Abstract
The objective of this study was to optimize the approach to obtain viable single flexor digitorum brevis (FDB) fibers following a collagenase digestion. A first aim was to determine the culture medium conditions for the collagenase digestion. The MEM yielded better fibers in terms of morphology and contractility than the DMEM. The addition of FBS to culture media was crucial to prevent fiber supercontraction. The addition of FBS to the physiological solution used during an experiment was also beneficial, especially during fatigue. Optimum FBS concentration in MEM was 10% (vol/vol), and for the physiological solution, it ranged between 0.2 and 1.0%. A second aim was to document the stability of single FDB fibers. If tested the day of the preparation, most fibers (∼80%) had stable contractions for up to 3 h, normal stimulus duration strength to elicit contractions, and normal and stable resting membrane potential during prolonged microelectrode penetration. A third aim was to document their fatigue kinetics. Major differences in fatigue resistance were observed between fibers as expected from the FDB fiber-type composition. All sarcoplasmic [Ca2+] and sarcomere length parameters returned to their prefatigue levels after a short recovery. The pCa-sarcomere shortening relationship of unfatigued fibers is very similar to the pCa-force curve reported in other studies. The pCa-sarcomere shortening from fatigue data is complicated by large decreases in sarcomere length between contractions. It is concluded that isolation of single fibers by a collagenase digestion is a viable preparation to study contractility and fatigue kinetics.
Keywords: calcium, culture medium, flexor digitorum brevis, membrane potential, sarcomere shortening
a variety of technical approaches have been used to study the physiological characteristics of skeletal muscle, including skinned fiber preparation, intact single fibers, and whole muscle in vitro and in situ. Although the major advantage of an in situ muscle preparation is the maintenance of an intact blood flow, a drawback of this technique is the difficulty of discerning properties intrinsic to the muscle itself from the effects resulting from changes in blood flow. In vitro, whole muscle preparations are not subject to such problems. However, preparations such as mouse extensor digitorum longus (EDL) and soleus are difficult to use at the physiological temperature of 37°C because of the presence of an anoxic core in the muscle center (4). This anoxic core is a major problem when studying muscle fatigue, as mouse EDL and soleus recovered only 50% and 80%, respectively, of their prefatigue tetanic force during a recovery period following fatigue (28, 36).
Recent studies have provided strong evidence for changes in KATP and ClC-1 Cl− channel activity at the onset of exercise and fatigue with major differences between fiber types (40, 41), and changes in K+ and Cl− conductance profoundly alter membrane excitability and contractility (25, 28, 36, 39, 42). Therefore, it is of great need to concomitantly study changes in membrane excitability, myoplasmic Ca2+ ([Ca2+]i), and contractility at the level of individual fiber types. The use of single muscle fibers provides such capability without any problems with blood flow and anoxic core. Mechanical dissection of single flexor digitorum brevis (FDB) fibers has been extensively used for such studies on muscle fatigue (15, 34, 37). It has, however, the disadvantage of limiting the number of fibers to be tested because of the lengthy dissection time, which can be as long as 3 h (21). This is an important consideration for two reasons. First, fibers can be damaged upon microelectrode penetration for cell membrane potential measurements, especially during contractions, increasing the need for a large number of available fibers. Secondly, FDB muscles comprise a spectrum of fiber types comprising primarily 6% I–IIA, 8% I–IIX, 19% IIA, 32% IIA–IIX, and 21% IIX (3). Associated with such a spectrum, we found that the fatigue kinetics of single FDB muscle fibers vary dramatically between fibers at 37°C (44). Consequently, a study on muscle fatigue among fiber types requires measurements of more than five fibers per experimental condition to acquire sufficient representation of each fiber type.
Another approach, which, in contrast, yields a large number of fibers, employs a collagenase digestion followed by trituration, an approach heavily used in the study of cardiac physiology. Such a preparation with skeletal muscle was first used by Bekoff and Betz (6, 7). It has been used to study ion channel properties under patch-clamp conditions (1, 46, 49), membrane potential, Ca2+ release, muscle contractility, and fatigue (2, 6, 7, 13, 18, 19, 26, 43, 48), as well as to study the intrinsic properties of muscle fibers in culture (6, 8, 38). However, although most studies showed that usable fibers can be obtained following a collagenase treatment, there is no documentation since the initial studies by Bekoff and Betz, about the extent of fiber viability and stability. Furthermore, when we first used this fiber preparation, our microelectrode penetrations resulted in massive membrane depolarization to −10 mV within 10 s; i.e., the method required improvement to ensure good excitability and resting potentials.
The overall purpose of this study was, therefore, to further outline properties and characteristics of the FDB single muscle fiber preparation following a collagenase treatment, and to establish standard parameters under which dependable results can be acquired. Three major aims were pursued. The methodology used for the collagenase digestion differs among various studies in which collagenase is either added to a saline physiological solution or to a culture medium supplemented with 10% FBS. Thus, the first aim was to determine which culture medium for the collagenase digestion yields the most viable fibers, as well as the importance of FBS in the culture medium, and the saline physiological solution used for membrane potential and contractile measurements. Recent studies have demonstrated a large production of reactive oxygen species (ROS) when the experimental temperature is 37°C. In whole muscles, the large ROS production is a major factor for the low force at 37°C (23), while single fibers can rapidly stop contracting during fatigue (37). Our measurements were conducted at 37°C. So, the second aim was to document the fiber stability at 37°C using fibers the day of their preparation and after a 24-h incubation period. In the absence of tendons, force or a pCa-force relationship cannot be measured. Instead, we measured changes in sarcomere length during contractions. Our third aim was to document the fatigue characteristics of these fibers in terms of 1) changes in [Ca2+]i and sarcomere length, 2) the pCa-sarcomere shortening relationship, and 3) the capacity to recover normal [Ca2+]i and sarcomere length after a recovery period.
METHODS
Animals
Experiments were carried out using single fibers from FDB muscles from CD-1 (Charles River, St. Constant, Canada) mice, which were 2–3-mo-old and weighed 20–30 g. Mice were fed ad libitum and housed according to the guidelines of the Canadian Council for Animal Care. The Animal Care Committee of the University of Ottawa approved all experimental procedures that were used in this study. Mice were anesthetized with a single intraperitoneal injection of 2.2 mg ketamine, 0.44 mg xylazine, and 0.22 mg acepromazine per 10 g of body weight. Subjects were then killed by cervical dislocation.
Single Fiber Preparation
Single fibers were isolated from FDB bundles by a method involving collagenase digestion. FDB muscles were incubated at 37°C in culture medium containing 0.2% (wt/vol) collagenase type I (Worthington, Lakewood, NJ), inactivated FBS (heat inactivated; Gibco, Burlington, ON, Canada) at the indicated concentrations, 100 units/ml of penicillin and 100 μg/ml of streptomycin (Gibco). Incubation time of 1 h was enough to obtain a small number of fibers, which can be used for experiments requiring a small number of fibers. Incubation time of 3 h was necessary if one required that all fibers be dissociated from one another. A large number of fibers was required for some measurements in this study, so all collagenase digestion lasted 3 h. Two culture media were tested: 1) MEM with Earle's salt and l-glutamine (MEM; Gibco) and 2) DMEM high glucose (DMEM; Gibco). Following incubation, fibers were separated by gentle trituration in 3 ml of collagenase-free culture medium. One hundred microliters of concentrated fiber-containing medium was placed on a Matrigel (VWR, Canada) precoated 12-mm diameter coverslip (VWR, Edmonton, Alberta, Canada). Fibers were then allowed 30 min to settle on the Matrigel and become fixed. Culture medium was then added to cover the entire coverslip, and fibers were incubated for at least another 30 min before the start of any experimental measurements.
Experimental Setup and Solutions
Coverslips containing single FDB fibers were mounted into a 370-μl chamber (model RC-25; Warner Instruments, Hamden, CT). Fibers were continuously superperfused with physiological solution at a rate of 5 ml/min. Experimental temperature was 37°C unless otherwise specified to be 25°C. The temperature was controlled for by simultaneously heating the plate in which the chamber was mounted, as well as the physiological solution running through the chamber, using a dual-channel heater controller (model TC-344B; Warner Instruments). The control physiological solution contained (in mM): 118.5 NaCl, 4.7 KCl, 2.4 CaCl2, 3.1 MgCl2, 25 NaHCO3, 2 NaH2PO4, and 5.5 d-glucose. All solutions were continuously bubbled with 95% O2-5% CO2 and had a pH of 7.4.
Protocols
Two major protocols were used. Protocol 1 was used for the experiments of our first aim, which was to test MEM vs. DMEM and the importance of FBS in culture media and physiological solution. In this case, the collagenase digestion was carried out in either the MEM or DMEM culture media with or without FBS. Morphological and contractile measurements were then carried out in physiological solution in the absence or presence of 0.2% FBS. Protocol 2 was used for our second aim, which was to obtain stability of a single fiber preparation, and our third aim, which was to measure fatigue kinetics. All collagenase digestion was carried out in MEM supplemented with 10% FBS, while measurements were carried out with physiological solution containing 0.2% FBS.
Fiber Stimulation
Fibers were stimulated using field stimulation, generated by two platinum electrodes that were 9 mm apart and running along each side of the chamber containing the fibers. The electrodes were connected to a Grass S88 stimulator and a SIU5 isolation unit (Grass Technologies, West Warwick, RI). Tetanic contractions were elicited with 200-ms trains of 0.3-ms, 10-V pulses at 100 Hz, unless otherwise indicated. Contraction frequency was one every 100 s, except when fatigue was elicited, for which the frequency was 1/s for 3 min.
