Abstract
CD40/CD40-ligand (CD40L) signalling is a key stimulatory pathway which triggers the tryptophan (Trp) catabolizing enzyme IDO in dendritic cells and is immunosuppressive in cancer. We reported IDO-induced Trp catabolism results in a T helper type 17 (Th17)/regulatory T cell (Treg) imbalance, and favours microbial translocation in HIV chronic infection. Here we assessed the link between sCD40L, Tregs and IDO activity in HIV-infected patients with different clinical outcomes. Plasmatic sCD40L and inflammatory cytokines were assessed in anti-retroviral therapy (ART)-naive, ART-successfully treated (ST), elite controllers (EC) and healthy subjects (HS). Plasma levels of Trp and its metabolite Kynurenine (Kyn) were measured by isotope dilution tandem mass spectrometry and sCD14 was assessed by enzyme-linked immunosorbent assay (ELISA). IDO-mRNA expression was quantified by reverse transcription–polymerase chain reaction (RT–PCR). The in-vitro functional assay of sCD40L on Treg induction and T cell activation were assessed on peripheral blood mononuclear cells (PBMCs) from HS. sCD40L levels in ART-naive subjects were significantly higher compared to ST and HS, whereas EC showed only a minor increase. In ART-naive alone, sCD40L was correlated with T cell activation, IDO-mRNA expression and CD4 T cell depletion but not with viral load. sCD40L was correlated positively with IDO enzymatic activity (Kyn/Trp ratio), Treg frequency, plasma sCD14 and inflammatory soluble factors in all HIV-infected patients. In-vitro functional sCD40L stimulation induced Treg expansion and favoured Treg differentiation by reducing central memory and increasing terminal effector Treg proportion. sCD40L also increased T cell activation measured by co-expression of CD38/human leucocyte antigen D-related (HLA-DR). These results indicate that elevated sCD40L induces immunosuppression in HIV infection by mediating IDO-induced Trp catabolism and Treg expansion.
Keywords: CD154, HIV, IDO, regulatory T cells (Tregs), soluble CD40-ligand (sCD40L)
Introduction
Chronic inflammation accompanied with a massive depletion of CD4 T cells in gut mucosa is a hallmark of HIV infection. HIV also alters mucosal immunity which results in microbial translocation from the gut into the periphery and is associated with immune dysfunction and persistent activation 1,2. Indoleamine 2,3-dioxygenase (IDO), expressed by professional antigen-presenting cells such as macrophages and dendritic cells (DCs), plays a pivotal role in the regulation of the immune system by catabolizing tryptophan (Trp) into kynurenine (Kyn), which favours the differentiation of immunosuppressive regulatory T cells (Treg) 3. Indeed, IDO-mediated catabolism of Trp limits immune responses in physiological or pathological conditions such as pregnancy, cancer and chronic viral illness 4–6. We and others have reported that IDO-induced Trp catabolism results in a T helper type 17 (Th17)/Treg imbalance and favours microbial translocation in HIV chronic infection 3,7,8. IDO induction in DCs is regulated by several stimulators such as interferons (IFNs), lipopolysaccharides (LPS), Toll-like receptors (TLRs) and cytotoxic T lymphocyte antigen-4 (CTLA-4) ligation 9–11.
More recently, induction of IDO has been observed through CD40L signalling 12,13. CD40L, also known as CD154, is a membrane glycoprotein and a functional trimer belonging to the tumour necrosis factor (TNF) superfamily, which is expressed on antigen-presenting cells, epithelial cells and haematopoietic progenitor cells 14,15. The soluble form of CD40L (sCD40L, sCD154) is a functional trimer retaining its ability to bind receptors 16. sCD40L is shed from activated T cells and platelets 17–19, and plays a crucial role in the co-stimulatory pathways involved in humoral B cell responses 20,21. The elevated CD40L plays a harmful role in the humoral response due to persistent chronic activation of B cells 22,23. Levels of sCD40L have been implicated in increasing platelet–monocyte complexes which are normally activated only during inflammatory conditions 24. Importantly, the CD40–CD40L pathway also contributes to the establishment of gut mucosal inflammation 25,26, as elevated levels of sCD40L are observed in inflammatory bowel disease 27. Interestingly, increased plasma levels of sCD40L have been reported in HIV-infected patients 14,28,29 with dementia 24,29,30 and impairing peripheral DCs innate immune responses 28. More recently, high plasma levels of sCD40L in cancer patients were shown to directly induce immunosuppression via the expansion of Treg and production of inflammatory cytokines 31.
