Abstract
Podocytes maintain the structure and function of the glomerular filtration barrier. However, podocytes have recently been implicated in the innate immune response, and their function as non-haematopoietic antigen-presenting cells was highlighted. We have shown previously that excessive expression of the chemokine CXCL13 is a distinctive early event for nephritis in a murine model of systemic lupus erythematosus (SLE). Furthermore, we found that CXCL13 is elevated significantly in the serum of patients with SLE-nephritis. In this study, we were able to show for the first time that (i) CXCL13 is expressed locally in glomeruli in a model for SLE-nephritis in mice and that (ii) incubation of human podocytes with CXCL13 induces receptor stimulation of CXCR5 with activation of signalling pathways, resulting in (iii) secretion of proinflammatory cytokines and chemokines in culture supernatant. This cytokine/chemokine cocktail can lead to (iv) a neutrophil respiratory burst in isolated human granulocytes. Taken together, our results provide further evidence that CXCL13 is involved in the pathogenesis of glomerulonephritis and that podocytes can play an active role in local proinflammatory immune responses. Thus, CXCL13 could be a direct target for the therapy of glomerulonephritis in general and for SLE-nephritis in particular.
Keywords: CXCL13, chemokines, podocytes, proinflammatory signaling, SLE-nephritis
Introduction
Inflammation is one of the leading reasons for the development of glomerulonephritis, a major cause of progressive kidney disease 1. The innate immune response is an old evolutionarily conserved non-specific defence system protecting the organism against infections. One of the major events occurring during an innate immune response is the recruitment of immune cells towards the site of required action via secretion of cytokines and chemokines. This leads to the removal of apoptotic cells and regeneration or wound healing 2. The balance between a protective immune response and an autoimmune reaction, where body cells are recognised as foreign, is very critical. There are many examples where infections and inflammation are described as a responsible trigger for autoimmunity 3. In this context, cross-reactivity between pathogen-derived and self-derived antigens plays an important role (for review see 4). The chemokine CXC ligand 13 protein (CXCL13), also known as B cell-attracting chemokine-1 (BAC-1) or B lymphocyte chemoattractant (BLC), is a CXC subtype member of the chemokine superfamily 5. CXCL13 is particularly important in the context of autoimmunity, as it has been demonstrated that CXCL13 is sufficient to induce secondary lymphoid tissues in peripheral organs. Via its receptor CXCR5, expressed on follicular dendritic cells and follicular T helper cells (Tfh), CXCL13 is crucial for germinal centre formation 6,7. We and others have linked CXCL13 expression to the onset of nephritis in a mouse model for SLE-nephritis 8,9. Of note, in the expression profile of 61 inflammatory molecules, CXCL13 was one of only a few markers that were elevated at a very early point of disease, suggesting a possible role of this chemokine for disease manifestation 10. Furthermore, we and others found that CXCL13 is elevated in the serum of patients with SLE-nephritis 11–15. This is of interest, as lupus nephritis belongs to the most severe complications of SLE, with unsatisfactory treatment strategies with respect to remission induction and unwanted toxic effects (for a review, see 16).
In the kidneys, the major cell type secreting CXCL13 was identified as non-resident dendritic cells 9,10,17,18. Podocytes, which are the epithelial cells at the kidney filtration barrier, have been linked to the innate immune response and are known to express several chemokine receptors 19,20. Recently, it was published that intrinsic proinflammatory signalling in podocytes contributes to podocyte damage and aggravated proteinuria in experimental glomerulonephritis in mice, indicating that production and secretion of proinflammatory chemokines by podocytes has a physiological role in this setting 21.
To our knowledge, we are the first to show that CXCL13 is expressed in the glomerula of a mouse model of SLE-nephritis and that CXCL13 acts directly on human podocytes. We are able to demonstrate that cultured human podocytes in vitro express the CXCL13 receptor CXCR5 and are responsive to even low concentrations of recombinant CXCL13 (500 pg/ml). This concentration corresponds to the concentration detected in the serum of patients with active, severe SLE-nephritis class IV. In addition, we show that stimulation of human cultured podocytes with CXCL13 results in a proinflammatory and chemoattractant signature. Furthermore, podocyte response to CXCL13 induces a respiratory burst in isolated human neutrophilic granulocytes. Collectively, our results suggest that CXCL13/CXCR5 signalling in podocytes can induce a proinflammatory milieu which could influence the development of leucocyte infiltration observed in severe (classes III and IV) lupus nephritis.
