Abstract
3-Hydroxy-4-pyridinones and 5-hydroxy-4-pyrimidinones were identified as inhibitors of catechol-O-methyltransferase (COMT) in a high-throughput screen. These heterocyclic catechol mimics exhibit potent inhibition of the enzyme and an improved toxicity profile versus the marketed nitrocatechol inhibitors tolcapone and entacapone. Optimization of the series was aided by X-ray cocrystal structures of the novel inhibitors in complex with COMT and cofactors SAM and Mg2+. The crystal structures suggest a mechanism of inhibition for these heterocyclic inhibitors distinct from previously disclosed COMT inhibitors.
Keywords: Catechol O-methyl transferase, schizophrenia, cognition, catechol mimic
Schizophrenia is a devastating neurodevelopmental disorder that affects approximately 1% of the population.1 Symptoms of schizophrenia are categorized into positive, negative, and cognitive. Negative symptoms and cognitive impairment are not well-treated with current drugs2 and account for much of the long-term disability associated with the disease.3 Therefore, new treatment options are needed, especially ones that target the negative and cognitive symptoms of schizophrenia.4
In contrast to the increase in midbrain dopaminergic signaling thought to be important in the etiology of positive symptoms,5 impaired dopamine activity in the prefrontal cortex is thought to contribute to the cognitive deficits in schizophrenia.6 One strategy to selectively modulate prefrontal cortex (PFC) dopamine is to take advantage of the differential modes of clearance of dopamine from different brain regions. In the midbrain, there is extensive expression of the dopamine transporter (DAT), which is thought to be primarily responsible for dopamine clearance from the synapse.7 In contrast, cortical regions exhibit only low levels of DAT expression, and dopamine is cleared primarily by enzymatic catabolism of dopamine, with a contribution from the norepinephrine transporter (NET).8,9 The primary enzymes responsible for dopamine catabolism in the PFC are monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT).
While MAO A/B inhibitors are clinically used to treat depression, they show only modest and variable effects on cognition.10 In contrast, COMT has been extensively characterized in terms of its role in modulating cognitive function. The enzyme exists in a membrane-bound (MB-COMT) form and a soluble form (S-COMT), with the MB form predominating in the brain.11 Genetic and pharmacological manipulation of COMT activity has demonstrated positive effects on cognition in rodent12 as well as human studies.13,14 These studies provide validation for COMT inhibition as a promising avenue for treatment of cognitive deficits in schizophrenia, although no distinctions have yet been made with regards to selective MB- or S-COMT inhibition. Both forms of COMT are Mg-dependent and use S-adenosylmethionine (SAM) as the methyl donor, and both forms exhibit selectivity for catechol-containing substrates.
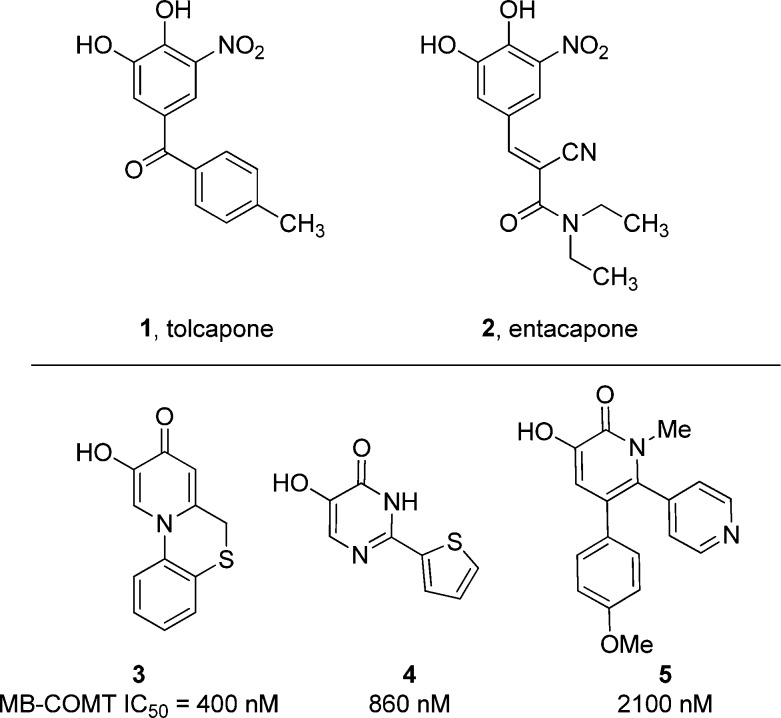
Small molecule inhibition of COMT has been extensively studied in the context of Parkinson’s disease.15,16 The clinically used nitrocatechol agents tolcapone 1 and entacapone 2 (Figure 1) were originally developed to improve the pharmacokinetics of exogenously administered L-DOPA.17 While they are effective in blocking peripheral COMT activity, entacapone has negligible brain penetration, and tolcapone has low but measurable levels in the brain.18 Despite low brain penetration, tolcapone improved cognition in schizophrenic patients in an early phase clinical study.14 However, its association with serious liver injury, including three deaths, and requirement for liver function monitoring during dosing limit its potential as a widespread treatment for schizophrenia.19 Since MB-COMT is the most prevalent isoform in human brain, selective inhibition of MB-COMT has potential for reducing the peripheral side effects of S-COMT inhibition.20−22 Further, MB-COMT is also reported to be overexpressed in schizophrenia patients.21 Therefore, a new potent, selective, and brain-penetrant COMT inhibitor has the potential for widespread usage to treat cognitive deficits in schizophrenia.
Figure 1.
Marketed COMT inhibitors and hits from high-throughput screening. For assay protocol, see refs (23) and (26).
