Abstract
Ovariectomized rodents model human menopause in that they rapidly gain weight, reduce spontaneous physical activity (SPA), and develop metabolic dysfunction, including insulin resistance. How contrasting aerobic fitness levels impacts ovariectomy (OVX)-associated metabolic dysfunction is not known. Female rats selectively bred for high and low intrinsic aerobic fitness [high-capacity runners (HCR) and low-capacity runners (LCR), respectively] were maintained under sedentary conditions for 39 wk. Midway through the observation period, OVX or sham (SHM) operations were performed providing HCR-SHM, HCR-OVX, LCR-SHM, and LCR-OVX groups. Glucose tolerance, energy expenditure, and SPA were measured before and 4 wk after surgery, while body composition via dual-energy X-ray absorptiometry and adipose tissue distribution, brown adipose tissue (BAT), and skeletal muscle phenotype, hepatic lipid content, insulin resistance via homeostatic assessment model of insulin resistance and AdipoIR, and blood lipids were assessed at death. Remarkably, HCR were protected from OVX-associated increases in adiposity and insulin resistance, observed only in LCR. HCR rats were ∼30% smaller, had ∼70% greater spontaneous physical activity (SPA), consumed ∼10% more relative energy, had greater skeletal muscle proliferator-activated receptor coactivator 1-alpha, and ∼40% more BAT. OVX did not increase energy intake and reduced SPA to the same extent in both HCR and LCR. LCR were particularly affected by an OVX-associated reduction in resting energy expenditure and experienced a reduction in relative BAT; resting energy expenditure correlated positively with BAT across all animals (r = 0.6; P < 0.001). In conclusion, despite reduced SPA following OVX, high intrinsic aerobic fitness protects against OVX-associated increases in adiposity and insulin resistance. The mechanism may involve preservation of resting energy expenditure.
Keywords: ovarian hormones, energy expenditure, brown adipose tissue, menopause, fitness
menopause, or the cessation of ovarian hormone production, is associated with weight gain in the form of white adipose tissue (WAT), which preferentially accumulates in the intra-abdominal region (42). This is associated with metabolic dysfunction and increases the risk of developing cardiovascular disease and Type 2 diabetes. Indeed, postmenopausal women are at an increased risk of these metabolic diseases compared with premenopausal women and age-matched men (21). Since women are now living longer than any other period in history and spending more time in the postmenopausal phase of life, it is essential to identify strategies that will facilitate healthy aging, especially in this population. The mechanisms driving the body composition and metabolic changes that occur with menopause are not completely understood, but rodent (17) and human (7) data suggest that reduced physical activity and energy expenditure likely play a major role, while hyperphagia does not appear to be a primary cause of weight gain in this population (28). A recent study found that postmenopausal women lost significantly less weight following gastric banding surgery compared with equally obese premenopausal women (32), suggestive of a menopause-related reduction in energy expenditure. Following menopause, women significantly reduce their physical activity levels (7), and reduced physical activity is known to reduce aerobic fitness (2). The idea that a reduction in physical activity and related reduction in aerobic fitness contributes significantly to postmenopausal metabolic dysfunction is evidenced by the fact that aerobic exercise training has a dose-dependent, independent protective effect on metabolic syndrome among postmenopausal women (8). In that study, improvements in V̇o2 max, an indicator of aerobic fitness, was related to improvements in metabolic syndrome variables, such as waist circumference, glucose regulation, and blood pressure. While low aerobic fitness is a powerful independent risk factor for all-cause mortality and metabolic disease risk (24), the impact of aerobic fitness on postmenopausal metabolic function per se is largely unknown. Animal studies have demonstrated that loss of ovarian hormones causes a reduction in physical activity and that exercise can offset these effects (18), but no studies have investigated the unique role played by intrinsic aerobic fitness, independent of exercise, on the changes in energy balance, body composition, and insulin resistance following ovariectomy (OVX). Accordingly, the purpose of the present study was to test the hypothesis that high intrinsic aerobic fitness protects against the metabolic dysfunction associated with OVX in rats.
We used a model system of contrasting rat lines that were developed by artificial selection for low- and high-endurance treadmill running capacity. The divergent lines are termed “low-capacity runners” (LCR) and “high-capacity runners” (HCR) and originated from a founder population of the genetically heterogeneous N:NIH rats (22). An important feature of the model is that they are not exposed to running except for a running capacity test at ∼11 wk of age. Therefore, they display contrasting phenotypes in a sedentary condition, allowing for the unique investigation of aerobic fitness independent of structured exercise training and offer a method for investigating the mechanism(s) by which aerobic fitness protects against disease. At generation 11, the HCR rats displayed 30% higher aerobic fitness (V̇o2 max) and 347% longer run time to exhaustion than the LCR (50). Compared with LCR rats, HCR rats have increased longevity (23), better metabolic function, and are protected from a high-fat diet (HFD)-induced weight gain (29). For the first time, we used this model to determine the relative role of intrinsic aerobic fitness on the metabolic consequences associated with loss of ovarian hormones and found that, despite no protection against an OVX-associated reduction in physical activity, HCR rats are protected from increased adiposity and insulin resistance with OVX, suggestive of a protective role of aerobic fitness per se, which may be related to a preservation of resting energy metabolism.
METHODS
Animals and diets.
Female rats were artificially selected to be either LCR or HCR, as originally described by Koch and Britton (22) and Wisloff et al. (50) For this study, 43 randomly cycling female HCR and LCR rats (generation 32) were housed under standard temperature (e.g., ∼22°C) and humidity on a 12:12-h light-dark cycle. Rats were tested for running capacity at the University of Michigan at 11 wk of age and shipped to the University of Missouri at 16 wk of age, where they remained for the next 18 wk until the completion of the study. Animals were provided with standard rodent chow (LabDiet 5001, Purina) and water ad libitum for the duration of the study. National Institutes of Health guidelines were strictly followed, and all procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri prior to their initiation.
Ovariectomy and sham surgeries.
When rats were ∼25 wk old, ovariectomy or sham (SHM) operations were performed, creating the following groups: HCR-SHM (n = 7), HCR-OVX (n = 7), LCR-SHM (n = 14), and LCR-OVX (n = 15). Unless otherwise indicated, data are representative of all animals. Briefly, rats were anesthetized using inhaled isoflurane and then bilateral ovariectomies, or SHM operations were performed. For the ovariectomy, the whole ovary, including the ovarian bursa and part of the oviduct, were removed. For SHM, the ovary was externalized and then replaced back inside the body cavity. For both types of surgery, the single lateral incision was closed with wound clips. OVX surgery effectiveness was determined at the conclusion of the study via verification of significant uterine atrophy.
Energy expenditure and spontaneous physical activity assessment.
A metabolic monitoring system (Promethion, Sable Systems International, Las Vegas, NV) using oxygen consumption, carbon dioxide production, and a multidimensional beam break system was employed to assess fuel utilization, cage spontaneous physical activity (SPA), and total energy expenditure (TEE), resting energy expenditure (REE), and activity energy expenditure over a 72-h period, 8 wk following surgery. Subcomponents of energy expenditure were determined using manufacturer-constructed analysis macros (Sable Systems International). Animals were singly housed in the metabolic chamber system and allowed to acclimate to the chamber environment for 1 day before data collection. Data were analyzed as daily and cycle averages (i.e., 24-h, as well as individual 12:12-h light-dark cycle averages) and were calculated per animal; these daily averages were then used to calculate group means.
Glucose tolerance tests.