Materials and methods
Human podocyte culture
For this study, immortalised human podocytes (gift from Moin Saleem, University of Bristol, UK) were cultured as described previously 22. The cells contain the tsSV40 T gene and are in a proliferative state when cultured in RPMI-1640 medium containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin at 33°C. Expression of the podocyte marker proteins WT-1 and synaptopodin was confirmed after 14 days of differentiation by thermoswitching to 37°C. For stimulation experiments, human podocytes were serum-starved in 1% FCS overnight.
Immunohistochemistry
Snap-frozen renal cryosections from New Zealand Black/White F1 mice with lupus nephritis and controls were used for immunohistochemistry. For indirect immunofluorescence, non-specific binding sites were blocked with 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min. Thereafter, sections were incubated with the primary CXCL13 polyclonal rat anti-mouse antibody (1:50; Biorbyt, San Francisco, CA, USA) or CD11c monoclonal hamster anti-mouse antibody (1:200; BD Pharmingen, Erembodegem, Belgium) for 1 h in a humid chamber at room temperature. For fluorescent visualisation of bound primary antibodies, sections were further incubated with Alexa Fluor 555 goat anti-rat immunoglobulin (Ig)G conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) for 1 h. Sections were analysed using a Leica imaging microscope by an investigator blinded for the animal group assignment. The semiquantitative scoring of glomerular expression was: 0 = no expression, 1 = mild expression < 20% of glomeruli stained, 2 = 21–50% of glomeruli stained, 3 = 50–75% of glomeruli stained and 4 = extensive glomerular staining of > 75% of glomeruli.
Western blot analysis
Whole cell protein lysates were harvested from differentiated cultured human podocytes, which were either left untreated or treated with 500 pg/ml recombinant human CXCL13 (R&D Systems, Wiesbaden, Germany) for the indicated time-points. Cells were lyzed on ice in radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris, pH 7·5, 150 mM NaCl, 0·5% sodium deoxycholate, 1% Nonidet P-40, 0·1% sodium dodecyl sulphate (SDS)] containing protease inhibitors (complete mini; Roche, Mannheim, Germany), 1 mM sodium orthovanadate, 50 mM NaF and 200 μg/l okadaic acid. Lysates were centrifuged for 15 min at 12 000 g, and aliquots of the supernatants were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA, USA). After probing with primary antibodies [mouse anti-p-p44/42 mitogen-activated protein kinase (MAPK) (phospho-extracellular-regulated kinase – ERK) or rabbit anti-p44/42 MAPK (total ERK1/2) (Cell Signaling Technology, Santa Cruz, CA, USA)], antigen–antibody complexes were detected with horseradish peroxidase-labelled anti-rabbit and anti-mouse secondary antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), respectively, and visualised using SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA), according to the manufacturer's protocol.
Fluorescence activated cell sorter (FACS) analysis
Flow cytometry was performed on a FACSCanto I (BD Biosciences, San Jose, CA, USA) with FACSDiva software version 6·0. Data were analysed using WinList™ software (Verity Software House, Topsham, ME, USA). Differentiated human podocytes were trypsinised and stained with an anti-human CXCR5 (R&D Systems) or an isotype matched control antibody followed by a fluorescein isothiocyanate (FITC)-labelled secondary antibody.
