Abstract
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction resulting in cyclic intermittent hypoxia (IH) during sleep in affected individuals. OSA occurs more frequently in postmenopausal than premenopausal women and the severity of OSA increases after menopause. Gonadal hormones can influence brain and behavior; testosterone and estrogens in particular can enhance spatial learning and memory. We hypothesized that estrogens may protect mice from IH-induced hippocampal morphological and behavioral changes. To test this hypothesis we exposed intact or gonadectomized male and female mice to room air or IH [15 cycles/h, 8 h/day, fraction of inspired oxygen (FiO2) nadir of 5%] for a total of 30 days. During the final 4 days of IH, mice were tested for anxiety- and depressive-like behaviors. After cessation of IH exposure mice were tested on the Barnes maze and passive avoidance tests to assess learning and memory. Ovariectomy paired with IH treatment, impaired spatial learning and memory compared to all other female groups. Intact male mice receiving IH treatment also had impaired learning and memory compared with intact or castrated male mice exposed to room air. Learning and memory changes were mirrored by changes in basilar dendritic length of the CA1 region of the hippocampus. These data suggest that estrogens provide protection against IH-induced deficits, whereas androgens partially exacerbate IH-induced deficits on learning and memory.
Keywords: obstructive sleep apnea, intermittent hypoxia, hormones, behavior
obstructive sleep apnea (OSA) is a condition characterized by repetitive upper airway obstructions resulting in cyclic intermittent hypoxia (IH) during sleep in affected individuals. OSA is an independent risk factor for diabetes, hypertension, heart disease, and stroke (25, 31, 43). In addition to health risks, people with OSA typically suffer from daytime fatigue and impaired memory (1, 26). Approximately half of people with OSA also report depression and anxiety (22).
OSA is a common health problem affecting about 9% of women and 17% of men in the United States between 50 and 70 years of age (30). OSA is more prevalent in men than premenopausal women, but the mechanism for the sex differences remains unclear (18). Sleep apnea is also more frequently reported in postmenopausal women than premenopausal women. Even after controlling for body mass index, both the prevalence and severity of OSA increase postmenopause, and hormone replacement appears to reduce OSA severity in this population (6, 41). This observation suggests hormones as one likely mechanism underlying sex differences in OSA (18).
Sex differences are also observed in the effects of IH in mouse models of OSA. Male mice increase oxidative biomarkers following chronic IH, whereas females do not exhibit these changes (20). Additionally, oxidant production in liver mitochondria increases with ovariectomy in female rats and treatment with estradiol reduces production to levels similar to intact females (5). Patients with OSA experience drowsiness; similarly, chronic IH exposure in male mice impairs wake times and sleep latencies, whereas females maintain normal wake times and sleep latencies (37). Elevated blood pressure is common after IH exposure; gonadally intact female rats are protected from IH-induced hypertension compared with ovariectomized females and males (15). Sex differences in ventilatory control are observed in mice following hypoxic exposure (28), and ventilation during hypoxia is increased by exogenous progesterone treatment of newborn rat pups (4). Thus sex differences in response to IH exist and appear to have a gonadal hormone component.
Sex hormones influence learning and memory, with both estrogens and androgens typically associated with enhanced learning and memory (12, 34). Naturally or artificially elevated estrogen concentrations in female rats enhances spatial learning in the object placement task (12). Aged, postestropausal female mice given estradiol improve performance in the Morris water maze compared with mice without hormone replacement (11). Reproductively photoperiodic rodents display seasonal changes in hippocampal volume and spatial learning and memory such that male short day white-footed mice are impaired compared with their long day counterparts (33). Short day decreases in hippocampal volume and spatial learning and memory correspond to lower concentrations of testosterone in males, and spatial learning and memory performance is enhanced in short day white-footed mice with the addition of testosterone (34). Although sex steroid hormones influence learning and memory, it remains unclear whether they influence the effects of IH on learning and memory performance.
We hypothesize that female mice are protected from IH-induced behavioral and hippocampal morphological changes in a hormone-dependent manner. If this hypothesis is true, then 1) gonadally intact females will be protected from IH-induced changes in learning and memory, as well as anxiety-like behavior compared with ovariectomized females; 2) intact females in IH will be protected compared with male mice; and 3) gonadectomized males should have similar IH-induced changes to intact females. These results could suggest hormonal manipulations that may be important in developing strategies for OSA management.
MATERIALS AND METHODS
Animals.
Forty-five male and 48 female Swiss-Webster mice (∼8 wk old) were obtained from Charles River Labs (Wilmington, MA). Mice were group housed, 3 to 5 same-sex individuals per cage in propylene cages (33 cm × 18 cm × 14 cm) at an ambient temperature of 22 ± 2°C and relative humidity of 50 ± 10%. Mice were provided Harlan Teklad 8640 food (Madison, WI) and filtered tap water ad libitum. Upon arrival mice were maintained under a 16 h:8 h light-dark cycle of illumination for 1 wk to allow acclimation to local conditions. Mice were then randomly assigned to either gonadectomy (n = 45) or a sham-gonadectomy (n = 48). Briefly, male mice were anesthetized and had a 1-cm2 patch shaved on the lower abdomen. For sham-castrated male mice (n = 24), testes were located and returned inside the cavity; for castration (n = 21) testes were separated from the fat pad and removed, then the vas deferens and spermatic blood vessels were cauterized. All males then had their muscle and skin sutured separately. Briefly, female mice were anesthetized and had a 1-cm2 patch shaved on the lower back. For sham-ovariectomized female mice (n = 24) ovaries were located and returned inside the cavity; for ovariectomized mice (n = 24), ovaries were separated from the fat pad and removed. All female mice then had their muscle and skin sutured separately. After surgery, all mice were allowed to recover for 2 wk before beginning air or IH treatment. All experimental procedures were approved by The Ohio State University Institutional Animal Care and Use Committee.
Hypoxia treatment.
Mice were randomly assigned to receive IH (n = 47) [15 cycles/h, 8 h/day, fraction of inspired oxygen (FiO2) nadir of 5%] or room air (RA) (n = 46) creating eight experimental groups [n = 12/group, except male gonadectomized mice in RA (n = 10) or IH (n = 11)]. FiO2 nadir of 5% corresponds to severe OSA, classified as >30 events of 10-s apneas per hour (7). Though the frequency of apneic events is reduced in the mouse model, oxygen levels are similar to those experienced by patients with severe OSA (27). Treatment occurred daily for 30 consecutive days. Mice were exposed to treatment in two 8-h shifts starting at the beginning of the light phase; intact mice were exposed separately from gonadectomized mice. The group that received treatment first and second shift were alternated every day. Additionally, male and female mice were run separately; sham and ovariectomized females were exposed to treatment on the same days and intact and gonadectomized male mice were exposed on the same days. During treatment, mice were moved to custom-designed Plexiglas chambers (31 cm × 19 cm × 18 cm) with a raised floor (6.5 cm), 10 mice were placed in one chamber at a time (35). Oxygen levels were controlled by connecting the cages via a regulator system to compressed air (14 l/min) and nitrogen tanks (9 l/min) that automatically switched throughout exposure; levels were checked with a flowmeter (23). Before the experiment began oxygen fluctuations in the chamber were checked using a portable data collection unit (DI-158U Series, DATAQ Instruments, Akron, OH). RA exposure mice were housed in a similar cage, without connections to nitrogen or air tanks. Treatment occurred during the light phase (when these animals typically sleep) and lasted for 30 days. Behavioral testing occurred during the final 4 days in IH or RA treatment during the dark phase and continued after the cessation of IH treatment.
