by staining with VE-cadherin antibody. Shown are merged images of F-actin (red) and VE-cadherin (green) staining. Bar graphs depict quantitative analysis of the area of VE-cadherin-positive adherens junctions and intercellular gaps in control and treated EC monolayers. Data are expressed as means ± SD of 3 independent experiments; *P < 0.05. Veh, vehicle.
Effects of OxPAPC on the EC cytoskeletal remodeling induced by HKSA were next examined by immunofluorescence staining and visualization of actin cytoskeleton and VE-cadherin-positive adherens junctions in pulmonary EC after 6 h of HKSA treatment, the time point corresponding to developed EC monolayer permeability increase. HKSA-induced formation of paracellular gaps (shown by arrows) and the disappearance of VE-cadherin from cell junctions in the lung EC, which reflects disruption of the EC monolayer integrity, was abolished by cotreatment with OxPAPC (Fig. 3B).
OxPAPC suppresses inflammatory signaling activated by HKSA.
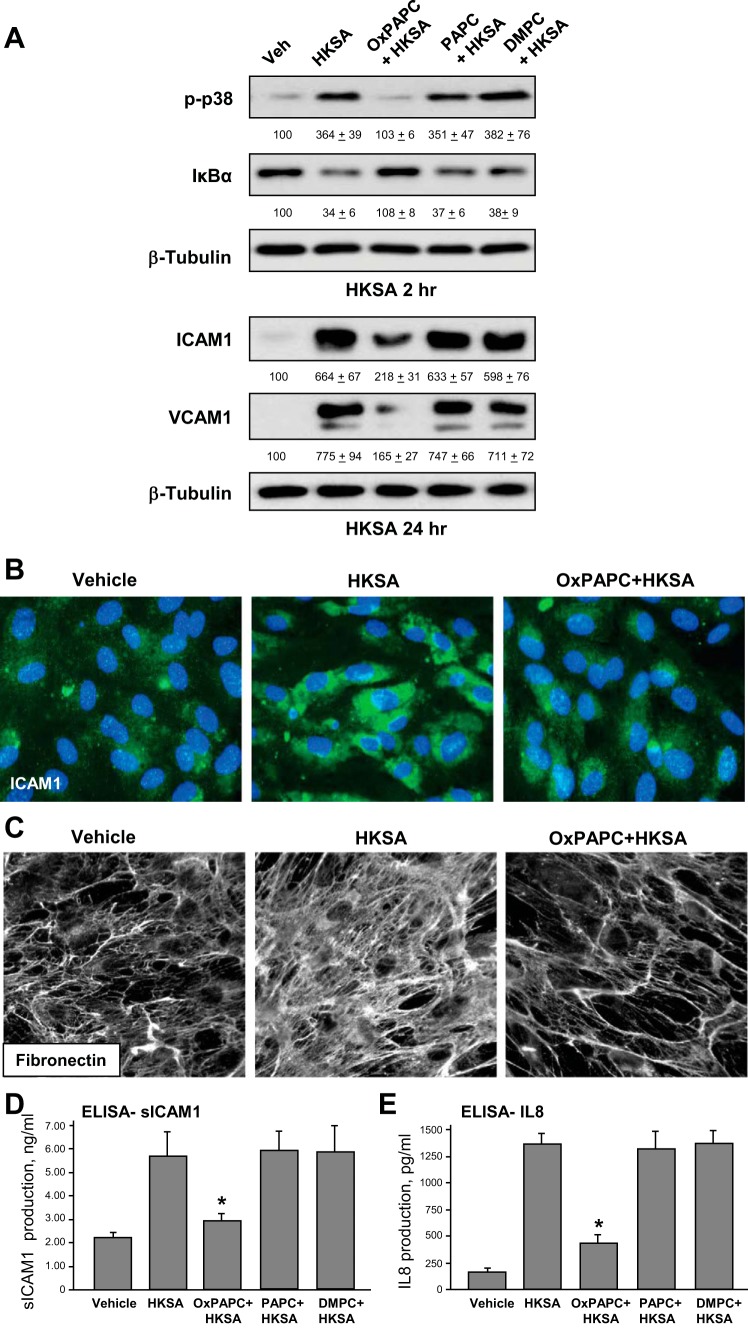
Protective effects of OxPAPC on pulmonary EC barrier were further linked to OxPAPC-induced attenuation of inflammatory signaling pathways activated by HKSA. EC were cotreated with OxPAPC and HKSA for 1, 24, or 48 h. Control experiments were performed with nonoxidized PAPC or DMPC described above. Effects of OxPAPC on activation of stress and inflammatory signaling were evaluated by Western blot. OxPAPC attenuated HKSA-induced p38 MAPK phosphorylation and IκBα subunit degradation after 2 h of HKSA treatment (Fig. 4A, top), and suppressed expression of adhesion molecules ICAM1 and VCAM1 after 24 h (Fig. 4A, middle). Of note, PAPC or DMPC administration failed to prevent EC inflammatory activation in response to HKSA. Increased cellular expression of ICAM1 by HKSA-stimulated EC was further confirmed by immunofluorescence staining of EC monolayers with an ICAM1 antibody (Fig. 4B). We next analyzed deposition of fibronectin