Abstract
At a cellular level, oxidative stress is known to increase telomere attrition, and hence cellular senescence and risk of disease. It has been proposed that dietary micronutrients play an important role in telomere protection due to their antioxidant properties. We experimentally manipulated dietary micronutrients during early life in zebra finches (Taeniopygia guttata). We found no effects of micronutrient intake on telomere loss during chick growth. However, females given a diet high in micronutrients during sexual maturation showed reduced telomere loss; there was no such effect in males. These results suggest that micronutrients may influence rates of cellular senescence, but differences in micronutrient requirement and allocation strategies, probably linked to the development of sexual coloration, may underlie sex differences in response.
Keywords: ageing, antioxidants, diet, oxidative stress, sexual maturation
1. Introduction
Telomeres, specialized nucleotide repeat sequences at the ends of eukaryotic chromosomes, play a pivotal role in maintaining genome stability [1]. There is good empirical evidence linking changes in telomere length (TL) with ageing and disease [2,3]. An important environmental factor thought to increase telomere loss is oxidative stress [4], a physiological state characterized by an imbalance between the production of pro-oxidant molecules and antioxidant defences [5]. These defences are a composite of endogenously produced antioxidants and those of dietary origin. Dietary micronutrients such as vitamins (e.g. E) and minerals (e.g. Mg, Se) are needed in small amounts but play essential roles in antioxidant protection and chromosome stability [5,6]. Hence, it has been recently suggested that the availability of dietary micronutrients might be a prominent environmental factor influencing telomere dynamics and thus potentially rates of ageing [7].
Telomeres shorten throughout life in long-lived organisms, but the rate of loss seems to be higher during growth and development [8], when cell division rates and exposure to oxidative stress are higher [5]. Dietary micronutrients might, therefore, help alleviate the oxidative costs of growth [9,10]. However, dietary micronutrients are also required for other physiological processes. During the development of sexual maturity, dietary antioxidants can be deployed in sexual coloration (reviewed in [6]). In sexually dimorphic species, investment of micronutrients in sexual ornamentation is often higher in males owing to their more pronounced sexual coloration [5,6].
In this study, we experimentally tested whether the availability of dietary micronutrients during growth and sexual maturation influenced telomere dynamics in male and female zebra finches (Taeniopygia guttata), a species showing marked sexual dimorphism. Importantly, males develop a more intense and antioxidant-demanding adult coloration than females [11–13]. We predicted that a rich micronutrient diet would alleviate the oxidative costs of growth, leading to a reduced rate of telomere loss and hence longer TL. During sexual maturation, a greater intake of dietary micronutrients might be predicted to have a greater effect on male TL, as dietary micronutrients should be more limiting in males than in females owing to the added demand of plumage and bill coloration. However, the opposite scenario might also be possible, if for instance, the sexes differentially prioritize the use of dietary nutrients between competing functions or traits such as sexual maturation and self-maintenance. Males might allocate additional micronutrients to secondary sexual coloration, while females allocate these to somatic maintenance.
2. Material and methods
In September 2012, we bred 50 pairs of zebra finches to produce the birds to be reared on variable micronutrient levels. Pairs were randomly assigned to the ‘high’ (H) or ‘low’ (L) micronutrient treatment 1 day after their chicks hatched. Just before starting the treatments, half of each brood was reciprocally swapped with a pair allocated to the other treatment whose chicks hatched at the same time (cross-foster dyad) to separate effects of genetic and rearing environment. Chick body mass did not differ between groups either at hatching (F1,124 = 0.573, p = 0.451) or after cross-fostering (day = 1; F1,122 = 0.141, p = 0.708). From hatching, chicks (n = 126, 36 families) had a diet either low (L) or high (H) in antioxidant micronutrients: all chicks received the same basic seed diet low in micronutrients but ‘H’ chicks were also given individually an oral aqueous dose of a specially formulated avian micronutrient dietary supplement (Feed Food Magic Antistress Mix, Feed-Food Ltd, UK) every 3 days from day 1 to day 40 (hereafter ‘growth period’). The main components of the supplement were vitamins A, C, E, B and K, and essential minerals Se and MgSO4. At the same times nestlings in the ‘L’ group received only an oral dose of water (see electronic supplementary material for further details).
