Abstract
Spiropyrazolopyridone 1 was identified, as a novel dengue virus (DENV) inhibitor, from a DENV serotype 2 (DENV-2) high-throughput phenotypic screen. As a general trend within this chemical class, chiral resolution of the racemate revealed that R enantiomer was significantly more potent than the S. Cell-based lead optimization of the spiropyrazolopyridones focusing on improving the physicochemical properties is described. As a result, an optimal compound 14a, with balanced in vitro potency and pharmacokinetic profile, achieved about 1.9 log viremia reduction at 3 × 50 mg/kg (bid) or 3 × 100 mg/kg (QD) oral doses in the dengue in vivo mouse efficacy model.
Keywords: Dengue virus (DENV), spiropyrazolopyridones, structure−activity relationship, lead optimization
Dengue fever is a febrile disease caused by dengue virus (DENV), which is transmitted by Aedes aegypti, a mosquito that feeds on humans.1 DENV threatens up to 2.5 billion people in more than 100 endemic countries. According to a World Health Organization (WHO) report,2 there are 50–100 million infections annually (and yet this number could be far underestimated),3 with approximately 500,000 cases of dengue hemorrhagic fever and 22,000 deaths globally.4,5 Despite the clear unmet medical need, currently there is no clinically approved vaccine or antiviral therapy available for treatment of dengue.6−10 Therefore, it is urgent to develop safe and effective therapeutics.
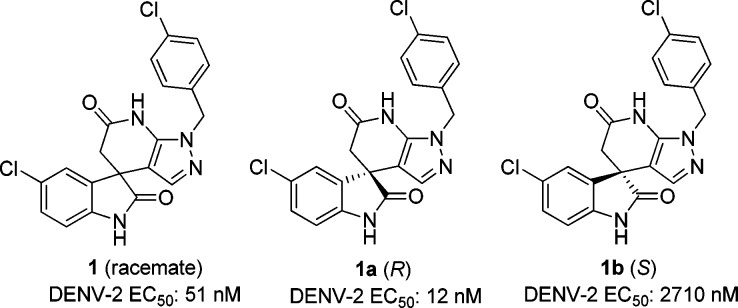
High-throughput phenotypic screening is a powerful tool to identify compounds that are active against DENV by inhibiting either host pathways and/or viral proteins, which are essential for viral replication.11−15 As a result of DENV-2 screening on Novartis compound library, a new chemical class spiropyrazolopyridone 1 was identified to be an interesting starting point for further optimization (Figure 1). As compound 1 was a racemate, two enantiomers were subsequently separated by chiral high-performance purification chromatography (HPLC). The structure of R enantiomer 1a was unambiguously determined by X-ray crystallography (Figure 2). Interestingly, when tested in the DENV-2 in vitro assay, these two individual enantiomers displayed remarkably different potency where R enantiomer 1a was greater than 200-fold more potent than the S enantiomer 1b (Figure 1). Very recently, genetic analysis demonstrated that mutations in dengue viral NS4B conferred the resistance of compound 1a, suggesting that this class of compounds inhibited DENV replication by targeting NS4B protein.11,16−18 In this report, lead optimization efforts to explore the structure–activity relationships (SARs) and improve the physicochemical properties are described.
Figure 1.
DENV-2 in vitro potency of spiropyrazolopyridone 1 and its enantiomers.
Figure 2.
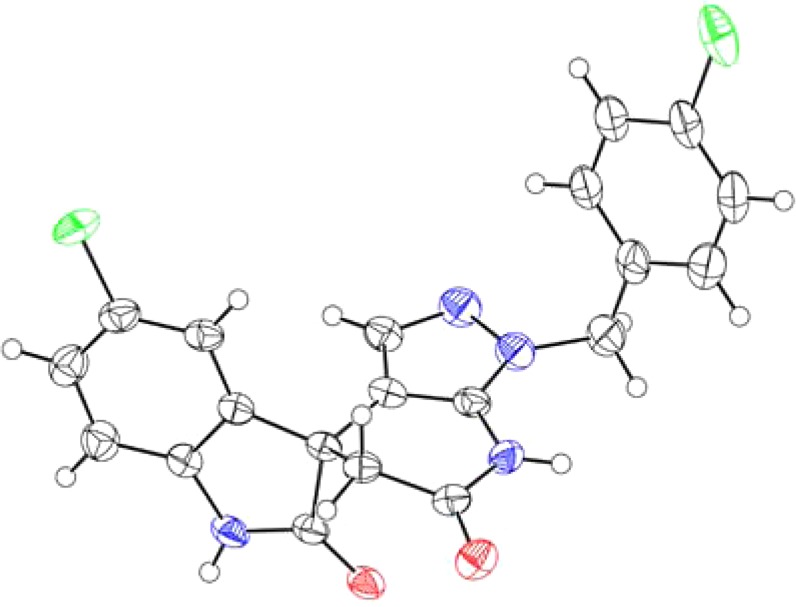
X-ray structure of the R enantiomer 1a.
