Abstract
The natural history of Epstein-Barr virus (EBV) infection in 556 infants born to 517 human immunodeficiency virus (HIV) type 1–infected mothers was studied in a prospective, multicenter, cohort study. HIV-1–infected children had a cumulative EBV infection rate similar to HIV-1–uninfected children at age 3 years (77.8% vs. 84.9%) but had more frequent oropharyngeal EBV shedding (50.4% vs. 28.2%; P < .001). The probability of shedding decreased with longer time from EBV seroconversion and was similar to that of HIV-1–uninfected children 3 years after seroconversion. HIV-1–infected children identified as rapid progressors shed EBV more frequently than nonrapid progressors (69.4% vs.41.0%; P = .01). HIV-1–infected children with EBV infection had higher mean CD8 cell counts. EBV infection did not have an independent effect on mean CD4 cell counts, percent CD4, IgG levels, HIV-1 RNA levels, lymphadenopathy, hepatomegaly, or splenomegaly. Early EBV infection is common in children born to HIV-1–infected mothers. Children with rapidly progressive HIV-1 disease have more frequent EBV shedding.
Epstein-Barr virus (EBV) infects up to 95% of the world’s adult population. In developing countries and in socioeconomically disadvantaged populations of industrialized countries, primary infection occurs principally during infancy and early childhood and is generally asymptomatic or only mildly symptomatic [1, 2]. In more affluent populations in industrialized countries, infection in early childhood is most common, but about one-third of infections occur during adolescence and early adulthood [1, 3, 4].
In persons with human immunodeficiency virus (HIV) type 1 infection and AIDS, EBV infection is causally associated with oral hairy leukoplakia [5, 6], lymphoid interstitial pneumonitis (LIP), especially in children [7–11], and increased cardiac morbidity and mortality in children with advanced HIV-1 infection [12]. In persons with AIDS and in other immunocompromised persons [13], EBV is associated with non-Hodgkin’s lymphoma [14] and leiomyosarcomas [15, 16].
The distinguishing predisposition to EBV-associated lesions of oral hairy leukoplakia, LIP, and cardiomyopathy, the increased incidence of EBV-associated lymphoid and smooth muscle tumors, and the bidirectional interactions between HIV-1 and EBV in vitro at the cellular and molecular levels [17–22] indicate the influence of EBV infection on the clinical expression of HIV-1 infection. We prospectively studied HIV-1–infected and –uninfected children born to HIV-1–infected mothers to determine the epidemiology, immune response, and clinical manifestations of EBV infection in these groups during the first 5 years of life. For HIV-1–infected children, we analyzed the consequences of EBV infection on the clinical symptoms and laboratory findings of AIDS.
Methods
The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV-1 Infection (P2C2) Study is a prospective study of vertically transmitted HIV-1 infection focusing primarily on the cardiopulmonary manifestations of vertically transmitted HIV-1 infection. Starting in 1990, pregnant HIV-1–infected women were enrolled at five US sites: Boston, Houston, Los Angeles, and New York City (two sites). The methodology and population in this study has been fully described [23].
Women infected with HIV-1, confirmed by Western immunoblotting, were enrolled during pregnancy and up to 28 days postpartum, provided that infants were not identified because of HIV-1–associated symptoms. Blood was obtained from the mothers for EBV serologies within 30 days of delivery. HIV-1 cultures and serologies of the children were performed at birth and at 3 and 6 months with confirmatory serology at age 15–18 months. At 3, 9, 15, 21, 30, 36, 42, and 48 months of age, the children had interval history, physical examination, EBV culture and serology, serum IgG level, complete blood count, and lymphocyte subsets (absolute cell counts and percent of CD4, CD8, and B [CD19 or 20]) determined by use of AIDS Clinical Trials Group (ACTG) consensus protocol [24] at ACTG-certified laboratories.
HIV-1 culture, serology, and quantitative RNA
HIV-1 cultures were performed according to the ACTG consensus protocol for qualitative peripheral blood mononuclear leukocyte HIV-1 cultures [25]. HIV-1 ELISA and Western immunoblotting were used to test for HIV-1 antibodies. Children were considered HIV-1 infected if they had two positive cultures, positive HIV-1 serology at ≥15 months of age, death from an HIV-1–associated condition, or AIDS [26]. Children were considered HIV-1 uninfected if they had two negative cultures, including one at >5 months or age, with no positive cultures, or negative HIV-1 serology at ≥15 months of age. Children who did not meet either definition were considered indeterminate. HIV-1–infected children were further classified as rapid progressors if diagnosed with an AIDS-defining condition (other than LIP) or with severe immunosuppression (<750 CD4 cells/mm3 or <15% of total lymphocytes) in the first year of life [26]. HIV-1 RNA was measured in serum by quantitative HIV-1 RNA polymerase chain reaction by Amplicor HIV-1 Monitor Test (Roche Diagnostic Systems, Branchburg, NJ) [27].
EBV culture and serologies
Culture specimens for EBV were obtained by swabbing the oropharynx with a cotton swab that was immediately placed in viral transport medium, stored at −70°C, and shipped to a central laboratory. EBV was cultured qualitatively by a standard transformation assay and quantitatively by a standard virus titration assay [28]. One negative control well was included with each culture plate. The 50% end-point titer was determined by the Reed-Muench formula [29]. EBV serology tests for antibody to viral capsid antigen (VCA)-IgM, VCA-IgG, early antigen (EA)–restricted and EA-diffuse (EA-D), and nuclear antigen (EBNA) were done using standard titered immunofluorescence methods [30].
Statistical analysis
For all analyses, children were defined as either HIV-1 infected or HIV-1 uninfected and were subclassified as rapid or nonrapid progressors. Children were defined as EBV infected with the first positive EBV culture at any age or a positive EBV serology at ≥12 months of age. Children were classified as EBV uninfected at a given age if no positive tests were obtained on or before that age, with one or more seronegative tests at ≥6 months of age. In some instances, EBV infection was identified by a 4-fold increase in EBV antibody titers for children infected in infancy before complete disappearance of maternal EBV antibodies. Serologies for children receiving intravenous immune globulin were excluded.
