Abstract
Objective
To explore the relationship of PET/CT parameters with HPV status of oropharyngeal (OP) and oral cavity (OC) squamous cell carcinomas (SCC).
Material and Methods
We retrospectively reviewed 39 patients with OC and OP SCC who underwent staging 18F-FDG PET/CT. PET/CT parameters were measured for the primary tumor and the hottest involved node, including maximum, mean, peak standardized uptake values (SUV max, SUV mean, SUV peak), metabolic tumor volume (MTV), total lesion glycolysis (TLG), standardized added metabolic activity (SAM), normalized standardized added metabolic activity (N SAM). Patient characteristics compared between HPV positive (HPV+) and negative (HPV−) groups. ROC analysis was used to dichotomize PET/CT parameters into high and low. Logistic regression models predicting HPV status were fit for each PET/CT parameter.
Results
The HPV+ group was comprised of 18 patients all with OP SCC; the HPV− group consisted of 21 patients, 4 OP cancer patients and 17 OC cancer patients. The HPV+ group had a higher proportion of N2 stage (94% vs 43%; p<0.001). Nodal PET/CT parameters were higher in the HPV+ group (p<0.01), this difference was not present for the primary lesion. After adjusting for sex and age, the association of higher nodal SUV max (OR 9.67), SUV mean (OR 10.48), SUV peak (OR 9.67), MTV (OR 14.52), TLG (OR 11.84) and SAM, N SAM (OR 16.21) with HPV+ status remained statistically significant (p<0.05).
Conclusion
Nodal PET/CT parameters predict HPV status. High nodal FDG uptake should raise suspicion for positive HPV status in the evaluation of the primary lesion.
Introduction
Squamous cell carcinoma of head and neck (SCCHN) is the sixth most common cancer with more than 50,000 new cases diagnosed each year1–3. Tobacco use, alcohol consumption and Human Papilloma virus (HPV) infection are the best-known risk factors associated with SCCHN4–5. With the decrease in smoking prevalence in the past decade, there has been a decline in the incidence of oral cavity, laryngeal and hypopharyngeal carcinomas in the United States6, however, there has been a steady increase in incidence of HPV+ oropharyngeal squamous cell carcinomas (OP SCC)7. The typical HPV+ OP SCC patient is a white male, non-smoker or infrequent smoker, and is younger than the average HPV− OP SCC patient. HPV associated OP SCC has a better prognosis than the non-HPV associated OP SCC4–5. There has been substantial research evaluating biological and clinical behavior of HPV+ OP SCCs, although research regarding imaging characteristics has been limited.
18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG- (PET/CT) imaging has been increasingly used in the assessment of SCCHN. PET/CT provides both functional and anatomic information that offers significant improvement in diagnostic accuracy both in the pretreatment and post treatment setting. Functional data from PET/CT can also provide quantitative information which can be used as biomarkers that may have prognostic and diagnostic value8–14. SUV max, the most commonly utilized PET parameter, is the maximum SUV for a voxel in VOIPET. SUV mean is the average SUV of all the voxels in VOIPET15. SUV peak is the local average of a 1 ml spherical volume centered on SUV max and has been proposed to be less impacted by image noise and more reproducible than SUV max because it is not calculated from a single voxel13,16. The role of SUV peak in head and neck carcinomas has not yet been studied13. Metabolic tumor volume (MTV, the volume of the tumor defined by metabolism instead of anatomical imaging) is the volume of VOIPET. Total lesion glycolysis (TLG) is the product of MTV and SUV mean and thus as a marker, incorporates both the tumor’s size and activity9,13,14.
Each of these parameters has limitations in practice. SUV max and SUV peak are subject to partial volume errors in small tumors, where the maximum pixel count in the PET scan varies with the distribution size and shape17,18. SUV mean, MTV and TLG will vary with the method used to determine the VOIPET, whether it be a gradient method as in this work, a contour based on a percentage of the maximum voxel value, or a manually drawn contour. For example, a larger VOIPET around the same tumor will increase the MTV and result in a decrease of SUV mean.
A recently reported measurement, standardized added metabolic activity (SAM), seeks to determine the total metabolic activity above background due to tumor uptake while avoiding partial volume effects and the influence of VOI size12. The goal in calculating SAM is to measure all of the activity due to the tumor in the measurement above the activity concentration of surrounding tissue. While partial volume effects reduce the maximum voxel counts in small tumors, the total counts due to a given tumor should be linear with total activity.
Although OC SCC and OP SCC differ in etiology, cumulatively they form most of HNCs19. The recent rise in number of OC SCCs could be related to misclassification of some of OP SCCs19. In advanced cases referred for PET/CT imaging it can be even more challenging to identify the primary site due to presence of tumor both in OP and OC regions19,20.
The primary aim of this study is to explore the relationship of quantitative PET/CT parameters of primary tumor and regional nodal metastasis with HPV status in OC and OP SCC. If a relationship between nodal PET/CT parameters and HPV status is present, the quantitative characteristics of nodal disease may also be useful to identify the primary site of origin between these two very different but anatomically very close and difficult to separate HNC subtypes.
Methods and materials
Patients
After institutional review board approval for this retrospective investigation, we performed a review of medical charts from January 2008 to March 2013 for treatment naïve patients with biopsy proven HNSCC who had had a baseline PET/CT as well as at least one follow up imaging study (either PET/CT or dedicated neck CT) examination performed in our institution.
Chart review targeted patient information, including any current or prior history of smoking and alcohol use, age at the time of diagnosis, ethnic origin and sex; the primary malignancy subsite, original clinical TNM staging, HPV status of the tumor; dates of treatment and treatment modality (chemotherapy, radiation therapy, surgery, or a combination thereof). Determination of the HPV status of the tumor at our institution was often done in a stepwise manner. The p16 immunohistochemical (IHC) stain was completed first, and when interpreted as positive, HPV 16/18 in situ hybridization (ISH) was subsequently completed to confirm evidence of transcriptionally active virus. When p16 IHC was negative, additional testing with HPV 16/18 ISH was not completed5.
