Abstract
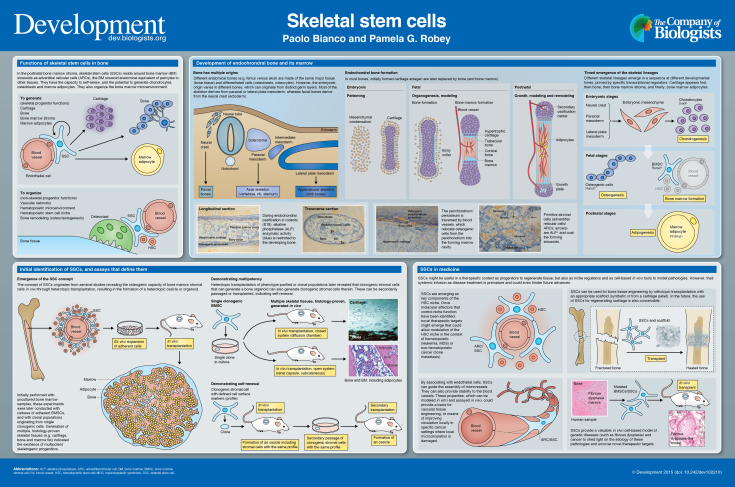
Skeletal stem cells (SSCs) reside in the postnatal bone marrow and give rise to cartilage, bone, hematopoiesis-supportive stroma and marrow adipocytes in defined in vivo assays. These lineages emerge in a specific sequence during embryonic development and post natal growth, and together comprise a continuous anatomical system, the bone-bone marrow organ. SSCs conjoin skeletal and hematopoietic physiology, and are a tool for understanding and ameliorating skeletal and hematopoietic disorders. Here and in the accompanying poster, we concisely discuss the biology of SSCs in the context of the development and postnatal physiology of skeletal lineages, to which their use in medicine must remain anchored.
Keywords: Bone marrow stromal cell, Bone, Cartilage, Hematopoiesis, In vivo transplantation, Skeletal stem cells
Summary: This poster article provides an overview of skeletal stem cells, which reside in the bone marrow and give rise to cartilage, bone, hematopoiesis-supportive stroma and marrow adipocytes.
Introduction
Bone marrow (BM) stroma includes self-renewing, multipotent progenitors for skeletal lineages (cartilage, bone, marrow adipocytes, fibroblasts). These progenitors (skeletal stem cells, SSCs) secure a reservoir of bone-forming cells for bone growth during development, bone modeling (sculpting of bone shape) and bone remodeling (life-long bone turnover); they generate adipocytes during growth and during BM remodeling; and under certain circumstances, they form cartilage. BM stromal cells (BMSCs), including SSCs, also shape and regulate the local microvascular network, regulate differentiation of osteoclasts (bone-resorbing cells), and establish and maintain the hematopoietic microenvironment (HME) necessary for growth and blood cell maturation. In addition, they might be essential for retaining long-term self-renewing hematopoietic stem cells (HSCs) (niche function) [reviewed by Bianco et al. (2013, 2008)]. SSCs, which function as skeletal progenitors and as organizers and regulators of the local BM microenvironment, physically exist as perivascular cells (adventitial reticular cells, ARCs) residing at the outer side of the endothelial lining of BM sinusoids, with a distinct phenotype and reticular morphology (Sacchetti et al., 2007; Bianco et al., 2013). Current evidence ascribes both stem cell and organizer functions to this specific cell subset, while leaving room for further refinement of potential subpopulations therein.
Here and in the accompanying poster, we summarize the emergence of skeletal lineages throughout embryonic development and postnatal growth and adaptation, as a frame necessary and suitable for understanding the biology of postnatal SSCs. We also briefly consider how the inherent properties of SSCs, sculpted through development, can guide their proper use in medicine, both for understanding and for treating diseases.