This potential prompted a high-throughput screen of the Merck compound collection using recombinantly expressed MB-COMT.23 This screen identified several classes of compounds related to the Mg2+-binding pharmacophore in tolcapone, including catechols and heterocyclic catechol mimics including 3-hydroxy-4-pyridinones (3), 5-hydroxy-4-pyrimidinones (4), and 3-hydroxy-2-pyridinones (5, see Figure 1). Hits 3–5 are related to weak heterocyclic COMT inhibitors first described in 1973.24 Because of the toxicity risks associated with catechols (potential to form reactive o-quinones)25 and the clinically observed hepatotoxicity of tolcapone, efforts were directed toward optimization of these noncatechol lead structures 3–5, which exhibit improved toxicity profiles versus 1 and 2 (tolcapone and entacapone).26 Specifically, 3–5 exhibit >1000-fold lower potency than tolcapone and entacapone in an assay measuring mitochondrial membrane potential disruption, which has been associated with nitrocatechol hepatotoxicity.26 S-COMT can be crystallized,27 and optimization was guided by a series of X-ray cocrystal structures of the human enzyme in complex with novel inhibitors.
Ring-opening of the thiomorpholine ring embedded in 3 provides N-aryl 4-pyridinone analogues, which retain COMT inhibition, and rapid analogue synthesis in this series was enabled via the condensation reactions shown in Supporting Information Scheme S1. The condensation of amines with a variety of known kojic acid derivatives28,29 proceeded in good yields and enabled the exploration of both the N-substituent as well as the 4-pyridinone ring substituent structure–activity relationship (SAR) in parallel. N-Alkyl and N-aryl derivatives were prepared in the 6-hydroxymethyl-4-pyridinone series (Table 1).
Table 1. Profiles of Compounds 6–18a.
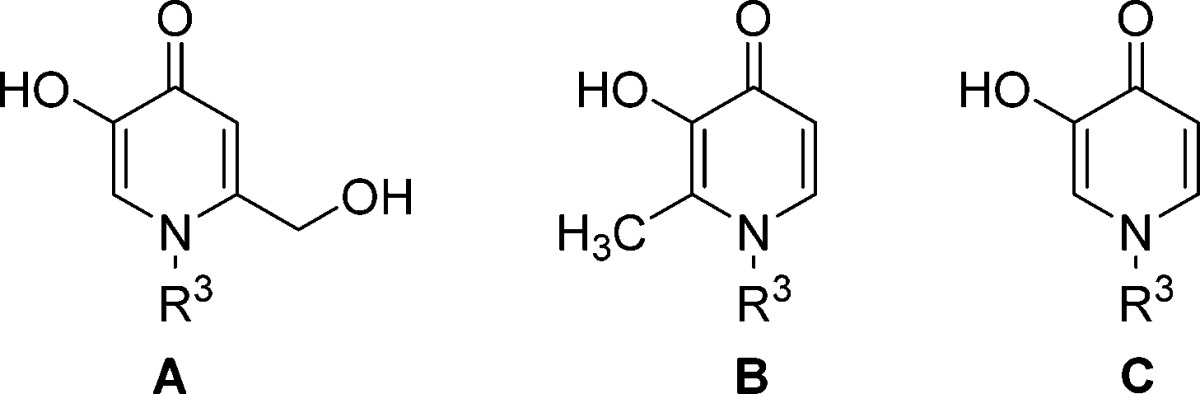
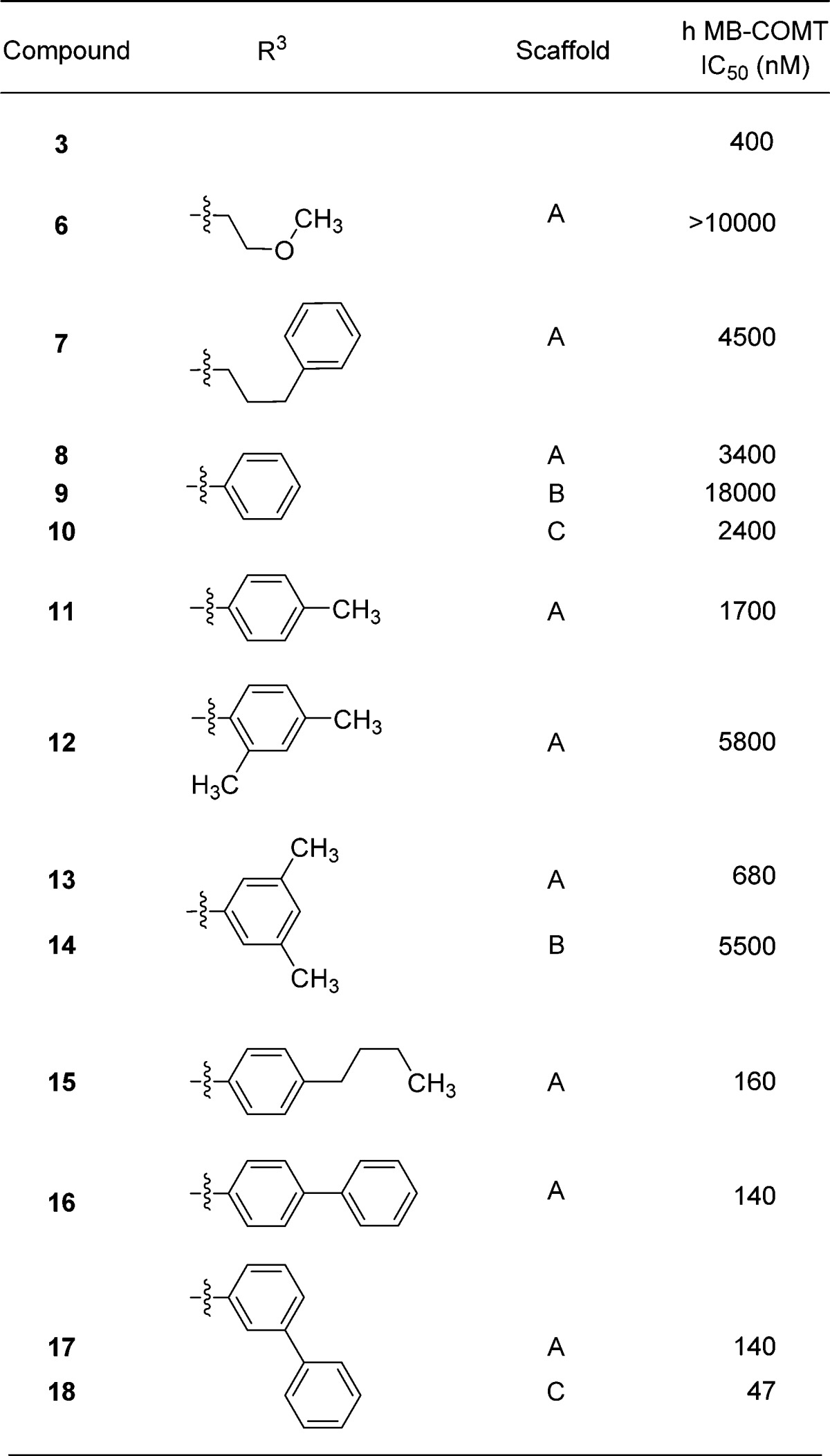
The N-alkyl analogues 6 and 7 lose most or all potency when compared with 3 (Table 1). Among the N-aryl analogues, p-methyl substitution provided an inhibitor approximately equipotent with the parent phenyl (compare 8 and 11), while o-substitution diminished potency (e.g., 12).
