Abstract
Insulin-like growth factor (IGF)-1 is associated with a higher risk of prostate cancer. IGF-binding protein (IGFBP)-1, a marker for insulin activity, also binds IGF-1 and inhibits its action. Data on IGFBP-1 and prostate cancer risk are sparse and whether the IGF and insulin axes interact to affect prostate cancer carcinogenesis is unknown. We evaluated the independent and joint influence of prediagnostic plasma levels of IGFBP-1 (fasting) and IGF-1 on risk of prostate cancer among 957 cases and 1021 controls with fasting levels of IGFBP-1 and 1709 cases and 1778 controls with IGF-1 nested within the Health Professionals Follow-up Study. Unconditional logistic regression adjusting for matching factors were used to estimate the odds ratio (OR) and 95% confidence interval (CI). Higher prediagnostic fasting IGFBP-1 levels were associated with lower risk of prostate cancer (highest vs lowest quartile OR=0.67, 95% CI 0.52-0.86, Ptrend=0.003), which remained similar after adjusting for IGF-1. Prediagnostic IGF-1 was associated with increased risk of prostate cancer (highest vs lowest quartile OR=1.28, 95% CI=1.05-1.56, Ptrend=0.01). The associations with each marker were primarily driven by lower-grade and non-advanced prostate cancer. Being low in IGFBP-1 and high in IGF-1 did not confer appreciable additional risk (Pinteraction=0.42). In summary, prediagnostic fasting IGFBP-1 may influence prostate cancer carcinogenesis. Being low in IGFBP-1 or high in IGF-1 is sufficient to elevate the risk of prostate cancer.
Keywords: IGFBP-1, IGF-1, insulin, prostate cancer, risk
Introduction
Prostate cancer is the most commonly diagnosed cancer in the western world. Insulin is involved in homeostatic regulation of glucose and energy metabolism, but also has potent mitogenic and growth-stimulatory effects on the prostate that may contribute to the development of malignancy.1 Since hepatic expression of the insulin-like growth factor-binding protein (IGFBP)-1 is down-regulated by insulin,2 plasma levels of IGFBP-1 are thought to robustly reflect end organ stimulation by insulin.3
In addition to reflecting target tissue insulin exposure, IGFBP-1 binds IGF-1 with high affinity and inhibits IGF-1 action, and thereby decreases free or bioactive IGF-1 levels.4 Although higher circulating IGF-1 levels are consistently associated with increased risk of prostate cancer in epidemiologic studies,5 especially low-grade disease,6 whether the insulin and IGF axes interact to affect prostate cancer carcinogenesis is unknown. Despite the potential importance of IGFBP-1, few studies have examined the association between circulating IGFBP-1 and risk of prostate cancer, with limited sample sizes.7-10
To address these, we examined the association between prediagnostic fasting plasma levels of IGFBP-1 and risk of prostate cancer among men in the Health Professionals Follow-up Study (HPFS), and the joint influence of IGFBP-1 and IGF-1. With 4 more years of follow-up and an additional 445 cases than our prior publication on IGF-1,6 we were also able to investigate the grade- (Gleason score 7 separately from 8-10) and stage-specific associations with more power.
Material and Methods
Study population
The HPFS is a cohort study of 51,529 US male health professionals aged 40-75 years at enrollment in 1986. Participants have been mailed questionnaires every 2 years since baseline to collect data on demographics, lifestyle factors, medical history, and disease outcomes, and every 4 years to report update in dietary intake. The overall follow-up rate was greater than 94% and ascertainment of deaths was more than 98% complete.11 This case-control study is nested in the HPFS blood cohort, which includes 18,018 (35%) participants who provided blood specimen between 1993 and 1995.
Prostate cancers were identified from self-reports on questionnaires or from death certificates and then confirmed by medical record review through 2008. Staging was classified using the TNM staging system. Histological grade was assessed using Gleason scores and summed. We used pathological stage and grade when available and clinical measures if pathological information was not available. Prostate cancer cases with T1a disease were excluded because small volume tumors incidentally detected during benign prostatic hyperplasia surgery are susceptible to detection bias. Biennial follow-up questionnaires were mailed to prostate cancer survivors to collect information on treatment and disease progression (e.g. metastases). Vitality status was assessed via repeated mailings, telephone calls, and searches of the National Death Index. Causes of deaths were confirmed through review of medical records and death certificates. Follow-up for prostate cancer-specific death was complete through March 31, 2012. All reviews were conducted blinded to exposure information.
Eligible controls were participants with an available blood sample, who were alive and free of prostate cancer at the time when the case participant was diagnosed. One randomly selected control was matched to each case on age (year of birth ± 1 year), PSA(Prostate-Specific Antigen) test before blood draw (yes vs no), and timing of blood collection (midnight to before 9 AM, 9 AM to before noon, noon to before 4 PM, and 4 PM to before midnight), season (winter, spring, summer, fall), and year. We also required controls to have a PSA test within 2.5 years before the date of diagnosis of their matched case to allow occult prostate cancers to be diagnosed.
For both cases and controls, we excluded anyone who had cancer (except nonmelanoma skin cancer) before blood draw. For IGFBP-1 analysis, we excluded cases (n=39) and controls (n=51) with diabetes before blood draw because IGFBP-1 levels among diabetics may not reflect their long-term insulin exposure12. We also limited the analysis to fasting samples (≥8 hours since last meal) as fasting IGFBP-1 levels are a good indicator of long-term insulin levels.13 This study was approved by the Human Subjects Committee at the Harvard School of Public Health.