Open field.
The open-field test in mice characterizes anxiety-like responses in a novel environment, as well as locomotor activity. Central tendency is the primary measure for anxiety-like responses and is defined as the proportion of time spent in the center of the open field. Locomotor activity was measured separately as the total number of beam breaks during testing. All data were collected from a photobeam activity system (PAS) (San Diego Instruments, San Diego, CA) contained in a chamber (Med Associates, St. Albans, VT). Mice were tested starting at the beginning of the dark phase and were allowed to acclimate to the room for 20 min before testing. Mice were tested for 20 min as previously described (9).
Barnes maze.
Mice were allowed a 24-h break between previous behavioral testing and the beginning of Barnes maze testing. The Barnes maze (San Diego Instruments) assesses spatial learning and memory using a brightly light circular arena with 20 evenly space holes, one hole leads to a dark escape box and the other holes are blocked off with black inserts (10). Mice were acclimated to the maze on day 1. During acclimation mice were guided from the center of the maze to the target hole. Once mice were in the target hole, mice were left undisturbed for 30 s. Then mice were trained for the next 5 consecutive days, consisting of three 90-s trials separated by 10-min intervals in a clean cage. Latency, number of errors, and path length were averaged for the three trials each day. One day after the last training trial, mice were given a 60-s probe trial, where they were allowed to search the Barnes maze without an escape box present. Latency to find the target hole, path length, and number of errors were scored during all trials by a video tracking program (HVS Image, Mountain View, CA).
Passive avoidance.
The following day mice were tested in the passive avoidance chamber. The passive avoidance test also assesses learning and memory by allowing mice to form an association between escaping from an aversive stimulus (light) and a foot shock. Retention of this pairing was assessed 24 h after the initial trial and latency to enter the dark chamber was used to assess retention. The following 2 days animals were placed in the passive avoidance chamber (Gemini Avoidance System, San Diego Instruments). Mice were placed in the right side of the chamber in a starting box; after 20 s the chamber was illuminated as an electrically operated door opened to expose a dark chamber on the other side. Mice had a maximum trial length of 300 s to enter the dark chamber after which point no shock was received and animals were removed from the apparatus. After animals entered the dark side, the door closed and mice received a 1.5-mA foot shock for 2 s. Then mice were removed from the chamber and returned to a clean cage. Twenty-four hours later mice were again placed in the chamber following the same procedure. Latency to enter the dark side of the chamber was automatically recorded by the passive avoidance chamber for both trials.
Tissue collection and processing.
Mice were euthanized during the light phase (0700 h EST) by rapid decapitation under isoflurane sedation. At necropsy heart, spleen, and inguinal fat pads were collected and weighed. Brain tissue was also collected and half of each brain placed in Golgi-Cox staining using Rapid GolgiStain Kit (FD NeuroTechnologies). The hemisphere of brain used for Golgi-Cox staining was randomly selected. Golgi-Cox-stained brains were stored, processed, and analyzed as described previously (3). Briefly, six representative CA1 pyramidal neurons were selected per mouse that met the following criteria: neurons were clearly stained, lacked truncated dendrites, and were not obscured by neighboring neurons. Neurons and dendrites were traced at ×20 (0.5). Dendritic spines were traced at ×100 (1.40) in 4 apical and 4 basilar, randomly selected, representative dendritic segments of at least 20 μm in length and at least 50 μm from the cell body, using Neurolucida 8 software (MicroBrightField, Williston, VT) for PC and a Nikon Eclipse, E800 microscope. Neurons, dendrites, and spine density were analyzed using Neurolucida Explorer software (MicroBrightField).
Statistical analysis.
Main effect of sex (female, male) was assessed. Main effects of gonadal status (intact, gonadectomized) and IH treatment (RA, IH) and interactions between the two factors were assessed with data split by sex. Multivariate ANOVAs were used to analyze open field, passive avoidance, Golgi-Cox-stained dendritic morphology, and organ masses with final body mass as a covariate. Change in body mass over the 4 wk of IH or RA treatment was analyzed as a univariate ANOVA. Change in body mass across the experiment, Barnes maze latency, path length, errors, and Sholl analysis of Golgi-Cox-stained tissue were analyzed with a repeated measures ANOVA. Barnes maze path length and errors were further analyzed using Mann-Whitney U nonparametric tests to assess group differences on each day due to unequal variance on individual days. Statistics were performed using SPSS 19 for Windows (IBM, New York, NY). Outliers determined by Z score (± 2 SE from mean) were removed from subsequent analysis, as mentioned specifically in the results. Mean differences were considered statistically significant when P was ≤0.05 and were followed up with Tukey's honest significant differences post hoc tests, except dendritic morphology and Sholl analysis were further analyzed with least significant difference post hoc tests.
RESULTS
Open field.
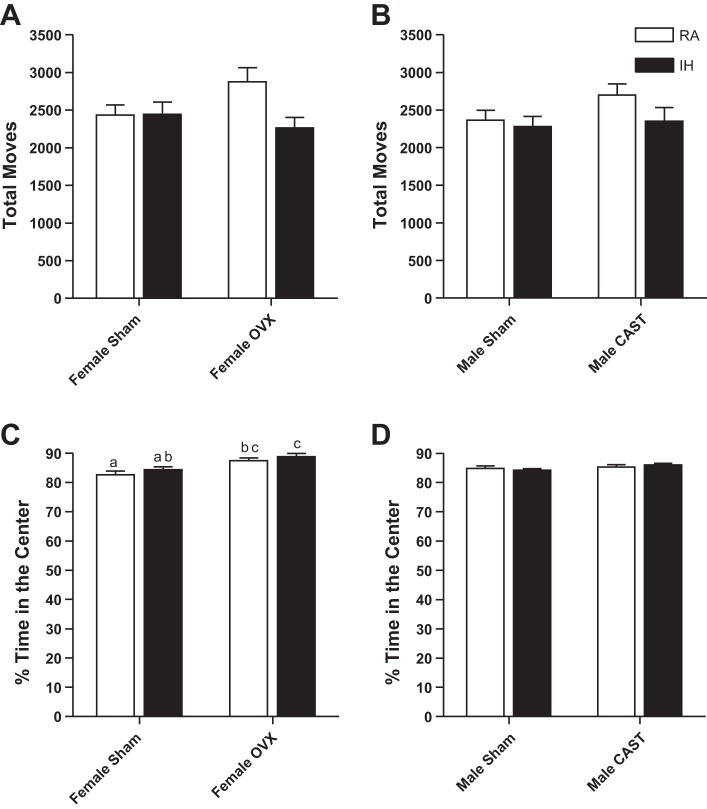
Male and female mice displayed similar total moves in the open field and spent a similar percentage of time in the center of the open field (P > 0.05, in each case, Fig. 1). Intact and gonadectomized female (P > 0.05, Fig. 1A) and male (P > 0.05, Fig. 1B) mice had similar levels of activity in the open field. RA- and IH-exposed female (P > 0.05, Fig. 1A) and male (P > 0.05, Fig. 1B) mice also had similar levels of activity in the open field. Ovariectomized female mice displayed fewer anxiety-like responses, spending a larger percentage of time in the center of the open field than intact mice (F1,44 = 18.59, P < 0.01, Fig. 1C). Castrated and intact male mice spent equivalent amounts of time in the center of the open field (P > 0.05, Fig. 1D). Treatment with RA or IH did not alter time spent in the center of the open field in female (P > 0.05, Fig. 1C) or male mice (P > 0.05, Fig. 1D).
