Abstract
Studies have documented that cancer patients with tumours which are highly infiltrated with cytotoxic T lymphocytes show enhanced survival rates. The ultimate goal of cancer immunotherapy is to elicit high-avidity tumour-specific T cells to migrate and kill malignant tumours. Novel antibody therapies such as ipilumimab (a cytotoxic T lymphocyte antigen-4 blocking antibody) show enhanced T cell infiltration into the tumour tissue and increased survival. More conventional therapies such as chemotherapy or anti-angiogenic therapy and recent therapies with oncolytic viruses have been shown to alter the tumour microenvironment and thereby lead to enhanced T cell infiltration. Understanding the mechanisms involved in the migration of high-avidity tumour-specific T cells into tumours will support and provide solutions for the optimization of therapeutic options in cancer immunotherapy.
Keywords: cancer, T cells, tumour immunology
Introduction
The presence of high numbers of cytotoxic T lymphocytes (CTLs) in the tumours of cancer patients correlates with enhanced survival 1–3. Several murine models have shown the capability of cancer immunotherapy to elicit strong avidity T cell responses in vivo after vaccination. In adoptive cell therapy experiments, high-avidity T cells were more effective at eliminating lung metastases from B16 melanoma than low-avidity T cells 4–6. The potential to stimulate the immune system and generate high-avidity effector T cells that localize and kill tumours is the ultimate goal of cancer immunotherapy. This review discusses the mechanisms behind T cell recruitment and infiltration to the tumour site and addresses current therapies that result in improved T cell infiltration. Clinical trials that monitor T cell infiltration are limited, and we highlight throughout the text whether the studies have been performed in animal models or in clinical trials and which cancer has been studied. The basis of our conclusions are that these findings may well apply to other tumour types.
Trafficking of T cells
Migrating lymphocytes are essential to regulate efficient immunological mechanisms. The initiation step of these cell-mediated immune responses includes T cell trafficking to specific tissues. In this context, naive T cells migrate through specialized endothelium of secondary lymphoid organs. In contrast, primed T cells exert their function by infiltration through post-capillary venules into the target tissues to their antigenic site. The activation and differentiation into effector or memory lymphocytes trigger the expression of specific receptors. This migration from the peripheral blood to the tissue is a process that includes tethering, rolling and adhesion followed by diapedesis or transmigration through the endothelial cell barrier, which covers the inner wall of blood vessels 7–14. The mechanisms of T cell extravasation from the blood to the site of infection have been covered in other reviews, and therefore will not be discussed in detail in this review 10–12,14,15.
Chemokines
Chemokines are involved in the recruitment of lymphocytes. The expression and secretion of these chemokines by the tissue or the endothelium has been shown to have an effect on specific T cell recruitment. During T cell activation, the chemokine environment plays a pivotal role and dictates the trafficking behaviour of lymphocytes. An example is the expression of the CCR5 and CXCR3 receptors on T effector cells within the T helper type 1 (Th1) subset. The CCR5 ligands, CCL5 and macrophage inflammatory proteins (MIP-1α), are known to be produced by activated dendritic cells. Enhanced CXCR3 expression on activated infiltrating lymphocytes has been described in inflammatory diseases. The CCR5 and CXCR3 chemokine receptors may therefore play a pivotal role in the regulation of leucocyte migration to inflammatory sites 1,16–18. The CCR3, CCR4, CCR8 and CXCR4 are shifted towards the Th2 subset. CXC chemokine ligand (CXCL)12 (SDF-1), which binds to the receptor CXCR4, has previously been shown to be chemotactic for a number of leucocyte populations, including neutrophils, monocytes, lymphocytes and, more recently, eosinophils 19.
Within the tumour environment, chemokine expression will have an effect not only on leucocyte migration but also on tumour metastasis, tumour angiogenesis and tumour cell proliferation 20. Tumours often over-express certain chemokines which dysregulate the immune response. For example, chemokine ligand (CCL)22 in ovarian and breast cancer has been shown to be responsible for the accumulation of regulatory T cells (Tregs) within tumours forming an immune suppressive microenvironment 21. CCL2 has been shown to increase infiltration of tumour-associated macrophages (TAMS) in colorectal cancer and to be associated with progression of the cancer 22.