Laboratory assays
The cases and matched controls were identified in five waves of follow-up resulting in five assay batches: blood-draw to 1996, 1996-1998, 1998-2000, 2000-2004 and 2004-2008. Plasma concentrations of IGF-1 and IGFBP-1 were measured in duplicate by ELISA (Diagnostic Systems Laboratory, Webster, TX) in the laboratory of Dr. Pollak and the mean of the replicates was used in the analysis. Cases and their matched controls were analyzed together but in random within-pair order, and laboratory personnel were blinded to case-control status. For IGFBP-1, the mean intrapair coefficients of variation (CV) calculated from blinded quality control samples were 13.2%, 17.2%, 13.1%, 2.2% and 14%, respectively. For IGF-1, the CVs were below 10% for all batches, except for the 1998 to 2000 batch (CV=13.1%). Measurement of other biomarkers included in this analysis (C-peptide and IGFBP-3) was described in detail elsewhere.6,14
Statistical analysis
We used t and Chi-square test to compare continuous and categorical characteristics of cases and controls, respectively. Partial Spearman correlation coefficients adjusted for age at blood draw and batch were used to examine the relationships among the various biomarkers, height, body-mass index (BMI) and vigorous physical activity among the controls. We used unconditional logistic regressions to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of prostate cancer by batch-specific quartiles of fasting IGFBP-1 and IGF-1 defined among the controls, adjusting for matching factors including age at blood draw, PSA test before blood draw, batch and season of blood draw, year and follow-up time. We also ran the corresponding conditional logistic regressions using the matched case and control pairs (707 pairs for IGFBP-1, and 1708 pairs for IGF-1); however, the results were similar, and the estimates from the unconditional models were more stable because of the inclusion of all controls. We therefore present the results for the unconditional models only. Tests for trend were done by treating the median concentration for each quartile as a continuous variable. We also estimated the OR per standard deviation using continuous IGFBP-1/IGF-1 standardized for batch.15 We considered the following potential confounders assessed before blood draw: BMI, family history of prostate cancer, height, history of vasectomy, vigorous physical activity, smoking status, coffee intake, and history of diabetes (only in the IGF-1 analysis). We also ran separate models adjusting for IGF-1 and C-peptide (another marker of insulin14) for the IGFBP-1 analysis, and IGFBP-3 for the analysis of IGF-1.
We assessed the associations between the two markers and prostate cancer by grade and stage. Cases were defined as low (Gleason score 2-6), intermediate (Gleason score 7) or high-grade (Gleason score 8-10), and non-advanced (T1b to T3a and N0M0) or advanced/lethal if participant had regionally invasive or metastatic disease (≥T3b, N1 or M1) at diagnosis or developed metastases or died from prostate cancer during follow-up. We also evaluated whether the associations were different by age at diagnosis (<65, ≥65 years). We tested for heterogeneity using a Chi-square statistic.
We investigated whether any association varied by BMI (<25, ≥25 kg/m2)16, 17 and height (<70, ≥70 inches),17, 18 as well as family history of prostate cancer (yes/no) to explore if association differed by genetic predisposition. Cross-product term of the above factors and continuous IGFBP-1 or IGF-1 were entered into the corresponding model along with the main effect terms and Wald test was used to evaluate interaction.
In the subset of cases and controls with fasting IGFBP-1, we cross-classified IGFBP-1 and IGF-1 to assess their joint association with prostate cancer risk, using the top tertile of IGFBP-1 and the bottom tertile of IGF-1 as the referent group. We assessed interaction by entering a product term of continuous IGFBP-1 and IGF-1, and the P value for interaction was determined by a Wald test.
To consider the possibility of reverse causation that undiagnosed tumor could potentially change IGFBP-1/IGF-1 levels, as sensitivity analysis, we excluded cases diagnosed within the first 2 years after blood draw. For IGFBP-1, we also conducted sensitivity analysis including cases and controls with diabetes before blood draw. All the analyses were performed using SAS v 9.3 (SAS Institute, Cary, NC), and the statistical tests were two-sided.
Results
We included a total of 1,709 men with incident prostate cancer diagnosed between 1993 and 2008 and 1,778 controls in the IGF-1 analysis, and 957 cases and 1,021 controls with fasting levels of IGFBP-1 in the IGFBP-1 analysis (Table 1). Few statistically significant differences of potential confounders were observed between cases and controls. IGFBP-1 was inversely associated with IGF-1 in fasting controls (Table 2). BMI and vigorous physical activity was associated with IGFBP-1 but not IGF-1.
Table 1. Characteristics of prostate cancer cases and controls in the Health Professionals Follow-up Study, 1993-2008.
