Abstract
Background
The American Heart Association/American Stroke Association (AHA/ ASA) recommended an expansion of the time window for acute ischemic stroke (AIS) reperfusion with intravenous (IV) recombinant tissue plasminogen activator (rt-PA) from 3 to 4.5 hours after symptom onset. We examine rates of IV and intraarterial (IA) reperfusion before and after the recommendations to track guideline adoption in community practice.
Methods
Patients with AIS in the Paul Coverdell National Acute Stroke Registry spanning years 2007-2012 were identified. Trends in rates of IV rt-PA versus IA therapy were examined. Outcomes included symptomatic intracerebral hemorrhage (sICH), in-hospital mortality, ability to ambulate at discharge, and discharge destination.
Results
From 2007 to 2012, there were 182,235 AIS patients (median age, 72 years; 51.5% women) in the database at the time of analysis. AIS patients receiving IV rt-PA increased significantly from 3.7% in 2007 to 5.1% in 2012 in the ≤3 hours time window and from .2% in 2007 to 1.3% in 2012 in the 3-4.5 hours time window (P <.001 for both). There was also a significant increase in the rate of IA therapy between 2007 and 2012 (P <.001). There was a significant decrease in the rate of sICH among patients who received any reperfusion between 2007 and 2012.
Conclusions
There was a trend for increased utilization of IV rt-PA in the 0-3 hours and the 3-4.5 hours time windows, which began around the same time as the publication of AHA/ASA recommendations in 2009. This increase was associated with an increase in IA treatment rates along with a decrease in overall sICH rates for patients receiving any reperfusion. Key Words: Expanded time window—ischemic stroke—thrombolysis—trend analysis.
Background
In May 2009, the American Heart Association/American Stroke Association (AHA/ASA) recommended an expansion of the time window for acute ischemic stroke (AIS) therapy with intravenous (IV) recombinant tissue plasminogen activator (rt-PA) from 3 to 4.5 hours after symptom onset based on the European Cooperative Acute Stroke Study III Trial and the Safe Implementation of Treatments in Stroke-International Stroke Thrombolysis Registry study.1-3 A pooled analysis found no differences between the 3-4.5 hours cohort and ≤3 hours cohort with respect to symptomatic intracerebral hemorrhage (sICH) rates, mortality, and modified Rankin Scale (mRS) score.4 National estimates of IV rt-PA utilization range from 1.8% to 5.2% as most AIS patients arrive outside the time window for IV thrombolysis.5 With expansion of the time window, a larger group of patients could become eligible for treatment. We examined rates of IV rt-PA use before and after the AHA/ASA recommendations for an expanded time window for IV thrombolysis to determine the impact of these guideline changes on community practice. Rates of intra-arterial (IA) therapies for AIS during this same period were also analyzed for shifts in response to the expanded IV time window.