In melanoma, the lack of certain chemokines (CCL2, CCL3, CCL4, CCL5, CXCL9 and CXCL10) in metastases has been associated with limited infiltration of antigen-specific T cells 23,24. This might represent an important barrier for effective T cell-mediated tumour rejection. Indeed, when a subset of melanoma cells producing a broad array of these chemokines was implanted as a xenograft in murine models, CD8+ T cells were recruited into the tumour 23. In their turn, macrophages, endothelial cells and recruited T cells are key mediators for chemokine secretion and can positively enhance the recruitment and infiltration of antigen-specific T cells into the tumour tissue 23.
Increased infiltration of CD4 and CD8 T cells in colorectal cancer has been associated with the chemokine CXCL16 and the receptor CXCR6, resulting in increased survival 25. Additionally, CXCL12 has been shown to be a T cell attractant which binds to the CXCR4 receptor. However, T cell infiltration is dependent upon the concentration of CXCL12 in the microenvironment, as it attracts T cells at low concentrations and repels them at high concentrations 26, a process known as ‘leucocyte fugetaxis’ 15. High concentrations of CXCL12 in cervical cancer have also been correlated with intratumoural accumulation of forkhead box protein 3 (FoxP3)+ regulatory T cells, leading to an immune-suppressive environment that correlates with tumour progression 17. However, CXCL12 has an impact not only on the T cells, but has also been associated with tumour metastasis 17,27–29, and high levels of CXCL12 in colorectal cancer have been shown to lead to reduced patient survival 30. In addition, CXCR4 is involved in tumour cell homing to specific organs and in tumour progression 29.
Therefore, patients' survival is dictated in part by which inflammatory cells infiltrate the tumour, and this is dependent upon which chemokines are expressed by the tumour and their effect on the tumour cells themselves.
Antigen-specific T cell recruitment to tissues
However, for an effective therapy it is not only the number of tumour-infiltrating T cells which are crucial; they also need to be antigen-specific. To enhance recruitment of antigen-specific T cells, Marelli-Berg proposed a ‘shop window’ model where endothelial cells display the contents of the underlying tissues on their major histocompatibility complex (MHC) as items for display in a shop. In this way, antigen-specific T cell migration is enhanced by the recognition of their specific peptides via MHC expressed on endothelial cells. Upon recognition of an antigen, primed T cells are able to access non-lymphoid tissues 7–11,21. Using an in-vivo model of HY-specific CD8 T cell transmigration into antigenic and non-antigenic tissue, up-regulation of MHC molecules by interferon (IFN)-γ in peritoneal mesothelium led to recruitment of HY-specific T cells in male but not in female mice. This MHC recognition was shown to enhance diapedesis without affecting rolling or adhesion 9. Marelli-Berg et al. hypothesized that this MHC recognition is essential for T cell recruitment to the specific target site and is a further mechanism of immunoregulation. Within this ‘shop window’ model the tissue-derived antigens are incorporated, processed and then presented via MHC on endothelial cells. If this were to apply in the tumour setting, then endothelial cells would have to present tumour antigens on their MHC. However, a major obstacle of this proposed model in a tumour setting is the dependence on the vasculature of the tumour itself. The endothelium within the tumour is constructed of a heterogeneous cell population. Here, the endothelium not only shows a higher rate in proliferation; the blood vessels are also dilated and fail to have a basement membrane.
Improving T cell infiltration into tumours
There is an increased recognition that many types of cancer treatment have an effect on the immune system. High-dose chemotherapy has a negative effect on the immune system by causing neutropenia and lymphopenia. However, recent work has highlighted the more positive effects of chemotherapy, ipilimumab and anti-angiogenic therapies on altering the tumour environment and improving T cell migration into tumours.