Prior to and 4 wk following surgery, glucose tolerance tests (GTT) were performed as a measure of insulin sensitivity. Following an overnight fast, glucose was sampled from the tail vein of each rat using a 23 g 3/4 blood collection set (Terumo Medical) and a hand-held glucometer, specifically designed for rodents (AlphaTrak; Abbott Laboratories, Chicago, IL). A sterile solution of glucose (2 g/kg body wt) was injected into the peritoneum of the fasted rat, and the blood glucose concentration was measured at 15, 30, 45, 60, and 120 min following the glucose administration. The parameter calculated for GTT is the area under the curve from T0 to T120 (AUC). Blood was collected at each GTT time point for analysis of the insulin response to glucose. Since it was difficult to obtain a blood sample for insulin analysis at every GTT time point, only 3–6 animals per group were used for the determination of insulin AUC during GTT; only animals for which blood was obtained at each GTT time point were used in the insulin AUC analysis. Insulin was measured from plasma collected during GTT using rat insulin ELISA kits per the manufacturer's instructions (Alpco Diagnostics, Salem NH).
Circulating metabolic markers.
At the end of the study, fasting (i.e., following an 8-h fast) serum and plasma samples were collected and stored at −80°C until analysis. The analysis of insulin, cholesterol, nonesterified fatty acids (NEFA), triglycerides (TG), and glucose was done using a clinical diagnostic service provided by the University of Missouri (Clinical Pathology Services, LLC), which uses an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) and commercially available assays, according to the manufacturer's guidelines.
Liver triglycerides.
Liver triacylglycerol concentration was determined using commercially available kits (F6428; Sigma, St. Louis, MO) and expressed per wet weight gram of tissue, as previously described (45).
Body composition assessment.
Body weight was measured weekly throughout the study. On the day of death, rats were assessed for body composition (i.e., total and % fat and lean mass and bone density) using dual-energy X-ray absorptiometry (DXA) (Hologic QDR-1000, calibrated for rodents). Following DXA, animals were killed, and to assess regional fat distribution, perigonadal (PGAT), perirenal (i.e., retroperitoneal, PRAT), omental (OAT), subcutaneous inguinal (SQAT), and interscapular brown adipose tissue (BAT) depots were removed and weighed to the nearest 0.01 g, as performed previously (34). To assess relative BAT, total BAT mass (g) was expressed relative to white adipose tissue (WAT) mass (BAT/WAT) and relative to body weight (BAT/BW). WAT mass was represented by the sum of all WAT depots (PGAT, PRAT, OAT, and SQAT).
Histological analysis.
Formalin-fixed BAT samples (n = 5/group) were processed through paraffin embedment, sectioned at 5 μm, and stained with anti-UCP-1 antibody produced in rabbit (1:1,200 dilution, U6382; Sigma), as previously described (4). Sections were examined using an Olympus BX60 photomicroscope (Olympus, Melville, NY) and photographed at ×20 magnification using with a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI). An investigator who was blinded to the experimental conditions collected four fields of view from each sample photograph. A representative photograph from one animal from each group was then selected. WAT samples from the PGAT, PRAT, and SQAT depots were processed similarly and stained with hematoxylin and eosin for the determination of mean adipocyte size. For cell size analysis, 100 random adipocytes, representing at least four fields of view, were analyzed per animal using ImageJ software (National Institutes of Health, public domain).
RNA extraction and real-time PCR.
Adipose tissue samples were homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT; Qiagen, Valencia, CA). Total RNA was isolated using the Qiagen's RNeasy lipid tissue kit and assayed using a Nanodrop spectrophotometer (ThermoScientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed, as previously described (34) using the ABI StepOne Plus sequence detection system (Applied Biosystems). Primer sequences (available upon request) were designed using the NCBI primer design tool. All primers were purchased from IDT (Coralville, IA). A 20-μl reaction mixture containing 10 μl iTaq UniverSYBR Green SMX (Bio-Rad, Hercules, CA) and the appropriate concentrations of gene-specific primers plus 2 μl of cDNA template were loaded in each well of a 96-well plate. All PCR reactions were performed in duplicate. PCR was performed with thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. 18S and/or GAPDH primers were used to amplify the endogenous control product (i.e., HK gene). mRNA expression values are presented as 2ΔCT, whereby ΔCT = HK CT − gene of interest CT. Data are expressed as the fold difference relative to the HCR-SHM group.
Western blot analysis.
Triton X-100 cell lysates were used to produce Western blot-ready Laemmli samples. Samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary antibodies. Proliferator-activated receptor coactivator 1-alpha (PGC1-α) antibody was purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ), UCP-1 antibody was obtained from Sigma-Aldrich (U6382; Sigma), and Ox Phos antibody cocktail from Abcam (ab110413; Cambridge, MA). Individual protein bands were quantified using a densitometer (Bio-Rad), and protein loading was corrected by 0.1% amido-black (Sigma) staining to determine total protein, as previously described (39).
Skeletal muscle citrate synthase activity.
Citrate synthase activity was determined using the method of Srere (43), as previously described by our group (39).
Statistical analysis.
All data were analyzed using SPSS 21.0. Statistical differences were determined using two-way ANOVA to determine whether line (i.e., HCR vs. LCR), treatment (i.e., OVX vs. SHM), and/or treatment × line interactions existed. When a significant treatment × line interaction was found, post hoc least significant difference tests were performed to determine between which pairs within the four groups (i.e., HCR-OVX, HCR-SHM, LCR-OVX, and LCR-SHM) differences occurred. Such post hoc differences are indicated by different letters or * if different from all other groups. For comparison of HCR and LCR prior to surgical treatments, Student's independent sample t-tests were performed. Pearson's bivariate two-tailed correlation analysis was performed to determine the relationship between relative BAT and REE. For weight gain over time, a two-way ANOVA with repeated measures was used; the significant treatment × line interaction was followed by post hoc tests to determine individual differences, which are indicated by different letters. Repeated-measures ANOVA was also used to determine how energy expenditure, SPA, and insulin resistance changed from presurgery to postsurgery. In all cases, P < 0.05 was considered statistically different and data are reported as means ± SE.
RESULTS
Compared to LCR, female HCR rats have better intrinsic aerobic capacity.
To determine running capacity (i.e., aerobic fitness), rats underwent three treadmill exercise trials (1 trial/day, every 2 days). After 32 generations of artificial selection for intrinsic aerobic fitness, HCR rats ran for significantly longer distance and duration (2,191.9 ± 82 m and 76.3 ± 2 min for HCR compared with 151.2 ± 8 m and 17.9 ± 0.4 min for LCR, P < 0.001 between HCR and LCR for both measures). These trials were conducted at 11 wk of age, after which time, the rats did not engage in any structured exercise training or exercise bouts.
HCR rats are protected from OVX-induced weight gain in the form of WAT.