extracellular matrix by activated EC using immunofluorescent staining of coverslips with a fibronectin antibody after the cell detachment step. Increased fibronectin deposition by HKSA-stimulated pulmonary EC was observed after 48 h of HKSA treatment; this effect was abolished by cotreatment with OxPAPC (Fig. 4C).
Fig. 4.
Effects of OxPAPC cotreatment on HKSA-induced inflammatory signaling. A: pulmonary EC were challenged with HKSA (5 × 108 particles/ml) with or without cotreatment with OxPAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), or dimirystoyl-sn-glycero-3-phosphorylcholine (DMPC) (15 μg/ml) and incubated for 2 or 24 h. Phosphorylation of p38 MAP kinase, degradation of IκBα, and expression of ICAM1 and VCAM1 were detected by immunoblotting with corresponding antibodies. Equal protein loading was verified by membrane probing with β-tubulin antibody. The results of Western blot quantitative densitometry are presented as means ± SD; n = 4. B: ICAM1 expression in HKSA-stimulated EC with and without OxPAPC cotreatment after 48 h of culture was visualized by immunofluorescence staining with ICAM1 antibody (green). Cell nuclei were visualized by DAPI staining (blue). C: fibronectin deposition by control stimulated EC after 48 h of culture was visualized by immunofluorescence staining of coverslips after cell detachment. D and E: EC were cultured for 24 h with vehicle, HKSA, with or without OxPAPC, PAPC, or DMPC. Secretion of soluble ICAM1 (D) or IL-8 (E) was evaluated by ELISA assay. Data are expressed as means ± SD of 5 independent experiments; *P < 0.05.
HKSA-induced EC inflammatory activation was additionally characterized by increased secretion of sICAM1 and IL-8 in cultured medium observed after 24 h of HKSA challenge. Cotreatment with OxPAPC abolished HKSA-induced sICAM1 and IL-8 production (Fig. 4D). Control treatment with PAPC or DMPC was without effect.
OxPAPC posttreatment attenuates EC barrier dysfunction.
