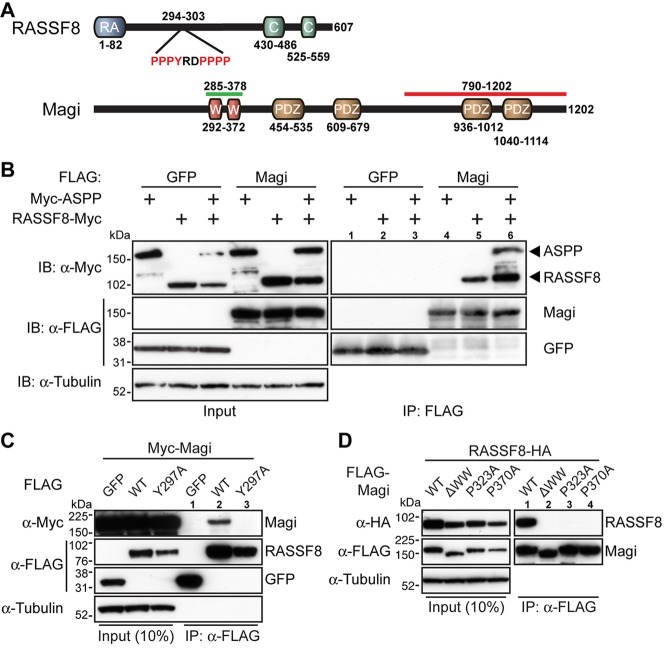
Fig. 2.
Magi binds to RASSF8. (A) Schematics of Drosophila RASSF8 and Magi structure. Highlighted are the RASSF8 residues 294-303 with a PPxY motif, the Magi residues 285-378 (green line) representing the common fragment of the Magi clones identified by two-hybrid analysis using RASSF8 as bait, and the Magi residues 790-1202 (red line) representing the fragment used to raise the anti-Magi antibody. The PDZ (PSD95/Dlg1/Zo1), WW, Ras association (RA) and coiled-coil (C) domains are shown. (B) RASSF8 co-immunoprecipitates with Magi. (Left) Western blot of cleared lysates from cells expressing either FLAG-tagged GFP (FLAG-GFP, negative control) or FLAG-Magi with Myc-ASPP or Myc-RASSF8, or both. (Right) Western blot following immunoprecipitation with anti-FLAG. Myc-RASSF8 co-immunoprecipitates only with FLAG-Magi (lanes 5 and 6) and not with FLAG-GFP (lanes 2 and 3). Myc-ASPP co-immunoprecipitates with FLAG-Magi only in the presence of RASSF8 (lane 6). (C) The PPxY motif in RASSF8 is required for the interaction with Magi. (Left) Western blot of cleared lysates from cells expressing Myc-Magi with either FLAG-GFP (negative control) or FLAG-RASSF8 (WT), or FLAG-RASSF8Y297A in which Y297 is substituted for alanine (Y297A). (Right) Western blot following immunoprecipitation with anti-FLAG. Myc-Magi co-immunoprecipitates only with FLAG-RASSF8 (lane 2) and not with FLAG-GFP (lane 1) or FLAG-RASSF8Y297A (lane 3). (D) The WW domains in Magi are required for the interaction with RASSF8. (Left) Western blot of cleared lysates from cells expressing HA-RASSF8 with different FLAG-Magi mutant constructs: wild-type Magi (WT), Magi deleted of the two WW domains (ΔWW), Magi in which P232 in the first WW is mutated (P232A), and Magi in which P370 in the second WW is mutated (P370A). (Right) Western blot following immunoprecipitation with anti-FLAG. HA-RASSF8 co-immunoprecipitates only with HA-Magi (WT, lane 1) and not with MagiΔWW (ΔWW, lane 2) or Magi with point mutations in the WW domains (lanes 3 and 4).