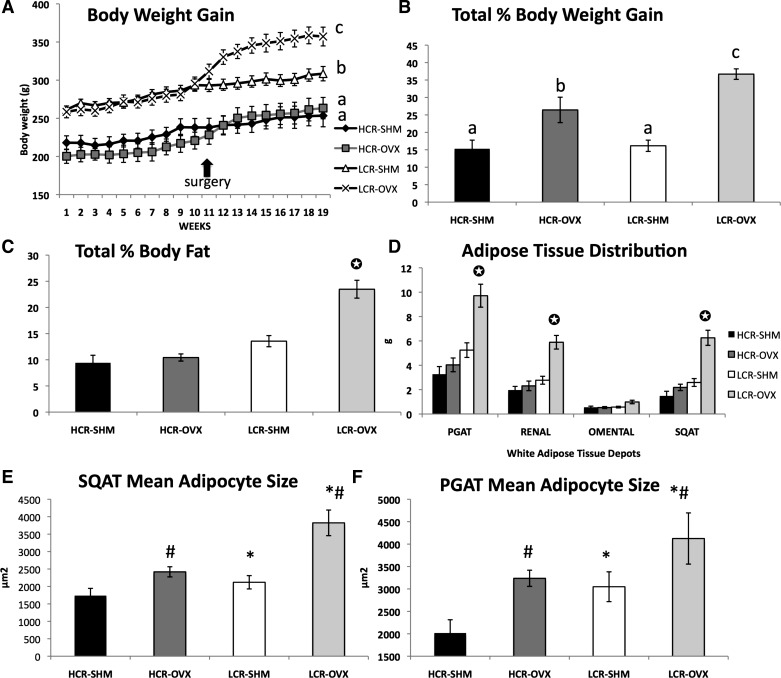
Rats were tracked weekly for body weight beginning at age 16 wk, upon arrival at the University of Missouri. Throughout the study, LCR rats were significantly heavier than HCR (Fig. 1A). Over the first 8 wk of observation, prior to OVX and SHM surgeries, LCR rats weighed ∼23% more than HCR [mean body weight for LCR compared with HCR were 268 and 208 g, respectively (P < 0.001)], yet the rate of weight gain was similar between HCR and LCR. HCR rats were protected from an OVX-associated increase in weight gain. Following OVX, LCR responded to OVX with greater weight gain. Thus, while OVX increased total % body weight gain from baseline in both lines, HCR were protected compared with LCR, as indicated by significant treatment × line interactions (both P < 0.03) for both weight gain over time and total weight gain (Fig. 1B). These differences coincided with comparable differences in adiposity, such that LCR responded to OVX with a greater body fat percentage increase (treatment × line interaction; P < 0.01), which resulted in LCR-OVX having a significantly greater percent body fat than the other three groups by the end of the study (Fig. 1C). Likewise, visceral (i.e., PGAT, PRAT, and OAT) and SQAT fat depots were heavier in LCR-OVX compared with LCR-SHM, whereas there were no differences in any of these fat depot weights between HCR-OVX and HCR-SHM (OVX × line interaction, P < 0.01 for PGAT, PRAT, and SQAT; OAT interaction, P = 0.087) (Fig. 1D). In addition, mean adipocyte size increased following OVX more so in LCR rats (Fig. 1, E and F). LCR also had greater total lean body mass than HCR, but OVX did not significantly alter lean mass in either line (HCR-SHM = 222 ± 23 g; LCR-SHM = 251 ± 24 g; HCR-OVX = 239 ± 35 g; LCR-OVX = 263 ± 31 g total lean mass, HCR vs. LCR main effect, P = 0.01). However, LCR but not HCR underwent a significant reduction in percent lean mass following OVX due to their significant gain in adiposity (OVX × line interaction; P = 0.002). HCR and LCR both underwent a similar reduction in bone mineral density following OVX with no differences between HCR and LCR (0.180 ± 0.0013, 0.173 ± 0.004, 0.183 ± 0.002, and 0.175 ± 0.002 g/cm2 for HCR-SHM, HCR-OVX, LCR-SHM, and LCR-OVX, respectively; OVX vs. SHM main effect, P < 0.01). As indication of successful OVX surgeries, all uteri were measured post mortem. Each OVX animal experienced significant uterine atrophy. That is, the mean uterine mass of OVX compared with SHM groups was significantly diminished (i.e., ∼73% reduction in mass) in both lines (1.03 ± 0.18 g, 0.30 ± 0.04 g, 1.00 ± 0.12 g, 0.26 ± 0.03 g for HCR-SHM, HCR-OVX, LCR-SHM, and LCR-OVX, respectively; for OVX vs. SHM main effect, P < 0.001).
Fig. 1.
Unlike low-capacity runner (LCR) rats, high-capacity runner (HCR) rats are protected against ovariectomy (OVX)-associated weight gain in the form of white adipose tissue. Body weight gain (A), % body weight gain from baseline (B), % body fat via dual-energy X-ray absorptiometry (DXA) (C), adipose tissue distribution via fat pad weights (D), and mean adipocyte size from the subcutaneous (SQAT) (E), and perigonadal (PGAT) depots (F). Total n = 43; two-way ANOVA for line (i.e., HCR vs. LCR), treatment [i.e., sham (SHM) vs. OVX], and treatment × line interaction effects were determined; significant interactions were followed by post hoc least significant difference tests to determine individual group differences; such differences, if found, are indicated by different letters or ✪ if different from all other groups. *HCR vs. LCR, P < 0.05; #OVX vs. SHM, P < 0.05. For adipocyte size analysis, n = 5/group.
Energy expenditure, not energy intake, associated with decreases post-OVX.
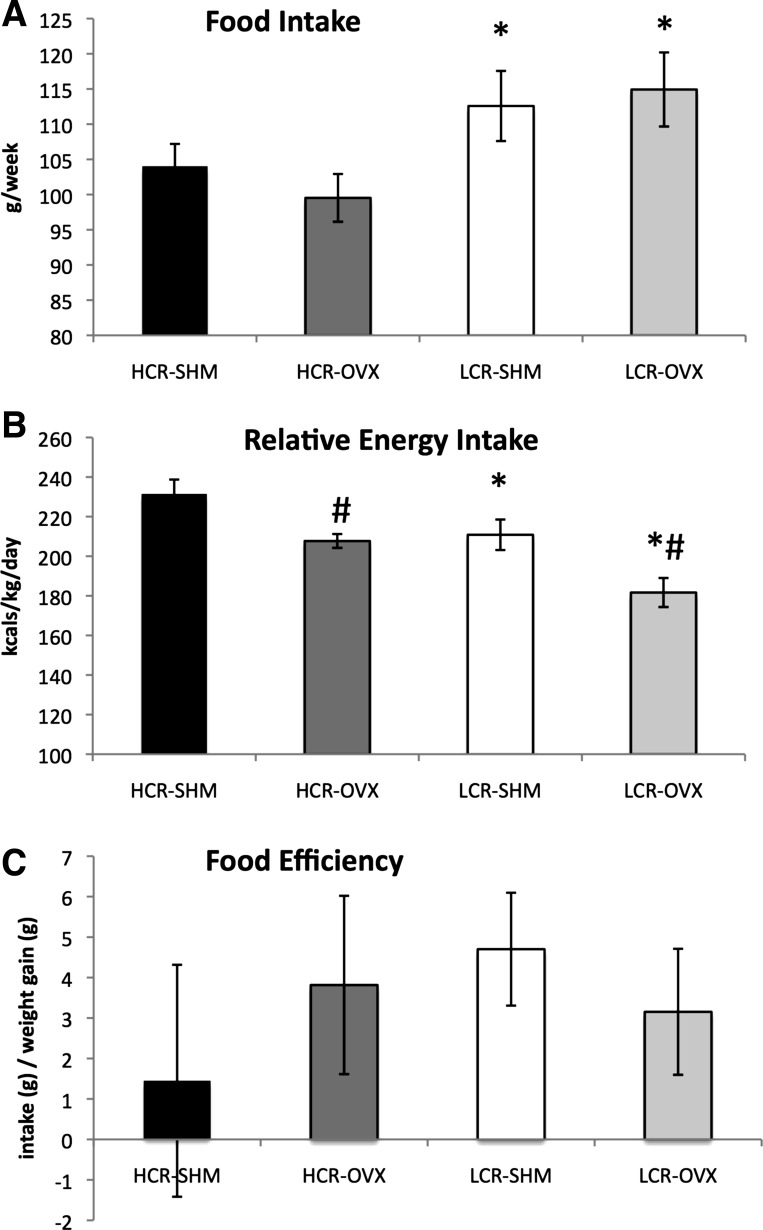
Prior to surgical treatments, total energy expenditure relative to body mass had a tendency to be greater in HCR than LCR (7.6 ± 0.3 vs. 6.9 ± 0.2 kcal·kg−1·h−1; P = 0.058). HCR rats were not significantly more physically active in their cages than LCR (21,626 ± 1,401 vs. 18,722 ± 2,362 total multidimensional beam breaks, P = 0.306) but did have higher resting energy expenditure (5.2 ± 0.2 vs. 4.7 ± 0.2 kcal·kg−1·h−1, P = 0.036), similar to results that have been reported previously in the model (31, 45). Prior to the intervention, LCR consumed more total food than HCR (87.8 ± 1.6 vs. 77.3 ± 3.8 g/wk, P < 0.001), but food intake relative to body weight (i.e., g food/g body wt) was greater for HCR (0.40 ± 0.02) vs. LCR (0.33 ± 0.01) (P < 0.001), suggestive of higher feeding efficiency in LCR.