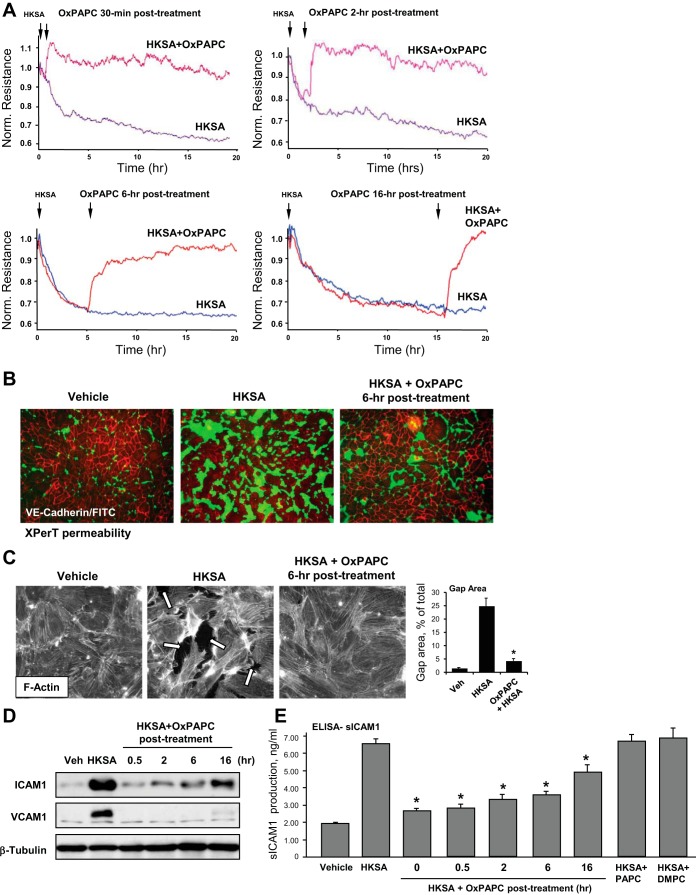
Because treatment of ongoing inflammation with protective compounds represents a more clinically relevant scenario of pharmacological intervention, the following studies evaluated the effects of OxPAPC posttreatment on HKSA-induced EC monolayer barrier dysfunction in the in vitro assays. Permeability changes in pulmonary EC cultures treated with OxPAPC at various time points after HKSA challenge were monitored by the measurements of TER. OxPAPC was added 30 min, 2 h, 6 h, or 16 h after HKSA stimulation. Remarkably, at all time points including 16 h of HKSA posttreatment OxPAPC exhibited potent protective effect (Fig. 5A).
Fig. 5.
Time-dependent effects of OxPAPC posttreatment on HKSA-induced EC permeability and inflammatory activation. A: pulmonary EC were challenged with HKSA (5 × 108 particles/ml) with or without posttreatment with OxPAPC (15 μg/ml) at different times after HKSA, and TER was measured over 20 h. B: EC grown in on glass coverslips with immobilized biotinylated gelatin were stimulated with HKSA (5 × 108 particles/ml) followed by posttreatment with OxPAPC (6 h after HKSA challenge) and incubation during 24 h. After addition of FITC-avidin for 5 min at the end of experiment, unbound FITC-avidin was removed, and sites of increased EC monolayer permeability were visualized by FITC fluorescence. C: EC monolayers were treated with HKSA (5 × 108 particles/ml) with or without OxPAPC posttreatment (6 h), and cytoskeletal remodeling was assessed by immunofluorescence staining for F-actin with Texas red phalloidin after 24 h of HKSA stimulation. Bar graphs depict quantitative analysis of gap area in control and HKSA-challenged EC monolayers with or without OxPAPC posttreatment (6 h). Data are expressed as means ± SD of 3 independent experiments; *P < 0.05. D: EC challenged with HKSA (5 × 108 particles/ml) were posttreated with OxPAPC at different times after HKSA addition, and expression of ICAM1 and VCAM1 was monitored by immunoblotting. Probing for β-tubulin was used as a normalization control. E: secretion of sICAM1 was measured by ELISA assay of EC conditioned media. Data are expressed as means ± SD of 6 independent experiments; *P < 0.05.
Visualization of EC monolayer permeability for FITC-avidin tracer using the XPerT permeability assay showed robust HKSA-induced EC barrier dysfunction, which was almost completely blocked by OxPAPC posttreatment 6 h after HKSA challenge (Fig. 5B). These effects were accompanied by disappearance of actin stress fibers and recovery of monolayer integrity in EC challenged with HKSA and posttreated with HKSA (Fig. 5C).