Membrane Potential
Resting membrane potentials (resting Em) were measured using microelectrodes filled with 1 or 3 M KCl, K citrate, or K acetate. Microelectrode resistance ranged between 10 and 100 MΩ. Microelectrodes were connected to an Axoclamp 2A (Axon Instruments, Sunnyvale, CA). Data were recorded on a Kipp Zonen chart recorder (model BD100, Becton Dickinson, Franklin Lakes, NJ).
[Ca2+]i Measurement
Fibers were loaded with Fura 2 by incubating fibers 30 min at 37°C in culture medium containing 5 μM Fura-2 AM (Molecular Probes, Burlington, ON, Canada), similar to the protocol described by Westerblad and Allen (47). Fura-2 was alternatively excited at wavelengths of 340 and 380 nm using the IonOptix dual fluorescence system (IonOptix, Milton, MA), and light emission was measured at 505 nm with a Hamamatsu photomultiplier tube (Hamamatsu, Japan). Filters used were as follows: 340 ± 12 nm, 380 ± 6 nm, and 505 ± 6 nm. Fibers were viewed using a Zeiss Axo Observer A1 (North York, ON, Canada) at a magnification of ×200. Data acquisition was set at 200 Hz with the IonOptix software. [Ca2+]i was calculated, as previously described (9) using the following equation: [Ca2+]i = Kd·[(R − Rmin)/(Rmax − R)]·β, where R is the ratio of the fluorescence from 340-nm excitation over the fluorescence from 380-nm excitation; Kd is the dissociation constant of Fura-2 for Ca2+ (37°C: 224 nM) (35); Rmin is the minimum ratio measured at low Ca2+, being 89 ± 0.7% (n = 7 fibers) of R measured in resting fibers; Rmax is the maximum ratio at saturating [Ca2+], being 126.1 ± 7.5% of R during a tetanic contraction; and β is the fluorescence at 380-nm excitation of Ca2+-free divided by Ca2+-bound Fura-2, being 3.17 ± 0.72. Values for Rmin, Rmax, and β were obtained, as described by Boudreault et al. (9). Unstimulated [Ca2+]i, the [Ca2+]i in the absence of any stimulation, was calculated by averaging the [Ca2+]i values over the 100 ms preceding a contraction. Tetanic [Ca2+]i, the maximum [Ca2+]i during a contraction, was calculated by averaging the [Ca2+]i during the plateau phase.
Sarcomere Length Measurement
A MyoCam-S high-speed contractility camera and the IonOptix SarcLen software were used to measure sarcomere length before and during contractions. Briefly, a rectangle-shaped region is selected in which 20–25 sarcomeres are present. SarcLen first measured the light intensity longitudinally along four lines within the rectangle-shaped region. A frequency spectrum was then calculated using a fast Fourier transformation. Because of the periodicity and symmetry of A and I bands, the spectrum always had one strong peak at a given frequency that was dependent on the I band width. The frequency of the peak was converted to a sarcomere length from a calibration that was obtained using a grid of vertical black bars 10 μm apart. Sarcomere length was recorded at a frequency of 250 Hz. Unstimulated sarcomere length was defined as the length measured 5 ms prior to a contraction; tetanic sarcomere length was defined as the minimum length during a tetanic contraction, and sarcomere shortening was calculated as the difference between unstimulated and tetanic length.
Statistical Analysis
ANOVA was used to determine the significance of differences. Split plot designs were used because fibers were tested at all time, voltage, or frequency levels. ANOVA calculations were made using the version 9.0 GLM (general linear model) procedures of the Statistical Analysis Software (SAS Institute, Cary, NC). When a main effect or an interaction was significant, the least-squares difference (LSD) was used to locate the significant differences (45). The word “significant” refers only to a statistical difference where P < 0.05.
RESULTS
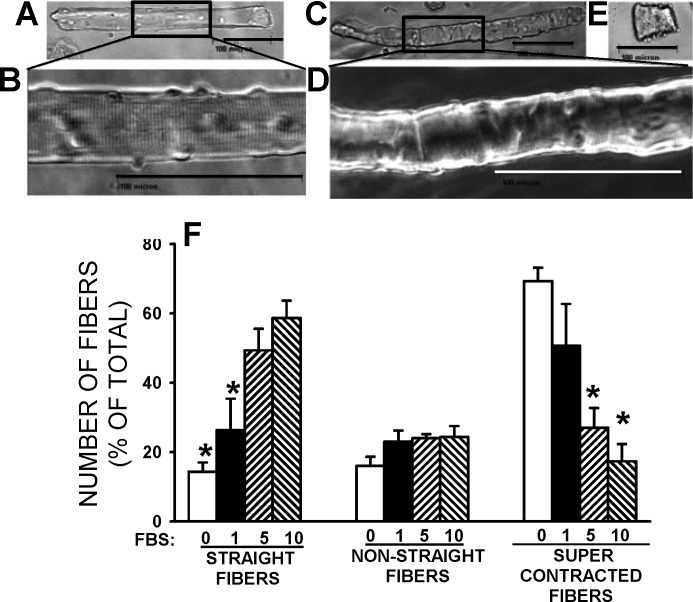
For any study of contractile properties, single fibers obtained using a collagenase digestion must be “viable”. In this study, viable fibers were first defined as fibers with “straight” appearance (Fig. 1A) with clearly discernible A and I bands (Fig. 1B), as opposed to less straight fibers (Fig. 1C) with A and I bands that are not easily discernible (Fig. 1D) or fibers that are supercontracted (Fig. 1E). Second, fiber viability was based on their contractility. For this study, three factors were taken into consideration: 1) the electrical stimulus that must be of a duration and strength similar to that used for intact muscles, mainly because pulse duration of 1 ms or longer causes a membrane depolarization large enough to directly stimulate Ca2+ release in the absence of action potentials (11); 2) the capacity to contract and contraction stability over a long time period; and 3) fibers must fully recover from a fatigue stress, as has been reported with mechanically dissected single muscle fibers (16).
Fig. 1.
Single-fiber appearance after a 3-h collagenase digestion in MEM culture medium containing the indicated FBS concentration (vol/vol). Example of a straight fiber (A), in which clear A and I bands (B) were observed. Example of a nonstraight fiber (C), in which membrane folds and not easily discernible A and I bands (D) are observed. E: example of a supercontracted fiber. A–E: scale bars = 100 μm. F: effects of varying FBS concentration on the number of single fibers with straight or nonstraight appearance or with supercontracted appearance. Vertical bars represent SE of 580–659 fibers from three mice. *Mean value significantly different from the mean value at 10% FBS, using ANOVA and least significant difference (LSD) test, P < 0.05.
Importance of Culture Medium Type and Presence of FBS
We first verified the importance of FBS during the collagenase digestion in MEM culture medium. In the complete absence of FBS, most fibers (69% of total) were supercontracted (Fig. 1F). As the FBS concentration was increased from 1 to 10% (vol/vol), the number of straight fibers increased from 26% to 59%, while the number of supercontracted fibers decreased from 51% to 17%. So, from this point on, all collagenase digestions were carried out using culture medium supplemented with 10% FBS.
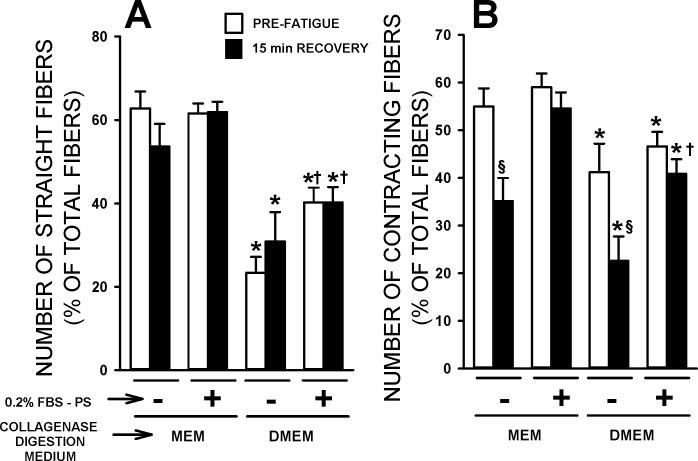
Next, we tested the fiber morphology and capacity to contract before and after fatigue in regard to 1) MEM vs. DMEM culture media during the collagenase digestion and 2) the necessity of adding FBS in the physiological solution. The proportion of straight fibers was significantly greater when the collagenase digestion was carried out in MEM compared with DMEM (Fig. 2A); this was observed in the absence or presence of FBS (physiological solution), as well as before fatigue and after 15 min of recovery following fatigue. Adding 10% FBS to the physiological solution caused a major foaming issue as the solution was constantly bubbled with 95% O2-5% CO2 to maintain a pH of 7.4. Therefore, we initially tested the impact of FBS in the physiological solution on fiber viability at a concentration of 0.2% (see section on resting Em for a FBS dose-response curve). The addition of 0.2% FBS in the physiological solution had no significant effect on the proportion of straight fibers when prepared in MEM. However, when fibers were prepared in DMEM, the presence of 0.2% FBS in the physiological solution significantly increased the proportion of straight fibers.
Fig. 2.