Total food intake was greater among LCR and was not affected by OVX in either line (Fig. 2A). Energy intake was also expressed relative to body weight to account for differences in body weight between LCR and HCR animals. Similar to presurgery, HCR consumed more energy relative to their body weight than LCR, and both lines experienced a similar reduction in relative energy intake following OVX compared with SHM (Fig. 2B), again suggesting that HCR had lower feed efficiency and that feed efficiency increased following OVX. Surprisingly, we observed no differences in feeding efficiency (i.e., food consumed relative to weight gained over a 1-wk period) either between HCR and LCR or in response to OVX; however, these data were only collected over 1 wk and may not be reflective of the entire intervention period (Fig. 2C).
Fig. 2.
Energy intake is not affected by OVX in HCR or LCR rats. Total grams of food consumed per week (A), daily energy intake relative to body weight (B), and food efficiency based on the ratio of food consumed and body weight gained over a 1-wk period (C). *HCR vs. LCR, P < 0.05; #OVX vs. SHM, P < 0.05.
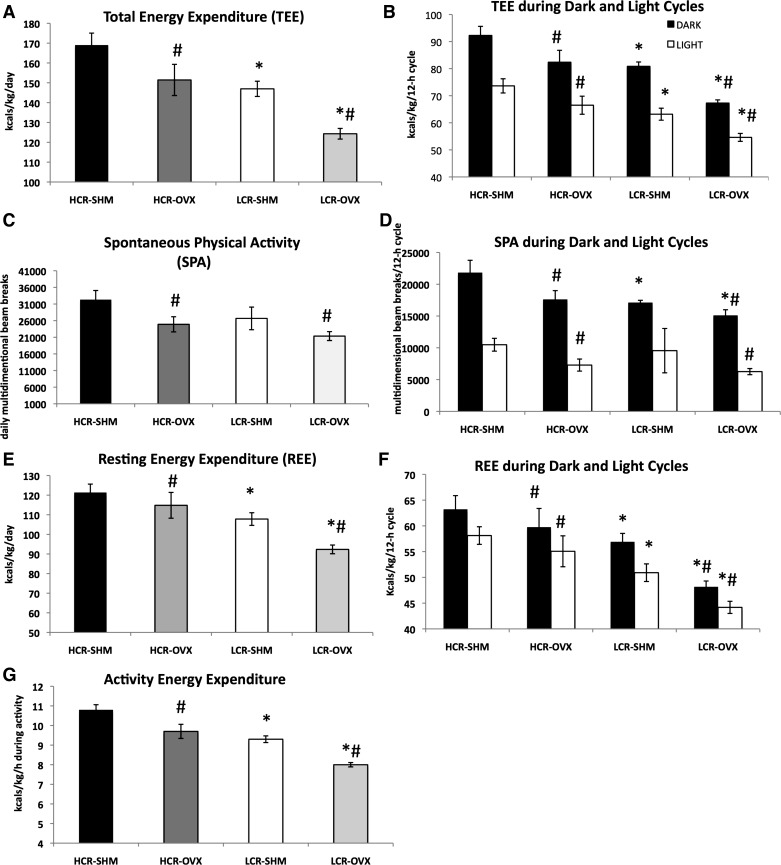
Similar to the reduction in relative energy intake, both HCR and LCR reduced total energy expenditure following OVX (OVX and line main effects, P < 0.01) (Fig. 3, A and B). Importantly, the LCR-OVX group had the lowest total energy expenditure compared with all other groups.
Fig. 3.
Low aerobic fitness and OVX reduces energy expenditure and physical activity. Total daily energy expenditure (TEE) relative to body weight and expressed as kilocalories per kilogram (A), TEE per average 12:12-h light-dark cycle (B), spontaneous physical activity (SPA) (C), SPA per average 12:12-h light-dark cycle (D), resting energy expenditure (REE) relative to BW and expressed as kilocalories per kilogram (E) REE per average 12:12-h light-dark cycle (F), and energy expended during activity (G) were measured over a 72-h period; For G, average values during activity in the dark cycle were used since this is when most activity occurs. *HCR vs. LCR, P < 0.05; #OVX vs. SHM, P < 0.05; n = 4–7/group.
Cage spontaneous physical activity (SPA) decreased by ∼20% in both OVX groups, while remaining ∼20% lower among LCR compared with HCR (HCR vs. LCR and OVX vs. SHM effects, both P < 0.01; no OVX × line interaction) (Fig. 3, C and D). In neither case were differences present in the light (i.e., rodent inactive period). The fact that SPA reduced similarly in HCR and LCR following OVX was confirmed by repeated-measures analysis of SPA measured presurgery and postsurgery. We observed a significant SPA × OVX effect (P = 0.042), but no SPA × line × OVX effect (P = 0.50), confirming that HCR and LCR reduced SPA equally following OVX compared with SHM.
Similar to TEE and SPA, REE was significantly reduced by OVX, as well as intrinsic aerobic fitness. LCR had significantly lower REE compared with HCR (P < 0.001), while both groups experienced a reduction following OVX (P = 0.01) (Fig. 3, E and F). Compared with HCR, LCR experienced a more robust OVX-associated reduction in REE (∼12% reduction in LCR compared with ∼4% reduction in HCR). Energy expended during activity was also significantly lower in LCR compared with HCR, and both lines experienced reductions post-OVX (HCR vs. LCR, P < 0.001 and OVX vs. SHM, P < 0.001), suggesting efficiency of activity was lower among HCR and increased with OVX (Fig. 3G).
Differences in skeletal muscle oxidative capacity between HCR and LCR.
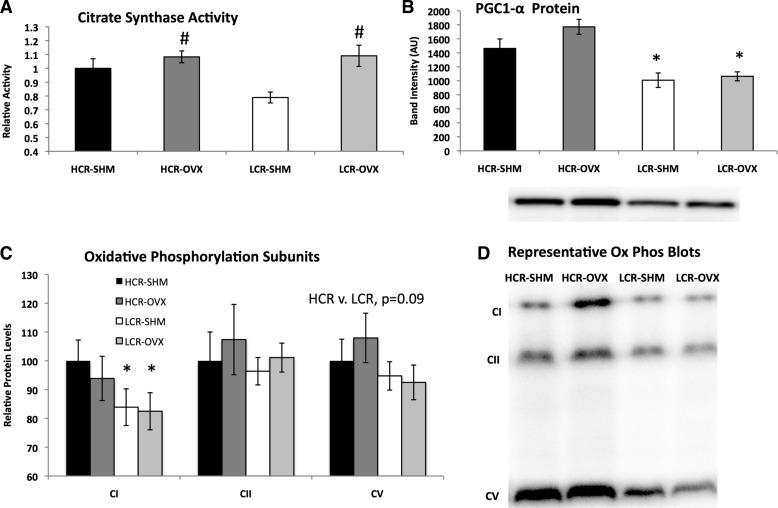
To determine whether there were inherent metabolic differences between skeletal muscle of HCR and LCR rats and whether OVX differentially affected these phenotypes, we measured citrate synthase activity (Fig. 4A); protein expression of PGC1-α, a transcriptional coactivator that plays a major role in controlling mitochondrial biogenesis and oxidative phosphorylation (Fig. 4B); and mitochondrial oxidative phosphorylation subunits CI, CII, and CV (Fig. 4C) in gastrocnemius skeletal muscle samples. Although citrate synthase activity was higher in HCR-SHM compared with LCR-SHM, OVX unexpectedly increased citrate synthase activity in both HCR and LCR. HCR rats also had greater expression of skeletal muscle PGC1-α, while neither line was significantly affected by OVX (HCR vs. LCR, P < 0.001; OVX vs. SHM, P = 0.085). Similarly, protein expression of individual subunits of the mitochondrial electron transport chain tended to be higher in HCR compared with LCR, although only statistically significant for CI; OVX did not significantly alter protein levels of any of the oxidative phosphorylation subunits measured (Fig. 4C).