| IGFBP-1 (fasting)1 | IGF-12 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cases (n=957) | Controls (n=1021) | P3 | Cases (n=1709) | Controls (n=1778) | P3 | |
| Sampling period, No.(%) | ||||||
| Blood draw(1993-1995) to 1996 | 118(12.3) | 123(12.1) | 207(12.1) | 208(11.7) | ||
| 1996-1998 | 151(15.8) | 158(15.5) | 257(15.0) | 257(14.5) | ||
| 1998-2000 | 123(12.9) | 125(12.2) | 229(13.4) | 235(13.2) | ||
| 2000-2004 | 310(32.4) | 361(35.4) | 571(33.4) | 630(35.4) | ||
| 2004-2008 | 255(26.7) | 254(24.9) | 445(26.0) | 448(25.2) | ||
| Age at blood collection (y), mean (SD) | 63.2(8.1) | 63.2(8.0) | 0.92 | 63.2(8.0) | 63.1(8.0) | 0.64 |
| Age at diagnosis (y), mean (SD) | 70.0(7.6) | --- | 70.0(7.4) | --- | ||
| Time to diagnosis from blood draw (y), median (IQR) | 6.5(3.5-9.9) | --- | 6.5(3.5-9.8) | --- | ||
| Family history of prostate cancer, % | 14.3 | 11.5 | 0.06 | 14.0 | 11.3 | 0.02 |
| Personal history of diabetes, % | 0 | 0 | 5.2 | 5.3 | 0.86 | |
| History of vasectomy, % | 29.7 | 29.1 | 0.77 | 28.2 | 29.5 | 0.39 |
| Current smoker, % | 3.8 | 3.2 | 0.52 | 3.6 | 3.4 | 0.68 |
| Height (inch), mean (SD) | 70.2(2.7) | 70.2(2.6) | 0.98 | 70.2(2.7) | 70.2(2.6) | 0.97 |
| BMI (kg/m2), mean (SD) | 25.8(3.3) | 25.8(3.5) | 0.84 | 25.8(3.3) | 25.8(3.4) | 0.63 |
| Vigorous physical activity (MET-hrs/wk), mean (SD) | 12.0(21.2) | 14.2(24.2) | 0.03 | 12.8(22.8) | 13.3(22.4) | 0.52 |
| Coffee intake (servings/d), mean (SD) | 1.9(1.6) | 1.9(1.6) | 0.63 | 1.9(1.6) | 2.0(1.6) | 0.36 |
| Fasting at blood draw, % | 100 | 100 | 59.6 | 61.0 | 0.40 | |
| Plasma IGFBP-1, median (IQR)4 | 18.5(10.5-25.7) | 19.5(11.0-28.4) | 0.01 | 15.6(6.70-25.1) | 15.9(7.43-25.3) | 0.07 |
| Plasma IGF-1, median (IQR)4 | 177(147-211) | 172(142-208) | 0.009 | 178(147-212) | 173(142-208) | 0.003 |
| Plasma IGFBP-3, median (IQR)4 | 3546(3065-4061) | 3445(2932-3956) | 0.001 | 3513(3078-4032) | 3469(2925-3972) | 0.002 |
| Plasma C-peptide, median (IQR)4 | 1.61(1.20-2.22) | 1.59(1.14-2.24) | 0.61 | 1.85(1.27-2.91) | 1.87(1.24-2.96) | 0.20 |
excluded participants with diabetes before blood draw.
189 cases and 195 controls did not have IGFBP-1 measurement, and 12 cases and 13 controls did not have C-peptide measurement.
t-test for continuous variables and Chi square test for categorical variables.
standardized for batch
Table 2. Partial Spearman correlation coefficients between IGFBP-1(fasting), IGF-1 and other variables among controls.
| IGFBP-1 | IGF-1 | IGFBP-3 | C-peptide | Height | BMI | Vigorous physical activity | ||
|---|---|---|---|---|---|---|---|---|
|
Controls in the IGFBP-1 analysis (fasting, n=1021) |
IGFBP-1 | 1.00 | ||||||
| IGF-1 | -0.181 | 1.00 | ||||||
| IGFBP-3 | -0.141 | 0.611 | 1.00 | |||||
| C-peptide | -0.561 | 0.101 | 0.111 | 1.00 | ||||
| Height | -0.081 | 0.071 | 0.04 | 0.071 | 1.00 | |||
| BMI | -0.471 | 0.01 | 0.02 | 0.471 | -0.01 | 1.00 | ||
| Vigorous physical Activity | 0.191 | 0.05 | 0.03 | -0.161 | 0.03 | -0.191 | 1.00 | |
|
Controls in the IGF-1 analysis
2 (n=1778) |
IGFBP-1 | 1.00 | ||||||
| IGF-1 | -0.151 | 1.00 | ||||||
| IGFBP-3 | -0.141 | 0.621 | 1.00 | |||||
| C-peptide | -0.561 | 0.101 | 0.081 | 1.00 | ||||
| Height | -0.061 | 0.051 | 0.003 | 0.07 | 1.00 | |||
| BMI | -0.421 | -0.01 | 0.04 | 0.36 | -0.01 | 1.00 | ||
| Vigorous physical Activity | 0.141 | 0.03 | 0.02 | -0.14 | 0.03 | -0.191 | 1.00 |
P<0.05.
195 controls did not have IGFBP-1 measurement, and 13 controls did not have C-peptide measurement.
Fasting IGFBP-1 was associated with lower risk of total prostate cancer (highest vs lowest quartile OR= 0.67, 95% CI 0.52-0.86, Ptrend=0.003) among non-diabetic men (Table 3). The association remained similar after additionally adjusting for confounders, IGF-1, or C-peptide, indicating the robustness of the inverse association and its independence of circulating IGF-1 and C-peptide levels. The associations were similar when we included cases and controls with history of diabetes (996 cases, 1072 controls; highest vs lowest quartile OR=0.71, 95% CI 0.55-0.91, Ptrend=0.009) or excluded cases diagnosed 2 years within blood draw (830 cases, 1021 controls; highest vs lowest quartile OR=0.66, 95% CI 0.51-0.87, Ptrend=0.004).