The collagenase digestion in MEM medium gave rise to a greater number of straight and contracting fibers than in DMEM medium, while the presence of 0.2% FBS in the physiological solution prevented the loss of contracting fibers following fatigue. Flexor digitorum brevis (FDB) fibers were prepared and tested according to protocol 1. Experimental temperature: 37°C. Contracting fibers were counted, while fibers were stimulated with a 200-ms train of 0.3 ms, 10-V pulses at 100 Hz. The numbers of straight fibers (A; as defined in Fig. 1A) and contracting fibers (B) are expressed as a percentage of the total number of fibers present on the coverslip. Prefatigue measurements were performed after a 15-min equilibration period. Fibers were fatigued with one 200-ms-long tetanic contraction every second for 3 min. Measurements were repeated after 15 min of recovery. Vertical bars represent SE of 109–297 fibers from six to eight mice. *Mean value for the DMEM condition was significantly different from the mean value for the MEM condition. §Mean value after 15 min of recovery was significantly different from the prefatigue mean value. †Mean value in 0.2% FBS was significantly different from the mean value in the absence of FBS, using ANOVA and LSD, P < 0.05.
For fiber contractility, the pulse duration was 0.3 ms long, which is similar to the duration used to elicit contraction in intact muscles (19, 36). Prior to fatigue and regardless of the FBS condition, the proportion of contracting fibers was significantly greater when prepared in MEM than in DMEM (Fig. 2B). The proportion of contracting fibers was not different between 0% and 0.2% FBS prior to fatigue. There was, however, a significant reduction in the number of contracting fibers after a fatigue bout and 15 min of recovery when FBS was absent, but not when it was present.
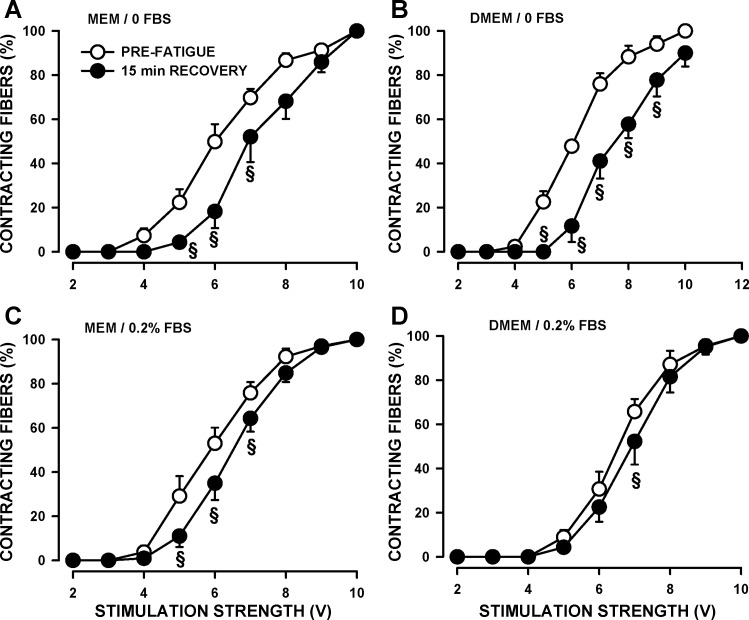
When stimulated, all fibers contracted in an all-or-none manner, but the threshold stimulus strength varied among fibers. Prior to fatigue, the stimulus strength that triggered a contraction in 50% of the fibers (SS50) was not different between fibers prepared in MEM or DMEM when FBS was absent from the physiological solution (Table 1). However, the addition of FBS significantly lowered SS50 for fibers prepared in MEM. By far, the greatest impact in regard to FBS was observed after the 3-min fatigue and after 15 min of recovery. That is, large and significant shifts toward higher SS50 were observed following fatigue when FBS was absent from the physiological solution regardless of the medium used during the collagenase digestion (Table 1, Fig. 3, A and B). Similar but smaller shifts were observed in the presence of 0.2% FBS (Fig. 3, C and D).
Table 1.
The effects of MEM, DMEM, and FBS (in the physiological solution) on the number of contracting fibers and stimulus strength relationship
| FBS | MEM | DMEM | |
|---|---|---|---|
| Prefatigue | 0% | 6.10 ± 0.24 (8) | 6.09 ± 0.08 (7) |
| 0.2% | 5.89 ± 0.27 (6) | 6.62 ± 0.20 (6)* | |
| 15-min Recovery | 0% | 7.08 ± 0.30 (6)§ | 7.50 ± 0.31 (5)§ |
| 0.2% | 6.51 ± 0.22 (6) | 6.94 ± 0.27 (6) |
Data are expressed as means ± SE (number in parentheses equals number of mice). The values in volts represent the stimulus strength that gave rise to 50% of fibers contracting (SS50). For each mouse, the number of contracting fibers and stimulus strengths were fitted by nonlinear regression analysis of a sigmoidal curve: No = Nmax/1 − e−(SS−SS50/Nmin), where No is the number of contracting fibers, SS is the stimulus strength, Nmax is the constant representing the maximum number of contracting fibers, Nmin is the constant representing the minimum number of contracting fibers, SS50 is the constant representing the stimulus strength that gave rise to 50% of contracting fibers.
Mean SS50 of DMEM was significantly different from mean SS50 of MEM.
Mean SS50 after 15 min of recovery was significantly different from prefatigue mean, ANOVA, and least significant difference, P < 0.05.
Fig. 3.
The presence of 0.2% FBS in the physiological solution significantly reduced the shift toward higher stimulus strength to elicit contraction after fatigue. FDB were prepared and tested, as described in Fig. 2. The numbers of contracting fibers are expressed as a percentage of the total number of contracting fibers when stimulated with a 200-ms train of 0.3 ms, 10-V pulses at 100 Hz. Error bars represent means ± SE of 109–297 fibers from six to eight mice. §Mean value after 15 min of recovery was significantly different from the prefatigue mean value, using ANOVA and LSD, P < 0.05.
Therefore, the results of the first aim suggested that single FDB fibers separated by trituration following a 3-h collagenase digestion in MEM containing 10% FBS and tested using a physiological solution containing 0.2% FBS have better morphology and contractile characteristics than when prepared in DMEM and with no FBS in the physiological solution.
Stability of Single FDB Fiber Preparation
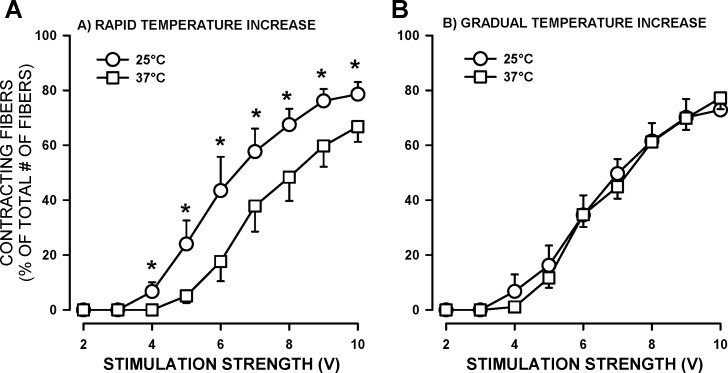
Single fibers were kept in an incubator set at 37°C until used. For an experiment, the protocol involved transfer of the fiber-containing coverslip from the incubator to the RC-25 chamber resulting in a decrease in temperature to room temperature. For consistency, the dual-channel heater controller was set for an initial experimental temperature of 25°C, and the temperature was then increased to a physiological 37°C. It was noted that raising the experimental temperature from 25° to 37°C in 2–3 min (fastest possible rate) caused a loss in the proportion of contracting fibers and a shift in SS50 toward higher strength (Fig. 4A). When a gradual approach was used (i.e., 2°C every 100 s), this reduction in contracting fibers and a shift in SS50 no longer occurred (Fig. 4B).
Fig. 4.
FDB single fibers maintained better contractility following gradual increase in temperature from 25°C to 37°C. Fibers were prepared and tested according to protocol 2. A: experimental temperature was increased from 25° to 37° within 2–3 min (fastest possible rate). B: experimental temperature was increased from 25° to 37°C at a rate of 2° every 100 s. For each experimental temperature, the numbers of contracting fibers (200-ms train of 0.3 ms, 10-V pulses at 100 Hz) were counted at different stimulation strengths and expressed as a percentage of the total number of fibers on the coverslip. Error bars represent means ± SE for eight fibers from three mice. *Mean percent of fibers contracting at 25°C was significantly different from the mean percent at 37°C. Using ANOVA and LSD, P < 0.05.
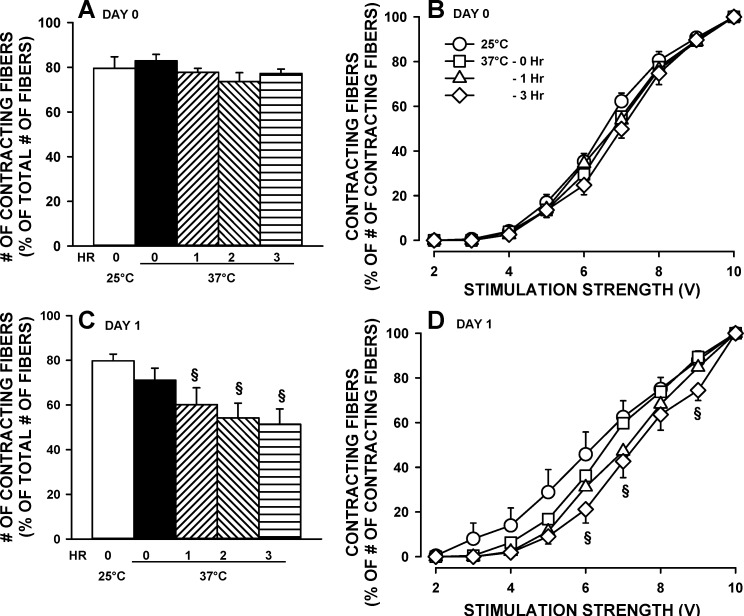
Single fibers kept in culture behave like denervated fibers; i.e., they atrophy while the cell membrane depolarizes (6, 7). It was, therefore, decided to determine the contractile stability of single fibers tested the same day (day 0) and day after (day 1) the preparation. For day 0, the number of contracting fibers remained constant, near 80%, and there was no shift in SS50 for up to 3 h (Fig. 5, A and B). For day 1, while the initial number of contracting fibers at 25°C was the same compared with day 0, that number significantly decreased as the experimental temperature was raised to 37°C and over a period of 3 h (Fig. 5C). There was also a shift in SS50 toward higher stimulation strength becoming significant by the 3rd h of testing (Fig. 5D).