Fig. 1.
Total activity in the open field was not altered by gonadal status or treatment in female (A) or male (B) mice. Percentage of time spent in the center of the open field was higher in ovariectomized (OVX) mice than intact female mice (C), indicating increased anxiety-like behavior. Values are means ± SE total moves in the open field for female (A) and male mice (B). OVX female mice spent a larger percentage of their time (means ± SE) in the center of the open field than intact mice (C). Percentage of time (means ± SE) in the center of the open field did not differ among male mice (D). Significant mean differences at P < 0.05 indicated by different letters above groups (e.g., a vs. b) as determined by Tukey's HSD post hoc. RA, room air; IH, intermittent hypoxia; CAST, castrated.
Barnes maze.
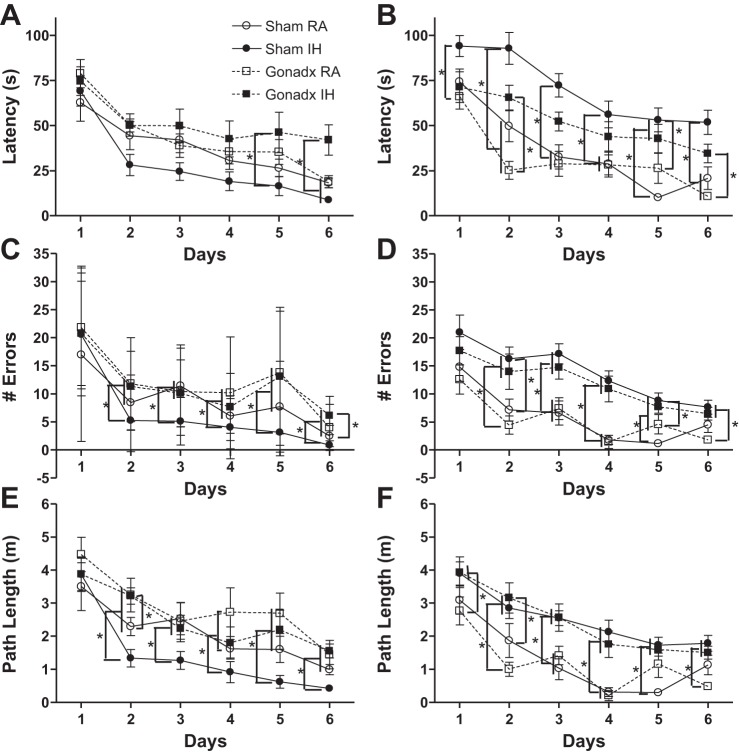
Latency to find the escape hole across days of testing was similar between female and male mice (P > 0.05, Fig. 2, A and B). Intact and ovariectomized mice had similar latencies to reach the escape hole across days (P > 0.05, Fig. 2A). RA- and IH-treated mice had similar latencies to reach the escape hole across days (P > 0.05, Fig. 2A). Gonadal status and RA/IH treatment interacted in female mice to affect latency to find the escape hole across days (F1,215 = 2.47, P < 0.05, Fig. 2A), such that ovariectomized mice exposed to IH had a longer latency than intact female mice exposed to IH (P < 0.05). Castrated mice had a shorter latency to find the escape hole than intact male mice across days (F1,175 = 2.75, P < 0.05, Fig. 2B). RA-exposed male mice had a shorter latency to find the escape hole than IH-exposed male mice (F1,175 = 2.88, P < 0.05, Fig. 2B). Gonadal status and RA/IH treatment did not interact to alter latency in male mice (P > 0.05). Seven mice were removed from all repeated measures Barnes analysis based on a latency z score ± 2 (1 female sham RA, 2 male sham RA, 1 male sham IH, 2 male gonadectomized RA, 1 male gonadectomized IH).
Fig. 2.
Intact female mice were protected against IH-induced spatial learning and memory deficits, whereas intact male mice exposed to IH had impaired learning and memory compared with RA-exposed males. Mean (± SE) latency to find the escape hole for female mice (A), number of errors for female mice (C), and path length for female mice (E) are shown. Mean (± SE) latency to find the escape hole for male mice (B), number of errors for male mice (D), and path length for male mice (F) are shown. *Significant mean differences among groups at P < 0.05 as determined by Tukey HSD post hoc. Gonadx, gonadectomized.
Female mice made more errors than males across days of training in the Barnes maze (F5,390 = 4.59, P < 0.01, Fig. 2, C and D, respectively). There were no main effects on the number of errors made or path length in female or male mice across days; however, because of sex differences and differences in latency we examined differences on each individual day of testing. Intact female mice made fewer errors than ovariectomized mice on days 2 (U = 161, P < 0.05), days 5 (U = 140, P < 0.05), and days 6 (U = 124, P < 0.05, Fig. 2C). RA or IH treatment did not alter the number of errors made by female mice on days 1–6 (P > 0.05, Fig. 2C). Castrated and intact male mice did not alter the number of errors made on days 1–6 (P > 0.05, Fig. 2D). RA-exposed male mice made fewer errors than IH-exposed male mice on days 1–6 (day 1, U = 114; day 2, U = 65; day 3, U = 54; day 4, U = 19; day 5, U = 46; day 6, U = 63, P < 0.05 in each case, Fig. 2D). Path length to reach the escape hole across days of testing was similar between female and male mice (P > 0.05, Fig. 2, E and F, respectively). Intact female mice had a shorter path length than ovariectomized mice on days 2 (U = 107, P < 0.05), days 5 (U = 150, P < 0.05), and days 6 (U = 137, P < 0.05, Fig. 2E). IH-exposed female mice had a shorter path length than RA-exposed female mice on days 4 (U = 176, P < 0.05) and days 5(U = 171, P < 0.05, Fig. 2E). Castrated and intact male mice had similar path lengths on days 1–6 (P > 0.05, Fig. 2F). IH-exposed male mice had a longer path length than RA-exposed male mice on days 1–6 (day 1, U = 98; day 2, U = 62; day 3. U = 62; day 4, U = 21; day 5. U = 54; day 6, U = 67, P < 0.05 in each case, Fig. 2F).