Multiplex analysis
Cell culture supernatants of human podocytes were harvested before and after stimulation with 500 pg/ml CXCL13 for 8 and 24 h. Unstimulated cell culture supernatants were harvested at matching time-points. Cytokine and chemokine content were analysed using the Luminex-based multiplex technique, according to the manufacturer's instructions (BioPlex assay, Bio-Rad, Hercules, CA, USA). In brief, 50 μl of culture supernatant and lyophilised cytokine and chemokine standard were incubated at room temperature (RT) for 30 min with a bead mixture coated with the antibodies for the respective cytokine or chemokine. Serial dilution series (1:4) were performed to generate standard curves for each protein. After three wash steps with 100 μl wash buffer, the mixture of secondary biotinylated antibodies was added for 30 min at RT, followed by three wash steps. Finally, streptavidin–phycoerythrin (SA–PE) was added for 10 min at RT (1:100 dilution) in order to detect the amount of bound secondary monoclonal antibody that correlated with the cytokine or chemokine concentration. After three final washing steps, the beads were resuspended in 125 μl assay buffer and acquired and analysed using the BioPlex Manager version 6·0 software that calculates standard curves using a five-parameter logistic plot formula, which allows the calculation of protein concentrations in the samples (pg/ml). Three independent experiments were analysed and each supernatant and each time-point was measured in duplicate on the multiplex protein array. Statistical analysis was performed by Student's t-test.
Preparation of human neutrophilic granulocytes
Peripheral venous blood was drawn from healthy volunteers after local ethics board approval (MHH 2010/807) and informed consent. Neutrophilic granulocytes were isolated by density gradient centrifugation, as described previously 23.
Measurement of respiratory burst by oxidation of dihydrorhodamine (DHR) to rhodamine
Isolated neutrophilic granulocytes were cultured at a concentration of 5 × 106 cells/ml for 24 h in podocyte supernatants recovered 8 h after stimulation with/without CXCL13. Cells were loaded with DHR (1 μM) for 10 min at 37°C followed by stimulation with recombinant human tumour (AE) necrosis factor (TNF)-alpha (20 ng/ml; Peprotech, Rocky Hill, NJ, USA) for 20 min. Mean fluorescence intensity was assessed by flow cytometry on a BD FACSCanto I (Becton Dickinson, Heidelberg, Germany), and data were analysed using FlowJo software (TreeStar Inc., Ashland, OR, USA). Mean fluorescence intensity (MFI, after correction for baseline) of cells cultured in medium of stimulated podocytes was compared to medium from unstimulated podocyte controls harvested at the same time-point and incubated with neutrophils from the same donor. Seven incubation experiments in isolated neutrophilic granulocytes were performed using cell culture supernatants from four independent podocyte stimulation experiments. Results are expressed as foldness of control MFI, as baseline levels varied in different donors. Values from control versus CXCL13-stimulated groups were compared by Mann–Whitney U-test; P < 0·05 was considered significant and is indicated by *.
Results
Dendritic cells and local production of CXCL13 are detectable in glomeruli of nephritic NZB/W-F1 mice
We have published previously that CXCL13 is expressed in inflammatory infiltrates of nephritic NZB/W F1 mice 9. Here we evaluated the local expression of CXCL13 in the glomeruli of nephritic mice by staining a set of nephritic SLE mice and non-nephritic control mice for CXCL13 and for the dendritic cell marker CD11c. We observed glomerular infiltration with dendritic cells and glomerular expression of CXCL13 protein in the majority of nephritic glomeruli (Fig. 1a–h). At the mRNA level we found a significant increase of CXCL13 expression in nephritic mice versus controls (Fig. 1i). Next, we wanted to address the question whether CXCL13, which is known to be expressed at the mRNA level long before the onset of lupus nephritis 11, has a potential influence on the disease process in lupus nephritis by triggering an intrinsic cell response in podocytes.
Fig 1.
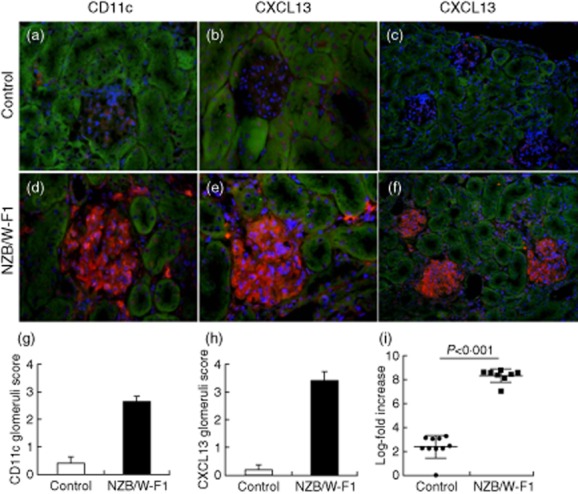
Dendritic cells and expression of CXCL13 in systemic lupus erythematosus (SLE) mice. Renal cortex sections of non-proteinuric control mice (a–c) and nephritic New Zealand Black/White (NZB/W) F1 mice (d–f) were stained with the dendritic cell marker CD11c (a,d) and for expression of CXCL13 (b,c,e,f) (red fluorescence; 4′,6-diamidino-2-phenylindole (DAPI) for nuclear counterstaining). While CD11c and CXCL13 staining was almost absent in control mice, the presence of significant numbers of dendritic cells and local production of CXCL13 was detectable in glomeruli of nephritic NZB/W F1 mice. Semiquantitative scoring of five mice in each group for CD11c is shown in (g) and for CXCL13 in (h) [magnification (a,b,d,e): 400-fold; overview in (c,f): 200-fold]. Total mRNA expression of CXCL13 shown as log-fold increase in non-nephritic control and nephritic NZB/W F1 mice (i).
