Abstract
A series of diterpenoid derivatives based on podocarpic acid were synthesized and evaluated as anti-influenza A virus agents. Several of the novel podocarpic acid derivatives exhibited nanomolar activities against an H1N1 influenza A virus (A/Puerto Rico/8/34) that was resistant to two anti-influenza drugs, oseltamivir and amantadine. This class of compounds inhibits the influenza virus by targeting the viral hemagglutinin-mediated membrane fusion. These results indicated that podocarpic acid derivatives may serve as potential drug candidates to fight drug-resistant influenza A virus infections.
Keywords: Influenza A, influenza inhibitor, podocarpic acid, totarol, hemagglutinin
Influenza virus infection causes a contagious respiratory illness known as the flu. During the peak of the flu season, 5–10% of adults and 20–30% of children may be affected worldwide. Severe illness and death can occur especially among high risk populations.1 Influenza type A virus (IAV) infections have caused several major pandemics in the past with grave impacts on global health.2−5 The IAV genome contains eight negative-stranded RNA segments that encode for 11 viral proteins including hemagglutinins (HA1; HA2), matrix 1 (M1), matrix 2 (M2), nucleoprotein (NP), nonstructural protein 1 (NSP), polymerase acidic protein (PA), polymerase basic proteins (PB1; PB2; PB1-F2), and neuraminidase (NA).6 IAVs are classified into subtypes based on combinations of the viral surface proteins HA and NA. The H1N1 and H3N2 are two currently circulating IAV subtypes. IAVs have evolved a promiscuous entry process that uses sialic acid-containing molecules as receptors.6 This allows the virus to infect host cells across various animal species. Avian influenza viruses, such as H5N1, have been circulating in recent years. Although the transmission of avian flu viruses to humans is inefficient, the viruses are highly pathogenic and may pose a threat if they acquire the ability of efficient human-to-human transmission.
Two NA inhibitors, oseltamivir and zanamivir, are the current FDA-approved drugs recommended by the US CDC for clinical use against recently circulating influenza viruses.7 However, emerging drug resistance and limited effectiveness associated with the NA inhibitors have been reported.8−10 Older drugs such as amantadine and rimantadine that target the M2 ion channel of the virus were approved for treatment and prevention of IAV infection. However, many strains of the influenza virus, such as the 2009 H1N1 influenza virus, are resistant to these two drugs.11 Thus, novel anti-influenza virus agents are needed to circumvent the limitations of currently available drugs.