Table 3. Plasma IGFBP-1(fasting), IGF-1 and risk of prostate cancer.
| Quartile | Ptrend | OR(95%CI) per SD |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 | Q2 | Q3 | Q4 | |||
| Fasting IGFBP-1 | ||||||
| Ca/Co (957/1021) | 287/253 | 235/256 | 237/258 | 198/254 | ||
| OR(95%CI)1 | 1.00(ref) | 0.81(0.63-1.04) | 0.79(0.62-1.01) | 0.67(0.52-0.86) | 0.003 | 0.88(0.80-0.97) |
| OR(95%CI)2 | 1.00(ref) | 0.79(0.62-1.02) | 0.79(0.61-1.03) | 0.67(0.51-0.89) | 0.01 | 0.89(0.81-0.98) |
| OR(95%CI)3 | 1.00(ref) | 0.81(0.64-1.04) | 0.81(0.63-1.04) | 0.69(0.53-0.90) | 0.01 | 0.89(0.81-0.98) |
| OR(95%CI)4 | 1.00(ref) | 0.77(0.60-1.00) | 0.74(0.57-0.97) | 0.63(0.47-0.85) | 0.004 | 0.87(0.79-0.96) |
| IGF-1 | ||||||
| Ca/Co (1709/1778) | 377/443 | 395/445 | 469/446 | 468/444 | ||
| OR(95%CI) 1 | 1.00(ref) | 1.06(0.87-1.28) | 1.27(1.04-1.54) | 1.28(1.05-1.56) | 0.01 | 1.12(1.05-1.21) |
| OR(95%CI) 2 | 1.00(ref) | 1.05(0.87-1.28) | 1.26(1.04-1.53) | 1.29(1.06-1.57) | 0.005 | 1.12(1.05-1.21) |
unconditional logistic regression adjusted for matching factors: age at blood collection (year), prostate-specific antigen test before blood collection (yes/no), year of blood collection, timing of blood collection (midnight to 9 am, 9 am to before noon, noon to before 4 pm, 4 pm to before midnight), batch (blood collection [1993-1995]-1996, 1996-1998, 1998-2000, 2000-2004, 2004-2008), season (winter, spring, summer, fall) and follow-up time; Quartile cutpoints for IGFBP-1 (ng/mL) were 14.2, 21.9 and 35.0 for batch 1; 15.8, 26.3 and 40.3 for batch 2; 10.6, 19.2 and 30.9 for batch 3; 12.5, 22.6 and 39.1 for batch 4; 2.1, 3.8 and 6.7 for batch 5; Quartile cutpoints for IGF-1 (ng/mL) were 129.5, 168.9 and 209.2 for batch 1; 138.0, 168.3 and 204.8 for batch 2; 153.8, 181.9 and 224.4 for batch 3; 176.3, 210.2 and 256.1 for batch 4; 106.0, 127.5 and 150.4 for batch 5.
in addition to matching factors, adjusted for potential confounders including BMI (<25,25-30, ≥30 kg/m2), history of diabetes (for IGF-1 analysis only), family history of prostate cancer, height, history of vasectomy, vigorous physical activity(quintiles), smoking status (never, past, current, unknown) and coffee intake (quartiles).
adjusted for IGF-1 in addition to matching factors.
adjusted for C-peptide in addition to matching factors.
Plasma IGF-1 levels were positively associated with risk of total prostate cancer (Table 3). ORs of total prostate cancer comparing highest to lowest quartile of IGF-1 were 1.28 (95% CI 1.05-1.56, Ptrend=0.01). When potential confounders were adjusted in addition to the matching factors, the association remained similar. When we additionally adjusted for IGFBP-3, the association for IGF-1 was attenuated (highest vs lowest quartile OR=1.10, 95% CI 0.86-1.40, Ptrend=0.30). After exclusion of the cases in the first 2 years of follow-up, the results were not remarkably different (1493 cases, 1788 controls; highest vs lowest quartile OR=1.32, 95% CI 1.07-1.62, Ptrend=0.009), suggesting that preclinical disease did not influence our results.
Fasting IGFBP-1 was strongly inversely associated with low- and intermediate-grade but not high-grade prostate cancer (Table 4). A statistically significant inverse association was also observed between fasting IGFBP-1 and non-advanced disease but not with advanced/lethal prostate cancer. Higher IGF-1 was significantly associated with increased risk of low-grade prostate cancer, but not intermediate- or high-grade prostate cancer. We also observed significant positive association between IGF-1 and non-advanced prostate cancer, but not with advanced/lethal prostate cancer. Tests of heterogeneity were not significant for the above comparisons (Table 4) and findings should be interpreted with caution because of small sample sizes in high-grade and non-advanced disease.
Table 4. Plasma IGF-1(fasting), IGFBP-1 and risk of prostate cancer by tumor characteristics.