Immortalised human podocytes in culture express CXCR5 that is responsive to CXCL13 stimulation
Expression of CXCR5 and other chemokine receptors in primary cultured podocytes has been described previously based on mRNA expression analysis 20. In the present work, we asked whether an immortalised human podocyte cell line in culture expresses a functional form of the receptor CXCR5 and if stimulation with CXCL13 can induce a specific signalling response in podocytes. To accomplish this, we first analysed the expression of CXCR5 on differentiated immortalised human podocytes by flow cytometry. As shown in Fig. 2a, roughly 20% of the cultured podocytes expressed CXCR5 on their surface. Next, we analysed the ERK1/2 phosphorylation response in podocytes as a classical downstream mediator of chemokine receptor activation 24. For stimulation of podocytes, we chose 500 pg/ml CXCL13, as this corresponds to the medium serum concentration that we detected in highly active SLE patients [SLE Disease Activity Index (SLEDAI) > 8] in our Hannover Medical School patient cohort (data not shown). When we performed a time–course experiment and stimulated the cells with 500 pg/ml CXCL13, we detected a clear ERK1/2 phosphorylation response in the stimulated cells as early as 4 h after CXCL13 exposure (Fig. 2b), indicating that a signalling response could be induced in podocytes. Of note, the used concentration of 500 pg/ml is 10-fold lower than the lowest range for the effective dose for 50% of the patients (ED50) ability of recombinant CXCL13 to chemoattract BaF3 mouse pro-B cells transfected with human CXCR5 as tested by the vendor (R&D Systems). Additional dose response experiments using up to 1000 pg/ml recombinant CXCL13 did not lead to a significant increase in ERK-phosphorylation response in podocytes (data not shown). Therefore, the concentration of 500 pg/ml was chosen for all following experiments.
Fig 2.
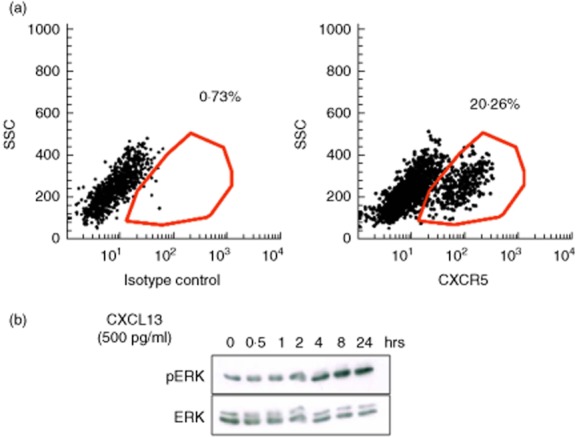
Functional CXCR5 is expressed on human podocytes in vitro. (a) Cell surface expression of CXCR5 on cultured human podocytes was detected by fluorescence activated cell sorter (FACS) analysis. Differentiated human podocytes were stained with CXCR5 or an isotype control antibody followed by fluorescein isothiocyanate (FITC)-labelled secondary antibody. (b) Stimulation of differentiated human podocytes with recombinant human CXCL13 induces an extracellular-regulated kinase (ERK)1/2 (42/44) phosphorylation response. Cells were stimulated for the time-points indicated. Following cell lysis, Western blots were performed using an anti phospho-ERK1/2 antibody. Total ERK1/2- was used as loading control.