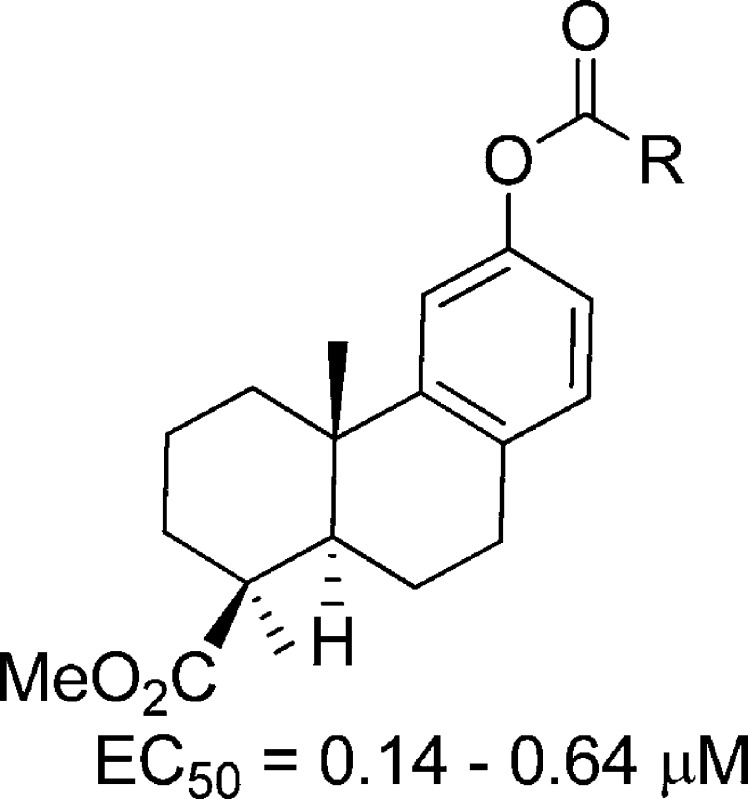
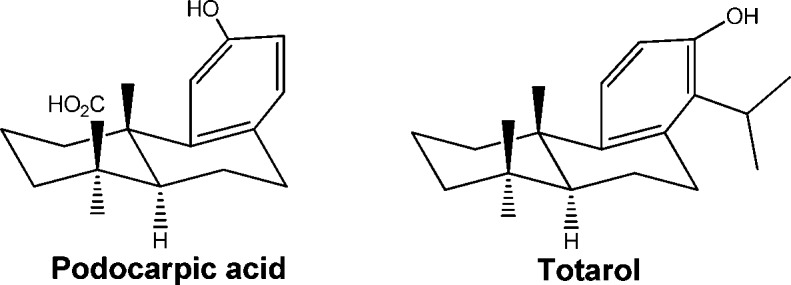
In an effort to identify new anti-IAV agents, we have screened over a thousand natural products for their potential activity against IAV. As a result, several classes of natural products were identified to have anti-IAV activity, including quinolizidine alkyloids.12 In this study, we found that two diterpenoids, (+)-podocarpic acid (PA) and (+)-totarol, could inhibit IAV at low micromolar concentrations. PA and totarol are both abietane type tricyclic phenolic diterpenoids (Figure 1) and can be found in resins from the New Zealand conifers Dacrydium cupressinum(13) and Podocarpus totara.(14) Several different biological activities were reported for these diterpenoids including liver X receptor agonist activity and cytokine release inhibition activity for PA derivatives.15−18 Totarol was reported to have antiplasmodial, antifungal, and antimicrobial activities.19−23 Methyl O-methyl-7-ketopodocarpate, a PA analogue, was identified as an inhibitor of influenza virus that targets the hemagglutinin-mediated membrane fusion.24 To further improve the anti-IAV activity of abietane-type diterpenoids, we synthesized and evaluated 22 derivatives for their anti-IAV activities, including a variety of esters and amides (2, 3, 22, 23) and O-acyl (4–9, 12–19) and O-alkyl derivatives (10, 11, 20, and 21).
Figure 1.
Structure of diterpenoids.
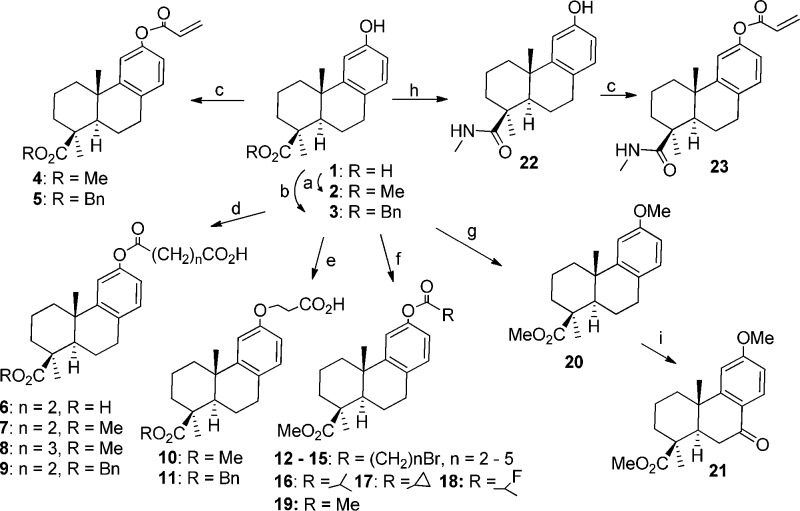
The synthesis of novel diterpenoid derivatives described herein is outlined in Scheme 1. PA derivatives 2 and 19–21 were known compounds synthesized by the previously reported methods.25,26 Benzyl ester (3) was prepared by treating PA with benzyl bromide in the presence of potassium carbonate under microwave heating. PA was converted into compound 22 by treatment with the coupling reagent BOP in the presence of methylamine hydrochloride and THF. Compounds 4, 5, and 23 were synthesized by coupling acrylic acid with either 2, 3, or 22. Compounds 6–9 were obtained by coupling the phenol with corresponding dicarboxylic acid anhydride under sodium hydride mediated-esterification. Treatment of methyl or benzyl podocarpate with 3-bromopropionic acid in the presence of sodium hydride resulted in phenolic ether compounds 10 and 11. Microwave heating of different ω-bromocarboxylic acid or other carboxylic acid reagents with methyl podocarpate (2) in the presence of DCC and DMAP resulted in corresponding esters 12–18. Compound 19 was synthesized by treating 2 with acetic anhydride in the presence of pyridine at room temperature.