In this study we assessed the immunosuppressive role of sCD40L, making the link between IDO enzyme activity, altered Th17/Treg balance and sCD14, a marker of microbial translocation in HIV-infected patients.
Material and methods
Study population
Peripheral blood mononuclear cells (PBMC) and plasma samples were collected from untreated ART-naive patients, ART-successfully treated patients (ST) and healthy subjects (HS) at the Chronic Viral Illness Service, McGill University Health Centre (MUHC), Montreal, QC, Canada. Samples from elite controllers (EC) were obtained from the FRQ-S slow progressor cohort, Montreal, QC, Canada (Table 1). All study subjects gave their written consent and the study was approved by the Research Ethics Board of MUHC.
Table 1.
Clinical and virological characteristics of study groups
| Characteristics | Study population n = 55 | |||
|---|---|---|---|---|
| Successfully treated (n = 14) | ART-naive (n = 14) | Elite controllers (n = 13) | Healthy controls (n = 14) | |
| Age (years) [mean ± s.d. (range)] | 46 ± 9 (29–62) | 38 ± 9 (23–52) | 47 ± 7 (40–60) | 46 ± 8 (30–60) |
| Male [n (%)] | 12 (86%) | 13 (93%) | 7 (54%) | 9 (64%) |
| Time since HIV-1 diagnosis (years) [mean ± s.d. (range)] | 9 ± 4 (3–18) | 6 ± 6·5 (0–19) | 13 ± 4 (4–20) | n.a. |
| CD4 T cell count (cells/μl) [mean ± s.d. (range)] | 660·0 ± 244 (403–1177) | 322 ± 183 (3–523) | 624 ± 247 (417–1341) | 988 ± 301·5 (519–1559) |
| CD8 T cell count (cells/μl) [mean ± s.d. (range)] | 816 ± 265 (452–1381) | 844 ± 354 (272–2180) | 596 ± 357 (162–1198) | 459 ± 159 (227–843) |
| CD4 : CD8 ratio [mean ± s.d. (range)] | 0·86 ± 0·32 (0·44–1·51) | 0·4 ± 0·328 (0·01–1·07) | 1·34 ± 0·68 (0·35–2·72) | 2·32 ± 0·85 (1·16–3·97) |
| Viral load (log10 copies/ml) [mean ± s.d. (range)] | <1·6 | 4·6 ± 0·8 (2·89–6·35) | <1·6 | n.a. |
| Time since start of ART (years) [mean ± s.d. (range)] | 7 ± 3 (3–14) | n.a. | n.a. | n.a. |
| Monocyte count (109/l) [mean ± s.d. (range)] | 0·44 ± 0·15 (0·20–0·71) | 0·53 ± 0·22 (0·19–0·91) | 0·52 ± 0·17 (0·26–0·90) | 0·53 ± 0·17 (0·24–0·76) |
| Platelet count (109/l) [mean ± s.d. (range)] | 234·1 ± 51·4 (178–363) | 209·6 ± 55·33 (122–303) | 282 ± 121·6 (144–511) | 246·5 ± 50·2 (145–323) |
| sCD40L (pg/ml) [mean ± s.d. (range)] | 502 ± 625 (92–2165) | 1279 ± 793 (119–3336) | 995 ± 977 (16–3049) | 601 ± 650 (105–2673) |
These include anti-retroviral therapy (ART) successfully treated (ST), ART-naive, elite controllers (EC) and healthy subjects (HS). Results are shown as mean ± standard deviation (s.d.) and (range); n.a. = not applicable.
Multiplex quantification of plasmatic soluble factors
Prior to analysis, all plasma samples were treated 10:1 with 5% Triton X-100 for 1 h at room temperature to inactivate the HIV-1 particles. Plasma levels of selected inflammatory soluble factors, including IL-1RA, IL-1β, IL-7, IL-8, IL-10, IFN-α2, IFN-γ, TNF-α, TNF-β, IP-10, sCD40L, macrophage inflammatory protein (MIP)-1α and MIP-1β, were measured in duplicate using a MILLIPLEX-MAP kit, according to the manufacturer's instructions (Millipore, Billerica, MA, USA). Mean fluorescence intensities for each analyte in each sample were detected using the MAGPIX instrument (Luminex, Austin, TX, USA) and the results were analysed using the Millipore Analyst software version 3·5 5.
