Abstract
Purpose
To determine the reproducibility of a multiplex bead assay for measuring cytokines in tears and correlations between ocular discomfort with or without contact lens wear and the concentration of cytokines in tears.
Methods
Ninety participants (divided into two groups) were enrolled in this prospective study. They were asked to rate their ocular comfort and collect their tears in the morning and just before sleep for 10 days with or without contact lenses. The participants collected their tears using a glass microcapillary tube for both stages. Galyfilcon A lenses were worn on a daily disposable basis during the contact lens stage, and comfort scores and tears were collected before lens insertion and prior to lens removal at the end of the day. Tears were analyzed for cytokine concentrations using a 27-plex multibead assay. Correlations were sought between cytokine concentrations and comfort.
Results
There was a significant (p<0.022) decrease in ocular comfort over the day with or without lens wear. The magnitude of ocular discomfort was significantly greater (p<0.009) with lens wear. The concentrations of 12 cytokines differed significantly between the groups; thus, these cytokines were not analyzed further. For the remaining 15 cytokines, interleukin-8 (IL-8) was the only cytokine that changed in both groups during the day without (reduced by >-0.5 Log pg/ml, p<0.001) or with lens wear (reduced by >-0.2 Log pg/ml, p<0.001). The change in the vascular endothelial growth factor (VEGF) concentration only in tears was correlated to ocular comfort, but this was not changed by contact lens wear.
Conclusions
Ocular comfort during the day is magnified by contact lens wear. However, the increase in the change in comfort during lens wear was not associated with changes in 15 cytokines in the tear film.
Introduction
The tear film is a dynamic fluid that contains various biological molecules, including proteins, lipids, and mucins. The human tear film has a complex multilayered fluid structure, consisting of an extensive aqueous layer situated between a mucin layer and a lipid layer. The function of the tear film is to hydrate and lubricate the ocular surface, to protect it from invading pathogens, and to provide a trophic environment for the corneal epithelium and a smooth optical surface for normal vision [1]. The aqueous component of tears comprises the major part by volume and is secreted by the lacrimal gland, accessory lacrimal glands as well as contribution from plasma leakage from conjunctival blood vessels. The aqueous layer contains electrolytes, minerals, enzymes, non-enzyme proteins, immunoglobulins, and growth factors. De Souza et al. [2] identified 491 proteins in the tear film of one individual, and Zhou et al. [3] identified 1,543 proteins in tears collected from four healthy non-contact lens wearers; 714 proteins were present in all samples.
Several studies have examined the tear film for changes in inflammatory mediators during dry-eye disease or contact lens-associated inflammation. Dry-eye disease is defined as being associated with inflammation of the ocular surface [4]. An increase in the concentration of several cytokines, including interleukin-1β (IL-1β), IL-5, IL-6, IL-8, interferon gamma (IFN-γ), and tumor necrosis factor-α (TNF-α), has been reported in the tears of people with dry-eye disease [5,6], and the IL-6 concentration correlates with corneal desiccation, surface damage, irritation, and symptom severity [6]. Studies have shown that contact lens wear can result in increases in the concentration of the cytokines IL-6, IL-8, TNF-α, and endothelial growth factor (EGF) in tears [7-11]. Furthermore, adverse events associated with infiltration of the cornea have been shown to result in increases in the IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations in tears [12]. Interestingly, there have been no reports of associations between the concentration of cytokines in tears and dryness or discomfort symptoms with contact lens wear.
Contact lens wear can result in symptoms of dryness and discomfort. The prevalence of dryness and discomfort ranges from approximately 30% up to 70% for different contact lens–wearing populations [13-16]. Furthermore, dryness or discomfort appears to increase during the day [17]. This study was designed to measure changes in comfort during the day with or without contact lenses, to determine whether these changes correlated with the level of a range of cytokines in the tear film, and to determine whether a multiplex bead assay for cytokine analysis was reproducible when used with tears.
Methods
Participants
A total of 90 participants in two groups (44 and 46 participants, respectively; see statistical analysis for power calculation for number of participants) were enrolled who met the inclusion and exclusion criteria. The two sets of participants were enrolled in the study approximately 1 month apart, and no participants were enrolled in both studies. The study followed the Declaration of Helsinki concerning the use of human participants, and all participants signed informed consent before enrollment in the study. Ethics approval for the study was granted by the Human Research Ethics Committee of the Brien Holden Vision Institute. Inclusion criteria were at least 18 years old, willing to comply with the wearing and clinical trial visit schedule as directed by the investigator, have ocular health findings considered “normal,” have vision correctable to at least 6/12 (20/40) or better in each eye with contact lenses, be experienced or inexperienced at wearing contact lenses, and able to demonstrate ability to collect tear samples on their own using equipment provided. Exclusion criteria were preexisting ocular irritation, injury, or condition of the cornea, conjunctiva, or eyelids that would preclude soft contact lens fitting and safe wearing of contact lenses; systemic disease that adversely affects ocular health; use of or a need for prescription ocular medication up to 12 weeks before and during the trial; use of or a need for any systemic medication or topical medications that may alter normal ocular findings or are known to affect a participant’s ocular health/physiology or contact lens performance (systemic antihistamines were allowed on an as needed basis, provided they were not used prophylactically during the trial); eye surgery within 12 weeks immediately before enrollment for this trial; previous corneal refractive surgery; contraindications to contact lens wear; currently enrolled in another clinical trial; participation in a clinical trial within the previous 2 weeks or participation in a short-term clinical test within the previous 48 h; use of or need for anticoagulants; any physical inability that may prevent them from collecting their own tears (e.g., epilepsy, Parkinson’s disease, etc.); or are pregnant or plan to become pregnant during the trial or currently breastfeeding.
Tear collection and ocular comfort ratings
Participants were first instructed not to wear contact lenses for 7 days before the beginning of the study if experienced lens wearers, and to collect their tears using a microcapillary tube [18] in the morning and in the evening (just before going to bed). During the non-contact lens wear stage, tears from both eyes were collected in the same Eppendorf tube, but different tubes were used for morning and evening collections and for each day. The participants were asked to collect approximately 5 μl of tears each day (this volume in the capillary tube was demonstrated to them in the clinic before the start of the tear collection), or as much tears as they could within a 10 min collection time, and to immediately place the tears in their home freezer (−20 °C). The participants were advised to immediately stop tear collection if any reflex tearing was stimulated, and simply freeze any amount of tears collected at that time. This collection period lasted for 10 days, and tears were collected each day. During this period, the tears were stored in Eppendorf tubes in the participants’ home freezers. At the end of the 10 days, the participants delivered the tears in an ice container to the laboratory where they were transferred to a −80 °C freezer before analysis. During the second stage of this study, the participants were fitted with contact lenses (Galyfilcon A; Johnson and Johnson Vision Care, Jacksonville, FL) under a daily disposable modality and told to collect tears each morning before lens insertion and each evening before lens removal for 10 days. Tears were treated as per the non-contact lens wearing stage. All the morning tears and all the evening tears were collected separately from an individual participant in each stage were pooled for each individual before laboratory analysis.