Scheme 1. Synthesis of Podocarpic Acid Derivatives.
Reagents and conditions: (a) NaOH, MeOH, Me2SO4, rt, 30 min, 70 °C, 15 min; (b) BnBr, K2CO3, Ac2O, 90 °C, MW, 30 min; (c) acrylic acid, DCC, DMAP, DCM, 90 °C, 15 min; (d) NaH, DMF, succinic or glutaric anhydride, 0 °C, 30 min; (e) NaH, DMF, BrCH2CH2COOH, rt, overnight; (f) RCOOH, DCC, DMAP, DCM, 110 °C, MW, 45 min or anhydride, pyridine, rt, overnight; (g) NaH, Me2SO4, 0 °C, 50 min, rt, overnight; (h) BOP, MeNH2·HCl, TEA, THF, rt, overnight; (i) CrO3, Ac2O, AcOH, 0 °C, 1 h, rt, overnight.
The new diterpenoid derivatives as well as PA and totarol were tested for their activity against a cell-line adapted influenza virus A/Puerto Rico/8/34 (H1N1) (PR8) infection of Madin-Darby canine kidney (MDCK) cells. The PR8 virus is resistant to both oseltamivir and amantidine with an EC50 greater than 20 μM, as shown in Table 1. PA inhibited the PR8 virus with moderate potency at an EC50 of 18.2 μM. The methyl podocarpate (2) and its O-acetyl derivative (19) improved anti-IAV potency over PA by approximately 100-fold with an EC50 at 0.16 and 0.14 μM, respectively. The benzyl ester (3), however, was only ten times more potent than PA. Amide modification on the carboxylic acid caused a great loss of activity as seen in 22. Modifications of the phenol group (R1) on compound 2 or 3 to an ester or ether did not improve anti-IAV potency, though the ester compounds (4, 5, 7, 8, 12–18) did remain active with EC50s ranging from 0.15 to 0.64 μM. The compounds with an ether substituent at R1 and with a propionic acid side chain similar to that of 7 suffered further loss of activity as seen in 10 and 11. Meanwhile, compound 20 with a methoxyl substituent at R1 remained relatively active with an EC50 at 0.73 μM. In addition, the previously reported C7 keto analogue (21)24 was about 5 times less active against the PR8 virus than its precursor 20. Totarol (24) possesses a similar abietane scaffold to that of PA but with different substitutions on C4 and the aromatic ring. Totarol was about 5 times more potent than PA against the PR8 virus.