Fig. 5.
Single FDB fibers were stable at 37°C over a 3-h period when tested the day they were prepared (day 0) but not on the following day (day 1). FDB fibers were prepared and tested according to protocol 2. Fibers tested on day 1 following their isolation were kept overnight at 37°C in MEM medium supplemented with 10% FBS. Fibers were elicited to contract every 100 s throughout the 3-h experimental period. A and C: numbers of contracting fibers at 10 V are expressed as a percentage of the total number of fibers on the coverslip. B and D: number of contracting fibers as a percentage of the total number of contracting fibers at 10 V vs. stimulus strength. Error bars represent the means ± SE of 290–312 fibers from seven mice. §Mean percent values significantly different the value at time 0. Using ANOVA and LSD, P < 0.05.
Characteristics of Single FDB Fibers
As discussed in the introduction, the major advantage of using single fibers is the capacity for measuring membrane potential, [Ca2+]i, and contractility from the same fibers. In the next section, we describe the best approach to measure resting Em, and in the subsequent two sections how [Ca2+]i and sarcomere length are affected by changes in stimulation frequencies and fatigue.
Resting membrane potential (resting Em).
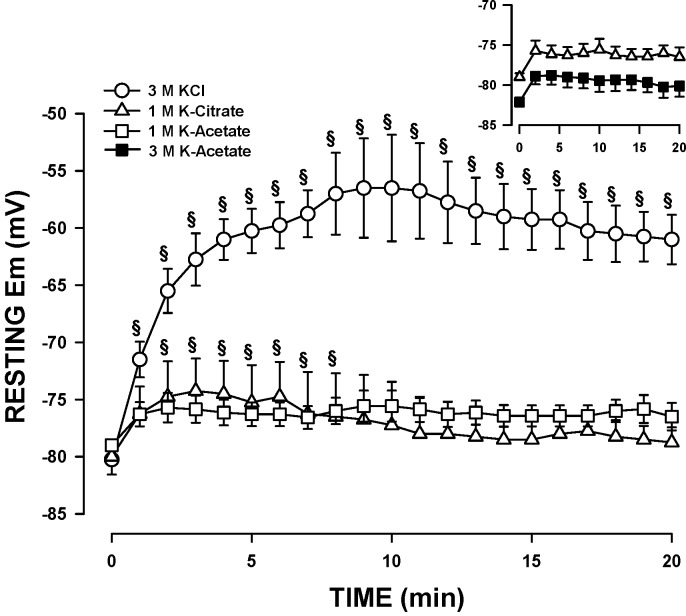
Our first approach for resting Em measurement was the use of conventional microelectrodes filled with 3 M KCl, as has been previously done for intact EDL and soleus muscles (28, 36). In the absence of FBS in the physiological solution, the membrane depolarized to –10 mV within 5 min (data not shown); the extent of the depolarization was reduced but not abolished if microelectrodes were dipped in FBS prior to their use (data not shown). So, we initially tested whether or not adding 1% FBS to the physiological solution would improve resting Em measurements with microelectrodes containing 3 M KCl. The mean resting Em upon penetration was −80 mV but was followed by a significant 24-mV depolarization (Fig. 6). The depolarization was largely suppressed by filling microelectrodes with 1 M K-citrate or K-acetate. With K-citrate, the initial resting Em was −80 mV and the depolarization just 6 mV. However, the variability among fibers was quite large. With K-acetate, the depolarization was not only the smallest (<4 mV), but the resting Em reached a steady state within 3 min and remained constant for 20 min. Resting Em was slightly higher when microelectrodes were filled with 3 M K-acetate as opposed to 1 M, but the differences were not significant (Fig. 6, inset).
Fig. 6.
Resting Em is best measured with microelectrodes filled with 3 M K-acetate. Fibers were prepared and tested, according to protocol 2, except that the physiological solution contained 1% FBS. Experimental temperature: 37°C. Microelectrodes were filled with KCl, K-citrate, or K-acetate. Error bars represent means ± SE of four to seven fibers from three to four mice. §Mean membrane potential (Em) was significantly different from the mean at time 0; using ANOVA and LSD, P < 0.05.
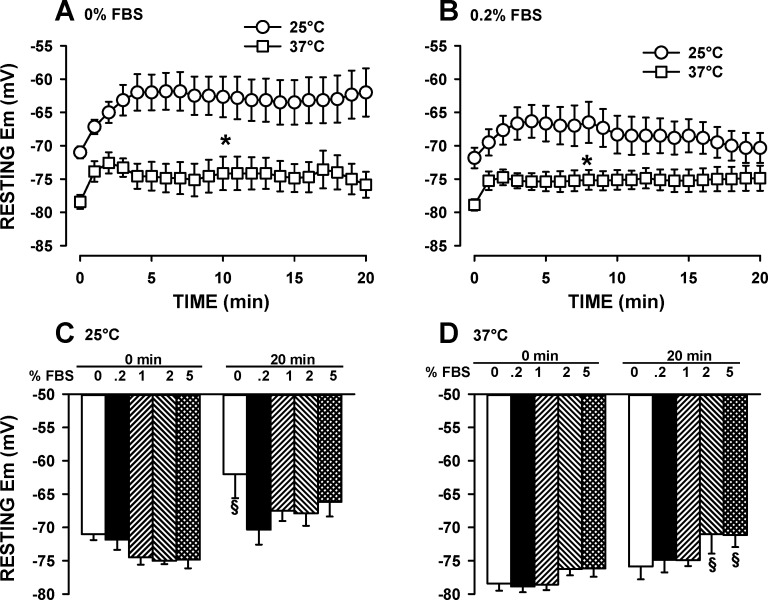
Since the addition of 0.2% FBS to the physiological solution improved the contractile characteristic, we endeavored to determine the optimum FBS concentration as indicated by Em measurements using microelectrodes filled with 1 M K-acetate. Furthermore, our resting Em values in Fig. 6 were much higher than those previously reported for FDB fibers at 25°C (6, 7, 43). So, to determine whether the differences were related to different experimental temperatures, we carried out the measurements at 25° and 37°C. In the absence of FBS, mean resting Em upon penetration at 25°C and 37°C were −71 and −78 mV, respectively (Fig. 7A). After 20 min of continuous recording, the membrane had depolarized by 9 mV at 25°C and by 5 mV at 37°C. Adding 0.2% FBS to the physiological solution, improved resting Em measurements at 25°C, but not at 37°C (Fig. 7B). However, at 37°C, the variability (i.e., SE) in resting Em between fibers was much less in the presence than in the absence of 0.2% FBS.
Fig. 7.
At 37°C, the optimum FBS concentration for resting Em measurements ranged from 0% to 1.0%. Fibers were prepared and tested according to protocol 2. Changes in resting Em over time when the physiological solution had no FBS (A) or contained 0.2% FBS (B). Variations in the FBS dose-response on resting Em at 25°C (C) and 37°C (D). Microelectrodes were filled with 1 M K-acetate. Error bars represent the SE of six or seven fibers from three or four mice. *At all times, mean Em at 37°C was significantly different from mean Em at 25°C. §Mean Em was significantly different from the mean Em at 0.2% FBS. Using ANOVA and LSD, P < 0.05.
Experiments were repeated at 1%, 2%, and 5% FBS. At 25°C, mean resting Em upon penetration was the highest when FDB was 1–5% FBS, whereas after 20 min of continuous penetration, resting Em was the highest with 0.2% FBS (Fig. 7C). At 37°C, the highest resting Em upon microelectrode penetration and after 20 min of continuous penetration was obtained while FBS was 0% to 1% (Fig. 7D).
Effect of stimulation frequencies on tetanic [Ca2+]i and sarcomere length.
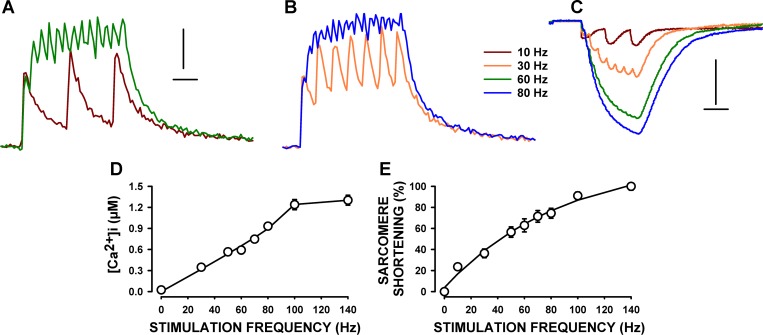
Stimulating a fiber at 10 Hz gave rise to three individual and transient [Ca2+]i increases and sarcomere shortening (Fig. 8, A–C). At 30 Hz, large fluctuations in tetanic [Ca2+]i were still present, while the change in tetanic sarcomere length was almost fused. Changes in sarcomere length were basically fused at 60 and 80 Hz, while fluctuations in tetanic [Ca2+]i could still be observed at 80 Hz. These fluctuations in [Ca2+]i were unlikely due to the noise associated with light measurements for two reasons. First, the number of peak Ca2+ clearly corresponded to the number of stimulation pulses up to 60 Hz. Second and more importantly, the magnitude of the fluctuations was greater than the variation prior to stimulation, including the changes in [Ca2+]i at 80 Hz. Thus, it appears that tetanic Ca2+ was still not completely fused at 80 Hz.