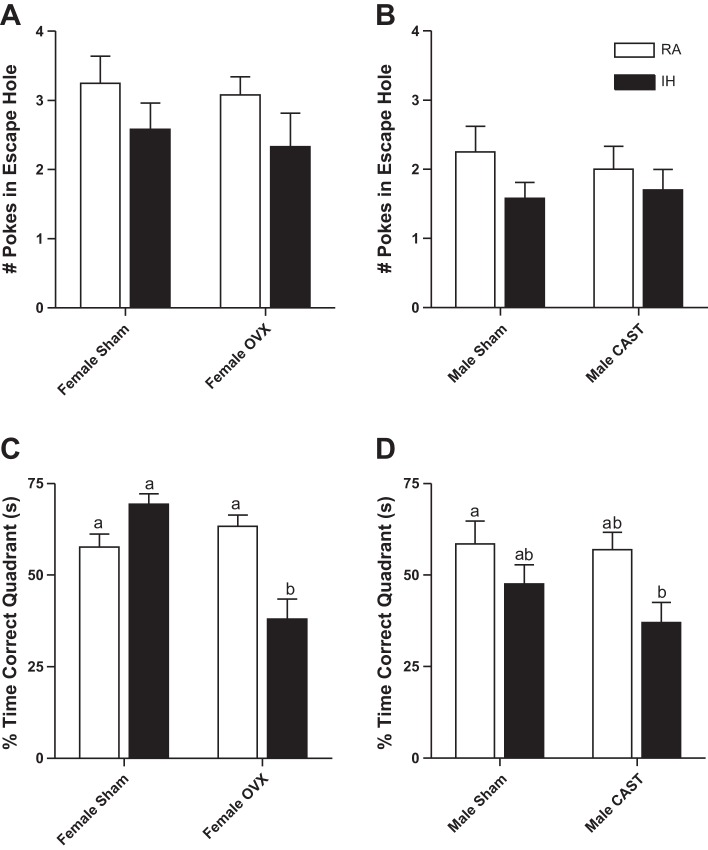
Male (Fig. 3B) mice made fewer pokes in the escape hole during the probe trial of the Barnes maze than female mice (F1,85 = 6.5, P < 0.01, Fig. 3A). Gonadectomized and intact female (P > 0.05, Fig. 3A) and male (P > 0.05, Fig. 3B) mice made a similar number of pokes in the escape hole during the probe trial. Female (P > 0.05, Fig. 3A) and male (P > 0.05, Fig. 3B) mice exposed to RA and IH made a similar number of pokes in the escape hole during the probe trial. Male and female mice spent a similar percentage of time in the correct quadrant during the probe trial of the Barnes maze [P > 0.05, Fig. 3, C (females) and D (males)]. Ovariectomized mice spent a smaller percentage of time in the correct quadrant of the Barnes maze than intact female mice (F1,44 = 11.01, P < 0.01, Fig. 3C). Female mice exposed to RA and IH spent a similar percentage of time in the correct quadrant of the Barnes maze (P > 0.05, Fig. 3C). Gonadal status and treatment interacted to alter time spent in the correct quadrant of the Barnes maze in female mice such that intact mice in RA and IH spent similar amounts of time in the correct quadrant of the Barnes maze, whereas ovariectomized mice in IH spent less time in the correct quadrant than ovariectomized mice in RA (F1,44 = 22.87, P < 0.01, Fig. 3C). Castrated mice spent a smaller percentage of time in the correct quadrant of the Barnes maze than intact male mice (F1,41 = 4.31, P < 0.05, Fig. 3D). Male mice in IH spent less time in the correct quadrant of the Barnes maze than mice exposed to RA (F1,41 = 5.56, P < 0.05, Fig. 3D). Gonadal status and treatment did not interact to alter percentage of time in the correct quadrant during the probe trial in male mice (P > 0.05).
Fig. 3.
OVX mice reduced time spent in the correct quadrant of the Barnes maze during the probe trial compared with intact female mice, particularly in IH-exposed mice (C). Male mice exposed to IH reduced time spent in the correct quadrant of the Barnes maze during the probe trial compared with RA-exposed mice (D), indicating impaired memory. Values are means ± SE for number of nose pokes into the escape hole in female (A) and male mice (B). Values are means ± SE for time spent in the correct quadrant of the Barnes maze during the probe trial in female mice (C) and male mice (D). Significant mean differences at P < 0.05 indicated by different letters above groups (e.g., a vs. b) as determined by Tukey's HSD post hoc.
Passive avoidance.
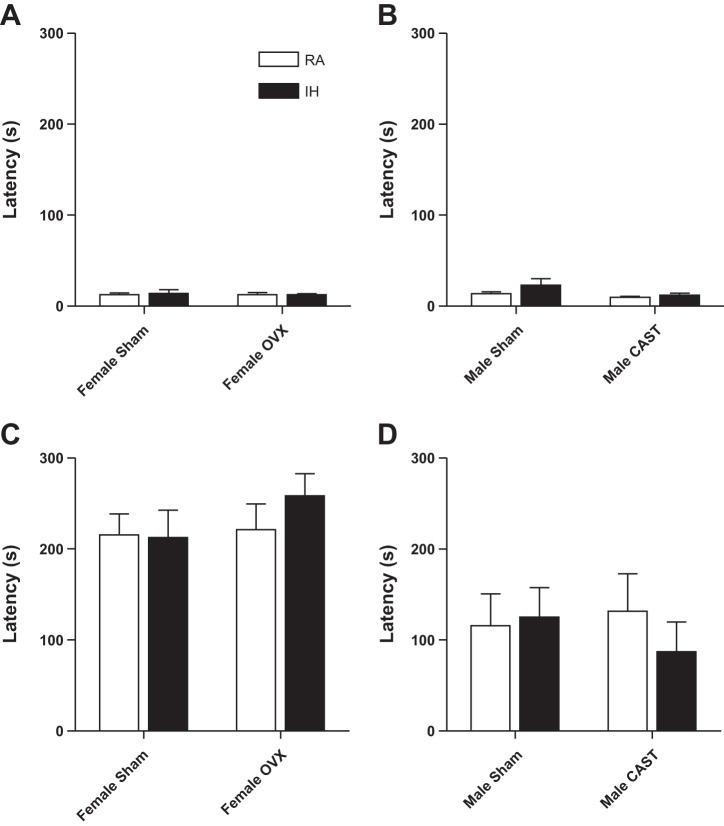
All groups had similar latencies to enter the dark chamber on day 1 [P > 0.05 in each case, Fig. 4, A (females) and B (males)]. Males had a reduced latency to enter the dark chamber on the second day of passive avoidance compared with female mice, indicating reduced learning and memory [F1,85 = 26.09, P < 0.01, Fig. 4, C (females) and D (males)]. Intact and gonadectomized female (P > 0.05, Fig. 4C) and male (P > 0.05, Fig. 4D) mice had similar latencies to enter the dark chamber on the second day of passive avoidance. Similarly, female (P > 0.05, Fig. 4C) and male (P > 0.05, Fig. 4D) mice exposed to RA and IH had similar latencies to enter the dark chamber on the second day.
