Abstract
Significant cancer health disparities exist in the United States and Puerto Rico. While numerous initiatives have been implemented to reduce cancer disparities, regional coordination of these efforts between institutions is often limited. To address cancer health disparities nationwide, a series of regional transdisciplinary networks through the Geographic Management Program (GMaP) and the Minority Biospecimen/Biobanking Geographic Management Program (BMaP) were established in six regions across the country. This paper describes the development of the Region 3 GMaP/BMaP network composed of over 100 investigators from nine institutions in five Southeastern states and Puerto Rico to develop a state-of-the-art network for cancer health disparities research and training.
We describe a series of partnership activities that led to the formation of the infrastructure for this network, recount the participatory processes utilized to develop and implement a needs and assets assessment and implementation plan, and describe our approach to data collection. Completion, by all nine institutions, of the needs and assets assessment resulted in several beneficial outcomes for Region 3 GMaP/BMaP. This network entails ongoing commitment from the institutions and institutional leaders, continuous participatory and engagement activities, and effective coordination and communication centered on team science goals.
Keywords: cancer health disparities, needs assessment, evaluation, implementation plan, multi-institutional, biospecimen, biobank, team science
INTRODUCTION1
Significant cancer health disparities exist in the Southeast region of the United States, which includes Alabama, Florida, Georgia, Louisiana, Mississippi, and Puerto Rico (Departamento de Salud, 2007; U.S. Cancer Statistics Working Group, 2010). The two racial/ethnic minority populations most affected by cancer health disparities in this region are African Americans and Hispanics (U.S. Cancer Statistics Working Group, 2010). A recent report from the American Cancer Society found that more Hispanics in the United States die of cancer each year than any other cause (American Cancer Society, 2012). Factors contributing to cancer health disparities in this region include a high proportion of individuals without health insurance, high poverty rates, large rural areas with limited access to quality cancer care, and communication and health literacy barriers (Author, et al., 2010a; Author, et al., 2011a; Haynes & Smedley, 1999; Author, et al., 2012a; Jacobs, Karavolos, Rathouz, Ferris, & Powell, 2005; Kaiser Family Foundation, 2009; Author, et al., 2005; Ryan & Siebens, 2012; Shin & Kominski, 2010).
Numerous initiatives in the Southeastern United States are underway to reduce cancer disparities and train future researchers from underrepresented groups, (Author, et al., 2011a; Author, et al., 2010b; Author, et al., 2005; Satcher, et al., 2006; Author, et al., 2009; Author, et al., 2011b; Author, et al., 2011c; Author, et al., 2012b; Author, et al., 2012c; Author et al., 2012d; Wynn, et al., 2011; Author, et al., 2006a) but with minimal regional collaborations or coordination. Strengthening cancer research across the continuum from basic science to population-based studies is critical to the advancement of cancer health disparities research. As such, a number of inter-institutional networks have been established to reduce cancer health disparities, including Community Network Program Centers (CNPCs). For instance, the Deep South Network, (Author, et al., 2006b; Author, et al., 2005; Wynn, et al., 2011) the Tampa Bay Community Cancer Network (Author, et al., 2011d), and the National Black Leadership Initiative on Cancer II: Network Project (Satcher, et al., 2006) are all inter-institutional networks. In addition to CNPCs, partnerships between minority institutions and cancer centers through the Partnerships to Advance Cancer Health Equity (PACHE) have a direct focus on cancer health disparities at an inter-institutional level (National Cancer Institute, 2012).
Population-based molecular studies are important to cancer health disparities research, and team science is vital to address these disparities. Moreover, development of biobanks is also essential for effective translational research by allowing researchers to uncover genetic causes of complex diseases and subsequently develop new therapies and prevention strategies (Author, et al. 2011e; Khoury, Millikan, Little, & Gwinn, 2004; Morente, Fernandez, & de Atava, 2008). By obtaining diverse samples (eg, disease status, racial/ethnic composition), biobanks may serve as key resources to address the issue of limited generalizability that plagues much of the current clinical and genomics research, and allow for powerful interpretation of differences between diverse racial/ethnic groups and their association with disease processes. Partnerships with minority-serving institutions may help to identify and overcome barriers to research, establish biobanking models unique from those at comprehensive cancer centers, and create opportunities for research, training and outreach (Author, et al., 2011e).
In an effort to better coordinate cancer disparities activities, the National Cancer Institute’s (NCI’s) Center to Reduce Cancer Health Disparities (CRCHD) issued a call through American Recovery and Reinvestment Act (ARRA) supplementary funding for regional transdisciplinary networks through the Geographic Management Program (GMaP) and the Minority Biospecimen/Biobanking - Geographic Management Program (BMaP). The purpose of GMaP/BMaP was to establish multi-institutional networks to develop infrastructure for research and training for the purpose of reducing cancer related health disparities. Specifically, for BMaP, development of a state-of-the-art network lays the needed foundation and infrastructure for ensuring the adequate and continuous supply of high-quality human biospecimens (neoplastic and nonneoplastic tissues) for cancer research that takes into account “cultural sensitivities of diverse communities” in the region (National Cancer Institute, 2009). This paper details efforts toward the development of the Region 3 GMaP/BMaP network composed of over 100 investigators from nine institutions in five Southeastern states (Florida, Georgia, Alabama, Mississippi, Louisiana) and Puerto Rico who have assiduously worked to develop a regional plan for tackling cancer health disparities.
Our goals in this paper are to: (1) describe a series of partnership activities leading to the formation of infrastructure for Region 3 GMaP/BMaP network, (2) recount the participatory processes used to develop and implement a Region 3 needs and assets assessment to inform a comprehensive regional implementation plan, and (3) report lessons learned. We detail the application of the principles of community-based participatory research to the implementation of the network and the assessment. The blueprint of ideas outlined in this paper may be useful for other institutions and researchers who seek to create regional plans for impacting health disparities.
METHODS
Infrastructure Development of Region 3 GMaP/BMaP Network
A regional GMaP/BMaP teleconference hosted by CRCHD in Spring 2009 initiated discussions between investigators in Region 3. Universities and cancer centers in Region 3 already had significant infrastructure to contribute to a regional network. Most of the institutions had CRCHD funding at the time, and the nine partner institutions were identified: Winship Cancer Institute of Emory University, H. Lee Moffitt Cancer Center & Research Institute, Morehouse School of Medicine, Ponce School of Medicine, Tulane University, Tuskegee University, University of Alabama at Birmingham, University of Mississippi Medical Center, and Xavier University of Louisiana. The partner institutions included those with a demonstrated excellence in cancer [one NCI-designated cancer center; two NCI-designated comprehensive cancer centers; and two Commission on Cancer (CoC) accredited programs], and four minority-serving institutions. This make-up of the partner institutions was intended to provide a base of expertise in cancer health disparities from biobanking to clinical trials to community engagement. A subsequent series of regional teleconferences among institutional leaders at the nine institutions was held to determine how Region 3 would respond to the call for applications. An Administrative Core of the leaders at each institution was formed through these teleconferences. Senior leadership, such as cancer center directors, deans and/or Principal Investigators of center grants in health disparities, made up the Administrative Core. While the title of the leaders may have varied, the qualifier of the institutional leadership across the network was that they be the responsible contact for cancer health disparities research at their institutions. In addition to institutional leaders, the Administrative Core consisted of the core leaders who had a strong track record of professional experience in the area of their core and included investigators from both cancer centers and minority serving institutions. Institutional leaders also served as contacts to their institution in identifying investigators that would be core members. While the program strived to have at least one investigator from each institution in each core, it was recognized that the base of investigators at minority-serving institutions and teaching demands limited the number of investigators and their time commitment. Even though the number of investigators participating in Region 3 GMaP and BMaP was not equal between each institution, an Administrative Core made up of institutional leaders from every institution in the network served to balance participation by having representation from all partners in a core with the responsibility for network-level decision-making. Also, decisions on next steps of the network, the needs and assets assessment, implementation plan, and general formation of the network, were brought up in plenary sessions of retreats for transparency and participation across the network.