Fig. 4.
Skeletal muscle oxidative capacity differences between HCR and LCR rats. Skeletal (gastrocnemious) muscle citrate synthase activity increased with OVX (P = 0.003) and tended to be higher in HCR (line; P = 0.098) (A); protein expression detected via Western blot analysis and adjusted for total protein content of proliferator-activated receptor coactivator 1-alpha (PGC-1α) was higher in HCR vs. LCR (line, P < 0.001) (B); protein expression of oxidative phosphorylation subunits adjusted for total protein content and expressed relative to HCR-SHM (C); one representative blot from C per group (n = 6–7/group) (D). *HCR vs. LCR, P < 0.05; #OVX vs. SHM, P < 0.05.
HCR rats are protected against OVX-induced increase in insulin resistance.
As shown in Table 1, LCR, but not HCR, experienced an OVX-induced increase in HOMA-IR (line × OVX interaction, P = 0.038). Repeated-measures ANOVA confirmed this, as indicated by a significant group × time interaction (P = 0.012) followed by post hoc tests, which revealed that only LCR-OVX experienced a significant increase in HOMA-IR from presurgery to postsurgery. The glucose response to GTT was not statistically different among groups, while insulin secretion during the GTT (i.e., in response to glucose) was greater in LCR compared with HCR, suggestive of peripheral insulin resistance (HCR vs. LCR; P = 0.020). Moreover, adipose tissue insulin resistance [i.e., fasting [NEFA] × [insulin] (25)] increased following OVX only in LCR (line × OVX, P = 0.031).
Table 1.
Insulin resistance, blood lipid profile, and liver triglyceride content
| HCR-SHM | HCR-OVX | LCR-SHM | LCR-OVX | Two-Way ANOVA Statistics | |
|---|---|---|---|---|---|
| Fasting serum insulin, ng/m | 2.06 ± 0.54 | 1.90 ± 0.29 | 1.47 ± 0.26 | 2.59 ± 0.25 | Interaction, P = 0.063 |
| Fasting serum glucose, mg/dl | 172.86 ± 18.9 | 153.28 ± 6.9 | 150.89 ± 5.5 | 166.73 ± 5.9 | Interaction, P = 0.057 |
| HOMA-IR index | 29.00 ± 11.91 | 20.62 ± 3.27 | 16.49 ± 3.05 | 30.93 ± 3.30a | Interaction, P = 0.038 |
| GTT glucose AUC, mg/dl | 32,155 ± 1485 | 29,480 ± 3428 | 32,291 ± 2454 | 27,104 ± 2016 | NS |
| GTT insulin AUC, ng/ml | 96.6 ± 19 | 97.2 ± 7 | 123.0 ± 8* | 163.5 ± 16* | HCR vs. LCR, P = 0.02 |
| AdipoIR | 0.48 ± 0.10 | 0.57 ± 0.11 | 0.65 ± 0.17 | 1.82 ± 0.52a | Interaction, P = 0.031 |
| Fasting serum TG, mg/dl | 30.57 ± 3.17 | 25.28 ± 2.54 | 45.32 ± 8.36* | 47.00 ± 4.93* | HCR vs. LCR, P = 0.04 |
| Fasting serum NEFA, mmol/l | 0.25 ± 0.02 | 0.28 ± 0.05 | 0.36 ± 0.06 | 1.10 ± 0.62 | NS |
| Fasting serum HDL, mg/dl | 24.00 ± 2.46 | 30.43 ± 2.19# | 27.86 ± 1.75 | 30.47 ± 1.85# | OVX vs. SHM, P = 0.044 |
| Liver TG, nmol/g | 0.34 ± 0.042 | 0.31 ± 0.051 | 0.32 ± 0.054 | 0.51 ± 0.088 | NS |
Metabolic markers were analyzed from fasted plasma samples. Two-way ANOVA was used to determine OVX, line, and/or OVX × line interaction effects; if interaction effects were found, individual group differences were determined by post hoc LSD tests and indicated by different letters. HOMA-IR, homeostatic assessment model of insulin resistance calculated using the formula [glucose (mmol/l) × insulin (mU/l)/22.5]; Glucose AUC, area under the curve calculated over the course of a 120-min glucose tolerance test (GTT) conducted 8 wk following surgery; n = 6 or 7/group; insulin AUC, area under the curve for insulin response during GTT; n = 3–6/group; AdipoIR, adipocyte insulin resistance calculated by the formula: fasting [NEFA] × fasting [insulin]; TG, triglycerides; NEFA, nonesterified fatty acids; HDL, high-density lipoprotein cholesterol. Low-density lipoprotein cholesterol (LDL) was measured but not detectable.
Significant HCR vs. LCR main effect.
Significant OVX vs. SHM main effect.
LCR had higher fasting circulating triglycerides than HCR, but neither group experienced increases post-OVX. On the other hand, while there were no differences in HDL cholesterol levels between HCR and LCR, both HCR and LCR experienced an increase following OVX (OVX vs. SHM main effect, P = 0.04). No differences were found in circulating levels of NEFAs, insulin, or glucose; however, LCR-OVX rats tended to increase liver TG accumulation, but this did not reach statistical significance (line × OVX interaction, P = 0.15) (Table 1).
Greater resting energy expenditure in HCR inversely associates with BAT.
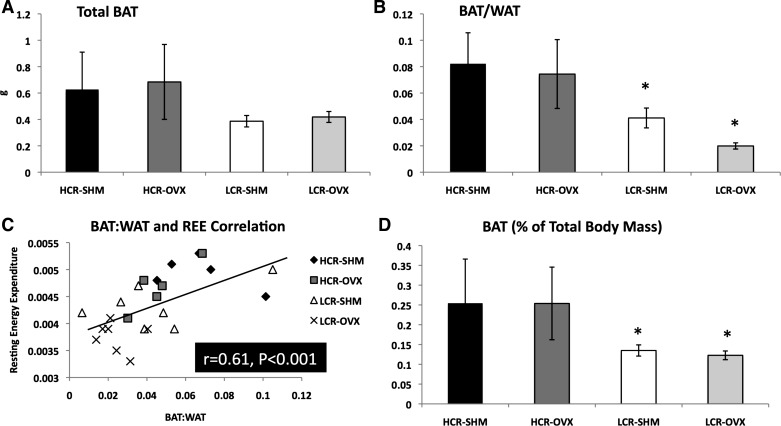
In stark contrast to the WAT depots, total BAT mass tended to be greater in HCR compared with LCR (line main effect, P = 0.08, Fig. 5A), despite HCR being smaller animals. Furthermore, while all WAT depots (i.e., PGAT, PRAT, OAT, and SQAT) increased in size following OVX in LCR (Fig. 1D), the BAT depot did not. The BAT/WAT ratio was significantly higher in HCR-SHM compared with LCR-SHM (0.082 ± 0.02 vs. 0.041 ± 0.01, P = 0.048). Not only did HCR have a greater BAT/WAT, they were protected from a decrease in BAT/WAT following OVX, unlike LCR (∼9% reduction post-OVX in HCR vs. ∼48% reduction post-OVX in LCR) (Fig. 5B). Since REE decreased following OVX, we determined whether it correlated with BAT/WAT and observed a positive and statistically significant correlation between REE and BAT/WAT across the entire study population (r = 0.60, P < 0.001) (Fig. 5C). When expressed as BAT/total body weight, HCR also had significantly more BAT relative to body weight, while this percentage did not change following OVX (HCR vs. LCR, P = 0.017) (Fig. 5D).
Fig. 5.