Fig. 4.
Male mice had impaired learning and memory in the passive avoidance test compared with female mice. Latency to enter the dark side of the chamber on the first day of testing in female (A) and male mice (B) is shown. Latency to enter the dark side of the chamber on the second day of testing in female (C) and male mice (D) is also shown. Significant mean differences at P < 0.05 indicated by different letters above groups (e.g., a vs. b) as determined by Tukey's HSD post hoc.
Body mass.
Changes in body mass during the 4 wk of RA or IH treatment were similar in male and female mice (P > 0.05 in each case, Table 1). Ovariectomized mice had a similar change in body mass as intact female mice (P > 0.05, Table 1). Castrated mice had a similar change in body mass as intact female mice (P > 0.05, Table 1). Mice in RA gained more mass than mice in IH for both female (F1,4 4= 49.09, P < 0.01, Table 1) and male mice (F1,40 = 105.76, P < 0.01, Table 1). Gonadal status and treatment interacted in female mice to alter change in body mass (F1,44 = 80.53, P < 0.01, Table 1), such that intact female mice in IH had a greater decrease in body mass than ovariectomized mice in IH.
Table 1.
Change in body mass between the start and end of the 4 wk of room air or intermittent hypoxia treatment
| Gonadal Status | RA | IH |
|---|---|---|
| Females | ||
| Intact (sham) | 1.80 ± 0.27 | −2.54 ± 0.27 |
| OVX | −0.47 ± 0.27 | 0.06 ± 0.27 |
| Males | ||
| Intact (sham) | 0.97 ± 0.27 | −2.06 ± 0.27 |
| CAST | 0.98 ± 0.30 | −1.16 ± 0.28 |
Values are means ± SE.
RA, room air; IH, intermittent hypoxia; OVX, ovariectomized; CAST, castrated.
Female mice had a lower body mass than male mice across the experiment (F5,415 = 2.82, P < 0.05, Fig. 5, A and B, respectively). Ovariectomized mice had a larger mass than intact female mice across the experiment (F5,220 = 9.61, P < 0.01, Fig. 5A). Female mice in IH had lower body mass than female mice in RA treatment across weeks of the experiment (F5,220 = 145.3, P < 0.01, Fig. 5A). Gonadal status and RA/IH treatment interacted to alter body mass across the experiment (F5,220 = 14.7, P < 0.01, Fig. 5A), such that intact female mice in IH lost more mass than ovariectomized mice. Castrated mice had a smaller body mass than intact male mice across the experiment (F5,200 = 15.9, P < 0.01, Fig. 5B). Male mice in IH had lower body mass than male mice in RA treatment across weeks of the experiment (F5,200 = 42.8, P < 0.01, Fig. 5B). Gonadal status and RA/IH treatment did not interact to alter body mass across the experiment in male mice (P > 0.05, Fig. 5B). One mouse was removed because it was an outlier based on Z score (one male sham mouse in IH).
Fig. 5.
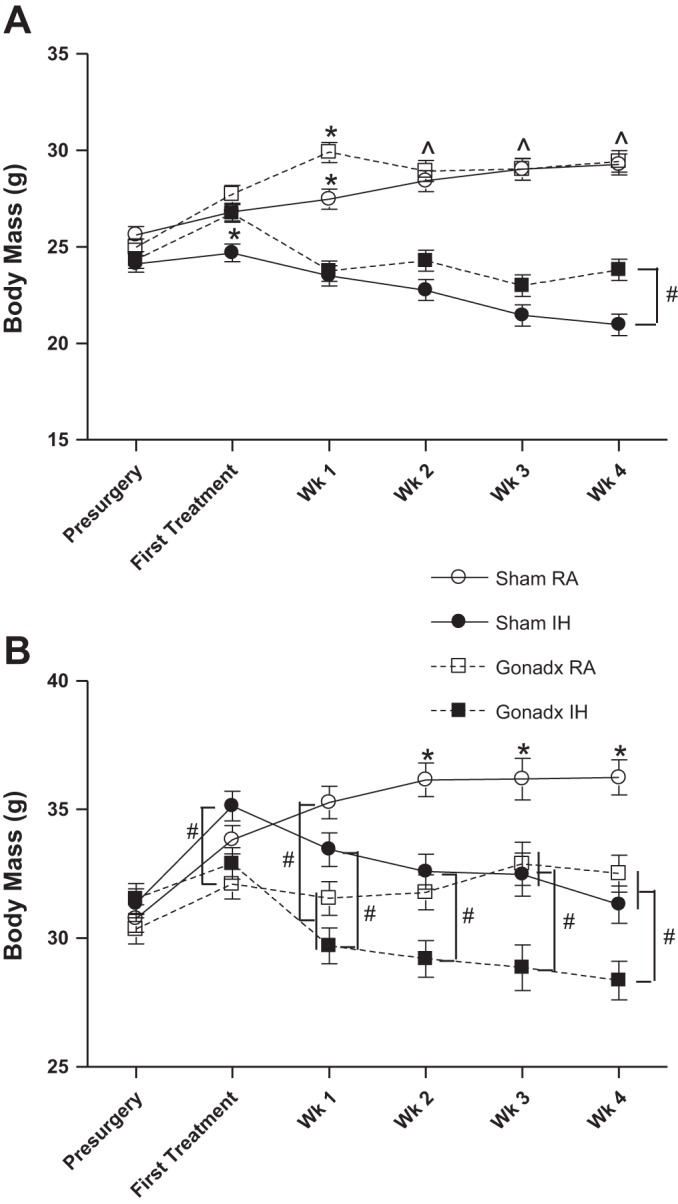
Male mice had a larger body mass than female mice. Male and female mice exposed to IH had a lower body mass than mice exposed to RA. OVX females had increased body mass compared with intact mice (A), whereas CAST mice had decreased body mass compared with intact mice (B). Values are means ± SE for female (A) and male (B) body mass across the experiment. *Significant mean differences at P < 0.05 from all other groups; ^mean differences from mice exposed to IH; #other mean differences among groups.
Organ masses.
Male mice had larger heart mass than female mice (F1,84 = 27.73, P < 0.01, Table 2). Male and female mice had similar spleen masses (P > 0.05). Female mice had larger fat pad mass than male mice (F1,84 = 19.55, P < 0.01, Table 2). Ovariectomized mice had similar heart, spleen, and fat pad masses compared with intact female mice (P > 0.05, Table 2). RA- and IH-exposed female mice had similar heart, spleen, and fat pad masses compared with intact female mice (P > 0.05, Table 2). Castrated mice had similar heart, spleen, and fat pad masses compared with intact male mice (P > 0.05, Table 2). RA- and IH-exposed male mice had similar heart, spleen, adrenal gland, and fat pad masses compared with intact male mice (P > 0.05, Table 2).