Table 1. Anti-IAV Activities of Diterpenoidsa.
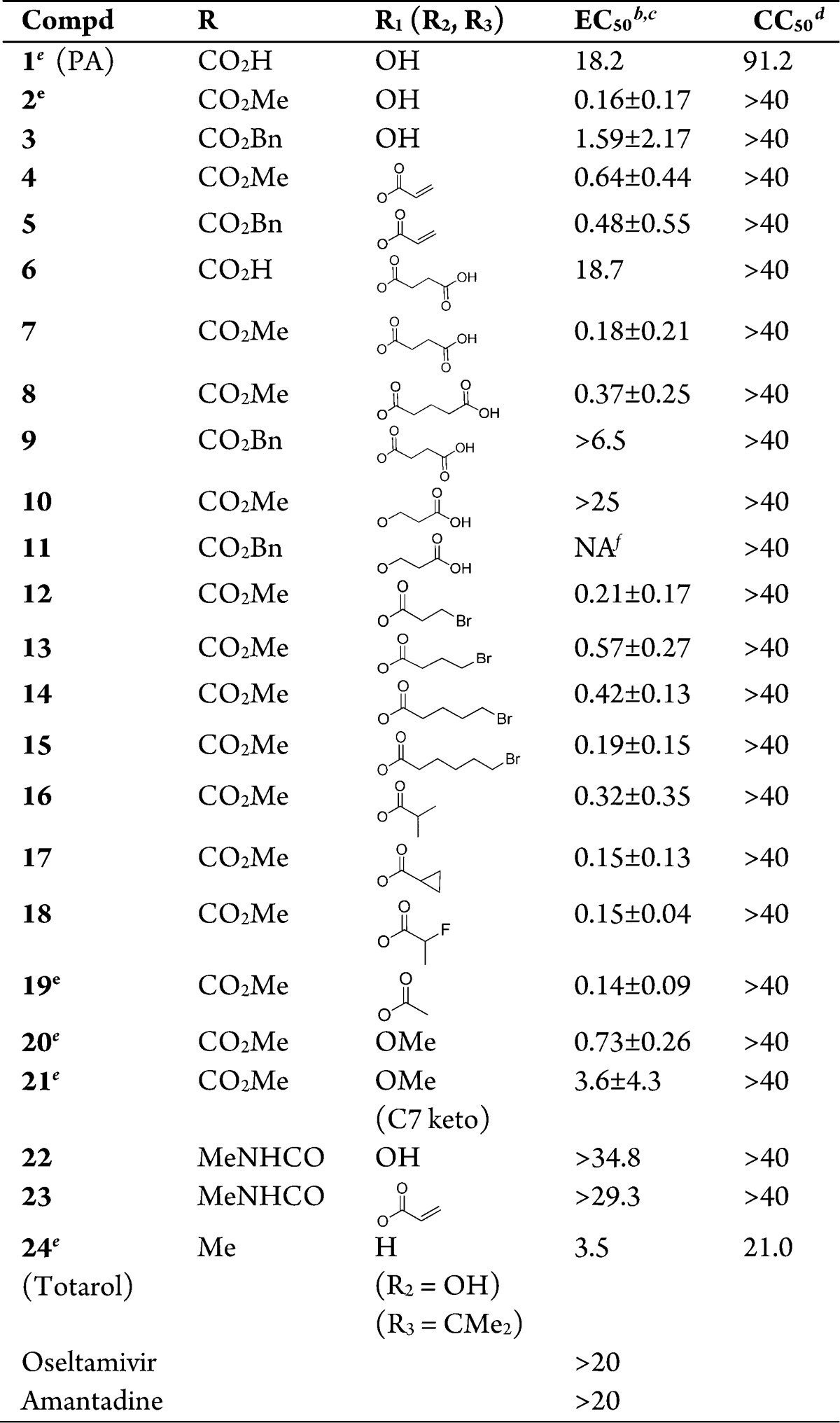
An influenza virus strain A/Puerto Rico/8/1934, PR8, was used in the assays.
Concentration (μM) required to protect cells from the cytocidal effect of PR8 by 50%.
The EC50 (μM) is presented as mean ± SD from at least three independent tests.
Concentration (μM) that reduced the viability of MDCK cells by 50%.
Known compounds.
NA, Not active.
We next investigated whether compounds that were active against the PR8 virus could also inhibit an H3N2 virus A/Hong Kong/8/68 (VR1679), an oseltamivir sensitive IAV. None of the tested podocarpate derivatives inhibited the VR1679 virus by greater than 50% at a concentration as high as 20 μM using the cytocidal protection assay described in Table 1.
Totarol was identified as having anti-VR1679 activity in a process of screening new anti-IAV natural products with structures similar to podocarpates. Because of the weak activity of podocarpate derivatives against the VR1679 virus, confocal microscopy was used to study the anti-IAV activity of the compounds and to compare their relative potency against the PR8 and VR1679 viruses. The viral infection was established in the presence of totarol or the PA derivative 12. The virus infected cells were stained green with an antibody against IAV NP. As shown in the confocal microscopic images in Figure 2, VR1679 efficiently infected MDCK cells in the absence of anti-IAV compounds (Control). Cells treated with 10 μM of 12 showed strong inhibition of PR8 infection and mild inhibition of VR1679. However, totarol was clearly more potent than 12 against VR1679 at the same concentration. Even though totarol was approximately 15-fold less potent than 12 against PR8 (Table 1), totarol was more potent than 12 in inhibiting the H3N2 VR1679. These results suggest that totarol displays less subtype specific antiviral activity against H1N1 and H3N2 viruses than compound 12.
Figure 2.