Posttreatment with OxPAPC 30 min, 2 h, 6 h, and 16 h after HKSA challenge markedly attenuated ICAM1 and VCAM1 expression by pulmonary EC (Fig. 5C). In complementary experiments, secretion of sICAM1 to the culture medium was measured at 0 min, 30 min, 2 h, 6 h, and 18 h of OxPAPC posttreatment after HKSA administration. OxPAPC suppressed sICAM1 production at all time points (Fig. 5D). Control posttreatment with nonoxidized PAPC or DMPC did not exhibit protective effects.
HKSA induces lung injury in vivo.
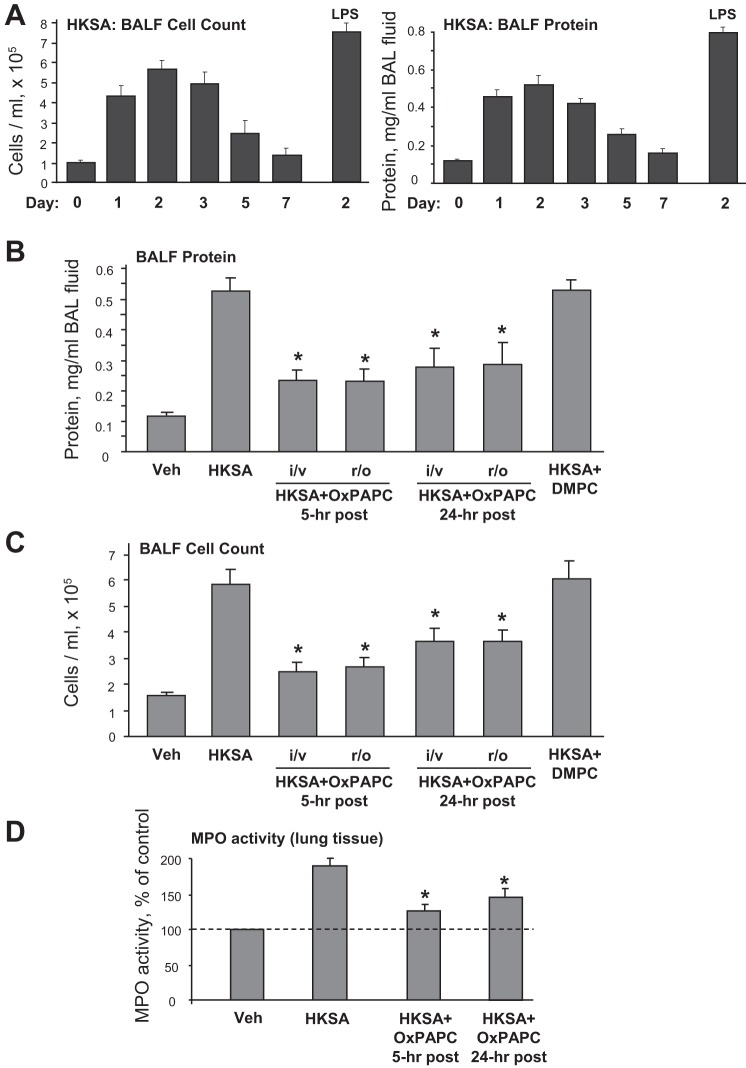
The time course of HKSA-induced lung injury was evaluated by the analysis of protein content and cell counts in the BAL samples taken from HKSA-challenged C57Bl/J6 mice. HKSA intratracheal administration caused prominent increases in both BAL cell counts (Fig. 6A) and protein concentration (Fig. 6B). The maximal effect was observed at day 2 after HKSA challenge, which was followed by a gradual decline and recovery by day 7. The day 2 time point of HKSA treatment was selected to evaluate the effects of OxPAPC posttreatment. The magnitude of changes in BAL protein and cell counts caused by HKSA was comparable to changes induced by LPS doses that were previously used in similar models of acute lung injury (ALI) (8).