At 40 days, half of the broods in each treatment were switched to the opposite treatment until 90 days of age. This second treatment period (40–90 days) corresponded with the period of sexual maturation [11]. This then produced four experimental groups (H–H, H–L, L–L, L–H). All birds were weighed (±0.01 g) and blood-sampled at 20, 40 and 90 days of age. Blood samples were centrifuged and the red blood cells (RBC) stored at −80°C. Body mass growth did not differ among experimental groups during the experiment (see electronic supplementary material).
The TL was quantified in RBC DNA by the qPCR method [14]. TL was calculated in relation to a reference gene (GAPDH) [14]. All samples were run in triplicate, and mean values used to calculate the relative TL (T : S ratio). Reaction efficiencies were always within an acceptable range (i.e. 100 ± 10%). The average intra- versus inter-plate coefficients of variation for the telomere and GAPDH assays were 1.71 versus 8.10% and 0.40 versus 5.74%, respectively.
We investigated whether dietary treatments affected the change in TL (after correcting for regression to the mean; [15]) during both (1) the growth period (between 20 and 40 days) and (2) sexual maturation (between 40 and 90 days) by using linear mixed effect models (LME) in SAS. In analysis (1), sex, treatment (growth period: ‘H’ or ‘L’) and their interaction were included as fixed factors, with body mass gain (20–40 days) and TL (day 20) as covariates. Analysis (2) included the factors sex, treatments (during both the growth period and sexual maturation) and their two- and three-way interactions, along with covariates body mass gain (between 40 and 90 days) and TL (day 40).
We also investigated the effect of diet treatment on TL at 20 days and at the end of both growth (day 40) and sexual maturation (day 90). The 20- and 40-day models of TL included sex, treatment (H or L) and their interaction as fixed factors and body mass as a covariate. The model of TL at the end of sexual maturation included sex, treatments (growth period and maturation) and corresponding two- and three-way interactions, with body mass as a covariate. All models included nest of origin and of rearing and the identity of the cross-foster dyad as random factors and were run with Satterthwaite's d.f.; post hoc comparisons were performed using Tukey's post hoc test.
3. Results
(a). Telomere dynamics during growth
The change in TL from 20 to 40 days did not differ between experimental groups or sexes (treatment: F1,14.4 = 4.40, p = 0.054; sex: F1,98.8 = 1.08, p = 0.300; treatment × sex: F1,91.9 = 2.71, p = 0.103) and was unaffected by TL at 20 days (F1,98.5 = 0.00, p = 0.956), body mass gain between 20 and 40 days (F1,108 = 0.08, p = 0.774) or the random factors (all p > 0.062). Average TL at 20 and at 40 days was also similar among experimental groups and sexes (TL 20 days—treatment: F1,18.7 = 1.126, p = 0.302; sex: F1,101 = 0.51, p = 0.477; treatment × sex: F1,102.4 = 0.93, p = 0.337; TL 40 days—treatment: F1,13.2 = 1.17, p = 0.298; sex: F1,94.5 = 2.11, p = 0.149; treatment × sex: F1,91.8 = 2.86, p = 0.094). TL at 20 days was not related to body mass (F1,112.6 = 1.07, p = 0.302). However, TL at 40 days was negatively related to body mass (table 1; figure 1). Random factors had no significant effect on TL (table 1; for TL 20 days, all p > 0.05).
Table 1.
Summary of the final linear mixed effect (LME) models of the change in TL during sexual maturation (40–90 days; adjusted for the regression to the mean), and TL at the end of both the growth period (day 40) and sexual maturation (day 90). In the minimal model on TL at 20 days and the change in TL during growth period only the intercept was retained (see Results for details). In all models, nest of origin and foster were nested within the cross-foster dyad.