Subjective ratings of ocular comfort were recorded at the time of tear collection. Participants were asked to rate their ocular comfort of each eye before tear collection on a 1–100 scale where 1=extremely uncomfortable and 100=extremely comfortable.
Cytokine assay
Twenty-seven cytokines (IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, -4, -5, -6, -7, -8, -9, -10, -12p70, -13, -15, -17, tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), granulocyte-monocyte colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), platelet-derived growth factor-BB (PDGF-BB), basic fibroblast growth factor (FGF-b), vascular endothelial growth factor (VEGF), monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein -1α, -1β (MIP-1α, MIP-1β), eotaxin, interferon-induced protein-10 (IP-10), and regulated upon activation, normal T-cell expressed and presumably secreted (RANTES)) in each tear sample were detected using multiplex bead analysis (Bio-Plex Human Cytokine 27-plex panel, Bio-Rad, Hercules, CA). The tear samples were diluted 20-fold using the sample diluents supplied in the kit. Standard curves were generated by using the reference cytokine sample supplied in the kit and were used to calculate the cytokine concentrations in tear samples. The lower limits of quantification for each cytokine are presented in Table 1. In addition to the multiplex assay, an enzyme-linked immunosorbent assay (ELISA; Bethyle Laboratories, Montgomery, AL; lower limit of detection=8 ng/ml) was used to measure the amount of immunoglobulin A (IgA) in tears as this concentration has been reported to change upon reflex tearing [18,19].
Table 1. Calculated lower limits of quantification for each cytokine assessed by Bio-Plex Human Cytokine 27-plex panel.
| CYTOKINES | Lower limit of detection (pg/ml) |
|---|---|
|
IL-1β |
3.2 |
|
IL-1Ra |
81.1 |
|
IL-2 |
2.1 |
|
IL-4 |
2.2 |
|
IL-5 |
3.1 |
|
IL-6 |
2.3 |
|
IL-7 |
3.1 |
|
IL-8 |
1.9 |
|
IL-9 |
2.1 |
|
IL-10 |
2.2 |
|
IL-12p70 |
3.3 |
|
IL-13 |
3.7 |
|
IL-15 |
2.1 |
|
IL-17 |
4.9 |
|
Eotaxin |
40.9 |
|
bFGF |
27.2 |
|
G-CSF |
2.4 |
|
GM-CSF |
63.3 |
|
IFN- γ |
92.6 |
|
IP-10 |
18.8 |
|
MCP-1 |
2.1 |
|
MIP-1α |
1.4 |
|
MIP-1β |
2.0 |
|
PDGF-BB |
7.0 |
|
RANTES |
2.2 |
|
TNF-α |
5.8 |
| VEGF | 5.5 |
Abbreviations: IL = interleukin; FGF = fibroblast growth factor; G-CSF = Granulocyte colony stimulating factor; GM-CSF = granulocyte monocyte colony stimulating facotr; IFN = interferon; IP = Interferon gamma-induced protein; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor
Statistical analysis
A sample size of 40 participants was required to determine a significant paired difference of 5±10 units for ocular comfort scores with 80% power at the 5% level of significance after adjusting for a possible dropout rate of 20%. To determine the repeatability of the cytokine and comfort values, data for the first group of participants was compared to that of the second group. Participants who commenced the study treatment were included in the analysis data set. The analysis of efficacy variables such as subjective ratings and inflammatory mediators used only scheduled and evaluable visits. Concentrations of different inflammatory mediators obtained from the tear analysis were quantified on a continuous scale and summarized as means ± standard deviations. Prior to statistical analysis, log transformation was applied to the inflammatory mediators due to their dynamic range. Subjective ratings of ocular comfort were also summarized as means ± standard deviations. The concentrations of tear components and subjective ratings were analyzed using mixed linear models with subject random intercepts and stages factored as repeated effects, to determine significant differences between the stages and morning to evening differences. Correlations between the concentration of single cytokines and change in comfort response magnitude were calculated with the Pearson correlation coefficient. Level of significance was set at 5%.
Results
Subject demographics
The demographics of the participants enrolled in each group are given in Table 2. The demographics factors assessed for each group were not significantly different.
Table 2. Subject demographics in the two participant groups.
| Variable | Group 1 | Group 2 | p value | |
|---|---|---|---|---|
| Gender |
% Female |
69 |
60 |
0.516 |
| Ethnicity |
% Asian |
40 |
40 |
0.439 |
| % Caucasian |
20 |
33 |
||
| % Indian |
18 |
13 |
||
| % Other |
22 |
15 |
||
| contact lens wear experience |
% neophyte |
13 |
17 |
0.775 |
| Age |
Years |
26.4±8.5 |
24.9±8.5 |
0.397 |
| Refraction |
Sphere (Diopters) |
−2.9±2.0 |
−2.9±2.2 |
0.910 |
| Cylinder (Diopters) |
−0.5±0.4 |
−0.6±0.4 |
0.166 |
|
| Keratometry | Flat Meridian (Diopters) |
43.1±1.3 |
43.4±1.3 |
0.269 |
| Steep Meridian (Diopters) | 43.9±1.2 | 44.2±1.2 | 0.231 | |
Change in ocular comfort ratings
There were no significant differences in comfort ratings between the two groups at any stage (Table 3). There was a significant morning to evening decrease in comfort ratings with and without contact lens wear in both groups (p≤0.021). However, the morning to evening decrease in comfort ratings was greater with contact lens wear compared to non-contact lens wear (p=0.008 and p=0.006 for groups 1 and 2, respectively). Within a participant, there was no difference in the comfort ratings over the course of the 10 days of data (and tear) collection.
Table 3. Comfort and dryness responses of the two participant groups.
| Variables | Time | Stage | Group 1 |
Group 2 |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||||
| Comfort 1–100 | Morning |
No contact lens |
43 |
84 |
13 |
46 |
86 |
10 |
0.706 |
| With contact lens |
43 |
83 |
14 |
44 |
83 |
11 |
0.848 |
||
| Evening | No contact lens |
44 |
81 |
16 |
46 |
82 |
13 |
0.443 |
|
| With contact lens | 43 | 76 | 16 | 44 | 74 | 16 | 0.637 | ||
Statistical analysis used mixed linear models as outlined in the methods section.