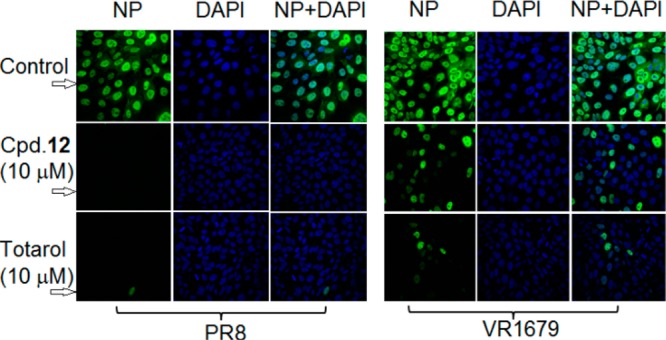
Effects of compound 12 and totarol on IAV infected MDCK cells. MDCK cells were infected with PR8 or VR1679 at MOI = 1 in the presence of 10 μM of 12 or totarol for 6 h. The cells were stained with FITC conjugated anti-NP antibodies (green) and DAPI (nuclear stain, blue). The top panel (control) shows viral infection in the absence of the compounds. The confocal images were acquired using a Nikon A1R confocal microscope.
Compound 21 is a podocarpate previously shown to target the HA of IAV for its moderate antiviral activity.24 To determine if the synthesized podocarpate derivatives also target the HA of IAV, compound 4 was tested in an HA-mediated hemolysis assay. Oseltamivir, a neuraminidase inhibitor, was used as a negative control. As expected, oseltamivir did not inhibit the PR8-HA mediated hemolysis. However, compound 4 significantly inhibited the HA-mediated hemolysis in a dose-dependent manner (Figure 3). This result suggests that compound 4 inhibited IAV PR8 by targeting HA.
Figure 3.
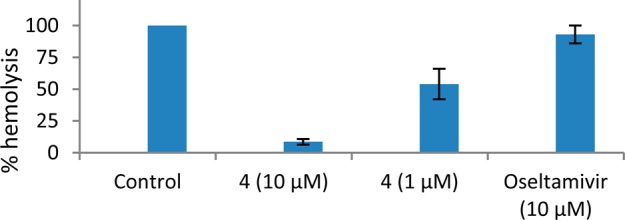
Podocarpates inhibited PR8-HA-mediated hemolysis. A hemolysis inhibition assay was carried out to evaluate the effects of PA derivatives on the HA-mediated hemolysis of chicken erythrocytes.27 Compound 4 or oseltamivir was mixed with the PR8 virus for 30 min at room temperature before adding chicken erythrocytes. The mixture was then incubated at 37 °C for another 30 min. To trigger hemolysis, the suspension containing the erythrocyte-virus-compound mixture was acidified to pH = 5.2 with a 0.15 M sodium acetate buffer. Hemolysis was quantified by measuring the OD540 of supernatants. The HA-mediated hemolysis in the absence of compounds is defined as 100% hemolysis. The results represent the average of two independent tests.
The synthesized podocarpic acid derivatives contain one or two ester linkages, which may be subjected to degradation by serum esterases. To test their plasma stabilities, two of the potent podocarpic acid derivatives, 2 (a mono ester) and 19 (a diester), were incubated with 80% human plasma at 37 °C for various durations shown in Figure 4. The initial and remaining quantities of parent compounds at various time points were determined using reverse phase HPLC. The results indicated that 2 with a methyl ester was relatively stable with 86% of the parent compound intact after 2 h of incubation in human plasma (Figure 4). However, the phenolic ester in 19 was unstable under this condition with only 30% of parental compound remaining after 10 min. This result suggests that evaluation of plasma stability of podocarpic acid derivatives may be important in selecting compounds for further preclinical drug development.
Figure 4.
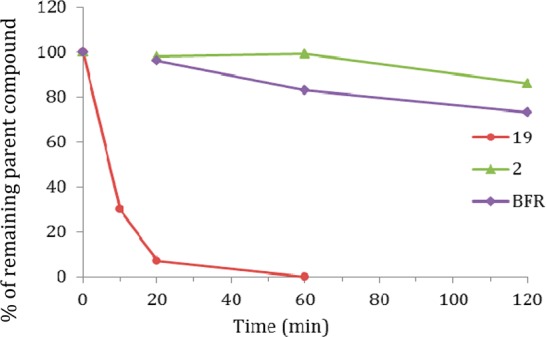
Stability of podocarpic acid derivatives in human plasma. Benfluorex (BFR) was used as a control.28 Each data point represents the average of a duplicated experiment. Detailed methodology was included in the Supporting Information.