| dependent variable | source of variation | parameter estimate | F or Z | d.f. | p-value |
|---|---|---|---|---|---|
| change in TL (40–90 days) | intercept | 0.120 | |||
| TL at day 40 | −0.1785 | 8.12 | 1,104 | 0.005 | |
| sex (female) | 0.186 | 3.91 | 1,98.9 | 0.051 | |
| treatment (sexual maturation) (L) | 0.027 | 4.92 | 1,4.92 | 0.030 | |
| sex × treatment (sexual maturation) | −0.218 | 7.24 | 1,99.7 | 0.008 | |
| random factors | |||||
| nest of origin | 0.009 | 1.60 | 0.054 | ||
| nest of rearing | 0.003 | 0.63 | 0.263 | ||
| cross-foster dyad | 0a | — | 1 | ||
| TL (day 40) | intercept | 1.845 | |||
| body mass | −0.054 | 4.68 | 1,105 | 0.032 | |
| random factors | |||||
| nest of origin | 0.020 | 1.38 | 0.084 | ||
| nest of rearing | 0.007 | 0.67 | 0.250 | ||
| cross-foster dyad | 0.035 | 1.61 | 0.054 | ||
| TL (day 90) | intercept | 1.351 | |||
| body mass | −0.030 | 6.58 | 1,85 | 0.012 | |
| sex (female) | 0.272 | 10.40 | 1,92.6 | 0.002 | |
| treatment (sexual maturation) (L) | 0.032 | 7.17 | 1,61.3 | 0.009 | |
| sex × treatment (sexual maturation) | −0.257 | 9.67 | 1,91.6 | 0.002 | |
| random factors | |||||
| nest of origin | 0.017 | 2.19 | 0.014 | ||
| nest of rearing | 0.010 | 1.39 | 0.082 | ||
| cross-foster dyad | 0a | — | 1 | ||
aParameter estimate bound at zero; hence no Z was estimated.
Figure 1.
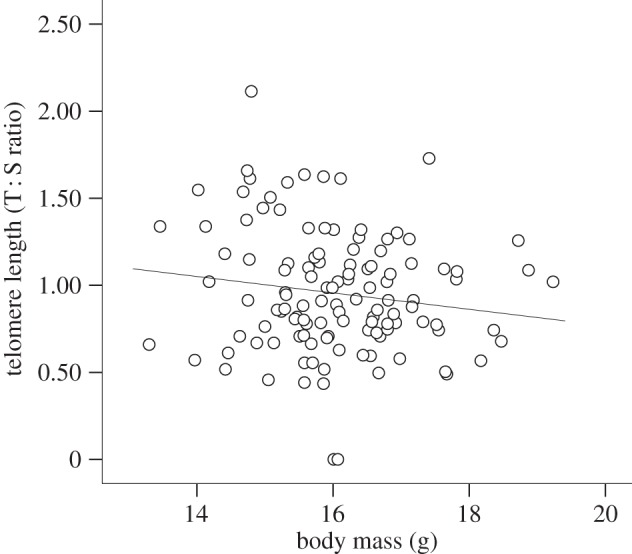
TL in relation to body mass at the end of the growth period (day 40). The fitted line is the linear regression.
(b). Telomere dynamics during sexual maturation
Birds with shorter TL at 40 days showed accelerated telomere loss during sexual maturation (40–90 days; table 1), which was unaffected by the dietary treatment during the growth period (F1,17.6 = 0.40, p = 0.536) or its interaction with sex (F1,91.7 = 1.20, p = 0.275). However, there was a significant interaction between the diet during sexual maturation and sex (table 1): both sexes lost a similar amount from their telomeres when on the low micronutrient diet (Tukey's test: p = 0.555; figure 2a; mean change in TL differed from zero in both sexes; one-sample t-test, p < 0.05). However, males and females showed different amounts of loss when on the high diet (Tukey's test; p = 0.002). Thus, whereas loss rates in males were similar irrespective of their diet (Tukey's test; p > 0.05), females on the high diet showed no change in TL during sexual maturation (change in TL in females; one-sample t-test, p > 0.05), and as a consequence, had significantly longer TL than any other group at the end of the experiment (Tukey's test; all p < 0.001; table 1; figure 2b). No other measured factors influenced telomere loss over this period (all p > 0.805; table 1). At the end of sexual maturation (day 90), TL was negatively related to the birds' body mass (table 1), but unrelated to their dietary treatment received during growth (F1,19.4 = 0.06, p = 0.816), and its interaction with sex (F1,86.8 = 2.57) or other two- and three-way interactions (all p > 0.894). Interestingly, our data indicate that genetic siblings showed a similar TL at 90 days but unrelated chicks reared in the same nest did not (table 1).
Figure 2.