In the morning, differences in comfort between contact lens wear and non-contact lens wear were not consistent between the two groups (p=0.313 and 0.025 for groups 1 and 2, respectively). However, in the evening, comfort rating with contact lens wear was significantly lower than without contact lens wear for both groups (p=0.006 and <0.001 for groups 1 and 2, respectively).
Differences in cytokine concentrations in tears between groups
Initially, the IgA concentration in tears was analyzed to determine whether the pooled tears resembled basal or reflex tears. The mean IgA concentration in tears collected during the morning without lens wear was 6.6±12.1 mg/ml, and during the evening without lens wear was 1.6±1.5 mg/ml. For tears collected during contact lens wear, the amount of IgA was 4.9±4.0 mg/ml for morning tears and 2.0±1.5 mg/ml for evening tears. Although the standard deviations for these results were large, the values were consistent with the range of values reported for IgA in basal and closed-eye tears [20-26] and much higher than those reported for reflex tears on average (0.1–0.4 mg/ml) [19,20,27,28]. This is consistent with basal tears being the predominant tear type collected in the pooled samples.
The cytokines detected in each tear sample were investigated (Table 4). As can be seen, IL-15, IL-17, eotaxin, FGF-b, and GM-CSF were found in the tears of fewer than 30% of the participants. The comparison between levels of cytokines demonstrated that there were differences between the two groups. The groups had significantly different concentrations of eotaxin, FGF-b, GM-CSF, IFN-γ, IL-10, IL-17, IL-2, IL-9, IP-10, MIP-1β, PDGF-BB, and RANTES and many were found only in a minority of tear samples (Table 4). Thus, these cytokines were not examined further.
Table 4. Positive detection rates of each mediator in the tested tear samples.
| Mediators | Percentage of tear samples which contained mediators in levels higher than the limit of quantification* |
|---|---|
|
PDGF-bb, IL-1Ra, IL-4, IL-6, IL-7, IL-8, IL-12p70, G-CSF, IFN-γ, IP-10, MCP-1, VEGF |
>60% |
|
IL-1β, IL-2, IL-5, IL-9, IL-10, IL-13, MIP-1α, MIP-1β, RANTES, TNF-α |
30%–60% |
| IL-15, IL-17, eotaxin, FGF-b, GM-CSF | <30% |
*, limits of quantification are given in Table 1. Abbreviations: IL = interleukin; FGF = fibroblast growth factor; G-CSF = Granulocyte colony stimulating factor; GM-CSF = granulocyte monocyte colony stimulating facotr; IFN = interferon; IP = Interferon gamma-induced protein; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor
The cytokines and growth factors consistent between the groups and used for further analysis were G-CSF, IL-12p70, IL-13, IL-15, IL-1β, IL-1Ra, IL-4, IL-5, IL-6, IL-7, IL-8, MIP-1α, TNF-α, and VEGF. Most were found in the majority of samples (Table 4). Results are presented within each group and as a combined sample using the percentage data. Table 5 provides the summary data of natural log-transformed cytokine or growth factor data from each group.
Table 5. Cytokine concentrations (Log pg/ml) in tears that were consistent between subject groups.
| Cytokine | Stage | Group 1 |
Group 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Morning |
Evening |
Combined |
Morning |
Evening |
Combined |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| G-CSF |
No contact lens |
3.6 |
0.8 |
3.7 |
0.8 |
3.7 |
0.8 |
3.2 |
1.0 |
3.2 |
1.1 |
3.2 |
1.1 |
| With contact lens wear |
3.5 |
0.9 |
3.5 |
0.8 |
3.5 |
0.8 |
3.3 |
0.7 |
2.8 |
1.2 |
3.1 |
1.1 |
|
| Both stages |
3.5 |
0.8 |
3.6 |
0.8 |
3.6 |
0.8 |
3.3 |
0.9 |
3.0 |
1.2 |
3.2 |
1.1 |
|
| IL-12p70 |
No contact lens |
5.0 |
0.7 |
5.2 |
0.6 |
5.1 |
0.7 |
4.7 |
1.0 |
5.0 |
0.7 |
4.9 |
0.9 |
| With contact lens wear |
4.9 |
0.7 |
4.9 |
0.9 |
4.9 |
0.8 |
4.8 |
0.6 |
4.6 |
0.7 |
4.7 |
0.7 |
|
| Both stages |
4.9 |
0.7 |
5.1 |
0.8 |
5.0 |
0.8 |
4.8 |
0.8 |
4.8 |
0.7 |
4.8 |
0.8 |
|
| IL-13 |
No contact lens |
3.8 |
0.7 |
4.1 |
0.6 |
4.0 |
0.7 |
3.3 |
1.5 |
3.9 |
1.0 |
3.6 |
1.3 |
| contact lens Wear |
3.7 |
0.7 |
3.9 |
0.9 |
3.8 |
0.8 |
3.4 |
1.3 |
3.2 |
1.2 |
3.3 |
1.2 |
|
| Both stages |
3.8 |
0.7 |
4.0 |
0.8 |
3.9 |
0.7 |
3.4 |
1.3 |
3.6 |
1.2 |
3.5 |
1.3 |
|
| IL-15 |
No contact lens |
2.2 |
1.1 |
2.3 |
1.2 |
2.2 |
1.1 |
1.2 |
2.3 |
1.7 |
3.1 |
1.5 |
2.7 |
| With contact lens wear |
1.8 |
1.2 |
2.1 |
1.1 |
2.0 |
1.2 |
1.1 |
2.2 |
1.0 |
1.9 |
1.0 |
2.0 |
|
| Both stages |