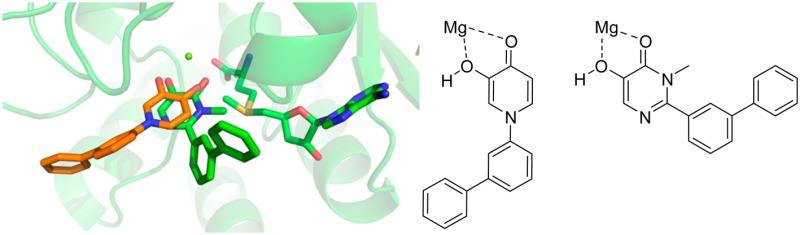
Crystallographic studies with human S-COMT, which confirm the Mg2+-binding properties of the HTS hits and thus their similarity to tolcapone27,30 (Figure 2A), may explain this loss in potency as the structure of 18 suggests that there is little room above and below the plane of the metal-binding pharmacophore to accommodate the o-substituent in 12 due to the presence of Trp 88 and Pro 224 (Figure 2B). Among the methyl-substituted analogues, 3,5-dimethyl 13 was most potent. Larger hydrophobic groups, both aromatic and aliphatic, provide an additional potency boost when incorporated at either meta or para positions (see 15–18). In the case of 18, this may be explained by the formation of hydrophobic contacts between the extended side chain of the inhibitor and residues Trp 88, Cys 223, Leu 248, Arg 251, and Val 253 (Figure 2B). The nature of these contacts differ from those observed with tolcapone30 (Figure 2A), which because of the geometry of the ketone linker between the aryl groups projects into solvent instead of making additional contacts with the protein.
Figure 2.
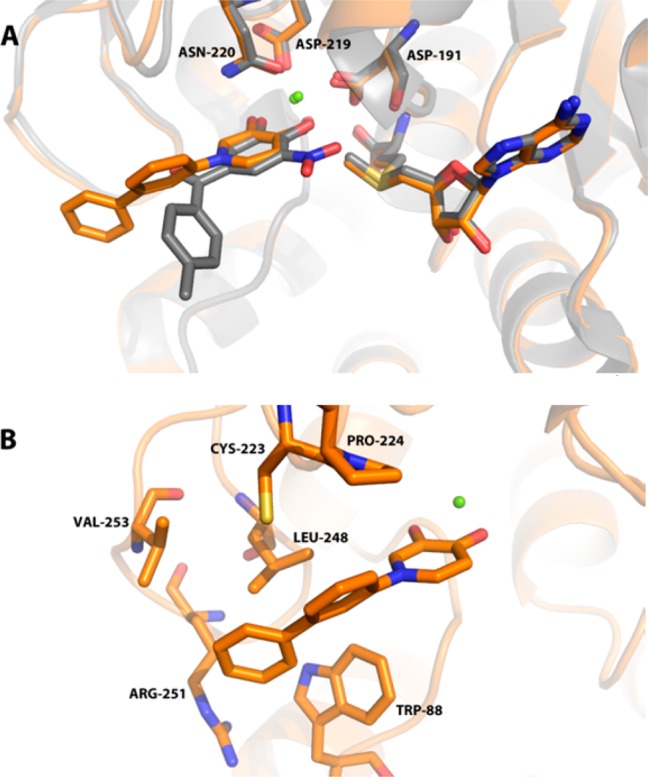
(A) Superposition of the 18/human S-COMT structure (orange, PDB 4XUC) and tolcapone (1)/rat S-COMT structure (gray, PDB 3S68). Human and rat S-COMT exhibit 74% sequence identity overall and 94% sequence identity within the catechol binding site (residues within 6 Å of tolcapone). Inhibitors are shown as sticks, together with Mg2+ (green spheres), the cofactor S-adenosylmethionine (SAM), and residues completing the octahedral complex around the Mg2+. The novel inhibitor 18 binds in an orientation very similar to tolcapone, with the two oxygen atoms of the hydroxypyridinone chelating the active site Mg2+ atom. (B) Detailed view of 18/human S-COMT complex (PDB 4XUC). The distal biaryl group of 18 makes contacts with hydrophobic residues at the edge of the S-COMT active site (shown as sticks).
Among the initial set of analogues prepared, the effect of 4-pyridinone ring substitution depended on the nature and position of the substituent and was similar across the N-aryl groups investigated. The effect on MB-COMT potency varied from minimal (compare 8 and 10) to approximately 8-fold (compare 8 or 10 versus 9 as well as 13 versus 14), and scaffolds A and C were preferred over B. This small data set suggested substitution may not be required for maximal potency, and a larger study of the 4-pyridinone 6-position substituent was undertaken to explore this further.