CXCL13 induces secretion of a variety of proinflammatory factors in human podocytes
Next, we performed multiplex protein arrays from cell culture supernatants of three independent experiments from human podocytes that were stimulated for 8 h and 24 h with CXCL13 in comparison to untreated controls to evaluate if stimulation with 500 pg/ml of recombinant CXCL13 could induce specific secretion of other proinflammatory cytokines and chemokines. One of the most significantly up-regulated factors in the culture supernatant was CXCL1 (Gro-α) (Fig. 3a). This chemokine is known for its ability to attract neutrophils to sites of inflammation 25,26. Further experiments showed an up-regulation of CXCL12 (SDF-1α) secretion following stimulation (Fig. 3b). CXCL12 is a chemokine that is highly efficient in inducing chemotaxis of lymphocytes and macrophages 27,28. We also found a significant up-regulation of macrophage migration inhibitory factor (MIF) (Fig. 3c), a proinflammatory cytokine normally secreted by white blood cells in response to bacterial antigens as well as leukaemia inhibitory factor (LIF) (Fig. 3d), a pleiotropic glycoprotein of the IL-6 family that has been shown to protect podocytes against oxidative stress 29. Furthermore, we detected soluble intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1) (Fig. 3e,f) in the culture supernatants. Both adhesion molecules are expressed in the inflammatory milieu and also indicate a more migratory phenotype of podocytes, which has been described previously as a key step in crescent formation in glomerulonephritis 30.
Fig 3.
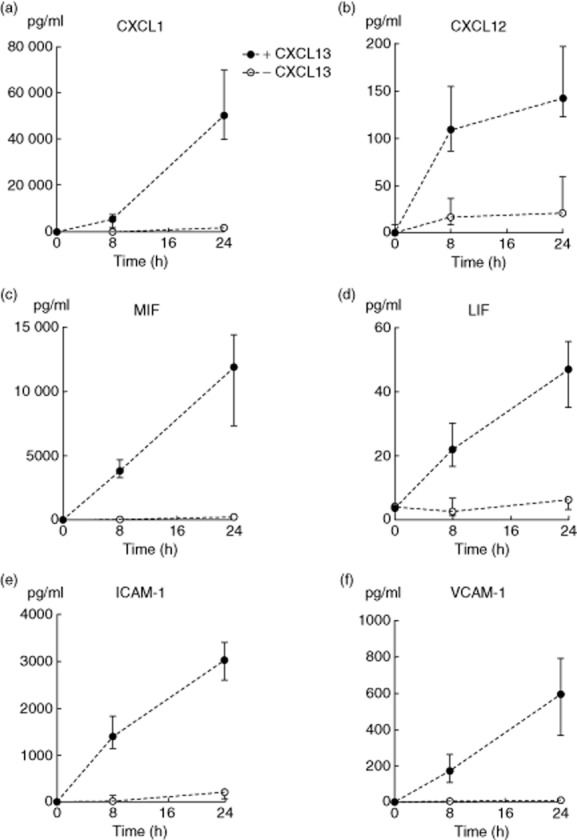
CXCL13 stimulation induces production of proinflammatory mediators in human podocytes. Differentiated podocytes were stimulated with 500 pg/ml of recombinant CXCL13 for the time-points indicated. Culture supernatants were harvested and analysed for cytokine and chemokine content using Multiplex analysis. Significantly up-regulated factors detectable in the culture supernatant include: (a) CXCL1 (*P < 0·001), (b) CXCL12 (**P < 0·001/*P < 0·003), (c) macrophage migration inhibitory factor (MIF) (**P < 0·01; *P < 0·05), (d) leukaemia inhibiting factor (LIF) (*P < 0·03; *P < 0·05), (e) intercellular adhesion molecule 1 (ICAM-1) (**P < 0·001; *P < 0·03) and (f) vascular cell adhesion protein 1 (VCAM-1) (**P < 0·01; *P < 0·05) (three independent experiments).