2.0 |
1.2 |
2.2 |
1.1 |
2.1 |
1.2 |
1.2 |
2.2 |
1.3 |
2.6 |
1.2 |
2.4 |
|
| IL-1β |
No contact lens |
3.4 |
0.5 |
3.3 |
0.6 |
3.4 |
0.5 |
3.1 |
1.4 |
3.0 |
1.7 |
3.0 |
1.5 |
| With contact lens wear |
3.2 |
0.6 |
3.1 |
0.9 |
3.1 |
0.7 |
2.8 |
1.2 |
2.4 |
1.3 |
2.6 |
1.3 |
|
| Both stages |
3.3 |
0.5 |
3.2 |
0.7 |
3.2 |
0.6 |
3.0 |
1.3 |
2.7 |
1.5 |
2.8 |
1.4 |
|
| IL-1Ra |
No contact lens |
8.0 |
0.6 |
8.0 |
0.7 |
8.0 |
0.7 |
8.1 |
1.2 |
7.8 |
1.0 |
7.9 |
1.1 |
| With contact lens wear |
7.9 |
0.7 |
7.9 |
0.7 |
7.9 |
0.7 |
7.7 |
0.9 |
7.7 |
1.3 |
7.7 |
1.1 |
|
| Both stages |
8.0 |
0.7 |
7.9 |
0.7 |
7.9 |
0.7 |
7.9 |
1.1 |
7.7 |
1.1 |
7.8 |
1.1 |
|
| IL-4 |
No contact lens |
3.7 |
0.8 |
3.9 |
0.7 |
3.8 |
0.8 |
3.2 |
1.3 |
3.6 |
1.1 |
3.4 |
1.2 |
| With contact lens wear |
3.5 |
0.9 |
3.7 |
1.0 |
3.6 |
0.9 |
3.2 |
1.3 |
3.0 |
1.4 |
3.1 |
1.4 |
|
| Both stages |
3.6 |
0.9 |
3.8 |
0.9 |
3.7 |
0.9 |
3.2 |
1.3 |
3.3 |
1.3 |
3.3 |
1.3 |
|
| IL-5 |
No contact lens |
3.8 |
1.0 |
4.1 |
0.8 |
4.0 |
0.9 |
3.5 |
1.2 |
4.0 |
1.1 |
3.8 |
1.2 |
| With contact lens wear |
3.7 |
0.9 |
3.9 |
0.8 |
3.8 |
0.9 |
3.4 |
1.3 |
3.3 |
1.3 |
3.3 |
1.3 |
|
| Both stages |
3.8 |
0.9 |
4.0 |
0.8 |
3.9 |
0.9 |
3.4 |
1.2 |
3.6 |
1.3 |
3.5 |
1.3 |
|
| IL-6 |
No contact lens |
4.1 |
0.7 |
4.4 |
0.6 |
4.3 |
0.7 |
4.0 |
1.2 |
3.8 |
1.4 |
3.9 |
1.3 |
| With contact lens wear |
3.9 |
0.8 |
4.0 |
0.9 |
4.0 |
0.8 |
4.1 |
0.9 |
3.6 |
1.3 |
3.8 |
1.1 |
|
| Both stages |
4.0 |
0.8 |
4.2 |
0.8 |
4.1 |
0.8 |
4.0 |
1.1 |
3.7 |
1.4 |
3.9 |
1.2 |
|
| IL-7 |
No contact lens |
7.0 |
0.4 |
7.1 |
0.4 |
7.1 |
0.4 |
7.2 |
0.5 |
7.1 |
0.5 |
7.2 |
0.5 |
| With contact lens wear |
6.8 |
0.5 |
6.7 |
1.4 |
6.8 |
1.0 |
7.1 |
0.4 |
7.1 |
0.5 |
7.1 |
0.5 |
|
| Both stages |
6.9 |
0.4 |
6.9 |
1.0 |
6.9 |
0.8 |
7.1 |
0.5 |
7.1 |
0.5 |
7.1 |
0.5 |
|
| IL-8 |
No contact lens |
6.5 |
0.8 |
5.9 |
0.5 |
6.2 |
0.7 |
6.4 |
1.5 |
5.3 |
0.8 |
5.9 |
1.3 |
| With contact lens wear |
6.2 |
0.9 |
5.9 |
0.6 |
6.0 |
0.8 |
6.0 |
1.1 |
5.4 |
1.2 |
5.7 |
1.1 |
|
| Both stages |
6.3 |
0.8 |
5.9 |
0.6 |
6.1 |
0.8 |
6.2 |
1.3 |
5.3 |
1.0 |
5.8 |
1.2 |
|
| MIP-1α |
No contact lens |
4.1 |
0.9 |
3.9 |
1.0 |
4.0 |
1.0 |
4.3 |
2.5 |
4.0 |
2.2 |
4.1 |
2.3 |
| With contact lens wear |
3.9 |
1.0 |
3.8 |
1.2 |
3.8 |
1.1 |
4.0 |
2.1 |
3.0 |
1.7 |
3.5 |
2.0 |
|
| Both stages |
4.0 |
1.0 |
3.8 |
1.1 |
3.9 |
1.0 |
4.1 |
2.3 |
3.5 |
2.0 |
3.8 |
2.2 |
|
| TNF-α |
No contact lens |
4.4 |
2.3 |
5.1 |
1.5 |
4.8 |
2.0 |
4.0 |
2.2 |
4.7 |
2.0 |
4.4 |
2.1 |
| With contact lens wear |
4.1 |
2.3 |
4.6 |
2.1 |
4.3 |
2.2 |
4.6 |
1.6 |
4.1 |
2.1 |
4.3 |
1.9 |
|
| Both stages |
4.2 |
2.3 |
4.8 |
1.8 |
4.5 |
2.1 |
4.3 |
2.0 |
4.4 |
2.0 |
4.3 |
2.0 |
|
| VEGF | No contact lens |
5.3 |
1.0 |
5.1 |
1.0 |
5.2 |
1.0 |
4.8 |
1.9 |
3.8 |
2.5 |
4.3 |
2.3 |
| With contact lens wear |
4.9 |
1.1 |
5.0 |
0.8 |
5.0 |
1.0 |
5.2 |
1.7 |
4.9 |
1.9 |
5.1 |
1.8 |
|
| Both stages | 5.1 | 1.1 | 5.1 | 0.9 | 5.1 | 1.0 | 5.0 | 1.8 | 4.4 | 2.3 | 4.7 | 2.1 | |
Abbreviations: IL = interleukin; FGF = fibroblast growth factor; G-CSF = Granulocyte colony stimulating factor; GM-CSF = granulocyte monocyte colony stimulating facotr; IFN = interferon; IP = Interferon gamma-induced protein; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor
As an alternate method for examining the cytokine concentrations in tears, and removing some of the inherent variability between groups, the data for individual cytokines in each group were converted into a percentage of the total cytokines examined. The cytokines below the limit of detection were excluded from the analyses. After this conversion, the percentage of eotaxin, GM-CSF, IP-10, and MCP-1 were significantly different between groups; thus, the data for these cytokines were not analyzed further. After conversion, the data for the remaining cytokines from the two participants groups were averaged and analyzed as percentage data.
Changes to cytokine concentrations during the day
In both groups, the IL-15, IL-1β, IL-1Ra, and MIP-1α concentrations in tears did not significantly change from the morning to evening tear collection. The only cytokine that consistently decreased in tears from morning to evening was IL-8 (p<0.001, for groups 1 and 2 and combined [% data]). There was almost a 50% decrease in IL-8 from morning to evening. This decrease was observed in the contact lens– and no-contact-lens-wearing stages.