During a teleconference of the institutional leaders, it was decided by consensus that the University of Alabama at Birmingham (UAB) would submit the GMaP supplement on behalf of Region 3 due to extensive experience of the institution’s investigators in health disparities research. Moffitt Cancer Center (MCC) was selected by consensus to submit the BMaP supplement because of prior work in biospecimen donation and biobanking through its Total Cancer Care® initiative and its establishment of the first cancer tissue biobank at a Hispanic-serving institution through collaboration with Ponce School of Medicine (Author, et al., 2011e). Each institution committed to provide to the Region 3 GMaP/BMaP network in-kind contributions which included financial assistance as well as the time and effort of the investigators. A quarterly expense template was created for institutions to document effort spent on the project. Such expenses include time spent on teleconferences, travel expenses for face to face meetings, and effort in completing necessary project tasks towards the deliverables.
Upon receipt of GMaP-3 and BMaP-3 supplementary funding, communication, planning, and developmental activities were managed through regularly scheduled teleconferences as well as two in-person retreats each year, which rotated among Region 3 institutions. Each retreat had specific tasks to accomplish, which led to the development of GMaP-3 and BMaP-3 Implementation Plans (Table 1).
Table 1.
Region 3 GMaP/BMaP Retreats
| Date | Location; Host Institution | Objectives | Number of Participants | Highlights of Outcomes/Decisions |
|---|---|---|---|---|
| January 14–15, 2010 | Birmingham, AL; University of Alabama at Birmingham |
|
50 |
|
| July 15–16, 2010 | Tampa, FL; H. Lee Moffitt Cancer Center & Research Institute |
|
56 |
|
| February 17–18, 2011 | New Orleans, LA; Tulane University |
|
57 |
|
| August 4–5, 2011 | Atlanta, GA; Emory University |
|
37 |
|
| April 23–24, 2012 | Jackson, MS; University of Mississippi Medical Center |
|
42 |
|
Abbreviations: CAT = Comprehensive Assessment Tool; TMAs = Tissue Microarrays
Organizational Structure of Region 3 GMaP/BMaP Network
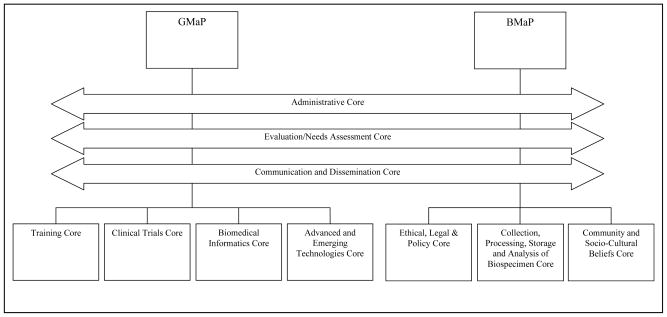
The organizational structure of Region 3 GMaP/BMaP network (Figure 1) has been coordinated around ten cores. There are three joint Region 3 GMaP/BMaP cores (administrative; evaluation/needs assessment; communication and dissemination; Figure 1). The Administrative Core is led by the GMaP-3 and BMaP-3 Project Leaders who, together with the GMaP-3 and BMaP-3 Regional Coordinators at UAB and MCC, are responsible for day-to-day conduct of the programs. GMaP-3 and BMaP-3 Project Leaders and Regional Coordinators meet through monthly teleconferences as a Coordinating Committee for both initiatives. GMaP-3 has four cores (training; clinical trials; biomedical informatics; and advanced and emerging technologies), and BMaP-3 has three cores (ethical, legal, and policy; collection, processing, storage, and analysis of biospecimens; and community and sociocultural beliefs). Each core has a core leader/co-leaders and core members across the nine partnering institutions to aid collaboration. A description of each core is provided in Table 2.
Figure 1. G/BMaP-3 Core Structure.
Note: The joint GMaP-3 and BMaP-3 cores are represented with left-right arrows to illustrate their roles across both programs.
Table 2.
Region 3 GMaP/BMaP Network Cores’ Descriptions and Selected Needs/Assets Assessment Template Objectives
| Region 3 GMaP/BMaP Network Cores | Overall Description of Core | Selected Needs/Assets Assessment Template Objectives |
|---|---|---|
| Communication and Dissemination Core | Provides assistance in external communication and dissemination through engagement of communities in Region 3, as well as internal communication and dissemination, or information exchange and resource sharing within the institutional partners. |
|
| Training Core | Develops a comprehensive plan for training the next generation of cancer researchers within the region, including strategies for recruitment, mentorship, and retention of faculty from diverse and underserved populations. |
|
| Biospecimen Science: Ethical, Legal, and Policy Core | Addresses issues related to access to biospecimens, informed consent, assessing the impact of sociocultural beliefs on biospecimen use among racially and ethnically diverse populations, intellectual property, regulatory compliance, privacy protection, and biospecimen custodianship or ownership. |
|
| Biospecimen Science: Collection, Processing, Storage, and Analysis of Biospecimens Core | Focuses on the scientific aspects of biospecimen donation and biobanking, including defining quality metrics, linking participants to education, training, and biobanking management resources. |
|
| Biospecimen Science: Community and Sociocultural Beliefs Core | Identifies the perceived community cultural, racial/ethnic, and social contexts in which biospecimen donation and biobanking research are viewed. |
|
| Clinical Trials Core | Develops strategies to enhance clinical trial recruitment of underserved populations, especially African-Americans and Hispanics. |
|
| Biomedical Informatics Core | Increases the bioinformatics capabilities and infrastructure within participating institutions relevant to cancer health disparities. |
|
| Advanced and Emerging Technologies Core | Enhances the understanding and application of advanced and emerging technologies to research through increased training, educational, and research opportunities. |
|
Note: The Biomedical Informatics and Advanced and Emerging Technologies Cores were merged after the February 2011 Face-to-face Retreat.
