Abstract
Seafloor integrity is threatened by disturbances owing to human activities. The capacity of the system to recover from disturbances, as well as maintain resilience and function, depends on dispersal. In soft-sediment systems, dispersal continues after larval settlement, but there are very few measurements of how far the post-settlers disperse in nature. Spatial scales of post-settlement dispersal are, however, likely to be similar to pelagic larval dispersal because of continued, frequent, small-scale dispersal over longer periods. The consequences of this dispersal may be more important for the maintenance of biodiversity and metacommunity dynamics than is pelagic larval dispersal, because of the greater size and competency of the dispersers. We argue that an increased empirical understanding of post-settlement dispersal processes is key for predicting how benthic communities will respond to local disturbances and shrinking regional species pools, with implications for monitoring, managing and conserving biodiversity.
Keywords: post-larval dispersal, disturbance-recovery, metacommunity, community ecology, dispersal scales, connectivity
1. Introduction
Dispersal is a fundamental process in all ecosystems and is the glue that connects communities across time and space. Unconsolidated soft-sediment ecosystems are the most extensive habitat on the planet and the communities that inhabit them play important roles in biogeochemical cycles, sustaining pelagic primary production as well as supporting fisheries [1]. In estuarine and coastal systems, many of these processes are driven by the macroinvertebrates, many of which are relatively sedentary as adults. In these systems, there is a loss of biodiversity resulting from escalating human impacts, such as fishing disturbance, eutrophication, hypoxia, sedimentation and marine construction [2]. Continuous dispersal, re-colonization and connectivity are integral for biodiversity maintenance, which is associated with the functions and resilience of these systems [3].
Current thinking emphasizes the importance of pelagic larvae as the primary agents of dispersal for macrobenthic invertebrates. This view stems from the rocky shore as a model system, with a focus on the larvae of barnacles, mussels and oysters. During a relatively short period (hours to weeks), these larvae can disperse in very high numbers with currents before settling and attaching to a hard substrate, after which their movement is limited [4]. In soft-sediment systems, however, post-settlement juveniles are still mobile. Potentially, all soft-sediment species can disperse as post-settlers, and a substantial proportion of species (40–60%) lack any pelagic larvae [5].
Evidence has been mounting for the importance of post-settlement dispersal in maintaining soft-sediment biodiversity. Over 20 years ago, the role of post-settlement dispersal in recovery after disturbance was suggested [6] and we have not advanced much since then. A Web of Science survey of benthic studies published between 1980 and 2014 with the search terms ‘larval dispersal’, ‘post-larval dispersal’ or ‘post-settlement dispersal’, yields 1252, 37 and 72 papers, respectively. Hence, papers that address larval dispersal dominate both the literature and our thinking about processes defining connectivity, perhaps because post-settler mobility often is considered to be local movement within a community rather than regional dispersal [4]. Here, we argue that the scales of post-settlement dispersal are similar to larval dispersal, and the competence of the recruits is higher. We discuss the consequences for biodiversity maintenance and offer suggestions for future research.
2. Spatial and temporal scales of post-settlement dispersal
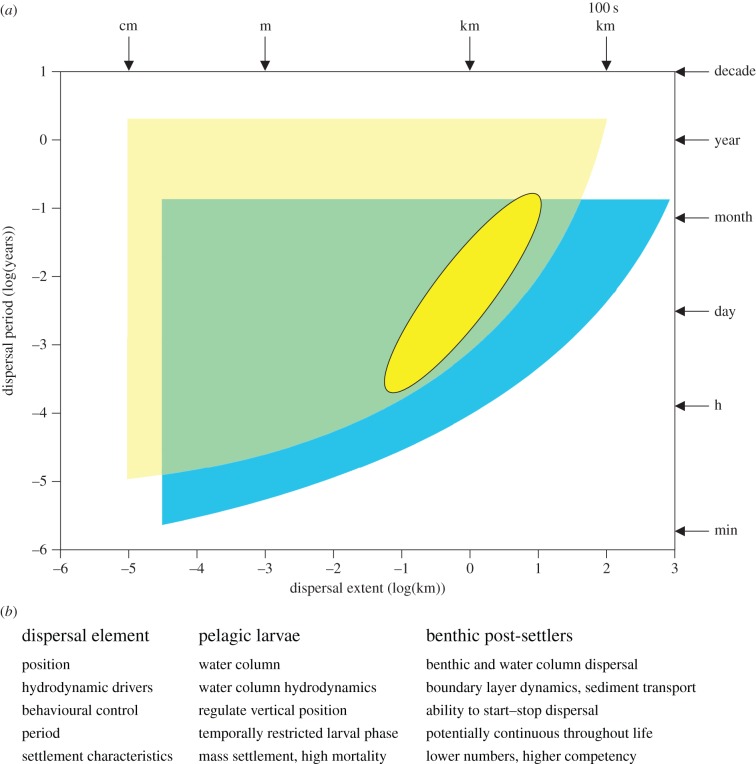
For benthic macroinvertebrates, dispersal across all life-history stages includes spatial scales from a few centimetres to thousands of kilometres, and temporal extents ranging from seconds to seasons to years [7]. Larval dispersal was previously thought to be potentially large-scale, with pelagic larval duration used as proxy for dispersal distance and connectivity. This view has been reinforced by studies of genetic homogeneity [8], but this requires only a few individuals arriving at infrequent intervals [9]. From an ecological perspective of connectivity and community maintenance, these individuals are not important. Moreover, it is now known in many species the larvae disperse less than a kilometre [10]. Thus, dispersal predicted from pelagic larval duration is much greater than reality. Importantly, larval dispersal to the settlement stage is limited in time. Benthic post-settlement dispersal (defined here as the period after initial settlement through to adulthood), however, can continue for considerably longer (figure 1).