Following the identification of compound 1, which was subsequently found to be poorly soluble in aqueous media, we initiated a lead optimization effort in an attempt to improve the solubility while maintaining the potency. The general synthetic route to the spiropyrazolopyridone derivatives is outlined in Scheme 1. The synthesis, featuring a three component condensation of aminopyrazoles, isatin derivatives, and Meldrum’s acid, provides the access to a series of spiropyrazolopyridone analogues for SAR investigation.19,20 Since isatin derivatives (9) and Meldrum’s acid (10) are largely commercially available, our main effort to diversify the class was to make aminopyrazole derivatives (6 and 7).
Scheme 1. Synthetic Route to the Spiropyrazolopyridones.
Reagents and conditions: (a) N2H4·H2O, EtOH, room temperature (rt); (b) i. di-tert-butylcarbazate, PPh3, THF, 0 °C; ii. HCl in dioxane, rt; (c) (E)-ethyl 2-cyano-3-ethoxyacrylate 5, EtOH, 80 °C; (d) CH3I, NaH, DMF, 0 °C to rt; (e) i. NaOH, EtOH/H2O, reflux; ii. AcOH, reflux; (f) BH3·Me2S, THF, rt.
Briefly, substituted hydrazine 4 was made either by substitution of chloride 2 with hydrazine or Mitsunobu reaction of the alcohol 3 with di-tert-butylcarbazate followed by deprotection in the presence of acid. Then, condensation of hydrazine 4 with (E)-ethyl 2-cyano-3-ethoxyacrylate 5 in ethanol afforded aminocarboxylate 6,21 which could be methylated to deliver compound 7. Lastly, hydrolysis of the esters 6 or 7 in the presence of NaOH gave the corresponding sodium salts, which were directly used for the three component condensation with substituted isatins 8 and Meldrum’s acid 9 in acetic acid at reflux to afford the spiropyrazolopyridones 1, 10–19, and 22–26. Reduction of the compound 1 in the presence of borane–dimethyl sulfide gave a mixture of compounds 20 and 21, which were separated by chromatography. Finally, those enantiomers mentioned in this report were obtained by chiral HPLC separation of their corresponding racemates.
The structure–activity relationship (SAR) of this spiropyrazolopyridone class is summarized in Table 1. For all the chiral separated pairs (compounds 13a/b, 14a/b, and 23a/b), similarly to what has been described for 1a and 1b, R enantiomers were markedly more potent than S enantiomers. So we believe that this phenomenon is a general trend within this class of compounds.22 As we explored the SAR around the R1 substitution, we found the para-chlorobenzyl was preferred, while meta- or ortho-chlorobenzyl (10 and 11) resulted in a loss of activity on DENV-2. In addition, for the para-benzyl substitutions, electron withdrawing group (13) was superior to electron donating group (12). Interestingly, 3-pyridyl (14) was tolerated, and the R enantiomer 14a maintained a similar level of potency in the secondary viral-titer reduction assay7 (EC50 = 42 nM) with lower lipophilicity (measured LogP = 2.8). However, the other R1 substitutions, such as 2-pyridyl (15), 3,5-pyrimidine (16), cyclohexyl (17), phenyl (18), or homobenzyl (19), resulted in significant loss of potency, suggesting a narrow range of substitutions are tolerated at the R1-position. Next, we started to investigate the SAR around the core modification. Both of the compounds 20 and 21 resulted in a loss of potency, indicating both of the amide carbonyl groups are important for the activity. Furthermore, compounds 22 and 23 with methyl on either of the amides maintained the same level of potency, indicating that neither of the NH is critical for the potency. In fact, R enantiomer 23a was the most potent compound identified so far; however, it suffered with high LogP and poor metabolic stability (Table 2). Finally, SAR around the R4 substitutions was investigated. Though bromo (24) was well tolerated, both electron-withdrawing (25) and electron-donating groups (26) diminished the potency, suggesting again a tight range of tolerated substitutions at this position.