Abbreviations: QA/QC = Quality Assurance/Quality Control; caGRID = underlying network for caBIG® (cancer biomedical informatics grid)
Development and Implementation of Region 3 GMaP/BMaP Network Needs and Assets Assessment
Development of the Comprehensive Assessment Tool (CAT)
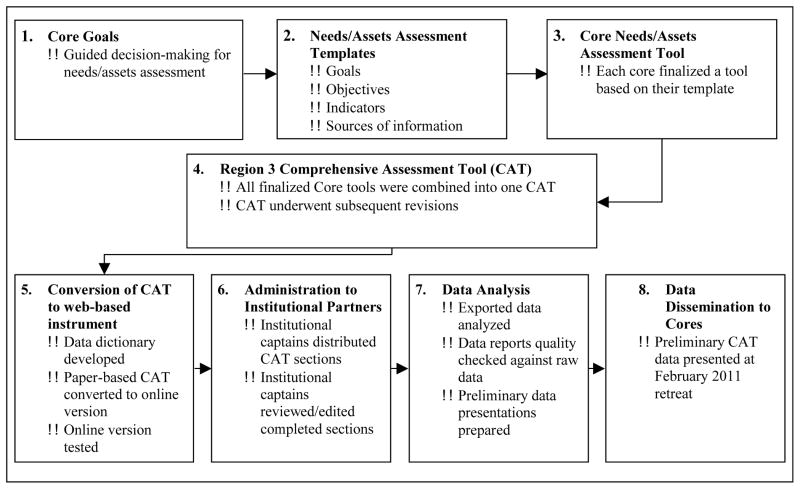
To develop a comprehensive strategy for building the Region 3 GMaP/BMaP network, an initial task was to assess regional strengths, weaknesses, capabilities, and resources at GMaP-3/BMaP-3 institutions. This was achieved through the development and implementation of the Comprehensive Assessment Tool (CAT), described below. The first step in creating the CAT entailed a face-to-face retreat of institutional and core leaders focused on establishment of the vision and goals for the Region 3 GMaP/BMaP network. At this initial retreat in January 2010, each core met for the first time and developed goals for their specific group (see Figure 2, step 1) that were consistent with the overall vision and goals of the network. The Evaluation/Needs Assessment Core developed a Needs/Assets Assessment Template to assist in designing questions for each core’s section of the needs assessment document. This template guided each core in developing measurable objectives for each goal, indicators of gaps and resources related to each of the objectives identified, and sources of data to evaluate objectives (See Figure 2, step 2; Table 2). The Administrative Core and the Evaluation/Needs Assessment Core did not complete templates as their roles were related to oversight and support of the needs assessment.
Figure 2.
Development of Region 3 Comprehensive Assessment Tool (CAT): From Core Goals to Data Dissemination
By March 2010, each core completed the template whereby members of the Evaluation/Needs Assessment Core generated items to measure each of the objectives of interest to the different cores. Drafts of these items were sent to each core and refined in an iterative manner with the Evaluation/Needs Assessment Core until a finalized draft of items was established for each core (Figure 2, step 3). The overall development of the CAT represented an iterative (step-wise) and participatory process whereby each core developed questions and an accompanying glossary of key terms pertinent to content domains of their core (Figure 1).
The Regional Coordinators combined the items and glossary terms into a first draft of the CAT for distribution to the Region 3 GMaP/BMaP investigators at the second face-to-face retreat (July 2010). During the second retreat, each network member was assigned a working group to review every item for one section of the CAT. In separate core sessions, members discussed feedback from the working groups that applied to their core’s section(s). In addition to providing feedback on the CAT, methods for collecting data were presented and finalized at the retreat. The MCC Survey Methods Core presented potential approaches for data collection, including paper (scannable) forms and web-based data entry. Following discussion of the advantages and disadvantages of each approach, it was decided the MCC Survey Methods Core (SMC) would develop a web-based data entry portal using Checkbox® 4.7 software.
After incorporating suggestions from the retreat, the final CAT included 181 closed and open-ended items and 32 tables divided into 12 sections (including Introduction & Instructions and the Glossary; Table 3). The revised CAT underwent a review by each core in August 2010 to address final conflicting recommendations (Figure 2, step 4). Steps three and four required multiple iterations of the CAT that were revised and reviewed by the cores, retreat attendees, and project leaders.
Table 3.
Region 3 Comprehensive Assessment Tool (CAT) Sections
| Section | Purpose | Core(s) that Contributed to Design of Sectiona | Example Items |
|---|---|---|---|
| Introduction and Instructions | Provide an explanation of Region 3 GMaP/BMaP and the CAT. | Administrative Core | N/A |
| Glossary | Provide definitions to terms used in CAT items that may be unfamiliar to the respondent. | Administrative Core | N/A |
| Institution Characteristics | Assess the partnerships and communication strategies that each institution employs. | Communication/Dissemination Core; Administrative Core | What type of language accommodation is provided to enrolled participants? |
| Research Regulatory Infrastructure | Address the research regulatory infrastructure at each institution which includes items on informed consent. | Ethical, Legal, and Policy Core | Does your institution have specific language that is mandatory to use in informed consent documents for biospecimen collection and biobanking? |
| Research Studies/Clinical Trials | Address cancer-related research studies/clinical trials conducted at each institution. | Clinical Trials Core | What strategies does your institution use to promote recruitment and retention to cancer-related epidemiologic, observational, or outcomes trials among African Americans and Hispanics/Latinos? |
| Biospecimen Donation and Biobanking: Collection, Processing, Storage and Analysis of Biospecimensb | Assess what types of biospecimens were stored; biobanking equipment available; personnel and administrative capacity; types of standard operating procedures; and other factors in biospecimen collection and biobanking. | Collection, Processing, Storage and Analysis of Biospecimens Core | Do you currently have an informatics system to collect and store data for your biospecimen collection? |
| Biospecimen Donation and Biobanking: Ethical, Legal and Policy | Address ethical, legal, and policy issues as they relate to biospecimen donation and biobanking. | Ethical, Legal, and Policy Core | At your institution, is research being conducted that is designed specifically to evaluate the ethical aspects of biospecimen research? |
| Biospecimen Donation and Biobanking: Community and Sociocultural Beliefs | Assess research involving community and socio-cultural beliefs in relation to biospecimen donation and biobanking. | Community and Sociocultural Beliefs; Communication/Dissemination Core | In what ways does your institution’s infrastructure address the inclusion of community and sociocultural beliefs in biospecimen/biobanking systems? |
| Advanced and Emerging Technologies | Assess the advanced and emerging technology cores, resources, and its application to cancer health disparities research. | Advanced and Emerging Technologies Core | Is data analysis a barrier to the use of advanced and emerging technologies in general health disparities research at your institution? |
| Biomedical Informatics | Address the biomedical informatics capacity at each institution that includes use of caBIG® modules. | Biomedical Informatics Core | Are inter-institutional agreements in place to allow or promote access to biomedical informatics resources? |
| Training | Assess the training capacity at each institution and the level of involvement of partnerships in training. | Training Core | List funding sources and resources for current training programs and strengths and barriers to providing training in linguistic and cultural competence at your institution. |
| Collaborative Research Grant Submissions | This section addresses research grants submitted in collaboration with other members of institutions in the Region 3 GMaP/BMaP network. | Administrative Core | Has your institution submitted research grants in collaboration with other members of institutions in the Region 3 GMaP/BMaP network in the past 5 years? |
The Evaluation/Needs Assessment Core contributed to the design of every section.
This section was divided into five sections for web-based completion. (I) Types of Biospecimens from Caucasian, African American and Hispanic/Latino Patients; (II) Equipment and Resources for Biospecimen Donation and Biobanking; (III) Personnel, Administrative Services, Procedures for Biobanking, Biospecimen Research, Data Elements and Training; (IV) Standard Operating Procedures; and (V) Outreach, Education, Biospecimen Core Committee and Financial Support.