Table 2.
Organ masses with final body mass as a covariate
| Females, g |
Males, g |
||||
|---|---|---|---|---|---|
| Tissue | Gonadal Status | Intact (sham) | OVX | Intact (sham) | CAST |
| Heart | RA | 0.114 ± 0.004 | 0.110 ± 0.004 | 0.143 ± 0.005 | 0.126 ± 0.004 |
| IH | 0.106 ± 0.005 | 0.108 ± 0.005 | 0.134 ± 0.005 | 0.135 ± 0.005 | |
| Spleen | RA | 0.185 ± 0.022 | 0.231 ± 0.022 | 0.140 ± 0.027 | 0.187 ± 0.023 |
| IH | 0.171 ± 0.027 | 0.166 ± 0.025 | 0.138 ± 0.024 | 0.153 ± 0.024 | |
| Fat pads | RA | 1.099 ± 0.089 | 1.131 ± 0.088 | 0.528 ± 0.112 | 0.715 ± 0.088 |
| IH | 0.926 ± 0.111 | 0.867 ± 0.104 | 0.500 ± 0.100 | 0.506 ± 0.093 | |
Values are means ± SE of organ masses with final body mass (g) as a covariate and presented after 4 wk of RA or IH treatment and 13 days after removal from RA and IH treatments.
Dendritic morphology.
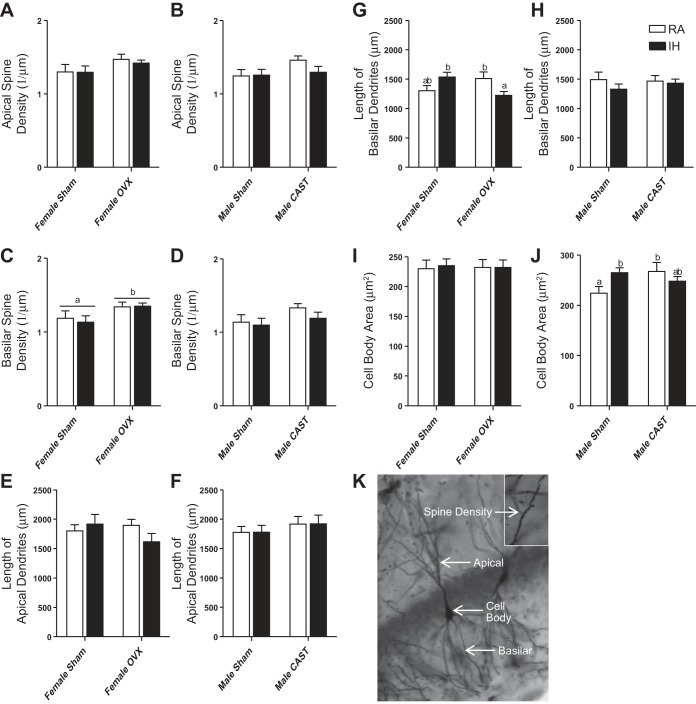
Male and female mice had similar apical and basilar lengths and spine densities (P > 0.05 in each case, Fig. 6, A–H). Male mice had a larger cell body area than female mice (F1,84=4.6, P < 0.05, Fig. 6, J and I, respectively). Neither gonadal status nor RA/IH treatment altered apical spine density in female mice (P > 0.05 in each case, Fig. 6A). Ovariectomized mice increased basilar spine density compared with intact female mice (F1,38 = 5.51, P < 0.05, Fig. 6C); basilar spine density was not altered by RA or IH treatment (P > 0.05). Gonadal status and RA/IH treatment did not alter apical dendritic length in female mice (P > 0.05 in each case, Fig. 6E). Gonadal status and RA/IH treatment alone did not alter basilar dendritic length in female mice (P > 0.05 in each case, Fig. 6G). Gonadal status and treatment interacted to alter basilar dendritic length in female mice (F1,38 = 9.01, P < 0.01, Fig. 6G), such that ovariectomized mice exposed to IH reduced basilar dendritic length compared with ovariectomized mice exposed to RA and intact female mice exposed to RA (P < 0.05). Cell body area was not altered by gonadal status or RA/IH treatment in female mice (P > 0.05 in each case, Fig. 6I). Gonadal status and RA/IH treatment did not alter apical spine density in male mice (P > 0.05 in each case, Fig. 6B). Gonadal status and RA/IH treatment did not alter basilar spine density (P > 0.05 in each case, Fig. 6D). Gonadal status and RA/IH treatment did not alter apical dendritic length in male mice (P > 0.05 in each case, Fig. 6F). Gonadal status and RA/IH treatment did not alter basilar dendritic length in male mice (P > 0.05 in each case, Fig. 6H). Cell body area was not altered by gonadal status or RA/IH treatment alone in male mice (P > 0.05 in each case, Fig. 6J). Gonadal status and RA/IH treatment interacted to alter cell body area in male mice (F1,38 = 5.55, P < 0.05, Fig. 6J), such that intact male mice in RA decreased cell body area compared with castrated mice in RA (P < 0.05). Nine mice were not included in any analysis of neuronal morphology because there was insufficient staining of the tissue or the brain was damaged during processing (1 female sham RA, 1 female sham IH, 2 female ovariectomized RA, 2 female ovariectomized IH, 1 male sham RA, and 2 male gonadectomized IH).
Fig. 6.
Overall, male mice had an increased cell body area compared with female mice as assessed by Golgi-Cox staining (J and I, respectively). Ovariectomy increased basilar spine density compared with intact female mice (C). Gonadal status and RA/IH treatment interacted in female mice to affect basilar dendritic length (G) such that OVX female mice exposed to IH decreased basilar dendritic length compared with intact mice exposed to IH. Gonadal status and RA/IH treatment interacted in male mice to affect cell body area (J) such that intact male mice exposed to IH increased cell body area compared with RA, but this did not occur in CAST mice. Values are means ± SE for apical spine density in female (A) and male mice (B), basilar spine density in female (C) and male mice (D), length of apical dendrites in female (E) and male mice (F), length of basilar dendrites in female (G) and male mice (H), and cell body area in female (I) and male mice (J). Representative neuron in the CA1 region from a CAST male exposed to IH, apical and basilar dendrites, cell body and spine density are indicated in K. This image was captured at ×20 and spine density inlay taken at ×100 (K). Significant mean differences at P < 0.05 indicated by different letters above groups (e.g., a vs. b) as determined by LSD post hoc test.
