Abstract
Oxidative stress is involved in the pathophysiology of rheumatoid arthritis (RA). We investigated the therapeutic potential of rebamipide, a gastroprotective agent with a property of reactive oxygen species scavenger, on the development of inflammatory polyarthritis and the pathophysiological mechanisms by which rebamipide might confer anti-arthritic effects in SKG mice, an animal model of RA. Intraperitoneal (i.p.) injection of rebamipide attenuated the severity of clinical and histological arthritis. Rebampide treatment reduced the number of T helper type 1 (Th1), Th2, Th17, inducible T cell co-stimulator (ICOS)+ follicular helper T (Tfh) transitional type (T2) and mature B cells in the spleen, but increased the number of regulatory T (Treg), CD19+ CD1dhigh CD5high, CD19+ CD25high forkhead box protein 3 (FoxP3)+ regulatory B (Breg) cells, memory B cells, and transitional type 1 (T1) B cells. In addition, flow cytometric analysis revealed significantly decreased populations of FAS+GL-7+ germinal centre B cells and B220− CD138+ plasma cells in the spleens of rebamipide-treated SKG mice compared to controls. Rebamipide decreased germinal centre B cells and reciprocally induced Breg cells in a dose-dependent manner in vitro. Rebamipide-induced Breg cells had more suppressive capacity in relation to T cell proliferation and also inhibited Th17 differentiation from murine CD4+ T cells. Together, these data show that i.p. administration of rebamipide suppresses arthritis severity by inducing Breg and Treg cells and suppressing Tfh and Th17 cells in a murine model of RA.
Keywords: arthritis (including rheumatoid arthritis), B cells, T cells
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune polyarthritis affecting ∼1% of the world population, resulting in the loss of joint function and progressive structural damage in affected joints. Reactive oxygen species (ROS) are normally present in all aerobic cells. Oxidative stress implies a disturbed cell state in which ROS production exceeds the neutralization capacity. In RA pathophysiology, oxidative stress produced by hypoxic–reperfusion injury may contribute to persistent synovial inflammation in rheumatoid joints 1. Changes in ROS levels contribute to autoantigen formation and the regulation of antigenic processing, cell-mediated immunity and apoptosis. Furthermore, post-translational modification of proteins by ROS increases their antigenicity for autoantibody production, suggesting a role for oxidized antigens in the aetiology of RA 2,3.
Although the aetiology of RA remains to be fully elucidated, interleukin (IL)-17-expressing CD4+ T cells, known as T helper type 17 (Th17) cells, are believed to play a critical role in the pathogenesis of RA 4,5. In contrast, regulatory T (Treg) cells are crucial for maintaining immune tolerance and suppressing certain proinflammatory processes in inflammatory diseases, including RA 6,7. In arthritis, antigen-presenting cells (APCs) along with proinflammatory cytokines, such as tumour necrosis factor (TNF)-α and IL-6, inhibit the differentiation and activation of Treg cells, thereby diminishing the immunosuppressive activity of Treg cells in vivo 8–11. This attenuation, combined with the overwhelming activation of pathogenic Th17 cells, can lead to chronic inflammation in rheumatoid joints.
In addition to the clear role of T cells in the pathogenesis of RA, recent evidence has suggested that B cells may also be involved in the development and progression of RA 12. With the help of T cells, activated B cells migrate into lymphoid follicles of lymphoid organs and form germinal centres (GCs) 13. Follicular helper T (Tfh) cells emerge as specialized local effector T cells and contribute to the development of B cell immunity 14. Although relatively little is known about Tfh cells in RA, a recent study demonstrated a significant increase in the number of CD4+ CXC chemokine receptor 5 (CXCR5)+ inducible T cell co-stimulator (ICOS)high Tfh cells in the peripheral blood of RA patients, compared to healthy controls. Furthermore, the amount of Tfh cells was correlated positively with anti-cyclic citrullinated peptide (anti-CCP) antibody titres, suggesting a potential role for Tfh cells in the pathogenesis of RA 15.
Rebamipide, a gastroprotective agent used for the treatment of gastritis and gastric ulcers, acts as scavenger of oxygen radicals 16,17. As rebamipide possesses both anti-oxidant and anti-inflammatory properties, we had previously examined the efficacy of this compound for the treatment of RA in collagen-induced arthritis mice (CIA), an established animal model of RA. Rebamipide effectively reduced both clinical and histological scores in these mice via reciprocal regulation of Th17 and Treg cells in vivo, suggesting a mechanism by which this compound inhibits autoimmune arthritis.
Several animal models of human RA are currently in use 18. Recently, Sakaguchi et al. 19 described a mouse model for chronic autoimmune polyarthritis caused by a spontaneous point mutation in BALB/c mice (SKG model). Arthritis in SKG mice is characterized by symmetric inflammation in small joints, elevation of rheumatoid factor and progressive joint destruction, all hallmarks of human RA 20. In the present study, we investigated whether rebamipide treatment may limit the severity of arthritis in SKG mice and the role that T and B cell subpopulations play in this model, particularly in the cross-talk between B and Tfh cells.