Origin of the concept of SSCs
The SSC concept originates from seminal studies whereby heterotopic transplants of intact postnatal BM specimens (devoid of bone) or total BM cell suspensions were found to form an ectopic ‘ossicle’, comprising multiple skeletal tissues and mimicking the architecture of the bone-bone marrow organ (Friedenstein et al., 1966; Tavassoli and Crosby, 1968). Subsequent work progressively ascribed this function to a specific cell population. Generation of an ossicle was first assigned to adherent (non-hematopoietic) BMSCs; then, to a clonogenic subset thereof, capable of density-insensitive growth (a progenitor), and, more precisely, to a multipotent clonogenic progenitor, giving rise to cartilage, bone and adipocytes, in vivo (Owen and Friedenstein, 1988). Finally, self-renewal and anatomical and phenotypic identity of clonogenic, multipotent progenitors (i.e. bona fide SSCs) were recognized (Méndez-Ferrer et al., 2010; Sacchetti et al., 2007; Zhou et al., 2014).
Skeletal, not ‘mesenchymal’, stem cells
Clarifying the relationship between SSCs and ‘mesenchymal stem cells’ is not just a matter of semantics. It is essential to elucidate the link between terms, concepts and biological objects, a link often blurred in recent years in the academic and lay usage of terminology. The term ‘mesenchymal stem cells’ (MSCs), currently used to denote cultures of fibroblastic cells from virtually every tissue or organ (da Silva Meirelles et al., 2006), was in fact first introduced to refer to cultures of BMSCs (Caplan, 1991; Pittenger et al., 1999). The concept of ‘MSCs’ as non-HSCs in BM was borrowed from the previously formulated hypothesis of an SSC, but was extended to non-skeletal tissues. To date, evidence from rigorous assays indicates that BMSCs include stem cells for skeletal tissues, and skeletal tissues only. Furthermore, such stem cells can be assayed in BM, and BM only. All the extra-skeletal tissues and organs in which ‘MSCs’ are said to exist (e.g. muscle, placenta, adipose tissue) are developmentally distinct from skeletal lineages, do not contribute to skeletal development or postnatal physiology, do not display skeletogenic properties assayable in vivo and are not generated by skeletal progenitors found in the BM. Cells that can be induced to undergo osteogenesis by BMP signaling [including ligand-independent signaling from constitutively active receptors, as in skeletal muscle in the condition fibrodysplasia ossificans progressiva (Medici et al., 2010; Shore et al., 2006)] do exist in other mesoderm derivatives. However, these ‘inducible’ progenitors must be considered distinct from ‘determined’ progenitors found in BM stroma, which natively express the osteogenic master gene, runt-related transcription factor 2 (Runx2), and require no reprogramming by exogenous BMPs to generate bone. Additionally, there is no evidence that a stem cell with the characteristics of embryonic mesenchyme, or with multipotency beyond the skeletal lineages, exists in postnatal BM or in the postnatal organism. We therefore believe that the term ‘MSC’ is a misnomer and should be abandoned.
Skeletal lineages
The ability to generate cartilage, bone, stroma and marrow adipocytes characterizes SSCs. These are all skeletal lineages: they are anatomically part of the skeleton. These lineages are related to each other in specific developmental processes and are linked to an assayable postnatal progenitor – the SSC. Cartilage, bone, stroma and marrow adipocytes, like individual bones, develop at different times (with cartilage forming first and adipocytes last) and from multiple embryonic lineages. Facial bones derive from neural crest (ectoderm), whereas the rest of the skeleton derives from paraxial or lateral plate mesoderm (Olsen et al., 2000). Thus, two distinct germ layers and multiple specifications of mesoderm give rise during development to the same range of tissues that are generated by postnatal SSCs. Regardless of developmental origin, BM stroma is found in all bones except auditory ossicles, and studies to date have not revealed significant divergence in the properties of BMSCs from different bones. During organogenesis, cartilage, bone, stroma and marrow adipocytes are generated through a sequence of different fate choices that recall binary choices at different developmental stages – with the early lineages forming exclusively at embryonic times, whereas genesis of later lineages extends into fetal, postnatal and adult life – as discussed further below.