Patients with distant metastatic disease (M1) at initial presentation, less than 18 years of age, or with a history of local and/or systemic therapy, including surgical intervention (excluding biopsy), before the baseline PET/CT were excluded. Patients were also excluded if HPV status was unknown, and in whom target tissues were not discretely separated from adjacent abnormal tissues.
FDG-PET/CT Protocol
All PET/CT studies were performed using one of 4 PET/CT systems available in our department (GE Discovery 690 Elite, 600, ST, or LS; General Electric, Milwaukee, Wis). Patients fasted at least 4 hours before the scan, and were imaged approximately 60 minutes after radiotracer injection. Scans were obtained from skull vertex to mid thigh with arms down. All PET data was reconstructed with and without CT-based attenuation correction. The emission scan lasted for 2–4 minutes for each bed position.
Image Analysis
All PET/CT studies were retrieved from the electronic archival system and reviewed on a MIMvista workstation (software version 6.1; MIM Software Inc) by two board certified radiologists (ATK, AC), with subspecialty training in Nuclear radiology and /Neuroradiology and in Neuroradiology with expertise in head and neck imaging, respectively. PET, and fused PET/CT images were reviewed in multiple planes. Volume of Interest (VOIPET) was determined as the volume of hypermetabolic FDG uptake using a gradient technique from commercially available software (PETedge; MIM Software, Inc.; Cleveland, Ohio).
The imaging biomarker measurements performed were SUV max, SUV mean, SUV peak, MTV, TLG, SAM and N SAM. All SUVs used in this study are normalized to body weight (as opposed to body surface area or lean body mass). Once the primary tumor and/or regional nodal disease were segmented, SUV max, SUV mean, SUV peak, MTV and TLG were automatically calculated by the MIMvista