Fig. 8.
Frequency-[Ca2+]i and frequency-sarcomere shortening relationships in single FDB fibers. Fibers were prepared and tested according to protocol 2. Examples of [Ca2+]i (A and B) and sarcomere length (C) during tetanic contractions. Horizontal lines represent 100 ms, while vertical lines represent 0.2 μM (A and B) and 0.2 μm (C). D: [Ca2+]i-stimulation frequency relationship (data at 10 Hz not included because of the individual Ca2+ peaks). E: frequency-sarcomere shortening relationship. Sarcomere shortenings were calculated as the difference between unstimulated and tetanic sarcomere length and are expressed as a percentage of the shortening at 140 Hz. Error bars, which, here, are smaller than the symbols, represent the means ± SE of six fibers from four mice.
For tetanic [Ca2+]i, we averaged all [Ca2+]i value from the 20th to the 200th ms. The resulting mean tetanic [Ca2+]i steadily increased from 0.35 μM at 30 Hz to 1.23 μM at 100 Hz (Fig. 8D). A stimulation frequency of 140 Hz caused a further, albeit smaller, increase to 1.30 μM. The mean sarcomere length at rest, or unstimulated sarcomere length, was 1.91 μm. Sarcomere shortening during contractions at 30 Hz was 0.14 μm, or 36%, of the maximum shortening at 140 Hz (Fig. 8E). The extent of sarcomere shortening increased as stimulation frequencies were increased reaching 0.60 μm at 140 Hz.
Fatigue kinetics.
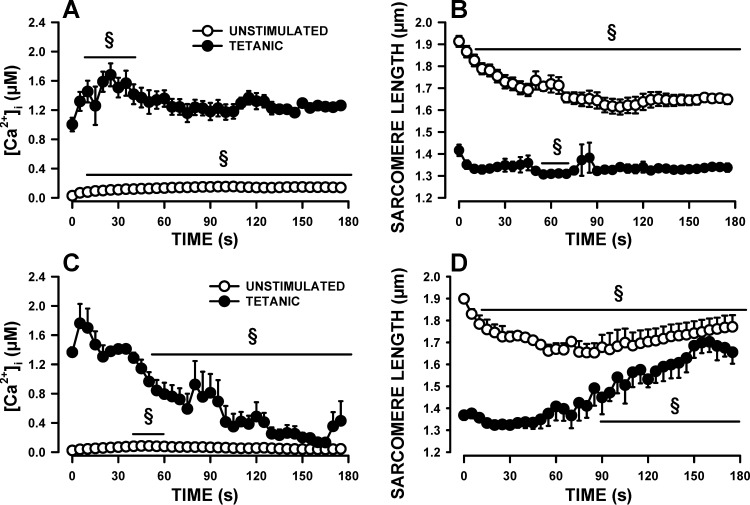
As a final test, we measured the effects of fatigue on [Ca2+]i and sarcomere length. Considering that no attachment maintains muscle fiber at constant length, one major aim here was to document the capacity of sarcomeres to return to normal unstimulated and tetanic length after a fatigue bout. We also noted major differences in the fatigue kinetics between fibers. A total of 18 fibers had been tested as part of a large study. For clarity, we report here examples of the more and less fatigue-resistant fibers. The more fatigue-resistant fibers (3 of the 18 fibers) were those for which the tetanic [Ca2+]i at the end of the fatigue period was still greater than the tetanic [Ca2+]i measured prior to fatigue, while the less fatigue-resistant fibers (3 of the 18 fibers) were those among several fibers [see Selvin and Renaud (44)] for which the decrease in tetanic [Ca2+]i were the largest.
For the more resistant fibers, mean unstimulated [Ca2+]i significantly rose from a resting value of 29 nM to 155 nM within 105 s, decreasing very slightly thereafter to 141 nM by 180 s (Fig. 9A). The unstimulated sarcomere length shortened from a resting value of 1.91 μm to 1.61 μm by 105, with no further changes thereafter (Fig. 9B), which coincided with the changes that occurred in unstimulated [Ca2+]i. Tetanic [Ca2+]i increased from 1.0 μM to 1.79 μM in 25 s before it declined slowly to 1.2 μM by the 65th s with no further changes until the end of the fatigue period (Fig. 9A). Mean tetanic sarcomere length decreased from 1.42 to 1.33 in 15 s, with no further changes thereafter, as expected from no changes in tetanic [Ca2+]i (Fig. 9B).
Fig. 9.
Changes in [Ca2+]i and sarcomere length during fatigue. Fibers were prepared and tested as per protocol 2. Fatigue was elicited with one 200-ms tetanic contraction every second for 3 min; data are shown every 5 s. Changes in unstimulated and tetanic [Ca2+]i (A) and sarcomere length (B) in the more fatigue-resistant fibers. Changes in unstimulated and tetanic [Ca2+]i (C) and sarcomere length (D) in the less fatigue-resistant fibers. Error bars represent means ± SE of three fibers for each category of fatigue-resistant fibers from four mice. §Mean value significantly different from the mean value before fatigue (time 0; using ANOVA and LSD, P < 0.05).
For the less fatigue-resistant fibers, mean resting unstimulated [Ca2+]i was 24 μM (Fig. 9C), a value similar to that of the more fatigue-resistant fibers. However, the initial increase in unstimulated [Ca2+]i was smaller for the less fatigue-resistant fibers reaching only 85 nM at 50 s compared with 155 nM for the more fatigue-resistant fibers. Another difference between the two fiber groups was a constant decrease in unstimulated [Ca2+]i in the less fatigue-resistant fibers, reaching a value of 60 nM by the end of the fatigue period compared with 141 nM for the more fatigue-resistant fibers. The changes in unstimulated sarcomere length mirrored those of the unstimulated [Ca2+]i. That is, while unstimulated [Ca2+]i increased the unstimulated sarcomere length shortened from 1.90 to 1.65 μm, and when unstimulated [Ca2+]i decreased to prefatigue levels, unstimulated sarcomere length increased reaching 1.77 μm by 180 s (Fig. 9D). Mean tetanic [Ca2+]i slightly increased from 1.37 to 1.41 μM (Fig. 9C). However, unlike the more fatigue-resistant fibers, tetanic [Ca2+]i of the less fatigue-resistant fibers rapidly decreased becoming significantly less than the prefatigue levels by 50 s. Thereafter, mean tetanic [Ca2+]i fluctuated as some fibers did not always release Ca2+ upon stimulation. Mean tetanic sarcomere length initially decreased from 1.37 μm to 1.32 μm (Fig. 9D). It remained stable for about 60 s. As fatigue progressed and as tetanic [Ca2+]i decreased, the capacity of shortening became less and tetanic sarcomere length started to increase reaching 1.66 μM s at 180 s.
For both the more and less fatigue-resistant fibers, mean sarcomere shortening, calculated as the difference between unstimulated and tetanic sarcomere length, was 0.52–0.53 μm prior to fatigue (Fig. 9, B and D). During the first 45 s, sarcomere shortening steadily declined to 0.37 μm; the decreases being mainly due to the large decreases in unstimulated sarcomere length, as tetanic sarcomere length was relatively constant during that time. Thereafter, mean sarcomere shortening remained constant for the more fatigue-resistant fibers as both unstimulated and tetanic sarcomere lengths were constant. However, for the less fatigue-resistant fibers, mean sarcomere shortening continued to decrease because of the large changes in tetanic sarcomere length. For both the more and the less fatigue-resistant fibers, all [Ca2+]i and sarcomere length parameters returned toward their prefatigue level after 10 min of recovery (data not shown).
pCa-sarcomere shortening relationship.