Materials and methods
Animals
SKG mice with the BALB/c background were kindly obtained from Professor Shimon Sakaguchi (Department of Experimental Immunology, World Premier International Immunology Frontier Research Center, Osaka University). The mice were maintained in a specific pathogen-free environment under climate-controlled conditions with a 12-h light/dark cycle at the Catholic University of Korea. They were fed standard mouse chow (Ralston Purina, St Louis, MO, USA) and water ad libitum. All experimental procedures were examined and approved by the Institutional Animal Care and Use Committee (IACUC) at the School of Medicine, Animal Research Ethics Committee of the Catholic University of Korea and were conducted in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals. This animal care and use protocol was reviewed and approved by the Catholic University of Korea.
Induction of arthritis and scoring of clinical signs
Arthritis was induced according to a previously published method 21. Briefly, zymosan A (Sigma, St Louis, MO, USA) was suspended in phosphate-buffered saline (PBS) and incubated for 10 min in boiling water. Then the zymosan A solution (2 mg/mice) was injected intraperitoneally (i.p.) into 7- or 8-week-old mice. Clinical scores, following a previously published system 19, were monitored weekly; 0, no swelling or redness; 0·1, swelling or redness of digit; 0·5, mild swelling and/or redness of wrists or ankle joint; and 1, severe swelling of larger joints. Scores of affected joints were totalled for each mouse.
Rebamipide treatment
Rebamipide was obtained from Hanlim Pharmaceutical Company (Seoul, Korea). Rebamipide was dissolved in 10% dimethyl sulphoxide solution and administered i.p. at a dose of 6 mg/kg body weight. Rebamipide was administered three times per week after induction of arthritis using zymosan A.
Histopathology of arthritis
Mouse joint tissues were fixed in 10% formalin, decalcified in ethylenediamine tetraacetic acid (EDTA) and embedded in paraffin. Sections were deparaffinized using xylene, dehydrated in a graded series of alcohol solution, and stained with Harris haematoxylin and eosin (H&E), safranin O and toluidine blue to detect proteoglycan contents. Inflammation and cartilage damage were scored as described previously 22.
Intracellular staining and flow cytometry
Cells were stained with anti-IL-4-phycoerythrin (PE), anti-CXCR5-PE, anti-ICOS-PE, anti-mouse immunoglobulin (Ig)M-biotin, anti-CD21/CD35-fluorescein isothiocyanate (FITC), anti-CD138-PE, anti-FAS(CD95)-PE, anti-mouse T and B cell activation antigen (GL7)-FITC (BD PharMingen, San Diego, CA, USA), anti-IL-10-allophycocyanin, anti-CD25-allophycocyanin, anti-B220-allophycocyanin, streptavidin-PE-cyanin 5·5 (Cy5·5), anti-CD196(CCR6)-allophycocyanin (Biolegend, San Diego, CA, USA), anti-IFN-γ-PE, anti-IL-17A-FITC, anti-forkhead box protein 3 (FoxP3)-PE, anti-CD4-peridinin chlorophyll (PerCP)Cy5·5, anti-IgD-FITC, anti-CD38-PE, anti-CD23-PE, anti-CD19-PerCP-Cy5·5, anti-mouse CD1d-PE, anti-CD5-FITC and anti-retinoic acid receptor-related orphan nuclear receptor gamma (ROR-γt)-PE (eBiosciences, San Diego, CA, USA). Intracellular staining was performed as described previously 23.
Real-time PCR
Total RNA was extracted using Trizol (Molecular Research Center, Cincinnati, OH, USA). Total RNA (2 μg) was reverse-transcribed using the SuperScript Reverse Transcription system (Takara, Shiga, Japan). Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) was performed with FastStart Universal SYBR Green Master (Roche, Basel, Switzerland) fluorescent dye using an ABI PCR machine. The following primers were used for mouse genes: IL-17 (forward: 5′-CCT CAA AGC TCA GCG TGT CC-3′, reverse: 5′-GAG CTC ACT TTT GCG CCA AG-3′), ROR-γt (forward: 5′-TGT CCT GGG CTA CCC TAC TG-3′, reverse: 5′-GTG CAG GAG TAG GCC ACA TT-3′), CCL20 (forward: 5′-CAG CTG TTG CCT CTC GTA CA-3′, reverse: 5′-CAC CCA GTT CTG CTT TGG AT-3′), FoxP3 (forward: 5′-GGC CCT TCT CCA GGA CAG A-3′, reverse: 5′-GCT GAT CAT GGC TGG GTT GT-3′), IL-10 (forward: 5′-AAG TGA TGC CCC AGG CA-3′, reverse: 5′-TCT CAC CCA GGG AAT TCA AA-3′), suppressor of cytokine signalling-3 (SOCS3) (forward: 5′-CCT TTG ACA AGC GGA CTC TC-3′, reverse: 5′-GCC AGC ATA AAA ACC CTT CA-3′), Blimp-1 (forward: 5′-CTG TCA GAA CGG GAT GAA CA-3′, reverse: 5′-TGG GGA CAC TCT TTG GGT AG-3′), XBP-1 (forward: 5′-GAT CCT GAC GAC GTT CCA GA-3′, reverse: 5′-ACA GGG TCC AAC TTG TCC AG-3′) and β-actin (forward: 5′-GAA ATC GTG CGT GAC ATC AAA G-3′, reverse: 5′-TGT AGT TTC ATG GAT GCC ACA G-3′). mRNA levels were normalized to that of β-actin.