HCR have more relative brown adipose tissue (BAT), which correlates significantly with resting energy expenditure (REE). Total interscapular BAT (HCR vs. LCR, P = 0.083) (A), BAT relative to white adipose tissue (WAT) (i.e., the sum of all visceral and SQAT WAT depots) (B), and the correlation between BAT/WAT and REE (C). *HCR vs. LCR, P < 0.05.
Ovariectomy increases UCP-1, but not other characteristic BAT markers.
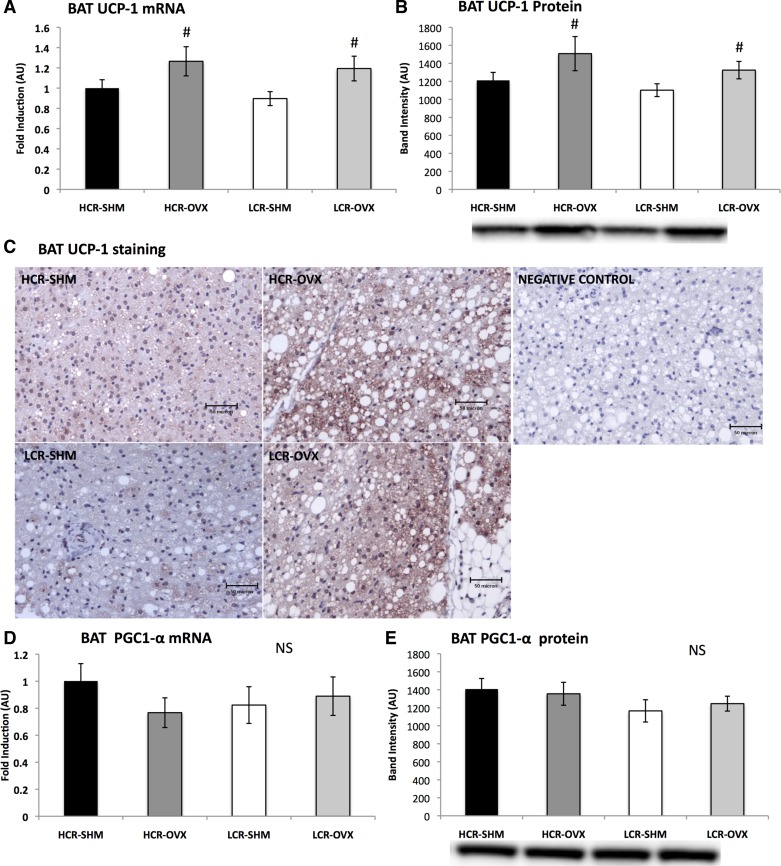
To assess BAT phenotype, markers characteristic of BAT (e.g., UCP-1 and PGC1-α) were measured at the levels of gene and protein expression (Fig. 6). BAT UCP-1 content was also qualitatively examined using immunostaining with anti-UCP-1 antibodies. As shown in Fig. 6A, OVX resulted in increased gene expression of UCP-1 in BAT in both lines (OVX vs. SHM, P = 0.01). This was confirmed with protein expression (Fig. 6B) and supported by immunohistological analysis (Fig. 6C). However, levels of PGC1-α were not different, either at the level of mRNA (Fig. 6D) or protein expression (Fig. 6E). To more comprehensively assess the effects of intrinsic fitness and OVX on BAT phenotype, other phenotypic BAT markers were measured at the gene expression level. Unlike UCP-1, none of the characteristic BAT markers that were measured (e.g., CIDEA, NRF1, Tfam) was different between groups (data not shown).
Fig. 6.
OVX augments BAT phenotype in both HCR and LCR rats. Gene expression of uncoupling protein-1 (UCP-1) relative to HCR-SHM using the housekeeping gene, 18s (A), protein expression of UCP-1 via Western blot analysis (B), representative photographs (×40 magnification) of immunological staining of BAT for UCP-1 (C), peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1-α) gene expression (D), and PGC1-α protein expression (E). In C, one representative image per group is shown; a total of five animals per group were assessed. In B and D, one representative Western blot band is shown below the corresponding group; blots were adjusted for total protein using amido-black staining. NS, not significant. #OVX vs. SHM, P < 0.05.
DISCUSSION
Herein, we demonstrate that rats selectively bred for high intrinsic aerobic fitness (i.e., HCR rats) are protected against insulin resistance and the accretion of body fat associated with loss of ovarian hormone production compared with rats bred for low aerobic fitness (i.e., LCR rats), despite experiencing the same ∼20% reduction in SPA. Several rodent models demonstrate that, when maintained under sedentary conditions and fed normal low-fat rodent chow diet, OVX is followed by rapid weight gain in the form of WAT (15, 40, 48). To our knowledge, no studies have shown protection from OVX-associated weight gain and/or insulin resistance in the absence of intervention. Some (19), but not all (15), have reported that exercise training can at least partially mitigate OVX-induced weight gain in rodents, but no prior studies have investigated the role of aerobic fitness per se on the metabolic response to OVX, which was the objective of this current study.
Insulin resistance develops after OVX in rodents (48, 54) and menopause or hysterectomy in humans (12, 36) via mechanisms that are incompletely understood but that likely involve increased accretion of WAT. In the current study, OVX caused a reduction in insulin sensitivity in LCR but not HCR rats. The insulin resistance that developed was not severe, as indicated by no differences in fasting glucose or glucose AUC during GTT. However, we did observe hypersecretion of insulin in response to glucose, an increase in insulin resistance via HOMA-IR, and an increase in adipoctye insulin resistance among the LCR-OVX rats. None of those indicators increased in HCR-OVX rats. Whether the increased insulin resistance following OVX in LCR rats was due to loss of ovarian hormones per se or the increased adiposity attributed to OVX is not known, but increased adiposity correlates strongly with insulin resistance and is an important predicting factor (13). Thus, it is probable that the protection among HCR against OVX-induced insulin resistance was at least partially explained by their protection against increased adiposity.
To determine what was responsible for the protection against OVX-associated weight gain observed in HCR rats, energy intake and all components of energy expenditure were compared among groups. Neither HCR nor LCR rats increased energy intake following OVX. The LCR-OVX actually consumed ∼15% less energy than the HCR-OVX rats relative to body weight yet gained a significant amount of weight in the form of WAT. Counter to our finding that OVX did not cause hyperphagia, other groups have reported OVX-induced hyperphagia in rats (20, 38), but this does not occur in mice (51). Similar to what we observed, others have also shown no effect of OVX on food intake in rats (46); thus, there is heterogeneity among species and even among rat strains in terms of the effect of OVX on energy intake. However, pair-feeding studies using hyperphagic OVX rats have shown that, when fed the same amount of food as SHM controls, OVX rats gain more weight (5). Together, those findings implicate energy expenditure as playing a more important role in OVX-associated weight gain than energy intake. In support of this hypothesis, a recent comprehensive longitudinal study in humans, the MONET study, demonstrated that weight gain during the early years following menopause was attributed to reduced physical activity and total energy expenditure (7), and not increased dietary intake (6).