Analogues varying the 4-pyridinone 6-position substituent were prepared from three routes including condensation of kojic acid derivatives (Supporting Information Scheme S1), nucleophilic addition to the aldehyde (Supporting Information Scheme S2), and de novo 4-pyridinone ring synthesis31 (Supporting Information Scheme S3). These synthetic routes provided analogues whose in vitro characterization is described in Table 2.
Table 2. Profiles of Compounds 19–26a.
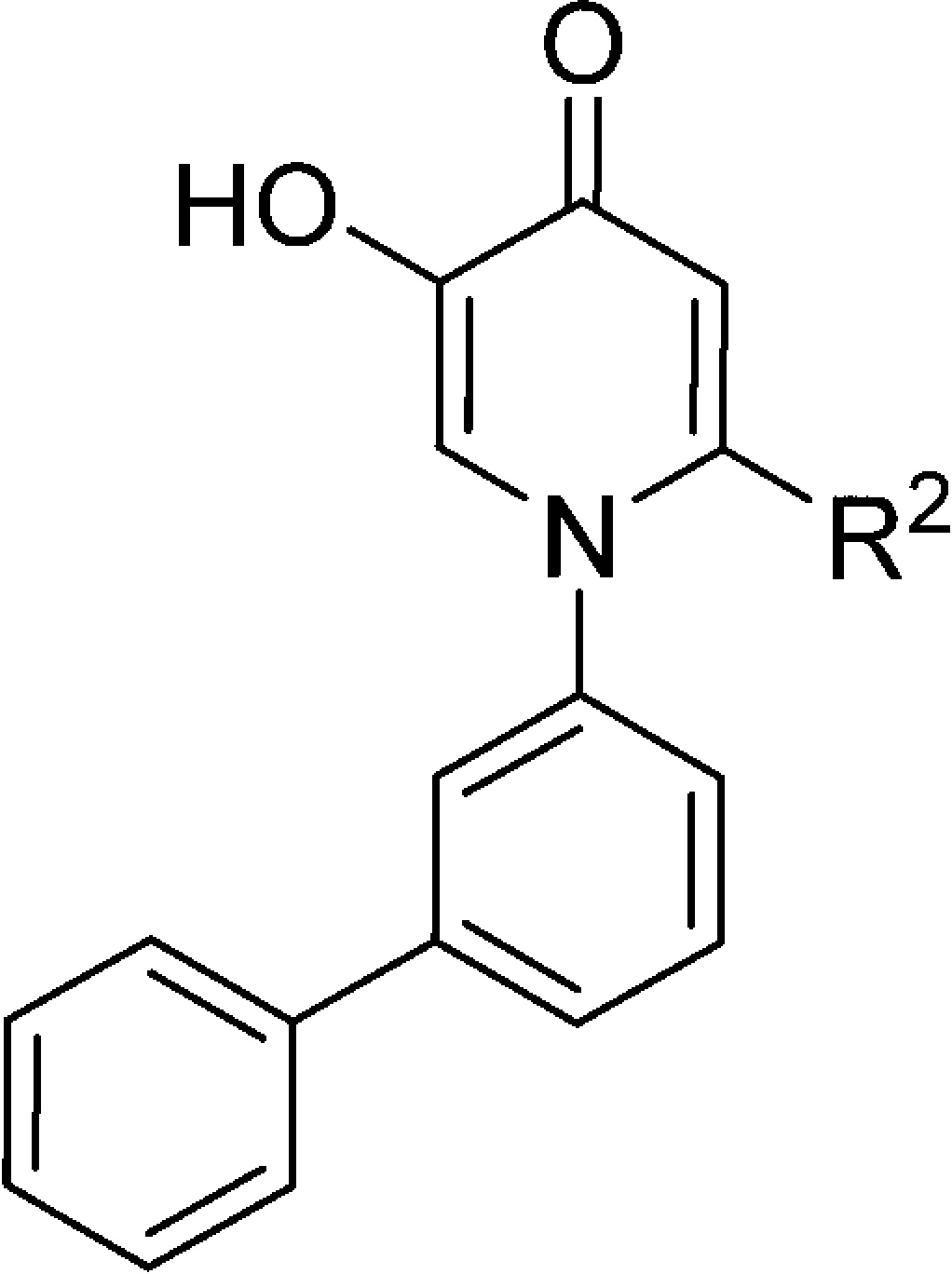
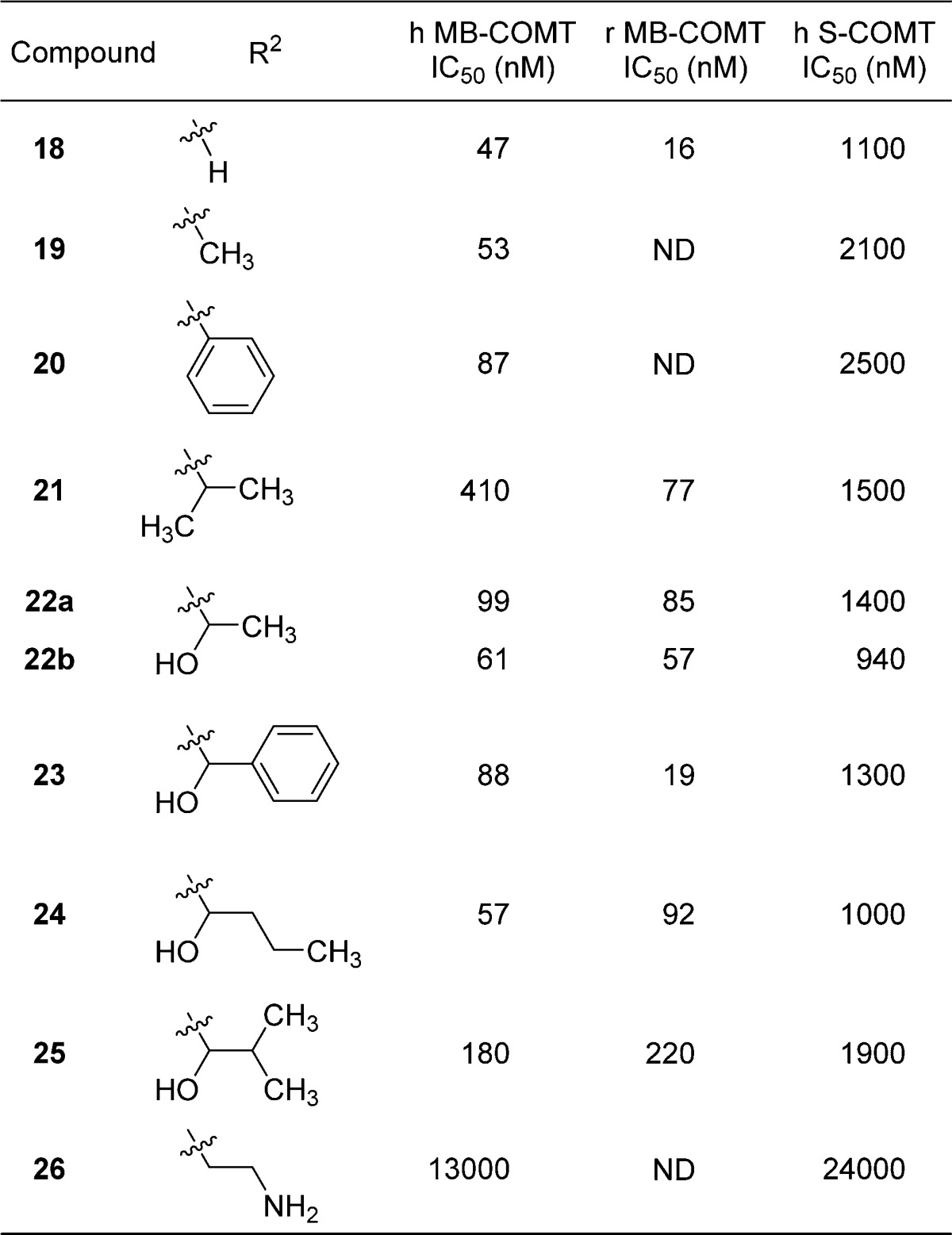
A range of substituents was explored in the 6-position, including no substitution (18), methyl (19), aryl (20), and 1-hydroxyalkyls (22–26). The observed SAR among these analogues is consistent with the shallow and solvent-exposed nature of the binding site and with the observation that 18 would direct 6-position substituents toward solvent in a relatively unencumbered manner. This is consistent with the observation that stereochemistry in the 1-hydroxyalkyls did not seem to affect potency, as enantiomers 22a and 22b exhibit nearly identical IC50 values. The basic amine in 26 was not well tolerated. Generally, rat MB-COMT activity tracked well with human MB-COMT activity, and compounds exhibited a 10- to 20-fold selectivity for human MB-COMT versus S-COMT, something not explained by our crystallographic studies with S-COMT.