Improving T cell infiltration with chemotherapy
Chemotherapy is often used as a first-line therapeutic option for most cancer-associated malignancies, but its interaction with the immune system is still not fully understood. Chemotherapeutics at high doses are immunosuppressive (e.g. cyclophosphamide) 24,31,32. However, the administration of low-dose chemotherapy in short intervals (metronomic chemotherapy) causes mild and short-lived side effects in comparison to other therapeutic regimens. Various chemotherapies given in a low-dose metronomic regimen promote disease eradication by stimulating anti-cancer immune reactions, selectively eliminating immunosuppressive cells 32, inducing an anti-angiogenic activity or modifying the tumour microenvironment by altering the chemokine profile.
Increased tumour-infiltrating lymphocytes have been observed in breast cancer after neoadjuvant paclitaxel chemotherapy 33, while dacarbazine treatment promotes stromal remodelling and enhances lymphocyte infiltration into cutaneous melanoma lesions due to the alteration of genes involved in the cell cycle, epidermis development, response to wounding and extracellular matrix (ECM) organization, and immune response (CD8A, CD2, granzymes, the chemokine receptor CXCR3, the chemokine CCL5, CD96, TNFRSF7, TNF and RAC2). As chemotherapies such as dacarbazine induce intratumoural expression of the chemokine CCL5, this may lead to the higher T cell infiltration observed 31. In melanoma tissue samples, an increased T cell infiltration was observed after chemotherapy treatment. CXCR3 and CCL5 ligands were identified as the main determinants of T cell infiltration. It was postulated that the induction of chemokine expression in cancer cells due to the therapy itself led to the higher infiltration of T cells 24.
The ability of the combination of chemotherapy and vaccines to increase immune responses was shown in a clinical trial in extensive stage small cell lung cancer. After the administration of a cancer vaccine (adenoviral vector including wild-type p53), 57·1% of the treated patients showed a p53-specific T cell response, while an additional chemotherapy regimen resulted in 75% of patients having an immune response to p53 34. Further combinations of immunotherapy and chemotherapy have been shown to prolong survival in clinical trials 32. However, analyses of T cell infiltration in clinical trials have been limited.
Murine models have allowed further exploration of the role of chemotherapy on the immune system. In a murine mammary tumour model (4T1), which can be compared to a human stage IV metastatic cancer, Alizadeh et al. demonstrated that the administration of doxorubicin led to the elimination of myeloid-derived suppressor cells (MDSCs) and increased proliferation and activation of T cells 35. Hu et al. observed an enhanced T cell infiltration after a doxorubicin and interleukin (IL)-12 treatment. They demonstrated that this combination led to an increased expression of natural killer group 2D (NKG2D) within the infiltrating T cells which was responsible for their accumulation within the tumour 35.
Also, a combination of cancer vaccines and chemotherapy may serve to activate T cells and lead to tumour infiltration. Doxorubicin and paclitaxel, in combination with a human epidermal growth factor receptor 2 (HER2)/neu vaccine in a murine mammary carcinoma model, showed enhanced efficacy and also increased antigen-specific CD8+ T cells. Similarly, in a granulocyte–macrophage colony-stimulating factor (GM-CSF) secreting whole cell vaccine in HER2/neu tolerized mice, doxorubicin and paclitaxel were shown to increase antigen-specific T cells and enhance the ability of the vaccine to delay tumour growth 36.
Therefore, as described, studies have demonstrated the effect of chemotherapy on T cell infiltration, but further research is necessary to determine the optimal chemotherapy regimen in combination with immunotherapy.
Regulatory T cell depletion (Tregs) by chemotherapy and other regimes
The depletion of Tregs has been associated with increased T cell infiltration. High endothelial venules (HEV) are specialized blood vessels found normally in secondary lymphoid tissues involved in T cell recruitment. The presence of HEV in tumours has been correlated with an increase in tumour infiltrating T cells 37. In a murine model, depletion of T regulatory cells resulted in increased numbers of HEV and an associated increase in T cell infiltration 38.
