Abstract
There is a limited understanding how of lung cancer cells evade cytotoxic attack. Previously, we have shown reduced production of the cytotoxic mediator granzyme B by CD8+ T cells in lung cancer tissue. We hypothesized that lung cancer would be further associated with decreased production of granzyme B, perforin and proinflammatory cytokines by other cytotoxic lymphocytes, natural killer (NK) T-like and NK cells, and that this would result from soluble mediators released by the cancer cells. Lung cancer and non-cancer tissue from five patients was identified by experienced pathologists. Tumour necrosis factor (TNF)-α, interferon (IFN)-γ, granzyme B and perforin were measured in CD4 and CD8+ T, NK T-like cells and NK cells by flow cytometry. Correlation between cancer stage and granzyme B was analysed retrospectively for 21 patients. The effects of soluble factors released by lung cancer cells on production of cytotoxic mediators and cytokines was assessed, and the role of prostaglandin E2 (PGE)2/COX investigated using indomethacin inhibition. There were significantly decreased percentages of T, NK T-like and NK cells expressing perforin, TNF-α and IFN-γ in cancer versus non-cancer tissue, and of CD8+ T cells and CD8+ NK T-like cells expressing granzyme B (e.g. NK T-like cells: non-cancer 30% ± 7 versus cancer 6% ± 2·5). Cancer cells released soluble factors that inhibited granzyme B, perforin and IFN-γ production that was partially associated with the PGE2/COX2 pathway. Thus, lung cancer is associated with decreased expression of granzyme B, perforin and IFN-γ by infiltrating T cells, NK T-like and NK cells, possibly as a result of soluble factors produced by the cancer cells including PGE2. This may be an important immune evasion mechanism.
Keywords: cytotoxicity, granzyme B, interferon gamma, lung cancer, NK and NK T-like cells
Introduction
Lung cancer is responsible for more cancer-related deaths than colon, breast and prostate cancers combined 1, and more than 80% of cases occur in smokers or ex-smokers 2. Smokers with chronic obstructive pulmonary disease (COPD) have a higher risk of developing lung cancer than do smokers without COPD 3, even with the same smoking history. The burden of COPD and the associated prevalence of lung cancer are projected to increase in the coming decades; therefore, there is an urgent need to understand more clearly the links between smoking, COPD and lung cancer to enable novel therapeutic or preventative strategies.
The average 5-year survival rate of lung cancer is only 15·5%, with non-small cell lung carcinoma (NSCLC) accounting for the majority of cases. Small cell lung carcinoma (SCLC) accounts for 15% 4. We and others have shown that lung cancers produce mediators [e.g. prostaglandin E2 (PGE2), transforming growth factor (TGF)-β] that assist tumour cell proliferation, anti-apoptotic properties, angiogenesis and chemotherapeutic resistance 5,6. Other immune mechanisms, such as silencing of the death receptor FAS, have been shown to confer resistance to apoptosis 7.
Cytotoxic cells, including CD8+ T cells, mount immune responses to cancer via by the release of cytolytic enzymes, including granzyme B, perforin and cytokines, including interferon (IFN)-γ. We have also shown that natural killer (NK) cells and NK T-like cells are potent producers of these cytotoxic mediators 8,12. Resistance to attack by these mediators is therefore essential for the survival and proliferation of lung cancer cells, and in this regard we have shown previously that there is significantly reduced granzyme B production by CD8+ T cells from cancer tissue obtained by lung resection surgery when compared to non-cancer tissue 9. We further showed that granzyme B production by CD8+ T cells was reduced in the presence of conditioned media from lung cancer cell lines, suggesting that the cancer cells themselves exert a suppressive effect on granzyme production and thus contribute to an ideal ‘pro-cancer’ environment, potentially acting as a further immune evasion mechanism by reducing the ability of these cells to cause apoptosis of the cancer cells.