Table 1. Structure–Activity Relationship and Cytotoxicity.
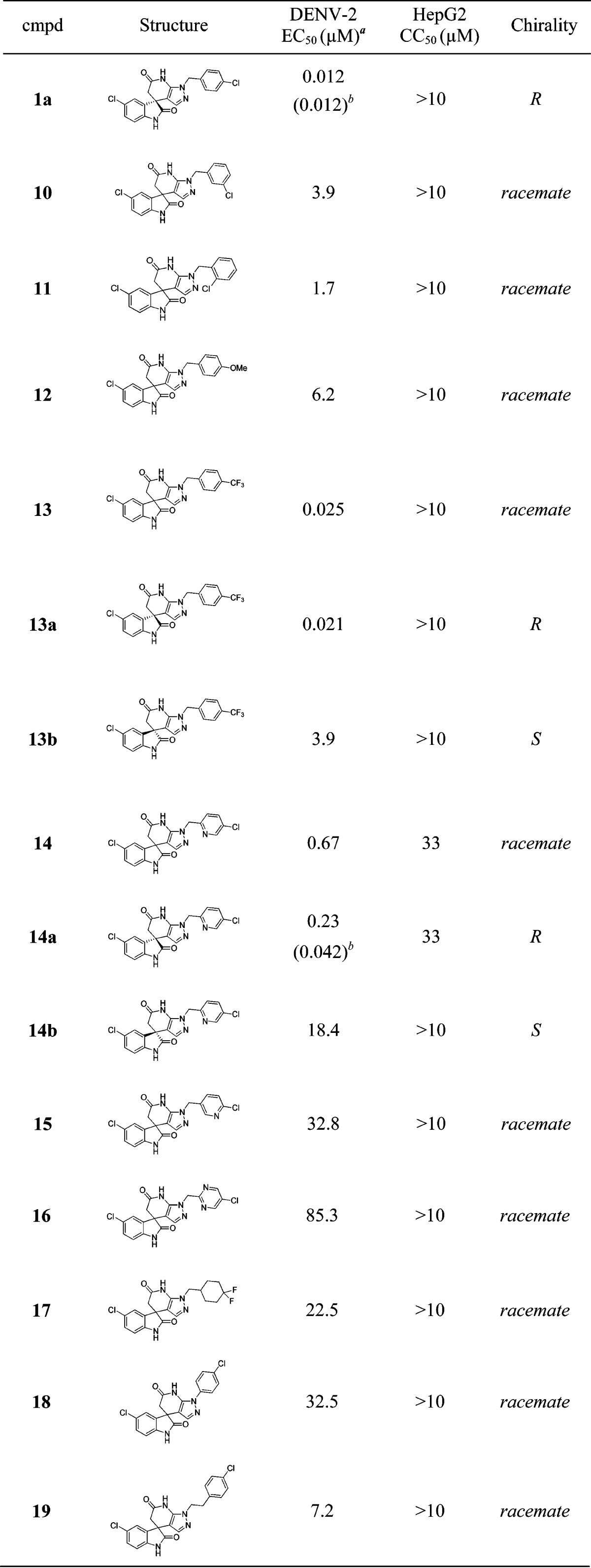
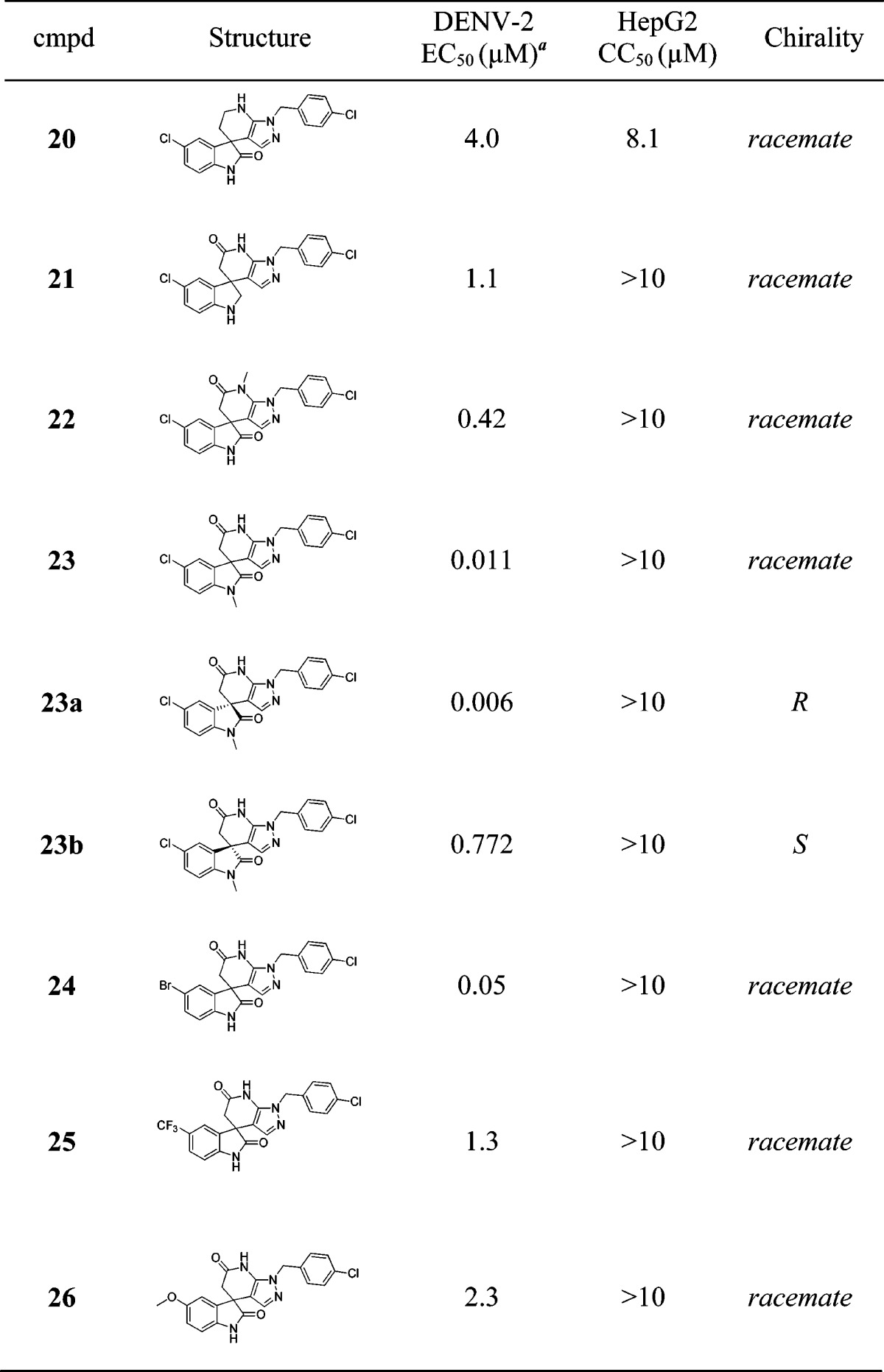
EC50 values are determined in the DENV-2 CFI assay (see Supporting Information).
Determined in the DENV-2 viral-titer reduction assay.7
Table 2. Summary of the Key in Vitro Properties.
| mouse
microsomal stability |
||||
|---|---|---|---|---|
| cmpd | solubility (μM, pH 6.8) | LogP | ERa (%) | T1/2 (min) |
| 1a | 11 | 4.9 | 66 | 31 |
| 13a | 27 | 4.1 | 49 | 62 |
| 23a | 161 | 4.6 | 91 | 5.8 |
| 14a | 504 | 2.8 | 71 | 24 |
Hepatic extraction ratio.