| Quartile | Ptrend | OR(95%CI) per SD | Pheterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Q1 | Q2 | Q3 | Q4 | ||||||||
|
| |||||||||||
| Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | ||||
| Fasting IGFBP-1 | |||||||||||
| Gleason score | |||||||||||
| Low (2-6; 453 cases) | 137/253 | 1.00(ref) | 106/256 | 0.77(0.56-1.05) | 122/258 | 0.86(0.64-1.17) | 88/254 | 0.63(0.45-0.87) | 0.01 | 0.87(0.77-0.98) | 0.81 |
| Intermediate (7; 339 cases) | 108/253 | 1.00(ref) | 87/256 | 0.82(0.58-1.14) | 81/258 | 0.75(0.53-1.05) | 63/254 | 0.61(0.42-0.88) | 0.01 | 0.85(0.74-0.97) | |
| High (8-10; 100 cases) | 29/253 | 1.00(ref) | 25/256 | 0.86(0.49-1.52) | 19/258 | 0.58(0.31-1.08) | 27/254 | 0.80(0.45-1.42) | 0.41 | 0.90(0.72-1.13) | |
| Stage | |||||||||||
| Non-advanced (799 cases) | 247/253 | 1.00(ref) | 195/256 | 0.80(0.62-1.03) | 205/258 | 0.81(0.63-1.05) | 152/254 | 0.61(0.47-0.81) | 0.001 | 0.85(0.76-0.94) | 0.41 |
| Advanced/lethal (119 cases) | 34/253 | 1.00(ref) | 31/256 | 0.87(0.51-1.48) | 24/258 | 0.63(0.36-1.13) | 30/254 | 0.74(0.43-1.29) | 0.26 | 0.92(0.76-1.11) | |
| Age at diagnosis | |||||||||||
| <65 (249 cases) | 91/253 | 1.00(ref) | 58/256 | 0.76(0.49-1.18) | 63/258 | 1.00(0.64-1.57) | 37/254 | 0.70(0.42-1.16) | 0.30 | 0.88(0.73-1.07) | 0.91 |
| ≥65 (708 cases) | 196/253 | 1.00(ref) | 177/256 | 0.80(0.61-1.06) | 174/258 | 0.69(0.52-0.92) | 161/254 | 0.61(0.46-0.82) | 0.001 | 0.85(0.77-0.94) | |
| IGF-1 | |||||||||||
| Gleason score | |||||||||||
| Low (2-6; 816 cases) | 164/443 | 1.00(ref) | 180/445 | 1.10(0.85-1.41) | 239/446 | 1.44(1.13-1.84) | 233/444 | 1.41(1.10-1.81) | 0.002 | 1.17(1.08-1.28) | 0.19 |
| Intermediate (7; 576 cases) | 128/443 | 1.00(ref) | 136/445 | 1.01(0.77-1.34) | 151/446 | 1.10(0.83-1.44) | 161/444 | 1.15(0.87-1.52) | 0.26 | 1.07(0.97-1.18) | |
| High (8-10; 188 cases) | 51/443 | 1.00(ref) | 43/445 | 0.92(0.60-1.42) | 44/446 | 1.01(0.65-1.56) | 50/444 | 1.19(0.78-1.83) | 0.36 | 1.06(0.90-1.24) | |
| Stage | |||||||||||
| Non-advanced (1417 cases) | 297/443 | 1.00(ref) | 323/445 | 1.07(0.87-1.32) | 387/446 | 1.27(1.04-1.56) | 412/444 | 1.35(1.09-1.66) | 0.002 | 1.14(1.06-1.23) | 0.07 |
| Advanced/lethal (207 cases) | 61/443 | 1.00(ref) | 46/445 | 0.83(0.55-1.25) | 57/446 | 1.09(0.73-1.62) | 41/444 | 0.88(0.57-1.37) | 0.83 | 1.01(0.87-1.18) | |
| Age at diagnosis | |||||||||||
| <65 (434 cases) | 52/443 | 1.00(ref) | 71/445 | 0.81(0.52-1.27) | 131/446 | 1.17(0.78-1.76) | 180/444 | 1.25(0.84-1.86) | 0.06 | 1.12(0.98-1.27) | 0.59 |
| ≥65 (1275 cases) | 325/443 | 1.00(ref) | 324/445 | 1.14(0.92-1.41) | 338/446 | 1.36(1.10-1.68) | 288/444 | 1.24(1.00-1.55) | 0.03 | 1.10(1.02-1.19) | |
Note: The models were adjusted for matching factors: age at blood collection, prostate-specific antigen test before blood collection, year of blood collection, timing of blood collection, batch, season and follow-up time.
Both the inverse and positive associations between fasting IGFBP-1, IGF-1 and total prostate cancer were slightly stronger for cases diagnosed at or above age 65 (Table 4). For both markers, we found no interactions with either BMI or height (Table 5 and Supplemental Table 1). For IGF-1, the association with prostate cancer was stronger among men with family history than among those without family history (Pinteraction=0.045).
Table 5. Plasma IGFBP-1(fasting) and risk of prostate cancer by BMI, height and family history of prostate cancer (joint analysis).
| Quartile of fasting IGFBP-1 | Pinteraction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Q1 | Q2 | Q3 | Q4 | ||||||
|
| |||||||||
| Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | Ca/Co | OR(95%CI) | ||
| BMI, kg/m2 | |||||||||
| <25 | 70/57 | 1.00(ref) | 91/91 | 0.81(0.51-1.28) | 119/134 | 0.70(0.46-1.08) | 135/181 | 0.59(0.39-0.90) | 0.13 |
| ≥25 | 217/196 | 0.91(0.61-1.35) | 144/165 | 0.72(0.47-1.09) | 118/124 | 0.76(0.49-1.18) | 63/73 | 0.69(0.42-1.12) | |
| Height, inch | |||||||||
| <70 | 97/78 | 1.00(ref) | 78/82 | 0.77(0.50-1.19) | 83/103 | 0.64(0.42-0.97) | 88/100 | 0.70(0.46-1.06) | 0.80 |
| ≥70 | 190/175 | 0.87(0.61-1.26) | 157/174 | 0.72(0.50-1.05) | 154/155 | 0.78(0.53-1.13) | 110/154 | 0.55(0.37-0.82) | |
| Family history | |||||||||
| No | 253/221 | 1.00(ref) | 197/229 | 0.75(0.58-0.98) | 200/228 | 0.75(0.57-0.97) | 170/226 | 0.64(0.48-0.84) | 0.75 |
| Yes | 34/32 | 0.95(0.56-1.59) | 38/27 | 1.24(0.73-2.11) | 37/30 | 1.08(0.64-1.81) | 28/28 | 0.85(0.49-1.49) | |
Note: The models were adjusted for matching factors: age at blood collection, prostate-specific antigen test before blood collection, year of blood collection, timing of blood collection, batch, season and follow-up time.