The relative expression of NK and NK T-like cells is unknown in cancer versus non-cancer tissue and their relative production of granzyme B, perforin and IFN-γ in lung cancer patients. Also unknown is whether the cancer cells exert a suppressive effect on the production of these mediators by the various immune cell types. In this proof-of-principle study we therefore investigated whether there was diminished activity of T, NK and NK T-like cells in cancer versus non-cancer areas of the resected lung tissue that could contribute to a reduced cytotoxic activity and the establishment of a pro-tumour environment. We retrospectively assessed the correlation between cancer stage and granzyme B and evaluated the effects of soluble factors, including PGE2, released by lung cancer cell lines on production of cytotoxic mediators.
Materials and methods
Reagents
The following monoclonal antibodies (mAbs) and immunological reagents were employed: for investigation of lymphocyte subsets and their production of granzyme B/perforin: CD45 (V500) (BD Biosciences (BD), San Jose, CA, USA), CD3 (perCP.Cy5·5) (BD) and CD8 [fluorescein isothiocyanate (FITC)] (BD) CD56 [allophycocyanin (APC)] (Immunotech/Coulter, Marseille, France), granzyme B [phycoerythrin (PE)] (BD), perforin (FITC), FACSPerm (BD), FACSLyse (BD) and IsoFlow (Beckman Coulter, Lane Cove, NSW, Australia).
For stimulation experiments for cytokine evaluation the following reagents and mAbs were employed: phorbol 12-myristate 13-acetate (PMA), brefeldin A (Sigma Aldrich, Sydney, Australia) and ionomycin (Calbiochem, La Jolla, CA, USA), CD8 (FITC) CD56 (APC), CD3 [peridinin chlorophyll cyanin 5·5 (perCP.Cy5·5)], IFN-γ (FITC) (BD), TNF-α (V450) (BD) and CD45 (V500) (BD).
Subject population and collection of samples
Lung tissue
For investigation of cytotoxic mediators and cytokines, both cancer and non-cancer lung tissues were obtained from five lung cancer patients undergoing curative-intent lobectomy at the Royal Adelaide Hospital. For correlation between cancer stage and granzyme B, data from a further 21 patients was assessed (the demographic details of these patients have been described previously 5,9). Lung cancer was diagnosed using World Health Organization (WHO) criteria 10. Lung cancer stage was assessed using the 7th edition Tumour, Node and Metastasis (TNM) Classification for Lung Cancer. Patients were categorized further on the presence of COPD using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) < 70%] with X-ray and clinical correlation 11. Cancer tissue was obtained from the resected lobe using a core biopsy needle, while non-cancer tissue was obtained from lung periphery as distal to the cancer site as possible 5,9. Lung cancer tissue was identified by experienced pathologists. Ethics approval was granted by the Royal Adelaide Hospital and all procedures were undertaken after fully informed consent.
Preparation of samples
Tissue was cut into 5 × 5 mm sections followed by mechanical disaggregation using a ‘Medimachine’ tissue disaggregator (BD) to achieve a single-cell suspension, as described previously 5,9.
T, NK and NK T-like cell expression of intracellular granzyme B and perforin in lung cancer tissue
Flow cytometry was applied to identify T, NK and NK T-like cells, as reported previously 8,12. Dissagregated tissue was stained with directly conjugated monoclonal antibodies to granzyme B, perforin, CD3 and CD56 or isotype control and acquired/analysed by flow cytometry as reported 8,12,13. Briefly, 200 μl aliquots of prepared blood, bronchoalveolar lavage (BAL) or airway brushing were added to fluorescence activated cell sorter (FACS) tubes. For blood, FACSlyse was added for 10 min. All cells were washed with Isoflow containing 10% fetal calf serum (FCS) (CSL, Melbourne, Australia), centrifuged at 1500 g for 2 min, and the supernatant discarded. Cell membranes were permeabilized with FACSperm for 10 min and then washed. Cells were then incubated for 20 min with directly conjugated monoclonal antibodies to granzyme B or perforin. Data were acquired using a FACSCanto II flow cytometer (BD).
T, NK and NK T-like cell production of intracellular IFN-γ in lung cancer tissue
Flow cytometry was applied to measure intracellular IFN-γ and TNF-α production by T, NK and NK T-like cells, as reported previously 12,14. Briefly, cells from dissagregated tissue were stimulated with PMA (25 ng/ml), ionomycin (1 mg/ml) and brefeldin A (1 mg/ml), treated with FACSLyse and FACSPerm, then stained with 5 μl of appropriately diluted CD8 FITC, CD56 APC, CD3 perCP/Cy5·5, IFN-γ FITC, TNF-α V450, CD45 V500 or isotype control were added for 15 min in the dark at room temperature. Cells were washed and events acquired immediately.