Confocal microscopy of immunostaining
For confocal staining, 7-μm spleen sections were stained using anti-CD4, anti-B220, anti-GL7, anti-ICOS, anti-FoxP3 and anti-CD138 (all from eBiosciences). Immunostaining was performed as described 23. Stained sections were analysed using a Zeiss microscope (LSM 510 Meta; Carl Zeiss, Oberkochen, Germany).
Cell isolation and culture
Splenocytes from normal SKG mice were cultured at a density of 5 × 106 cells/well in six-well flat-bottomed plates containing 100 ng/ml lipopolysaccharide (LPS) in the presence or absence of 300 μM rebamipide for 72 h. CD19+ B cells were isolated using the CD19+ B cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Magnetic-activated cell sorted (MACS) CD19+ cells were stained with anti-CD25 allophycocyanin for 30 min at 4°C. Cells were sorted using a fluorescence activated cell sorter (FACS) Aria III sorter (BD Bioscience, San Jose, CA). CD4+ T cells were isolated from normal SKG mice using the CD4+ T cell isolation kit (Miltenyi Biotec), according to the manufacturer's instructions. Negatively selected non-CD4+ cells were considered APCs. After each type of cell was harvested, the CD19+ CD25+ regulatory B (Breg)-like cells (4 × 104 cells/well) were co-cultured with CD4+ T cells (2 × 105 cells/well) and APCs (2 × 105 cells/well) in 48-well plates. APCs were irradiated (5000 rad) before co-culture. For Th17-polarizing conditions, co-cultured cells were stimulated with anti-IFN-γ (2 μg/ml), anti-IL-4 (2 μg/ml), IL-6 (20 ng/ml) (all from R&D Systems, Minneapolis, MN, USA) and human transforming growth factor (TGF)-β (2 ng/ml) (PeproTech, Rocky Hill, NJ, USA).
Proliferation assay
CD19+CD25+ Breg-like cells (2 × 104 cells/well) were co-cultured with CD4+ T cells (1 × 105 cells/well) and APCs (1 × 105 cells/well) in 96-well plates and stimulated with 0·5 μg/ml plate-bound anti-CD3 monoclonal antibodies (mAb) (BD Biosciences) for 3 days. During the last 16–18 h of the 72 h assay, cells were pulsed with 1 μCi of [3H]-thymidine (Perkin-Elmer, Wellesley, MA, USA) per well. The incorporation of [3H]-thymidine was determined using a Betaplate scintillation counter (Perkin-Elmer).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 4 for Windows; GraphPad Software). The experimental values are presented as means ± standard deviation (s.d.). Comparisons of numerical data between two groups were performed using Student's t-test or Mann–Whitney U-test. Differences in categorical variables were analysed using analysis of variance (anova) with a post-hoc test. P-values < 0·05 (two-tailed) were considered significant.
Results
Rebamipide attenuates zymosan-induced arthritis via Th17/Treg regulation in SKG mice
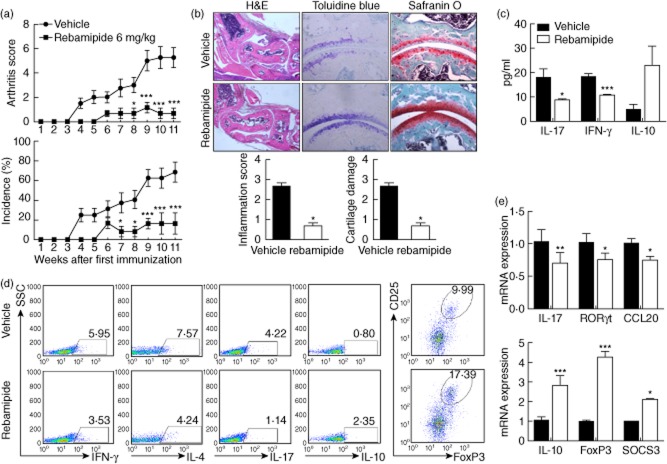
First, we investigated whether treatment with rebamipide would suppress the rheumatoid inflammation and joint destruction seen in zymosan-induced arthritis in SKG mice. In zymosan-induced SKG mice, the joints showed a pervasive infiltration of inflammatory cells, synovial hyperplasia and the destruction of the articular cartilage and bone. Injection of rebamipide (6 mg/kg) reduced the severity and incidence of arthritis compared to vehicle controls (Fig. 1a), including a statistically significant reduction in inflammation scores. It also resulted in a significant reduction in cartilage loss 77 days after immunization, as assessed by safranin O staining (Fig. 1b). Also, the IFN-γ and IL-17 levels were decreased, whereas the IL-10 level was increased in sera from rebamipide-treated mice compared to vehicle-treated mice (Fig. 1c).
Fig 1.