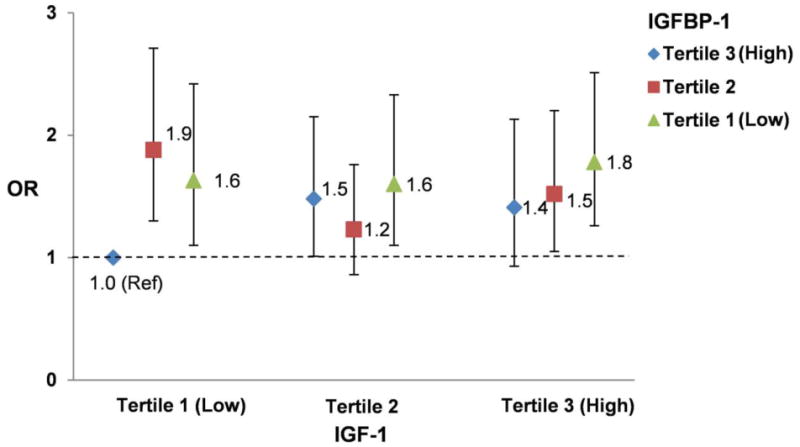
In fasting samples, IGF-1 was similarly associated with prostate cancer as in the total samples after adjusting for matching factors and potential confounders (highest vs lowest quartile OR =1.28, Ptrend=0.04). We did not observe significant multiplicative interaction between fasting IGFBP-1 and IGF-1 levels for their associations with total prostate cancer. Men in the bottom IGFBP-1 and top IGF-1 tertiles had 78% increased risk of prostate cancer compared to men in the top IGFBP-1 and bottom IGF-1 tertiles (OR=1.78, 95% CI 1.26-2.51; Pinteraction=0.42) (Figure 1). Being low in IGFBP-1 or high in IGF-1 was sufficient to elevate the risk of prostate cancer.
Figure 1. Joint association of plasma fasting IGFBP-1 and IGF-1 and risk of prostate cancer.
Discussion
In this large prospective nested case-control study, we found evidence for an inverse association between fasting plasma IGFBP-1 and risk of prostate cancer. The association was independent of circulating IGF-1 and C-peptide, not confounded by known risk factors of prostate cancer, and primarily driven by low- and intermediate-grade and non-advanced prostate cancer than by high-grade and advanced/lethal prostate cancer. With extended follow-up, we also confirmed the positive association between circulating IGF-1 and prostate cancer risk, primarily driven by low-grade and non-advanced prostate cancer. Being low in IGFBP-1 or high in IGF-1 was sufficient to elevate the risk of prostate cancer, and being low in IGFBP-1 and high in IGF-1 did not confer appreciable additional risk.
Previous studies on circulating IGFBP-1 and risk of prostate cancer were conducted in European9, 10 or non-white populations (African, Japanese-American and Latino8 or Chinese7) with mixed findings (two non-significant but inverse associations,7, 10 one null,8 and one positive association9). Limitations of these studies included small number of cases and controls,7, 9, 10 using blood samples collected 4-6 weeks after case diagnosis9 or right before treatment.7
It is unclear whether fasting IGFBP-1 influences prostate cancer development through reflecting insulin levels, modulating IGF-1 activity, and/or exerting independent effects. However, direct epidemiologic evidence linking circulating insulin and prostate cancer risk is limited and mixed: a case-control19 and a recent case-cohort study20 observed positive associations, whereas three other prospective studies observed no association.10, 21, 22 In addition, findings are inconclusive between C-peptide, a marker for insulin secretion, and risk of prostate cancer,14, 23-25 including a recent analysis in HPFS (highest vs lowest quartile OR=1.05, 95% CI 0.82-1.34, Ptrend=0.95) .14
The modulation of IGF-1 activity by IGFBP-1 is complex. In fasting state, IGFBP-1 levels are high due to the low inhibitory effect of insulin and the stimulatory effect of cortisol and glucagon on hepatic IGFBP-1 transcription. Because the affinity of IGFBP-1 for IGF-1 exceeds that of IGF-1 for the type-1 IGF-receptor, high IGFBP-1 levels may reduce IGF-1 available to bind to IGF-1 receptors and thus reduce the insulin-like activity of IGF-1 on peripheral metabolism.12 Preclinical studies also suggest that IGFBP-1 may exert inhibitory effects on cancer cell growth and migration in an IGF-1-dependent manner.4, 26, 27Among a cohort of men participating in a low-fat diet and exercise program, the addition of IGFBP-1 to their baseline serum induced a significant reduction in cell growth and increased apoptosis of LNCaP cell cultures, and IGFBP-1 antagonized the IGF-I growth stimulatory effects on LNCaP cell growth and induced apoptosis.27 Although we observed minimal multiplicative interaction between fasting IGFBP-1 and IGF-1 levels on risk of total prostate cancer, more research of the joint influence of IGFBP-1 and IGF-1, particularly by subtypes of prostate cancer, is needed.
In addition, evidence has emerged showing that IGFBP-1 could activate cell-surface receptors directly, and IGF-1 independent stimulation of integrin-FAK(focal adhesion kinase)-ILK(integrin-linked kinase) -PI3k-Akt constitutes a putative mechanism by which IGFBP-1 modulates insulin sensitivity28, 29 and influences cancer development.30
Our findings on IGF-1 and overall risk of prostate cancer are consistent with a previous meta-analysis,5 The stronger association with low-grade prostate cancer was observed in a pooled analysis of 12 prospective studies,31 and in a recently updated report from EPIC (European Prospective Investigation into Cancer and Nutrition).32 The additional 445 cases than our prior publication 6 allowed us to evaluate Gleason score 7 (intermediate-grade) separately from Gleason score 8-10 (high-grade) and found that the association between IGF-1 and prostate cancer was primarily driven by low-grade disease compared to intermediate and to high-grade prostate cancer although p value for heterogeneity was not significant and power was limited. This finding further supports our previous hypothesis that growth of poorly differentiated cancers may be more autonomous because the PI3k-Akt pathway is constitutively active due to molecular defects (e.g. loss of PTEN, which is associated with higher-grade tumors). Thus, high-grade prostate cancers may be less sensitive to the action of IGF-1 than low-grade cancers.6 In fact, a recent pooling analysis among 2,424 prostate cancer patients also supported that IGF-1 was minimally involved in prostate cancer progression,33 although results from other studies had mixed findings.34, 35 A recent report from a large case-control study from UK and meta-analysis also suggested that IGF-1 was associated with routinely but not PSA detected disease,36 therefore the role of IGF-1 in prostate cancer progression thus could not be completely ruled out and requires further research.