Metronomic low-dose chemotherapeutic treatment (MCT) is able to enhance anti-tumour immune responses due to the depletion or neutralization of Tregs and offers a novel concept in cancer treatment. A metronomic low-dose application of cyclophosphamide over a longer period of time inhibits the reformation of Tregs in vivo 32. Cyclophosphamide has also been used in a murine model to deplete Tregs which lead to enhanced high-avidity T cell infiltration and tumour rejection. High- and low-avidity T cells against the breast cancer antigen HER-2/neu were developed and transferred adoptively into HER-2/neu transgenic mice. Only the high-avidity neu-specific T cells were inhibited by Tregs. After Treg depletion through cyclophosphamide treatment, these high-avidity T cells secreted IFN-γ and infiltrated the tumour tissue 39.
Li et al. analysed the effect of Treg depletion and cytotoxic T cell infiltration in murine models. A novel transgenic FoxP3.LuciDTR mouse model was developed where Tregs express luciferase and the human diphtheria toxin receptor (DTR), which can be used for Treg depletion by diphtheria toxin (DT) administration 40. This study showed that a depletion of approximately 90% of the Tregs in the tumour microenvironment led to normalization of the tumour vasculature and T cell infiltration which resulted in tumour rejection. Fifteen per cent of the infiltrated CD8 cells secreted IFN-γ and 10% of these infiltrated T cells were antigen-specific (ovalbumin). Furthermore, CD31+ endothelial cells from the Treg-depleted tumours expressed an increased level of vascular cell adhesion protein 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1), which play a pivotal role in T cell adhesion and infiltration 36. Several clinical studies have shown that a lympho-depleting regimen before the administration of therapeutic cancer vaccines significantly enhances the therapeutic outcome of the patient 24,32,41. Soriano et al. showed that a metronomic cyclophosphamide and methotrexate administration in combination with a 1E10 anti-idiotype vaccine [specific to Ab1 monoclonal antibody (mAb) which reacts with NeuGc-containing gangliosides] in metastatic breast cancer enhanced the median progression time and the overall survival of the patients receiving the metronomic chemotherapy and the vaccine in comparison to the patients who received chemotherapy alone 32. Here, the combination of the depletion of regulatory T cells and the restoration of available homeostatic cytokines enhance T cell infiltration 32,42.
In addition to abrogating the function of regulatory cells by blocking their differentiation, accumulation of regulatory cells in the tumour microenvironment can be reduced by targeting chemokine pathways. CCL2, for instance, is an important chemoattractant for myeloid suppressor cells and its neutralization could augment the anti-tumour activity of vaccine or adoptive CTL transfer. Treg recruitment, through CCL17 and CCL22, could also be inhibited using a small molecule antagonist of CCR4 32,41,43.
Blockade of checkpoint proteins and increased T cell migration
Checkpoint proteins such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death 1 (PD-1) are part of the normal suppressive mechanism for down-regulating immune responses. These receptors have been shown to be up-regulated on tumour-infiltrating T cells and are exploited by the tumour in order to down-regulate T cell responses. There has been a wide amount of interest in the blockage of these immunosuppressive pathways after studies documented the improvements in patients' survival with ipilimumab (targeting CTLA-4) and antibodies targeting PD-1 and its ligand PD-L1 44. There is controversy over how these CTLA-4 blocking antibodies mediate anti-tumour responses. While papers have documented an increase in T cells within the tumour 45, these results could be due to the expansion of T cells as opposed to increased T cell infiltration. Indeed, recent papers point to a mechanism of T cell depletion of Tregs expressing CTLA-4 while blocking inhibitor mechanisms on effector T cells 17,40,45,46. Blockage of PD-1 has been demonstrated to enhance T cell migration by increasing expression of IFN-γ and CXCL10 at the tumour site 47. Similarly, Wu et al. have also shown in murine models that a combination therapy of blocking anti-CTLA-4 between cycles of chemotherapy increases CD4 and CD8 infiltrating T cells within the tumour with increased up-regulation of the genes for IL-2, IFN-γ, granzyme B, perforin and inducible T cell co-stimulator (ICOS), suggesting that the presence of IFN-γ may be responsible for the increase in T cell migration 48.