A possible origin of MB-/S-COMT selectivity, though speculative, is contact between the transmembrane domain of MB-COMT and the binding site for these inhibitors as suggested by modeling (see Figure 9B in ref (32)). Such interactions could be affected by the nature of the aromatic group projecting from the Mg binder and are consistent with a preference for hydrophobic groups in what appears, from the S-COMT structures, to be a solvent-exposed area.
On the basis of the attractive profile of 18, related analogues 27a and 27b were prepared according to the routes in Supporting Information Scheme S4. Generation of the 4-pyrimidinone was accomplished via selective nucleophilic addition of allyl alcohol, followed by allyl deprotection and methylation (for 27b). Suzuki reaction and methyl ether deprotection with BBr3 provided 27a and 27b.
These structures incorporate a meta-biaryl with a hydroxypyrimidinone-based metal binder similar to that in the HTS lead 4 (Table 3). Unsubstituted pyrimidinone 27a is a substrate of human COMT; its N-Me analogue 27b is not (vide infra) and exhibits potency similar to the hydroxy-4-pyridinone 18 even though their substitution patterns differ, i.e., the biaryl is para to the pyrimidinone hydroxyl group in 27b, while it is meta in 4-pyridinone 18. A difference in the orientation of the aryl substituents in 18 and 27b, rather than an alteration in the orientation of the hydroxyl groups through rotation of the hydroxypyrimidinone scaffold, was observed crystallographically (Figure 3A). Although we cannot discount the effects of crystal packing, this may suggest that hydrogen bonds involving the phenolic group of 27b and residues Asn 220 and Glu 249 (Figure 3B) may help orient inhibitors in the COMT binding site.33
Table 3. Profiles of Compounds 27a, 27b, and 28a.