The key physicochemical properties of the representative spiropyrazolopyridones are summarized in Table 2. Compound 13a displayed similar profile to compound 1a with marginal improvement of solubility, while compound 23a suffered from poor metabolic stability with high mouse hepatic extraction ratio and short half-life. Gratifying, compound 14a with lower LogP showed improved solubility, though the microsomal stability was slightly compromised. In order to determine if the improved solubility would translate to a better in vivo PK profile, compounds 1a and 14a were evaluated in rats by oral (p.o.) and intravenous (i.v.) routes at 25 and 5 mg/kg, respectively (Table 3). Indeed, compared to compound 1a, optimized compound 14a showed higher Cmax and exposure (AUC), as well as increased oral bioavailability (63%).
Table 3. Pharmacokinetic Parameters Following 25 mg/kg Oral and 5 mg/kg Intravenous Dosing in Ratsa.
| oral PKb parameters |
intravenous PKc parameters |
||||||
|---|---|---|---|---|---|---|---|
| cmpd | Cmax (μg/mL) | Tmax (h) | AUC (h·μg/mL) | F (%) | Vss (L/kg) | CL (mL/min/kg) | T1/2 (h) |
| 1a | 0.78 | 2.0 | 9.5 | 33 | 4.3 | 15.1 | 3.8 |
| 14a | 3.86 | 0.17 | 13.8 | 63 | 3.0 | 18.8 | 1.3 |
Cmax, maximum concentration of drug in plasma; Tmax, time to maximum concentration of drug in plasma; AUC, area under the curve (t = 0 to 24 h); Vss, volume of distribution at steady state; CL, plasma clearance; T1/2, terminal half-life; F, absolute oral bioavailability.
Formulation for oral dose: suspension of 0.5% Tween80 and 0.5% methylcellulose in phosphate buffer, pH 6.8.
Formulation for intravenous dose: 10% NMP and 90% rat plasma.
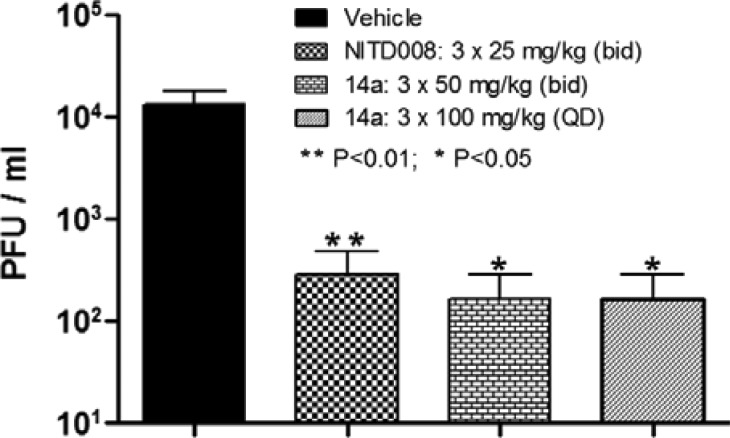
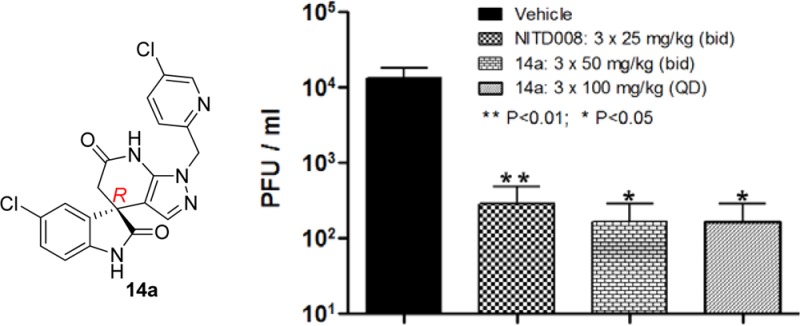
Next, in order to determine the in vivo efficacy of this class of compounds, compound 14a was tested in a DENV-2 viremia mouse model (Figure 3).23 When compound 14a was administrated orally at 50 mg/kg twice daily (bid) for 3 days, compared to vehicle control group, significant viremia reduction (about 1.9 log, P value <0.05) was achieved. Similar efficacy was observed when compound 14a was orally dosed at 100 mg/kg once daily (QD) for 3 days. These results were similar if not better than the positive control group, which was treated with nucleoside analogue NITD008 (25 mg/kg bid for 3 days).24 In summary, this class of compounds clearly demonstrated a good in vivo mouse efficacy and proved that DENV NS4B protein is a druggable target for dengue drug discovery.