Murine models combining CTLA-4 monoclonal antibodies with indoleamine 2,3-dioxygenase (IDO) inhibitors 49, cryoablation 50 and cancer vaccines 46 have also shown increases in T cells infiltrating tumours and hold great promise for the future in terms of potential therapeutic options.
Improving T cell infiltration with anti-angiogenic agents
Blood vessel formation within solid tumours is necessary for tumour survival. Tumour vasculature displays an abnormal architecture and is characterized by dilated and fragile vessels, which result in leaking, hypoxia, acidosis and a high interstitial pressure within the tumour. The normalization of this vasculature by specific therapies, such as chemotherapy, irradiation or anti-vascular endothelial growth factor (VEGF) antibody, leads to increased T cell infiltration, and therefore might be of relevance in tumour immunotherapy.
The angiogenic switch within solid tumours is triggered by VEGF, which is secreted by the tumour cells and binds to VEGF receptors expressed on the endothelial cells from pre-existing capillaries, as well as on circulating endothelial precursor cells derived from the bone marrow.
In a normal setting, VEGF is secreted by endothelial cells to enhance and restore blood vessel growth. It has been shown that VEGF has immune-modulatory properties when secreted by solid tumours and suppresses immune cells within the tumour microenvironment 51–54. It also regulates antigen presentation by dendritic cells and shifts mature dendritic cells to immature precursor cells. In addition, in high concentrations, VEGF induces apoptotic pathways in cytotoxic CD8 cells and activates regulatory T cells. Mulligan et al. have shown in oral squamous carcinoma that VEGF triggers endothelial cells to secrete prostaglandin E2 (PGE2) which is an immune suppressant and disrupts T cell activation and suppresses T cell proliferation and cytotoxic functions 54. In ovarian carcinoma the expression of VEGF was correlated negatively with infiltrating T cells 51,52.
The integrity of tumour vasculature affects lymphocyte migration from blood vessels into the tumour stroma. Anti-angiogenic antibodies such as VEGF blockers (Bevacizumab) can improve T cell infiltration into solid tumours and enhance the efficiency of immunotherapy 51–53. Not only do anti-angiogenic agents inhibit tumour growth and restore microvessel density, these drugs also up-regulate endothelial adhesion molecules in tumour vessels which lead to enhanced T cell infiltration. In this context, Dirkx et al. have shown in two murine models (colon carcinoma LS174T/nude mice and B16F10 melanoma/C57bl/6) that the angiogenesis inhibitors anginex, endostatin, angiostatin and the chemotherapeutic drug paclitaxel enhance the leucocyte-vessel wall interaction by up-regulation of adhesion molecules. These up-regulated interactions lead to an enhanced T cell infiltration into the tumour tissue 51,52. The blockage of VEGF normalizes the tumour vasculature and significantly enhances the intravascular components in the stroma. Recent studies show that the normalization of the tumour vasculature by anti-angiogenic drugs, such as anti-VEGF antibody and thalidomide, enhances drug penetration and also results in an increased percentage of T cells in the tumour tissue 51–53.
Treatment of metastatic melanoma with BRAF inhibitors results in increased T cell infiltration into the tumours 55. In a murine model the use of a BRAF inhibitor in combination with adoptive T cell transfer (ACT) demonstrated that the down-regulation of VEGF was responsible for the increase in T cell infiltration 56.
Chemotherapies have also been shown to affect angiogenesis in clinical trials. In a metastatic breast cancer trial, metronomic combination therapy of dalteparin/cyclophosphamide and methotrexate resulted in a decrease in VEGF after 2 weeks of therapy. Although the T cell infiltration was not monitored within this study, other results from preclinical data might indicate that the patients' increased survival was due to increased T cell infiltration 57. Chemotherapeutic drugs, therefore, act as key modulators of the tumour environment and this can be exploited to increase T cell infiltration.