In summary, the step-wise/iterative and systematic process allowed broad goals set by each core to eventually be transformed into specific items making up the CAT instrument. These steps were also shaped by the principles of community-based participatory research. The completion of the needs/assets assessment template provided a “check” on whether a goal could be measured through an assessment tool and, overall, provided an organized manner for core leaders and members not necessarily experienced in evaluation to have a guide in developing their section. Steps 1–3 were accomplished through the participation of each core in the creation and approval of the items that would form a section of the CAT. In step 4, the CAT was reviewed by retreat attendees from the nine institutions. Subsequently, each core approved their section of the CAT.
Data Collection Using the CAT
By November 2010, the finalized paper-based CAT and corresponding data dictionary detailing over 1,000 response variables were sent to MCC Survey Methods Core (SMC) for conversion of the CAT to a web-based format using a site license for Checkbox® (Figure 2, step 5). A functional online test version of the CAT was quality checked before the online CAT was distributed to partner institutions. To check the quality of the CAT web survey, links to the CAT sections were sent to individuals at Moffitt and UAB familiar with health disparities. These individuals were asked to review each section and provide feedback on: (a) terms that may not be understandable or confusing; (b) questions that are not easily understood; (c) misleading or confusing items; and (d) any technical bugs. This final quality check allowed individuals who had not been involved in the CAT design to provide an “outsider” perspective. Having the quality check completed by individuals not familiar with the network was important because it was not expected that individuals completing the web survey would necessarily be the same personnel who had been involved in the development of the CAT as a Region 3 GMaP/BMaP core leader or member and thus would not be familiar with its purpose and content. The final version of the CAT instrument is available upon request.
Using the CAT matrix, each institutional leader was asked to identify an institutional captain who would take responsibility for distribution of the sections and ultimate completion of the CAT at each of the Region 3 institutions. Sections (eg, training) would then be assigned for completion to individuals at each institution who were most knowledgeable about a particular topic. Because each section of the CAT would be assigned to particular respondent(s) at an institution, the SMC staff created a section-specific login and password using the Checkbox® software. Each respondent was instructed to login and answer the questions in the CAT in reference to their institution, not from an individual perspective. The regional coordinators and institutional leaders were able to track the progress made on the completion of the CAT through email alerts when a section was submitted. Also, regional coordinators had the ability to log into a Checkbox® portal in order to download responses to a particular section completed at an institution in real-time. All nine institutions uploaded CAT responses by February 2011 (Figure 2, step 6). CAT findings as they related to the original goals and objectives previously identified in the Needs/Assets Assessment Templates (Figure 2, step 7) were provided to Region 3 investigators at the February 2011 face-to-face retreat (Figure 2, step 8). During this retreat, each core reviewed and discussed their specific core’s data during break-out sessions, and subsequently discussed their interpretation of the data during a “report-out” meeting of the network investigators attending the retreat. In all, Figure 2 provides a systematic strategy for implementing a needs and assets assessment within a multi-institutional network that is geared towards addressing multiple specialized areas (by splitting them by core) and addressing needs, strengths, capacities and capabilities (by using a standard template and having the involvement of the Evaluation/Needs Assessment Core in the development of the assessment).
RESULTS
Region 3 GMaP/BMaP Network Infrastructure
The infrastructure of Region 3 GMaP/BMaP evolved over time through a series of discussions among investigators at each partnership institution. Partnership activities leading to the formation of the infrastructure for the network included (1) the initial establishment of the network partners and institutional leaders; and (2) the finalization of a core structure and goals at the first in-person retreat. As described in the Methods section, the initial communication of the network partners began with a teleconference hosted by CRCHD. Following this initial teleconference, a series of additional teleconferences between the institutional leadership led to aspects of the collaboration being defined (eg, which institutions would be lead sites for Region 3 GMaP and BMaP; which investigators at the institutions would serve as institutional leaders, core leaders, core members, etc). The first in-person retreat of the network brought together institutional and core leaders (ie, Administrative Core) and core members in solidifying the Region 3 infrastructure (Figure 1), individual goals, and the needs and assets timeline and proposed template. A retreat format of breakout sessions by each core, followed by open discussion during a plenary session was established as a satisfactory and participatory manner of structuring the retreats. Equal participation across cores from all nine institutions was a challenge of the network, as cancer centers typically had a larger base of investigators to draw from in comparison to minority serving-institutions. This was addressed by ensuring that core leadership and membership still had a mix of investigators across institutions.
Participatory Processes for the Development of the Needs and Assets Assessment and Regional Implementation Plan
The principles of community-based participatory research guided the processes for the participatory design and completion of the needs and assets assessment and the Implementation Plan. Especially (a) identifying and building on strengths and resources within the network; (b) fostering collaborative, equitable involvement of all partners in all phases of the research; (c) disseminating findings to partners; and (d) long-term commitment by all partners (Israel, et al., 1998). The assessment was designed to not only examine needs of the partner institutions but also the resources, capacities and capabilities so that ultimately the findings could inform how the network as a whole could strengthen areas of opportunity and address gaps. The process outlined in Figure 2 was participatory throughout, involving core leaders and members in the development and design of the needs and assets assessment. In step 1 core members defined goals the informed the needs/assets assessment templates (the outline of which had been presented by the evaluation/needs assessment core leaders during a plenary session of the first retreat). In step 2 a template was completed by each applicable core through core teleconferences of members across the region and the involvement of a member of the evaluation/needs assessment core for technical assistance. In step 3, each core reviewed and revised the tool that had been created by from their needs and assets template. At this point, each core had been working in isolation (other than the involvement of the evaluation/needs assessment core) on the CAT. Thus, the in-person retreat in July 2011 was implemented so that the entire CAT (all sections combined into one document) could be reviewed in plenary sessions and in breakout sessions (with breakout members across cores) to provide feedback for each core to consider about their section of the CAT (step 4). At this retreat, the decision on administering the CAT through an online instrument was also made during a plenary session (implemented in step 5). Each partner also had a participatory role in the administration of the CAT (step 6) where each institution had a “captain” who identified the most appropriate individual(s) to complete each section at their institution and also monitor its completion. Once the CAT was completed, findings were summarized and then presented at the Region 3 retreat where open discussion on the interpretation and implications of the findings took place (steps 7–8). The systematic and sequential process of the CAT development was replicated in the development of the Implementation Plans. Each core completed a template, thus involving investigators from every institution within the network. The project leaders and every core leader signed final Implementation Plans submitted to NCI to reflect the continued involvement of the network partners in every phase of the program. Further, the long-term commitment by all of the partner institutions cannot be underestimated. As described in Future Directions, the commitment continues through Region 3 GMaP and BMaP pilot studies.
Benefits of Region 3 GMaP/BMaP Network Needs and Assets Assessment
Completion of the CAT resulted in several beneficial outcomes for Region 3 GMaP/BMaP. First, the resulting CAT data on strengths, weaknesses, capabilities, and resources provided cores with information to develop GMaP-3 and BMaP-3 Implementation Plans, which serve as a future roadmap for full-scale implementation of Region 3 GMaP/BMaP, including core services. The same methodology that had proven effective for designing the CAT was used and included the following steps: (1) designing templates; (2) having each core complete a template corresponding to their section of the plan; and (3) incorporating these templates into Implementation Plans that were further revised as a whole. The Region 3 GMaP/BMaP Implementation Plans were completed and delivered to NCI in December 2011 to inform future funding opportunities. Second, completion of the CAT served to further solidify network infrastructure as core leaders, core members, and institutional leaders participated in development of the CAT. CAT development and completion tested the feasibility of the Region 3 GMaP/BMaP network to accomplish a standardized multi-site endeavor. Third, analysis of CAT data provided the basis for planning GMaP-3 and BMaP-3 pilot projects, which are being implemented as part of the second phase of GMaP/BMaP funding (described below). It is beyond the intent or scope of this paper to present the CAT data, however, in Table 4 we highlight a few pertinent findings as examples to illustrate for the reader the richness of data obtained in a participatory manner.