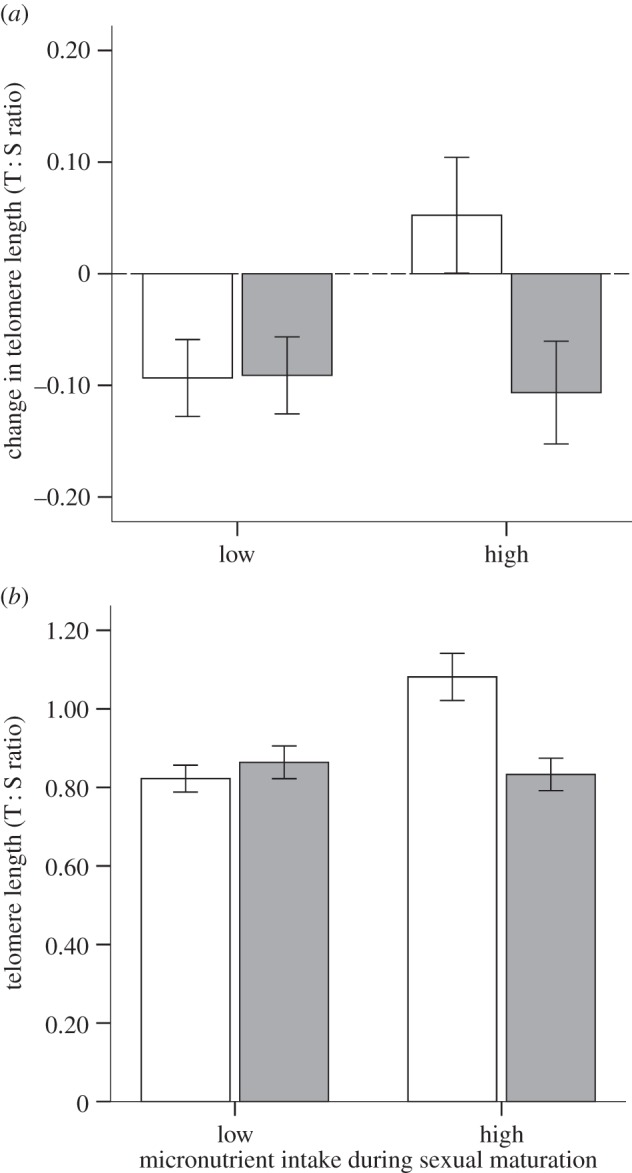
(a) Change in TL (adjusted for the regression to the mean; mean ± s.e.) during sexual maturation (40–90 days) and (b) TL at 90 days of age (mean ± s.e.) of male (grey bars) and female zebra finches (white bars) reared on a low or high micronutrient diet.
4. Discussion
While dietary micronutrients had no influence on the telomere loss of zebra finches during the main period of growth, they influenced telomere dynamics during sexual maturation in a sex-specific way: both sexes showed similar telomere loss when the micronutrient supply was low, but, when the supply was relatively high, telomere loss was negligible in females but significant in males, resulting in females having longer telomeres on average than males by 90 days of age.
Previous studies have demonstrated a positive link between growth rate and levels of oxidative stress [5,9], an important factor influencing TL [4]. We also found that young zebra finches that had the heaviest body mass at the end of the growth period (40 days) had the shortest telomeres (which was still the case by the completion of sexual maturation). However, contrary to our predictions, we found no effect of dietary micronutrients on growth rates or telomere dynamics during the growth period. There are at least three possible explanations of this result. Firstly, oxidative stress during growth could be relatively low, and thus not influenced by dietary antioxidants, the reduced TL in faster-growing chicks reflecting an increased cell division rate. Alternatively, growing chicks might be able to upregulate endogenously produced antioxidants to compensate for the reduced dietary intake. A third possibility is that those receiving higher levels of micronutrients might have prioritized allocation of these to other micronutrient-demanding physiological functions (e.g. immune system [5,6]).