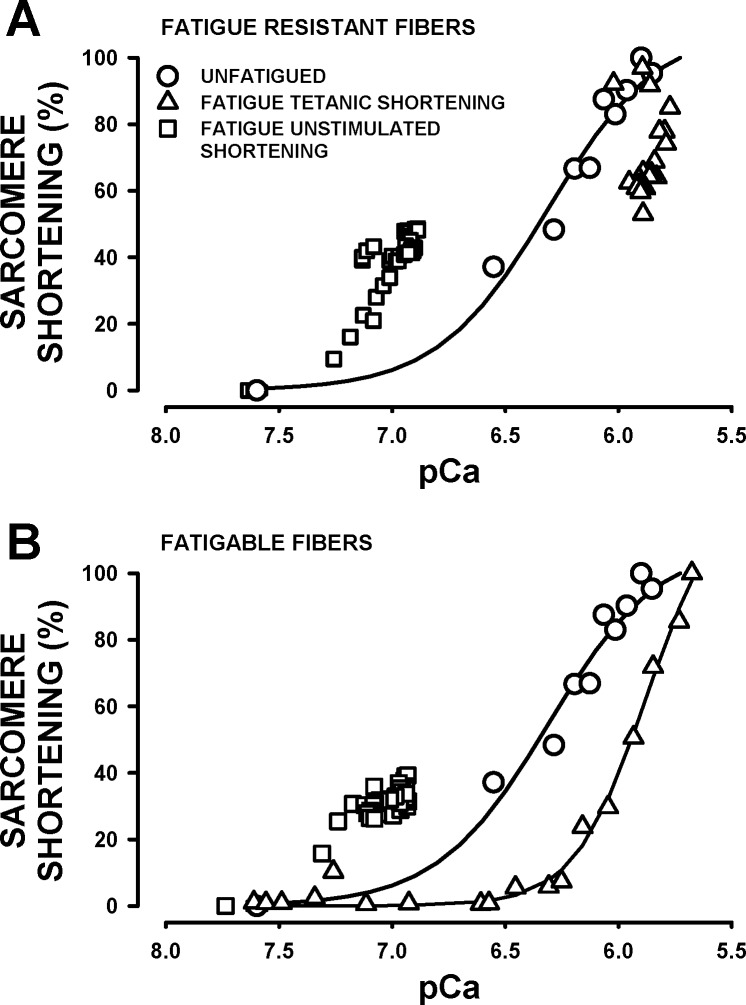
In the absence of any tendon, it is not possible to measure force and, therefore, construct a pCa-force relationship. However, as shown in Fig. 8 for unfatigued fibers, both tetanic [Ca2+]i and sarcomere shortening increased, as we increased the stimulation frequency. So, we plotted a [Ca2+]i-sarcomere shortening relationship using the mean [Ca2+]i and sarcomere shortening data from Fig. 8, D and E, similar to how it was done for the pCa-force relationship in mechanically dissected single FDB fibers (15), taking into account the following considerations. First, the pCa-force relationship was obtained at 22°C (15), a temperature at which tetanic [Ca2+]i and force reached a steady state at all stimulation frequencies, whereas it was not always the case at 37°C. At a stimulation frequency of 30 Hz, tetanic [Ca2+]i fluctuated to a greater extent than the sarcomere length (Fig. 8, A and B), suggesting that the on-off kinetics between Ca2+ and Fura-2 are faster than between Ca2+ and troponin; i.e., sarcomere shortening is expected to depend more on the mean rather than the peak tetanic [Ca2+]i. At higher stimulation frequencies (i.e., 80–140 Hz), tetanic [Ca2+]i increased rapidly for 8–12 ms and then at a much slower rate until it reached a plateau at 100 ms. For consistency, tetanic [Ca2+]i was calculated by averaging all [Ca2+]i values between 20 and 200 ms for all stimulation frequencies. Second, [Ca2+]i data were acquired at 200 Hz, a frequency that is too low to exactly follow the shape of the [Ca2+]i fluctuations when fibers were stimulated between 30 and 60 Hz. Measuring peak values from 30, 50, and 100 Hz, sine waves acquired at 200 Hz resulted in underestimated values. However, calculating the means of all data points over a 180-ms period resulted in the same value for all sine wave frequencies whether the sine waves were acquired at 200 Hz or 5 kHz (a frequency that was high enough to properly follow 30-, 50-, and 100-Hz sine waves). So, considering that we calculated mean tetanic [Ca2+]i over a 180-ms period, our mean [Ca2+]i values for stimulation frequencies between 30 and 60 Hz were not expected to be underestimated because the acquisition was set at 200 Hz. Third, calculating tetanic [Ca2+]i between 20 and 200 ms for stimulation frequencies between 80 and 140 Hz introduced a small error because of the small increase between 20 and 100 ms. However, the difference in tetanic [Ca2+]i for the 20–200 ms and 100–200 ms was 0.06 μM, representing a difference of 5%. Fourth, sarcomere shortenings did not reach a plateau by the end of the 200-ms-long tetanus. However, longer tetani are detrimental to single FDB fibers at 37°C because of large reactive oxygen species generation especially during fatigue (Bourassa F and Renaud JM, unpublished data and Ref. 37). So, for the sarcomere shortenings, we measured the largest shortening values from a 200-ms tetanus.
For unfatigued fibers, there was no difference in the pCa-sarcomere shortening relationship between the more and less fatigue-resistant fibers, so the data were grouped together. The relationship appeared sigmoidal with a pCa50 of 6.3 (Fig. 10A). We then plotted a pCa-sarcomere shortening relationship using the tetanic [Ca2+]i and sarcomere shortening measured during fatigue to investigate whether the capacity to shorten upon stimulation for a given pCa was the same or lower in fatigued fibers compared with unfatigued fibers. For the more fatigue-resistant fibers, the first three pCa-sarcomere shortening data points from the first 15 s of fatigue fell on the relationship of unfatigued fibers. All other data points then appeared shifted toward lower pCa, decreasing from 100% to as low as 53% despite a relatively constant pCa of 5.8–5.9 (Fig. 10A). This situation occurred because while tetanic [Ca2+]i and sarcomere length remained constant for most of the fatigue period, the large decreases in unstimulated sarcomere length during the first 90 s of fatigue (Fig. 9B) resulted in shorter sarcomere shortening. A similar situation was observed for the less fatigue-resistant fibers, where most of the decreases in sarcomere shortening at pCa lower than 6.0 were related to lower unstimulated sarcomere length (Fig. 10B). Between pCa of 7.5 and 6.5 (obtained during the last minute of the fatigue period) sarcomere shortening was zero for fatigued fibers compared with 36% at pCa 6.4 for unfatigued fibers, which suggests a decreased Ca2+ sensitivity at the end of fatigue.
Fig. 10.
pCa-sarcomere shortening relationships of the more fatigue-resistant fibers (A) and the less fatigue-resistant fibers (B). Symbols: ● (unfatigued); data are from the mean [Ca2+]i and sarcomere shortening from Fig. 8, D and E (same curve as in A and B); ▲ (fatigue tetanic shortening), pCa-sarcomere shortening using the tetanic [Ca2+]i and the extent of shortening upon stimulation during the fatigue period, the sarcomere shortening was calculated as the difference in unstimulated sarcomere length just prior to a contraction and the tetanic sarcomere length and expressed as a percentage of the largest sarcomere shortening observed during the fatigue period; ■ (fatigue unstimulated shortening), pCa-sarcomere shortening using the unstimulated [Ca2+]i and the differences in unstimulated sarcomere length prior to a contraction during fatigue and the unstimulated sarcomere length prior to fatigue; these differences represent the extent of the decreases in unstimulated sarcomere length between contractions during the fatigue period.
Finally, we investigated whether the decrease in unstimulated sarcomere length was solely related to the increase in unstimulated [Ca2+]i. For this, we calculated the difference in unstimulated sarcomere length just before a contraction and the unstimulated sarcomere length prior to fatigue. We then plotted the calculated differences vs. the pCa value calculated from the unstimulated [Ca2+]i just before a contraction. Notably, the unstimulated pCa-sarcomere shortening from the fatigue data was shifted to higher pCa (i.e., lower [Ca2+]i) for both the more and less fatigue-resistant fibers (Fig. 10, A and B). So for example, at pCa 7.0, the extent of unstimulated shortening sarcomere was greater during fatigue than the sarcomere shortening elicited by stimulation in unfatigued fibers.
DISCUSSION
The major findings of this study were 1) MEM was a better culture media for the collagenase digestion than DMEM, yielding single muscle fibers with better morphology (i.e., straight fiber with visible A and I bands) and contractile characteristics (i.e., greater number of contracting fibers, lower stimulation strength required for contractions before and after fatigue); 2) increasing the FBS concentration from 0% to 10% in MEM reduced the number of supercontracted fibers; 3) the presence of 0.2% FBS (vol/vol) in the physiological solution during experiments improved fiber contractility; 4) single FDB fibers maintained stable contractility for at least 3 h if tested the same day as the preparation was done, but not the day after; 5) good and stable membrane potentials were measured with microelectrodes filled with 1 M K-acetate and a physiological solution with an optimum FBS concentration between 0.2% and 1%; 6) large differences in fatigue kinetics can be observed between fibers, which were also able to fully recover their normal [Ca2+]i and sarcomere length during a short recovery period after fatigue; and 7) the pCa-sarcomere shortening relationship of unfatigued fibers has a sigmoidal shape, but as fatigue developed, the relationship became complex.
Importance of the Culture Medium and FBS
In terms of culture medium, the number of straight fibers with clear A and I bands and of contracting fibers were higher with MEM as opposed to DMEM, suggesting improved viability of the fibers with MEM. The improvement in viability was further supported by findings that with MEM, the stimulus strengths required to elicit contractions and the shift toward higher stimulus strengths following fatigue were smaller. Although these observations cannot be directly explained from our results, it is possible that they stem from differences in osmolarity between culture mediums. Compared with MEM, DMEM has extra nutrients (i.e., glycine, serine, ferric nitrate, and pyruvate) and greater glucose concentrations. The calculated osmolarity for MEM and DMEM are 319 and 369 mosmol/l, respectively. Considering an intracellular osmolarity of 290 mosmol/l, then DMEM is hyperosmotic to the myoplasm. Exposing rat FDB single fibers to 404 mosmol/l by adding sucrose leads to membrane depolarization and sarcoplasmic reticulum Ca2± release (43). Thus, it is possible that fiber morphology and contractility are affected by DMEM because of a decreased membrane excitability related to the depolarization (50) and fiber damage associated with large [Ca2+]i (27, 32).