Cartilage and bone
Individual skeletal segments form through two distinct processes, referred to as membranous and endochondral ossification. In the latter, which applies to the vast majority of bones, a cartilage anlage is replaced by bone and BM from within. In the former, a cartilage anlage does not form, or is replaced by bone through a different process, as briefly discussed below. The earliest stage of endochondral ossification during embryonic development is the appearance of mesenchymal condensations. Subsequently, chondrogenesis generates cartilage, which forms anlagen for endochondral bones (Hall, 2005; Kronenberg, 2003). In each condensation, central cells transition to cartilage and then mature further into hypertrophic cartilage, later replaced by bone and BM. The most peripheral mesenchymal cells form an envelope, from which the perichondrium (a layer of connective tissue) and articular soft tissues develop (Hall, 2005). The primitive perichondrium gives rise to both chondrocytes and osteogenic cells, and the two lineages are spatially overlapping at specific sites (Ranvier's grooves) (Hall, 2005). As ossification centers and growth plates are established, peripheral (‘borderline’) and hypertrophic chondrocytes (HCs) appear to contribute subsets of bone cells (Bianco et al., 1998a; Riminucci et al., 1998; Yang et al., 2014).
In most membranous bones, chondrogenesis is abortive, reflected only by transient expression of type II collagen (Nah et al., 2000). However, a latent chondrogenic phase in membranous ossification can be revealed under specific experimental conditions (Jacenko and Tuan, 1986). Moreover, true cartilage anlagen do form in the development of certain membranous bones (e.g. parietal bones), only to be replaced by bone via a unique process: the anlagen regress by MT1-MMP-driven matrix degradation and apoptosis of resident chondrocytes, while the definitive bone forms outside (rather than inside, as in endochondral ossification) of the regressing cartilage (Holmbeck et al., 1999, 2003).
Bone and BM stroma
Bone tissue forms before a BM cavity is established: bone cells therefore appear before BM osteoprogenitors. Proliferating progenitors of bone-forming cells occupy the outermost part of the perichondrium/periosteum, generating osteoblasts that deposit the ‘bony collar’ during early ossification. The osteogenic perichondrium includes the presumptive BM stromal osteoprogenitors (Arai et al., 2002; Bianco et al., 1993), as confirmed by lineage-tracing studies (Maes et al., 2010). As bone-resorbing osteoclasts perforate the bony collar and underlying HCs, blood vessels from the osteogenic perichondrium/periosteum invade the forming marrow cavity. Periosteal osteoprogenitors, recruited to a perivascular position, relocate to the developing BM (Arai et al., 2002; Bianco et al., 1993; Streeter, 1949). The primitive, pre-hematopoietic BM is formed of large-caliber sinusoid-type blood vessels in an atmosphere of osteogenic cells, some of which reside at the abluminal surface of blood vessels (Bianco et al., 1993). Blood-borne hematopoietic progenitors then seed this environment (Bianco et al., 1999). Throughout prenatal and postnatal bone growth, the BM stroma found at the metaphyses of long bones retains active proliferation; at the same site, angiogenesis fuels growth of the sinusoidal network, and blood-borne hematopoietic progenitors might continue replenishing the locally growing marrow (Wang et al., 2013). Growth of BM stroma at these sites is regulated by parathyroid hormone (PTH) and PTH-related protein (PTHrP), known players in bone development and postnatal physiology. As bones elongate, site-specific downregulation of the PTH1 receptor (PTH1R) might be necessary for abating local ossification, thus making space for steady-state hematopoiesis (Kuznetsov et al., 2004).