Rebamipide attenuates zymosan-induced arthritis by regulating T helper type 17 (Th17)/regulatory T cells (Treg) in SKG mice. Rebamipide was given intraperitoneally three times per week at a dose of 6 mg/kg (n = 5 mice/group) after first immunization. (a) Arthritis severity was recorded as the mean arthritis index score and incidence score. (b) Ankle joint tissues were obtained from SKG mice with zymosan-induced arthritis treated with rebamipide 11 weeks after the start of the experiment, and stained with toluidine blue, safranin O and haematoxylin and eosin (H&E). Inflammation and cartilage scores are shown in bar graphs (right; magnification × 200). The mice were treated as described in (a); (c) 35 days after first immunization, serum levels of interferon (IFN)-γ, interleukin (IL)-17 and IL-10 cytokine measured in each group. (d) Splenocytes were collected from each group of mice, and CD4+ populations were analysed for Th1 (IFN-γ), Th2 (IL-4), Th17 (IL-17), IL-10, and regulatory T cell (Treg) [CD25high, forkhead box protein 3 (FoxP3+)] expression. The fluorescence activated cell sorter (FACS) plots show representative data. (e) Expression of IL-17, retinoic acid receptor-related orphan nuclear receptor gamma (ROR)-γt, CCL20, IL-10, FoxP3 and suppressor of cytokine signalling 3 (SOCS3) was determined using real-time polymerase chain reaction (PCR) in splenocytes obtained from each mouse, and normalized to β-actin expression. Data are presented as the mean ± standard deviation of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, compared to control mice.
To investigate the mechanisms by which rebamipide attenuates arthritis severity in SKG mice, IFN-γ-expressing (mainly Th1), IL-4-expressing (Th2), IL-17-expressing (Th17), IL-10-expressing and CD25high FoxP3+ (Treg) CD4+ T cells were isolated from spleens of rebamipide-treated and control mice. Th1, Th2 and Th17 cell populations were all lower in rebamipide-treated zymosan-induced mice compared to arthritis control mice, whereas the number of IL-10-expressing and Treg cells was higher (Fig. 1d).
Next, we compared mRNA expression levels for IL-17, ROR-γt, CCL20, IL-10, FoxP3 and SOCS3 in splenocytes of rebamipide-treated mice and untreated controls. mRNA expression of Th17-associated molecules such as IL-17, ROR-γt and CCL20 was significantly lower in splenocytes of rebamipide-treated mice, while expression of Treg-associated molecules such as IL-10, FoxP3 and SOCS3 was higher (Fig. 1e). These results suggest that rebamipide attenuates zymosan-induced arthritis through reciprocal regulation of Th17 and FoxP3+ Treg cell populations.
Rebamipide reduces Tfh cell subsets
Tfh cells are a subset of effector CD4+ T cells with B cell helper function 24, characterized by markedly elevated levels of CXCR5 and ICOS in mice 25,26. Having established a role for both Th17 and Treg cells in zymosan-induced arthritis, we next examined whether rebamipide could also regulate the Tfh subpopulation in vivo. Spleens isolated from rebamipide-treated SKG mice showed a reduction in Tfh cell populations expressing ICOS (Fig. 2a).
Fig 2.
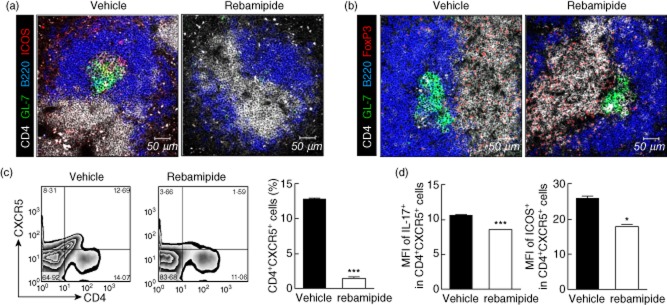
Effects of rebamipide on follicular helper T (Tfh) cell subsets. Spleens from rebamipide-treated and control mice were examined by immunofluorescence for staining with mononuclear cells (mAbs) (original magnification × 400) against (a) CD4 (white), B220 (blue), GL-7 (green), inducible T cell co-stimulator (ICOS) (red) and (b) CD4 (white), B220 (blue), GL-7 (green), and forkhead box protein 3 (FoxP3+) (red) (B) (original magnification × 400). (c) Left, representative fluorescence activated cell sorter (FACS) plots of CD4+ CXCR5+ cells are shown. Right, CD4+ CXCR5+ cells quantified as percentages. (d) Left, mean fluorescence intensities (MFIs) of interleukin (IL)-17+ measured using flow cytometry; right, MFIs of ICOS+ Tfh cells. Data are presented as the mean ± standard deviation of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, compared to control mice.
Next, we determined whether these changes were associated with populations of follicular regulatory T (Tfr) cells. Tfr cells preserve autoimmune tolerance via GC formation, and regulate immune activation by limiting the proliferation of Tfh and GC B cells in vivo 27. The population of FoxP3-expressing Tfr cells was larger in spleens of rebamipide-treated mice than in vehicle-treated SKG mice (Fig. 2b). Flow cytometric analysis of isolated splenocytes revealed smaller populations of CD4+CXCR5+ Tfh cells (Fig. 2c), as well as ICOS and IL-17-expressing Tfh cells (Fig. 2d) in rebamipide-treated mice. These results suggest that the inhibition of ICOS and IL-17-expressing Tfh cells, and a reciprocal increase in FoxP3-expressing Tfr cells, may be involved in the anti-inflammatory and anti-arthritic properties of rebamipide in SKG mice.