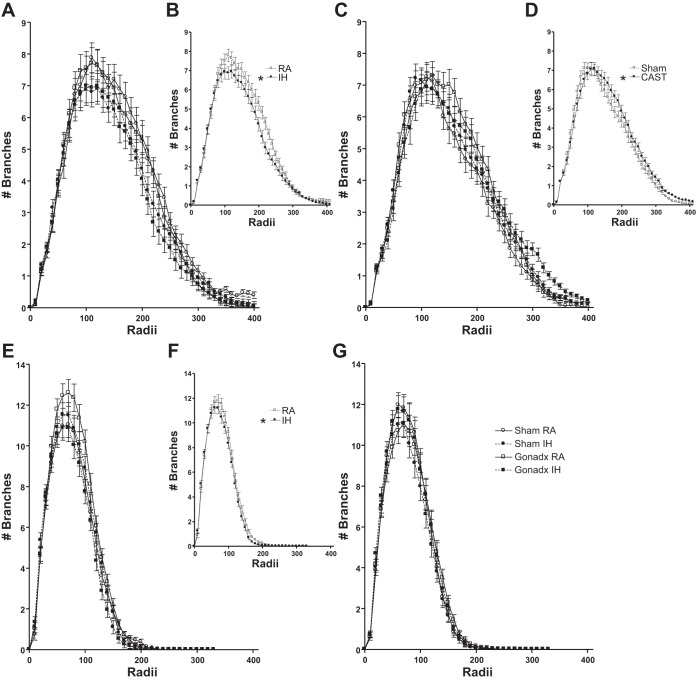
Female mice had a similar number of branches at each apical and basilar radii as male mice (P > 0.05, Fig. 7, A and C and E and G, respectively). Ovariectomized and intact female mice had similar apical radii (P > 0.05, Fig. 7A). IH treatment decreased apical radii compared with RA treatment in female mice (F45,1710 = 2.04, P < 0.05, Fig. 7, A and B). Castrated mice increased apical radii compared with intact male mice (P > 0.05, Fig. 7, C and D). IH and RA treatment did not alter apical radii in male mice (P > 0.05, Fig. 7C). Ovariectomized and intact female mice had similar basilar radii (P > 0.05, Fig. 7E). IH treatment decreased basilar radii compared with RA treatment in female mice (F33,1254 = 1.45, P = 0.05, Fig. 7, E and F). Gonadal status and RA/IH treatment interacted to alter basilar radii in female mice (F33,1254 = 3.04, P < 0.05, Fig. 7E), such that ovariectomized mice exposed to IH decreased basilar radii compared with ovariectomized RA exposed mice (P < 0.05). Neither gonadal status nor treatment altered basilar radii in male mice (P > 0.05 in each case, Fig. 7G).
Fig. 7.
Overall IH treatment in female mice reduced the number of apical (B) and basilar (F) branches indicated by Sholl analysis. Castration overall increased apical branching (D). Gonadal status and RA/IH treatment interacted in female mice (E) such that OVX mice exposed to RA increased the number of basilar branches compared with OVX mice exposed to IH (determined by LSD post hoc test). The number of apical branches at each radius in female mice (A) comparing grouped RA and IH in female mice (B) is shown. The number of apical branches at each radius in male mice (D) comparing grouped intact and CAST mice (D) is shown. The number of basilar branches at each radius in female mice (E) comparing grouped RA and IH in female mice (F) is shown. The number of basilar branches at each radius in male mice (G) is shown. *Significant mean differences at P < 0.05.
DISCUSSION
OSA is more prevalent and severity seems to be affected in a hormone-dependent manner such that more men are affected than premenopausal women, although the sex difference in both prevalence and severity wanes when postmenopausal women are compared with men (6, 18). Intact female mice are protected from the negative cellular consequences of IH and ovariectomy removes this protection (15). Additionally, gonadal hormones can enhance spatial learning and memory in mice. Therefore, we hypothesized that estrogens protect mice from IH-induced behavioral and hippocampal morphological changes. IH reduced body mass as previously described, indicating model validity (2). Ovariectomy paired with IH treatment impaired spatial learning and memory compared with all other female groups. Intact male mice receiving IH treatment also significantly impaired learning and memory compared with intact or castrated male mice exposed to RA. Basilar dendritic length in female mice was reduced in ovariectomized and IH-exposed mice compared with intact females exposed to IH, similar to changes observed in Barnes maze learning and memory. Additionally, IH treatment in female mice reduced branching in both apical and basilar dendrites compared with RA. These data suggest that estrogens provide protection against IH-induced deficits, whereas androgens partially exacerbate IH-induced deficits.
Just as patients with OSA have impaired cognition, IH induces impairments in the Barnes maze (8, 36, 42). Both estrogens and androgens are typically associated with enhanced learning and memory (12, 34). However, despite the increase in prevalence of OSA in men compared with women and the increase in prevalence of OSA in postmenopausal women, the influence of estrogens and androgens is not widely studied. As mentioned, intact female mice are protected from the negative cellular consequences of IH and ovariectomy removes this protection (15). Similarly, intact female mice were protected against the IH-induced spatial learning and memory deficits observed in ovariectomized mice. Intact male mice in IH had impaired learning and memory compared with all RA-exposed male mice. Estrogens and androgens are associated with enhanced learning and memory (12, 34). Although hormone concentrations were not assessed in the current study, there are previously reported hormone-dependent sex differences in response to IH. Because overall IH-exposed mice exhibited similar latencies and path lengths as RA-exposed mice, activity differences are probably not present after the cessation of daily IH exposure. Observed differences in learning and memory are likely independent of the effects of sleep deprivation. Sleep architecture during the initial days of IH treatment is disrupted but normalized by the end of the 14-day treatment (13); however, rats exposed to IH still exhibit impairments in the Morris water maze suggesting a role for IH in impairing learning and memory independent of sleep deprivation (13). These data suggest a protective role for estrogens against IH-induced spatial learning and memory deficits, whereas androgens may exacerbate IH-induced deficits in spatial learning and memory.
The passive avoidance test did not indicate learning and memory differences. However, passive avoidance was tested a week after removal from IH or RA treatment, possibly the negative consequences are reversible and prolonged removal from IH allows recovery of function. Additionally, passive avoidance requires amygdala-based fear learning, whereas spatial learning and memory is hippocampus based. The Barnes maze requires learning and retention over a 7-day span, whereas the passive avoidance task examined retention 24 h later. These results suggest that IH and gonadal status may not affect shorter-term memory but might influence long-term memory consolidation (40, 45).
Given that changes in learning and memory are associated with the hippocampus, we examined changes in hippocampal dendritic morphology. IH-induced changes in hippocampal morphology can return to normoxic levels following 21 days of IH exposure (24, 39). Changes can occur with prolonged exposure to IH. Reduced apical spine density in the CA3 was observed in a similar IH set up with 21 days of 8 h/day IH exposure (2). In the current study IH decreased apical and basilar dendritic branching in female mice in cornu Ammonis (CA) 1. Similarly, basilar dendritic length was reduced in ovariectomized mice exposed to IH compared with ovariectomized RA-exposed mice and intact female mice exposed to IH. These changes in basilar dendritic length correspond to the decreased spatial learning and memory observed in the ovariectomized mice exposed to IH. IH-based changes in dendritic morphology corresponding to learning and memory deficits were not observed in male mice. One consideration for these results is that collection of the brains occurred 2 wk after removal from IH or RA treatment. The timing of tissue collection gave mice ample time in normoxic conditions for dendritic morphology to completely or partially recover, as dendritic morphology changes rapidly differences might have been missed (17, 29). Dendritic morphology was affected in a hormone-specific manner in female mice.