Methods
The study population included patients admitted with a diagnosis of AIS from 2007 to 2012. The Paul Coverdell National Acute Stroke Registry (PCNASR) is an ongoing acute stroke quality improvement program funded by the Centers for Disease Control and Prevention (CDC). The PCNASR provides feedback to states on adherence to guidelines of care to improve care quality for hospitalized patients with stroke and transient ischemic attack. During our study period hospitals across 6 states (Georgia, Massachusetts, Michigan, Minnesota, North Carolina, and Ohio) participated in the PCNASR. Hospital participation is voluntary. Trained abstractors from participating hospitals collect detailed information on stroke and transient ischemic attack admissions concurrent with or soon after patient care using standard data definitions provided by the CDC.6,7
Trends by year were examined for rates of IV and IA rt-PA. Patients receiving either of these therapies were divided as follows: (1) treatment ≤3 hours from symptom onset; (2) treatment 3-4.5 hours from symptom onset; and (3) treatment >4.5 hours from symptom onset. Outcome measures included rates of sICH, in-hospital mortality, ability to ambulate at discharge, and discharge destination. Categoric variables were compared across treatment groups using 2-tailed Fisher exact or chi-square tests. Continuous variables were compared using the Wilcoxon–Mann–Whitney rank test or the Kruskal–Wallis test. Statistical analyses were performed at the CDC using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
From 2007 to 2012, there were 182,235 patients (Table 1; median age, 72 years; 51.5% women) admitted with AIS in the PCNASR database. The proportion of all AIS patients receiving IVrt-PA increased significantly from 3.7% in 2007 to 5.1% in 2012 in the ≤3 hours and from .2% in 2007 to 1.3% in 2012 in the 3-4.5 hours time windows, excluding patients who received IV rt-PA before transfer (P <.001 for both, Table 2, Fig 1). Patients who received IV rt-PA before transfer to another hospital increased from 1% in 2007 to 2.6% in 2012 (P <.001). There was a significant change in IA therapy rates between 2007 (.4%) and 2012 (2%), including those who received IA therapy only and those who received both IV and IA rt-PA (P <.001, Table 2). We have included analyses of those patients who were documented to have baseline National Institutes of Health Stroke Scale (NIHSS) scores. Among these patients, the trend for using IV rt-PA in the ≤3 hours window and the 3-4.5 hours window increased significantly. However, it is noted that there was no significant change in the utilization of IA therapy among those with a documented NIHSS score (Table 2).
Table 1.
Demographic and baseline characteristics among acute ischemic stroke patients, 2007-2012
| N (%) or statistics by year |
||||||
|---|---|---|---|---|---|---|
| Variables | Year 2007 (n = 13,499) |
Year 2008 (n = 22,086) |
Year 2009 (n = 27,963) |
Year 2010 (n = 35,289) |
Year 2011 (n = 40,148) |
Year 2012 (n = 43,250) |
| Age | ||||||
| Median | 72 | 73 | 72 | 71 | 71 | 71 |
| Range | 18-105 | 18-107 | 18-106 | 18-111 | 18-119 | 18-106 |
| Mean (SE) | 70.42 (.13) | 70.43 (.10) | 70.55 (.09) | 70.03 (.08) | 69.99 (.07) | 69.95 (.07) |
| Gender | ||||||
| Female | 7007 (51.9) | 11,608 (52.6) | 14,509 (51.9) | 18,121 (51.4) | 20,695 (51.5) | 21,986 (50.8) |
| Race | ||||||
| Black | 2931 (21.7) | 4397 (19.9) | 5598 (20.0) | 7667 (21.7) | 8579 (21.4) | 9230(21.3) |
| White | 10,018 (74.2) | 16,418 (74.3) | 20,790 (74.3) | 25,589 (72.5) | 29,239 (72.8) | 31,640(73.2) |
| Other | 550 (4.1) | 1271 (5.8) | 1575 (5.6) | 2033 (5.8) | 2330 (5.8) | 2380 (5.5) |
| Admission NIHSS in groups | ||||||
| NIHSS < 10 | 3419 (25.3) | 5959 (27.0) | 8810 (31.5) | 14,256 (40.4) | 18,348 (45.7) | 23,244 (53.7) |
| NIHSS 10-19 | 887 (6.6) | 1655 (7.5) | 2227 (8.0) | 3539 (10.0) | 4169 (10.4) | 5003 (11.6) |
| NIHSS > 20 | 457 (3.4) | 749 (3.4) | 1107 (4.0) | 1885 (5.3) | 2186 (5.4) | 2677 (6.2) |
| Missing | 8736 (64.7) | 13,723 (62.1) | 15,819 (56.6) | 15,609 (44.2) | 15,445 (38.5) | 12,326 (28.5) |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; SE, standard error.
Table 2.