B cell regulation by rebamipide
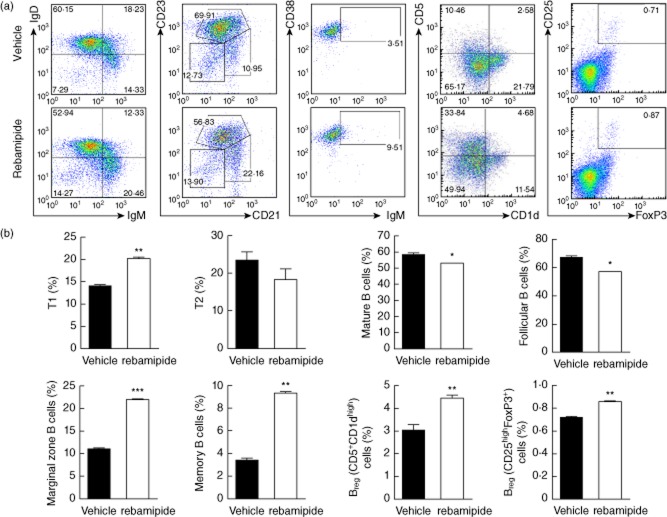
Tfh cells play an important role in antibody production by inducing differentiation of both plasma and memory B cells 28. Therefore, we examined whether there were any changes in B cell phenotypes in the rebamipide-treated mice. Compared to controls, rebamipide-treated mice had larger populations of T1 (IgMhighIgDlow), CD21highCD23low marginal zone B cells, and memory B cell (CD38highIgMhigh) and smaller populations of T2 (IgMhighIgDhigh) B cells and mature B cells (IgMlowIgDhigh). Breg cell subsets were identified as CD19+ CD1dhighCD5high 29 and CD19+CD25highFoxP3+ 30, both of which were more abundant in the spleens of rebamipide-treated SKG mice. We have previously investigated the function of the D19+CD25highFoxP3+ Breg cell subset (to be published). FACS plots show representative data. Graphs shown are the means ± standard errors of three independent experiments (Fig. 3a,b).
Fig 3.
Increase in regulatory B (Breg) cells caused by rebamipide. Cells were harvested and stained immediately for different cell surface markers. (a) Splenocytes isolated from zymosan-induced arthritis SKG or rebamipide-treated mice were stained for B220, immunoglobulin (Ig)M and IgD or B220, CD23, and CD21 and then analysed by flow cytometry. Data are shown as the average percentages of T1 (IgMhigh IgDlow), T2 (IgMhigh IgDhigh), and mature (IgMlow IgDhigh) B cells as well as follicular B cells (CD21low CD23high), marginal zone B cells (CD21high CD23high) and memory B cell (CD38high IgMhigh) from the B220+ gate in the spleen. Regulatory and putative B cell subsets from the CD19+ gated population of splenocytes were identified as B10 cells (CD1dhigh CD5high) or Breg cells [CD25high forkhead box protein 3 (FoxP3+)] as shown in each gate on the dot-plot. (b) Graphs depict the percentage of B cell subsets remaining in the B220+ or CD19+ gated population of the spleen following therapeutic treatment with rebamipide (6 mg/kg). Data are presented as the mean ± standard deviation of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, compared to control mice.
To investigate whether i.p. injection of rebamipide may affect GC formation and the production of plasma cells, spleens were isolated from each group of mice and examined by immunofluorescence. Both GL-7+ GC B cells and B220− CD138+ plasma cells were significantly less abundant in spleens of rebamipide-treated SKG mice (Fig. 4a). Flow cytometric analysis also showed significant decreases in the populations of both FAS+GL-7+ GC B cells and B220−CD138+ plasma cells in rebamipide-treated mice (Fig. 4b,c).
Fig 4.
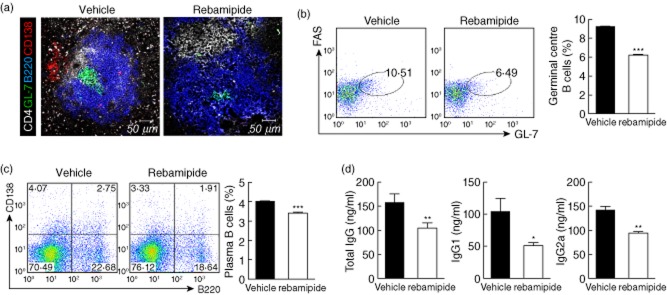
Decrease in plasma and germinal centre B cells caused by rebamipide. (a) Spleens from rebamipide-treated and control mice were stained with monoclonal antibodies (mAbs) against CD4 (white), B220 (blue), GL-7 (green) or CD138 (red), and examined by immunofluorescence (original magnification × 400). Flow cytometric analysis of germinal centre (FAS+ GL-7+) (b) and plasma (B220+ CD138−). (c) B cell populations in the spleen following treatment with rebamipide (6 mg/kg). Fluorescence activated cell sorter (FACS) plots show representative data. Graphs shown are the means ± standard errors of three independent experiments. (d) Serum was obtained from each group; concentrations of total immunoglobulin (Ig)G, IgG1 and IgG2a were determined by enzyme-linked immunosorbent assay (ELISA). Data are presented as the mean ± standard deviation of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, compared to control mice.
To determine the capacity of antibody production, serum levels of total IgG, IgG1 and IgG2a were measured in each group. They were all decreased significantly by rebamipide treatment (Fig. 4d).