Table 4.
Example of Selected Findings from Data Analysis of CAT and Implications
| Assets | Needs | How Findings Applied |
|---|---|---|
| Seven of nine participating institutions provide treatment to cancer patients | Dissemination of successful recruitment/retention strategies/materials | In GMaP-3 Implementation Plan, the Research Studies/Clinical Trials Core objectives included increasing the number of individuals trained on recruitment/retention of racial/ethnic minorities to therapeutic and non-therapeutic cancer-related studies, particularly African American and Hispanics/Latinos with Region 3. |
| Largest numbers of samples were from lung and breast tissue | Share biospecimens with investigators (external to institution) | In the BMaP-3 Implementation Plan, the Collection, Processing, Storage and Analysis of Biospecimen Core goals included the development of a plan to ensure that biospecimen collection and storage in BMaP-3 academic institutions as well as community hospitals would be done in a uniform fashion. This would facilitate sharing of prospectively collected samples. The BMaP-3 Pilot included the retrospective collection of breast biospecimens from partner institutions which were used to create a tissue microarray that is available to researchers at the partner institutions. Currently, pilot projects at different partner institutions are utilizing the tissue microarray sections.An accompanying database was also developed with accounts access by investigators at the nine institutions. |
| Well established and functional core facilities with an extensive array of advanced and emerging technology capabilities | Correspondence of regional data management to support inter-institutional research | The GMaP-3 Implementation Plan, the Biomedical Informatics and Advanced and Emerging Technologies Core proposed a Data Coordinating Center that would manage and coordinate datasets from the network using a standards-based approach to data integration for health disparities research. |
DISCUSSION
In 2009, a regional inter-institutional network was established to address cancer disparities in the Southeastern United States. Over the past four years, the nine institutions that comprise Region 3 have worked toward that goal. While previous inter-institutional partnerships focused on reducing cancer disparities, (Carey, et al., 2005; Author, et al. 2011e; Goldmon, et al., 2008; Author, et al., 2011a; Author, et al., 2005; Wynn, et al., 2011) to date there have not been any published articles describing the development of a partnership as large as the Region 3 GMaP/BMaP network that specifically centered on health disparities and biobanking/biospecimen collection.
Lessons Learned
During the development of the Region 3 network several lessons were learned. First, establishing the Region 3 GMaP/BMaP network required significant participation and commitment of each of the nine institutions and nine institutional leaders involved. From the beginning of Region 3 GMaP/BMaP, the investigators at the partner institutions were engaged as key stakeholders in the infrastructure building and needs/assets assessment process. Using a core structure, each institutional leader played an important role in identification of core leaders and core members from their institution (totaling approximately 100 investigators), development of the needs and assets assessment, evaluation of the needs and assets assessment data, and development of the implementation plan. The importance of having each core composed of members across the partner institutions is critical in maintaining the participatory aspect, continued momentum, and engagement of this multi-institutional initiative. At each juncture, the project leaders sought the equitable participation across institutions, especially a balance across the types of institutions represented in teleconferences, and to have retreats attended by investigators representing all network institutions. While needs, strengths, capabilities and capacities vary between institutions in the network, they complement a common goal to ultimately create a state-of-the-art network for cancer health disparities research, training and care. This common link focused on cancer health disparities created a bond between the institutions largely responsible for the momentum and engagement. It is also important to stress the strategy of viewing the network of approximately 100 investigators as a unit of identity, and from the perspective of the project leaders and institutional leaders, as a group whose engagement and perspective must be considered in the decision-making and communication of the network.
Second, the process of decision-making in the network was instrumental to development of the Region 3 GMaP/BMaP network. In general, decision-making has been implemented through a group consensus approach. Issues pertaining to the needs and assets assessment, implementation plan, and general formation of the network were discussed at in-person retreats that rotated through different Region 3 institutions. Having institutional leaders provide input into decisions has been central in developing the Region 3 GMaP/BMaP network. Decision-making was also carried out, especially at retreats, in an atmosphere of equity. This was agreed upon in the first retreat. Also, having all core minutes and materials equally available and having cores report on their activities through Administrative Core teleconferences and at retreats kept a transparent environment to the conduct of the program. Decision-making during the scope of the program described in this paper was not governed by an agreement, although specific research pilot activities in September 2011 and forward did involve the development of a charter, memoranda of understanding, and a collaboration agreement. A specific example of the decision-making process within the region was the consideration of implementing a Region 3 Consortium Institutional Review Board (IRB). The BMaP-3 Ethical, Legal & Policy Core had assessed the willingness for institutions to participate in a Region 3 Consortium IRB. During the third retreat, the results were reported for the CAT, including that a majority (n=7) of the institutions were willing to consider this idea. During the Ethical, Legal & Policy Core session the possibility of proposing the implementation of a Consortium IRB was discussed among the multi-institutional Core membership. After weighing the advantages and disadvantages, the Core proposed during their report-out in the plenary session to not pursue the idea given the challenges in implementation. This was agreed by all the members during discussion. This approach of having cores with the respective expertise weigh decisions served as a transparent and participatory approach to handling decision-making within the network. Another example of decision-making in the network which demonstrated the flexibility of the network was the decisions made to restructure the organizational structure of the network. At one of the first regional retreats, it had been proposed to merge the Communication & Dissemination Cores. During plenary session of the retreat this was brought for a vote to the participants and the decision was finalized to combine both cores as a merged GMaP-3/BMaP-3 Communication/Dissemination Core as both had overlapping roles and goals and members. The organizational structure was revisited again in merging the Advanced/Emerging Technologies Core with the Biomedical Informatics Core.
Third, effective coordination and communication between the geographically diverse institutions and institutional leaders have been instrumental in creating the network. Using the core structure, GMaP/BMaP Region 3 network information has been communicated back and forth between cores and project leaders via multiple modes of communication. These modes of communication specifically were (a) teleconferences for core meetings needed to accomplish tasks between in-person retreats, (b) a SharePoint website for access to program documents, (c) email for day-to-day communication and monthly email blasts to 100 investigators and leaders which detail network announcements such as funding opportunities and conferences in health disparities/team science (investigators are invited to contribute to email blasts), and (d) in-person meetings to provide for networking between investigators across the region, regional decision-making and provide the momentum to move the program from one phase to the next. Each in-person retreat was structured to meet specific goals of communicating results of previous efforts, obtaining participant feedback, and establishing objectives for future activities. Two regional coordinators provide support in these efforts.