Type of rt-PA given among all patients and among those with NIHSS recorded
| N (%) or statistics by year | ||||||
|---|---|---|---|---|---|---|
| Variables | Year 2007 | Year 2008 | Year 2009 | Year 2010 | Year 2011 | Year 2012 |
| Total | 13,499 | 22,086 | 27,963 | 35,289 | 40,148 | 43,250 |
| rt-PA group | ||||||
| IV therapy* | 576 (4.3) | 1028 (4.7) | 1482 (5.3) | 2162 (6.1) | 2434 (6.1) | 2923 (6.8)† |
| IA therapy* | 38 (.3) | 178 (.8) | 272 (1.0) | 366 (1.0) | 538 (1.3) | 649 (1.5)† |
| IV + IA therapy* | 10 (.1) | 47 (.2) | 75 (.3) | 124 (.4) | 158 (.4) | 226 (.5)† |
| IV therapy‡ | 130(1.0) | 298 (1.3) | 491 (1.8) | 735 (2.1) | 900 (2.2) | 1109(2.6)† |
| No rt-PA | 12,745 (94.4) | 20,535 (93.0) | 25,643 (91.7) | 31,902 (90.4) | 36,118 (90.0) | 38,343 (88.7) |
| Any rt-PA | 754 (5.6) | 1551 (7.0) | 2320 (8.3) | 3387 (9.6) | 4030 (10.0) | 4907 (11.3)† |
| IV rt-PA only: Symptom onset to treatment time | ||||||
| ≤3 hours | 506 (3.7) | 851 (3.9) | 1163(4.2) | 1657 (4.7) | 1821 (4.5) | 2215 (5.1)† |
| 3-4.5 hours | 33 (.2) | 85 (.4) | 201 (.7) | 373 (1.1) | 463 (1.2) | 547 (1.3)† |
| Beyond 4.5 hours | 3 (.0) | 7 (.0) | 18 (.1) | 38 (.1) | 45 (.1) | 58 (.1) |
| Missing time | 34 (.3) | 85 (.4) | 100 (.4) | 94 (.3) | 105 (.3) | 103 (.2) |
| No IV rt-PA | 12,923 (95.7) | 21,058 (95.3) | 26,481 (94.7) | 33,127 (93.9) | 37,714 (93.9) | 40,327 (93.2) |
| IA rt-PA only: Symptom onset to treatment time | ||||||
| ≤3 hours | 2 (.0) | 15 (.1) | 15 (.1) | 21 (.1) | 31 (.1) | 44 (.1) |
| 3-4.5 hours | 8 (.1) | 28 (.1) | 32 (.1) | 40 (.1) | 53 (.1) | 97 (.2) |
| Beyond 4.5 hours | 10 (.1) | 71 (.3) | 78 (.3) | 81 (.2) | 119 (.3) | 208 (.5)† |
| Missing time | 18 (.1) | 64 (.3) | 147 (.5) | 224 (.6) | 335 (.8) | 300 (.7) |
| No IA rt-PA | 13,461 (99.7) | 21,908 (99.2) | 27,691 (99.0) | 34,923 (99.0) | 39,610 (98.7) | 42,601 (98.5) |
| Among those with NIHSS recorded | ||||||
| Total | 4763 | 8363 | 12,144 | 19,680 | 24,703 | 30,924 |
| IV rt-PA only: Symptom onset to treatment time | ||||||
| ≤3 hours | 384 (8.1) | 666 (8.0) | 857 (7.1) | 1428 (7.3) | 1631 (6.6) | 2109 (6.8)† |
| 3-4.5 hours | 27 (.6) | 58 (.7) | 133 (1.1) | 333 (1.7) | 430 (1.7) | 523 (1.7)† |
| Beyond 4.5 hours | 2 (.0) | 4 (.0) | 12 (.1) | 33 (.2) | 37 (.1) | 55 (.2) |
| Missing time | 21 (.4) | 52 (.6) | 65 (.5) | 82 (.4) | 93 (.4) | 97 (.3) |
| No IV rt-PA | 4329 (90.9) | 7583 (90.7) | 11077 (91.2) | 17804 (90.5) | 22512 (91.1) | 28140 (91.0) |
| IA rt-PA only: Symptom onset to treatment time | ||||||
| ≤3 hours | 1 (.0) | 8 (.1) | 14 (.1) | 20 (.1) | 27 (.1) | 41 (.1) |
| 3-4.5 hours | 6 (.1) | 17 (.2) | 28 (.2) | 37 (.2) | 50 (.2) | 92 (.3) |
| Beyond 4.5 hours | 7 (.1) | 45 (.5) | 60 (.5) | 71 (.4) | 113 (.5) | 190 (.6) |
| Missing time | 11 (.2) | 36 (.4) | 108 (.9) | 181 (.9) | 280 (1.1) | 265 (.9) |
| No IA rt-PA | 4738 (99.5) | 8257 (98.7) | 11,934 (98.3) | 19,371 (98.4) | 24,233 (98.1) | 30,336 (98.1) |
Abbreviations: IA, intra-arterial; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale; rt-PA, recombinant tissue plasminogen activator.