The cumulative rates (and 95% confidence intervals [CIs]) of EBV infection were estimated by fitting a Weibull model to the interval-censored times to EBV infection. A logistic regression model using generalized estimating equations that adjusted for the correlation between multiple samples from each child was used to estimate and compare the EBV shedding rates by age and by time since seroconversion (determined as the midpoint of the censoring interval for seroconversion) for EBV-seropositive children. Similar logistic regression was used to examine the relationships of lymphadenopathy, hepatomegaly, and splenomegaly with HIV-1 infection, EBV infection, EBV shedding, and age.
Longitudinal repeated-measures analyses were performed for serum IgG, CD4 cell count and percent, CD8 cell count, and HIV-1 RNA. A linear model using restricted maximum-likelihood estimation and a Huynh-Feldt covariance structure among repeated measurements was fitted for each outcome. Covariate adjustment was made for HIV-1 infection, EBV infection, and age. EBV infection status was a time-dependent covariate, since at any age the EBV status could change irrevocably from uninfected to infected. The results were summarized with adjusted means and 95% CIs. Data transformations used for analysis included the cube-root transformation for CD4 and CD8 cell counts and a logarithmic scale for IgG (natural log) and HIV-1 RNA (base 10).
The first detectable antibody titers in the children were summarized with geometric mean titers ± SE and compared by HIV-1 infection status with a Wilcoxon rank sum test. Relationships between antibody titers and EBV shedding were examined by logistic regression with generalized estimating equations. Linear relationships between the antibody titers and culture titer, IgG, and CD4 percent of total lymphocytes were examined using the first available pair of measurements made within 1 month of each other for each child. The coefficient of determination (r2) or, when age adjustment was necessary, the coefficient of partial determination was used to quantify the linear relationships.
Results
In total 600 liveborn infants born to 555 HIV-1–infected mothers were enrolled, with 556 infants born to 517 mothers (93 HIV-1–infected infants born to 92 mothers, 463 HIV-1–uninfected infants born to 433 mothers, including 8 mothers with 1 HIV-1–infected and 1 HIV-1–uninfected child) included in the analyses. Forty-four infants of indeterminate HIV-1 infection status born to 41 mothers were excluded: 35 were lost to follow-up and 9 died before the required virologic testing (e.g., only 1 positive HIV-1 culture with ≥1 negative HIV-1 cultures or awaiting a confirmatory antibody test at age ≥15 months). Most children were African American (n = 309, 51.5%) or Hispanic (n = 182, 30.3%); 52.7% were male. Race and gender did not differ by HIV-1 infection. Of the 93 HIV-1–infected children, 45 (48.4%) were classified as rapid progressors on the basis of low CD4 cell count or CD4 percent (33.3%), development of CDC class C symptoms (24.4%), or both (42.2%). All 343 mothers of the 356 infants for whom maternal serum samples at term were available were seropositive for EBV infection [31].
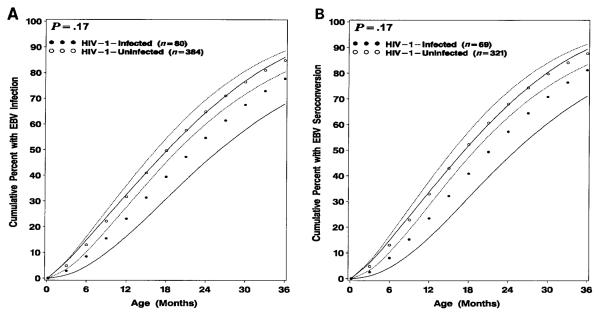
Both HIV-1–infected and –uninfected children had comparably high rates of acquisition of EBV in early childhood (figure 1). By use of a definition of primary EBV infection of the youngest age of either the first oropharyngeal shedding or the age at EBV seroconversion (P = .17) or the age at seroconversion alone (P = .17), there was no significant difference in the age of primary EBV infection between HIV-1–infected and –uninfected children. The incidence of EBV infection, defined by oropharyngeal shedding or seroconversion, for HIV-1–infected children was 23.2% (95% CI, 16.3%–32.6%) at age 1 year, 54.7% (95% CI, 45.4%–64.6%) at age 2 years, and 77.8% (95% CI, 68.0%–86.2%) at age 3 years. The incidence for HIV-1–uninfected children was 31.7% (95% CI, 27.7%–36.2%) at age 1 year, 64.9% (95% CI, 60.1%–69.6%) at age 2 years, and 84.9% (95% CI, 80.5%–88.7%) at age 3 years. There was no significant difference between HIV-1–infected and –uninfected children in the initial identification of EBV infection by positive culture alone, seroconversion alone, or both simultaneously (table 1). Of the children who had EBV culture or serologies available, 3 (3.8%) of 80 were HIV-1 infected, and 48 (12.5%) of 384 were HIV-1 uninfected (overall: 51 [11%] of 464) with a sustained 4-fold rise in EBV antibody titers before complete disappearance of maternal EBV antibodies.
Figure 1.
Cumulative rates (and 95% confidence intervals) of (A) Epstein-Barr virus (EBV) infection (defined as youngest age of either first oropharyngeal shedding or age at EBV seroconversion) and (B) EBV seroconversion alone of human immunodeficiency virus type 1 (HIV-1)–infected and –uninfected children for first 3 years of life born to HIV-1–infected mothers.
Table 1.