TEE is made up of physical activity and REE, with resting accounting for the vast majority (∼65%) (37). In the present study, TEE was significantly lower in LCR compared with HCR before, and especially after, OVX (Fig. 3). Both HCR and LCR experienced a similar reduction in SPA relative to their respective SHM controls, an effect that has been widely reported in other rodent OVX models (9, 16, 48). That the HCR rats were not impervious to the reduction in SPA yet were protected from weight gain suggests that the protection was due to differences in resting energy expenditure. Indeed, LCR were more profoundly affected by an OVX-associated reduction in resting energy expenditure. REE is somewhat determined by lean body mass (55). However, increased lean mass did not account for the higher energy expenditure in the HCR rats studied here since the LCR rats actually had greater total lean mass. Thus, we next sought to determine whether there were inherent differences in skeletal muscle oxidative capacity (i.e., qualitative differences in lean mass) between HCR and LCR rats. Skeletal muscle protein content of a major regulator of mitochondrial biogenesis, PGC1-α, was indeed higher in HCR; the same trend was observed for individual electron transport chain subunits (i.e., CI was higher in HCR compared with LCR) (Fig. 4). However, there was no indication that these mitochondrial indicators were differentially affected by OVX. Citrate synthase, indicative of mitochondrial activity, followed a similar trend in that HCR-SHM had greater activity compared with LCR-SHM. Unexpectedly, we found that OVX increased citrate synthase activity in both groups; this may represent a compensatory mechanism among OVX rats. Naples et al. (27) also showed a trend for greater skeletal muscle mitochondrial function in female HCR compared with LCR rats, and Seifert et al. (41) confirmed this finding, showing that mitochondrial oxidative capacity is, indeed, greater in skeletal muscle of HCR compared with LCR rats. Thus, enhanced skeletal muscle mitochondrial function may play a role in the higher resting energy expenditure and protection against OVX-associated metabolic dysfunction witnessed in the HCR rats. A limitation is that mitochondrial function was not directly assessed in this study; further studies are, therefore, warranted to determine the role of skeletal muscle mitochondrial function in the protection against OVX-induced metabolic dysfunction in HCR rats.
It is becoming increasingly evident that adipose tissue also plays a significant role in determining TEE (10). One study revealed that there are sex differences in the contribution of adipose tissue to TEE, such that adipose from premenopausal women contributes a greater percentage compared with that of age-matched men (30). Notably, the age-related reduction is independent of changes in body composition, implicating an important role of female sex hormones (14). Although the mechanism behind this sex difference is not clear, WAT from the women in that study had greater expression levels of genes associated with mitochondrial function/oxygen consumption. It may be hypothesized that OVX reduces WAT oxidative capacity and that aerobic fitness protects against this reduction. Unfortunately, WAT oxidative capacity was not assessed in the present study. Future studies should determine the effects of aerobic fitness and OVX on WAT oxidative capacity.
Only since 2007, when it was appreciated that humans, like rodents, harbor appreciable amounts of BAT, has there been excitement over the possibility of targeting BAT as an antiobesity target (3). BAT content and activity are inversely related to obesity but positively with total energy expenditure (49). Although the amount of BAT in humans maintained through adulthood is small, it is not insignificant, as estimates indicate that 50 g of BAT will burn 125 kcal/day in humans (33). And, aside from its contribution to energy expenditure, recent findings demonstrate that BAT also affects glucose homeostasis and insulin sensitivity (44). Here, we provide the first evidence that HCR rats have more BAT relative to both WAT and total body weight than LCR rats and that BAT/WAT is reduced following OVX only in LCR rats (Fig. 5). Remarkably, the significant positive correlation that we observed between relative BAT and resting energy expenditure (r = 0.604, P < 0.001) is comparable to that observed by Teule and colleagues (47) (r = 0.56, P = 0.005), where they revealed a relationship in humans between BAT activity and resting energy expenditure (47). We also assessed the relationship between skeletal muscle mitochondrial PGC1-α protein expression and resting energy expenditure and found this correlation (r = 0.488, P = 0.025) to be significant but weaker than the BAT/WAT-REE relationship. Because the animals in this current study were not exposed to cold or chemical BAT activation, BAT activity could not be assessed. UCP-1 uncouples oxidative phosphorylation and is the characteristic feature of BAT. Animal studies have shown that estrogen increases BAT UCP-1 (53), which led us to hypothesize that OVX would decrease BAT UCP-1. BAT UCP-1 and PGC1-α gene and protein expression were measured, and UCP-1 content was qualitatively assessed via immunohistological UCP-1 staining. Counter to our hypothesis, no differences in BAT UCP-1 gene or protein expression levels existed between lines, while, unexpectedly, levels increased in both lines following OVX; this was supported by an increase in UCP-1 staining. This finding is strikingly similar to that of Nadal-Casellas et al. (26), who also found increased basal UCP-1 gene expression in BAT following OVX in Wistar rats. Moreover, those authors confirmed the increase in UCP-1 gene expression with protein expression data. Neither of our groups demonstrated differences in BAT PGC1-α. However, PGC1-α may not be necessary for UCP-1 regulation (54). Nadal-Casellas et al. (26) showed that the oxidative capacity of BAT following OVX was reduced, while other groups have demonstrated OVX-associated reductions in UCP-1 gene expression in BAT (35). In that study, BAT was investigated only 5 wk following OVX; it may be that the OVX effects on BAT UCP-1 expression are time-dependent, such that expression is reduced early following OVX, and compensatory increases occur later. Nigro et al. (28) recently hypothesized that reduced BAT activity is an early event leading to OVX-induced obesity. Those authors comprehensively analyzed BAT function in OVX and SHM Wistar rats 21 days following surgery by measuring citrate synthase activity in isolated mitochondria, as well as oxygen consumption, ATP production, and heat production. They found no evidence of reduced BAT function following OVX, nor did they see any changes in basal BAT expression of UCP-1. It may be that OVX in rats induces an increase in UCP-1, but not until several weeks following surgery; perhaps this is a compensatory mechanism that occurs over time. The extent to which the weight gain associated with menopause and/or estrogen loss in rodents can be explained by a reduction in BAT function or a decrease in the oxidative capacity of WAT, is not known and warrants further study.
The UCP-1 upregulation in BAT with OVX was unexpected. Interestingly, chronic high fat diet (HFD) feeding also increases UCP-1 expression in BAT (52). Since both OVX and HFD lead to a positive energy balance state, an induction in BAT UCP-1 may serve as a compensatory mechanism to increase energy expenditure. We found that BAT lipid droplet accumulation appeared to increase in both groups after OVX. The Nadal-Casellas group (26) did not measure lipid content of BAT, but they reported that the BAT from OVX animals contained less total protein and less mitochondrial DNA, implying greater lipid content. Whether the increase in BAT lipid accumulation following OVX observed in the present study is related to increased BAT activity or changes in lipid handling is not clear and should be investigated. Our findings suggest that intrinsic aerobic fitness is associated with greater BAT mass and protects against OVX-associated reductions in relative BAT.
A limitation of this study is that the HCR rats also have higher SPA than the LCR rats. That is, it is unclear to what extent the protection observed is due to increased activity vs. enhanced aerobic fitness. Since both lines experienced the same ∼20% reduction in SPA in response to OVX, yet the LCR responded with greater weight gain despite consuming fewer calories relative to body mass compared with HCR, it appears that the differences observed are inherent to fitness and not activity per se. The HCR rats also had greater energy expenditure during activity compared with LCR, a finding that was recently replicated in this model (11). Admittedly, there is a link that is difficult to separate between aerobic fitness and physical activity. Nonetheless, the HCR/LCR model has emerged as an important model of the “aerobic hypothesis,” which states that aerobic capacity per se directly predicts optimal metabolic health and survivability (50).
Perspectives and Significance
Menopause leads to weight gain and insulin resistance, which greatly increases the risk of development of cardiometabolic diseases among women (7). While hormone replacement therapy (HRT) reverses some of the acute symptoms of menopause (e.g., hot flashes, sleep disturbances), it has been shown to adversely affect cardiometabolic disease outcomes in some, but not all, women (1). In light of the controversy over HRT, it is imperative to identify alternative strategies that may protect the metabolic health of women throughout the aging process. Increasing aerobic fitness through exercise prior to and during menopause may be one such strategy. Menopause in women and OVX in rodents reduces physical activity (40, 48). Meanwhile, a recent study demonstrated a dose-dependent protective effect of cardiorespiratory fitness on metabolic symptoms among postmenopausal women (8). No previous studies have investigated how intrinsic aerobic fitness, independent of exercise training, may offer protection against OVX-associated metabolic dysfunction. Our current findings reveal that selective breeding for high intrinsic aerobic fitness protects rats against OVX-induced weight gain in the form of WAT and preserves insulin sensitivity, even while highly fit rats experience the classical OVX-associated reduction in physical activity. Moreover, it appears that protection against positive energy balance following OVX in HCR rats may be related to the maintenance of REE, which may be attributed to an enhancement of skeletal muscle oxidative capacity, and/or higher relative level of BAT. The present findings extend the growing body of literature demonstrating the beneficial health benefits of high aerobic capacity to include protection against metabolic dysfunction associated with the loss of ovarian hormones.