In zebra finches, sexual maturation is likely to be more micronutrient-demanding for males than for females due to their faster gonadal maturation and redder bill coloration and adult plumage [11], traits that will have a higher micronutrient demand [5,6,13]. An extra source of dietary micronutrients might, therefore, be expected to have a more pronounced ‘rescue effect’ on the TL of males. This was not the case. Intriguingly, the TL of males did not differ between treatments, whereas females on the high micronutrient diet showed no telomere loss, and had longer telomeres. Although this result might seem counterintuitive, it would indeed be predicted if, for instance, dietary micronutrients were a limiting resource for both sexes even in the high treatment group. Since it is more important for relatively short-lived species like zebra finches to reproduce than to survive, the males in our experiment may have allocated any additional micronutrients into enhancing sexual attractiveness and the speed of maturation (and so increasing their chance of breeding), whereas females (whose reproductive chances are less affected by their appearance) invested the additional micronutrients in somatic maintenance, preserving TL. The lack of a sex difference in telomere dynamics in the low micronutrient group might indicate that both sexes favoured the allocation of micronutrients to somatic maintenance over sexual investment [16].
In conclusion, our results clearly show that high levels of dietary micronutrients have the potential to influence telomere dynamics by reducing telomere loss. This was evident during sexual maturation, but not during growth. During sexual maturation, a protective effect was found in females but not in males, presumably as a consequence of sex-specific differences in resource requirements for secondary sexual characteristics and thereby different allocation strategies. Males appear to have prioritized the allocation of micronutrients to sexual maturation, whereas females prioritized self-maintenance. This result should encourage sex-specific analyses of telomere dynamics in future studies. Furthermore, since an early loss of TL might reduce lifespan [8], our results stress the importance of investigating whether such variation in telomere loss might be translated into changes in life-history trajectories, for instance via changes to future reproductive investment.
Supplementary Material
Acknowledgements
We thank referees for helpful comments, the animal care staff for their support and Peter Surai for providing the micronutrient complex.
Ethics statement
The study was supported by University of Glasgow project licence no. 60/4109 and subjected to local ethical review.
Data accessibility
Additional details are available in the electronic supplementary material.
Funding statement
J.C.N. was funded by Marie Curie Actions (PIEF-GA-2011–301093) and later by AXA Research fund, N.B.M. by ERC Advanced grant no. 322784 and P.M. and W.B. by ERC Advanced grant no. 268926.
Authors' contribution
J.C.N. carried out the experiment, statistical analyses and drafted the manuscript. W.B. participated in the laboratory analyses. P.M. and N.B.M. conceived the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
References
- 1.O'Sullivan RJ, Karlseder J. 2010. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11, 171–181. ( 10.1038/nrm2848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. ( 10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 3.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. NY Acad. Sci. 1206, 130–142. ( 10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 4.Von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. ( 10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 6.Catoni C, Peters A, Martin Schaefer H. 2008. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 76, 1107–1119. ( 10.1016/j.anbehav.2008.05.027) [DOI] [Google Scholar]
- 7.Paul L. 2011. Diet, nutrition and telomere length. J. Nutr. Biochem. 22, 895–901. ( 10.1016/j.jnutbio.2010.12.001) [DOI] [PubMed] [Google Scholar]
- 8.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S-Y, Noguera JC, Morales J, Velando A. 2011. Quantitative genetic evidence for trade-off between growth and resistance to oxidative stress in a wild bird. Evol. Ecol. 25, 461–472. ( 10.1007/s10682-010-9426-x) [DOI] [Google Scholar]
- 10.Noguera JC, Lores M, Alonso-Álvarez C, Velando A. 2011. Thrifty development: early-life diet restriction reduces oxidative damage during later growth. Funct. Ecol. 25, 1144–1153. ( 10.1111/j.1365-2435.2011.01856.x) [DOI] [Google Scholar]
- 11.Zann RA, Bamford M. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127. ( 10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- 13.Hill GE. 1996. Redness as a measure of the production cost of ornamental coloration. Ethol. Ecol. Evol. 8, 157–175. ( 10.1080/08927014.1996.9522926) [DOI] [Google Scholar]
- 14.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 15.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur. J. Epidemiol. 28, 859–866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 16.McNamara JM, Houston AI, Barta Z, Scheuerlein A, Fromhage L. 2009. Deterioration, death and the evolution of reproductive restraint in late life. Proc. R. Soc. B 276, 4061–4066 ( 10.1098/rspb.2009.0959 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional details are available in the electronic supplementary material.