Improving T cell infiltration with oncolytic virotherapy
In the past few years oncolytic viruses have been investigated as a novel therapeutic option for cancer in preclinical models as well as in clinical trials 58. Oncolytic viruses can be seen as tumour-selective, multi-mechanistic anti-tumour agents. They are able to kill cancer and associated endothelial cells via direct oncolysis, and uninfected cells via tumour vasculature targeting and bystander effect.
Interestingly, a local administration of oncolytic viral particles genetically engineered to express ligands for Toll-like receptors were able to alter the function of tumour infiltrating CD8+ T cells which are normally inhibited by the immunosuppressive tumour microenvironment. These can also be used in combination with checkpoint inhibitors. A combination therapy with localized NDV (Newcastle disease virus) and systemic CTLA-4 or PD-1 blockade led to antigen-dependent distant tumour rejection in both NDV-susceptible (B16) and -resistant [transgenic adenocarcinoma of the mouse prostate (TRAMP) C2 and CT26] tumour models, and protection from further tumour rechallenge. This effect was associated with marked distant tumour infiltration with activated effector CD8 and NK cells, but not Tregs. This work provides a strong rationale for clinical exploration of combination therapies of engineered oncolytic viruses with agents targeting immune checkpoints 58,59.
Conclusions
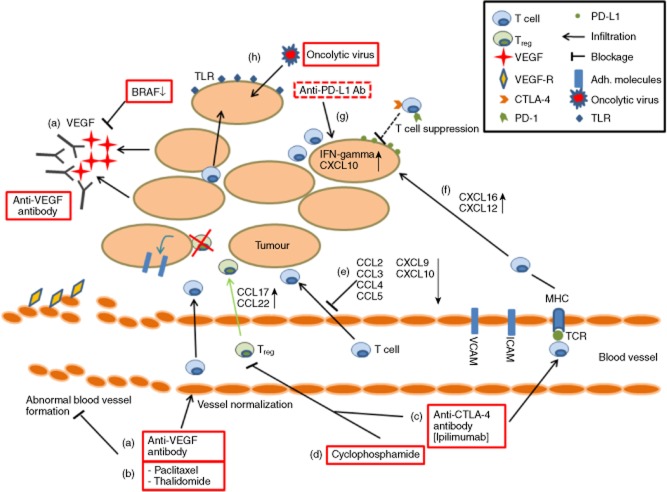
The presence of T cells within tumours correlates with favourable clinical outcomes (increased patient survival), but it is still not fully understood how the infiltration of T cells can be enhanced to aid in tumour rejection or prevent recurrence. Furthermore, cancer immunotherapy must overcome the immunosuppressive tumour microenvironment and elicit strong immune responses against certain tumour specific antigens. Because T cell avidity is an important factor to generate an immune response against cancer, it is the authors' opinion that optimal therapeutic options are required to elicit high-avidity T cell responses. This review has discussed strategies to enhance T cell migration and infiltration into tumour tissue and the effects of the tumour environment on this process. This is summarized in Fig. 1.
Fig 1.
Strategies to enhance T cell infiltration into tumour tissue. (a) Tumours secreting vascular endothelial growth factor (VEGF) leads to an abnormal blood vessel formation and decreased T cell infiltration. Anti-VEGF antibody and BRAF inhibitors decrease VEGF and normalize blood vessel formation; (b) treatment with paclitaxel and thalidomide also leads to blood vessel normalization and increased T cell infiltration; (c) anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibodies increase T cell infiltration into tumours and decrease regulatory T cells (Tregs); (d) up-regulation of chemokine ligand (CCL)17 and CCL22 leads to Treg accumulation within the tumours; cyclophosphamide treatment has been associated with decreased Treg cell infiltration; suppression of Tregs in the tumour microenvironment can increase the expression of adhesion molecules (VCAM-I and ICAM-I), leading to enhanced T cell infiltration; (e) down-regulation of chemokines in the tumour environment has been associated with decreased T cell infiltration; (f) expression of CXC chemokine ligand (CXCL)16 and CXCL12 enhance T cell infiltration to the tumour site; (g) anti-PD-L1 therapy has been shown to enhance interferon (IFN)-gamma and CXCL10 expression leading to T cell infiltration; (h) oncolytic virus therapy encoding Toll-like receptor (TLR) can enhance T cell infiltration through up-regulated expression of TLR on tumour cells.