Therapy given at participating hospital.
F-value for trend was significant at a .05 threshold.
IV rt-PA given at an outside hospital and received in transfer by participating hospital.
Figure 1.
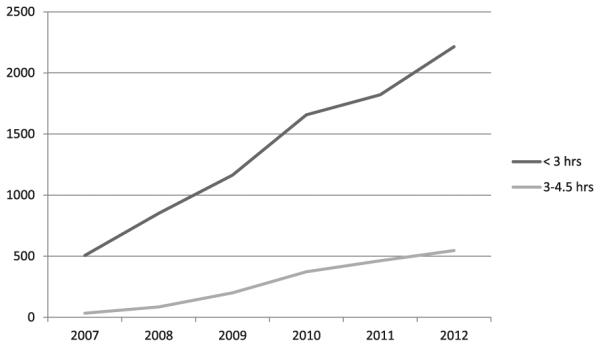
Number of patients receiving intravenous thrombolytic treatment based on time to treatment by year.
*Rates of treatment in the < 3 hour window did not decline in response to expansiion of treatment time window
Overall, patients receiving combined IV and IA therapy had the highest rates of sICH, life-threatening complications, and in-hospital mortality. The overall rates of sICH among those who received either IV only treatment or IA only treatment decreased significantly over time (P =.003 for both, Table 3). When stratified by NIHSS score ,10 and ≥10, patients receiving IV rt-PA only had the highest rates of ambulation at time of discharge and discharge to home compared with those who received either IA therapy or both IV and IA therapies (Table 4).
Table 3.
Outcome comparison among patients who received IV, IA, or combined IV + IA treatments
| N (%) by year | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Year 2007 | Year 2008 | Year 2009 | Year 2010 | Year 2011 | Year 2012 | P value for trend |
| Symptomatic ICH | |||||||
| IV therapy | * | 60 (5.8) | 76 (5.1) | 85 (3.9) | 107 (4.4) | 107 (3.7) | .003 |
| IA therapy | * | 14 (7.9) | 21 (7.7) | 27 (7.4) | 20 (3.7) | 28 (4.3) | .003 |
| IV + IA therapy | * | 6 (12.8) | 3 (4.0) | 14(11.3) | 14 (8.9) | 16 (7.1) | NS |
| Life-threatening complication | |||||||
| IV therapy | * | 14 (1.4) | 12 (.8) | 19 (.9) | 18 (.7) | 22 (.8) | .13 |
| IA therapy | * | 3 (1.7) | 3(1.1) | 2 (.5) | 5 (.9) | 3 (.5) | NS |
| IV + IA therapy | * | 1 (2.1) | 3 (4.0) | 3 (2.4) | 2(1.3) | 0 | NS |
| In-hospital mortality | |||||||
| IV therapy | 55 (9.5) | 88 (8.6) | 133 (9.0) | 174 (8.0) | 169 (6.9) | 167 (5.7) | <.001 |
| IA therapy | 8 (21.1) | 33 (18.5) | 48 (17.6) | 62 (16.9) | 97 (18.0) | 95 (14.6) | NS |
| IV + IA therapy | 4 (40.0) | 6 (12.8) | 21 (28.0) | 21 (16.9) | 29 (18.4) | 24 (10.6) | NS |
| Ambulating at discharge | |||||||
| IV therapy | 177 (30.7) | 342 (33.3) | 529 (35.7) | 747 (34.6) | 902 (37.1) | 1182(40.4) | <.001 |
| IA therapy | 6 (15.8) | 38 (21.3) | 49 (18.0) | 59 (16.1) | 110(20.4) | 146 (22.5) | NS |
| IV + IA therapy | 3 (30.0) | 15 (31.9) | 11 (14.7) | 20(16.1) | 36 (22.8) | 63 (27.9) | NS |
| Discharge home | |||||||
| IV therapy | 175 (30.4) | 335 (32.6) | 534 (36.0) | 884 (40.9) | 930 (38.2) | 1091 (37.3) | .0005 |
| IA therapy | 7 (18.4) | 38 (21.3) | 52(19.1) | 71 (19.4) | 103 (19.1) | 127 (19.6) | NS |