Effect of rebamipide on B cell development in vitro
To investigate the effects of rebamipide on B cell development, CD19+ B cells were isolated from the spleens of SKG mice and then stimulated with LPS in the presence or absence of rebamipide. Rebamipide treatment significantly decreased the proportion of FAS+GL-7+ GC B cells in conditions favouring B cell development (LPS stimulation; Fig. 5a). To further identify changes in plasma cell development conferred by rebamipide, we measured the concentrations of total IgG and IgG1 in the supernatants of treated cells. Rebamipide was found to inhibit the production of both total IgG and IgG1 in a dose-dependent manner (Fig. 5b).
Fig 5.
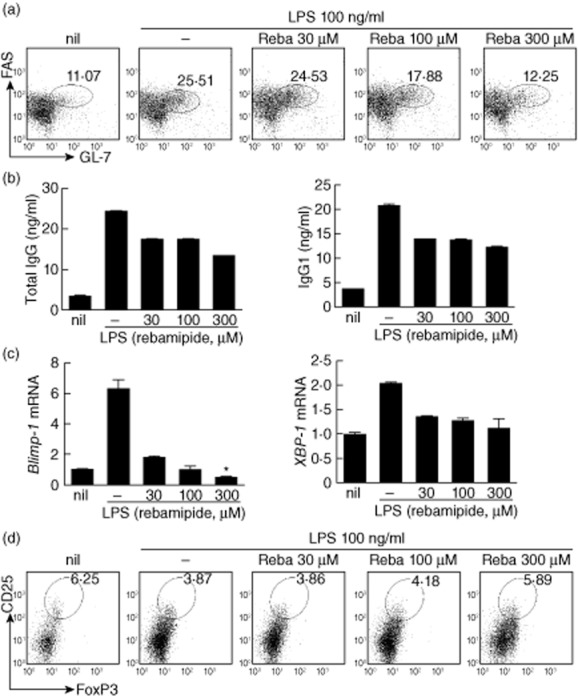
Rebamipide-induced regulatory B (Breg) cells in vitro. CD19+ B cells were isolated from the spleens of SKG mice, treated with different concentrations of rebamipide and incubated for 3 days in the presence of 100 ng/ml lipopolysaccharide (LPS). (a) Spleen CD19+ B cells stained with GL-7, FAS and CD19 monoclonal antibodies (mAbs), and analysed by flow cytometry. (b) Enzyme-linked immunosorbent assay (ELISA)of total immunoglobulin (Ig)G and IgG1 in supernatants. (c) Polymerase chain reaction (PCR) analysis of Blimp1, XBP-1 mRNA expression. (d) Spleen CD19+ B cells stained with CD25 mAb, permeabilized, stained with forkhead box protein 3 (FoxP3+) mAb and analysed by flow cytometry. Fluorescence activated cell sorter (FACS) plots show data representative of three independent experiments with similar results. Data are presented as the mean ± standard deviation of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001.
Terminal differentiation of B cells into plasma cells is regulated by Blimp-1 and X-box binding protein-1 (XBP-1) 31,32. Rebamipide reduced mRNA expression of both Blimp-1 and XBP-1 in B cells, indicating an inhibitory effect on plasma cell differentiation (Fig. 5c).
As described above, populations of CD19+CD1dhighCD5high and CD19+CD25highFoxP3+ regulatory B cells were increased significantly in rebamipide-treated mice (Fig. 3c,d). Therefore, we examined whether rebamipide induces Breg cells in vitro. Rebamipide treatment increased Breg cell populations in a dose-dependent manner, consistent with the in-vivo observations (Fig. 5d).
Finally, cells were tested for viability using an MTT assay, to determine whether reductions in B cell populations were the result of decreased cell viability. No changes in cell viability were observed following treatment with rebamipide (data not shown). Taken together, these data show that rebamipide treatment is able to suppress B cell development and induce Breg populations both in vivo and in vitro.
Suppression of T cell activation via induction of Breg cells by rebamipide
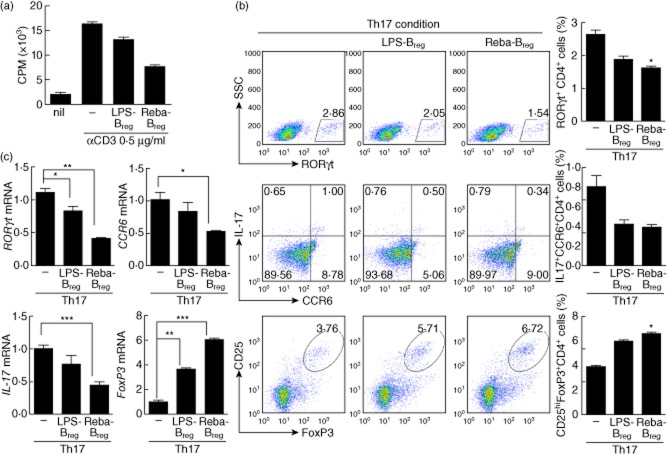
Splenocytes isolated from SKG mice were incubated for 3 days in the presence of LPS (100 ng/ml) with or without 300 μM rebamipide (Reba Breg and LPS Breg, respectively). Then CD19+ CD25+ Breg cells were isolated by flow cytometry, and co-cultured with CD4+ T cells and irradiated APCs under anti-CD3 antibody stimulation. The proliferative responses of T cells were determined using a [3H]-thymidine incorporation assay. Rebamipide treatment was found to enhance the ability of Breg cells to suppress T cell proliferation (Fig. 6a).