BM stroma and adipocytes
Marrow adipocytes develop postnatally from alkaline phosphatase (ALP)-expressing BMSCs located at the outer aspect of sinusoids (anatomically defined as ARCs) (Bianco et al., 1988). ARCs form during fetal development, when committed osteogenic cells associate with blood vessels; these can be isolated as SSCs at postnatal stages (Bianco, 2011). ARCs share their anatomical location (directly beneath the endothelial layer) with cells called pericytes in other tissues, although BM sinusoids are not the same as capillaries in other tissues. ARCs also express markers otherwise expressed in pericytes of other tissues (Bianco, 2014; Sacchetti et al., 2007). Specific regions of the skeleton (apophyses of long bones, short bones, facial bones in humans; tail vertebrae and short bones in mice) are entirely filled with adipocytes at an early age (yellow marrow); the rest of the skeleton is initially filled with hematopoietically active (red) marrow with few or no adipocytes; the number of adipocytes at these sites increases progressively during skeletal growth and aging, but is also modulated by hematopoietic homeostatic needs (Bianco and Riminucci, 1998). Marrow adipocytes are metabolically distinct from extramedullary fat; they contribute to the regulation of blood flow within BM, and, in this way, to hematopoietic activity (Bianco, 2011). They might represent negative regulators of the HSC niche (Naveiras et al., 2009), as much as SSCs/ARCs appear to be a positive regulator. In postnatal BM, a reciprocal relationship (balance) exists between osteogenesis and adipogenesis (Abdallah and Kassem, 2012; Beresford et al., 1992), which is relevant to studies on osteoporosis and bone aging (Justesen et al., 2001). The direct contribution of postnatal skeletal progenitors to remodeling of bone and BM is in need of more intensive studies (Kassem and Marie, 2011).
Regulation of lineage commitment
Developmental commitment to chondrogenesis, osteogenesis and adipogenesis is regulated by three master transcription factors: sex-determining region Y-box 9 (Sox9; chondrogenic) (Bi et al., 1999), Runx2 (osteogenic) (Komori et al., 1997) and peroxisome proliferator-activated receptor γ2 (PPARγ2; adipogenic) (Muruganandan et al., 2009). The levels of these factors are controlled by members of the transforming growth factor β/bone morphogenetic protein (TGFβ/BMP) superfamily, Wnts (wingless type MMTV integration site) and hedgehogs, with extensive crosstalk [reviewed by Cook and Genever (2013)]. In osteogenic commitment, BMP acts via Msh homeobox/distal-less-related (Msx/Dlx) homeoproteins to increase Runx2 expression, which subsequently increases osterix expression. BMPs also play a role in chondrogenesis by upregulating Sox9. In addition, sonic hedgehog (SHH) signaling increases Nkx3.2, which reduces Runx2, allowing for cartilage induction. Wnt signaling prevents cartilage induction, but controls progression of cartilage hypertrophy along with indian hedgehog (IHH) and PTHrP [reviewed by Cook and Genever (2013)]. Adipogenesis is stimulated by hormonal upregulation of CCAAT enhancer-binding protein (C/EBP) β and δ, which in turn induces PPARγ2. PPARγ2 upregulates C/EBPα, which maintains PPARγ2 expression via a positive-feedback loop. Of note, Wnt signaling promotes osteogenesis by stimulating Runx2 and by inhibiting C/EBPα, thereby inhibiting adipogenesis [reviewed by Cook and Genever (2013)]. In addition, TAZ (transcriptional coactivator with PDZ-binding motif) co-activates Runx2-dependent gene transcription while repressing PPARγ-dependent gene transcription (Hong et al., 2005).
Generating skeletal progenitors from pluripotent stem cells
Study of pluripotent stem cells (PSCs; including embryonic stem cells, ESCs; and induced pluripotent stem cells, iPSCs) provides a way to dissect out the regulatory circuits governing human bone and marrow development, including the origin of SSCs. Many studies have aimed at differentiating ESCs or iPSCs into skeletal tissues [e.g. Harkness et al. (2011); Mahmood et al. (2010)]; several have shown that putative PSC-generated skeletal progenitors could establish histology-proven bone in vivo, but failed to recreate a BM cavity and stroma, and therefore SSCs (Harkness et al., 2011; Hong et al., 2014; Mahmood et al., 2010; Phillips et al., 2014). This finding resonates with the current inability to generate genuine HSCs while generating hematopoietic cells from PSCs (Kaufman, 2009). It is also worth noting that BM stroma and SSCs comprised therein emerge late during bone organogenesis (see above), suggesting that current protocols for generating SSCs from PSCs might fall short of recapitulating the entire process of skeletogenesis, particularly its late events. More generally, the specific characterization of the properties of skeletal progenitors at specific points in developmental time and space stand as an important task ahead.