| IV + IA therapy | 3 (30.0) | 12 (25.5) | 11 (14.7) | 19 (15.3) | 37 (23.4) | 52 (23.0) | NS |
Abbreviations: IA, intra-arterial; ICH, intracerebral hemorrhage; IV, intravenous; NS, not significant.
Data not available.
Table 4.
Comparison of in-hospital outcomes between patients who received IV treatment and those who received IA or combined IV + IA treatments by NIHSS scores
| N (%) by NIHSS | ||||
|---|---|---|---|---|
| Variables | NIHSS < 10 | P value | NIHSS ≥ 10 | P value |
| In-hospital mortality | ||||
| IV therapy only | 72 (1.7) | <.0001 | 549(11.1) | <.0001 |
| IA therapy only | 27 (6.7) | 240 (18.4) | ||
| IV + IA therapy | 6 (7.6) | 86 (16.7) | ||
| Ambulated at discharge | ||||
| IV therapy only | 2310(55.1) | <.0001 | 1116(22.6) | <.0001 |
| IA therapy only | 163 (40.3) | 175 (13.4) | ||
| IV + IA therapy | 32 (40.5) | 111 (21.6) | ||
| Discharge home | ||||
| IV therapy only | 2329 (55.5) | <.0001 | 1136 (23.0) | <.0001 |
| IA therapy only | 160 (39.6) | 161 (12.3) | ||
| IV + IA therapy | 35 (44.3) | 95 (18.4) | ||
Abbreviations: IA, intra-arterial; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Rates of IV rt-PA use between 3 and 4.5 hours after symptom onset significantly increased after publication of the AHA/ASA recommendations in 2009; however, this increase began before the publication of the updated guidelines. Of interest, there was also a significantly higher rate of IV rt-PA use in the ≤3 hours group, which began in 2007 also. Because of known time lag for diffusion of innovation, it maybe too early to see additional trends that might be related to the updated guidelines as opposed to those from pre-existing secular trends. The benefit of rt-PA is time dependent, and it is most effective when given in the first 90 minutes after symptom onset.1,2,8 It has been suggested that patients who are eligible to receive IV rt-PA within the 3 hours time window might experience delays in receiving it, possibly because of a reduced sense of urgency after the time window expansion.8,9 However, our data do not support evidence of reduced usage in the ≤3 hours time window in favor of the extended time window. In fact, the rate of increase in IV rt-PA use from 2007 to 2012 was identical for both time windows.
The Food and Drug Administration has yet to approve IV rt-PA use for AIS beyond 3 hours from symptom onset.10 We have shown that community practice is following guideline recommendations regarding the extended time window although its use is much less than in the standard time window. We demonstrated evidence of improved outcomes over time.