Fig. 6.
Effects of OxPAPC posttreatment on HKSA-induced changes in bronchoalveolar lavage fluid (BALF) and MPO activity. A: C57BL/6J mice were treated with HKSA (2 × 108 cells/mouse it), and analysis of BALF cell counts and protein content was performed at different time points after HKSA challenge. Intratracheal injection of LPS (0.7 mg/kg) used in previous studies served as a reference control. B–D: after intratracheal injection of HKSA, 1.5 mg/kg OxPAPC was injected into jugular vein (i/v) or into retro-orbital sinus (r/o) after 5 h or 24 h of HKSA treatment. BALF protein content (B), BALF protein count (C) and lung tissue MPO activity (D) were measured as described in methods. Data are expressed as means ± SD of 4 independent experiments; *P < 0.05.
OxPAPC attenuates lung injury induced by HKSA in vivo.
We performed intravenous administration of OxPAPC in these experiments using injection into the jugular vein or into the retro-orbital venous sinus 5 or 24 h after intratracheal HKSA administration. Control experiments were performed with DMPC, which was administered 5 h after HKSA instillation. OxPAPC posttreatment significantly reduced protein content (Fig. 6B) and total cell count (Fig. 6C) in BAL fluid of HKSA-challenged mice. In contrast, DMPC had no protective effect on HKSA-induced lung injury. Of note, both, intrajugular and retro-orbital routes of OxPAPC administration were equally efficient, suggesting future use of retro-orbital injection as a more convenient experimental manipulation.
Severity of lung injury and inflammation was further monitored by measurements of myeloperoxidase (MPO) activity, a marker of neutrophil activation and tissue oxidative stress. HKSA significantly increased MPO activity measured in tissue homogenates, whereas OxPAPC posttreatment was protective at both time points, 5 and 24 h, of HKSA instillation (Fig. 6D).
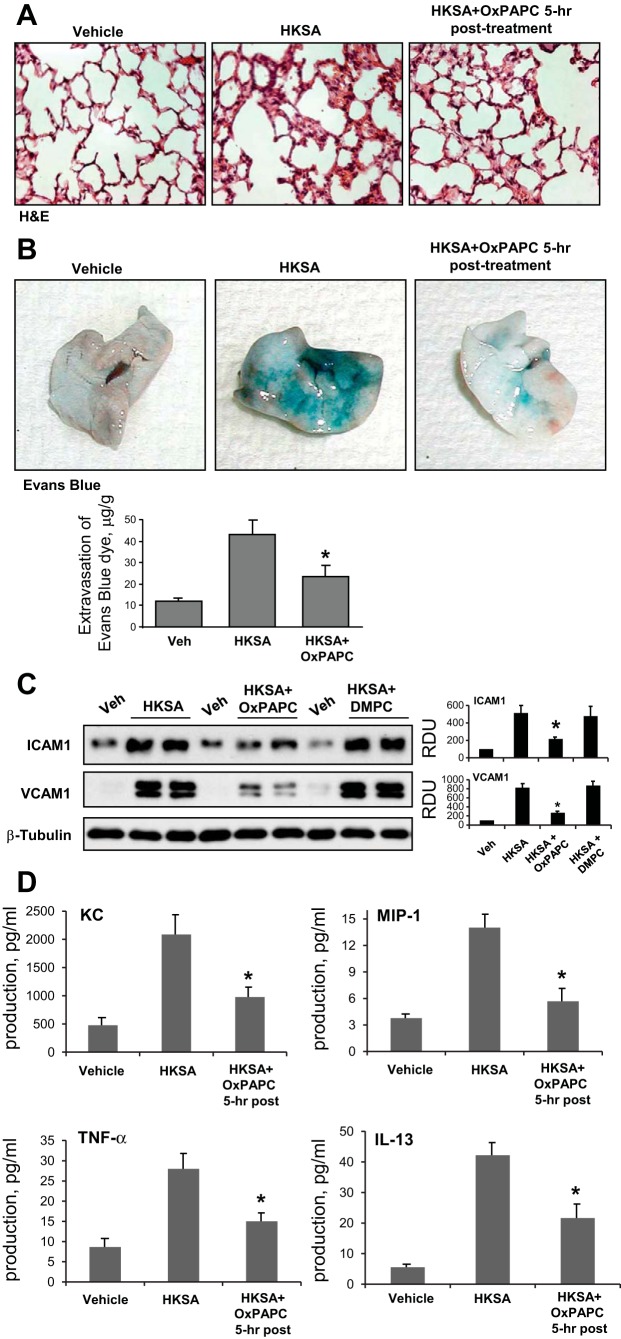
Histological analysis of lung tissue sections stained with hematoxylin and eosin showed that the treatment with HKSA induced neutrophil infiltration in the lung parenchyma and caused appearance of areas with alveolar hemorrhage indicative of vascular disruption. In consistence with the BAL data, OxPAPC posttreatment 5 h after HKSA abolished these effects (Fig. 7A).