Figure 1.
(a) Potential spatial and temporal scales of dispersing pelagic larvae (blue/DARKER FIELD) and post-settlers (yellow/LIGHTER FIELD) marine macroinvertebrates. Range of known post-settlement dispersal is shown in dark yellow (OVAL). (b) Summary of dispersal characteristics for pelagic larvae and post-settlers. (Online version in colour.)
There are very few measurements of how far post-settlers disperse in nature, but known estimates indicate that the distances can be on the same order as for larval dispersal. For example, juvenile bivalves that are several millimetres in size disperse distances of tens to thousands of metres on temporal scales from hours to weeks [11–13]. The net sum of frequent small-scale dispersal over longer temporal scales, up to a couple of years, may therefore be just as important as singular larval dispersal events (figure 1).
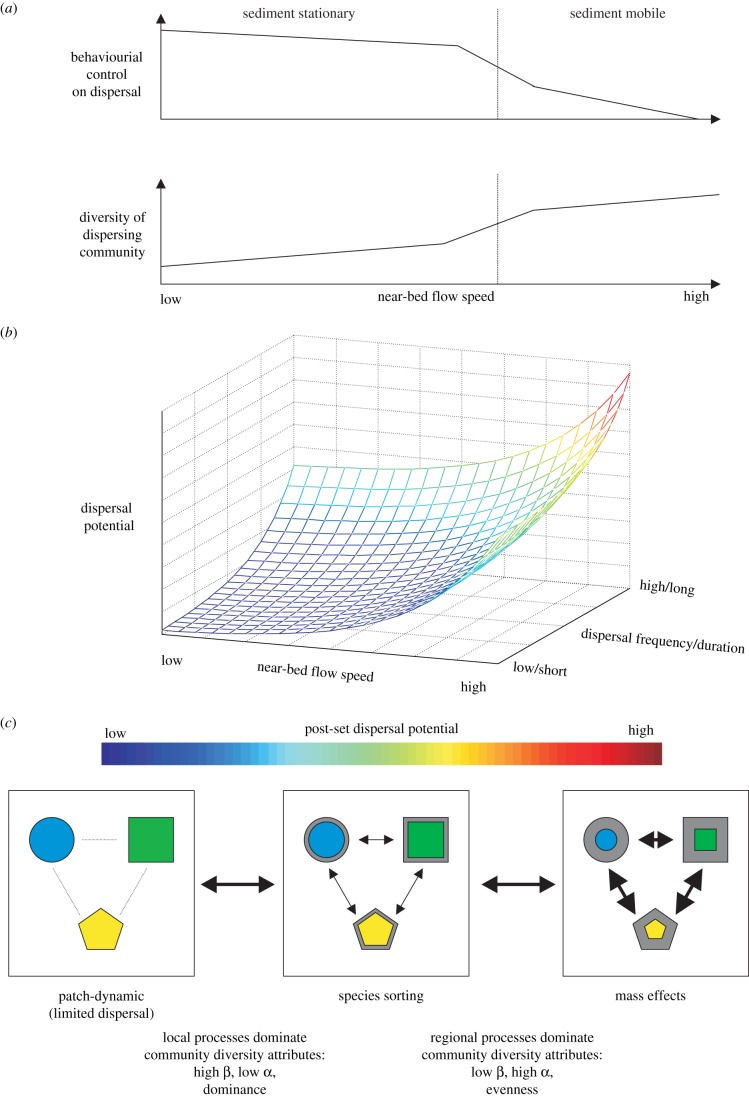
The realized dispersal distance is a combination of the interactions between near-bed hydrodynamics, sediment transport and species-specific traits [14]. While field studies have demonstrated correlations between sediment transport, dispersal rate and the diversity of the dispersing community [15], suggesting a passive process based on hydrodynamic forcing, there is also a behavioural element. Behaviours that promote dispersal include, in addition to active crawling and swimming, emergence from the sediment and the release of mucous or byssal threads providing lift, enabling post-settlers and even adults to disperse. Active behaviours that reduce dispersal include burrowing and attaching to the substrate with byssal fibres [7] (figure 2).