software for the primary lesion and for the hottest lymph node, or if there are many nodes with similar FDG activity, we chose the node with the largest axial dimension (Figure 1,2).
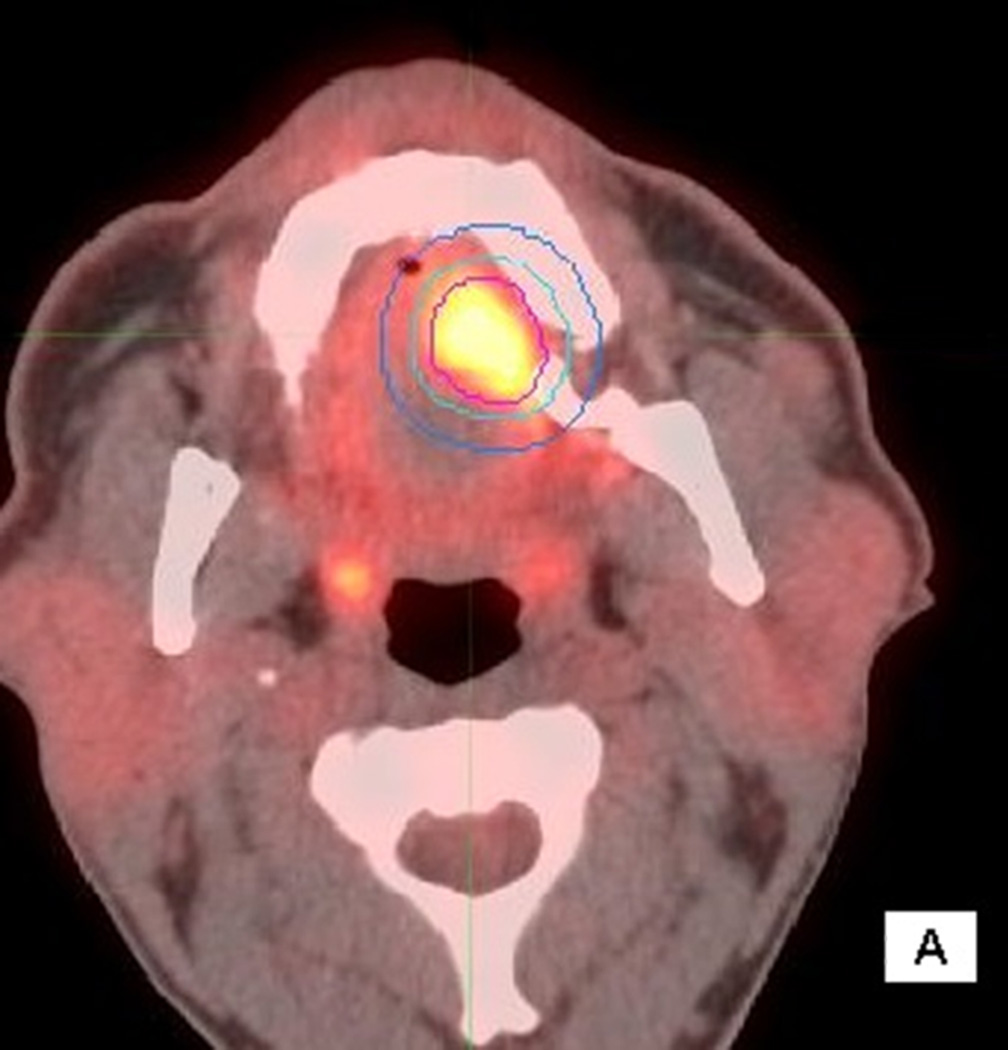
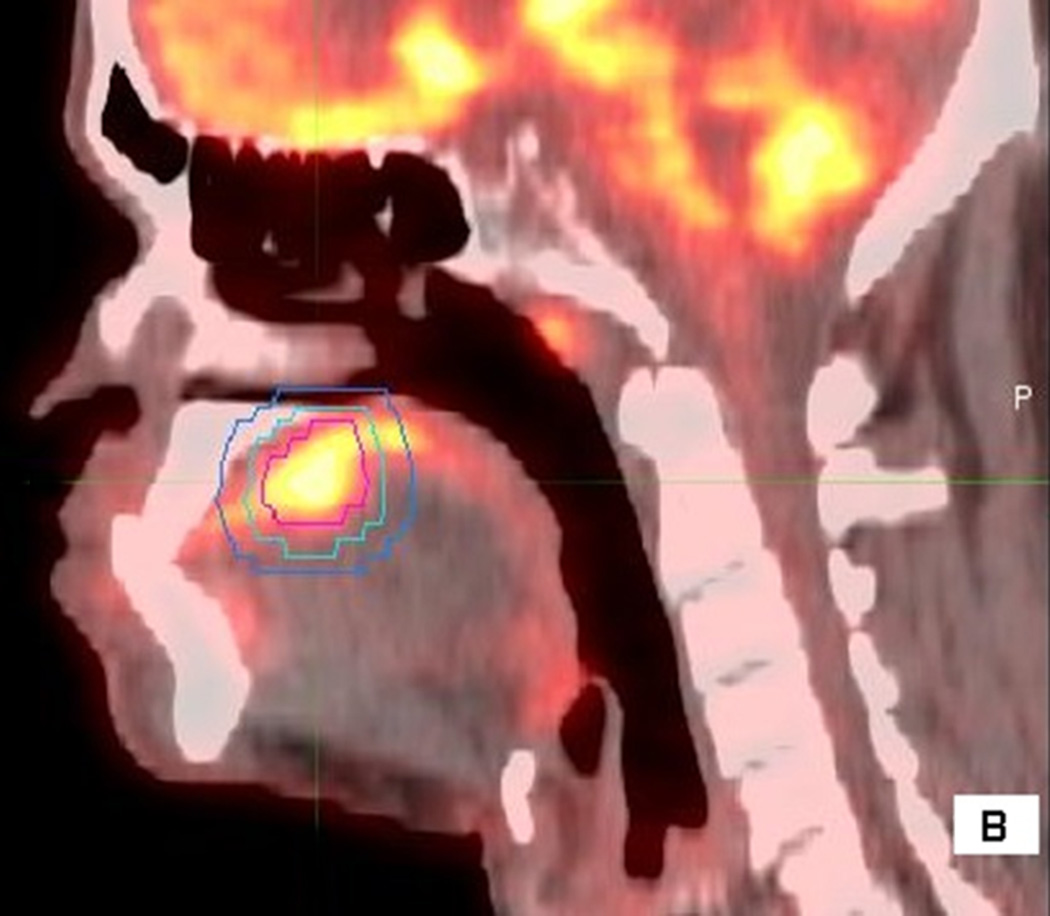
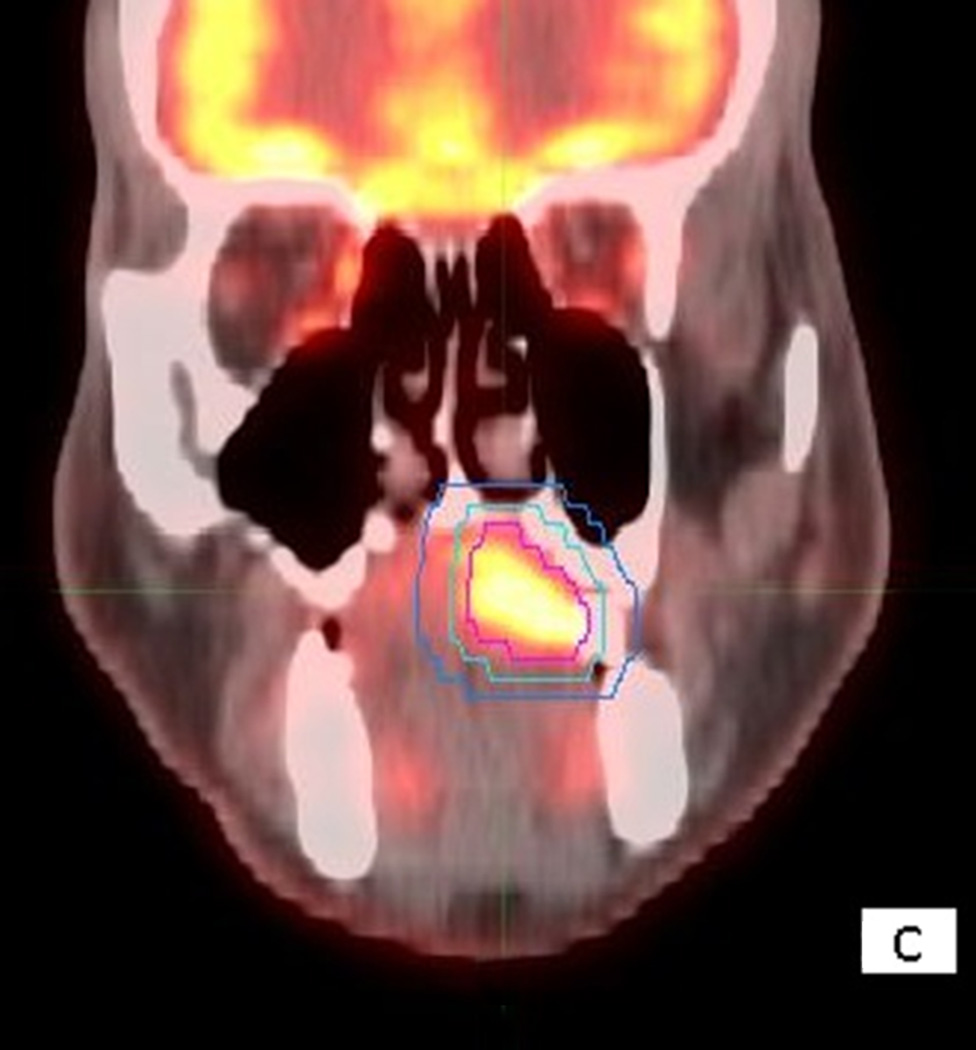
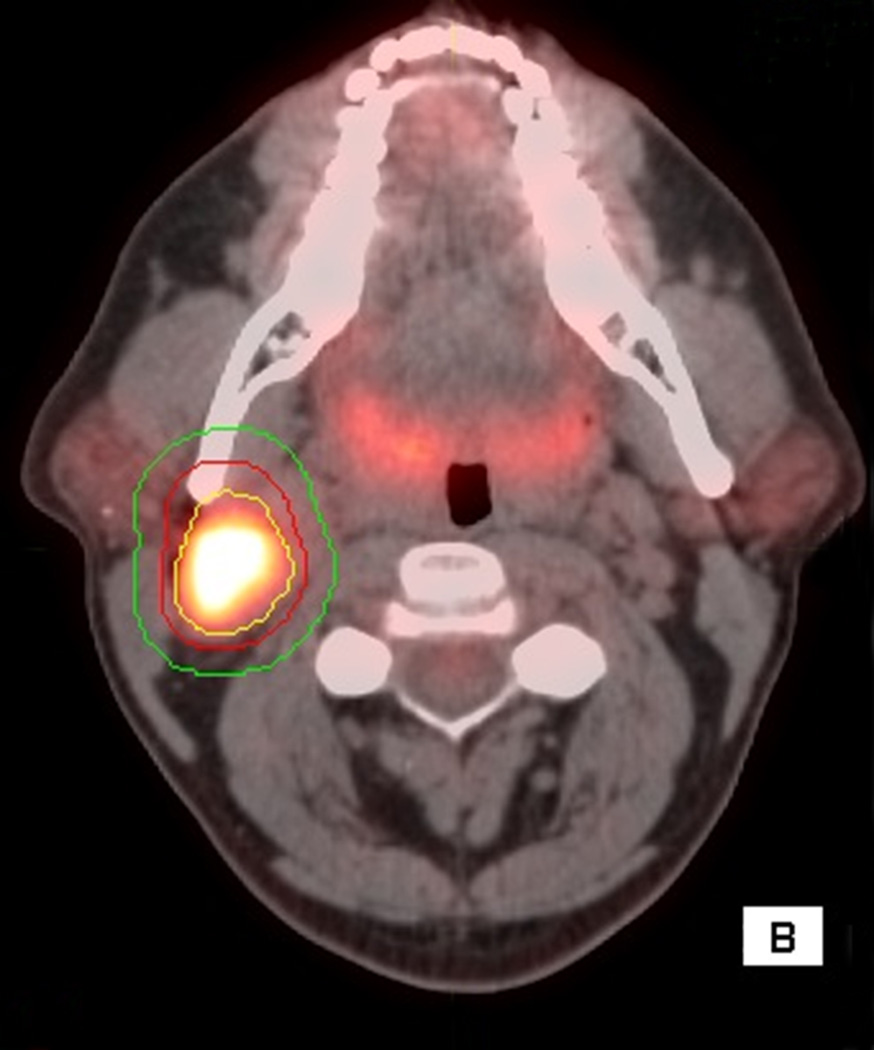
Figure 1.
56 year old male with T4aN2cM0 OC SCC. Axial (1A), sagittal (1B) and coronal (1C) fused PET/CT images. The figure shows the primary VOI (red) and two outer VOIs (light blue and dark blue). SUV max, SUV mean, MTV, and TLG were calculated from VOIPET (defined by a gradient method using the PET uptake, pink contour). SUV peak was centered on the voxel defined by SUV max. SAM was calculated as the total SUVs from VOISAM less the normal tissue SUVs calculated from the volume between VOISAM (light blue) and VOIBKG (dark blue contour).
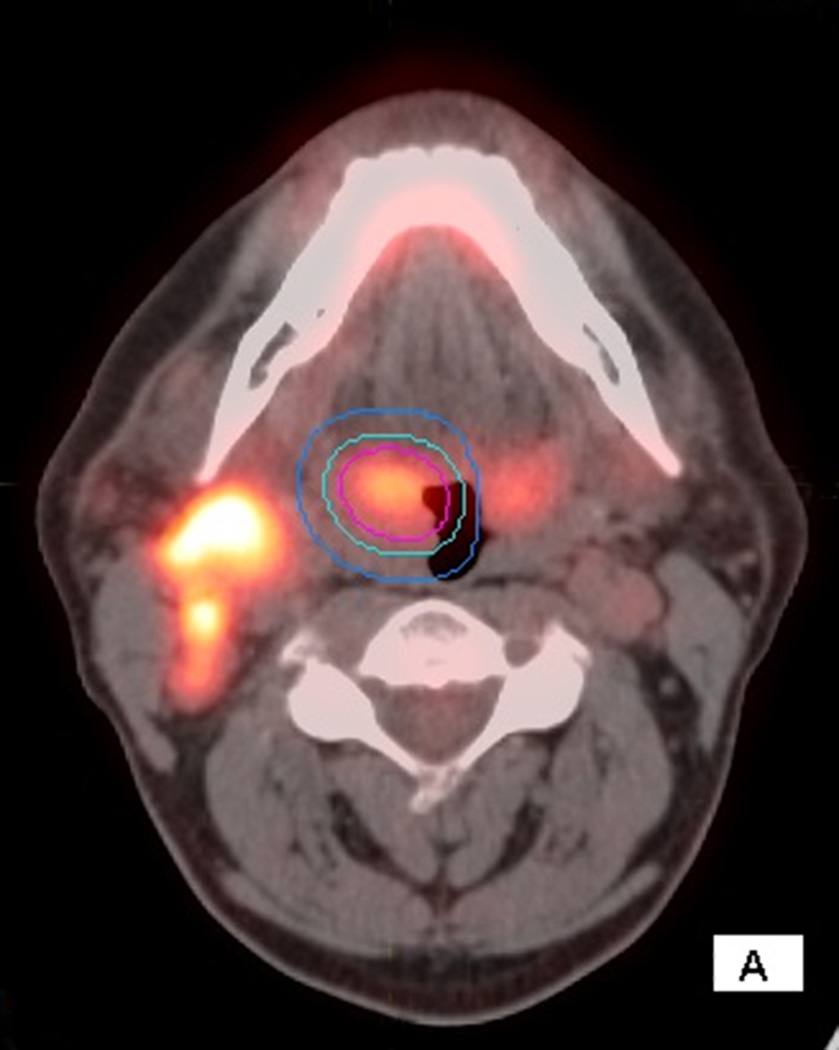
Figure 2.