In summary, among the synthesized PA derivatives, methyl podocarpate (2) and its O-acetyl derivative (19) displayed the most potent activity against the oseltamivir-resistant PR8 infection of MDCK cells with an EC50 of about 160 and 140 nM, respectively. The potency of these two compounds is approximately 20-fold greater than that of the previously reported podocarpate compound 21. The target of PA derivatives appears to be the HA of the influenza virus. This is consistent with a previous study by Staschke et al. that the podocarpate, compound 21, inhibited influenza viruses by blocking HA-mediated membrane fusion.24 The hemagglutinin protein is crucial for viral entry. Although the synthesized PA derivatives could potently inhibit the PR8 virus, they were significantly less active against the H3N2 virus VR1979. Totarol, however, likely has a broader spectrum of anti-IAV activity. Therefore, totarol is a promising new scaffold for the synthesis of anti-IAV agents with increased potency and breadth of anti-IAV activity.
Glossary
ABBREVIATIONS
- IAV
influenza A virus
- NP
nucleoprotein
- HA
hemagglutinin
- NA
neuraminidase
- DAPI
diamidino-2-phenylindole
- PA
podocarpic acid
Supporting Information Available
Experimental procedures for biological assays, synthesis, and analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by NIH/NIAID Grants AI108347 (to L.H.) and AI65310 (to C.H.C.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- World Health Organization. Health Topics on Influenza. http://www.who.int/topics/influenza/en/. [Google Scholar]
- Das K. Antivirals targeting influenza A virus. J. Med. Chem. 2012, 55, 6263–6277. [DOI] [PubMed] [Google Scholar]
- Reid A. H.; Taubenberger J. K.; Fanning T. G. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001, 3, 81–87. [DOI] [PubMed] [Google Scholar]
- Dawood F. S.; Iuliano A. D.; Reed C.; Meltzer M. I.; Shay D. K.; Cheng P. Y.; Bandaranayake D.; Breiman R. F.; Brooks W. A.; Buchy P.; Feikin D. R.; Fowler K. B.; Gordon A.; Hien N. T.; Horby P.; Huang Q. S.; Katz M. A.; Krishnan A.; Lal R.; Montgomery J. M.; Molbak K.; Pebody R.; Presanis A. M.; Razuri H.; Steens A.; Tinoco Y. O.; Wallinga J.; Yu H. J.; Vong S.; Bresee J.; Widdowson M. A. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [DOI] [PubMed] [Google Scholar]
- Cox N. J.; Subbarao K. Global epidemiology of influenza: Past and present. Annu. Rev. Med. 2000, 51, 407–421. [DOI] [PubMed] [Google Scholar]
- Das J. M.; Aramini J. M.; Ma L. C.; Krug R. M.; Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010, 17, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Influenza Antiviral Medications: Summary for Clinicians, 2014. http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
- WHO. Global Influenza Programme, 2012. http://www.who.int/influenza/en/.
- Ebell M. H.; Call M.; Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam. Pract. 2013, 30, 125–133. [DOI] [PubMed] [Google Scholar]
- Jefferson T.; Jones M. A.; Doshi P.; De l.; Mar C. B.; Hama R.; Thompson M. J.; Spencer E. A.; Onakpoya I.; Mahtani K. R.; Nunan D.; Howick J.; Heneghan C. J. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst. Rev. 2014, 4, CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian A.; Mercorelli B.; Nannetti G.; Compagnin C.; Palù G. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell. Mol. Life Sci. 2014, 71, 3659–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z.; Jung K.; Zhu L.; Lai W. H.; Xie H.; Lee K. H.; Huang L.; Chen C. H. Identification and synthesis of quinolizidines with anti-influenza A virus activity. ACS Med. Chem. Lett. 2014, 5, 942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambie R. C.; Rutledge P. S.; Woodgate P. D. Transformations of podocarpic acid. Aust. J. Chem. 1993, 46, 1447–1471. [Google Scholar]