Figure 2.
Linking post-settlement dispersal to community diversity. (a) When flow exceeds sediment entrainment thresholds, post-settlers are passively transported with little behavioural control. The propensity for dispersal is species-specific, with implications for the diversity of the dispersing community. (b) Species-specific dispersal potential increases with flow speed and the frequency/duration of behavioural/flow-driven dispersal events. (c) In a metacommunity context, competent post-settlers may dominate connectivity between spatially distinct communities influencing diversity. Limited dispersal results in low connectivity and a small pool of shared species (outline of shapes) but as connectivity increases, behaviourally mediated dispersal will contribute to species sorting and opportunistic population increases following disturbance. At high rates of dispersal, mass effects will dominate and disturbance recovery will be rapid. Transitions between low and high connectivity states will be driven by variability in near-bed flows. (Online version in colour.)
High mortality is typical of the larval phase, transition to the benthic environment and early post-settlement periods [16]. The fitness and probability of survival increase with age [17], and dispersal allows for competent individuals to move into areas more suitable for growth, escape adverse conditions and colonize disturbed areas [18]. They have also reached a size where they start to influence ecosystem function, for example through tube building or bioturbation affecting sediment stability and biogeochemistry. Trap studies show that competent individuals of multiple species are dispersing more or less continuously [19], but the contribution of this flux to seafloor integrity and biodiversity maintenance is poorly known.
3. Role of post-settlement dispersal in biodiversity maintenance
Much of our present insight into the role of post-settlement dispersal derives from disturbance-recovery studies, where the rates and patterns of recovery are both scale-dependent and influenced by site-specific environmental factors [20–23]. Conceptual models dictate that larval stages dominate when the spatial scales of disturbance are large, whereas post-settlers are more important when scales of disturbance are small [6,24] and when larvae are scarce (i.e. outside main larval recruitment periods). The post-settlers, however, may be more important than previously thought, as their scales of dispersal easily match those of larvae, and their competency is greater. Post-settler responses to disturbance are species-specific, with potential for opportunistic population increases in response to release of available food resources or release from predation or competition pressure [25]. Importantly, competent dispersers that rapidly respond to available resources can facilitate community assembly.
A substantial proportion of a soft-sediment community is constantly dispersing, in both quiescent and energetic hydrodynamic conditions [26,27], depending on species-specific behaviour and dispersal potential (figure 2). Consequently, turnover rates of individuals in local communities can be very high, even though the species composition remains more or less constant [11,28]. Because of this constant dispersal, we need to think of the local community as consisting of those species whose distribution happens to co-occur at a particular locality [29]. How well the local community is connected to its metacommunity depends on the temporal frequency and spatial extent of dispersal in the region [30] (figure 2). To understand spatial variation in community structure, it has been suggested that the amount of dispersal between communities within a metacommunity can be used to differentiate between the relative importance of the patch-dynamic (limited dispersal; colonization–competition trade-offs drive community patterns), species sorting (efficient dispersal allows species to track variation in environmental conditions) and mass effect (high dispersal, promoting higher diversity even in suboptimal conditions) models [31] (figure 2). In marine systems across natural and anthropogenic environmental gradients, dispersal will allow species to track environmental variation in space, and thus the importance of species sorting for metacommunity organization is apparently high [32]. However, mass effects may be relatively more important when post-larval dispersal is frequent, because of their competency and ability to continue moving after initial recruitment (figure 2c). This challenges the traditional view that soft-sediment communities remain relatively static. Generally, only dispersal during the larval phase has been considered, but post-larval dispersal likely plays a critical role in differentiating between various metacommunity models. A formal quantification of these ideas remains a challenge.