The IL-13 (p=0.02), IL-5 (p=0.007), and TNFα (p=0.002) concentrations were significantly increased in tears in the evening relative to morning in Group 1 but not Group 2. This increase was observed in both stages. In the combined sample (% data), TNF-α (p=0.003) increased in the evening in both stages. In the combined sample, the increase in TNF-α in the evening was approximately 30% more than the morning tears. The increase in the other two cytokines, IL-13 (p=0.001) and IL-15 (p=0.001), was observed only in the non-contact lens wearing stage when the samples were combined (% data).
The IL-6 (p=0.046) and VEGF (p=0.033) concentrations were significantly decreased in the evening compared to the morning tears in Group 2 without contact lens wear. This change was not observed in either Group 1 or the combined (% data) sample.
With the combined (% data) sample, evening tears showed higher IL-7 (p=0.007), IL-4 (p=0.001), IL-12P70 (p=0.002), and G-CSF (p=0.032) concentrations particularly in the no-contact lens wearing stage. However, these changes were not significant in each group.
Changes to cytokine concentrations in tears with contact lens wear
The only cytokines that consistently decreased in tears with contact lens wear were IL-1β and IL-12p70. The IL-1β concentrations decreased with contact lens wear in the morning and evening tear samples (p=0.031, p=0.022, p=0.05 for groups 1 and 2 and combined (% data)). In the combined sample, the decrease in IL-1β was almost 45% with contact lens wear compared to non-contact lens wear. In Group 1, the IL-12p70 concentration decreased with contact lens wear (p=0.011) in the morning and evening tear samples. However, in Group 2 and in the combined (% data) sample, this decrease was observed in the evening tear sample only (p=0.005 and p=0.011 for Group 2 and combined (% data) respectively).
The IL-4 (p=0.009, p=0.016), IL-5 (p=0.001, p=0.002), and IL-13 (p=0.001, p=0.006) concentrations significantly decreased in tears in the evening tear sample only with contact lens wear in Group 2 and in the combined (% data) sample. This decrease was not significant in Group 1. For the combined samples, the cytokines measured in the contact lens wearers were lower than that of the non-contact lens wearers. Additionally, the IL-8 concentration (p=0.001, p=0.016) significantly decreased in tears only in the morning tear sample with contact lens wear in Group 2 and in the combined sample (% data). This decrease was not significant in Group 1 (p=0.326).
The IL-6 (p=0.009), IL-7 (p=0.037), and TNF-α (p=0.025) concentrations were significantly decreased with contact lens wear compared to the no-contact lens stage in Group 1. This change was not observed in either Group 2 or the combined sample (% data). Additionally, VEGF (p=0.014) concentrations significantly increased with contact lens wear compared to the non-contact lens stage in Group 2 only.
With the combined sample (% data), contact lens wear showed lower IL-1Ra (p=0.044) and G-CSF (p=0.014; in the evening tear sample only) concentrations. However, these changes were not observed in the individual groups.
Correlations between cytokine concentrations and comfort responses
Using the absolute concentration of cytokines in tears in the individual groups, there was no correlation between any cytokine and ocular comfort scores of the participants in either group. Nor were there any correlations when the percentage concentrations of cytokines were analyzed.
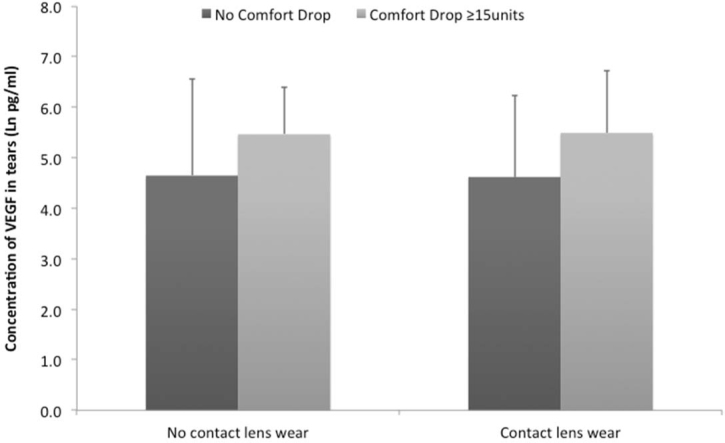
Finally, participants in groups 1 and 2 were combined and split into those who had no ocular comfort decrease during lens wear, those who had a <15 point ocular comfort decrease, and those who had a ≥15 point ocular comfort decrease. Comparisons between the cytokine levels in tears for those with no drop in comfort during the day and the ≥15 point ocular comfort decrease group (Figure 1) showed that the level of VEGF in tears was significantly increased (using the log converted data) in tears of the ≥15 point group either with (p=0.031) or without (p=0.014) contact lens wear, and the magnitude of the difference in VEGF was the same with or without lens wear. Analysis for correlations with cytokine levels showed that for the log-converted data or the % data there was a correlation with the VEGF concentration and ocular comfort for those with ≥15 point decrease (Pearson’s r=0.4, p=0.004; r=0.339, p=0.016, respectively). This implies that participants who had a large ocular comfort decrease during the day had an increased VEGF concentration in their tears, but lens wear did not change this effect.
Figure 1.
Differences in the vascular endothelial growth factor (VEGF) concentration in tears of symptomatic and asymptomatic participants. Statistical analysis using mixed linear models demonstrated that there were significant differences between the two populations.
Discussion
The current study aimed to demonstrate whether contact lens discomfort over the course of a day was associated with changes to inflammatory mediators in tears. Our results revealed two important findings, namely, that the decrease in ocular comfort associated with contact lens wear (over and above the decrease in ocular comfort that occurs normally during the day without lenses) is not related to changes in at least 15 cytokines, chemokines, and growth factors in tears and that the multiplex bead assay used in the current study is not reproducible for a set of 12 tear cytokines.
In the absence of contact lens wear, the ocular comfort response of both groups significantly decreased during the day by approximately the same amount (3–4 units). In the presence of contact lens wear, comfort decreased for both groups by a greater extent (6–10 units). These numbers correspond to the 7–8 point difference reported as the just-noticeable difference in ocular comfort responses for contact lens wearers [29]. The decrease in ocular comfort during contact lens wear has been shown in previous studies [17,30] as has the decrease in ocular comfort in the absence of lens wear [17]. The increase in ocular discomfort during contact lens wear has been postulated as one of the major reasons for contact lens drop-out [31].