- Bendall J.; Cambie R. C. Totarol: A non-conventional diterpenoid. Aust. J. Chem. 1995, 48, 883–917. [Google Scholar]
- Liu W.; Chen S.; Dropinski J.; Colwell L.; Robins M.; Szymonifka M.; Hayes N.; Sharma N.; MacNaul K.; Hernandez M.; Burton C.; Sparrow C. P.; Menke J. G.; Singh S. B. Design, synthesis, and structure-activity relationship of podocarpic acid amides as liver X receptor agonists for potential treatment of atherosclerosis. Bioorg. Med. Chem. Lett. 2005, 15, 4574–4578. [DOI] [PubMed] [Google Scholar]
- Singh S. B.; Ondeyka J. G.; Liu W.; Chen S.; Chen T. S.; Li X.; Bouffard A.; Dropinski J.; Jones A. B.; McCormick S.; Hayes N.; Wang J.; Sharma N.; Macnaul K.; Hernandez M.; Chao Y. S.; Baffic J.; Lam M. H.; Burton C.; Sparrow C. P.; Menke J. G. Discovery and development of dimeric podocarpic acid leads as potent agonists of liver X receptor with HDL cholesterol raising activity in mice and hamsters. Bioorg. Med. Chem. Lett. 2005, 15, 2824–2828. [DOI] [PubMed] [Google Scholar]
- He W.; Gavai A.; Huang F. C.; Regan J.; Hanney B.; Poli G.; Bruno J.; Chan W. K.; Djuric S. W.; Yu K. T.; Zilberstein A. Novel cytokine release inhibitors. Part IV: analogs of podocarpic acid. Bioorg. Med. Chem. Lett. 1999, 9, 469–474. [DOI] [PubMed] [Google Scholar]
- Cui Y. M.; Yasutomi E.; Otani Y.; Yoshinaga T.; Ido K.; Sawada K.; Ohwada T. Design, synthesis and characterization of podocarpate derivatives as openers of BK channels. Bioorg. Med. Chem. Lett. 2008, 18, 5197–5200. [DOI] [PubMed] [Google Scholar]
- Yamaji K.; Mori S.; Akiyama M.; Kato A.; Nakashima T. The antifungal compound totarol of Thujopsis dolabrata var. hondai seeds selects for fungi on seedling root surfaces. J. Chem. Ecol. 2007, 33, 2254–2265. [DOI] [PubMed] [Google Scholar]
- Clarkson C.; Campbell W. E.; Smith P. In vitro antiplasmodial activity of abietane and totarane diterpenes isolated from Harpagophytum procumbens (Devil’s Claw). Planta Med. 2003, 69, 720–724. [DOI] [PubMed] [Google Scholar]
- Muroi H.; Kubo I. Antibacterial activity of anacardic acid and toatarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [DOI] [PubMed] [Google Scholar]
- Kubo I.; Muroi H.; Himejima M. Antibacterial activity of totarol and its potentiation. J. Nat. Prod. 1992, 55, 1436–1440. [DOI] [PubMed] [Google Scholar]
- Nicolson K.; Evans G.; O’Toole P. W. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes. FEMS Microbiol. Lett. 1999, 179, 233–239. [DOI] [PubMed] [Google Scholar]
- Staschke K. A.; Hatch S. D.; Tang J. C.; Hornback W. J.; Munroe J. E.; Colacino J. M.; Muesing M. A. Inhibition of influenza virus hemagglutinin-mediated membrane fusion by a compound related to podocarpic acid. Virology 1998, 248, 264–274. [DOI] [PubMed] [Google Scholar]
- Nguyen D. M.; Miles D. H. Copper (I)-catalyzed cycloaddition of methyl O-propargylpodocarpate and azides at room temperature. Synth. Commun. 2011, 41, 1759–1771. [Google Scholar]
- Ohwada T.; Tani N.; Sakamaki Y.; Kabasawa Y.; Otani Y.; Kawahata M.; Yamaguchi K. Stereochemical evidence for stabilization of a nitrogen cation by neighboring chlorine or bromine. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 4206–4211. [Google Scholar]
- Zhu L.; Li Y.; Li S.; Li H.; Qiu Z.; Lee C.; Lu H.; Lin X.; Zhao R.; Chen L.; Wu J. Z.; Tang G.; Yang W. Inhibition of influenza A virus (H1N1) fusion by benzenesulfonamide derivatives targeting viral hemagglutinin. PLoS One 2011, 6, e29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L.; Kerns E. H.; Hong Y.; Chen H. Development and application of high throughput plasma stability assay for drug discovery. Int. J. Pharm. 2005, 297, 110–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.