Isolation and assessment of skeletal stem/progenitor cells
Skeletal progenitors can be isolated based either on surface markers (‘prospectively’) or by establishing clonal adherent cultures. As long as clonogenicity assays remain the mainstay of characterization of cells isolated based on surface markers, and as long as cell culture remains necessary prior to transplantation in vivo, isolation by either surface marker or by adherence and clonogenicity yield essentially identical results. Multipotency can only be assayed at the single cell level; i.e. in clones originating from a single colony-forming unit-fibroblast (CFU-F). All analyses of single clones have confirmed a major diversity across clones (growth and differentiation capacity) [e.g. Kuznetsov et al. (1997)], possibly reflecting a hierarchy (Muraglia et al., 2000). Although hugely popular, assessment of immunophenotype in vitro and assays of multipotency in non-clonal cultures do not define a culture as a culture of SSCs or of stem cells (Bianco et al., 2013), and significantly confound the assessment of stem cell growth, differentiation and self-renewal.
Cell proliferation and colony growth
Regardless of phenotypic homogeneity, all cultures of non-transformed cells are heterogeneous, as their growth kinetics are necessarily asymmetrical: further proliferation, differentiation and senescence of the progeny of a single cell do not occur uniformly, synchronously or at the same rate for all cells in a growing colony. Furthermore, fully symmetrical growth kinetics of a population originating from a single stem cell would expand the stem cell number without generating differentiating cells; or, conversely, would generate differentiating cells without increasing the number of stem cells. As we have no clue about the relative frequency of symmetric versus asymmetric cell divisions in the cultures, the actual content of true stem cells, or progenitors, in a culture cannot be controlled at this time. Thus, referring to any culture of BMSCs as a culture of ‘stem cells’ or of ‘mesenchymal stem cells’ (as in a copious amount of literature) remains ungrounded and misleading.
Differentiation
Heterotopic transplantation of non-doctored, non-induced cultures remains the mainstay for assessing the differentiation capacity of SSCs/BMSCs (Bianco et al., 2008). Surrogate in vitro assays are highly artificial and prone to artifact. This applies particularly to osteogenic and adipogenic differentiation assays, even if combined with limited analyses of gene expression. Cartilage formation in micromass or pellet cultures is more reliable, as it rests on ultimate histological proof of genuine cartilage. However, like the osteogenic and adipogenic assays, loose interpretation of pellet culture results plagues its use (Robey et al., 2014). Cartilage pellets can in turn be transplanted, to the effect of generating ossicles through a unique developmental sequence (Serafini et al., 2014).
Self-renewal
Self-renewal can only be assessed by proving the generation, in vivo, of a cell compartment that is anatomically, phenotypically and functionally equivalent to the one originally explanted, along with differentiated compartments. This requires: (a) definition of the anatomy and phenotype of the isolated population; (b) evidence that the same population is established in vivo; and (c) serial passaging and transplantation (Sacchetti et al., 2007). Extensive numbers of population doublings (PDs) do not prove self-renewal of post-natal cells, but simply a high proliferation capacity.
SSCs in medicine
There are four ways in which SSCs might be useful in therapeutic contexts, including patient treatment: (1) as progenitors, for example, of bone cells for bone tissue engineering (Robey, 2011); (2) as non-progenitors, for example, by guiding microvessel assembly and networking, as key components of the HME/niche or as suppliers of factors and cytokines in the context of specific tissue-engineering strategies (Bianco, 2011; Bianco et al., 2013); (3) as modeling tools in stem cell-based in vivo models of disease (Bianco et al., 1998b); and (4) as a conceptual tool, by revealing specific pathological changes in tissues as a result of dynamics in stem cell biology. It is important to understand these dynamics for therapeutic purposes, as drug-targetable processes might be operating in the stem or progenitor cells rather than in the differentiated cells (Bianco, 2014; Bianco and Riminucci, 1998). All of these modes of SSC impact in medicine need to be harnessed in specific ways, yet to be devised. A premature, empirical rush to clinical application (e.g. by systemic infusion of cells that are not amenable to systemic infusion) will not provide advances and might even prevent them (Bianco et al., 2013).