Effects of conditioned media from SBC-1 cell line on T, NK and NK T-like cell production of intracellular TNF-α and IFN-γ
Cell culture
A lung small cell carcinoma (SBC-1) cell line (Health Science Research Resources Bank, Osaka, Osaka Prefecture, Japan) was grown in culture media. SBC-1 was selected based on our previous studies. In one study, we showed that SBC-1 lung cancer cell supernatants have a superior effect on CD8+ cell granzyme B expression and cytotoxicity when compared to some other lung cancer cell lines 9. In addition, when we previously treated SBC1 cells with the cyclooxygenase (COX) inhibitor indomethacin to investigate the potential effects of PGE2 produced by lung cancer cells on efferocytosis we found that they had the greatest effect on efferocytosis, with no negative effects on cell growth or viability of the SBC-1 cells at the concentrations used 5. A normal human bronchoepithelial cell line, 16HBE, was used as a control. Cells were cultured in uncoated 75 cm2 plastic flasks in 95% air and 5% CO2 at 37°C. The medium was refreshed every 2–3 days. To obtain tumour cell-conditioned media, the cells were cultured at a density of 5 × 105 cells/ml. After 24 h supernatant were collected and stored at −70°C.
Preparation of peripheral blood mononuclear cells (PBMCs)
Mononuclear cells were isolated from peripheral blood of three healthy human donors using Lymphoprep (Axis-Shield, Oslo, Norway), according to the manufacturer's instructions, and experiments performed in triplicate. The mononuclear cell layer was washed in culture medium at a concentration of 4 × 105 cells/ml.
Effect of lung cancer cell-conditioned media on cytokine production
Supernatants from 16HBE epithelial cells and tumour cell-conditioned media were cultured with PBMCs for 24 h at 37°C in an atmospheric pressure of 5% CO2, then for a further 16 h with SBC-1 or 16HBE prepared cell culture media or RPMI as a control in the presence of 25 ng/ml PMA, 1 μg/ml ionomycin and 1 μg/ml brefeldin A, as reported previously 8,12. The pellet containing mononuclear cells was then resuspended and stained for intracellular proinflammatory T cell cytokines (IFN-γ and TNF-α) by T, NK and NK T-like cells, as described above and as reported previously 12,14.
Effect of neutralizing the COX2/PGE2 pathway on cytokine production by conditioned media from SBC-1 cell line
To assess the potential effects of PGE2 produced by lung cancer cells, PBMC were treated with SBC-1 cell-conditioned medium ± the COX inhibitor indomethacin (−10 μM), as reported 5. The cells were grown for 48 h and the medium was removed, filtered and stored immediately at −70°C before assessing the effects on production of IFN-γ and TNF-α by T, NK and NK T-like cells.
Correlation of lung cancer stage with granzyme B expression
Retrospective correlation of cancer stage and intracellular CD8+ production of granzyme B was performed for 21 patients with NSCLC who had been recruited for previous studies 5,9. Demographic details have been published previously 5,9. Cancer stage was characterized using the 7th edition TNM Classification for Lung Cancer recommendations and ranged from 1A (n = 5), 1B (n = 9), 2A (n = 2) and 2B (n = 3) to 3A (n = 2).
Statistical analysis
Data were analysed using spss software and Mann–Whitney or one-way analysis of variance (anova) with post-hoc for non-parametric analyses. Correlations were performed using Spearman's rank test. Analyses were performed using spss software. P-values < 0·05 were considered significant.
Results
Clinical characteristics of lung cancer patients
Lung tissue was collected from five patients with lung cancer: two females and three males, with a median age of 63 years [± standard error of the mean (s.e.m.) 5·5]. Four patients were ex-smokers, one currently smoking, with a median pack year of 40 ± s.e.m. 11 years. Three patients were diagnosed with COPD (median FEV1 66% ± s.e.m. 2%; FEV1/FVC 58% ± s.e.m. 2%) and two had normal lung function. Four patients presented with NSCLC (poorly differentiated adenocarcinoma) and one with metastatic melanoma. Patients with NSCLC had cancer stage 1B–3A.