The first laboratory evidence (positive culture, seroconversion, or both simultaneously) of Epstein-Barr virus (EBV) infection in human immunodeficiency virus type 1 (HIV-1)–infected and HIV-1–uninfected children born to HIV-1–infected mothers.
| Group | Positive culture alone (%) | P | Seroconversion alone (%) | P | Both simultaneously (%) | P |
|---|---|---|---|---|---|---|
| HIV-1 infected | 20/56 (36) | 22/56 (39) | 14/56 (25) | |||
| HIV-1 uninfected | 63/234 (27) | .19 | 122/234 (52) | .08 | 49/234 (21) | .51 |
| HIV-1 infected | ||||||
| Rapid progressors | 7/19 (37) | 4/19 (21) | 8/19 (42) | |||
| Nonrapid progressors | 13/37 (35) | .90 | 18/37 (49) | .05 | 6/37 (16) | .03 |
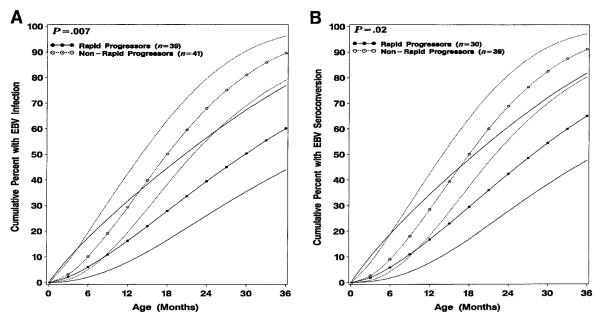
HIV-1–infected children identified as rapid progressors acquired EBV infection at significantly later age than nonrapid progressors, whether EBV infection was defined by youngest age of first oropharyngeal shedding or age at EBV seroconversion (P = .007) or age at seroconversion alone (P = .02; figure 2). By use of either oropharyngeal shedding or seroconversion to define EBV infection, 50.2% (95% CI, 38.3%–63.4%) of nonrapid progressors had EBV infection at age 18 months compared with only 27.9% (95% CI, 16.5%–44.7%) of rapid progressors, who had an incidence of EBV infection of 50.4% (95% CI, 35.5%–67.5%) at age 30 months. The cumulative mortality is higher for rapid progressors, which could account for the later acquisition of EBV. However, a similar analysis excluding those who died before age 18 months did not substantially change the results (data not shown).
Figure 2.
Cumulative rates (and 95% confidence intervals) of (A) Epstein-Barr virus (EBV) infection (defined as youngest age of either first oropharyngeal shedding or age at EBV seroconversion) and (B) EBV seroconversion alone of human immunodeficiency virus type 1–infected children identified as rapid progressors or as nonrapid progressors for first 3 years of life.
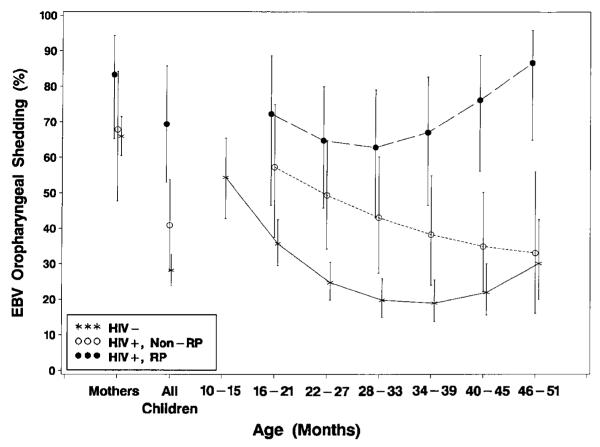
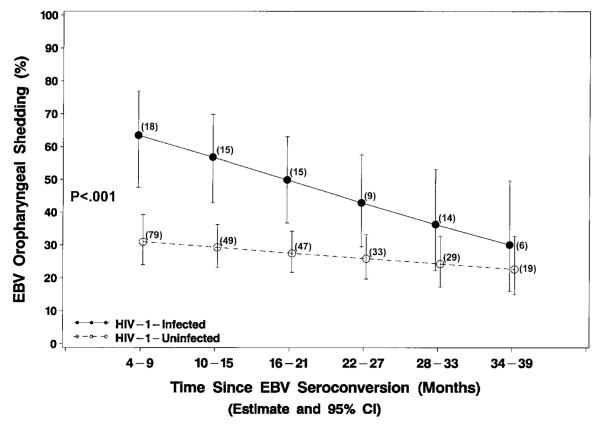
Among children who were seropositive for EBV infection, those who were HIV-1 infected had a significantly higher overall rate of EBV oropharyngeal shedding (50.4%; 95% CI, 39.7%–61.1%) through the first 4 years of life compared with HIV-1–uninfected children (28.2%; 95% CI, 23.7%–32.7%, P < .001; figure 3). EBV shedding in HIV-1–uninfected children was highest in the youngest EBV-infected children (54.4%; 95% CI, 42.8%–65.5% at age 12 months) and decreased to the lowest point at age 3 years (19.0%; 95% CI, 13.8%–25.5%), with a possible increase at age 4 years (30.1%; 95% CI, 20.0%–42.6%). EBV shedding steadily decreased in HIV-1–infected nonrapid progressors (49.4%, 95% CI, 34.2%–64.8% at age 2 years; 33.1%, 95% CI, 16.1%–56.1% at age 4 years) and in rapid progressors decreased until ~30 months of age but then diverged and steadily increased (64.8%, 95% CI, 45.8%–80.0% at age 2 years; 86.7%, 95% CI, 64.9%–95.9% at age 4 years). There were significant differences in overall EBV shedding between rapid progressors (69.4%; 95% CI, 53.0%–85.8%) and nonrapid progressors (40.9%, 95% CI, 28.1%–53.7%; P = .01) and between nonrapid progressors and HIV-1–uninfected children (28.2%, 95% CI, 23.8%–32.6%; P = .05). Oropharyngeal EBV shedding for HIV-1–infected children overall was highest shortly after acute infection (63.5%; 95% CI, 47.6%–76.8% at age 6 months), but the observed percentages and model estimates of probability of oropharyngeal shedding after seroconversion declined to 30.1% ( 95% CI, 15.9%–49.6%) 3 years after EBV seroconversion, becoming indistinguishable from the relatively stable levels in HIV-1–uninfected children (figure 4).
Figure 3.