Concluding remarks
The skeleton provides a key example of a low-turnover system in which bona fide stem cells have been proven to exist and have been characterized in humans and mice (Méndez-Ferrer et al., 2010; Sacchetti et al., 2007). This was carried out using experimental approaches germane to those that revealed the biology of other stem cells systems (i.e. hematopoietic), such as transplantation and single-cell analysis. Recent developments of classical approaches have been instrumental in identifying the SSCs, and remain crucial to the endeavor of probing their functions and regulation in humans. Very recently, advances towards identifying SSCs in the mouse have been reported (Chan et al., 2015; Worthley et al., 2015). These studies reveal novel facets of the ontogeny of the stromal system. Together with a more thorough appreciation of the specific developmental processes briefly discussed herein, these new data might contribute to a broader understanding of the stromal system in mammals.
SSCs have a unique role: they function at the same time as stem cells and as niche cells, as progenitors and as organizers of neighboring cells and tissues that they do not generate, such as nascent blood vessels and the HME. This singularity might rest on cellular properties and regulatory circuitries still to be elucidated. As central to the physiology and disease of two major systems such as the skeleton and blood, SSCs might provide conceptual and practical advances in medicine, provided their specificities are kept in mind.
Acknowledgements
The authors wish to acknowledge all members of their laboratories, past and present, that have contributed to the concepts presented.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by Telethon [GGP09227], the Italian Ministry of Education, Universities and Research (MIUR); Fondazione Roma; Fondazione Institut Pasteur-Cenci Bolognetti; the Italian Ministry of Health; the European Union (PluriMes consortium) and Sapienza University of Rome, Italy (to P.B.); and by the DIR, the National Institute of Dental and Craniofacial Research (NIDCR), a part of the Intramural Research Program (IRP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS) (to P.G.R.). Deposited in PMC for release after 12 months.
Development at a Glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.102210/-/DC1.
References
- Abdallah B. M. and Kassem M. (2012). New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone 50, 540-545 10.1016/j.bone.2011.06.030 [DOI] [PubMed] [Google Scholar]
- Arai F., Ohneda O., Miyamoto T., Zhang X. Q. and Suda T. (2002). Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J. Exp. Med. 195, 1549-1563 10.1084/jem.20011700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford J. N., Bennett J. H., Devlin C., Leboy P. S. and Owen M. E. (1992). Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 102, 341-351. [DOI] [PubMed] [Google Scholar]
- Bi W., Deng J. M., Zhang Z., Behringer R. R. and de Crombrugghe B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85-89 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Bianco P. (2011). Bone and the hematopoietic niche: a tale of two stem cells. Blood 117, 5281-5288 10.1182/blood-2011-01-315069 [DOI] [PubMed] [Google Scholar]
- Bianco P. (2014). “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 30, 677-704 10.1146/annurev-cellbio-100913-013132 [DOI] [PubMed] [Google Scholar]
- Bianco P. and Riminucci M. (1998). The bone marrow stroma in vivo: ontogeny, structure, cellular composition and changes in disease. In Marrow Stromal Cell Culture (ed. Beresford J. N. and Owen M. E.), pp. 10-25 Cambridge, UK: Cambridge University Press. [Google Scholar]
- Bianco P., Costantini M., Dearden L. C. and Bonucci E. (1988). Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br. J. Haematol. 68, 401-403 10.1111/j.1365-2141.1988.tb04225.x [DOI] [PubMed] [Google Scholar]
- Bianco P., Riminucci M., Bonucci E., Termine J. D. and Robey P. G. (1993). Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J. Histochem. Cytochem. 41, 183-191 10.1177/41.2.8419458 [DOI] [PubMed] [Google Scholar]
- Bianco P., Cancedda F. D., Riminucci M. and Cancedda R. (1998a). Bone formation via cartilage models: the “borderline” chondrocyte. Matrix Biol. 17, 185-192 10.1016/S0945-053X(98)90057-9 [DOI] [PubMed] [Google Scholar]
- Bianco P., Kuznetsov S. A., Riminucci M., Fisher L. W., Spiegel A. M. and Robey P. G. (1998b). Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J. Clin. Invest. 101, 1737-1744 10.1172/JCI2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Riminucci M., Kuznetsov S. and Robey P. G. (1999). Multipotential cells in the bone marrow stroma: regulation in the context of organ physiology. Crit. Rev. Eukaryot. Gene Expr. 9, 159-173 10.1615/CritRevEukarGeneExpr.v9.i2.30 [DOI] [PubMed] [Google Scholar]