For correlation between cancer stage and expression of granzyme B by CD8+ T cells in cancer tissue, data from 21 patients with COPD and lung cancer were analysed retrospectively. The demographic details of these patients have been described previously 5,9
T, NK and NK T-like cell production of intracellular granzyme B and perforin in lung cancer tissue
Granzyme B
Consistent with our previous report in cancer tissue 9, there was a decrease in the percentage of CD8+ and CD4+ T cells expressing granzyme B compared with non-cancer lung tissue. Both CD4+ and CD8+ NK T-like cells, and NK cells from cancer tissue showed a similar significant decrease in intracellular granzyme B versus non-cancer lung tissue (Fig. 1).
Fig 1.
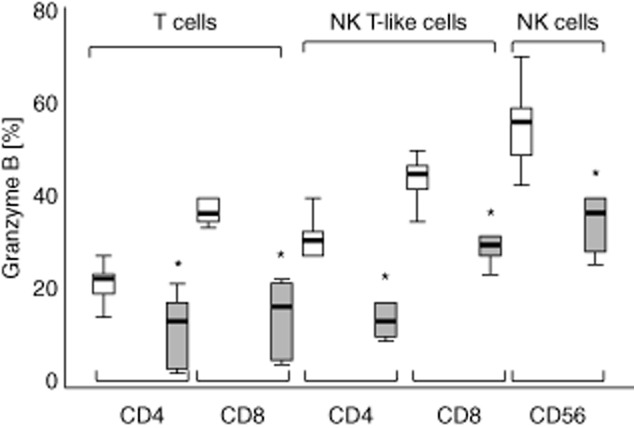
Intracellular granzyme B expression in CD4+ and CD8+ T cell and natural killer (NK) T-like subsets and NK cells from normal (clear bars) and cancer tissue (grey bars). There was a significant decrease in the percentage of CD8+ T cells expressing granzyme B from cancer tissue compared with normal tissue. There was also a decrease in the percentage of CD8+ NK T-like cells and NK cells expressing granzyme B compared with normal tissue. Box-plots present median ± 25th and 75th percentiles (solid box) with the 10th and 90th percentiles shown by whiskers outside the box (*P < 0·05).
Perforin
There was a decrease in the percentage of CD4+ and CD8+ T cells and NK cells expressing perforin compared with non-cancer lung tissue (Fig. 2). For NK T-like cells there was a significant decrease in the percentage of CD8+ cells expressing perforin compared with non-cancer lung tissue, and a non-significant trend for decreased expression in the CD4+ subset.
Fig 2.
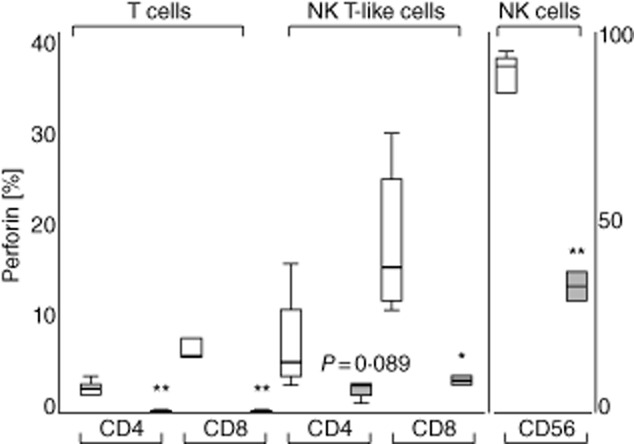
Perforin expression in CD4+ and CD8+ T cell and natural killer (NK) T-like subsets and NK cells from normal (clear bars) and cancer tissue (grey bars). There was a decrease in the percentage of CD4+ and CD8+ T cells expressing perforin from cancer tissue compared with normal tissue. There was also a decrease in the percentage of CD8+ NK T-like cells and NK cells expressing perforin compared with normal tissue. Data presented in box-plots as described in Fig. 1 (*P < 0·05; **P < 0·01).