From the work by Marelli-Berg et al. we would hypothesize that tumour-specific T cells would need to recognize cognate antigen presented by MHC on endothelial cells to enhance their migration through non-specific tissues in order to exert their cytotoxic potential. Moreover, the tumour microenvironment plays a pivotal role in T cell recruitment and infiltration into the tumour tissue. Treatment with therapies (such as low-dose metronomic chemotherapy) which increase cytokines such as IFN-γ and chemokines has been shown to enhance T cell infiltration. In addition, normalizing the tumour vasculature has shown positive results in increasing the recruitment of T cells to the tumour. However, the mechanism responsible for T cell infiltration needs further investigation, along with a more extensive study of the effect of cancer therapies on immune infiltration in cancer patients. It is encouraging to know that clinical trials to further investigate T cell infiltration with chemotherapy are in progress 60,61; further clinical trials are also required to investigate T cell infiltration with anti-angiogenic inhibitors. Understanding the roles that chemotherapy, anti-angiogenic therapies and blockage of checkpoint proteins have on chemokine modulation, Treg depletion and normalization of blood vessels will allow the optimization of these procedures in order to enhance T cell recruitment and infiltration into tumours. As the chemotherapeutic drugs, VEGF blockers and ipilimumab, are already approved and used as first-line therapies, this might lead to their increased use in combination with immunotherapy (e.g. cancer vaccines, oncolytic virotherapy) and thereby the realization of increased patient survival.
Disclosure
There are no financial or commercial conflicts of interest.
References
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin AM, Henriksson ML, Van GB, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- de la Cruz-Merino L, Henao CF, Vicente BD, et al. Immune microenvironment in colorectal cancer: a new hallmark to change old paradigms. Clin Dev Immunol. 2011;2011:174149. doi: 10.1155/2011/174149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutoit V, Rubio-Godoy V, Dietrich PY, et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. [PubMed] [Google Scholar]
- Dutoit V, Guillaume P, Romero P, Cerottini JC, Valmori D. Functional analysis of HLA-A*0201/Melan-A peptide multimer+ CD8+ T cells isolated from an HLA-A*. Cancer Immun. 2002;2:7–19. [PubMed] [Google Scholar]
- Dutoit V, Rubio-Godoy V, Pittet MJ, et al. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-a peptide multimer(+) CD8(+) T cells in humans. J Exp Med. 2002;196:207–216. doi: 10.1084/jem.20020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli-Berg FM, Frasca L, Weng L, Lombardi G, Lechler RI. Antigen recognition influences transendothelial migration of CD4+ T cells. J Immunol. 1999;162:696–703. [PubMed] [Google Scholar]
- Marelli-Berg FM, Scott D, Bartok I, Peek E, Dyson J, Lechler RI. Activated murine endothelial cells have reduced immunogenicity for CD8+ T cells: a mechanism of immunoregulation? J Immunol. 2000;165:4182–4189. doi: 10.4049/jimmunol.165.8.4182. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, James MJ, Dangerfield J, et al. Cognate recognition of the endothelium induces HY-specific CD8+ T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood. 2004;103:3111–3116. doi: 10.1182/blood-2003-08-2717. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Jarmin SJ. Antigen presentation by the endothelium: a green light for antigen-specific T cell trafficking? Immunol Lett. 2004;93:109–113. doi: 10.1016/j.imlet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–189. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Fu H, Vianello F, Tokoyoda K, Hamann A. Memory T-cell trafficking: new directions for busy commuters. Immunology. 2010;130:158–165. doi: 10.1111/j.1365-2567.2010.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- Weninger W, Manjunath N, von Andrian UH. Migration and differentiation of CD8+ T cells. Immunol Rev. 2002;186:221–233. doi: 10.1034/j.1600-065x.2002.18618.x. [DOI] [PubMed] [Google Scholar]
- Vianello F, Olszak IT, Poznansky MC. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J Mol Med (Berl) 2005;83:752–763. doi: 10.1007/s00109-005-0675-z. [DOI] [PubMed] [Google Scholar]
- Hamann A, Syrbe U. T-cell trafficking into sites of inflammation. Rheumatology (Oxf) 2000;39:696–699. doi: 10.1093/rheumatology/39.7.696. [DOI] [PubMed] [Google Scholar]