- Bianco P., Robey P. G. and Simmons P. J. (2008). Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2, 313-319 10.1016/j.stem.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Cao X., Frenette P. S., Mao J. J., Robey P. G., Simmons P. J. and Wang C.-Y. (2013). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 19, 35-42 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. I. (1991). Mesenchymal stem cells. J. Orthop. Res. 9, 641-650 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- Chan C. K. F., Seo E. Y., Chen J. Y., Lo D., McArdle A., Sinha R., Tevlin R., Seita J., Vincent-Tompkins J., Wearda T. et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell 160, 285-298 10.1016/j.cell.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. and Genever P. (2013). Regulation of mesenchymal stem cell differentiation. Adv. Exp. Med. Biol. 786, 213-229 10.1007/978-94-007-6621-1_12 [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L., Chagastelles P. C. and Nardi N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119, 2204-2213 10.1242/jcs.02932 [DOI] [PubMed] [Google Scholar]
- Friedenstein A. J., Piatetzky S. II and Petrakova K. V. (1966). Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 16, 381-390. [PubMed] [Google Scholar]
- Hall B. K. (2005). Bones and Cartilage. San Diego: Elsevier. [Google Scholar]
- Harkness L., Mahmood A., Ditzel N., Abdallah B. M., Nygaard J. V. and Kassem M. (2011). Selective isolation and differentiation of a stromal population of human embryonic stem cells with osteogenic potential. Bone 48, 231-241 10.1016/j.bone.2010.09.023 [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I. et al. (1999). MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81-92 10.1016/S0092-8674(00)80064-1 [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Chrysovergis K., Yamada S. and Birkedal-Hansen H. (2003). MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J. Cell Biol. 163, 661-671 10.1083/jcb.200307061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.-H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A. et al. (2005). TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074-1078 10.1126/science.1110955 [DOI] [PubMed] [Google Scholar]
- Hong S. G., Winkler T., Wu C., Guo V., Pittaluga S., Nicolae A., Donahue R. E., Metzger M. E., Price S. D., Uchida N. et al. (2014). Path to the clinic: assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 7, 1298-1309 10.1016/j.celrep.2014.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacenko O. and Tuan R. S. (1986). Calcium deficiency induces expression of cartilage-like phenotype in chick embryonic calvaria. Dev. Biol. 115, 215-232 10.1016/0012-1606(86)90242-3 [DOI] [PubMed] [Google Scholar]
- Justesen J., Stenderup K., Ebbesen E. N., Mosekilde L., Steiniche T. and Kassem M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165-171 10.1023/A:1011513223894 [DOI] [PubMed] [Google Scholar]
- Kassem M. and Marie P. J. (2011). Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 10, 191-197 10.1111/j.1474-9726.2011.00669.x [DOI] [PubMed] [Google Scholar]
- Kaufman D. S. (2009). Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood 114, 3513-3523 10.1182/blood-2009-03-191304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y.-H., Inada M. et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755-764 10.1016/S0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Krebsbach P. H., Satomura K., Kerr J., Riminucci M., Benayahu D. and Robey P. G. (1997). Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 12, 1335-1347 10.1359/jbmr.1997.12.9.1335 [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Riminucci M., Ziran N., Tsutsui T. W., Corsi A., Calvi L., Kronenberg H. M., Schipani E., Robey P. G. and Bianco P. (2004). The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J. Cell Biol. 167, 1113-1122 10.1083/jcb.200408079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C., Kobayashi T., Selig M. K., Torrekens S., Roth S. I., Mackem S., Carmeliet G. and Kronenberg H. M. (2010). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329-344 10.1016/j.devcel.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A., Harkness L., Schrøder H. D., Abdallah B. M. and Kassem M. (2010). Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. J. Bone Miner. Res. 25, 1216-1233 10.1002/jbmr.34 [DOI] [PubMed] [Google Scholar]