Model-based estimates (and 95% confidence intervals) of oropharyngeal Epstein-Barr virus (EBV) shedding by age of human immunodeficiency virus type 1 (HIV-1)–infected rapid progressors (RP; n = 17; 59 samples), HIV-1–infected nonrapid progressors (non-RP; n = 33; 121 samples), and HIV-1–uninfected (n = 195; 579 samples) EBV-seropositive children. Significant differences overall in oropharyngeal shedding were found between HIV-1–infected children (50.4% overall) vs. HIV-1–uninfected children (28.2% overall; P < .001), RP (69.4% overall) vs. non-RP (40.9% overall; P = .01), RP vs. HIV-1–uninfected children (P < .001), and non-RP vs. HIV-1–uninfected children (P = .05).
Figure 4.
Model-based estimates (and 95% confidence intervals [CIs]) of probability of oropharyngeal Epstein-Barr virus (EBV) shedding for 3 years after EBV seroconversion of human immunodeficiency virus type 1 (HIV-1)–infected (n = 36) and HIV-1–uninfected children (n = 108) born to HIV-1–infected mothers. No. of children at each time point is indicated.
Elevated VCA and EA titers are frequently found in conditions characterized by ongoing EBV replication. HIV-1–infected compared with –uninfected children had modestly higher initial VCA-IgG titers with a wide range in both groups (table 2). Both groups showed diversity of the predominant component of the first detectable EA. Significantly more HIV-1–infected children had antibody directed against EA-D than did HIV-1–uninfected children. HIV-1–infected children compared with HIV-1–uninfected children had similar EA titers and identical median EA titers (1:160) but with a greater range; 5 (13.5%) of 37 HIV-1–infected children had EA titers >1:1280. HIV-1–infected and –uninfected children had similar VCA-IgM and EBNA titers.
Table 2.
Characterization of the first detectable antibody titers to Epstein-Barr virus (EBV) viral capsid antigen (VCA), early antigen (EA), and nuclear antigen (EBNA) in human immunodeficiency virus (HIV)–infected and HIV-uninfected children born to HIV-infected mothers.
| Group and antigen | Geometric mean titer ± SE |
Median (range) |
|---|---|---|
| HIV-1 infected | ||
| VCA | ||
| VCA-IgMa | 20 ± 5 | 16 (8–256) |
| VCA-IgGb | 403 ± 81 | 320 (10–20,480) |
| EAs (n = 37) | 157 ± 51e | 160 (10–20,480) |
| EA-D (n = 12; 32%)c | 359 ± 251 | 400 (10–20,480) |
| EA-R (n = 20; 54%) | 72 ± 23 | 40 (10–2560) |
| EA-DR (n = 5; 14%) | 485 ± 312 | 320 (160–5120) |
| EBNAd | 13 ± 3 | 10 (2.5–160) |
| HIV-1 uninfected | ||
| VCA | ||
| VCA-IgMa | 18 ± 3 | 16 (8–512) |
| VCA-IgGb | 271 ± 32 | 640 (10–10,240) |
| EAs (n = 193) | 112 ± 8e | 160 (10–1280) |
| EA-D (n = 19; 10%)c | 50 ± 10 | 40 (10–320) |
| EA-R (n = 130; 67%) | 92 ± 7 | 80 (10–640) |
| EA-DR (n = 44; 23%) | 281 ± 33 | 320 (40–1280) |
| EBNAd | 14 ± 1 | 10 (2.5–640) |
NOTE. All P values obtained by Wilcoxon rank sum test. EA-D, -R, -DR5EA-diffuse, -restricted, and -diffuse restricted, respectively.
P = .53.
P = .57.
P = .02.
P = .59.
P = .76.
There was significant negative association between the presence of EBV shedding and the height of the VCA-IgG titer in an age-adjusted model for both HIV-1–infected children (1:646 ± 72 with shedding vs. 1:1342 ± 205 without shedding, P = .02) and HIV-1–uninfected children (1:596 ± 38 vs. 1: 688 ± 28, P = .03). In HIV-1–uninfected children, there was a positive correlation of culture titer with VCA-IgM titer (P = .05, r2 = 0.24, n = 16) and a negative correlation with VCA-IgG titer (P = .06, r2 = .05, n = 70) but not in HIV-1–infected children (P = .62 and .27, respectively). There was no correlation of the culture titer with other antibody titers (data not shown).
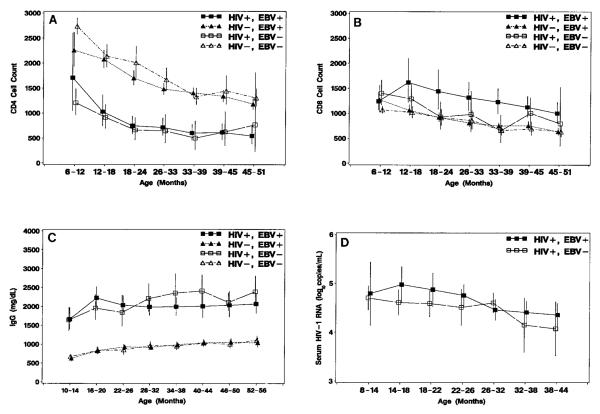
Acute EBV infection is usually associated with transient reversal of the normal CD4:CD8 cell ratio due to an increase in the relative as well as absolute number of CD8 cells [32]. In this study, EBV infection appeared to have no acute or long-term effects on the total lymphocyte counts, mean CD4 cell counts, or percent CD4 during the first 4 years of life (figure 5A). EBV infection was associated with elevated mean CD8 cell counts in HIV-1–infected children with significant differences at ages 21 (P = .02) and 36 months (P = .003; figure 5B). There was no significant association of EBV infection with mean CD8 cell counts of HIV-1–uninfected children.
Figure 5.