In terms of the needs and assets assessment process, there are lessons learned from the evaluation practice perspective as well. A participatory process is productive only to the point to which it (a) is initiated from inception of planning; (b) allows for sufficient time for interactions between participants; and (c) is held in an atmosphere of “mutual respect and trust” (Green & Kreuter, 2005). Interactions between network institutions began from time of the funding announcement, resulting in a mutual decision on lead institutions that would be funded for GMaP-3 (UAB) and BMaP-3 (MCC). We have found that a truly participatory approach requires significant time from all network partners from the design of the CAT instrument to data collection and interpretation of results. Time, respect, and effective communication were essential components of this transdisciplinary evaluation process, meaning that no method in the evaluation was taken for granted as common knowledge.
Limitations
As Green and Kreuter (2005) state, while generalizability and external validity are standards for judging sound science, it is recognized that program planning research yields no results that can be concluded for a larger population, but a generalizable “process for planning.” Thus, results from the CAT assessment are not necessarily generalizable outside of the nine institution network. Yet, results were, and continue to be, informative for internal network development. Also, the needs/assets assessment was not triangulated with other methods or sources. In part, this was due to funding constraints as other methods for verifying/corroborating responses (such as a document review or site visit) were beyond the resources for Region 3 GMaP/BMaP. In summary, the participatory design and intent of the CAT allowed identification of future areas of synergy and collaboration, guided decision-making on cancer health disparities research priorities, and fueled ideas to create a regional biobanking network.
Region 3 GMaP/BMaP Network Future Directions
Future steps for the Region 3 GMaP/BMaP network include development of investigator-initiated cross-institutional team science research studies and training programs to address gaps identified in Region 3. In addition, the Region 3 BMaP network began a biospecimen pilot project in Fiscal Year 2011, as a proof-of-principle for collaborative minority biospecimen/biobanking in Region 3. This pilot involves the retrospective collection of formalin-fixed paraffin embedded breast cancer tissues from Caucasian, African American, and Hispanic/Latino patients and associated de-identified data from multiple institutions for the construction of a tissue microarray (TMA) that can be used by researchers in the network for collaborative projects. To date, samples from 259 African American and Caucasian patients have been retrieved from four institutions and used to create a TMA governed by the Region 3 BMaP Tissue Advisory Board which has one voting member per institution. Two collaborative (multi-institutional) developmental pilots (projects must have PI’s from two Region 3 institutions) were reviewed, approved by the TAB, funded through Region 3 BMaP and are currently utilizing slides from the TMA. Furthermore, a Region 3 statistical training workshop on the analysis of TMA’s was recently held. These initial regional efforts demonstrate strong collaborations across institutions and lend high support for achieving initial network outcomes. Future efforts will be made to increase participation in the network by community members served by the institutions participating in the Region 3 GMaP/BMaP network, and to work towards the inclusion of additional institutions within the network. While we hope the network will reduce cancer health disparities, we currently do not have data to indicate whether this has happened or not. However, our efforts to date suggest that our original goal of forming a supportive and enriching network that would produce engaging and productive collaborations (development of CAT, interactions, pilot projects, etc) suggest much promise for future and sustained collaborations. Future efforts will be made to increase participation in the network by community members served by the institutions participating in Region 3 GMaP/BMaP network and to work towards the inclusion of additional institutions within the network.
CONCLUSIONS
The Region 3 GMaP/BMaP network entails ongoing commitment from the institutions and institutional leaders, continuous participatory and engagement activities, and effective coordination and communication centered on team science goals. While the ultimate goal is the establishment of infrastructure, the work in this paper represents the first three years where the foundation for the network is established. With continued support, interest, and commitment, this network will continue to seek to refine a state-of-the-art network for cancer health disparities research and training.
Highlights.
We detail the efforts behind the development of the Region 3 GMaP/BMaP network.
The network is comprised of over 100 investigators from nine institutions.
We describe development & implementation of participatory needs/assets assessment.
Acknowledgments
This work was supported by the NCI at the National Institutes of Health (U54 CA118948 [University of Alabama at Birmingham Comprehensive Cancer Center] and 3U56 CA118809-14S2 [H. Lee Moffitt Cancer Center & Research Institute]). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI. The work contained within this publication was supported in part by the Survey Methods and Biostatistics Cores at the Moffitt Cancer Center. Significant in-kind support for development of the partnership was provided by the nine partner institutions: Emory Winship Cancer Center, H. Lee Moffitt Cancer Center & Research Institute, Morehouse School of Medicine, Ponce School of Medicine, Tulane University, Tuskegee University, University of Alabama at Birmingham, University of Mississippi Medical Center, and Xavier University of Louisiana. The authors would also like to especially thank Dr. Mary Ann Van Duyn, Program Director for GMaP-3 and BMaP-3 at the NCI and Ms. Ivana Sehovic, Research Coordinator at the H. Lee Moffitt Cancer Center & Research Institute.
Biographies
Vitae
Kristen J. Wells, PhD, MPH, is an Assistant Professor in the Department of Psychology at San Diego State University and Participating Member at Moores Cancer Center. She has been engaged in public health research focused on improving the quality of health care delivered to underserved populations. Her work in public health has included leading observational and complex interventional studies focused on quality of health care and quality of life.
Diana S. Lima, MPH, is a Clinical Research Coordinator at H. Lee Moffitt Cancer Center & Research Institute. She has experience in designing and implementing evaluations for health promotion programs and served as Regional Coordinator for the Minority Biospecimen/Biobanking Geographic Management Program for Region 3 (BMaP-3).
Cathy D. Meade, PhD, RN, FAAN, is a Senior Member in the Division of Cancer Prevention & Control, Health Outcomes & Behavior Program, at H. Lee Moffitt Cancer Center & Research Institute, and provides leadership for a multitude of NCI funded health disparities research and education activities. She is a nationally recognized expert in the areas of health disparities, cancer communications, literacy and cancer education, is well versed in community-based participatory methods and especially skilled in building and sustaining community partnerships and multi-institutional relationships.
Clement K. Gwede, PhD, MPH, RN, is an Associate Member in the Department of Health Outcomes and Behavior, Division of Population Sciences at Moffitt Cancer Center, and Associate Professor in the Department of Oncologic Sciences at the University of South Florida College of Medicine. His research focuses on reducing cancer health disparities among medically underserved multi-ethnic/diverse populations and improving quality of life for cancer patients during treatment and survivorship. His funded research uses community-based participatory research (CBPR) methods and client-directed interventions to increase community access to colorectal and prostate cancer screening and informed decision making.
Mona Fouad, MD, MPH, is Professor of Medicine and Director of the Division of Preventive Medicine at UAB, and Director of the UAB Minority Health and Health Disparities Research Center. She serves as PI for NCI-funded CanCORS and UAB Minority Screening Center of the PLCO Cancer Screening Trial. She is PI for the CDC-funded project, REACH 2010, and its continuation, REACH US, a model that will serve as an infrastructure to implement, evaluate, and disseminate locally, regionally and nationally a community action plan to reduce cancer disparities between African American and white women.
Michelle Lacey, PhD, currently holds appointments as Associate Professor in the Tulane Department of Mathematics and Adjunct Associate Professor in the Tulane Department of Biostatistics. She has extensive experience in the modeling and analysis of biological data, particularly in the areas of phylogenetics, epigenetics, and genomics. She has a long history of collaboration with both basic and clinical biological researchers and has received funding from the NIH, the Office of Naval Research and the Louisiana Cancer Research Consortium.