CXCL13 stimulated podocytes can induce respiratory burst in human neutrophils
We tested the respiratory burst activity of isolated human neutrophils after pre-incubation with cell culture supernatants of podocytes stimulated with 500 pg/ml CXCL13 for 8 h. The isolated neutrophils were cultured at a concentration of 5 × 106/ml for 24 h in freshly recovered podocyte supernatants. After loading the cells with DHR, cells were stimulated with recombinant human TNF-α (20 ng/ml) for 20 min. DHR is reduced to rhodamine in the presence of reactive oxygen species (ROS). When we compared the fluorescence intensity of cells by flow cytometry, we discovered a significantly enhanced respiratory burst in neutrophils that were pre-incubated with culture supernatants of podocytes after CXCL13 stimulation, indicating a proinflammatory milieu of the culture medium (Fig. 4).
Fig 4.
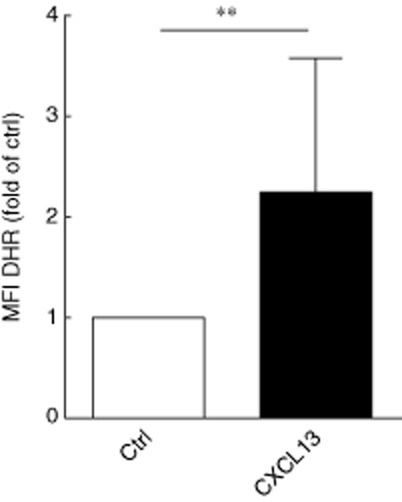
Podocyte supernatant after exposure to CXCL13 increases neutrophil respiratory burst. Neutrophil respiratory burst in response to tumour necrosis factor (TNF)-alpha was assessed after 24 h in control (ctrl) and CXCL13-treated podocyte supernatants (seven neutrophil exposure experiments, **P = 0·02).
Discussion
In SLE patients, circulating CXCL13 can function as a marker of disease activity and B cell activation and is elevated significantly in patients with lupus nephritis 11–14. However, the relevance of CXCL13 in the process of the development of lupus nephritis has so far remained elusive.
Podocytes are highly specialised glomerular epithelial cells that play a key role for kidney function 31. Different studies have proved over the last decades that podocyte damage initiates progression of many glomerular diseases that can lead to proteinuria and chronic kidney failure. The role of podocytes in disease progression is manifold: one major reason is a destabilisation of the glomerular filtration barrier due to podocyte alterations 32,33. Recently, a new role of podocytes has been highlighted. There is growing evidence that podocytes also play a relevant role in immunological reactions. Reiser et al. were able to show for the first time that, under pathological conditions, podocytes up-regulate B7-1 (CD80), a molecule that was known to be expressed mainly by antigen-presenting cells (APC) and to induce T cell co-stimulation 34,35. Despite this, podocyte structures can function as antigens, as shown with different podocyte autoantibodies in membranous nephropathy 36,37. Interestingly, it has been published recently that treatment of patients with B7-1-positive glomerular diseases by a co-stimulatory inhibitor that targets B7-1 (CD80) induces partial or complete remission of proteinuria 24.
In view of this speculated new function of podocytes, and our knowledge that the chemokine CXCL13 is elevated significantly on protein level in serum of patients with lupus nephritis 11, we aimed to study whether or not CXCL13 can modulate podocyte function and induce an immunological response in these cells.
Here we were able to show for the first time, using immunohistological studies, that there is local expression of CXCL13 in the glomeruli of nephritic mice. As Ishikawa et al. previously described dendritic cells as the major source of CXCL13 production 17, we stained glomeruli with a dendritic cell marker, and we indeed detected a significant amount of infiltrating CD11c-positive cells in the glomeruli of nephritic animals. Well in line with our previous studies, we also detected strong expression of CXCL13 in CD11c containing perivascular infiltrates in nephritic NZB/W F1 mice (data not shown) 9.
Using FACS analysis, we were able to confirm that differentiated human podocytes in cell culture express CXCR5, the receptor of CXCL13. To prove that incubation of differentiated human podocytes with CXCL13 leads to a CXCR5-mediated signalling event in these cells, we measured the ERK phosphorylation response in podocytes after CXCL13 stimulation. ERK phosphorylation is a classical downstream mediator of chemokine receptors 38. As expected, CXCL13 induced a strong ERK phosphorylation response over time, indicating responsiveness of podocytes to chemokine stimulation. This is well in line with a previously published study on ERK signalling in response to CXCL13 in other epithelial cells 39.