The 27-plex kit reproducibly measured the concentrations of 15 cytokines in the two populations studied. Thus, the hypothesis that changes to ocular comfort with and without contact lens wear during the day could be correlated with the concentration of these cytokines in tears was tested. Interestingly, the majority of the cytokines in tears (G-CSF, IL-12p70, IL-13, IL-15, IL-1β, IL-1Ra, IL-4, IL-5, IL-6, IL-7, IL-8, MCP-1, MIP-1α, and TNF-α) were not related to changes in ocular comfort. Some cytokines changed in the tears of one population but not the other, or did not change at all. This emphasizes the need to test different populations using different batches of test kits. Even when the data were normalized by using concentrations of individual cytokines as a percentage of the total cytokine concentration, there were no correlations between these cytokines and comfort. The only correlation with comfort was for the VEGF concentration in tears, either the absolute concentration or the relative concentration. The VEGF concentration was high when there was a high level of discomfort. Interestingly, when there was a change in the concentration of cytokines during the day, the change was often of a smaller magnitude than without lens wear.
VEGF is a pleiotropic growth factor that can promote the growth of vascular endothelial cells, induce vascular leakage and vasodilation (among many other potential activities) [32]. A study by Papas et al. [33] demonstrated that in the absence of lens wear, limbal redness increases during sleep but does not change during the day. During wear of low oxygen transmissibility lenses, limbal redness increases dramatically after only 4 h and remains high [33]. In contrast, eyes wearing a highly oxygen transmissible contact lens showed almost the same response as those wearing no contact lens [33]. The current study used a relatively high oxygen permeable lens, Galyfilcon A. Despite the lack of redness seen clinically, it may be that VEGF is active during the day and during contact lens wear promoting sub-clinical vessel leakage that then leads to symptoms of ocular discomfort. The finding that ocular comfort during the day was associated with increased VEGF concentration in tears but was not modulated by contact lens wear implies that the increased discomfort seen during contact lens wear is not mediated by VEGF concentrations in tears. The fact that the group with <15 point decrease in ocular comfort showed no correlation with VEGF may imply that changes to VEGF in tears are secondary to ocular comfort changes and not a causative effect, as it might be expected that there would be a gradual increase in VEGF as the level of discomfort increased. The correlation between VEGF and ocular comfort may be simply that, a correlate and not a causative event.
Several studies have shown that cytokines can be found in tears during frank inflammation [34-36] and during dry-eye disease [5,37-42], which may also be an inflammatory disease. These have often used non-multiplex assays, or multiplex assays from other manufacturers. However, they also have not been tested for reproducibility between groups or kits. Assuming at least some of the mediators that have been assayed in these studies are reproducible, as some are in the current study, contact lens wear and associated ocular discomfort are not likely to be an inflammatory condition since the current study did not find evidence of an increase in cytokines in tears during contact lens wear. If there is any contribution of an inflammatory response, it is likely to be at levels much reduced compared to dry-eye or other inflammatory conditions. When there were differences in the concentrations of cytokines between the morning and evening in the absence of contact lens wear, when lenses were worn these differences were muted.
The current study used the Bio-Plex Human Cytokine 27-plex panel bead-assay kit. This antibody-based assay was designed to measure 27 cytokines and chemokines or growth factors, and has been optimized to work for serum, plasma, and tissue culture supernatants (see BioPlex Pro; accessed on November 22, 2013). This assay has been used previously to examine the concentration of cytokines in tears of normals and diabetic patients [43], and a similar multiplex kit from the same manufacturer was used to measure the concentration of 17 cytokines in the tears of patients with glaucoma and control individuals [44]. A study also used a single-plex assay from the same manufacturer to measure the IL-1β concentration in tears [45]. However, unlike the current study, none of these previous studies examined the reproducibility of the assay between groups of different participants. The finding in the current study that several cytokines did not give reproducible results using the 27-plex kit is important to keep in mind when reviewing those previous reports. Examinations of the reported cytokine concentrations in tears [46] and the effect of contact lens wear have demonstrated that the concentrations vary widely between studies [47], and this may be due to inherent deficiencies in the methods used to analyze the cytokines. However, without analysis of the reproducibility of the tests between subject groups, or between batches of tests, the reproducibility of other test kits is unknown. Using a different multiplex bead assay from Luminex (Austin, TX), Huang et al. [48] found good (≥0.70) interclass correlations between 18 tear proteins, including the cytokines IL-15, IL-1β, eotaxin, IL-17, MCP-1, IL-12p70, VEGF, IL-1α, IL-8, and Il-1Ra when the researchers tested the same participants on different days or between eyes. This differs in some respects with the analysis in the current study, although both studies found reproducible concentrations of IL-15, IL-1β, MCP-1, IL-12p70, IL-8, IL-1Ra, and VEGF. Unlike in the current study, there was relatively poor reproducibility for the IL-6 concentration in tears in the study by Huang et al. [48] Tears can contain substances that may interfere with enzyme-linked immunosorbent assays such as immunoglobulin components [49] or lysozyme [50], and these may also be interfering with some of the analytes used in different protocols or kits. A study used the same 27-plex kit as used in the current study [51] and reported that the concentration many cytokines in tears that were tested on two groups varied widely when different contact lens multipurpose disinfecting solutions were used. When the second group was retested for changes to tear cytokines using the same disinfecting solution as a previous group, apparent changes to tear cytokines concentrations were not seen with the second group. The authors [51] also measured comfort responses during lens wear with different lenses, but did not attempt to correlate comfort with cytokine concentrations. Perhaps the issue was that the kits could not reproducibly measure the level of cytokines in tears. The level of the cytokines RANTES (CCL5), Eotaxin (CCL11), GM-CSF, IFN-γ, IL-2, and IL-17 did not appear to be reproducible in the paper by Kalsow [51] or in the current study. Comparison of the data from the current study to that of Liu et al. from the same laboratory [43] is also illuminating. These two studies used identical multiplex assay kits from the same manufacturer (Bio-Rad). Liu et al. [43] reported low positive detection rates for IL-17 (20%), TNF-α (27%), and MIP1-α (60%). Although the present study is in agreement regarding the low positive detection rate of these cytokines, the present study also found many other cytokines, reported to be in most samples from the Liu study, at a much reduced positive rate. The reason for this discrepancy is unknown, but may be related to different populations, or the effect of tear storage (discussed in more detail below). However, the concentrations of all the reproducible cytokines in the current study were at approximately the same concentration in the study of Liu et al. [43], with the exception of G-CSF, which was approximately 7–8 times less concentrated in the present study. The study by Liu et al. [43] using the same multiplex kit demonstrated excellent recovery rates (68–113%) for spiked samples for most of the reproducible cytokines from the current study, with the exception of C-CSF which had a recovery rate of only 27% (they did not report recovery rates for TNF-α, IL-17, or MIP-1α) [43].