Model-based means (and 95% confidence intervals) for (A) CD4 and (B) CD8 cells/mm3 (n = 76 human immunodeficiency virus type 1 (HIV-1)–infected and n = 311 HIV-1–uninfected children), (C) IgG levels (mg/dL; n = 69 HIV-1–infected and n = 266 HIV-1–uninfected children), and (D) serum log10 HIV-1 RNA levels (HIV-1 RNA genome copies/mL; n = 67 HIV-1–infected children) by age according to HIV-1 infection status and Epstein-Barr virus infection status at each age. Means for CD4 and CD8 cell counts are cube-root transformations; IgG level is a geometric mean.
Children with AIDS characteristically have elevated IgG levels, lymphadenopathy, hepatomegaly, and splenomegaly, possibly resulting from concomitant secondary infections such as EBV. In this study, HIV-1–infected children had sustained elevated IgG levels compared with HIV-1–uninfected children (figure 5C). There was no discernible effect of EBV infection on IgG levels in either HIV-1–infected children or –uninfected children. The IgG levels in HIV-1–infected children, adjusted linearly for age, positively correlated with the initial VCA-IgG titer (P < .001, partial coefficient of determination [r2] = 0.27, n = 52) and EA titer (P < .001, r2 = 0.36, n = 35) but not VCA-IgM or EBNA titers. The IgG levels in HIV-1–uninfected children correlated with the initial VCA-IgG titer (P < .001, r2 = 0.08, n = 201) but not with VCA-IgM, EA, or EBNA titers.
There was no consistent difference (P = .13) due to EBV infection on the mean serum levels of HIV-1 RNA (figure 5D).
HIV-1 infection was significantly associated with lymphadenopathy at any site (P < .001), hepatomegaly (>3 cm below the right costal margin; P < .001), and splenomegaly (>1 cm below the left costal margin; P < .001). After adjusting for age, neither EBV infection nor oropharyngeal shedding was associated with presence of lymphadenopathy, hepatomegaly, or splenomegaly in HIV-1–infected or –uninfected children (data not shown). Among the 93 HIV-1–infected children, 1 child (1.1%) had LIP, and 4 (4.3%) had presumptive LIP. No cases of LIP or presumptive LIP were found in HIV-1–uninfected children.
Discussion
Infection with EBV in children born to HIV-1–infected mothers occurred in most children during the first 3 years of life regardless of the child’s HIV-1–infection status. HIV-1–infected children acquired EBV infection at a similar, and certainly not higher, rate to HIV-1–uninfected children (figure 1). All of the mothers were seropositive for EBV, consistent with the high EBV seroprevalence (98.5%) in women of child-bearing age [33–35] and in HIV-1–infected persons [36]; 67.7% of the mothers had oropharyngeal EBV shedding at the time of delivery [31]. Maternal EBV shedding has been identified as an independent risk factor for vertical HIV-1 transmission [31]. The mothers of HIV-1–infected children had significantly higher mean VCA-IgG titers (1:831 vs. 1:628; P = .03), although both groups had a median VCA-IgG titer of 1:640 [31]. The slightly higher VCA-IgG titers in mothers of HIV-1–infected children may have provided the children with longer passive protection against acquisition of EBV.
There was no significant difference between HIV-1–infected and –uninfected children in the initial manifestation of EBV infection by culture alone, seroconversion alone, or both simultaneously (table 1). The slightly later age in HIV-1–infected children of EBV infection defined by seroconversion alone (figure 1B) suggested the possibility of impaired production of antibody resulting from HIV-1 immunodeficiency, but similar results were observed using viral culture to identify acquisition of EBV infection (figure 1A). The indication of possibly later age of primary EBV infection in HIV-1–infected children compared with HIV-1–uninfected children (figure 1) and the significantly later age of EBV infection in rapid progressors compared with nonrapid progressors (figure 2) suggest that HIV-1 infection may be protective for acquisition of EBV infection. The comparable results using either oropharyngeal culture or seroconversion, or seroconversion alone, suggest that this is not the result of impaired antibody production. There is decreased expression of the receptor for EBV, CD21 (CR2 or C3d), on the surface of B lymphocytes of persons coinfected with HIV-1 [37]. Increased circulating soluble CD21 may also inhibit EBV infection [38].
The significantly higher rate of EBV oropharyngeal shedding in HIV-1–infected children (50.4%) compared with HIV-1–uninfected children (28.2%; figure 3) is similar to the rates (49%–78%) reported in HIV-infected adults [39, 40] and other immunocompromised patients [41, 42]. In this study, increased EBV oropharyngeal shedding at age 4 years was observed only in children identified as rapid progressors (86.7%); the rate for nonrapid progressors was 33.1%, comparable to that of HIV-1–uninfected children (30.1%) and similar to that in asymptomatic healthy seropositive adults (12%–33%) [43, 44]. This may be due to HIV-1–associated immunosuppression and may be attributed in part to the later age of acquisition of EBV infection in rapid progressors compared with nonrapid progressors (figure 2) and the decline in EBV shedding that occurs over time in HIV-1–infected children (figure 4).
The association of higher VCA-IgG titer with decreased frequency of oropharyngeal shedding in both HIV-1–infected (P = .02) and HIV-1–uninfected children (P = .03) reflects the immunologic response to control latent EBV infection. In HIV-1–uninfected children, higher oropharyngeal culture titers were associated with higher VCA-IgM titer (P = .05,r2 = 0.24), consistent with increased shedding at the time of acute EBV infection, and lower culture titers were associated with higher VCA-IgG titers (P = .06, r2 = 0.05), also indicative of immunologic control of infection. In HIV-1–infected children, there was no relationship between oropharyngeal culture titers and EBV serology titers. The higher prevalence of oropharyngeal shedding in HIV-1–infected children, the quantitatively increased shedding long after acute infection, and the continued heightened levels of EBV replication despite high levels of VCA-IgG document persistent and elevated EBV replication associated with HIV-1-infection even in the presence of a specific EBV antibody response.