66 year old male with T2N2c HPV+ OPC. Staging PET/CT with axial fused images show the primary VOI and two outer VOIs around the right tonsil mass (2A) and adenopathy(2B)
To calculate SAM the VOIPET was determined as noted above. A second VOI (VOISAM) was created by expanding VOIPET by 3mm in three dimensions. VOISAM should contain all of the counts due to the tumor but also includes some counts from surrounding tissue. A third VOI (VOIBKG) was created by expanding VOISAM, again by 3 mm, excluding other structures with high activity. Average SUV in the volume between VOISAM and VOIBKG was calculated and used to subtract the SUVs that would be present in VOISAM in the absence of tumor. Thus SAM represents the total SUVs above background for the tumor. Normalized SAM (N SAM) is calculated as SAM/(Average normal tissue SUV) and seeks to remove errors due to some of the factors included in the SUV calculation such as scanner calibration and body weight12.
The nodal PET/CT parameters were considered to be zero for patients with undetectable nodal disease at PET/CT.
Statistical Analysis
Statistical analysis was conducted using SAS Version 9.3. Descriptive statistics were reported for the total sample and also for each HPV group. Differences between groups were assessed using the chi-square or Fisher’s exact test, where appropriate, for categorical variables; and analysis of variance (ANOVA) for numeric variables. PET/CT parameters were first examined as continuous measures. Boxplots were produced comparing the PET/CT parameters between the HPV+ and HPV− groups. In order to categorize the PET/CT parameters into two groups, a receiver operating characteristic (ROC) analysis was conducted to predict HPV status. The cut points that maximized sensitivity and specificity were chosen.
Logistic regression models predicting HPV status were fit separately for each PET/CT parameter. Firth’s penalized maximum likelihood estimation was used to reduce bias in the parameter estimates. Covariates that had a marginally significant association (p-value<0.10) with HPV in unadjusted analysis were included in the models. However, N stage was excluded due to a strong collinearity with the nodal parameters.
Results
Patient characteristics
Thirty-nine patients met the eligibility and inclusion criteria. Patient demographics and tumor characteristics are listed in Table 1. Twenty-eight patients were men, eleven patients were women; the average age was 64 years (SD+/−- 10.3). Thirty-seven patients were white, one patient was African American and one patient was Indian.
Table 1.
Patient Characteristics
| Variable | Level | Total N=39 (%) |
HPV Negative N=21 (%) |
HPV Positive N=18 (%) |
P-value* |
|---|---|---|---|---|---|
| Sex | Female | 11 (28.2) | 9 (42.86) | 2 (11.11) | 0.028 |
| Male | 28 (71.8) | 12 (57.14) | 16 (88.89) | ||
| Smoking | No | 18 (46.2) | 8 (38.1) | 10 (55.56) | 0.276 |
| Yes | 21 (53.8) | 13 (61.9) | 8 (44.44) | ||
| Alcohol | No | 28 (71.8) | 14 (66.67) | 14 (77.78) | 0.442 |
| Yes | 11 (28.2) | 7 (33.33) | 4 (22.22) | ||
| AJCC Stage | 2–3 | 8 (20.5) | 6 (28.57) | 2 (11.11) | 0.247 |
| 4 | 31 (79.5) | 15 (71.43) | 16 (88.89) | ||
| T Stage | T1/T2 | 20 (51.3) | 9 (42.86) | 11 (61.11) | 0.256 |
| T3/T4 | 19 (48.7) | 12 (57.14) | 7 (38.89) | ||
| N Stage | N0/N1 | 13 (33.3) | 12 (57.14) | 1 (5.56) | <.001 |
| N2 | 26 (66.7) | 9 (42.86) | 17 (94.44) | ||
| Tumor Site | Oropharynx | 22 (56.4) | 4 (19.05) | 18 (100) | <.001 |
| Oral Cavity | 17 (43.6) | 17 (80.95) | 0 (0) | ||
| Age | Mean (SD) | 63.95 (10.3) | 68.29 (9.97) | 58.89 (8.45) | 0.003 |
The p-value is calculated by chi-square test or Fisher's exact test for categorical covariates, where appropriate; and analysis of variance (ANOVA) for age.
HPV, human papillomavirus; AJCC, American Joint Committee on Cancer; SD, standard deviation.
10/39 patients were scanned on the GE Discovery 690 Elite in 3D mode, 11/39 on the GE Discovery 600 in 3D mode, 14/39 on the GE Discovery ST in 2D mode, and 4/39 on the GE Discovery LS. The mean patient blood glucose level was 105 (SD±20.65) mg/dl. Patients were injected with a mean of 14.5 (SD±2.25) mCi of 18F-FDG.