T, NK and NK T-like cell production of intracellular IFN-γ in lung cancer tissue
There were significant decreases in the production of IFN-γ by CD4+ and CD8+ T cells, CD4+ and CD8+ NK T-like cells and NK cells from cancer tissue compared with non-cancer lung tissue (Fig. 3).
Fig 3.
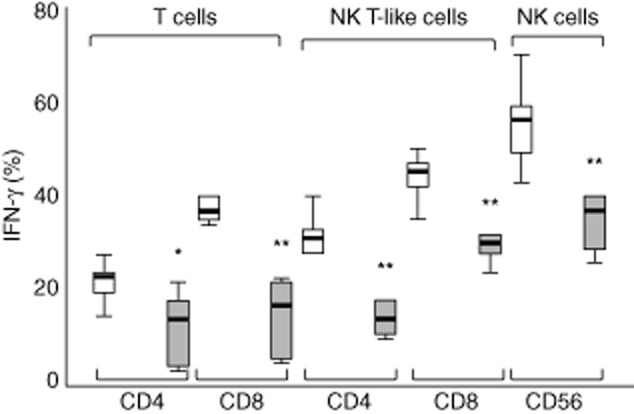
Interferon (IFN)-γ production in CD4+ and CD8+ T cell and natural killer (NK) T-like subsets and NK cells from normal (clear bars) and cancer tissue (grey bars). There was a decrease in the percentage of CD4+ and CD8+ T cells, CD4+ and CD8+ NK T-like cells and NK cells producing IFN-γ from cancer tissue compared with normal tissue. Data presented in box-plots as described in Fig. 1 (*P < 0·05; **P < 0·01).
Effects of conditioned media from SBC-1 cell line on T, NK and NK T-like cell production of intracellular TNF-α and IFN-γ
Inhibition of cytokine production by conditioned media from SBC-1 cell line supernatant
There was a significant inhibitory effect on TNF-α and IFN-γ production by CD4+ and CD8+ T cells, CD8+ NK T-like cells and NK cells in the presence of SBC-1 cancer cell line supernatant (Fig. 4), but no effect with supernatant from the 16HBE normal cell line compared with media alone (data not shown).
Fig 4.
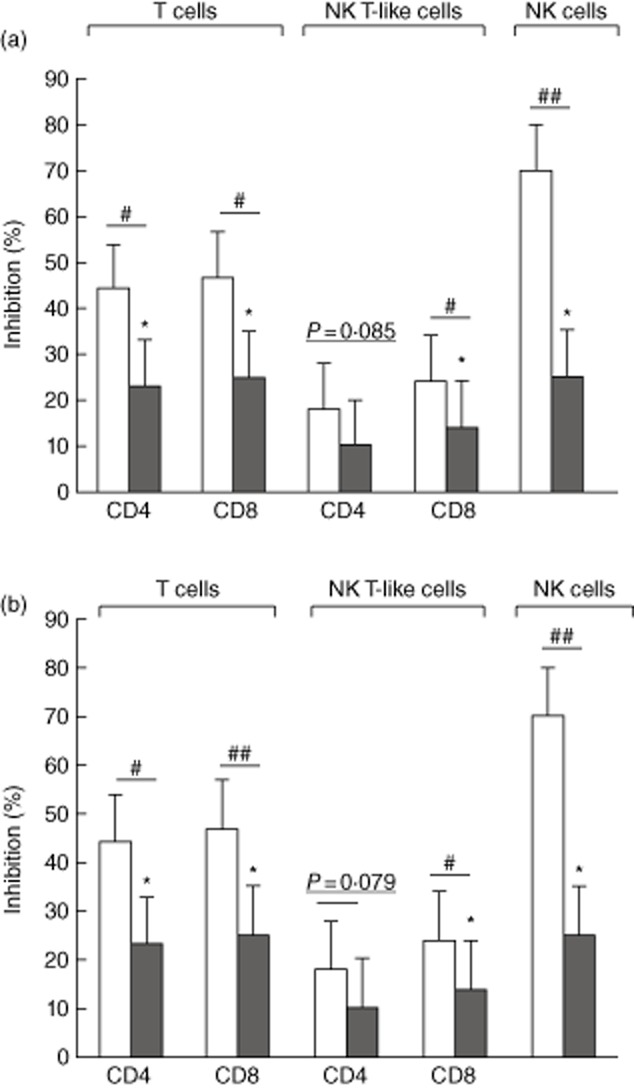
Inhibition of (a) interferon (IFN)-γ production and (b) tumour necrosis factor (TNF)-α in CD4+ and CD8+ T cells, natural killer (NK) T-like and NK cells (clear bars) in the presence of conditioned media from small-cell carcinoma (SBC)-1 cells compared with supernatant from the 16HBE normal human bronchoepithelial cell line (control). Data for three separate experiments (n = 3) are presented as % change compared to control. Significant decrease (*P < 0·05) in the percentage of lymphocyte subsets producing cytokines in the presence of SBC-1 supernatants. Neutralizing PGE2 with 10 μM indomethacin (grey bars) significantly (#P < 0·05; ##P < 0·01) diminished the inhibitory effect of SBC-1 cancer cell line supernatant on IFN-γ and TNF-α production (three separate experiments, performed in triplicate).