Debra W. Christie, MBA, RHIA, CTR, CCRP, is Director, Cancer Research & Registry at the University of Mississippi Medical Center. Her experience includes 30 plus years of conducting and overseeing cancer related treatment, cancer control and prevention clinical trials. This includes the regulatory aspects of clinical trials, recruiting/enrolling participants on studies, and data management for studies. She chaired the Clinical Research Associates Committee of the Southwest Oncology Group, an NCI funded cooperative group, for almost 20 years. Other activities include participation in NCI grant review committees for conducting cancer related clinical trials.
Teresita Muñoz-Antonia, PhD, is an Associate Member in the Molecular Oncology Program at H. Lee Moffitt Cancer Center & Research Institute, and her research focus is on TGFβ receptor regulation at the molecular and cellular levels. In addition to her interest in basic science, Dr. Muñoz-Antonia has been involved in many initiatives to reduce cancer health disparities. Specifically, she has served as assistant Director of Institutional Diversity, Hispanic Initiatives from 2005 to 2008, at H. Lee Moffitt Cancer Center & Research Institute, and currently has a leadership role in several projects that involve collaborations with institutions in Puerto Rico.
Isabel Scarinci, PhD, MPH, is a Professor and Associate Director for Faculty Development and Education in the UAB Division of Preventive Medicine. Her primary area of interest is cancer prevention and control among low-income, minority and immigrant populations (particularly Latinos and African Americans). She works primarily in the areas of breast and cervical cancer and tobacco control among women.
Allison McGuire, MPH, is a Program Manager with the University of Alabama at Birmingham Division of Preventive Medicine. She has extensive experience in managing large, multifaceted research/educational grants focusing on cancer health disparities and prevention among low-income minorities, particularly Latino immigrants. She has directed several successful, community-based, culturally relevant behavioral research initiatives contributing to her proficiency in project planning, organization, and implementation.
W. Jack Pledger, PhD, serves as Associate Center Director for Research and Deputy Director at Gibbs Cancer Center. His research interests focus on the mechanisms that regulate cellular proliferation and differentiation with special emphasis on tumor development and metastasis. His research has been seminal in our understanding of the regulation of cellular proliferation and oncogenesis.
Edward Partridge, MD, currently serves as Director of the UAB Comprehensive Cancer Center. He is also Professor in the Department of Obstetrics and Gynecology, Division of Gynecologic Oncology and holds the Evalina B. Spencer Endowed Chair in Oncology. Dr. Partridge has demonstrated exceptional leadership ability in both research and administrative posts. His clinical interests are cancer control and prevention; cervical cancer; community based research; gynecology oncology; minority health disparities; and ovarian cancer.
Joseph Lipscomb, PhD, is Professor, Department of Health Policy and Management, Rollins School of Public Health at Emory University and a Georgia Cancer Coalition Distinguished Cancer Scholar. He is Associate Director of Population Sciences in the Winship Cancer Institute. His research has focused recently on patterns and quality of cancer care in Georgia, health care disparities in urban areas, and building data resources for comparative effectiveness research and quality-of-care assessment.
Roland Matthews, MD, Chair of the Department of Obstetrics and Gynecology at The Morehouse School of Medicine, is a board certified obstetrician and gynecologist, with subspecialty certification in gynecologic oncology. Currently, he holds appointments at Grady Memorial Hospital, Emory Crawford Long Hospital and South Fulton Medical Center. His area of special interest in Gynecologic Oncology is preinvasive cervical disease. He is currently working to understand protein biomarkers of cervical cancer risk and studying the gene prohibitin and its functional significance as a tumor suppressor in women with gynecologic cancers.
Jaime Matta, PhD, Professor, Department of Pharmacology and Toxicology has expertise is in the area of toxicology. He also has a dual appointment in the Department of Surgery and serves as the contact PI for the Ponce School of Medicine U54 Partnership with Moffitt Cancer Center. His research is primarily focused on the role of DNA repair as a risk and prognostic factor for breast and cancer. His laboratory has made important contributions on the factors that are associated with dysregulation of DNA repair in cancer.
Idhaliz Flores, PhD, serves as Professor in the Department of Obstetrics and Gynecology at Ponce School of Medicine (PSM). She has worked in endometriosis research for the past ten years, specifically looking at molecular biomarkers and genetic factors associated with this disease. She currently directs the Endometriosis Research Program (ERP) at PSM. Under her direction, the ERP has successfully established a unique biorepository of tissues, serum and nucleic acids, as well as demographic and clinical obtained from patients with endometriosis and controls.
Roy Weiner, MD, is Associate Dean for Clinical Research and Training at Tulane University School of Medicine. His research expertise is in cancer education, medical student cultural competence, clinical research in breast cancer including triple-negative breast cancer, and community outreach for risk reduction.
Timothy Turner, PhD, Professor of Biology, is the Deputy Director of Research and Training in the Tuskegee University National Center for Bioethics in Research and Health Care and the Lead Principal Investigator for the Morehouse School of Medicine/Tuskegee University/University of Alabama at Birmingham U54 Cancer Partnership. His research interests focus on identifying and disrupting signaling mechanisms involved the progression of prostate cancer to its invasive and metastatic stages. Within this approach, his lab has utilized luteinizing hormone releasing hormone receptors as the tumor target for the delivery of cancer drugs to prostate cancer cells.
Lucio Miele, MD, PhD, is Director of the University of Mississippi Medical Center (UMMC) Cancer Institute and Ergon Professor, Departments of Medicine, Pharmacology, Biochemistry and Radiation Oncology. His research interests include: breast cancer drug development and biomarker validation; notch and other developmental pathways as therapeutic targets in cancer; and cancer stem cells-targeted therapeutics. The Miele laboratory has been focusing on cancer experimental therapeutics for 17 years. The Miele laboratory studies the Notch signaling pathway, which mediates communication between contiguous cells and regulates cell fate during embryonic development and postnatal life.
Thomas E. Wiese, PhD, is an Associate Professor in the Division of Basic Pharmaceutical Sciences in the College of Pharmacy at Xavier University of Louisiana. He teaches molecular biology-biotechnology, medicinal chemistry and cancer biology lecture and laboratory courses and also maintains an active research laboratory focusing on the molecular mechanisms involved in nuclear receptor mediated endocrine disruption in cancer promotion and progression. Since 2007, Dr. Wiese has served as the Xavier Associate Director of the Louisiana Cancer Research Consortium (LCRC) that includes the cancer centers at Tulane and LSU Health Science Centers as well as Ochsner Clinic Foundation.
Carlos S. Moreno, PhD, is Associate Professor of Pathology & Laboratory Medicine at Emory University, where he is a member of the Winship Cancer Institute. He has served as Director of the Winship Microarray Core Facility, Co-Director of the Emory Biomarker Service Center, and Scientific Director of the Cancer Genomics Shared Resource. He specializes in cancer bioinformatics, systems biology, and whole genome expression profiling, having authored 50 peer-reviewed publications, the majority of which have used microarrays to study cancer biology. He has used high throughput technologies to analyze gene expression patterns in prostate, breast, ovarian, and brain tumors.