Fig. 7.
Effects of OxPAPC posttreatment on HKSA-induced tissue injury Evans blue extravasation and inflammatory markers. C57BL/6J mice were challenged with vehicle or HKSA (2 × 108 cells/mouse it) with or without OxPAPC posttreatment (1.5 mg/kg, 5 h after HKSA). Analysis of lung injury was performed 48 h after HKSA challenge. A: histological analysis of lung tissue by hematoxylin and eosin staining (×40 magnification). B: Evans blue dye (30 ml/kg iv) was injected 2 h before termination of the experiment. Lung vascular permeability was assessed by Evans blue accumulation in the lung tissue. The quantitative analysis of Evans blue-labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples; *P < 0.05 vs. HKSA alone; n = 3. C: ICAM1 and VCAM1 protein expression in lung tissue samples was evaluated by immunoblotting analysis. Membrane probing with β-tubulin antibody was used as a normalization control. The results of Western blot quantitative densitometry are presented as means ± SD; n = 4. D: levels of mouse cytokines KC, MIP-1, TNF-α, and IL-13 were measured in BALF samples by ELISA assay. Data are expressed as means ± SD of 4 independent experiments; *P < 0.05.
Effects of OxPAPC posttreatment on the HKSA-induced lung vascular leak were evaluated by measurements of Evans blue extravasation into the lung tissue. Evans blue dye was intravenously injected 2 h before termination of the experiment. HKSA administration induced prominent Evans blue leakage from the vascular space into the lung parenchyma, which was suppressed by a single injection of OxPAPC after 5 h of HKSA (Fig. 7B). Quantitative analysis of Evans blue-labeled albumin extravasation in the lung tissue confirmed these results. OxPAPC posttreatment attenuated HKSA-induced ICAM1 and VCAM1 expression in the lung, as detected by Western blot analysis of lung tissue homogenates (Fig. 7C). DMPC had no protective effects.
Because HKSA treatment stimulated IL-8 production by human pulmonary EC cultures in vitro, effects of OxPAPC on HKSA-induced cytokine production were further tested in the murine model of ALI. The levels of mouse cytokines were measured in BAL samples by use of a multiplex cytokine ELISA assay. HKSA administration significantly increased KC, MIP-1, TNF-α, and IL-13 levels detected in BAL samples (Fig. 7D), whereas OxPAPC injection 5 h after HKSA significantly attenuated cytokine production. Altogether, these data demonstrate potent protective effects of OxPAPC posttreatment against lung inflammation and barrier dysfunction induced by gram-positive pathogens.