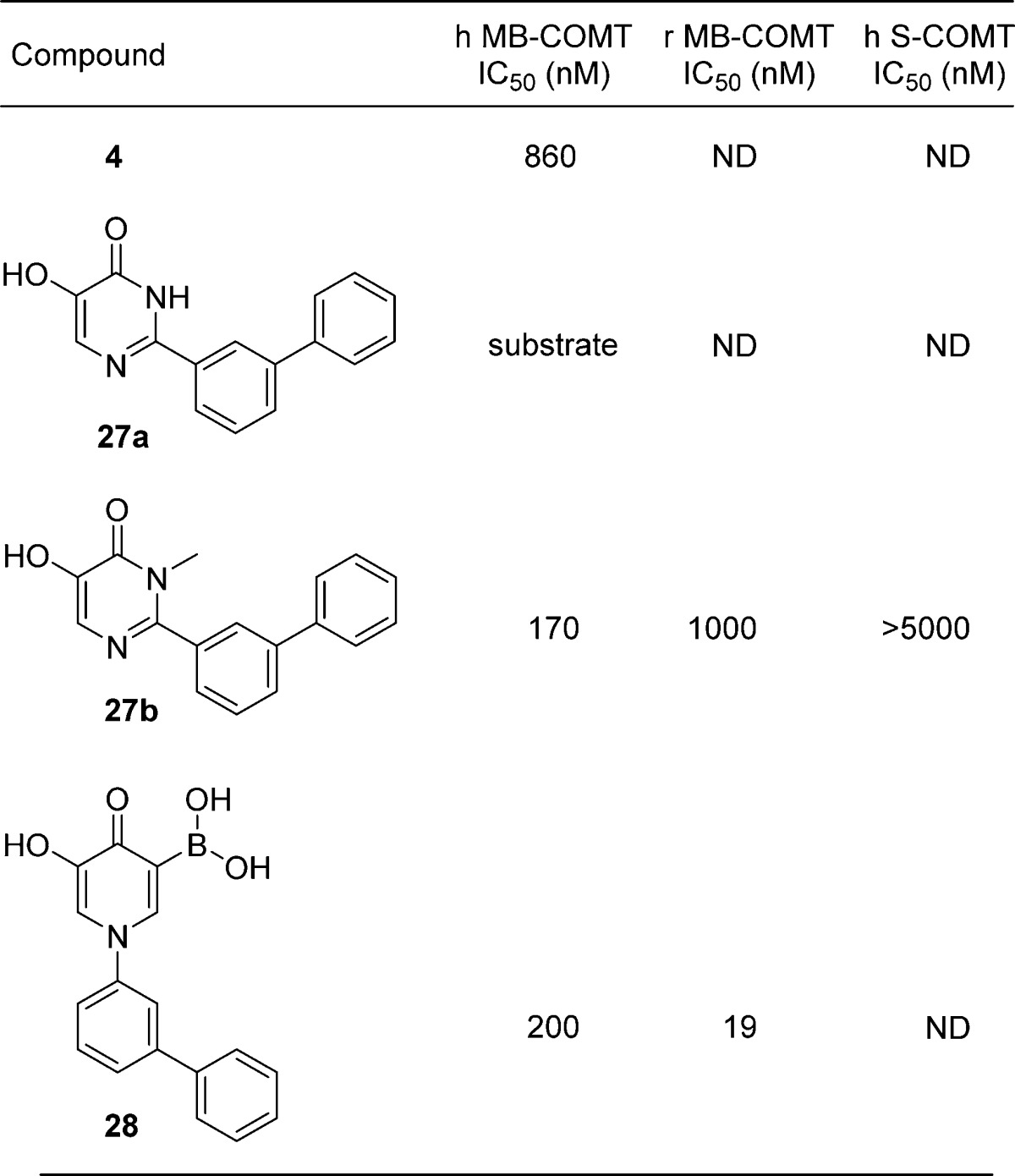
Figure 3.
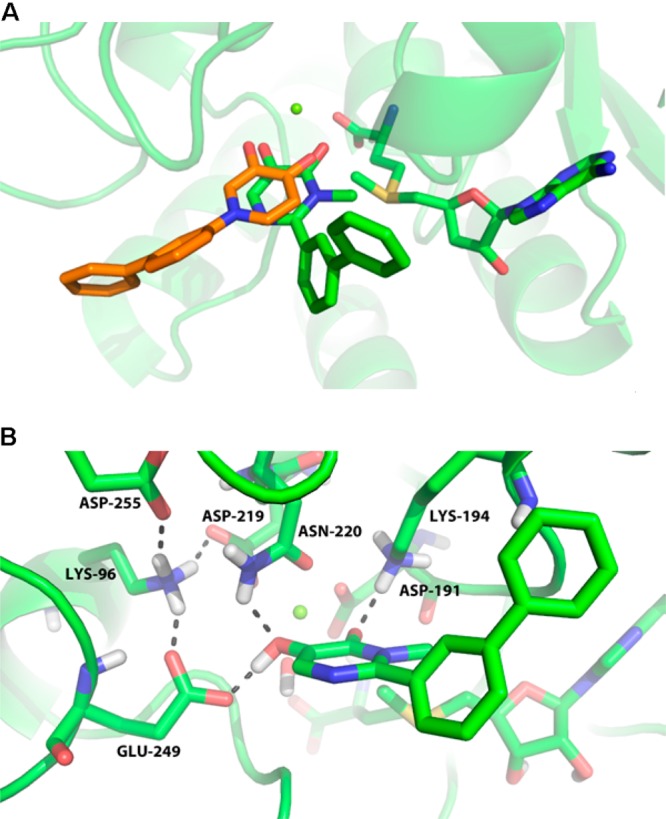
(A) Superposition of the novel heterocyclic inhibitors 18 (orange) and 27b (green) in complex with human S-COMT. As with other inhibitors, 27b chelates the active site Mg2+ (PDB 4XUE). (B) Putative hydrogen bonding network between 27b and S-COMT is shown as black dashed lines (PDB 4XUE). Compound 27b is believed to donate a hydrogen bond to Glu 249 and accept a hydrogen bond from Asn 220. This is thought to result in the biaryl substituent being oriented very differently than in 18 although it affects the active site residues only slightly. Hydrogen atoms were added and optimized using the Schrödinger Protein Preparation Wizard (Suite 2012: Maestro, version 9.3, Schrödinger, LLC, New York, 2012.).
The putative H-bond to Glu249 is significant because it appears to orient 27a and 27b such that the carbonyl oxygen atom in these inhibitors is in close proximity to the methyl group of SAM. This arrangement suggests a rationale for the difference in COMT-catalyzed methylation susceptibility of 27a and 27b since the nucleophilicity of the carbonyl oxygen atoms in 27a and 27b differ. In 27a, the contribution of a dihydroxypyrimidine tautomer renders the oxygen more nucleophilic, and methylation by COMT is observed experimentally. In 27b, the dihydroxypyrimidine tautomer is not accessible since the pyrimidinone N-methyl group maintains the carbonyl oxygen atom in the keto form; this reduces its nucleophilicity and renders the compound a COMT inhibitor and nonsubstrate. This proposed mechanism is consistent with the observed differences between 27a and 27b and is distinct from the nitrocatechols such as tolcapone (1), where O-alkylation does not occur due to the electron-withdrawing nitro substituent.