Effect of neutralizing the COX2/PGE2 pathway on cytokine production by conditioned media from SBC-1 cell line
Neutralizing the COX2/PGE2 pathway with 10 μM indomethacin significantly reduced the inhibitory effect of SBC-1 cancer cell line supernatant on production of IFN-γ and TNF-α by CD4 and CD8 T cells, CD8+ NK T-like cells and NK cells (Fig. 4).
Correlation of lung cancer stage with granzyme B expression
A retrospective correlation of cancer stage and CD8+ production of granzyme B was performed for 21 patients with NSCLC who had been recruited for previous studies 5,9. Cancer stage ranged from 1A to 3A. We showed a significant negative correlation between granzyme expression by CD8+ T cells and cancer stage (n = 21, correlation coefficient – 0·508, P = 0 019).
Discussion
Lung cancer remains the highest cause of cancer-related mortality, with the vast majority of cases associated with tobacco smoking. Anti-smoking campaigns have played an important role in slowing the rate of lung cancer (at least in men), but the incidence is projected to remain high for many decades. Only about 15% of cancers are suitable for attempted cure by resection, and the outlook for unresectable cancer remains poor due to limited efficacy of current therapies. A better understanding of tumour evasion mechanisms is needed to identify new therapeutic targets.
Cytotoxicity is an important component of the immune system and is a highly regulated, multi-factorial process carried out by different cytotoxic cells of the immune system. Cytotoxic CD8+ T cells, NK and NK-like T cells mount immune responses to cancer via pathways that include the cytotoxic granzyme B/perforin pathway and by release of cytokines including IFN-γ and TNF-α. Resistance to attacks by these cytotoxic cells by immune evasion mechanisms is essential for the survival and proliferation of lung cancer cells.
We have previously shown decreased CD8+ T cell granzyme B expression in lung cancer tissue compared to non-cancer tissue 9. In the present study we extended our previous findings to investigate another important cytotoxic cell, CD3(+)CD56(+) NK T-like cells that, like NK cells, can participate in tumour rejection via direct cytolysis. Consistent with our previous report in cancer tissue there was a decrease in the percentage of CD8+ and CD4+ T cells expressing granzyme B compared with non-cancer lung tissue. In addition, both CD4+ and CD8+ NK T-like cells, and NK cells from cancer tissue showed a similar significant decrease in intracellular granzyme B versus non-cancer lung tissue, suggesting a combined deficiency in the anti-tumour response of the various lymphocyte subsets.