DISCUSSION
This study showed that HKSA-induced EC permeability and inflammation was abolished by posttreatment with OxPAPC. OxPAPC showed a strong anti-inflammatory effect and blunted HKSA-induced p38 MAP kinase, NF-κB signaling, and inhibited HKSA-induced fibronectin deposition, IL-8 production, and release of soluble ICAM1 by pulmonary EC. Cotreatment of HKSA-challenged cells with OxPAPC prevented disruption of VE-cadherin-positive adherens junctions, formation of intercellular gaps, and cytoskeletal remodeling. The anti-inflammatory and barrier-protective activities were attributed to oxidized phospholipids, because treatments with nonoxidized PAPC or oxidation-resistant phospholipid, DMPC, did not affect HKSA-induced injury.
OxPAPC was also effective in the animal model of HKSA-induced lung injury. Of note, the utilization of separate routes of HKSA and OxPAPC administration in the in vivo studies allowed to avoid direct competitive inhibition of HKSA interaction with TLR receptors by OxPAPC. Concurrent intravenous administration of OxPAPC attenuated the HKSA-induced inflammatory and barrier-disruptive response, suggesting a major role of the pulmonary vascular endothelium in the pathogenesis of ALI caused by inhaled pathogens.
Many studies screening for novel protective compounds in cells and animal models of ALI utilize preventive or concurrent treatment protocols. Although the pretreatment approach remains valid for targeting of mechanisms preventing activation of injurious signaling and may still lead to identification of new protective molecules, mechanisms contributing to the suppression or reversal of the ongoing pathological process remain poorly understood. This study demonstrates for the first time that single intravenous injection of OxPAPC as posttreatment after HKSA challenge in the in vivo ALI model and in pulmonary EC culture significantly attenuated lung barrier dysfunction and acute inflammation. Unlike many other protective treatments, which become ineffective if applied later than 2–3 h after an inflammatory insult, OxPAPC posttreatment even after 16–24 h of HKSA administration induced pronounced EC barrier recovery and improved lung function. OxPAPC posttreatment also reversed HKSA-induced induction of ICAM1 and VCAM1 expression by pulmonary EC, as well as HKSA-induced expression of adhesion molecules in the lung tissue and elevation of inflammatory cytokines KC, MIP-1, TNF-α, and IL-13 in BAL samples. Of note, live bacteria may trigger additional inflammatory cascades and engage other cells types and cellular processes such as bacteria killing and phagocytosis. Thus further studies are required for the comprehensive evaluation of the beneficial effects of OxPAPC in the models of lung dysfunction caused by S. aureus.
Although the Rho pathway of vascular endothelial permeability is most common for endogenous vasoactive mediators (2, 19, 24, 40, 44, 47), mechanisms of EC barrier dysfunction caused by bacterial pathogens and involvement of Rho signaling remain much less understood. A recent study showed that both Src and Rho were required for full NF-κB activation caused by TLR2 ligands. Rho activation was reported downstream of TLR2-MyD88 (32). TLR2-dependent Rho activation was achieved via interaction with the Rho-specific GEF AKAP13 recruited by activated TLR2 (32). Because OxPAPC inhibits agonist-induced Rho signaling via negative Rac-Rho GTPase cross talk (16, 36), OxPAPC-dependent control of inflammatory signaling via negative regulation of Rho may be a plausible mechanism, which warrants further investigation.
The results of our present study strongly suggest a dual nature of OxPAPC protective effects toward lung barrier function: first, via blunting of Toll-like receptor/NF-κB proinflammatory signaling cascade characterized in previous studies (18), and second, via direct effects on cytoskeletal remodeling and enhancement of cell-cell interactions driven by Rac1- and Rap1-mediated mechanisms described by our group (10, 14, 17). Appearance of oxidized phospholipids in inflamed and injured tissues (25, 27, 43) suggests an intriguing mechanism of self-regulation of tissue inflammation and recovery, whereby endogenous oxidized phospholipids may act as a negative feedback mechanism serving to attenuate the acute immune response to endotoxin, which involves activation of Toll-like receptors and also directly enhance lung vascular barrier properties.