- Medici D., Shore E. M., Lounev V. Y., Kaplan F. S., Kalluri R. and Olsen B. R. (2010). Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 16, 1400-1406 10.1038/nm.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., MacArthur B. D., Lira S. A., Scadden D. T., Ma'ayan A., Enikolopov G. N. and Frenette P. S. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829-834 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraglia A., Cancedda R. and Quarto R. (2000). Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 113, 1161-1166. [DOI] [PubMed] [Google Scholar]
- Muruganandan S., Roman A. A. and Sinal C. J. (2009). Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 66, 236-253 10.1007/s00018-008-8429-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah H.-D., Pacifici M., Gerstenfeld L. C., Adams S. L. and Kirsch T. (2000). Transient chondrogenic phase in the intramembranous pathway during normal skeletal development. J. Bone Miner. Res. 15, 522-533 10.1359/jbmr.2000.15.3.522 [DOI] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P. L., Hauschka P. V., Fahey F. and Daley G. Q. (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259-263 10.1038/nature08099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Reginato A. M. and Wang W. (2000). Bone development. Annu. Rev. Cell Dev. Biol. 16, 191-220 10.1146/annurev.cellbio.16.1.191 [DOI] [PubMed] [Google Scholar]
- Owen M. and Friedenstein A. J. (1988). Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found. Symp. 136, 42-60. [DOI] [PubMed] [Google Scholar]
- Phillips M. D., Kuznetsov S. A., Cherman N., Park K., Chen K. G., McClendon B. N., Hamilton R. S., McKay R. D. G., Chenoweth J. G., Mallon B. S. et al. (2014). Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays. Stem Cells Transl. Med. 3, 867-878 10.5966/sctm.2013-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S. and Marshak D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-147 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- Riminucci M., Bradbeer J. N., Corsi A., Gentili C., Descalzi F., Cancedda R. and Bianco P. (1998). Vis-à-vis cells and the priming of bone formation. J. Bone Miner. Res. 13, 1852-1861 10.1359/jbmr.1998.13.12.1852 [DOI] [PubMed] [Google Scholar]
- Robey P. G. (2011). Cell sources for bone regeneration: the good, the bad, and the ugly (but promising). Tissue Eng. B Rev. 17, 423-430 10.1089/ten.teb.2011.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Kuznetsov S. A., Riminucci M. and Bianco P. (2014). Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol. Biol. 1130, 279-293 10.1007/978-1-62703-989-5_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M. et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324-336 10.1016/j.cell.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Serafini M., Sacchetti B., Pievani A., Redaelli D., Remoli C., Biondi A., Riminucci M. and Bianco P. (2014). Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 12, 659-672 10.1016/j.scr.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Shore E. M., Xu M., Feldman G. J., Fenstermacher D. A., Cho T. J., Choi I. H., Connor J. M., Delai P., Glaser D. L., LeMerrer M. et al. (2006). A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38, 525-527 10.1038/ng1783 [DOI] [PubMed] [Google Scholar]
- Streeter G. L. (1949). Developmental horizons in human embryos (fourth issue): a review of the histogenesis of cartilage and bone. Contr. Embryol. Carneg. Instn. 33, 149-141 167. [PubMed] [Google Scholar]
- Tavassoli M. and Crosby W. H. (1968). Transplantation of marrow to extramedullary sites. Science 161, 54-56 10.1126/science.161.3836.54 [DOI] [PubMed] [Google Scholar]
- Wang L., Benedito R., Bixel M. G., Zeuschner D., Stehling M., Sävendahl L., Haigh J. J., Snippert H., Clevers H., Breier G. et al. (2013). Identification of a clonally expanding haematopoietic compartment in bone marrow. EMBO J. 32, 219-230 10.1038/emboj.2012.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley D. L., Churchill M., Compton J. T., Tailor Y., Rao M., Si Y., Levin D., Schwartz M. G., Uygur A., Hayakawa Y. et al. (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160, 269-284 10.1016/j.cell.2014.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tsang K. Y., Tang H. C., Chan D. and Cheah K. S. E. (2014). Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 111, 12097-12102 10.1073/pnas.1302703111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. O., Yue R., Murphy M. M., Peyer J. G. and Morrison S. J. (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154-168 10.1016/j.stem.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]