This study had several limitations. There is, of course, the possibility that participants induced reflex tearing on some occasions when they were collecting their tears. Although we did perform rigorous training in tear collection, and asked participants to immediately stop collection if they felt they were producing reflex tears, since the tears were collected by the participants in their own homes we could not control for this. As stated, the tears were analyzed for the secretory immunoglobulin A (sIgA) concentration in tears as the concentration of this protein is known to vary between reflex and basal tears [18]. Even if the concentration of cytokines was normalized to the concentration of sIgA, the cytokines that showed inconsistencies were still inconsistent. Thus, although reflex tearing may be a problem inherent in the study design, our best efforts to control for this did not seem to affect the problems encountered with concentrations of certain cytokines between groups. Tear samples were pooled over the 10 days of collection into those collected in the morning and those collected in the evening for each individual. The decision to pool tears collected in the morning or evening from individuals was taken so that the tears could also be used in other assays (not reported herein) that required more sample volume than the multiplex bead assay. This may have masked some variations that could have resulted in additional correlations to comfort. However, the fact that the change in comfort response for an individual during the day over the 10 days showed no significant differences suggest that a participant’s comfort response over the 10 days was consistent. Thus, we assumed that there should then be consistent changes in cytokine levels if they were related to the comfort response. This means that it would be expected that, if changes in cytokine concentrations are correlated with changes in comfort, the changes over the 10-day collection period should be similar, since the comfort change for an individual was similar.
The study examined comfort and changes in tears over several days, and thus, participants were required to store their tears in their home freezers. These may have been of the “frost-free” variety where freezers go through freeze–thaw cycles to reduce the buildup on ice. This may have affected the quality of the tears collected. However, since the tear cytokines IL-6, IL-12p70, IL-13, IL-15, IL-1β, IL-1Ra, IL-8, MCP-1, and VEGF were within the same concentration range as a previous study where tears were collected and stored at the clinic [43], and the IgA concentration was within the normal range expected, this may not a major concern. Nonetheless, the discrepancies between the percentage of samples positive for cytokines between the current study and a previous study from the same group where samples were collected in the clinic [43] may indicate some cause for concern. An alternative explanation for the differences between percentage positive samples is that the current study used predominantly Caucasian participants living in Australia, whereas the study by Liu et al. [43] used exclusively Chinese participants living in China. The IgA level detected in tears had a large range between participants (i.e., large standard deviation in the populations). This may imply that some participants collected reflex tears, but even when the concentration of cytokines was calculated per milligram of IgA, this did not produce new correlations with comfort scores. However, in future experiments it may be desirable to collect tears individually (avoiding pooling) and collecting in the clinic so that tears could be stored at −80 °C immediately to avoid any issues, if present, with collecting reflex tears and freeze–thawing of the samples during storage.
In conclusion, this study has shown that ocular comfort decreases during the day and that contact lens wear exacerbates this decrease in comfort. However, there was no evidence that this change in ocular comfort, or contact lens wear in general, was associated with a change in the concentrations of 15 cytokines, chemokines, or growth factors in tears. Contact lens wear might induce changes to levels of these inflammatory mediators in the ocular tissues that are not released into the tear film. However, it seems equally likely that contact lens wear is not associated with increases in these mediators, and thus not associated with the production of inflammation. Even when cytokine levels increased during the day, the difference in concentration (percentage) of the cytokines between morning and evening tears was less than when lenses were worn. In addition, this study emphasizes the need to perform tests on the reproducibility of methods for measuring cytokine concentrations, and possibly other proteins, in tears.
Acknowledgments
This work was funded by the Brien Holden Vision Institute and Vision CRC, and the study was performed while the first author was an employee of the University of New South Wales and a part-time employee of the Brien Holden Vision Institute.
References
- 1.Tiffany JM. The normal tear film. Dev Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- 2.de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72. doi: 10.1186/gb-2006-7-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW. In-depth analysis of the human tear proteome. J Proteomics. 2012;75:3877–85. doi: 10.1016/j.jprot.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 4.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 5.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–7. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 6.Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Perez J, Villa-Collar C, Sobrino Moreiras T, Lema Gesto I, Gonzalez-Meijome JM, Rodriguez-Ares MT, Parafita M. Tear film inflammatory mediators during continuous wear of contact lenses and corneal refractive therapy. Br J Ophthalmol. 2012;96:1092–8. doi: 10.1136/bjophthalmol-2012-301527. [DOI] [PubMed] [Google Scholar]
- 8.Lema I, Duran JA, Ruiz C, Diez-Feijoo E, Acera A, Merayo J. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008;27:758–63. doi: 10.1097/ICO.0b013e31816a3591. [DOI] [PubMed] [Google Scholar]
- 9.Acar BT, Vural ET, Acar S. Effects of contact lenses on the ocular surface in patients with keratoconus: piggyback versus ClearKone hybrid lenses. Eye Contact Lens. 2012;38:43–8. doi: 10.1097/ICL.0b013e31823ff181. [DOI] [PubMed] [Google Scholar]
- 10.Schultz CL, Kunert KS. Interleukin-6 levels in tears of contact lens wearers. J Interferon Cytokine Res. 2000;20:309–10. doi: 10.1089/107999000312441. [DOI] [PubMed] [Google Scholar]
- 11.Thakur A, Willcox MD. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res. 2000;70:255–9. doi: 10.1006/exer.1999.0767. [DOI] [PubMed] [Google Scholar]
- 12.Thakur A, Willcox MD. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67:9–19. doi: 10.1006/exer.1998.0480. [DOI] [PubMed] [Google Scholar]
- 13.Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74:624–31. doi: 10.1097/00006324-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Chen H, Wu X. Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong Province, China. Ophthalmic Epidemiol. 2012;19:226–30. doi: 10.3109/09286586.2012.670742. [DOI] [PubMed] [Google Scholar]