Associations with HPV status
Patient characteristics were compared between HPV+ and HPV− patients. The HPV+ group was comprised of 18 OP SCC patients. The HPV− group consisted of 21 patients, 4 OP (4/21), and 17 OC SCC. The HPV+ group had a higher proportion of males (89% vs. 57%; p=0.028) and N2 stage (94% vs. 43%; p<0.001) compared to the HPV− group (Table 1). Those in the HPV+ group were also younger (59 vs. 68; p=0.003). All HPV+ patients were white.
Eleven patients did not have detectable nodal disease at PET/CT. The other 28 patients had both primary and nodal disease.
When comparing the PET/CT parameters between HPV groups, nodal PET/CT parameters (SUV max, SUV mean, SUV peak, TLG, MTV, SAM and N SAM) were significantly higher in the HPV+ group (p<0.01), though this difference was not present for the primary lesion.
Since nodal quantitative values were noted to be significant, an ROC analysis was carried out which demonstrates that with a nodal SUV max cutoff of 7.66, HPV positivity can be predicted with 83% sensitivity and 81% specificity. The cutoff values for all nodal PET/CT parameters and performance estimates are provided in Table 2.
Table 2.
ROC Results for Nodal PET/CT Parameters in the Prediction of HPV Status
| PET/CT parameters | ROC analysis cutoff value |
Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Nodal SUV max | 7.66 | 83 | 81 |
| Nodal SUV mean | 4.47 | 78 | 86 |
| Nodal SUV peak | 5.93 | 83 | 81 |
| Nodal MTV | 2.5 | 94 | 71 |
| Nodal TLG | 9.0 | 89 | 76 |
| Nodal N SAM | 7.8 | 89 | 81 |
| Nodal SAM | 7.7 | 89 | 81 |
Results for tumor PET/CT parameters are not shown since cut off values were not significant predictors of HPV status.
ROC, receiver operating characteristic; HPV, human papillomavirus; SUV, standardized uptake values; MTV, metabolic tumor volume; TLG, total lesion glycolysis; N SAM, normalized standardized added metabolic activity; SAM, standardized added metabolic activity.
After adjusting for sex and age in the logistic regression models, the association of higher nodal SUV max (OR 9.67), SUV mean (OR 10.48), SUV peak (OR 9.67), MTV (OR 14.52), TLG (OR 11.84) and SAM, N SAM (OR 16.21) with HPV+ status remained statistically significant on adjusted analysis (p<0.05) (Table 3).
Table 3.
PET/CT Parameters and Adjusted Odds of HPV+ Status (n=39)
| PET/CT Parameters High vs. Low |
Odds Ratio (95% CI) | P-value |
|---|---|---|
| Tumor SUV max | 0.4 (0.09–1.78) | 0.231 |
| Tumor SUV mean | 0.49 (0.11–2.09) | 0.336 |
| Tumor SUV peak | 0.49 (0.09–2.64) | 0.404 |
| Tumor MTV | 0.52 (0.1–2.75) | 0.439 |
| Tumor TLG | 0.81 (0.17–3.84) | 0.788 |
| Tumor N SAM | 0.13 (0.01–1.23) | 0.075 |
| Tumor SAM | 0.56 (0.11–2.85) | 0.484 |
| Nodal SUV max | 9.67 (1.61–58.03) | 0.013 |
| Nodal SUV mean | 10.48 (1.82–60.36) | 0.009 |
| Nodal SUV peak | 9.67 (1.61–58.03) | 0.013 |
| Nodal MTV | 14.52 (2.05–102.98) | 0.007 |
| Nodal TLG | 11.84 (1.50–93.74) | 0.019 |
| Nodal N SAM | 16.21 (2.09–125.61) | 0.008 |
| Nodal SAM | 16.21 (2.09–125.61) | 0.008 |
Separate models were run for each PET/CT parameter adjusting for sex and age.
HPV, human papillomavirus; SUV, standardized uptake values; MTV, metabolic tumor volume; TLG, total lesion glycolysis; N SAM, normalized standardized added metabolic activity; SAM, standardized added metabolic activity; CI, confidence interval.
Discussion
In our study we evaluated PET/CT parameters from analysis of FDG PET/CT of primary and regional nodal disease. We compared two groups: HPV+ and HPV−. We found that the HPV+ subgroup presents with more advanced N stage and high nodal PET/CT parameters (SUV max, SUV mean, SUV peak, TLG, MTV, SAM and N SAM), though this difference was not present for the primary lesion. After adjusting for sex and age, the association of higher nodal PET/CT parameters with HPV+ status remained statistically significant.
As the incidence of HPV + OP SCC increases, experience with imaging characteristics is also growing. Goldenberg21 demonstrated an association with cystic/necrotic nodal metastasis with HPV+ OP SCCs. Recently Cantrell22 retrospectively reviewed pretreatment CT images of HPV+ and HPV− OP SCC patients and mentioned that HPV+ OP SCCs often had primary lesions with well defined borders and “cystic” nodal metastasis which is a well known reason for false negative results in PET/CT23.
Recently, two publications explored associations between PET/CT findings and tumor HPV status. Joo24 studied 78 patients with OP SCC (28 HPV+ and 50 HPV−) and Tahari25 studied 123 patients (98 HPV+ and 25 HPV−) with OP SCC. Joo24 found that a median SUV max value of 7.1 for the primary can be used as an adjuvant diagnostic tool for high risk HPV infection, with a sensitivity of 78% and specificity of 68%. They also suggested that HPV negativity is associated with higher median SUV max of the primary tumor. Regional nodal disease was not evaluated in their study. Tahari25 also revealed that HPV− primary tumors have higher PET/CT parameters, including morphologic and glycolytic indices. They noted higher PET parameters in HPV+ nodal metastasis, however without statistical significance. In our investigation, we did not demonstrate a significant relationship between quantitative parameters for the primary lesion, but did report a significant relationship for nodal parameters and HPV status. This discrepancy for significance of quantitative parameters for the primary lesion may be due to differences in patient populations in each study. Our finding of advanced nodal disease in HPV+ OP SCCs is also in concordance with recent literature2,26.