Figure 3.
DENV-2 in vivo mouse efficacy of compound 14a.
In conclusion, a novel spiropyrazolopyridone scaffold was identified from dengue phenotypic screening. Chiral HPLC separation of the racemates revealed that R enantiomers were significantly more potent than S enantiomers. After exploring the SARs, we identified compound 14a with optimal in vitro potency and physicochemical properties, which translated into good in vivo PK and efficacy. However, the main drawback for this class of compounds is the relatively weak potency in DENV-1 and -4, though the potency of DENV-3 tracks well with the DENV-2 activity.16 Further optimization to acquire potency of all four serotypes will be reported in due course.
Acknowledgments
We would like to thank all the other NITD colleagues for their support during the course of this study. We thank Ms. Joefina Lim for high-resolution mass spectrometry (HRMS) measurement.
Supporting Information Available
Full experimental details for compounds synthesized and descriptions of assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Simmons C. P.; Farrar J. J.; van Vinh Chau N.; Wills B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1 http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [PubMed]
- Bhatt S.; Gething P. W.; Brady O. J.; Messina J. P.; Farlow A. W.; Moyes C. L.; Drake J. M.; Brownstein J. S.; Hoen A. G.; Sankoh O.; Myers M. F.; George D. B.; Jaenisch T.; Wint G. R. W.; Simmons C. P.; Scott T. W.; Farrar J. J.; Hay S. I. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M. G.; Kouri G. Dengue: an update. Lancet Infect. Dis. 2002, 2, 33–42. [DOI] [PubMed] [Google Scholar]
- Gubler D. J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002, 33, 330–342. [DOI] [PubMed] [Google Scholar]
- Fink K.; Shi P. Y. Live attenuated vaccine: the first clinically approved dengue vaccine?. Expert Rev. Vaccines 2014, 13, 185–188. [DOI] [PubMed] [Google Scholar]
- Stevens A. J.; Gahan M. E.; Mahalingam S.; Keller P. A. The Medicinal Chemistry of Dengue Fever. J. Med. Chem. 2009, 52, 7911–7926. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Strategies for the treatment of dengue virus infections: a narrative account. Future Med. Chem. 2010, 2, 601–608. [DOI] [PubMed] [Google Scholar]
- Nitsche C.; Holloway S.; Schirmeister T.; Klein C. D. Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem. Rev. 2014, 114, 11348–11381. [DOI] [PubMed] [Google Scholar]
- Beesetti H.; Khanna N.; Swaminathan S. Drugs for Dengue: a Patent Review (2010–2014). Expert Opin. Ther. Pat. 2014, 24, 1171–1184. [DOI] [PubMed] [Google Scholar]
- Xie X.; Wang Q. Y.; Xu H. Y.; Qing M.; Kramer L.; Yuan Z.; Shi P. Y. Inhibition of Dengue Virus by Targeting viral NS4B protein. J. Virol. 2011, 85, 11183–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd C. M.; Dai D.; Grosenbach D. W.; Berhanu A.; Jones K. F.; Cardwell K. B.; Schneider C.; Wineinger K. A.; Page J. M.; Harver C.; Stavale E.; Tyavanagimatt S.; Stone M. A.; Bartenschlager R.; Scaturro P.; Hruby D. E.; Jordan R. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd C. M.; Grosenbach D. W.; Berhanu A.; Dai D.; Jones K. F.; Cardwell K. B.; Schneider C.; Yang G.; Tyavanagimatt S.; Harver C.; Wineinger K. A.; Page J.; Stavale E.; Stone M. A.; Fuller K. P.; Lovejoy C.; Leeds J.; Hruby D. E.; Jordan R. Novel benzoxazole inhibitor of dengue virus replication that targets the NS3 helicase. Antimicrob. Agents Chemother. 2013, 57, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardiot D.; Carlens G.; Dallmeier K.; Kaptein S.; McNaughton M.; Marchand A.; Neyts J.; Smets W. Viral replication inhibitors. WO2013/045516 A1.