Fig 6.
Suppression of T cell activation by regulatory B cells induced by rebamipide. Splenocytes were isolated from SKG mice, and incubated for 3 days in the presence of lipopolysaccharide (LPS) 100 ng/ml regulatory B cells (Breg) or LPS 100 ng/ml + rebamipide 300 μM (Reba Breg). Then CD19+ B cells were isolated using microbeads and stained with CD25 monoclonal antibodies (mAbs). CD25+ cells were sorted, and isolated Breg cells (1 × 105 cells/well) were co-cultured with CD4+ T cells (5 × 105 cells/well) and irradiated APCs (5 × 105 cells/well) from SKG mice. Cells were cultured with or without 0·5 μg/ml anti-CD3 mAb for 3 days. The proliferative responses of T cells were determined using a [3H]-thymidine incorporation assay. Data are presented as the mean counts per min (a). Cells were incubated for 3 days under T helper type 17 (Th17)-polarizing conditions. The number of CD4+ retinoic acid receptor-related orphan nuclear receptor gamma (ROR)-γt+, CD4+ IL-17+ CCR6+ or CD4+ CD25high forkhead box protein 3 (FoxP3+) cells was determined by intracellular flow cytometry (b). Real-time polymerase chain reaction (PCR) analysis of ROR-γt, CCR6, interleukin (IL)-17A, and FoxP3 mRNA expression (c). Data are presented as the mean ± standard deviation of three independent experiments *P < 0·05; **P < 0·01; ***P < 0·001, compared to the control mice.
The immunoregulatory capacity of Breg cells under Th17-polarizing conditions was also investigated. CD4+ T cells were cultured under conditions favouring Th17 differentiation with either LPS-Breg or Reba-Breg. The production of CD4+ROR-γt+ and CD4+IL-17+CCR6+ effector T cells was inhibited significantly by Reba-Breg, whereas populations of CD4+CD25highFoxP3+ Treg cells were increased (Fig. 6b). Expression of ROR-γt, CCR6 and IL-17A mRNA was also decreased in these cells. In contrast, FoxP3 mRNA expression was increased significantly by Reba-Breg (Fig. 6c). These results indicate that rebamipide treatment of induced Breg cells can suppress Th17 differentiation, and reciprocally increase Treg cells through the induction of FoxP3.
Discussion
We have demonstrated that i.p. injection of rebamipide effectively reduced both clinical and histological scores in zymosan-induced arthritis in SKG mice, a murine model of RA; several mechanisms by which rebamipide exert these anti-arthritic effects were also shown. Among CD4+ T cell subsets, Th1, Th2 and Th17 cell populations were all decreased significantly in the spleens of rebamipide-treated SKG mice compared to vehicle controls, while Treg cells were increased. CIA, an animal model of RA, is the most commonly studied to prove the mechanisms of disease pathogenesis. It is induced in this model by immunization with type II collagen in adjuvant and associated with strong and sustained T and B cell response to type II collagen 33,34. SKG mice has a point mutation in the gene encoding an SH2 domain of ZAP-70, and this genetic defect causes production of arthritogenic T cells and Th17 cells and develops spontaneous chronic autoimmune arthritis similar to human RA 19.
Additional effects on antibody production were also examined, with i.p. administration of rebamipide inhibiting ICOS+ Tfh differentiation, combined with a reciprocal induction of CD19+CD1dhighCD5high and CD19+CD25high FoxP3+ Breg populations. In vitro, rebamipide regulated terminal differentiation of B cells into plasma cells in a dose-dependent manner through inhibition of Blimp-1 and XBP-1, and significantly induced Breg differentiation under conditions favouring B cell differentiation. Furthermore, rebamipide-induced Breg cells showed greater immunoregulatory capacity compared to LPS-induced Breg cells.
Tfh cells are a distinct subpopulation of CD4+ T cells present in the GCs of lymphoid organs that help regulate B cell immunity 35. CXCR5 is expressed in Tfh cells, with CXCR+ Tfh cells influencing both B cell expansion and GC recruitment 36. Tfh cells also aid B cell activation and antibody production. The presence of GC Tfh cells among dense follicular dendritic cell networks suggests a role for these cells in the differentiation of high-affinity GC B cells into memory B cells 37. Although little is known about the role Tfh cells play in RA, patients with RA tend to have more circulating CD4+CXCR5+ICOShigh Tfh cells than healthy controls 15.
RA is a chronic and symmetric inflammatory polyarthritis, the serological features of which include circulating autoantibodies against targets such as anti-cyclic citrullinated peptide (CCP) antibody. The involvement of anti-CCP antibodies in the progression of joint inflammation was recently elucidated in CIA mice, suggesting a pathophysiological role for these antibodies in the pathogenesis of autoimmune arthritis 38. Although B cells are essential for the production of autoantibodies, relatively little is known about other immune cells that may aid B cell development and autoantibody production in RA.