- Jaafar F, Righi E, Lindstrom V, et al. Correlation of CXCL12 expression and FoxP3+ cell infiltration with human papillomavirus infection and clinicopathological progression of cervical cancer. Am J Pathol. 2009;175:1525–1535. doi: 10.2353/ajpath.2009.090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molon B, Ugel S, Del PF, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol. 2002;160:1353–1360. doi: 10.1016/S0002-9440(10)62562-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Bailey C, Negus R, Morris A, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24:121–130. doi: 10.1007/s10585-007-9060-3. [DOI] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- Hojo S, Koizumi K, Tsuneyama K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67:4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- Poznansky MC, Olszak IT, Foxall R, et al. Active movement of T cells away from a chemokine. Nat Med. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- Schimanski CC, Galle PR, Moehler M. Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol. 2008;14:4721–4724. doi: 10.3748/wjg.14.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake N, Fukui H, Yamagishi H, et al. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–1689. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popple A, Durrant LG, Spendlove I, et al. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br J Cancer. 2012;106:1306–1313. doi: 10.1038/bjc.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin A, Wong WC, Tow C, et al. Dacarbazine promotes stromal remodeling and lymphocyte infiltration in cutaneous melanoma lesions. J Invest Dermatol. 2011;131:1896–1905. doi: 10.1038/jid.2011.128. [DOI] [PubMed] [Google Scholar]
- Soriano JL, Batista N, Santiesteban E, et al. Metronomic cyclophosphamide and methotrexate chemotherapy combined with 1E10 anti-idiotype vaccine in metastatic breast cancer. Int J Breast Cancer. 2011;2011:710292. doi: 10.4061/2011/710292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Volm MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–118. doi: 10.1158/0008-5472.CAN-13-1545. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels JP1, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- Hindley JP, Jones E, Smart K, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 2012;72:5473–5482. doi: 10.1158/0008-5472.CAN-12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss VL, Lee TH, Song H, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLOS ONE. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kostareli E, Suffner J, Garbi N, Hammerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–3335. doi: 10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- Roselli M, Cereda V, di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2:e27025. doi: 10.4161/onci.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, David R, Glennie S, et al. Differential effects of immunosuppressive drugs on T-cell motility. Am J Transplant. 2006;6:2871–2883. doi: 10.1111/j.1600-6143.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology. 2013;2:e25961. doi: 10.4161/onci.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS1, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RR, Jalil J, Economou JS, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17:4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Liu C, Xu C, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11:1809–1819. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–439. doi: 10.1158/0008-5472.CAN-11-1782. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Kuijpers MJ, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–2329. [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Castermans K, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan JK, Day TA, Gillespie MB, Rosenzweig SA, Young MR. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375–382. doi: 10.1016/j.humimm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmott JS, Scolyer RA, Long GV, Hersey P. Combined targeted therapy and immunotherapy in the treatment of advanced melanoma. Oncoimmunology. 2012;1:997–999. doi: 10.4161/onci.19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Peng W, Xu C, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NS, Buckman RA, Clemons M, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–730. doi: 10.1200/JCO.2009.24.0143. doi: 10.1200/JCO.2009.24.0143. [DOI] [PubMed] [Google Scholar]
- Lemay CG, Rintoul JL, Kus A, et al. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol Ther. 2012;20:1791–1799. doi: 10.1038/mt.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, Song XT. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther. 2014;22:102–111. doi: 10.1038/mt.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical trial: Tumor-Infiltrating Lymphocytes After Combination Chemotherapy in Treating Patients With Metastatic Melanoma, NCT01807182.
- Huang RR1, Jalil J, Economou JS, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17:4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]