A greater frequency of EA antibody directed principally against the EA-D component was found in HIV-1–infected children compared with HIV-1–uninfected children (32% vs. 10%; P = .02) but with similar EA titers (1:157 ± 51 vs. 1:112 ± 8; P = .76; table 2). EA-D is usually suppressed during latent infection and is detectable with lytic replication [45]. The higher frequency of EA-D and the higher EA and EA-D titers in HIV-1–infected children indicate enhanced EBV replication compared with that in HIV-1–uninfected children.
EBV infection did not affect the mean CD4 (figure 5A) or mean CD8 (figure 5B) cell counts in HIV-1–uninfected children. The greatest difference was on mean CD8 cell counts in HIV-1–infected children during the first 2 years of life. The similar CD8 cell counts in HIV-1–infected and –uninfected children during later childhood may result from diminishing influence of EBV infection with latent rather than acute infection and the inability to discern a difference due to the gradual decline in CD8 cells that occurs after age 6–9 months [46, 47] and the lymphopenia associated with HIV-1 infection. There was a significant overall correlation of increasing VCA-IgG titer with low CD4 percent of total lymphocytes but not the absolute mean CD4 cell counts. Adults with HIV-1 infection have increasing VCA-IgG titers and decreasing EBNA titers with lower absolute CD4 cell counts [36, 48]. The immunologic manifestations of the primary and recent EBV infections in this young population may differ from that found with the reactivated EBV infections in adults [49]. The specific analysis of effect of EBV infection on absolute CD4 cell counts during early childhood is confounded by the normal age-related variability of absolute CD4 cell counts [46, 47].
HIV-1–infected children had elevated mean IgG levels compared with HIV-1–uninfected children, with similar profiles regardless of EBV infection (figure 5C). There were significant correlations of IgG levels with VCA-IgG titers in both HIV-1–infected and –uninfected children and with EA titers in the HIV-1–infected children. Elevated EBV antibody titers in adults with AIDS are independent of IgG levels [36]. In children, a part of the heightened antibody response to EBV infection may represent nonspecific IgG elevation resulting from HIV-1 infection. HIV-1–infected children during the first 6 years of life appear competent to mount a strong antibody immune response against EBV infection.
In HIV-1–infected homosexual men, higher EBV antibody titers are found in those with lymphadenopathy, suggesting that concomitant EBV infection may contribute to the lymphadenopathy characteristic of AIDS [36]. In the children studied, EBV infection was not an independent risk factor in addition to HIV-1 infection for development of lymphadenopathy, hepatomegaly, or splenomegaly.
These studies provide a comprehensive view of the effects of EBV infection during acute infection and in the subsequent 4 years on HIV-1 infection in children who acquire HIV-1 from their mothers and in children born to HIV-1–infected mothers but are ultimately proved to be HIV-1 uninfected. The role of EBV as a cofactor in AIDS-associated lesions of oral hairy leukoplakia [5, 6] LIP [7–11], AIDS-associated lymphoma [13, 14], and leiomyosarcoma [15, 16] has been more easily discernible than the subtle effects on the immune system and contribution to HIV-1 disease progression. EBV infection does not appear to independently contribute significantly to the lymphadenopathy, elevated IgG levels, and lymphocyte perturbations characteristic of AIDS in children. Whether EBV infection contributes substantively to clinical progression of AIDS in children, especially in the subset of children identified as rapid progressors, or serves only as a sentinel marker of advancing disease, remains enigmatic and will require studies extending beyond early childhood. Elucidation of the basis for delayed EBV infection in HIV-1–infected rapid progressors should provide insight into the interaction of these two viruses in vivo and may suggest a possible means to prevent EBV infection.
Acknowledgments
Acknowledgment is made to the following P2C2 HIV-1 study investigators (a complete list of study participants is in [23])—National Heart, Lung and Blood Institute: Hannah Peavy, project officer; Anthony Kalica, Elaine Sloand, George Sopko, and Margaret Wu; steering committee, chairman: Robert Mellins.
Clinical centers. Baylor College of Medicine, Houston: William Shearer,* Marilyn Doyle, Linda Davis, Teresa Tonsberg; Children’s Hospital–Harvard Medical School, Boston: Steven Lipshultz,* Ellen Cooper, Janice Hunter; Mount Sinai School of Medicine, New York: Meyer Kattan,* Stephen Heaton, David Hodes, Diane Carp, Mary Anne Worth; Presbyterian Hospital (New York City)–Columbia University: Robert Mellins,* Kimberly Geromanos; UCLA School of Medicine, Los Angeles: Samuel Kaplan,* Yvonne Bryson, Joseph Church, Andrea Kovacs, Helene Cohen, Lynn Fukushima, Eileen Garratty, Lucy Kunzman, Toni Ziolkowski.
Clinical coordinating center. Cleveland Clinic Foundation: Michael Kutner,* Mark Schluchter* (through April 1998), Johanna Goldfarb, Richard Martin (Case Western Reserve University), Douglas Moodie, Cindy Chen, Kirk Easley, Scott Ansak, Sunil Rao, Amrik Shah, Victoria Konig, Paul Sartori, Lori Schnur, Susan Sunkle.
Central laboratory for Epstein-Barr virus testing. University of Texas Health Science Center at San Antonio: Hal B. Jenson,* Yasmin Ench.
Policy, Data, and Safety Monitoring Board. Henrique Rigatto (chairman), Edward B. Clark, Robert B. Cotton, Vijay V. Joshi, Paul S. Levy, Norman S. Talner, Patricia Taylor, Robert Tepper, Janet Wittes, Robert H. Yolken, Peter E. Vink.
*Principal Investigator.
Financial support: NIH (contracts HR-96037, -96038, -96039, -96040, -96041, -96042, -96043; grants RR-00188, -02172, -00533, -00071, -00645, -00865, -00043).
Footnotes
Presented in part: Society for Pediatric Research and annual meeting of the Pediatric Academic Societies, New Orleans, May 1998 (abstract 853); 36th annual meeting of the Infectious Diseases Society of America, Denver, November 1998 (paper/board 410).
Informed consent was obtained from parents or guardians of all patients. Human experimentation guidelines of the US Department of Health and Human Services and of the participating institutions were followed in the conduct of this clinical research.