Accounting for life-stage-specific dispersal traits may help quantify the importance of the post-settlement dispersal for biodiversity. Previous studies have explored spatial patterns of beta-diversity, i.e. the turnover component of diversity, to infer contributions of different reproductive modes of marine benthic invertebrates to abundance–occupancy relationships using additive partitioning of diversity [33]. A similar approach can be adopted using community data differentiated by life-stage, because beta should be higher for less-dispersive (adults) than for more-dispersive stages (post-settlers). Variation partitioning is another method that can be used to address the relative contribution of niche-based and dispersal-based factors to community structure [32,34,35]. Such an approach could be used to compare variation in post-settler and adult community structure explained by (i) dispersal traits, (ii) environmental factors, (iii) spatial factors and/or (iv) their shared effects. These analyses could further be embedded along gradients of physical forcing, environmental heterogeneity and disturbance to provide information on metacommunity structuring. Importantly, as post-settlers have a prolonged dispersal period, accounting for not only spatial but also temporal dynamics is essential.
4. Conclusion
The capacity of the seafloor ecosystem to maintain resilience and function after human disturbances is dependent on dispersal. We need to recognize that dispersal in soft-sediment macroinvertebrates is not restricted to a short larval phase, that the spatial scales of post-settlement dispersal may be similar to those of larval dispersal and that the impact of post-settlement dispersal is potentially more important because of the greater size and competency of the dispersers. An increased empirical understanding of post-settlement dispersal processes is essential for: (i) identifying disturbance thresholds for when the spatial extent of disturbance exceeds the dispersal potential of concurrently shrinking regional species pools, and (ii) effective marine spatial planning and the design of marine protected areas. See table 1 for a summary of recommendations for future research.
Table 1.
Recommendations for research to quantify the role of post-settlement dispersal in soft-sediment community dynamics.
| knowledge gap | recommendations |
|---|---|
| post-settlement dispersal traits of many benthic taxa | studies that address the interaction between behaviour and environmental conditions (including flow) to promote or limit dispersal across post-settlement life-stages. Example studies include [13,19,26]. |
| dispersal potential of post-settlers | develop coupled physical–biological models to estimate post-settlement dispersal trajectories of multiple species. These models must include interactions between near-bed hydrodynamics, sediment transport and species-specific behaviours, which will regulate modes and rates of dispersal. See [12,36] for a starting point for such an approach. |
| contribution of post-settlement dispersal to recovery from disturbances, connectivity and biodiversity maintenance | use scale-dependent disturbance-recovery experiments nested within environmental gradients of hydrodynamic forcing and background biodiversity to quantify the role of post-settlement dispersal in maintaining local and regional biodiversity. The study designs of Thrush et al. [22], Norkko et al. [21] and Norkko et al. [25] provide a conceptual framework for such studies. analyse spatial and temporal beta-diversity patterns along environmental gradients (e.g. disturbance, hydrodynamic forcing) with an emphasis on life-stage-based dispersal traits. Apply the approaches of Josefson & Göke [33] and Grönroos et al. [34] to determine the relative amount of variation in community structure split up by key dispersal traits explained by spatial, environmental or shared effects. This should help determine the degree of connectivity among spatially discrete communities and test metacommunity models. |
Acknowledgements
Comments by Sally Woodin, Kim King and an anonymous reviewer greatly improved the manuscript.
Author contributions
All authors contributed to the ideas presented in this opinion paper and the drafting of the manuscript.
Funding statement
We thank the Walter and Andrée de Nottbeck Foundation, the University of Helsinki (3-year grant to J.N.), the Maj and Tor Nessling Foundation, the University of Waikato and the Academy of Finland for support.
Conflict of interests
We have no competing interests.