Hormonal influences from estrous cycles, gonadectomy, IH, and food composition must be considered in the current experiment. A recent meta-analysis suggests that variability on individual measures was the same for randomly cycling females as males; the results suggest constant monitoring of estrous cycles is unnecessary when using female mice (32). Female performance on the T-maze is not altered by proestrus or estrus, whereas castration decreases accuracy during training (14). Similarly, ovariectomy is associated with memory impairments compared with intact females (38). A few days of IH exposure increases testosterone (16); however, a 17-day exposure to IH reduces testosterone concentrations compared with male mice in normal air conditions (44). Many changes associated with short IH exposure alone normalize over prolonged exposure, such as changes in corticosterone (2) or dendritic morphology (24); a similar phenomenon may occur with testosterone concentrations. The Harlan 8640 diet used in this study has soybean meal, which contain phytoestrogens that can act as both an estrogen agonist and antagonist. A diet rich in phytoestrogens improves visual spatial memory performance in females compared with a diet free of phytoestrogens, whereas males perform better on the diet free of phytoestrogens (19). Similarly, ovariectomized females on a diet rich in phytoestrogens improve performance on a spatial memory task and increase spine density in the CA1 region of the hippocampus (21). Because all of the mice received the same diet, diet is not likely a factor in group differences within sexes and no differences in Barnes maze were observed between the sexes. However, diet may explain the reduced learning and memory performance observed in males compared with females on the passive avoidance test. Overall, comparing both sexes likely provides the most accurate picture of hormone-dependent effects of IH exposure.
In conclusion, these data suggest cycling female sex hormones provide protection against IH-induced deficits, whereas androgens partially exacerbate IH-induced deficits in male mice. Our conclusions support clinical observations that women seem to be protected from OSA until menopause and that hormone replacement following menopause reduces severity of OSA (6, 41). Additionally, these data suggest that hormone replacement might be an important intervention to help ameliorate cognitive impairments associated with OSA particularly in postmenopausal women.
GRANTS
T. G. Aubrecht was supported by a National Institute of Dental and Craniofacial Research Grant T32 DE014320. The behavioral data were collected with the support of the National Institute of Neurological Disorders and Stroke Grant P30 NS045758.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.G.A., U.J.M., and R.J.N. conception and design of research; T.G.A. and R.J.N. performed experiments; T.G.A. analyzed data; T.G.A. and R.J.N. interpreted results of experiments; T.G.A. prepared figures; T.G.A. drafted manuscript; T.G.A., U.J.M., and R.J.N. edited and revised manuscript; T.G.A., R.J., U.J.M., and R.J.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anupama Suresh, Shan Chen, Yasmine Cisse, Nick Queen, Celynn Vaughn, and Sarah Zornes for help with behavior, collecting, slicing, and tracing Golgi-Cox-stained brains.
REFERENCES
- 1.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med 163: 1626–1631, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Aubrecht TG, Weil ZM, Magalang UJ, Nelson RJ. Dim light at night interacts with intermittent hypoxia to alter cognitive and affective responses. Am J Physiol Regul Integr Comp Physiol 305: R78–R86, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubrecht TG, Weil ZM, Nelson RJ. Melatonin treatment during early life interacts with restraint to alter neuronal morphology and provoke depressive-like responses. Behav Brain Res 263: 90–97, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairam A, Lumbroso D, Joseph V. Effect of progesterone on respiratory response to moderate hypoxia and apnea frequency in developing rats. Respir Physiol Neurobiol 185: 515–525, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34: 546–552, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V. Impact of menopause on the prevalence and severity of sleep apnea. Chest 120: 151–155, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med 7: 459–465B, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, Cappa SF. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 61: 87–92, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res 205: 349–354, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 27: 319–327, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115: 547–558, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem 88: 208–216, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav 105: 1014–1020, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hwang GS, Chen ST, Chen TJ, Wang SW. Effects of hypoxia on testosterone release in rat Leydig cells. Am J Physiol Endocrinol Metab 297: E1039–E1045, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Ikeno T, Weil ZM, Nelson RJ. Timing of light pulses and photoperiod on the diurnal rhythm of hippocampal neuronal morphology of Siberian hamsters. Neuroscience 270: 69–75, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome. Part 2: mechanisms. Sleep 25: 499–506, 2002. [PubMed] [Google Scholar]
- 19.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol 24: 5–16, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Li QY, Li M, Feng Y, Guo Q, Gu SY, Liu JL, Zhang RF, Wan HY. Chronic intermittent hypoxia induces thioredoxin system changes in a gender-specific fashion in mice. Am J Med Sci 343: 458–461, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res 1126: 183–187, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Macey PM, Woo MA, Kumar R, Cross RL, Harper RM. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLos One 5: e10211, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes 117: 129–134, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Maiti P, Muthuraju S, Ilavazhagan G, Singh SB. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Brain Res 189: 233–243, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Naegele B, Launois SH, Mazza S, Feuerstein C, Pepin JL, Levy P. Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep 29: 533–544, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLos One 6: e19847, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Lewis SJ. Hypoxia-induced ventilatory responses in conscious mice: gender differences in ventilatory roll-off and facilitation. Respir Physiol Neurobiol 185: 497–505, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis 3: 215–227, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177: 1006–1014, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem 385: 217–221, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus). J Neurosci 25: 4521–4526, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyter LM, Trainor BC, Nelson RJ. Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus). Eur J Neurosci 23: 3056–3062, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Ray AD, Magalang UJ, Michlin CP, Ogasa T, Krasney JA, Gosselin LE, Farkas GA. Intermittent hypoxia reduces upper airway stability in lean but not obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 293: R372–R378, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res 177: 308–314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanfilippo-Cohn B, Lai S, Zhan G, Fenik P, Pratico D, Mazza E, Veasey SC. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep 29: 152–159, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Su J, Sripanidkulchai K, Hu Y, Wyss JM, Sripanidkulchai B. The effect of ovariectomy on learning and memory and relationship to changes in brain volume and neuronal density. Int J Neurosci 122: 549–559, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Titus AD, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, Raju TR. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145: 265–278, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res 1294: 128–137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesstrom J, Ulfberg J, Nilsson S. Sleep apnea and hormone replacement therapy: a pilot study and a literature review. Acta Obstet Gynecol Scand 84: 54–57, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Xie H, Yung WH. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: a role for brain-derived neurotrophic factor. Acta Pharmacol Sin 33: 5–10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Zhang GL, Dai DZ, Zhang C, Dai Y. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3beta-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes. Reprod Toxicol 36: 60–70, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JX, Lu XJ, Wang XC, Li W, Du JZ. Intermittent hypoxia impairs performance of adult mice in the two-way shuttle box but not in the Morris water maze. J Neurosci Res 84: 228–235, 2006. [DOI] [PubMed] [Google Scholar]