GRANTS
The LCR-HCR rat model system was funded by the National Center for Research Resources grant. The LCR-HCR rat model system was funded by the National Center for Research Resources Grant R24RR017718 and subsequently supported by the Office of Research Infrastructure Programs/OD Grant R24OD010950 and by Grant R01DK099034 (to L. G. Koch and S. L. Britton) from the National Institutes of Health (NIH). S. L. Britton was also supported by NIH Grants R01DK-077200 and R01GM-104194. This work was also supported by NIH R01DK-088940 to J. P. Thyfault and a University of Missouri Research Council grant (to V. Vieira-Potter). J. Padilla is supported by National Heart, Lung, and Blood Institute K01HL-125503.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.J.V.-P. and J.P.T. conception and design of research; V.J.V.-P., J.P., Y.-m.P., R.J.W., R.J.S., S.L.B., L.G.K., N.T.J., J.M.C., T.Z., and G.M.M. performed experiments; V.J.V.-P. analyzed data; V.J.V.-P., J.P., N.T.J., E.M.M., and J.P.T. interpreted results of experiments; V.J.V.-P. prepared figures; V.J.V.-P. drafted manuscript; V.J.V.-P., J.P., Y.-m.P., S.L.B., N.T.J., J.M.C., E.M.M., and J.P.T. edited and revised manuscript; V.J.V.-P., J.P., Y.-m.P., R.J.W., R.J.S., L.G.K., N.T.J., J.M.C., T.Z., E.M.M., G.M.M., and J.P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact L. G. Koch (lgkoch@med.umich.edu) or S. L. Britton (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, Michigan.
REFERENCES
- 1.Bassuk SS, Manson JE. Menopausal hormone therapy and cardiovascular disease risk: utility of biomarkers and clinical factors for risk stratification. Clin Chem 60: 68–77, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) 111: 1497–1504, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev 15: 92–106, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, Rector RS, Thyfault JP, Laughlin MH, Padilla J. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regul Integr Comp Physiol 306: R596–R606, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35,983–35,991, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Duval K, Prud'homme D, Rabasa-Lhoret R, Strychar I, Brochu M, Lavoie JM, Doucet E. Effects of the menopausal transition on dietary intake and appetite: a MONET Group study. Eur J Clin Nutr 68: 271–276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duval K, Prud'homme D, Rabasa-Lhoret R, Strychar I, Brochu M, Lavoie JM, Doucet E. Effects of the menopausal transition on energy expenditure: a MONET Group Study. Eur J Clin Nutr 67: 407–411, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earnest CP, Johannsen NM, Swift DL, Lavie CJ, Blair SN, Church TS. Dose effect of cardiorespiratory exercise on metabolic syndrome in postmenopausal women. Am J Cardiol 111: 1805–1811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira JA, Foley AM, Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur J Appl Physiol 112: 3007–3018, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta 1831: 986–1003, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab 306: E635–E647, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Cadarso-Suarez C, Garcia F, De Francisco A. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract 94: 146–155, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev 34: 463–500, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Hunter GR, Weinsier RL, Gower BA, Wetzstein C. Age-related decrease in resting energy expenditure in sedentary white women: effects of regional differences in lean and fat mass. Am J Clin Nutr 73: 333–337, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Ignacio DL, Fortunato RS, Neto RA, da Silva Silvestre DH, Nigro M, Frankenfeld TG, Werneck-de-Castro JP, Carvalho DP. Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats. Horm Metab Res 44: 797–803, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Izumo N, Ishibashi Y, Ohba M, Morikawa T, Manabe T. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res 227: 1–6, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Izumo N, Ishibashi Y, Ohba M, Morikawa T, Manabe T. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res 227: 1–6, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Jackson KC, Wohlers LM, Valencia AP, Cilenti M, Borengasser SJ, Thyfault JP, Spangenburg EE. Wheel running prevents the accumulation of monounsaturated fatty acids in the liver of ovariectomized mice by attenuating changes in SCD-1 content. Appl Physiol Nutr Metab 36: 798–810, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Jeong S, Yoon M. Swimming's prevention of ovariectomy-induced obesity through activation of skeletal-muscle PPARα. Int J Sport Nutr Exerc Metab 22: 1–10, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Jiang JM, Sacco SM, Ward WE. Ovariectomy-induced hyperphagia does not modulate bone mineral density or bone strength in rats. J Nutr 138: 2106–2110, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Womens Health 8: 155–167, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 109: 1162–1172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Nadal-Casellas A, Proenza AM, Llado I, Gianotti M. Effects of ovariectomy and 17-β estradiol replacement on rat brown adipose tissue mitochondrial function. Steroids 76: 1051–1056, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab 35: 151–162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigro M, Santos AT, Barthem CS, Louzada RA, Fortunato RS, Ketzer LA, Carvalho DP, de Meis L. A change in liver metabolism but not in brown adipose tissue thermogenesis is an early event in ovariectomy-induced obesity in rats. Endocrinology 155: 2881–2891, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E31–E41, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Nookaew I, Svensson PA, Jacobson P, Jernas M, Taube M, Larsson I, Andersson-Assarsson JC, Sjostrom L, Froguel P, Walley A, Nielsen J, Carlsson LM. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab 98: E370–E378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav 58: 355–367, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochner CN, Teixeira J, Geary N, Asarian L. Greater short-term weight loss in women 20–45 versus 55–65 years of age following bariatric surgery. Obes Surg 23: 1650–1654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun 288: 191–197, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Pirimoglu ZM, Arslan C, Buyukbayrak EE, Kars B, Karsidag YK, Unal O, Turan MC. Glucose tolerance of premenopausal women after menopause due to surgical removal of ovaries. Climacteric 14: 453–457, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Poehlman ET. A review: exercise and its influence on resting energy metabolism in man. Med Sci Sports Exerc 21: 515–525, 1989. [PubMed] [Google Scholar]
- 38.Prasannarong M, Vichaiwong K, Saengsirisuwan V. Calorie restriction prevents the development of insulin resistance and impaired insulin signaling in skeletal muscle of ovariectomized rats. Biochim Biophys Acta 1822: 1051–1061, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Rogers NH, Perfield JW 2nd Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert EL, Bastianelli M, Aguer C, Moffat C, Estey C, Koch LG, Britton SL, Harper ME. Intrinsic aerobic capacity correlates with greater inherent mitochondrial oxidative and H2O2 emission capacities without major shifts in myosin heavy chain isoform. J Appl Physiol (1985) 113: 1624–1634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spritzer PM, Oppermann K. Weight gain and abdominal obesity at menopause. Climacteric 16: 292, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Srere PA. The enzymology of the formation and breakdown of citrate. Adv Enzymol Relat Areas Mol Biol 43: 57–101, 1975. [DOI] [PubMed] [Google Scholar]
- 44.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trevaskis JL, Turek VF, Wittmer C, Griffin PS, Wilson JK, Reynolds JM, Zhao Y, Mack CM, Parkes DG, Roth JD. Enhanced amylin-mediated body weight loss in estradiol-deficient diet-induced obese rats. Endocrinology 151: 5657–5668, 2010. [DOI] [PubMed] [Google Scholar]
- 47.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 153: 4266–4277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, Brans B, van Marken Lichtenbelt WD. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. PLoS One 8: e77221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao L, Heuser-Baker J, Herlea-Pana O, Zhang N, Szweda LI, Griffin TM, Barlic-Dicen J. Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J 28: 4534–4550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, Sasahara M, Tsuneki H, Saito S, Sasaoka T. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab 303: E445–E456, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63: 514–525, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86: 1423–1427, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]