References
- 1.Niederman JC, Evans AS. Epstein-Barr virus. In: Evans AS, Kaslow RA, editors. Viral infections of humans: Epidemiology and control. 4th ed Plenum; New York: 1997. pp. 253–83. [Google Scholar]
- 2.Wang PS, Evans AS. Prevalence of antibodies to Epstein-Barr virus and cytomegalovirus in sera from a group of children in the People’s Republic of China. J Infect Dis. 1986;153:150–2. doi: 10.1093/infdis/153.1.150. [DOI] [PubMed] [Google Scholar]
- 3.Evans AS, Niederman JC, McCollum RW. Seroepidemiologic studies of infectious mononucleosis with EB virus. N Engl J Med. 1968;279:1121–7. doi: 10.1056/NEJM196811212792101. [DOI] [PubMed] [Google Scholar]
- 4.Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence, and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282:361–5. doi: 10.1056/NEJM197002122820704. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan JS, Greenspan D, Lennette ET, et al. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–71. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 6.Sciubba J, Brandsma J, Schwartz M, Barrezueta N. Hairy leukoplakia: an AIDS-associated opportunistic infection. Oral Surg Oral Med Oral Pathol. 1989;67:404–10. doi: 10.1016/0030-4220(89)90382-4. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med. 1992;86:49–52. doi: 10.1016/s0954-6111(06)80148-4. [DOI] [PubMed] [Google Scholar]
- 8.Andiman WA, Eastman R, Martin K, et al. Opportunistic lymphoproliferations associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet. 1985;2:1390–3. doi: 10.1016/s0140-6736(85)92557-7. [DOI] [PubMed] [Google Scholar]
- 9.Katz BZ, Berkman AB, Shapiro ED. Serologic evidence of active Epstein-Barr virus infection in Epstein-Barr virus–associated lymphoproliferative disorders of children with acquired immunodeficiency syndrome. J Pediatr. 1992;120:228–32. doi: 10.1016/s0022-3476(05)80432-9. [DOI] [PubMed] [Google Scholar]
- 10.Barberà JA, Hayashi S, Hegele RG, Hogg JC. Detection of Epstein-Barr virus in lymphocytic interstitial pneumonia by in situ hybridization. Am Rev Respir Dis. 1992;145:940–6. doi: 10.1164/ajrccm/145.4_Pt_1.940. [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Fox CH, Devaney KO, et al. Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis. Hum Pathol. 1992;23:529–41. doi: 10.1016/0046-8177(92)90130-u. [DOI] [PubMed] [Google Scholar]
- 12.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269:2869–75. [PubMed] [Google Scholar]
- 13.Levine AM. Lymphoma complicating immunodeficiency disorders. Ann Oncol. 1994;5(suppl 2):29–35. doi: 10.1093/annonc/5.suppl_2.s29. [DOI] [PubMed] [Google Scholar]
- 14.Rabkin CS, Biggar RJ, Horm JW. Increasing incidence of cancers associated with the human immunodeficiency virus epidemic. Int J Cancer. 1991;47:692–6. doi: 10.1002/ijc.2910470511. [DOI] [PubMed] [Google Scholar]
- 15.McClain KL, Leach CT, Jenson HB, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med. 1995;332:12–8. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- 16.Jenson HB, Leach CT, McClain KL, et al. Benign and malignant smooth muscle tumors containing Epstein-Barr virus in children with AIDS. Leuk Lymphoma. 1997;27:303–14. doi: 10.3109/10428199709059684. [DOI] [PubMed] [Google Scholar]
- 17.Astrin SM, Laurence J. Human immunodeficiency virus activates c-myc and Epstein-Barr virus in human B lymphocytes. Ann NY Acad Sci. 1992;651:422–32. doi: 10.1111/j.1749-6632.1992.tb24642.x. [DOI] [PubMed] [Google Scholar]
- 18.Henderson EE, Yang JY, Zhang RD, Bealer M. Altered HIV expression and EBV-induced transformation in coinfected PBLs and PBL subpopulations. Virology. 1991;182:186–98. doi: 10.1016/0042-6822(91)90662-u. [DOI] [PubMed] [Google Scholar]
- 19.Tozzi V, Britton S, Ehrnst A, Lenkei R, Strannegård Ö . Persistent productive HIV infection in EBV-transformed B lymphocytes. J Med Virol. 1989;27:19–24. doi: 10.1002/jmv.1890270105. [DOI] [PubMed] [Google Scholar]
- 20.Pagano JS, Kenney S, Markovitz D, Kamine J. Epstein-Barr virus and interactions with human retroviruses. J Virol Methods. 1988;21:229–39. doi: 10.1016/0166-0934(88)90069-9. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg RS, Paradis TJ, Crawford D, Byington RE, Hirsch MS, Schooley RT. Effects of human immunodeficiency virus (HIV) on the cytotoxic response to Epstein Barr virus (EBV) transformed B lymphocytes. AIDS Res Hum Retroviruses. 1987;3:303–15. doi: 10.1089/aid.1987.3.303. [DOI] [PubMed] [Google Scholar]
- 22.Kenney S, Kamine J, Markovitz D, Fenrick R, Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci USA. 1988;85:1652–6. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The P2C2 HIV Study Group The pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus (P2C2 HIV) infection study: design and methods. J Clin Epidemiol. 1996;49:1285–94. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–15. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 25.Hollinger FB, Bremer JW, Myers LE, Gold JW, McQuay L. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. The NIH/NIAID/DAIDS/ACTG Virology Laboratories. J Clin Microbiol. 1992;30:1787–94. doi: 10.1128/jcm.30.7.1787-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention 1994 revised classificationsystem for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 27.Griffith BP, Rigsby MO, Garner RB, Gordon MM, Chacko TM. Comparison of the Amplicor HIV-1 monitor test and the nucleic acid sequence-based amplification assay for quantitation of human immunodeficiency virus RNA in plasma, serum, and plasma subjected to freeze-thaw cycles. J Clin Microbiol. 1997;35:3288–91. doi: 10.1128/jcm.35.12.3288-3291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennette ET. Epstein-Barr virus (EBV) In: Lennette EH, Lennette DA, Lennette ET, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 7th ed American Public Health Association; Washington, DC: 1995. pp. 299–312. [Google Scholar]