In immune cells, CXCL13 stimulation leads to a release of cytokines and an enhancement of the immunological signal. To assess whether podocytes can act similarly, we analysed the culture supernatants of podocytes after stimulation with CXCL13 for cytokine and chemokine content. This is of special interest, as glomerular-produced chemokines are not only necessary for recruitment of non-resident inflammatory cells, but are known to alter functions of resident glomerular cells, such as the formation of extracellular matrix, ROS production and apoptosis 40. In our study, several chemokines and cytokines were secreted in the supernatant of stimulated podocytes. Of interest, we found two chemokines, CXCL1 and CXCL12, that are both highly efficient for the recruitment of neutrophils and lymphocytes, a classical feature of lupus nephritis classes III and IV 25–28. Even though the expression of CXCL1 by podocytes has not been described previously, CXCL1 and CXCL12 have been mentioned in the context of different glomerular diseases 41,42. In our multiplex cytokine array experiments we also detected significant expression of the proinflammatory cytokines MIF and LIF and of the adhesion molecules ICAM-1 and VCAM-1. MIF and LIF are known to be increased in kidney diseases, and blockade of MIF or its receptor protects from experimental kidney injury 43. Well in line with our data, the expression of ICAM-1 by podocytes has been described previously in kidney sections of mice after induction of experimental crescentic glomerulonephritis 30. Despite this, ICAM-1 and VCAM-1 secretion by podocytes could result in the retention of lymphocytes, macrophages and neutrophils in the kidney supporting local immune reactions.
It is of note that ICAM-1 and VCAM-1 are required for the retention and clustering of lymphoid tissue inducer cells (CD3–CD4+CD45+ cells) and other haematopoietic cells at the sites of lymphoid organogenesis and neogenesis 44. Therefore, it is tempting to speculate that secretion of ICAM-1 and VCAM-1 by podocytes due to the CXCL13 signalling response could also be a relevant event contributing to the migration of inflammatory cells into the kidney. In addition, we demonstrate that the chemokine/cytokine mix secreted by podocytes has a functional role. We found that culture supernatants from CXCL13-stimulated podocytes can induce a neutrophilic burst which is known to be sufficient to trigger a local proinflammatory response 45. A direct effect of recombinant CXCL13 on neutrophils could be excluded, as after 8 h free CXCL13 was no longer detectable in the culture medium (data not shown); this also indicates indirectly that CXCL13 is bound or absorbed by the cultured podocytes.
Taken together, our results provide further evidence that CXCL13 is involved in the pathogenesis of lupus nephritis and that podocytes play an active role in immunological mechanisms after CXCL13 stimulation. Thus, CXCL13 blockade may be an effective therapy for different types of podocyte-related kidney diseases in general and for lupus nephritis in particular.
Acknowledgments
We thank Heike Lührs, Herle Chlebusch and Barbara Hertel for excellent technical support. This work was supported by the IFB-Tx (BMBF 01EO1302).
Disclosure
The authors declare no financial or commercial conflicts of interest.
Author contributions
L. S. and M. S. designed the study, K. W., A. D.-M., F. W. and C. F. performed the experiments, L. S., M. S., K. W., H. H., F. G., A. D., T. W., S.v.V. and C. F. evaluated the data and wrote the manuscript.