HPV/p16 status is routinely determined for OP SCC in our institution but not for non OP SCCHN, as HPV+ SCCHN in sites other than OP is still rare. A recent study by Combes20 mentioned that the fraction of HPV+ OC SCC is about 3%. Due to the possibility of having p16 positive cases in OC SCC, we decided to obtain p16 assessment for our OC study population. None of our OC cases had p16 positivity and they formed the majority of HPV− group in our study.
The OP SCC group of our study was predominantly HPV+. The reason could be related to the fact that HPV+ OP SCC patients mostly present with clinically detectable advanced nodal disease more than HPV− counterparts. Hence PET/CT was mostly preferred as an imaging modality during staging of these patients due to presentation with advanced nodal disease. The other reason for a very high number of HPV+ cases in OP SCC patients in this study could be related to demographics of our patient population. All of OP SCCs patients were white in our study. Most of the OP SCC patients were male. As there is higher proportion of HPV+ OP SCCs among white males, presence of a large number of HPV+ OP SCCs in our study was expected19.
There are recent reports questioning the prognostic value of advanced nodal disease in the era of HPV+ OP SCC27. As the immune system plays an important role in viral oncogenesis, current evidence supports that the improved prognosis in HPV+ cancers is attributable to a nearly intact cell cycle that is mildly disrupted by HPV oncoproteins, and can be easily fixed after exposure to chemoradiation, unlike HPV− tumors6. The viral load is also correlated with higher failure rates, which may suggest impaired immune regulation is a risk factor for poor outcome6. Therefore we suggest that the presence of large nodes with marked FDG activity may not reflect poor prognosis in HPV+ OP SCCs, and in fact could be a sign of a functionally intact and responding immune system with reactive changes, leading to high glycolytic activity to the nodal tissue, resulting in increased glycolytic indices in PET/CT13.
PET/CT parameters should be interpreted cautiously in SCCHN, especially in OP SCC by correlating with the HPV status of the patient. While our study found an association between high nodal PET/CT parameters and HPV+ OP SCCs, this will be only supportive to other findings and needs to be further evaluated.
Assessment of neck nodal disease with clinical carcinoma of unknown primary (CUP) with PET/CT is an accepted approach in clinical practice23. Most of the time the CUP is localized to tonsils, however there is marked variation in identifying the primary site by imaging23. Presence of cystic/necrotic cervical adenopathy is suggestive for HPV+ OP SCC, and this is a common reason for false negatives in PET/CT23. In the absence of cystic/necrotic adenopathy, our finding of high nodal PET/CT parameters, specifically SUV max of 7.66 or higher in association with HPV+ OP SCC may be a useful tool when evaluating CUP cases as well by directing attention to the oropharynx. Although recent literature regarding CUP is somewhat contradictive28,29, Vent28 reported that p16 positive nodal disease in CUP is most likely to be OP SCC.
Limitations of our study include a relatively small patient population, retrospective analysis, as well as heterogeneity of PET scanners. In addition, the HPV− group was mostly composed of OC SCCs. Prospective studies with larger patient populations are needed.
Conclusion
Presence of cervical nodal disease with a median SUV max of 7.66 or higher is suggestive for HPV+ OP SCC. Recognition of regional nodal disease with high nodal FDG activity should not necessarily be interpreted as a bad prognostic factor in head and neck cancer. The benefits of recognizing imaging characteristics of HPV+ cases will become more important as new immunotherapies and de-escalation treatments specific for this patient group are adopted.
In addition, in patients with CUP presenting with advanced nodal disease and high nodal PET/CT parameters attention should be directed to OP sites in the search for the primary tumor as most of HPV+ cases originate from OP sites.
Acknowledgments
Grant Support: Research reported in this publication was supported in part by the Biostatistics & Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Drs. Mark El-Deiry (ME), Trad Wadsworth (TW), from head and neck surgery, Drs. Nabil Saba (NS), Jonathan Beitler (JB), Kristin Higgins (KH) from oncology, Dr. Kelly Magliocca (KM) from pathology, Drs. Amanda Corey (AC), Patricia Hudgins (PH), David Schuster (DS), James Galt (JG) and myself (ATK) from radiology and imaging sciences and Dana Nickleach (DN) from biostatistics & bioinformatics.
All authors contributed to the review of literature, writing and organization of the manuscript.
Authorship statement: ATK, KM, AC, JG, NS, DS, conceived the study, collected data, and reviewed data/literature and writing of the manuscript; NS, JB, KH, ME, TW treated patients with head and neck carcinoma, and reviewed data/literature and writing of the manuscript; PH reviewed data/literature and writing of the manuscript; DN performed statistical analysis/reviewed data/literature and writing of the manuscript..
Contributor Information
K Magliocca, Email: kmagliocca@emory.edu.
A Corey, Email: acorey@emory.edu.
DC Nickleach, Email: dana.nickleach@emory.edu.
J Galt, Email: jgalt@emory.edu.
K Higgins, Email: Kristin.higgins@emory.edu.
JJ Beitler, Email: jjbeitl@emory.edu.
MW El-Deiry, Email: mark.w.el-deiry@emory.edu.
JT Wadsworth, Email: jwadswo@emory.edu.
PA Hudgins, Email: phudgin@emory.edu.
NF Saba, Email: nfsaba@emory.edu.
DM Schuster, Email: dschust@emory.edu.