- 29.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 30.Jenson HB, Ench Y, Sumaya CV. Epstein-Barr virus. In: Rose NR, de Macario EC, Folds H, Lane HC, Nakamura RM, editors. Manual of clinical laboratory immunology. 5th ed American Society for Microbiology Press; Washington, DC: 1997. pp. 634–43. [Google Scholar]
- 31.Pitt J, Schluchter M, Jenson H, et al. Maternal and perinatal factors related to maternal-infant transmission of HIV-1 in the P2C2 HIV study: the role of EBV shedding. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:462–70. doi: 10.1097/00042560-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Tomkinson BE, Maziarz R, Sullivan JL. Characterization of the T cell–mediated cellular cytotoxicity during acute infectious mononucleosis. J Immunol. 1989;143:660–70. [PubMed] [Google Scholar]
- 33.Fleisher G, Bologonese R. Epstein-Barr virus infections in pregnancy: a prospective study. J Pediatr. 1984;104:374–9. doi: 10.1016/s0022-3476(84)81098-7. [DOI] [PubMed] [Google Scholar]
- 34.Le CT, Chang RS, Lipson MH. Epstein-Barr virus infections during pregnancy. A prospective study and review of the literature. Am J Dis Child. 1983;137:466–8. doi: 10.1001/archpedi.1983.02140310048014. [DOI] [PubMed] [Google Scholar]
- 35.Hunter K, Stagno S, Capps E, Smith RJ. Prenatal screening of pregnant women for infections caused by cytomegalovirus, Epstein-Barr virus, herpesvirus, rubella, and Toxoplasma gondii. Am J Obstet Gynecol. 1983;145:269–73. doi: 10.1016/0002-9378(83)90709-3. [DOI] [PubMed] [Google Scholar]
- 36.Rahman MA, Kingsley LA, Breinig MK, et al. Enhanced antibody responses to Epstein-Barr virus in HIV-infected homosexual men. J Infect Dis. 1989;159:472–9. doi: 10.1093/infdis/159.3.472. [DOI] [PubMed] [Google Scholar]
- 37.Scott ME, Landay AL, Lint TF, Spear GT. In vivo decrease in the expression of complement receptor 2 on B-cells in HIV infection. AIDS. 1993;7:37–41. doi: 10.1097/00002030-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Nemerow GR, Mullen JJ, Dickson PW, Cooper NR. Soluble recombinant CR2 (CD21) inhibits Epstein-Barr virus infection. J Virol. 1990;64:1348–52. doi: 10.1128/jvi.64.3.1348-1352.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferbas J, Rahman MA, Kingsley LA, et al. Frequent oropharyngeal shedding of Epstein-Barr virus in homosexual men during early HIV infection. AIDS. 1992;6:1273–8. doi: 10.1097/00002030-199211000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Lucht E, Biberfeld P, Linde A. Epstein-Barr virus (EBV) DNA in saliva and EBV serology of HIV-1–infected persons with and without hairy leukoplakia. J Infect. 1995;31:189–94. doi: 10.1016/s0163-4453(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 41.Chang RS, Lewis JP, Reynolds RD, Sullivan MJ, Neuman J. Oropharyngeal excretion of Epstein-Barr virus by patients with lymphoproliferative disorders and by recipients of renal homografts. Ann Intern Med. 1978;88:34–40. doi: 10.7326/0003-4819-88-1-34. [DOI] [PubMed] [Google Scholar]
- 42.Chatterjee SN, Chang RS. A prospective study of oropharyngeal excretion of Epstein-Barr virus in renal homograft recipients. Scand J Infect Dis. 1982;14:95–8. doi: 10.3109/inf.1982.14.issue-2.03. [DOI] [PubMed] [Google Scholar]
- 43.Miller G, Niederman JC, Andrews LL. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973;288:229–32. doi: 10.1056/NEJM197302012880503. [DOI] [PubMed] [Google Scholar]
- 44.Niederman JC, Miller G, Pearson HA, Pagano JS, Dowaliby JM. Infectious mononucleosis. Epstein-Barr virus shedding in saliva and the oropharynx. N Engl J Med. 1976;294:1355–9. doi: 10.1056/NEJM197606172942501. [DOI] [PubMed] [Google Scholar]
- 45.Oudejans JJ, Jiwa M, van den Brule AJ, et al. Detection of heterogeneous Epstein-Barr virus gene expression patterns within individual post-transplantation lymphoproliferative disorders. Am J Pathol. 1995;147:923–33. [PMC free article] [PubMed] [Google Scholar]
- 46.Waecker NJJ, Ascher DP, Robb ML, et al. Age-adjusted CD4+ lymphocyte parameters in healthy children at risk for infection with the human immunodeficiency virus. The Military Pediatric HIV Consortium. Clin Infect Dis. 1993;17:123–5. doi: 10.1093/clinids/17.1.123. [DOI] [PubMed] [Google Scholar]
- 47.The European Collaborative Study Age-related standards for T lymphocyte subsets based on uninfected children born to human immunodeficiency virus 1–infected women. Pediatr Infect Dis J. 1992;11:1018–26. [PubMed] [Google Scholar]
- 48.Quesnel A, Pozzetto B, Touraine F, et al. Antibodies to Epstein-Barr virus and cytomegalovirus in relation to CD4 cell number in human immunodeficiency virus 1 infection. J Med Virol. 1992;36:60–4. doi: 10.1002/jmv.1890360112. [DOI] [PubMed] [Google Scholar]
- 49.Rahman MA, Kingsley LA, Atchison RW, et al. Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. J Clin Microbiol. 1991;29:1215–20. doi: 10.1128/jcm.29.6.1215-1220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]