The strengths of this study include its prospective nature and large sample size, strict quality control for measurements of biomarkers, the use of methods to handle absolute differences in the biomarkers among the analytical batches (batch-specific quartiles and continuous measures adjusting for batch), the adjustment for a number of potential confounders, and the ability to investigate associations by Gleason score and tumor stage, as well as the joint association of IGFBP-1 and IGF-1. The study also has some limitations, including a single measurement of the biomarkers, which may not reflect long-term circulating levels, potential measurement errors and Gleason grading errors, and limited power to assess heterogeneity by stage and grade and to evaluate the joint influence of both biomarkers by subtypes of prostate cancer. In addition, lower IGFBP-1 and/or higher IGF-1 may be associated with increased risk of enlarged prostate, which may lead to elevated PSA levels and detection of prostate cancer. However, enlarged prostate in general would make cancer detection harder.
In summary, higher prediagnostic fasting IGFBP-1 levels were associated with lower risk of prostate cancer, whereas higher IGF-1 levels were associated with increased prostate cancer risk. The associations were primarily driven by lower-grade and non-advanced prostate cancer. Since high BMI, sedentary life style and consumption of Western diet lead to low levels of IGFBP-1,37 our findings imply that dietary and lifestyle modifications may play an important role for prostate cancer prevention. Research to better understand the role of IGFBP-1 as well as interaction of the insulin and IGF axes in prostate cancer development, in particular by subtypes of prostate cancer, is warranted.
Supplementary Material
Novelty & Impact Statements.
Data on IGFBP-1 and prostate cancer risk are sparse and whether the IGF and insulin axes interact to affect prostate cancer carcinogenesis is unknown. In a large prospective case-control study, prediagnostic fasting plasma IGFBP-1 levels were inversely associated with risk of prostate cancer. Being low in IGFBP-1 or high in IGF-1 was sufficient to elevate the risk of prostate cancer, and being low in IGFBP-1 and high in IGF-1 did not confer appreciable additional risk.
Acknowledgments
We thank the participants of the Health Professionals Follow-up Study for their continued dedication and commitment to the study. The authors also acknowledge Lauren McLaughlin and other staff of the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. We also thank Yuzhen Tao in the Dr. Pollak's laboratory for running the assays.
Grant sponsor: This work was funded by Public Health Service research grants R01 CA55075, R01 CA141298, and R01 CA133891 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations
- IGF-1
insulin-like growth factor-1
- IGFBP-1
insulin-like growth factor binding protein-1
- HPFS
Health Professionals Follow-up Study
- OR
odds ratio
- CI
confidence interval
Footnotes
Conflicts of interest: none.
References
- 1.Argiles JM, Lopez-Soriano FJ. Insulin and cancer (Review) International journal of oncology. 2001;18:683–7. [PubMed] [Google Scholar]
- 2.Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. The Journal of biological chemistry. 1991;266:18868–76. [PubMed] [Google Scholar]
- 3.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocrine reviews. 1997;18:801–31. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. The Journal of endocrinology. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 5.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. International journal of cancer Journal international du cancer. 2009;124:2416–29. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimptsch K, Platz EA, Pollak MN, Kenfield SA, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor 1 is positively associated with low-grade prostate cancer in the Health Professionals Follow-up Study 1993-2004. International journal of cancer Journal international du cancer. 2011;128:660–7. doi: 10.1002/ijc.25381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chokkalingam AP, Pollak M, Fillmore CM, Gao YT, Stanczyk FZ, Deng J, Sesterhenn IA, Mostofi FK, Fears TR, Madigan MP, Ziegler RG, Fraumeni JF, Jr, et al. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:421–7. [PubMed] [Google Scholar]
- 8.Gill JK, Wilkens LR, Pollak MN, Stanczyk FZ, Kolonel LN. Androgens, growth factors, and risk of prostate cancer: the Multiethnic Cohort. The Prostate. 2010;70:906–15. doi: 10.1002/pros.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Signorello LB, Brismar K, Bergstrom R, Andersson SO, Wolk A, Trichopoulos D, Adami HO. Insulin-like growth factor-binding protein-1 and prostate cancer. Journal of the National Cancer Institute. 1999;91:1965–7. doi: 10.1093/jnci/91.22.1965. [DOI] [PubMed] [Google Scholar]
- 10.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. Journal of the National Cancer Institute. 2000;92:1910–7. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Colditz GA, Giovannucci E, Willett WC. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. American journal of epidemiology. 1990;131:1068–71. doi: 10.1093/oxfordjournals.aje.a115598. [DOI] [PubMed] [Google Scholar]
- 12.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends in endocrinology and metabolism: TEM. 2009;20:153–62. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Bereket A, Wilson TA, Blethen SL, Fan J, Frost RA, Gelato MC, Lang CH. Effect of short-term fasting on free/dissociable insulin-like growth factor I concentrations in normal human serum. The Journal of clinical endocrinology and metabolism. 1996;81:4379–84. doi: 10.1210/jcem.81.12.8954045. [DOI] [PubMed] [Google Scholar]