References
- 1.Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 2.Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, Kim MS. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012;34:10–14. doi: 10.1002/hed.21697. [DOI] [PubMed] [Google Scholar]
- 3.Joo YH, Yoo IeR, Cho KJ, Park JO, Nam IC, Kim MS. Extracapsular spread in hypopharyngeal squamous cell carcinoma: Diagnostic value of FDG PET/CT. Head Neck. 2013;35:1771–1776. doi: 10.1002/hed.23239. [DOI] [PubMed] [Google Scholar]
- 4.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:758–762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 5.Howard JD, Chung CH. Biology of human papillomavirus-related oropharyngeal cancer. Semin Radiat Oncol. 2012;22:187–193. doi: 10.1016/j.semradonc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson E, Li R, Eisele D, Fakhry C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2013 Oct 14; doi: 10.1016/j.oraloncology.2013.09.008. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straetmans JM, Speel EJ, Kremer B. Value of human papillomavirus testing in the diagnostic workup of lymph node metastases from an unknown primary tumor to the neck. Head Neck. 2012;34:1819–1820. doi: 10.1002/hed.23166. [DOI] [PubMed] [Google Scholar]
- 8.Kurland BF, Gersner ER, Mountz JM, et al. Promise and pitfalls of quantitative imaging in oncology clinical trials. Magnetic Resonance Imaging. 2012;30:1301–1312. doi: 10.1016/j.mri.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zazien Y, Azuma K, Kurata S, et al. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur J Radiol. 2012;81:4179–4184. doi: 10.1016/j.ejrad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Murphy JD, La TH, Chu K, et al. Post radiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiation Oncology Biol. Phys. 2011;80:514–521. doi: 10.1016/j.ijrobp.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Lin WY, Kao CH, Yen KY, Chen SW, Yeh JJ. Prognostic value of preoperative metabolic tumor volumes on PET-CT in predicting disease free survival of patients with stage 1 Non small cell lung cancer. Anticancer Research. 2012;32:5087–5092. [PubMed] [Google Scholar]
- 12.Mertens J, Dobbeleir A, Ham H, D’Asseler Y, Goethals I, Van de Wiele C. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastasis. Eur J Nucl Med Mol Imaging. 2012;39:1441–1448. doi: 10.1007/s00259-012-2166-0. [DOI] [PubMed] [Google Scholar]
- 13.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arslan N, Tuncel M, Kuzhan O, et al. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-(18F) fluoro-D-glucose with positron emission tomography in patients with small cell lung cancer. Ann Nucl Med. 2011;25:406–413. doi: 10.1007/s12149-011-0478-y. [DOI] [PubMed] [Google Scholar]
- 15.Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun. 2004;25:651–656. doi: 10.1097/01.mnm.0000134329.30912.49. [DOI] [PubMed] [Google Scholar]
- 16.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography, 1: effect of object size. J Comput Assist Tomogr. 1979;3:299–308. doi: 10.1097/00004728-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Soret M, Bacharach S, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 19.Saba NF, Goodman M, Ward K, Flowers C, Ramalingam S, Owonikoko T, Chen A, Grist W, Wadsworth T, Beitler JJ, Khuri FR, Shin DM. Gender and Ethnic Disparities in Incidence and Survival of Squamous Cell Carcinoma of the Oral Tongue, Base of Tongue, and Tonsils: A Surveillance, Epidemiology and End Results Program-Based Analysis. Oncology. 2011;81:12–20. doi: 10.1159/000330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50:370–379. doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, Califano JA, Tufano RP, Koch WM. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 22.Cantrell SC, Peck BW, Li G, Wei Q, Sturgis EM, Ginsberg LE. Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol. 2013;34:2005–2009. doi: 10.3174/ajnr.A3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxyglucose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am J Neuroradiol. 2010;31:598–604. doi: 10.3174/ajnr.A1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo YH, Yoo IeR, Cho KJ, Park JO, Nam IC, Kim MS. Preoperative 18F-FDG PET/CT and high-risk HPV in patients with oropharyngeal squamous cell carcinoma. Head Neck. 2014;36:323–327. doi: 10.1002/hed.23296. [DOI] [PubMed] [Google Scholar]
- 25.Tahari AK, Alluri KC, Quon H, Koch W, Wahl RL, Subramaniam RM. FDG PET/CT Imaging of Oropharyngeal Squamous Cell Carcinoma: Characteristics of Human Papillomavirus-Positive and -Negative Tumors. Clin Nucl Med. 2014;39:225–231. doi: 10.1097/RLU.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam RM, Alluri KC, Tahari AK, Aygun N. Quon PET/CT Imaging and Human Papilloma Virus-Positive Oropharyngeal Squamous Cell Cancer: Evolving Clinical Imaging Paradigm. J Nuc Med. 2014;55:431–438. doi: 10.2967/jnumed.113.125542. [DOI] [PubMed] [Google Scholar]
- 27.Vila PM, Stucken CL, Morris LG, Posner MR, Genden EM, Boffetta P, Sikora AG. Reduced impact of nodal metastases as a prognostic factor for tonsil cancer in the HPV era. Eur Arch Otorhinolaryngol. 2013 Nov 5; doi: 10.1007/s00405-013-2796-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vent J, Haidle B, Wedemeyer I, Huebbers C, Siefer O, Semrau R, Preuss SF, Klussmann J. p16 expression in carcinoma of unknown primary: diagnostic indicator and prognostic marker. Head Neck. 2013;35:1521–1526. doi: 10.1002/hed.23190. [DOI] [PubMed] [Google Scholar]
- 29.Beadle BD, William WN, McLemore MS, Sturgis EM, Williams WD. P16 expression in cutaneous squamous carcinomas with neck metastasis: A potential pitfall in identifying unknown primaries of the head and neck. Head Neck. 2013;35:127–133. doi: 10.1002/hed.23188. [DOI] [PubMed] [Google Scholar]