The interaction of granzyme B with perforin, located in cytolytic granules within the cytotoxic cells, is important for effective cytolytic function. Perforin helps to create a channel through the membrane, allowing granzyme B to enter the target cell. Like granzyme B, perforin has been shown to be an anti-cancer mediator, controlling the growth and spread of tumours in mice 15,16. Consistent with these effects and the reduced expression of granzyme B, we also noted a decrease in the expression of perforin by CD4+ and CD8+ T cells, NK cells and NK T-like cells from cancer compared with non-cancer lung tissue. There were also significant decreases in the production of IFN-γ by CD4+ and CD8+ T cells, CD4+ and CD8+ NK T like cells and NK cells from cancer tissue compared with non-cancer lung tissue. The decreased IFN-γ is likely to contribute to the suppressive pro-tumour microenvironment and the survival of cancer cells, as this cytokine inhibits angiogenesis and cellular proliferation and promotes apoptosis of the cancer cells. In addition, it activates the adaptive immune system, and thus contributes to effective antigen processing and presentation.
There are limited studies of the role of NK T-like cells in cancer and their interaction with tumour cells. Wang et al. assessed the cytotoxic function of NK T-like cells isolated from human ovarian cancer and prostate cancer patients 17. They reported that the cytotoxic function of these NK T-like cells was compromised in these patients, due at least partially to release of soluble major histocompatibility complex (MHC) class I chain-related molecules by cancer cells impairing the lytic activity via down-regulation of the NKG2D receptor 17.
Although independent of the observations related to CD8 cells, it is possible that the low expression of granzyme B and perforin in CD4 cells could also be due at least partially to an increase in the regulatory T cell (Treg) population, rather than to changes in expression in other CD4 cells. These cells have been found in increased numbers in lung cancer, and are thought to play a major role in suppressing local immune responses. Potential drawbacks of the present study were the small numbers tested (paired cancer and non-cancer lung tissue from five patients), although we were able to demonstrate significant changes in all patients, negating the need for more samples. Tissues from different types of cancers with and without accompanying COPD were used, but the data were still very robust. Of the approximately 200 patients with NSCLC who our unit see annually, only a small percentage (∼15%) are considered for surgery (stages I and II), and of those many are medically inoperable, precluding the testing of later stage lung cancer. However, we analysed retrospectively data collected from cancer lung cancer tissues from 21 subjects. We showed a significant negative correlation between granzyme expression by CD8+ T cells and cancer stage. These findings are consistent with our previous report of increased expression of the specific granzyme inhibitor, PI-9 and NSCLC stage 9.
As curative lobectomy is not applied routinely for the management of SCLC, it was not possible in this study to assess any correlation between expression of the cytotoxic/inflammatory mediators and cancer type, although among the lung cancer cell lines we found previously that the more aggressive SCLC lines (SBC1 and, to a greater extent, SBC3) showed higher expression of granzyme inhibitor, PI-9, than NSCLC lines 9.
The precise reasons for the suppressive effects noted in our study are not known, but are likely to result from soluble mediators secreted by the cancer cells. Consistent with a report by Wang et al. [17], we have reported previously that soluble mediators secreted by various lung cancer cell lines could inhibit and thus contribute to the decreased granzyme B by CD8+ T cells and a reduction in their cytotoxic potential 9. We further showed that the released soluble mediators also inhibited innate immune responses, including macrophage phagocytic ability 5. We previously applied electrospray ionization tandem mass spectrometry (LC-ESI-MSMS) analysis for AA metabolites in lung cancer cells and showed that the soluble mediators included arachidonic acid (AA) metabolites including PGE 5. In the present study we investigated the effects of conditioned media from a SCLC cell line on production of granzyme B, perforin, IFN-γ by T, NK T-like and NK cells. Lung cancers arise from the respiratory epithelium, thus ‘normal’ airway epithelial cells are the most suitable controls for this type of study, and we have previously validated similar expression of cytotoxic mediators by a normal cell line and primary large airway epithelial cells taken from ‘normal’ volunteers 9. We found that the cancer cells released soluble factors that inhibited expression of all cytotoxic/proinflammatory mediators tested. No significant effects were noted using the normal epithelial cell line. PGE is released in cells expressing constitutive COX-2, regulated by liberation of AA by phospholipase A2 followed by metabolism by COX. Although we did not selectively inhibit PGE2, we applied a COX-22 inhibitor, indomethacin, and showed that the effects of the lung cancer cell-conditioned media on the cytotoxic lymphocytes resulted at least partially from the COX-2 pathway, implicating the presence of PGE2.