References
- 1.Snelgrove PVR, Thrush SF, Wall DH, Norkko A. 2014. Real world biodiversity–ecosystem functioning: a seafloor perspective. Trends Ecol. Evol. 29, 398–405. ( 10.1016/j.tree.2014.05.002) [DOI] [PubMed] [Google Scholar]
- 2.Lotze HK, et al. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. ( 10.1126/science.1128035) [DOI] [PubMed] [Google Scholar]
- 3.Thrush SF, Hewitt JE, Dayton PD, Coco G, Lohrer AM, Norkko A, Norkko J, Chiantore M. 2009. Forecasting the limits of resilience: integrating empirical research with theory. Proc. R. Soc. B 276, 3209–3217. ( 10.1098/rspb.2009.0661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer MA, Allan JD, Butman CA. 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol. Evol. 11, 322–326. ( 10.1016/0169-5347(96)10038-0) [DOI] [PubMed] [Google Scholar]
- 5.Grantham BA, Eckert GL, Shanks AL. 2003. Dispersal potential of marine invertebrates in diverse habitats. Ecol. Appl. 13, S108–S116. ( 10.1890/1051-0761(2003)013[0108:DPOMII]2.0.CO;2) [DOI] [Google Scholar]
- 6.Günther C-P. 1992. Dispersal of intertidal invertebrates: a strategy to react to disturbances of different scales? Neth. J. Sea Res. 30, 45–56. ( 10.1016/0077-7579(92)90044-F) [DOI] [Google Scholar]
- 7.Armonies W. 1994. Drifting meio- and macrobenthic invertebrates on tidal flats in Königshafen: a review. Helgoländer Meeresun. 48, 299–318. ( 10.1007/BF02367043) [DOI] [Google Scholar]
- 8.Bradbury IR, Laurel B, Snelgrove PVR, Bentzen P, Campana SE. 2008. Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B 275, 1803–1809. ( 10.1098/rspb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin LA. 2006. Recent progress in understanding larval dispersal: new directions and digressions. Integr. Comp. Biol. 46, 282–297. ( 10.1093/icb/icj024) [DOI] [PubMed] [Google Scholar]
- 10.Shanks AL. 2009. Pelagic larval duration and dispersal distance revisited. Biol. Bull. 216, 373–385. [DOI] [PubMed] [Google Scholar]
- 11.Norkko A, Cummings VJ, Thrush SF, Hewitt JE, Hume T. 2001. Local dispersal of juvenile bivalves: implications for sandflat ecology. Mar. Ecol. Prog. Ser. 212, 131–144. ( 10.3354/meps212131) [DOI] [Google Scholar]
- 12.Hunt HL, Fugate DC, Chant RJ. 2009. Modeling bedload transport of juvenile bivalves: predicted changes in distribution and scale of postlarval dispersal. Estuar. Coast. 32, 1090–1102. ( 10.1007/s12237-009-9205-5) [DOI] [Google Scholar]
- 13.Petuha ET, Lundquist CJ, Pilditch CA. 2006. Estimating spatial scale of post-settlement transport potential of Macomona liliana on an intertidal sandflat. N. Z. J. Mar. Freshwat. Res. 40, 487–502. ( 10.1080/00288330.2006.9517438) [DOI] [Google Scholar]
- 14.Turner SJ, Grant J, Pridmore RD, Hewitt JE, Wilkinson MR, Hume TM, Morissey DJ. 1997. Bedload and water-column transport and colonization processes by post-settlement benthic macrofauna: does infaunal density matter? J. Exp. Mar. Biol. Ecol. 216, 51–75. ( 10.1016/S0022-0981(97)00090-7) [DOI] [Google Scholar]
- 15.Emerson CW, Grant J. 1991. The control of soft-shell clam (Mya arenaria) recruitment on intertidal sandflats by bedload sediment transport. Limnol. Oceanogr. 36, 1288–1300. ( 10.4319/lo.1991.36.7.1288) [DOI] [Google Scholar]
- 16.Pedersen TM, Hansen JLS, Josefson AB, Hansen BW. 2008. Mortality through ontogeny of soft-bottom marine invertebrates with planktonic larvae. J. Mar. Syst. 73, 185–207. ( 10.1016/j.jmarsys.2007.10.008) [DOI] [Google Scholar]
- 17.Gosselin LA, Qian P-Y. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282. ( 10.3354/meps146265) [DOI] [Google Scholar]
- 18.Woodin SA. 1991. Recruitment of infauna: positive or negative cues? Am. Zool. 31, 797–807. [Google Scholar]
- 19.Valanko S, Norkko A, Norkko J. 2010. Strategies of post-larval dispersal in non-tidal soft-sediment communities. J. Exp. Mar. Biol. Ecol. 384, 51–60. ( 10.1016/j.jembe.2009.12.012) [DOI] [Google Scholar]