Eboni G. Price-Haywood, MD, MPH, is a General Internist, Associate Professor of Medicine, Chief Medical Officer (CMO) of Tulane’s Community Health Centers, contributing faculty to the Tulane Cancer Center, and committee member for the Louisiana Healthcare Quality Forum (medical home & health information technology). Her research program focuses on developing strategies to train health professionals to effectively engage in culturally-tailored cancer risk communication and health education with African Americans and patients with limited health literacy.
Gwendolyn P. Quinn, PhD, is a Senior Member of Moffitt Cancer Center in the Health Outcomes and Behavior Program and a Senior Professor at the University of South Florida College of Medicine, Department of Oncologic Sciences. She is Director of the Moffitt Survey Methods Core. She is a health psychologist whose research focuses on assessing the behavioral determinants of consumer decisions and choices about health. She is director of the National Training Collaborative for Social Marketing that trains health care professionals in the field of social marketing.
Domenico Coppola, MD, is a board-certified anatomic and clinical pathologist, Professor of Pathology and Senior Member of the Anatomic Pathology Department and GI Oncology Program at the H. Lee Moffitt Cancer Center. He is the Scientific Director of the Tissue Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, and a member of the ISBER Biospecimen Working Group. Dr. Coppola has a strong background in General Pathology and in Surgical and Molecular Pathology. Dr. Coppola has expertise with the intricacies and the challenges of managing a Tissue Biorepository.
Stephen O. Sodeke, PhD, MT (ASCP), MS, MA, contributes regularly to Bioethics education and training at Tuskegee University. He currently serves as Associate Director of the Tuskegee University National Center for Bioethics. He is Professor of Allied Health, and chairs the Tuskegee University IRB. Dr. Sodeke’s research interests include community bioethics, research ethics, health and human rights, ethical issues raised by clinical trials, health disparities, and by research with vulnerable populations in the United States and in the developing world.
B. Lee Green, PhD, is Vice President, Moffitt Diversity; Senior Member, Health Outcomes and Behavior at H. Lee Moffitt Cancer Center & Research Institute and Co-Director of the NIH-funded Center for Equal Health. His research expertise is in cancer health disparities with particular interest in education, knowledge and awareness among minority populations. His research interest also includes community based participatory research as well as minority participation in clinical trials and research studies. The research has been focused on enhancing theoretical models and methods for community-based health promotion and disease prevention among underrepresented populations.
Maureen Y. Lichtveld, MD, MPH, Professor and Chair, is Freeport McMoRan Chair of Environmental Policy, Associate Director Population Sciences, Louisiana Cancer Research Consortium at Tulane University School of Public Health and Tropical Medicine, Department of Environmental Health Sciences. Her background is in environmental public health and medicine. Her research expertise is in community based participatory research in cancer health disparities; and evaluating the role of the environment in its broadest sense as factors influencing the cancer care continuum. Her expertise also includes evaluation and training.
Footnotes
Abbreviations: Geographic Management Program (GMaP); Minority Biospecimen/Biobanking Geographic Management Program (BMaP); Quality Assurance/Quality Control (QA/QC); underlying network for caBIG® or the cancer biomedical informatics grid (caGRID); Comprehensive Assessment Tool (CAT); Tissue Microarrays (TMAs); Commission on Cancer (CoC); National Cancer Institute (NCI); Community Network Program Centers (CNPCs); Partnerships to Advance Cancer Health Equity (PACHE); Center to Reduce Cancer Health Disparities (CRCHD); American Recovery and Reinvestment Act (ARRA); Minority Institutions/Cancer Center Partnership (MI/CCP); Continuing Umbrella of Research Experiences (CURE); University of Alabama at Birmingham (UAB); Moffitt Cancer Center (MCC)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. Cancer Facts & Figures for Hispancis/Latinos 2012–2014. Atlanta: Publishing; 2012. [Google Scholar]
- Author et al. (2005).
- Author et al. (2006a).
- Author et al. (2006b).
- Author et al. (2009).
- Author et al. (2010a).
- Author et al. (2010b).
- Author et al. (2011a).
- Author et al. (2011b).
- Author et al. (2011c).
- Author et al. (2011d).
- Author et al. (2011e).
- Author et al. (2012a).
- Author et al. (2012b).
- Author et al. (2012c).
- Author et al. (2012d).
- Carey TS, Howard DL, Goldmon M, Roberson JT, Godley PA, Ammerman A. Developing effective interuniversity partnerships and community-based research to address health disparities. Acad Med. 2005;80:1039–1045. doi: 10.1097/00001888-200511000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Departamento de Salud. Figueroa N, De la Torre T, Ortiz K, Pérez J, Torres M, editors. [Date of access: June 8 2011];Datos de Cáncer. 2007 http://www.salud.gov.pr/RCancer/Reports/Pages/default.aspx.
- Goldmon M, Roberson JT, Carey T, Godley P, Howard DL, Boyd C, et al. The data collection/data distribution center: building a sustainable African-American church-based research network. Prog Community Health Partnersh. 2008;2:205–224. doi: 10.1353/cpr.0.0023. [DOI] [PubMed] [Google Scholar]
- Green LW, Kreuter MW. Health program planning: an educational and ecological approach. 4. New York: McGraw-Hill; 2005. [Google Scholar]
- Haynes MA, Smedley BD. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, D.C: National Academy Press; 1999. [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Karavolos K, Rathouz PJ, Ferris TG, Powell LH. Limited English proficiency and breast and cervical cancer screening in a multiethnic population. Am J Public Health. 2005;95:1410–1416. doi: 10.2105/AJPH.2004.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation. [Date of access: April 19, 2012];Key Health and Health Care Indicators by Race/Ethnicity and State. 2009 http://www.kff.org/minorityhealth/upload/7633-02.pdf.
- Khoury MJ, Millikan R, Little J, Gwinn M. The emergence of epidemiology in the genomics age. Int J Epidemiol. 2004;33:936–944. doi: 10.1093/ije/dyh278. [DOI] [PubMed] [Google Scholar]
- Morente MM, Fernandez PL, de Atava E. Biobanking: old activity or young discipline? Semin Diagn Pathol. 2008;25:317–322. doi: 10.1053/j.semdp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. [Date of access: June 8, 2011];NCI Guidelines for Administrative Supplements for Minority Biospecimen/Biobanking - Geographic Management Program (BMaP) 2009 http://www.cancer.gov/researchandfunding/fundingsources/administrative_supplement_bmap.pdf.
- National Cancer Institute. [Date of access: December 28, 2012];Partnerships to Advance Cancer Health Equity. 2012 http://crchd.cancer.gov/research/pache-overview.html.
- Ryan CL, Siebens J. Educational Attainment in the United States: 2009. Washington, D.C: US Census Bureau; 2012. [Google Scholar]
- Satcher D, Sullivan LW, Douglas HE, Mason T, Phillips RF, Sheats JQ, et al. Enhancing cancer control programmatic and research opportunities for African-Americans through technical assistance training. Cancer. 2006;107:1955–1961. doi: 10.1002/cncr.22159. [DOI] [PubMed] [Google Scholar]
- Shin HB, Kominski RA. Language Use in the United States: 2007. Washington, D.C: US Census Bureau; 2010. [Google Scholar]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- Wynn TA, Anderson-Lewis C, Johnson R, Hardy C, Hardin G, Walker S, et al. Developing a community action plan to eliminate cancer disparities: lessons learned. Prog Community Health Partnersh. 2011;5:161–168. doi: 10.1353/cpr.2011.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]