The efficacy of intravenous OxPAPC in attenuating ALI caused by intratracheal HKSA suggests a possible therapeutic role for this group of compounds. Moreover, the recent identification of specific oxidized phospholipid species exhibiting potent barrier-protective properties along with anti-inflammatory effects (13, 39) strongly supports the clinical significance of oxidized phospholipids as a potential new group of therapeutic agents. Further research toward development of stable synthetic molecules recapitulating biological activities of protective OxPAPC compounds will be important to more precisely characterize specific mechanisms of action of OxPAPC in vivo and increase their stability. Future studies in this direction may uncover a new treatment strategy utilizing OxPAPC as dual-function compounds with anti-inflammatory and barrier-protective properties for treatment of various conditions leading to ALI and/or sepsis.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL87823 and HL076259 for K. G. Birukov and HL089257 and HL107920 for A. A. Birukova.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M., F.M., Y.T., and N.S. performed experiments; A.M., F.M., Y.T., A.A.B., and K.G.B. analyzed data; A.M., G.M.M., A.A.B., and K.G.B. interpreted results of experiments; A.M. and K.G.B. drafted manuscript; A.M., F.M., Y.T., N.S., G.M.M., A.A.B., and K.G.B. approved final version of manuscript; F.M., Y.T., A.A.B., and K.G.B. prepared figures; G.M.M., A.A.B., and K.G.B. edited and revised manuscript; A.A.B. and K.G.B. conception and design of research.
REFERENCES
- 1.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103: 40–55, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290: L540–L548, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 295: L593–L602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, Birukov KG. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J Cell Physiol 227: 1883–1890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 211: 608–617, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 293: L199–L211, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res 161: 495–504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24: 2678–2688, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, Birukov KG. VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost. J Cell Physiol 227: 3405–3416, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res 317: 859–872, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol 226: 2052–2062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 419: 77–81, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-β-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest 86: 9–22, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612–624, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces lung injury through syndecan-1. Am J Pathol 174: 509–518, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCα and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 289: L176–L185, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kagan VE, Quinn PJ. Toward oxidative lipidomics of cell signaling. Antioxid Redox Signal 6: 199–202, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun 73: 7428–7435, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lechner AJ, Ryerse JS, Matuschak GM. Acute lung injury during bacterial or fungal sepsis. Microsc Res Tech 26: 444–456, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med 165: 1445–1450, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Luhrmann A, Deiters U, Skokowa J, Hanke M, Gessner JE, Muhlradt PF, Pabst R, Tschernig T. In vivo effects of a synthetic 2-kilodalton macrophage-activating lipopeptide of Mycoplasma fermentans after pulmonary application. Infect Immun 70: 3785–3792, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 286: L808–L816, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol 182: 3522–3529, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care 13: R28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res 150: 253–265, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol 75: 749–755, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonas SA, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JG, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med 173: 1130–1138, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinner RW, Teutsch SM, Simonsen L, Klug LA, Graber JM, Clarke MJ, Berkelman RL. Trends in infectious diseases mortality in the United States. JAMA 275: 189–193, 1996. [PubMed] [Google Scholar]
- 39.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol 20: 2248–2254, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13: 237–247, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Takeda K, Akira S. TLR signaling pathways. Semin Immunol 16: 3–9, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tian X, Tian Y, Gawlak G, Sarich N, Wu T, Birukova AA. Control of vascular permeability by atrial natriuretic peptide via GEF-H1-dependent mechanism. J Biol Chem 289: 5168–5183, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyurina YY, Tyurin VA, Kapralova VI, Amoscato AA, Epperly MW, Greenberger JS, Kagan VE. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol 580: 153–183, 2009. [DOI] [PubMed] [Google Scholar]
- 44.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 20: 402–414, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272: 13597–13607, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Xiaolu D, Jing P, Fang H, Lifen Y, Liwen W, Ciliu Z, Fei Y. Role of p115RhoGEF in lipopolysaccharide-induced mouse brain microvascular endothelial barrier dysfunction. Brain Res 1387: 1–7, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol 169: 4905–4912, 2002. [DOI] [PubMed] [Google Scholar]