The culture media used in this study were supplemented with a standard 10% (vol/vol) FBS concentration, which increased the number of viable fibers. Adding FBS to the physiological solution also resulted in improved fiber viability; that is, better resting Em, as well as lower loss of contracting fibers and shift to higher stimulus strength following fatigue. Interestingly, the optimum FBS concentration for culture media was 10%, whereas for the physiological solution, it ranged between 0.2 and 1.0% (at least for resting Em). The exact reason for the difference in FBS optima cannot be explained from our results, but the beneficial effect of FBS may be related to the transcription factor serum response factor (SRF). SRF is activated when serum is present in the extracellular milieu and modulates the expression of a multitude of genes involved in the maintenance of the sarcoplasmic reticulum and sarcomere, as well as being important during stress or following fiber damage (14, 30, 33). Thus, FBS can improve fiber viability during the collagenase digestion and fatigue, but its action remains to be determined.
Fiber Stability
The viability of fibers also depends on their stability during an experiment. Here, this stability was examined, with two issues becoming relevant: the length of time fibers are kept in culture and the experimental temperature. Single fibers were stable, i.e., maintain constant contractility for up to 3 h if used the day of the preparation, but consistently deteriorated if tested after being kept overnight at 37°C. That is, the SS50 continuously shifted over a 3-h period. Bekoff and Betz (6, 7) reported that single fibers maintained in culture behave like denervated fibers; i.e., they atrophy and the cell membrane depolarizes. Therefore, the decreased stability of single fibers after 24 h incubation may be a result of that process.
Cifelli et al. (19) have argued that an experimental temperature of 37°C is favorable when conducting in vitro research, as it most closely approximates physiological conditions. However, if the experimental temperature was raised to 37°C too quickly, such as within 2 min instead of 2°C every 100 s, some fibers stopped contracting. Conversely, when the experimental temperature is slowly increased, the stimulus strength to elicit contractions was the same at 25° and 37°C. The reduction in contractility cannot be explained by the development of an anoxic condition because at a rate of one tetanic contraction per second, intact whole EDL and soleus muscles maintain constant force over a long time period at 37°C (29, 36).
A possible explanation for the loss of contracting single fibers is a sudden increase in ROS. Edwards et al. (23) reported marginal increases in ROS production from 22°C to 32°C, but a five-fold increase from 32° to 37°C in rat and mouse EDL. They also reported large (∼50%) decreases in EDL tetanic force when they increased the temperature from 22°C to 37°C, and the decreases were less in the presence of ROS scavengers. In their experiments, EDL muscles were kept in 3 ml of physiological solution. When muscles are well superfused, with a rate such as 15 ml/min, EDL tetanic force actually increases when temperature is increased from 25°C to 37°C (Lucas B and Renaud JM, unpublished results). Mechanically dissected single FDB fibers contract properly at 37°C under nonfatiguing conditions, but during fatigue they rapidly lose their capacity to release Ca2+ and to contract unless exposed to ROS scavengers (37). Therefore, it is possible that a sudden and detrimental increase in ROS occurs when the experimental temperature is rapidly increased from 25° to 37°C, while a slower increase in temperature allows for the fiber defense mechanisms to better take effect, keeping ROS content at a normal level.
Resting Em Measurements
Resting Em and action potentials are easily measured from the surface fibers of mouse EDL and soleus when one uses microelectrodes filled with 3 M KCl (10, 28, 36). In single fibers, microelectrode filled with KCl was the least effective for recording stable resting Em due to very large membrane depolarization upon penetration. At rest, the sarcolemma is nine times more permeable to Cl− than to K+ (40). Furthermore, reducing extracellular Cl− concentration or adding 9-AC, a ClC-1 Cl− channel blocker, always hyperpolarized the cell membrane of FDB, EDL, and soleus muscles by about 15–30 mV (22, 43). The authors of the two studies had suggested that under normal conditions, Cl− is not in equilibrium with the resting Em and a net Cl− efflux constantly depolarizes the membrane. Thus, the large depolarization with KCl microelectrodes can be explained by similar K+ and Cl− diffusions out of the microelectrode into the myoplasm followed by much greater Cl− than K+ efflux to the extracellular space. K-citrate and K-acetate-filled microelectrodes gave rise to smaller depolarization following penetration because citrate and acetate have much lower membrane permeability than Cl−. Of the two filling solutions, K-acetate gave rise to the most stable resting Em with the smallest variations.
Membrane Excitability and Contractile Characteristics
Membrane excitability.
In terms of viability, it is critical that isolated single fibers exhibit characteristics similar to those measured from whole muscle bundles. Here, single fibers were elicited to contract with a stimulation pulse of 0.3 ms and stimulation strength from 2 to 10 V, which are values consistent with those used to elicit contractions in intact muscles (10, 11, 18). Under steady-state conditions, mean resting Em was −62 mV at 25°C, which is similar to reported values for FDB fibers tested at similar temperatures (6, 7, 43). However, at a more physiological temperature of 37°C, mean resting Em was −75 mV. The lumbrical muscle is another muscle for which resting Em is 10 mV more negative at 35°C than at 26°C (26). This is in contrast with EDL and soleus muscles for which resting Em is −80 mV at either 25°C or 37°C (10, 12, 28, 36, 40, 50). Resting Em of FDB and lumbrical fibers, thus, appear more sensitive to temperature than other hindlimb muscles, most likely because 1) Na+-K+-ATPase pump is more active at 37°C (20, 24) and 2) having much shorter muscle fibers, the electrogenic current generated by the pump may contribute more to the resting Em than do the much longer EDL and soleus fibers, in which the pump current is rapidly dissipated along the fiber length. Finally, the similarities in voltage strength and resting Em of FDB fibers to those of EDL and soleus suggest that the action potential threshold and membrane excitability are normal in our single FDB muscle fiber preparation.
Fatigue kinetics.
The next issue is how the fatigue kinetics measured under isotonic conditions with our fiber preparation compared with previous studies in which force was measured; albeit one must be cautious about such comparison because of differences in energy demand between isometric and isotonic contractions. One important observation is that with isotonic contractions and an experimental temperature of 37°C, we clearly demonstrated a wide range of fatigue kinetics. In this study, we showed examples of kinetics from the more fatigue-resistant fibers, which after 3-min tetanic [Ca2+]i and sarcomere length, were not different from prefatigue values, and of the less fatigue-resistant fibers, in which large and significant decreases in tetanic [Ca2+]i and sarcomere length were observed [further details can be found in Selvin and Renaud study (44)]. Such differences are, of course, not surprising considering that FDB muscle is primarily composed of the more fatigue-resistant fibers type I–IIA, I–IIX, and possibly IIA fibers, as well as the less fatigue-resistant type IIA–IIX and IIX fibers (3). Thus, our fiber preparation provides an excellent opportunity to study differences in the etiology of muscle fatigue between fiber types.
Many studies on muscle fatigue using mechanically dissected single FDB fibers have been carried out at room temperature (15–17). In one study carried out at 37°C (37), tetanic contractions were 400 ms long, and under those conditions, fibers stopped contracting within 1.5 s because of a large ROS production. We have actually observed a similar loss of contraction when the duration was 400 ms (Bourassa F and Renaud JM, unpublished observation), a situation that does not happen with a 200-ms duration (this study and Ref. 19).
Further comparisons can be done with the Cifelli et al. (18, 19) studies, in which small FDB bundles were used under the same fatigue protocol at 37°C. For FDB bundles, tetanic force, defined as the force generated upon stimulation, decreases at the onset of the fatigue stimulation with most of the decrease (70%) occurring within 60 s. For the less fatigue-resistant fibers, tetanic sarcomere length slightly decreased at first, remained constant for 60 s, and then increased as tetanic [Ca2+]i became submaximal. So, FDB bundles fatigue much sooner than single fibers. From an energy point of view, one would expect faster fatigue onset under isotonic than isometric contraction because the energy demand during shortening is greater than during any isometric contractions (5, 31). However, this effect is most likely masked by the development of hypoxia in the middle core of FDB bundles (4), accelerating the decrease in force, a situation that is either completely absent or of smaller amplitude for single fibers.
Under isometric conditions, small FDB bundles do not fully relax between contractions, resulting in the development of unstimulated force, defined as the force just prior to a contraction. Under control conditions, unstimulated force reaches a maximum by 60 s; it then decreases slowly until the end of the fatigue period. It is, therefore, not surprising that under isotonic conditions (this study), unstimulated sarcomere length also decreases during fatigue. Furthermore, for the more fatigable fibers, the decrease was continuous for 60 s before unstimulated sarcomere length started to increase again toward prefatigue level; i.e., the time course for the changes in unstimulated force and sarcomere length is, thus, very similar. However, while the increase in unstimulated force is most likely related to an increased unstimulated [Ca2+]i (19), the same is not true for the decrease in unstimulated sarcomere length. This is because the pCa-sarcomere shortening relationship obtained from unstimulated [Ca2+]i and sarcomere length values from the fatigue period is shifted toward lower [Ca2+]i compared with the relationship obtained from unfatigued fibers, suggesting that other factors are preventing the sarcomere length to fully return back to the resting level during the fatigue period.
pCa-sarcomere shortening relationship.