An additional 4-pyridinone analogue of 18 was prepared incorporating a boronic acid substituent ortho to the metal binding keto group. Synthesis of this analogue (28) from O-benzyl 18 proceeded with selective bromination followed by Mg–bromine exchange, borylation, and deprotection (Supporting Information Scheme S5). The boronic acid in 28 was designed to mimic the nitro group found in numerous COMT inhibitors (for a review of recent applications of boronic acids in medicinal chemistry, see ref (34)) and was found to be tolerated (compare 28 and 18), but not potency-enhancing for human or rat MB-COMT. Our crystallography studies indeed confirm that the boronic acid is isosteric with the nitro group from tolcapone (Figure 4), but we expect its electronic effect to deviate from nitro significantly (Hammett constants σp for −B(OH)2 and −NO2 are 0.12 and 0.78, respectively).35
Figure 4.
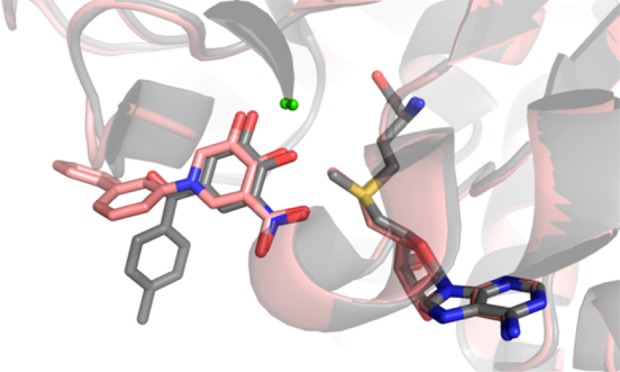
Superposition of 28/human S-COMT structure (pink, PDB 4XUD) and tolcapone (1)/rat S-COMT structure (gray, PDB 3S68). Compound 28 was designed to replace the nitro group found in tolcapone and other nitrocatechol inhibitors with an isostere carrying a reduced risk of toxicity. The boronic acid group of 28 superimposes on the nitro group of tolcapone at the same position, and the O–B–O is coplanar with the aromatic ring. The active site residues are shifted only slightly, suggesting that, in this case, the boronic acid is an acceptable isostere.
In conclusion, the HTS leads 3 and 4 were identified as heterocyclic catechol mimics and exhibit potent inhibition of MB-COMT without the requirement for an aromatic nitro substituent. Optimization of the distal aryl groups in these two series identified biaryl as a preferred group that could be oriented either meta or para to the chelating hydroxyl group of the inhibitors. X-ray cocrystal structures of the novel inhibitors in complex with COMT and cofactors SAM and Mg2+ suggest a mechanism of inhibition for these heterocyclic inhibitors distinct from nitrocatechol COMT inhibitors (enforced keto tautomer of a carbonyl oxygen versus strong electron withdrawing effect adjacent to a deprotonated phenolic oxygen). Further optimization and characterization within these series, from research efforts at Merck and Cerecor, Inc., will be reported in due course.36
Acknowledgments
We gratefully acknowledge Ray McClain, Anna Dudkina, April Cox, and Semhal Berhane for purifying novel analogues and Michael Bogusky and Charles Ross, III for NMR and HRMS determinations. We thank Stephen M. Soisson for assistance depositing crystal structure coordinates.
Supporting Information Available
X-ray crystallography, syntheses, and characterization data for key compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
⊥ Lieber Institute for Brain Development, Johns Hopkins School of Medicine, Room 336, John G. Rangos, Sr., Building, 855 North Wolfe Street, Baltimore, Maryland 21205, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- Saha S.; Chant D.; Welham J.; McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser K. T.; McGurk S. R. Schizophrenia. Lancet 2004, 363, 2063–2072. [DOI] [PubMed] [Google Scholar]
- Mackin R. S.; Delucchi K. L.; Bennett R. W.; Arean P. A. The effect of cognitive impairment on mental healthcare costs for individuals with severe psychiatric illness. Am. J. Geriatr. Psychiatry 2011, 19, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.; Enna S. J. Prospects for neurodegenerative and psychiatric disorder drug discovery. Expert Opin. Drug Discovery 2011, 6, 457–463. [DOI] [PubMed] [Google Scholar]
- Howes O. D.; Kapur S. The dopamine hypothesis of schizophrenia: version III - the final common pathway. Schizophrenia Bull. 2009, 35, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. S.; Castner S. A.; Svensson T. H.; Siever L. J.; Williams G. V. Targeting the dopamine D-1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology 2004, 174, 3–16. [DOI] [PubMed] [Google Scholar]
- Ciliax B. J.; Heilman C.; Demchyshyn L. L.; Pristupa Z. B.; Ince E.; Hersch S. M.; Niznik H. B.; Levey A. I. The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci. 1995, 15, 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaenmaki M.; Tammimaki A.; Myohanen T.; Pakarinen K.; Amberg C.; Karayiorgou M.; Gogos J. A.; Mannisto P. T. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J. Neurochem. 2010, 114, 1745–55. [DOI] [PubMed] [Google Scholar]
- Yavich L.; Forsberg M. M.; Karayiorgou M.; Gogos J. A.; Mannisto P. T. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 2007, 27, 10196–10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohey K. D.; Hieber R.; Nelson L. A. The role of selegiline in the treatment of negative symptoms associated with schizophrenia. Ann. Pharmacother. 2007, 41, 851–856. [DOI] [PubMed] [Google Scholar]
- Reenila I.; Mannisto P. T. Catecholamine metabolism in the brain by membrane-bound and soluble catechol-o-methyltransferase (COMT) estimated by enzyme kinetic values. Med. Hypotheses 2001, 57, 628–632. [DOI] [PubMed] [Google Scholar]