B cells may provide a critical link between the development of tertiary lymphoid tissues within the inflamed synovium and the propagation of inflammatory processes; the presence of GC-like structures within the RA synovium may support these associations. Interestingly, in a previous study, RA patients who received an anti-tumour necrosis factor (TNF) agent (etanercept) displayed a paucity of follicular dendritic cells and little GC structure in lymphoid tissue, suggesting that anti-TNF treatment may disrupt GC formation 39. Based on the idea that autoantibody-producing B cells could perpetuate joint inflammation, drugs that deplete B cells, such as rituximab, have been widely used in patients with RA. Despite its widespread clinical use, rituximab, which targets CD19+ B cells, may also deplete immunoregulatory B cell subsets. Recent studies have identified Bregs as important regulatory populations in both humans and mice. Negative correlations between Breg numbers and disease activity in patients with newly diagnosed RA have suggested a potential role for Breg cells in controlling RA disease activity 40. Taken together, these data suggest that selective regulation of B cell subpopulations may represent a more rational approach to RA treatment than B cell-depletion strategies. In the present study, i.p. administration of rebamipide attenuated Tfh cell populations and reciprocally induced GC Breg cells. Regarding B cell development, terminal differentiation of B cells into plasma cells was inhibited significantly by rebamipide treatment. Follicular B cell populations were decreased in spleens of rebamipide-treated SKG mice, whereas marginal zone B cell populations were profoundly expanded, suggesting a potent immunoregulatory capacity for rebamipide across multiple stages of B cell development and differentiation.
It has been known that rebamipide attenuated the pain severity and cartilage destruction, and reduced oxidative stress in articular cartilage and the subchondral bone region in an animal model of osteoarthritis (OA) 41. In addition, our results showed that rebamipide induces Breg and Treg cells while suppressing Tfh and Th17 cells in RA. Therefore, rebamipide, which was proved to modulate the imbalance of Th17 and Treg as well as the activation of B cells, could lead to the suppression of inflammation and cartilage damage.
In conclusion, the present study shows that i.p. administration of rebamipide in SKG mice reduces clinical arthritis severity, histological inflammation and cartilage destruction. These anti-arthritic effects may be associated with reciprocal regulation of Tfh and Breg cells. Furthermore, rebamipide treatment shifted the proportion of B lymphocytes from autoantibody-producing pathogenic cells to those conveying immunoregulatory properties.
Acknowledgments
We thank Professor Shimon Sakaguchi, Osaka University, Japan, for supplying the SKG mice. This study was supported by Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0016).
Disclosures
The authors have no conflicts of interest to disclose.
References
- Blake DR, Merry P, Unsworth J, et al. Hypoxic–reperfusion injury in the inflamed human joint. Lancet. 1989;1:289–293. doi: 10.1016/s0140-6736(89)91305-6. [DOI] [PubMed] [Google Scholar]
- Griffiths HR. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun Rev. 2008;7:544–549. doi: 10.1016/j.autrev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Nissim A, Winyard PG, Corrigall V, et al. Generation of neoantigenic epitopes after posttranslational modification of type II collagen by factors present within the inflamed joint. Arthritis Rheum. 2005;52:3829–3838. doi: 10.1002/art.21479. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens EJ, Mijnheer G, Duurland CL, et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood. 2011;118:3538–3548. doi: 10.1182/blood-2010-12-328187. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunology. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- van Amelsfort JM, van Roon JA, Noordegraaf M, et al. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- Chaiamnuay S, Bridges SL., Jr The role of B cells and autoantibodies in rheumatoid arthritis. Pathophysiology. 2005;12:203–216. doi: 10.1016/j.pathophys.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhu C, Ma B, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Yoshikawa T, Tanigawa T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Zinchuk VS, Garcia del Saz E, et al. Suppressive effect of rebamipide, an antiulcer agent, against activation of human neutrophils exposed to formyl-methionyl-leucyl-phenylalanine. Histol Histopathol. 2000;15:1067–1076. doi: 10.14670/HH-15.1067. [DOI] [PubMed] [Google Scholar]
- Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Takahashi T, Hata H, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- Hata H, Sakaguchi N, Yoshitomi H, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruutu M, Thomas G, Steck R, et al. beta-glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–2222. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Park MK, Park JS, Cho ML, et al. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3(+) regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis. Immunol Lett. 2011;135:50–58. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- Kessel A, Haj T, Peri R, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11:670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol. 2007;19:156–162. doi: 10.1016/j.coi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Glimcher LH. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev. 2003;194:29–38. doi: 10.1034/j.1600-065x.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. doi: 10.1385/1-59259-771-8:207. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Criado G, Medghalchi M, et al. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res Ther. 2007;9:R113. doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Junt T, Fink K, Forster R, et al. CXCR5-dependent seeding of follicular niches by B and Th cells augments antiviral B cell responses. J Immunol. 2005;175:7109–7116. doi: 10.4049/jimmunol.175.11.7109. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Kuhn KA, Kulik L, Tomooka B, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston B, Palanichamy A, Anolik JH. B cells in the pathogenesis and treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2010;22:307–315. doi: 10.1097/BOR.0b013e3283369cb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Liu B, Jiang Z, Jiang Y. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol. 2014;33:187–85. doi: 10.1007/s10067-013-2359-3. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Woo YJ, Jeong JH, et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthritis Cartilage. 2012;20:1426–1438. doi: 10.1016/j.joca.2012.08.002. [DOI] [PubMed] [Google Scholar]