We hypothesized that IA treatment would decline because of the expanded IV time window. IA therapy is traditionally used in circumstances where patients are ineligible for IV rt-PA with the most common reason being delay in presentation from symptom onset. IA treatment rates were increased across the observation period, but there was no change in favorable outcomes among those who received IA treatment over time. The more favorable outcomes among those who received IV thrombolysis are likely related to earlier time to treatments or lower initial stroke severity compared with those who received IA reperfusion. We note, however, that the rates of sICH among those receiving IV or IA therapies had reduced significantly over our study period; this maybe the result of a higher number of patients presenting with less severe strokes as defined by NIHSS ,10 later in the study period compared with the beginning.
The Interventional Management of Stroke III (IMS III), Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE), and Local Versus Systemic Thrombolysis for Acute Ischemic Stroke Expansion (SYNTHESIS) trials reported in early 2013 that IA therapies are not superior to traditional interventions. It was suggested that the prolonged time to treatment from symptom onset maybe the reason for the inability to show improved rates of disability and mortality despite the ability to recanalize occluded vessels.11-13 This may lead to an increased trend in administering IV rt-PA within the 3-4.5 hours window.
Study strengths are the large number of patients and the multistate data from a variety of hospitals gathered during regular care delivery rather than a clinical trial. A weakness includes outcome assessment by clinical features rather than formal scales such as the mRS score. However, it has been shown that discharge destination could act as a surrogate for mRS score and have high predictive values of determining rates of death and disability.14 Baseline NIHSS scores were only recorded in 55.2% of patients in our cohort, which causes difficulties in ascertaining baseline disability in a minority of patients; however, the rate of reporting NIHSS scores in our cohort continued to increase substantially over time.
Conclusion
We conclude that guideline recommendations on IV reperfusion after AIS in an expanded time window are slowly being adopted into community practice. The rates of thrombolysis in the original ≤3 hours window did not decrease in response to the recommended time window expansion. The increased IV use has coincided with increased rates of IA reperfusion. Future studies can examine further trends in IV and IA treatments for AIS now that recent studies have shown nonsuperiority of IA therapies to traditional interventions.
Acknowledgments
This work was supported by the Minnesota Stroke Registry, part of the Paul Coverdell National Acute Stroke Registry: Centers for Disease Control and Prevention U58 DP000857 and Institutional Review Board 0803E29268. K.L. has been supported by National Institutes of Health grant K23NS051377.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The authors do not report any conflicts of interest for this article.
References
- 1.del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITSISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.de los Rios la Rosa F, Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke. 2012;43:1591–1595. doi: 10.1161/STROKEAHA.111.645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George MG, Tong X, McGruder H, et al. Paul Coverdell National Acute Stroke Registry Surveillance four states, 2005-2007. MMWR Surveill Summ. 2009;58:1–23. [PubMed] [Google Scholar]
- 7.George MG, Tong X, Yoon PW. Use of a registry to improve acute stroke care–seven states, 2005-2009. MMWR Morb Mortal Wkly Rep. 2011;60:206–210. [PubMed] [Google Scholar]
- 8.Pitt M, Monks T, Agarwal P, et al. Will delays in treatment jeopardize the population benefit from extending the time window for stroke thrombolysis? Stroke. 2012;43:2992–2997. doi: 10.1161/STROKEAHA.111.638650. [DOI] [PubMed] [Google Scholar]
- 9.Asplund K, Glader EL, Norrving B, Eriksson M. Effects of extending the time window of thrombolysis to 4.5 hours: observations in the Swedish stroke register (riks-stroke) Stroke. 2011;42:2492–2497. doi: 10.1161/STROKEAHA.111.618587. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler LW, Jovin TG. Intravenous recombinant tissuetype plasminogen activator in the extended time window and the US Food and Drug Administration: confused about the time. Stroke. 2012;43:2517–2519. doi: 10.1161/STROKEAHA.112.670554. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. New Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. New Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