Taken together, our findings represent a suppressive environment in the presence of lung cancer, with significantly decreased production of cytotoxic mediators and IFN-γ by CD8+, NK and NK T-like cells. This environment is likely to significantly reduce the effective adaptive immune responses to the cancer, creating a tumour microenvironment that impairs effector T cell functionality and supports the escape phase of cancer.
Acknowledgments
This study was supported by the National Health and Medical Research Council (NHMRC) and Boehringer Ingelheim/Australian Lung Foundation.
Disclosure
The authors have no competing interests to declare.
References
- Fong K, Sekido Y, Gazdar A, Minna J. Lung cancer. 9: molecular biology of lung cancer: clinical implications. Thorax. 2003;58:892–900. doi: 10.1136/thorax.58.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tockman M, Anthonisen NR, Wright EC, Donithang G. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- Skillrud D. COPD: causes, treatment, and risk for lung cancer. Compr Ther. 1986;12:13–16. [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehle FC, Mukaro VR, Jurisevic C, et al. Defective efferocytosis in patients with lung cancer with or without COPD – mediated by PGE2 produced by lung cancer cells? PLOS ONE. 2013;8:e61573. doi: 10.1371/journal.pone.0061573. . Available at: http://dx.plos.org/10.1371/journal.pone.0061573 (accessed 26 April 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31:61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Burkhardt BR, Lyle R, Qian K, Arnold AS, Cheng H, Atkinson MA, Zhang YC. Efficient delivery of siRNA into cytokine-stimulated insulinoma cells silences Fas expression and inhibits Fas-mediated apoptosis. FEBS Lett. 2006;580:553–560. doi: 10.1016/j.febslet.2005.12.068. [DOI] [PubMed] [Google Scholar]
- Hodge G, Mukaro V, Holmes M, Reynolds PN, Hodge S. Enhanced cytotoxic function of natural killer and natural killer T-like cells associated with decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway. Respirology. 2013;18:369–376. doi: 10.1111/j.1440-1843.2012.02287.x. [DOI] [PubMed] [Google Scholar]
- Soriano C, Mukaro V, Hodge G, et al. Increased proteinase inhibitor-9 (PI-9) and reduced granzyme B in lung cancer: mechanism for immune evasion? Lung Cancer. 2012;77:38–45. doi: 10.1016/j.lungcan.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. 2011. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease ). Available at: http://www.goldcopd.org/ (accessed 28 January 2012)
- Hodge G, Hodge S, Li-Liew C, Reynolds PN, Holmes M. Increased natural killer T-like cells are a major source of pro-inflammatory cytokines and granzymes in lung transplant recipients. Respirology. 2012;17:155–163. doi: 10.1111/j.1440-1843.2011.02075.x. [DOI] [PubMed] [Google Scholar]
- Hodge S, Hodge G, Nairn J, Holmes M, Reynolds PN. Increased airway granzyme b and perforin in current and ex-smoking COPD subjects. COPD. 2006;3:1–9. doi: 10.1080/15412550600976868. [DOI] [PubMed] [Google Scholar]
- Hodge G, Reynolds PN, Holmes M, Hodge S. Differential expression of pro-inflammatory cytokines in intra-epithelial T cells between trachea and bronchi distinguishes severity of COPD. Cytokine. 2012;60:843–848. doi: 10.1016/j.cyto.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Hou R, Goloubeva O, Neuberg DS, Strominger JL, Wilson SB. Interleukin-12 and interleukin-2-induced invariant natural killer T-cell cytokine secretion and perforin expression independent of T-cell receptor activation. Immunology. 2003;110:30–37. doi: 10.1046/j.1365-2567.2003.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Chehimi J, Ghavimi D, Campbell D, Sullivan KE. Decreased natural killer (NK) cell function in chronic NK cell lymphocytosis associated with decreased surface expression of CD11b. Clin Immunol. 2001;99:53–64. doi: 10.1006/clim.2001.5002. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang D, Xu W, et al. Tumor-derived soluble MICs impair CD3(+)CD56(+) NKT-like cell cytotoxicity in cancer patients. Immunol Lett. 2008;120:65–71. doi: 10.1016/j.imlet.2008.07.001. [DOI] [PubMed] [Google Scholar]