- 15.Brennan NA, Efron N. Symptomatology of HEMA contact lens wear. Optom Vis Sci. 1989;66:834–8. doi: 10.1097/00006324-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Begley CG, Chalmers RL, Mitchell GL, Nichols KK, Caffery B, Simpson T, DuToit R, Portello J, Davis L. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20:610–8. doi: 10.1097/00003226-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Santodomingo-Rubido J, Barrado-Navascues E, Rubido-Crespo MJ. Ocular surface comfort during the day assessed by instant reporting in different types of contact and non-contact lens wearers. Eye Contact Lens. 2010;36:96–100. doi: 10.1097/ICL.0b013e3181d1d5a5. [DOI] [PubMed] [Google Scholar]
- 18.Fullard RJ, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci. 1990;31:1119–26. [PubMed] [Google Scholar]
- 19.Fullard RJ, Tucker DL. Changes in human tear protein levels with progressively increasing stimulus. Invest Ophthalmol Vis Sci. 1991;32:2290–301. [PubMed] [Google Scholar]
- 20.Sack RA, Sathe S, Beaton A. Tear turnover and immune and inflammatory processes in the open-eye and closed-eye environments: relationship to extended wear contact lens use. Eye Contact Lens. 2003;29:S80–2. doi: 10.1097/00140068-200301001-00022. [DOI] [PubMed] [Google Scholar]
- 21.Choy CK, Cho P, Benzie IF, Ng V. Effect of one overnight wear of orthokeratology lenses on tear composition. Optom Vis Sci. 2004;81:414–20. doi: 10.1097/01.opx.0000135094.15125.4c. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian SA, Pye DC, Willcox MD. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res. 2012;96:132–7. doi: 10.1016/j.exer.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Pearce DJ, Demirci G, Willcox MD. Secretory IgA epitopes in basal tears of extended-wear soft contact lens wearers and in non-lens wearers. Aust N Z J Ophthalmol. 1999;27:221–3. doi: 10.1046/j.1440-1606.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoshino M, Shoji J, Inada N, Sawa M, Kato H. Clinical evaluation of a measurement method for secretory IgA in tears. Nippon Ganka Gakkai Zasshi. 2006;110:276–81. [PubMed] [Google Scholar]
- 25.Vinding T, Eriksen JS, Nielsen NV. The concentration of lysozyme and secretory IgA in tears from healthy persons with and without contact lens use. Acta Ophthalmol (Copenh) 1987;65:23–6. doi: 10.1111/j.1755-3768.1987.tb08485.x. [DOI] [PubMed] [Google Scholar]
- 26.Willcox MD, Lan J. Secretory immunoglobulin A in tears:functions and changes during contact lens wear. Clin Exp Optom. 1999;82:1–3. doi: 10.1111/j.1444-0938.1999.tb06777.x. [DOI] [PubMed] [Google Scholar]
- 27.Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre-ocular tear layer. Prog Retin Eye Res. 2000;19:649–68. doi: 10.1016/s1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- 28.McClellan KA, Cripps AW, Clancy RL, Billson FA. The effect of successful contact lens wear on mucosal immunity of the eye. Ophthalmology. 1998;105:1471–7. doi: 10.1016/S0161-6420(98)98031-9. [DOI] [PubMed] [Google Scholar]
- 29.Papas EB, Keay L, Golebiowski B. Estimating a just-noticeable difference for ocular comfort in contact lens wearers. Invest Ophthalmol Vis Sci. 2011;52:4390–4. doi: 10.1167/iovs.10-7051. [DOI] [PubMed] [Google Scholar]
- 30.Stahl U, Willcox MD, Naduvilath T, Stapleton F. Influence of tear film and contact lens osmolality on ocular comfort in contact lens wear. Optom Vis Sci. 2009;86:857–67. doi: 10.1097/OPX.0b013e3181ae027b. [DOI] [PubMed] [Google Scholar]
- 31.Dumbleton K, Caffery B, Dogru M, Hickson-Curran S, Kern J, Kojima T, Morgan PB, Purslow C, Robertson DM, Nelson JD. members of the TIWoCLD. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 2013;54:TFOS20–36. doi: 10.1167/iovs.13-13125. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 33.Papas EB, Vajdic CM, Austen R, Holden BA. High-oxygen-transmissibility soft contact lenses do not induce limbal hyperaemia. Curr Eye Res. 1997;16:942–8. doi: 10.1076/ceyr.16.9.942.5049. [DOI] [PubMed] [Google Scholar]
- 34.Barton K, Monroy DC, Nava A, Pflugfelder SC. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997;104:1868–74. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M, Mishima H, Otori T. Detection of interleukin-1 beta in the tear fluid of patients with corneal disease with or without conjunctival involvement. Jpn J Ophthalmol. 1997;41:63–6. doi: 10.1016/s0021-5155(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 36.Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–84. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 37.Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernandez I, Carreno E, Garcia-Vazquez C, Herreras JM, Calonge M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci. 1994;35:3493–504. [PubMed] [Google Scholar]
- 39.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 41.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 42.Tishler M, Yaron I, Geyer O, Shirazi I, Naftaliev E, Yaron M. Elevated tear interleukin-6 levels in patients with Sjogren syndrome. Ophthalmology. 1998;105:2327–9. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Shi BY, He SX, Yao XL, Willcox MDP, Zhao ZJ. Changes to tear cytokines of type 2 diabetic patients with or without retinopathy. Mol Vis. 2010;16:2931–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Malvitte L, Montange T, Vejux A, Baudouin C, Bron AM, Creuzot-Garcher C, Lizard G. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91:29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayanan S, Miller WL, McDermott AM. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest Ophthalmol Vis Sci. 2006;47:2445–50. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- 46.Hagan S, Tomlinson A. Tear fluid biomarker profiling: A review of multiplex bead analysis. Ocul Surf. 2013;11:219–35. doi: 10.1016/j.jtos.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Craig JP, Willcox MD, Argueso P, Maissa C, Stahl U, Tomlinson A, Wang J, Yokoi N, Stapleton F. members of TIWoCLD. The TFOS International Workshop on Contact Lens Discomfort: Report of the Contact Lens Interactions With the Tear Film Subcommittee. Invest Ophthalmol Vis Sci 2013; 54(11):TFOS123–56. [DOI] [PubMed] [Google Scholar]
- 48.Huang D, Xu N, Song Y, Wang P, Yang H. Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2012;250:619–25. doi: 10.1007/s00417-011-1863-x. [DOI] [PubMed] [Google Scholar]
- 49.Sitaramamma T, Willcox MDP, Sack RA, Shivaji S, Morris CA. Homeostatic mechanisms that operate in the tear film during eye closure – identification of tear borne complement regulators. In: Husband AJ, Cripps AW, Beagley KW, editors. Mucosal Solutions: Advances in Mucosal Immunology. Vol 1: University of Sydney; 1997. p. 337–46. [Google Scholar]
- 50.Essink AW, Arkesteijn GJ, Notermans S. Interference of lysozyme in the sandwich enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1985;80:91–6. doi: 10.1016/0022-1759(85)90167-x. [DOI] [PubMed] [Google Scholar]
- 51.Kalsow CM, Reindel WT, Merchea MM, Bateman KM, Barr JT. Tear cytokine response to multipurpose solutions for contact lenses. Clin Ophthalmol. 2013;7:1291–302. doi: 10.2147/OPTH.S44642. [DOI] [PMC free article] [PubMed] [Google Scholar]