- 14.Lai GY, Giovannucci EL, Pollak MN, Peskoe SB, Stampfer MJ, Willett WC, Platz EA. Association of C-peptide and leptin with prostate cancer incidence in the Health Professionals Follow-up Study. Cancer causes & control : CCC. 2014;25:625–32. doi: 10.1007/s10552-014-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American journal of epidemiology. 2008;167:653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States) Cancer causes & control : CCC. 2005;16:917–27. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 17.Kajantie E, Fall CH, Seppala M, Koistinen R, Dunkel L, Yliharsila H, Osmond C, Andersson S, Barker DJ, Forsen T, Holt RI, Phillips DI, et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-1 in elderly people: relationships with cardiovascular risk factors, body composition, size at birth, and childhood growth. The Journal of clinical endocrinology and metabolism. 2003;88:1059–65. doi: 10.1210/jc.2002-021380. [DOI] [PubMed] [Google Scholar]
- 18.Signorello LB, Kuper H, Lagiou P, Wuu J, Mucci LA, Trichopoulos D, Adami HO. Lifestyle factors and insulin-like growth factor 1 levels among elderly men. Eur J Cancer Prev. 2000;9:173–8. [PubMed] [Google Scholar]
- 19.Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. Journal of the National Cancer Institute. 2001;93:783–9. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 20.Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, Virtamo J. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. Journal of the National Cancer Institute. 2009;101:1272–9. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard JS, Rohrmann S, Landis PK, Metter EJ, Muller DC, Andres R, Carter HB, Platz EA. Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology. 2004;63:253–8. doi: 10.1016/j.urology.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103:76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 23.Stocks T, Lukanova A, Rinaldi S, Biessy C, Dossus L, Lindahl B, Hallmans G, Kaaks R, Stattin P. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. International journal of cancer Journal international du cancer. 2007;120:2678–86. doi: 10.1002/ijc.22587. [DOI] [PubMed] [Google Scholar]
- 24.Borugian MJ, Spinelli JJ, Sun Z, Kolonel LN, Oakley-Girvan I, Pollak MD, Whittemore AS, Wu AH, Gallagher RP. Prediagnostic C-peptide and risk of prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:2164–5. doi: 10.1158/1055-9965.EPI-07-0495. [DOI] [PubMed] [Google Scholar]
- 25.Lai GY, Helzlsouer KJ, Clipp SL, Rifai N, Platz EA. Association between C-peptide concentration and prostate cancer incidence in the CLUE II cohort study. Cancer Prev Res (Phila) 2010;3:1334–41. doi: 10.1158/1940-6207.CAPR-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nature reviews Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 27.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–24. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 28.Jones JI, Gockerman A, Busby WH, Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10553–7. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang D, Cheung AT, Parsons JT, Bryer-Ash M. Focal adhesion kinase (FAK) regulates insulin-stimulated glycogen synthesis in hepatocytes. The Journal of biological chemistry. 2002;277:18151–60. doi: 10.1074/jbc.M104252200. [DOI] [PubMed] [Google Scholar]
- 30.Perks CM, Newcomb PV, Norman MR, Holly JM. Effect of insulin-like growth factor binding protein-1 on integrin signalling and the induction of apoptosis in human breast cancer cells. Journal of molecular endocrinology. 1999;22:141–50. doi: 10.1677/jme.0.0220141. [DOI] [PubMed] [Google Scholar]
- 31.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, Hsing AW, Lacey JV, Jr, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Annals of internal medicine. 2008;149:461–71. W83–8. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price AJ, Allen NE, Appleby PN, Crowe FL, Travis RC, Tipper SJ, Overvad K, Gronbaek H, Tjonneland A, Johnsen NF, Rinaldi S, Kaaks R, et al. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1531–41. doi: 10.1158/1055-9965.EPI-12-0481-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y, Lindstrom S, Schumacher F, Stevens VL, Albanes D, Berndt S, Boeing H, Bueno-de-Mesquita HB, Canzian F, Chamosa S, Chanock SJ, Diver WR, et al. Insulin-like growth factor pathway genetic polymorphisms, circulating IGF1 and IGFBP3, and prostate cancer survival. Journal of the National Cancer Institute. 2014;106:dju085. doi: 10.1093/jnci/dju085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowlands MA, Holly JM, Hamdy F, Phillips J, Goodwin L, Marsden G, Gunnell D, Donovan J, Neal DE, Martin RM. Serum insulin-like growth factors and mortality in localised and advanced clinically detected prostate cancer. Cancer causes & control : CCC. 2012;23:347–54. doi: 10.1007/s10552-011-9883-8. [DOI] [PubMed] [Google Scholar]
- 35.Rowlands MA, Tilling K, Holly JM, Metcalfe C, Gunnell D, Lane A, Davis M, Donovan J, Hamdy F, Neal DE, Martin RM. Insulin-like growth factors (IGFs) and IGF-binding proteins in active monitoring of localized prostate cancer: a population-based observational study. Cancer causes & control : CCC. 2013;24:39–45. doi: 10.1007/s10552-012-0087-7. [DOI] [PubMed] [Google Scholar]
- 36.Rowlands MA, Holly JM, Gunnell D, Donovan J, Lane JA, Hamdy F, Neal DE, Oliver S, Smith GD, Martin RM. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer research. 2012;72:503–15. doi: 10.1158/0008-5472.CAN-11-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen NE, Appleby PN, Kaaks R, Rinaldi S, Davey GK, Key TJ. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer causes & control : CCC. 2003;14:65–74. doi: 10.1023/a:1022518321634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