References
- Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol. 2011;22:802–809. doi: 10.1681/ASN.2010050510. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Sener AG, Afsar I. Infection and autoimmune disease. Rheumatol Int. 2012;32:3331–3338. doi: 10.1007/s00296-012-2451-z. [DOI] [PubMed] [Google Scholar]
- Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. [Aberrant high expression of B lymphocyte chemoattractant (BLC/CXCL13) in a murine model for SLE] Tanpakushitsu Kakusan Koso. 2002;47:2369–2374. [PubMed] [Google Scholar]
- Schiffer L, Sinha J, Wang X, et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Bethunaickan R, Ramanujam M, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer L, Kumpers P, Davalos-Misslitz AM, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE) Nephrol Dial Transplant. 2009;24:3708–3712. doi: 10.1093/ndt/gfp343. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Kielstein JT, Haubitz M, et al. Elevation of serum CXCL13 in SLE as well as in sepsis. Lupus. 2011;20:507–511. doi: 10.1177/0961203310383301. [DOI] [PubMed] [Google Scholar]
- Ezzat M, El-Gammasy T, Shaheen K, Shokr E. Elevated production of serum B-cell-attracting chemokine-1 (BCA-1/CXCL13) is correlated with childhood-onset lupus disease activity, severity, and renal involvement. Lupus. 2011;20:845–854. doi: 10.1177/0961203311398513. [DOI] [PubMed] [Google Scholar]
- Lee HT, Shiao YM, Wu TH, et al. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol. 2010;37:45–52. doi: 10.3899/jrheum.090450. [DOI] [PubMed] [Google Scholar]
- Wong CK, Wong PT, Tam LS, Li EK, Chen DP, Lam CW. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30:45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- Sprangers B, Monahan M, Appel GB. Diagnosis and treatment of lupus nephritis flares – an update. Nat Rev Nephrol. 2012;8:709–717. doi: 10.1038/nrneph.2012.220. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Sato T, Abe M, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–1402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Nagai S, Sato T, et al. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur J Immunol. 2002;32:1881–1887. doi: 10.1002/1521-4141(200207)32:7<1881::AID-IMMU1881>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Reinhardt HC, Exner M, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168:6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- Brahler S, Ising C, Hagmann H, et al. Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am J Physiol Renal Physiol. 2012;303:F1473–F1485. doi: 10.1152/ajprenal.00031.2012. [DOI] [PubMed] [Google Scholar]
- Saleem MA, O'Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- von Vietinghoff S, Choi M, Rolle S, Luft FC, Kettritz R. Febrile temperatures control antineutrophil cytoplasmic autoantibody-induced neutrophil activation via inhibition of phosphatidylinositol 3-kinase/Akt. Arthritis Rheum. 2007;56:3149–3158. doi: 10.1002/art.22832. [DOI] [PubMed] [Google Scholar]
- Yu CC, Fornoni A, Weins A, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. High-and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci USA. 1992;89:10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Z, Xu P, Yang Z. Protective effects of leukemia inhibitory factor against oxidative stress during high glucose-induced apoptosis in podocytes. Cell Stress Chaperones. 2012;17:485–493. doi: 10.1007/s12192-012-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse-Eschmann V, Le HM, Endlich N, Endlich K. Alteration of podocytes in a murine model of crescentic glomerulonephritis. Histochem Cell Biol. 2004;122:139–149. doi: 10.1007/s00418-004-0683-z. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Saleem MA. The podocyte cytoskeleton – key to a functioning glomerulus in health and disease. Nat Rev Nephrol. 2012;8:14–21. doi: 10.1038/nrneph.2011.151. [DOI] [PubMed] [Google Scholar]
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- Henry J, Miller MM, Pontarotti P. Structure and evolution of the extended B7 family. Immunol Today. 1999;20:285–288. doi: 10.1016/s0167-5699(98)01418-2. [DOI] [PubMed] [Google Scholar]
- Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtas C, Bruschi M, Candiano G, et al. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:1394–1400. doi: 10.2215/CJN.02170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec H, Hanoy M, Francois A, et al. Recurrent membranous nephropathy in an allograft caused by IgG3kappa targeting the PLA2 receptor. J Am Soc Nephrol. 2012;23:1949–1954. doi: 10.1681/ASN.2012060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- El-Haibi CP, Singh R, Gupta P, et al. Antibody microarray analysis of signaling networks regulated by Cxcl13 and Cxcr5 in prostate cancer. J Proteomics Bioinform. 2012;5:177–184. doi: 10.4172/jpb.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Minto AW, Dorf ME, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Czech KA, Arend LJ, Witte DP, Devarajan P, Potter SS. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol. 2007;107:e30–e40. doi: 10.1159/000106775. [DOI] [PubMed] [Google Scholar]
- Sayyed SG, Hagele H, Kulkarni OP, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52:2445–2454. doi: 10.1007/s00125-009-1493-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, et al. MIF, CD74 and other partners in kidney disease: tales of a promiscuous couple. Cytokine Growth Factor Rev. 2013;24:23–40. doi: 10.1016/j.cytogfr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;5:959–966. [PubMed] [Google Scholar]