- Wang Q. Y.; Kondreddi R. R.; Xie X.; Rao R.; Nilar S.; Xu H. Y.; Qing M.; Chang D.; Dong H.; Yokokawa F.; Lakshminarayana S. B.; Goh A.; Schul W.; Kramer L.; Keller T. H.; Shi P. Y. A translation inhibitor that suppresses dengue virus in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 4072–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qang Q. Y.; Bushell S.; Qing M.; Xu H. Y.; Bonavia A.; Nunes S.; Zhou J.; Poh M. K.; de Sessions P. F.; Niyomrattanakit P.; Dong H.; Hoffmaster K.; Goh A.; Nilar S.; Schul W.; Jones S.; Kramer L.; Compton T.; Shi P. Y. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J. Viol. 2011, 85, 6548–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuscript describing the mechanism of action studies for this class of compounds was submitted.
- van Cleef K. W.; Overheul G. J.; Thomassen M. C.; Kaptein S. J.; Davidson A. D.; Jacobs M.; Neyts J.; van Kuppeveld F. J.; van Rij R. P. Antiviral Res. 2013, 99, 165–171. [DOI] [PubMed] [Google Scholar]
- Bardiot D.; Carlens G.; Dallmeier K.; Kaptein S.; Koukni M.; Marchand A.; Neyts J.; Smets W.. WO2014/154682 A1.
- Lichitsky B. V.; Komogortsev A. N.; Dudinov A. A.; Krayushkin M. M. Three-component condensation of 5-aminopyrazole derivatives with isatins and Meldrum’s acid. Synthesis of 1,7-dihydrosprio[pyrazolo[3,4-b]-pyridine-4,3′-indole]-2′,6(1′H, 5H)-diones. Russ. Chem. Bull., Int. Ed. 2009, 58, 1504–1508. [Google Scholar]
- Zou B.; Chen C.; Leong S. Y.; Ding M.; Smith P. W. An efficient synthesis of 4,6-dihydrospiro[azepion[4,3,2-cd]indole-3,3′-indoline]-2′,5(1H)-diones via multi-component reaction. Tetrahedron 2014, 70, 578–582. [Google Scholar]
- Gatta F.; Pomponi M.; Marta M. Synthesis of 7,8-dihydro-6H-pyrzolo[3,4-b]quinolin-5-ones and related derivatives. J. Heterocycl. Chem. 1991, 28, 1301–1307. [Google Scholar]
- Similar phenomenon observed in antimalarial spiroindolone scaffold, see:Yeung B. K. S.; Zou B.; Rottmann M.; Lakshminarayana S. B.; Ang S. H.; Leong S. Y.; Tan J.; Wong J.; Keller-Maerki S.; Fischli C.; Goh A.; Schmitt E. K.; Krastel P.; Francotte E.; Kuhen K.; Plouffe D.; Henson K.; Wagner T.; Winzeler E. A.; Petersen F.; Brun R.; Dartois V.; Diagana T. T.; Keller T. H. Spirotetrahydro β-carbolines (spiroindolones): A new class of potent and orally efficacious compounds for the treatment of malaria. J. Med. Chem. 2010, 53, 5155–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul W.; Liu W.; Xu H. Y.; Flamand M.; Vasudevan S. G. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007, 195, 665–674. [DOI] [PubMed] [Google Scholar]
- Yin Z.; Chen Y. L.; Schul W.; Wang Q. Y.; Gu F.; Duraiswamy J.; Kondreddi R. R.; Niyomrattanakit P.; Lakshminarayana S. B.; Goh A.; Xu H. Y.; Liu W.; Liu B.; Lim J. Y.; Ng C. Y.; Qing M.; Lim C. C.; Yip A.; Wang G.; Chan W. L.; Tan H. P.; Lin K.; Zhang B.; Zou G.; Bernard K. A.; Garrett C.; Beltz K.; Dong M.; Weaver M.; He H.; Pichota A.; Dartoris V.; Keller T. H.; Shi P. Y. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 20435–20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.