- 20.Thrush SF, Whitlatch RB. 2001. Recovery dynamics in benthic communities: balancing detail with simplification. In Ecological studies (ed. Reise K.), pp. 297–316. Berlin, Germany: Springer. [Google Scholar]
- 21.Norkko J, Norkko A, Thrush SF, Valanko S, Suurkuukka H. 2010. Conditional responses to increasing scales of disturbance, and potential implications for threshold dynamics in soft-sediment communities. Mar. Ecol. Prog. Ser. 413, 253–266. ( 10.3354/meps08745) [DOI] [Google Scholar]
- 22.Thrush SF, Halliday J, Hewitt JE, Lohrer AM. 2008. The effects of habitat loss, fragmentation, and community homogenization on resilience in estuaries. Ecol. Appl. 18, 12–21. ( 10.1890/07-0436.1) [DOI] [PubMed] [Google Scholar]
- 23.McCall PL. 1978. Spatial–temporal distributions of Long Island sound infauna: the role of bottom disturbance in a nearshore marine habitat. In Estuarine interactions (ed. Wiley ML.), pp. 191–219. New York, NY: Academic Press. [Google Scholar]
- 24.Whitlatch RB, Lohrer AM, Thrush SF, Pridmore RD, Hewitt JE, Cummings VJ, Zajac RN. 1998. Scale-dependent benthic recolonization dynamics: life stage-based dispersal and demographic consequences. Hydrobiologia 375/376, 217–226. ( 10.1023/A:1017084217011) [DOI] [Google Scholar]
- 25.Norkko A, Rosenberg R, Thrush SF, Whitlatch RB. 2006. Scale- and intensity-dependent disturbance determines the magnitude of opportunistic response. J. Exp. Mar. Biol. Ecol. 330, 195–207. ( 10.1016/j.jembe.2005.12.027) [DOI] [Google Scholar]
- 26.Lundquist CJ, Thrush SF, Hewitt JE, Halliday J, MacDonald I, Cummings VJ. 2006. Spatial variability in recolonisation potential: influence of organism behaviour and hydrodynamics on the distribution of macrofaunal colonists. Mar. Ecol. Prog. Ser. 324, 67–81. ( 10.3354/meps324067) [DOI] [Google Scholar]
- 27.Valanko S, Norkko J, Norkko A. 2015. Does stability in local community composition depend on temporal variation in rates of dispersal and connectivity? J. Sea Res. ( 10.1016/j.seares.2014.09.001) [DOI] [Google Scholar]
- 28.Hewitt JE, Pridmore RD, Thrush SF, Cummings VJ. 1997. Assessing the short-term stability of spatial patterns of macrobenthos in a dynamic estuarine system. Limnol. Oceanogr. 42, 282–288. ( 10.4319/lo.1997.42.2.0282) [DOI] [Google Scholar]
- 29.Ricklefs RE. 2008. Disintegration of the ecological community. Am. Nat. 172, 741–750. ( 10.1086/593002) [DOI] [PubMed] [Google Scholar]
- 30.Matias MG, Mouquet N, Chase JM. 2013. Dispersal stochasticity mediates species richness in source–sink metacommunities. Oikos 122, 395–402. ( 10.1111/j.1600-0706.2012.20479.x) [DOI] [Google Scholar]
- 31.Winegardner AK, Jones BK, Ng ISY, Siqueira T. 2012. The terminology of metacommunity ecology. Trends Ecol. Evol. 27, 253–254. ( 10.1016/j.tree.2012.01.007) [DOI] [PubMed] [Google Scholar]
- 32.Moritz C, Meynard CN, Devictor V, Guizien K, Labrune C, Guarini JM, Mouquet N. 2013. Disentangling the role of connectivity, environmental filtering, and spatial structure on metacommunity dynamics. Oikos 122, 1401–1410. [Google Scholar]
- 33.Josefson AB, Göke C. 2013. Disentangling the effects of dispersal and salinity on beta diversity in estuarine benthic invertebrate assemblages. J. Biogeogr. 40, 1000–1009. ( 10.1111/jbi.12047) [DOI] [Google Scholar]
- 34.Grönroos M, Heino J, Siqueira T, Landeiro VL, Kotanen J, Bini LM. 2013. Metacommunity structuring in stream networks: roles of dispersal mode, distance type, and regional environmental context. Ecol. Evol. 3, 4473–4487. ( 10.1002/ece3.834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM. 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw. Biol. ( 10.1111/fwb.12533) [DOI] [Google Scholar]
- 36.Cowen RK, Paris CB, Srinivasan A. 2006. Scaling of connectivity in marine populations. Science 311, 522–527. ( 10.1126/science.1122039) [DOI] [PubMed] [Google Scholar]