- Tunbridge E. M.; Bannerman D. M.; Sharp T.; Harrison P. J. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J. Neurosci. 2004, 24, 5331–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F.; Goldberg T. E.; Kolachana B. S.; Callicott J. H.; Mazzanti C. M.; Straub R. E.; Goldman D.; Weinberger D. R. Effect of COMT Val(108/158) Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapakis E. M.; Giakoumaki S.; Roussos P.; Chrysoulaki A.; Kopsahili I.; Koudas V.; Bitsios P. One week tolcapone treatment in psychotic patients: effects on gating, working memory and clinical picture. Schizophrenia Res. 2010, 117, 493–493. [Google Scholar]
- Learmonth D. A.; Kiss L. E.; Soares-da Silva P. The chemistry of catechol O-methyltransferase inhibitors. Int. Rev. Neurobiol. 2010, 95, 119–162. [DOI] [PubMed] [Google Scholar]
- Kiss L. E.; Soares-da Silva P. Medicinal chemistry of catechol O-methyltransferase (COMT) inhibitors and their therapeutic utility. J. Med. Chem. 2014, 57, 8692–8717. [DOI] [PubMed] [Google Scholar]
- Bonifacio M. J.; Palma P. N.; Almeida L.; Soares-Da-Silva P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007, 13, 352–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ H.; Muller T.; Woitalla D.; Rahbar A.; Hahn J.; Kuhn W. Detection of tolcapone in the cerebrospinal fluid of parkinsonian subjects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 360, 719–720. [DOI] [PubMed] [Google Scholar]
- Olanow C. W.; Watkins P. B. Tolcapone. Clin. Neuropharmacol. 2007, 30, 287–294. [DOI] [PubMed] [Google Scholar]
- Parashos S. A.; Wielinski C. L.; Kern J. A. Frequency, reasons, and risk factors of entacapone discontinuation in Parkinson disease. Clin. Neuropharmacol. 2004, 27, 119–123. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky H. M.; Cheng K. H.; Faraone S. V.; Wilcox M.; Glatt S. J.; Gao F. M.; Smith C. L.; Shafa R.; Aeali B.; Carnevale J.; Pan H. J.; Papageorgis P.; Ponte J. F.; Sivaraman V.; Tsuang M. T.; Thiagalingam S. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum. Mol. Genet. 2006, 15, 3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Song J.; Yuan P.; Tian Q.; Ji Y.; Ren-Patterson R.; Liu G.; Sei Y.; Weinberger D. R. Orientation and cellular distribution of membrane-bound catechol-o-methyltransferase in cortical neurons: implications for drug development. J. Biol. Chem. 2011, 286, 34752–34760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela M.; Siiskonen A.; Finel M.; Tammela P.; Taskinen J.; Vuorela P. Microplate screening assay to identify inhibitors of human catechol-O-methyltransferase. Anal. Biochem. 2004, 331, 198–200. [DOI] [PubMed] [Google Scholar]
- Borchard R. T. Catechol O-Methyltransferase 4. In-Vitro Inhibition by 3-hydroxy-4-pyrones, 3-hydroxy-2-pyridones, and 3-hydroxy-4-pyridones. J. Med. Chem. 1973, 16, 581–583. [DOI] [PubMed] [Google Scholar]
- Monks T. J.; Jones D. C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug Metab. 2002, 3, 425–438. [DOI] [PubMed] [Google Scholar]
- Robinson R. G.; Smith S. M.; Wolkenberg S. E.; Kandebo M.; Yao L. H.; Gibson C. R.; Harrison S. T.; Polsky-Fisher S.; Barrow J. C.; Manley P. J.; Mulhearn J. J.; Nanda K. K.; Schubert J. W.; Trotter B. W.; Zhao Z. J.; Sanders J. M.; Smith R. F.; McLoughlin D.; Sharma S.; Hall D. L.; Walker T. L.; Kershner J. L.; Bhandari N.; Hutson P. H.; Sachs N. A. Characterization of non-nitrocatechol Pan and isoform specific catechol-O-methyltransferase inhibitors and substrates. ACS Chem. Neurosci. 2012, 3, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler A.; Benz J.; Schlatter D.; Rudolph M. G. Mapping the conformational space accessible to catechol-O-methyltransferase. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2014, 70, 2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. G.; Girard Y.; Rokach J.; Rooney C. S.; McFarlane C. S.; Rackham A.; Share N. N. Kojic amine - a novel gamma-aminobutyric acid analogue. J. Med. Chem. 1979, 22, 99–106. [DOI] [PubMed] [Google Scholar]
- Ozturk G.; Erol D. D.; Aytemir M. D.; Uzbay T. New analgesic and antiinflammatory agents 4(1H)-pyridinone derivatives. Eur. J. Med. Chem. 2002, 37, 829–834. [DOI] [PubMed] [Google Scholar]
- Ellermann M.; Lerner C.; Burgy G.; Ehler A.; Bissantz C.; Jakob-Roetne R.; Paulini R.; Allemann O.; Tissot H.; Grunstein D.; Stihle M.; Diederich F.; Rudolph M. G. Catechol-O-methyltransferase in complex with substituted 3′-deoxyribose bisubstrate inhibitors. Acta Crystallogr. Sect. D: Biol. Crystallogr. 2012, 68, 253–260. [DOI] [PubMed] [Google Scholar]
- Wustrow D. J.; Wierenga W. Synthesis of a 3-acetoxy-6-phenylpyrone and its conversion to a pyrido[1,4]benzodiazepine. Heterocycles 1989, 29, 1721–1728. [Google Scholar]
- Bai H. W.; Shim J. Y.; Yu J.; Zhu B. T. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem. Res. Toxicol. 2007, 20, 1409–1425. [DOI] [PubMed] [Google Scholar]
- Vidgren J.; Svensson L. A.; Liljas A. Crystal structure of catechol O-methyltransferase. Nature 1994, 368, 354–358. [DOI] [PubMed] [Google Scholar]
- Trippier P. C.; McGuigan C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. Med. Chem. Commun. 2010, 1, 183–198. [Google Scholar]
- Hansch C.; Leo A.; Taft R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar]
- Cerecor Acquires Rights to Merck COMT Inhibitors. http://www.cerecor.com/news-publications/news-3-20-2013.php.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.