The last important point is whether we can determine when tetanic [Ca2+]i becomes submaximal during fatigue using a pCa-sarcomere shortening relationship, similar to how it was done for force measurement using a pCa-force relationship (15) with the considerations mentioned in results. For unfatigued fibers, the pCa-sarcomere shortening relationship had similar characteristics to those of the pCa-force relationship. It had a sigmoidal shape with a pCa of 6.3 for half-maximum tetanic sarcomere shortening (Ca50) and a pCa of 5.9 for maximum tetanic sarcomere shortening, which are values comparable to the respective values of 6.2 and 6.1 measured from the pCa-force curve of mechanically dissected single FDB fibers at 37°C (37). The pCa-sarcomere shortening relationship from the fatigue data was, however, complex. For the less fatigue-resistant fibers, it appeared to be shifted to higher [Ca2+]i. It is, however, unlikely that the apparent shift at pCa above 6.0 is related to a decrease Ca2+ sensitivity because the same was observed for the more fatigue-resistant fibers, and in that case, the decreases in sarcomere shortening were not due to lower capacity to shorten because the tetanic sarcomere length remained constant; it was, in fact, due to large decreases in unstimulated sarcomere length. Only the portion between pCa 7.5 and 6.5 can be interpreted as a decrease in Ca2+ sensitivity for sarcomere shortening because in this range, there is no shortening upon stimulation for fatigued fibers compared with a shortening of 40% of maximum at pCa 6.4 in unfatigued fibers. Consequently, to determine whether tetanic [Ca2+]i becomes submaximal during fatigue, we must use the pCa sarcomere shortening measured in unfatigued fibers and not compare the relationship between unfatigued and fatigued conditions.
In conclusion, this study establishes the parameters under which large numbers of single fibers isolated by trituration following a collagenase digestion can be used reliably for a variety of experimental purposes, as well as elucidating some of the benchmark characteristics that can be expected to be observed when using this protocol. Incubating FDB muscles in MEM culture medium containing 0.2% collagenase, and 10% FBS yields viable fibers that can be kept in MEM containing 10% FBS until they are used for experiments during which they are to be superfused with a saline physiological solution containing 0.2% FBS. The resulting fibers display clear A and I bands, stable contractions for up to 3 h if tested the day of the preparation, normal stimulus duration and strength to elicit contractions, normal and stable resting Em, and a normal capacity to fully recover their prefatigue contractility following a fatiguing stimulation. While the lack of tendons prevents direct force measurement, tetanic sarcomere length during contractions is a function of [Ca2+]i, allowing the possibility for many future experimenters to study the relationship between Em, [Ca2+]i, and sarcomere length during fatigue. Finally, our single FDB fiber preparations can be used for the study of muscle fatigue, as most fatigue kinetics are very similar to those reported in previous studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.S., E.H., and J.M.R. conception and design of research; D.S. and E.H. performed experiments; D.S., E.H., and J.M.R. analyzed data; D.S., E.H., and J.M.R. interpreted results of experiments; D.S., E.H., and J.M.R. prepared figures; D.S. and E.H. drafted manuscript; D.S., E.H., and J.M.R. edited and revised manuscript; D.S., E.H., and J.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by a National Science and Engineering Research Council of Canada (NSERC) grant to J.-M. Renaud.
REFERENCES
- 1.Allard B, Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflügers Arch 422: 185–192, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Bakker AJ, Head SI, Williams DA, Stephenson DG. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J Physiol 460: 1–13, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas K, Clow C, Jasmin BJ, Renaud JM. The KATP channel Kir6.2 subunit protein content is higher in glycolytic than in oxidative skeletel muscle fibers. Am J Physiol Regul Integr Comp Physiol 301: R916–R925, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Barclay CJ. Modeling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil 26: 225–235, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Barclay CJ, Constable JK, Gibbs CL. Energetics of fast- and slow-twitch muscle of the mouse. J Physiol 472: 61–80, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekoff A, Betz W. Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol 271: 537–547, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekoff A, Betz WJ. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult muscle. J Physiol 271: 25–40, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115: 129–139, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Boudreault L, Cifelli C, Bourassa F, Scott K, Renaud JM. Fatigue preconditioning increases fatigue resistance in mouse flexor digitorum brevis muscles with non-functioning KATP channels. J Physiol 588: 4549–4562, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns SP, Buller SJ, Loiselle DS, Renaud JM. Changes of action potentials and force at lowered [Na+]o in mouse skeletal muscle: implication for fatigue. Am J Physiol Cell Physiol 285: C1131–C1141, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cairns SP, Chin ER, Renaud JM. Stimulation pulse characteristics and electrode configuration determine site of excitation in isolated mammalian skeletal muscle: implications for fatigue. J Appl Physiol (1985) 103: 359–368, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cairns SP, Ruzhynsky V, Renaud JM. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am J Physiol Cell Physiol 287: C762–C770, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Carroll SL, Klein MG, Schneider MF. Calcium transients in intact rat skeletal muscle fibers in agarose gel. Am J Physiol Cell Physiol 269: C28–C34, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol 53: 147–157, 2002. [PubMed] [Google Scholar]
- 15.Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol 491: 813–824, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin ER, Allen DG. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol 498: 17–29, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin ER, Allen DG. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol 512: 831–840, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud JM. Contractile dysfunctions in KATP channel deficient mouse FDB during fatigue involve Ca2+ influx through L-type Ca2+ channels. Exp Physiol 93: 1126–1138, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Cifelli C, Bourassa F, Gariépy L, Banas K, Benkhalti M, Renaud JM. KATP channel deficiency in mouse FDB causes fiber damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol 582: 843–857, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen T, Flatman JA. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol 270: 383–414, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delbono O, Meissner G. Sarcoplasmic reticulum Ca2+ release in rat slow- and fast-twitch muscles. J Membr Biol 151: 123–130, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Dulhunty AF. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J Physiol 276: 67–82, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards JN, Macdonald WA, van der Poel C, Stephenson DG. O2·− production at 37°C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am J Physiol Cell Physiol 293: C650–C660, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Everts ME, Retterstol K, Clausen T. Effects of adrenaline on excitation-induced stimulation of the sodium-potassium pump in rat skeletal muscle. Acta Physiol Scand 134: 189–198, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Fraser JA, Huang CL, Pedersen TH. Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J Gen Physiol 138: 95–116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geukes Foppen RJ. In skeletal muscle the relaxation of the resting membrane potential induced by K+ permeability changes depends on Cl− transport. Pflügers Arch 447: 416–425, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Gissel H, Clausen T. Excitation-induced Ca2+ influx in rat soleus and EDL muscle: mechanisms and effects on cellular integrity. Am J Physiol Regul Integr Comp Physiol 279: R917–R924, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Gong B, Legault D, Miki T, Seino S, Renaud JM. KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus. Am J Physiol Cell Physiol 285: C1464–C1474, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Gong B, Miki T, Seino S, Renaud JM. A K+ATP deficiency affects resting tension not contractile force during fatigue in skeletal muscle. Am J Physiol Cell Physiol 279: C1351–C1358, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg ME, Ziff EB. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311: 433–437, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond Ser B 126: 136–195, 1938. [Google Scholar]
- 32.Jackson MJ, Jones DA, Edwards RHT. Experimental skeletal muscle damage: the nature of the calcium-activated degenerative processes. Eur J Clin Invest 14: 369–374, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Lamon S, Wallace MA, Russell AP. The STARS signaling pathways: a key regulator of skeletal muscle function. Pflügers Arch 466: 1659–1671, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Lee JA, Westerblad H, Allen DG. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol 433: 307–326, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Zhengyan Z, Xielai Z, Suhang L. The effect of lead on intracellualr Ca2+ in mouse lymphocytes. Toxic In Vitro 22: 1815–1819, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Matar W, Nosek TM, Wong D, Renaud JM. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during fatigue in skeletal muscle. Am J Physiol Cell Physiol 278: C404–C416, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing skeletal muscle at 37°C. J Physiol 564: 189–199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel J, Rannou F, Talarmin H, Giroux-Metges MA, Pennec JP, Dorange G, Gueret G. Sodium channel NaV1.5 expression is enhanced in cultured adult rat skeletal muscle fibers. J Membr Biol 235: 109–119, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol 125: 237–246, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen TH, de Paoli FV, Flatman JA, Nielsen OB. Regulation of CLC-1 and KATP channels in action potential-firing fast-twitch muscle fibers. J Gen Physiol 134: 309–322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen TH, Macdonald WA, de Paoli FV, Gurung IS, Nielsen OB. Comparison of regulated passive membrane conductance in action potential-firing fast- and slow-twitch muscle. J Gen Physiol 134: 323–337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science 305: 1144–1147, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Pickering Jd, White E, Duke AM, Steele DS. DHPR activation underlies SR Ca2+ release induced by osmotic stress in isolated rat skeletal muscle fibers. J Gen Physiol 133: 511–524, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvin D, Renaud JM. Changes in myoplasmic Ca2+ during fatigue differ between fibers, between glibenclamide exposed and Kir6.2−/− fibers and are further modulated by verapamil. Physiological Reports In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steel RGD, Torrie JH. Principles and Procedures of Statistics. A Biometrical Approach New York: McGraw-Hill, 1980. [Google Scholar]
- 46.Weik R, Neumcke B. Effects of potassium channel openers on single potassium channels in mouse skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol 342: 258–263, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Westerblad H, Allen DG. Changes of myoplasmic calcium during fatigue in single mouse muscle fibers. J Gen Physiol 98: 615–635, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DA, Head SI, Bakker AJ, Stephenson DG. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol 428: 243–256, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woll KH, Lonnendonker U, Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: different modes of blockage by internal cations, ATP and tolbutamide. Pflügers Arch 414: 622–628, 1989. [DOI] [PubMed] [Google Scholar]
- 50.Yensen C, Matar W, Renaud JM. The K+-induced twitch potentiation is not due to longer action potential. Am J Physiol Cell Physiol 283: C169–C177, 2002. [DOI] [PubMed] [Google Scholar]