Quantification of IDO-1 mRNA
Total RNA from PBMCs were extracted with RNeasymini kits (Qiagen, Hilden, Germany). cDNA was synthesized from total RNA (500 ng) with oligo-dT and random hexamer primers (Applied Biosystems, Carlsbad, CA, USA) using an Omniscript reverse transcriptase kit (Qiagen). Then, specific mRNAs were amplified using the following primers: IDO-1 (forward: CGCTGTTGGAAATAGCTTCTTGC; reverse: CTTCCCAGAACCCTTCATACACC), and 18 s ribosomal RNA (forward: ATCAACTTTCGATGGTAGTCG; reverse: TCCTTGGATGTGGTAGCCG). Quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed by Rotorgene 3000 thermal cycler (Corbett Life Science, Sydney, Australia) and Quantitect Sybr Green PCR kit (Qiagen). The cycling conditions were 10 min at 95°C, then 35 cycles of 30 s at 94°C, 45 s at 60°C and 30 s at 72°C for 18 s rRNA. For IDO-1 amplification, 40 cycles were performed with 60 s annealing at 55°C followed by 30 s at 72°C. A final melting curve was obtained from 72 to 95°C. The expression of each gene was quantified relative to the housekeeping gene 18 s rRNA.
In-vitro characterization of Th17 cells
PBMCs were cultured in 48-well culture plates at 0·5–1 × 106 cells/ml per well and stimulated with 5 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (both from Sigma Aldrich, Oakville, ON, Canada) for 2 h at 37°C. Two μg/ml brefeldin A (Sigma) was then added to block cytokine secretion and cells were cultured for 18 h at 37°C. peripheral blood mononuclear cells (PBMCs), then underwent surface staining followed by fixation and permeabilization using a Cytofix/Cytoperm Permeabilization kit (BD Bioscience, Mississauga, ON, Canada) for intracellular staining with IL-17A and IFN-γ (positive control).
Flow cytometry
Flow cytometry was performed using a four-laser LSRII flow cytometer (BD Bioscience). The following antibodies were used: CD3-Pacific blue, CD4-peridinin chlorophyll cyanin 5·5 (PerCpCy5·5), CD4-phycoerythrin (PE)Cy5, CD8-Alexa700, CD25-PE, CD127-PECy7, CD27-Alexa700, human leucocyte antigen D-related (HLA-DR)-allophycocyanin (APC)-Cy7, CD38-APC and IFN-γ-Alexa700 (BD Bioscience); CD45RA-ECD from Beckman Coulter (Mississauga, Ontario, Canada) and IL-17-PE, CD8-APCeFluor780, forkhead box protein 3 (FoxP3)-Alexa488 and FoxP3 staining buffer from eBioscience (San Diego, CA, USA). The viability marker Vivid (Invitrogen, Burlington, ON, Canada) was used in all our cytometry panels to exclude the dead cell population from our analysis. Data were analysed using FlowJo software (version 7·6.5; TreeStar Inc., Ashland, OR, USA). Tregs were characterized as CD3+CD4+CD25highCD127lowFoxP3high and Th17 cells as CD3+CD4+IL-17a+ upon PMA/ionomycin stimulation (Supporting information, Fig. S1).
Measurement of plasma levels of IL-6, sCD14 and IL-10
Plasma levels of IL-6 and sCD14 were measured using commercially available human sCD14 and IL-6 enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, Minneapolis, MN, USA). We quantified IL-10 production in the supernatant of in-vitro-stimulated PBMCs using an IL-10 ELISA kit (eBiosciences, San Diego, CA, USA).
In-vitro sCD40L stimulation of PBMCs
PBMCs from 10 healthy subjects were cultured in RPMI containing 10% fetal bovine serum (FBS) and 10 ng/ml recombinant IL-2 (R&D Systems, Minneapolis, MN, USA) with or without 2 μg/ml recombinant soluble CD40 ligand (sCD40L) (Enzo Life Sciences, Farmingdale, NY, USA) for 5 days at 37°C. The cells were cultured at 0·5 × 106 cells/ml per well in a 48-well culture plate. At day 2, the plate was centrifuged at 400 g for 5 min and half the medium was removed and boosted with the same concentrations of IL-2, with or without sCD40L. At day 5, the cells were transferred into fluorescence activated cell sorter (FACS) tubes, then washed and stained for Treg expression and T cell activation.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software version 5. The Kruskal–Wallis test was performed for comparison between study groups, and Mann–Whitney U-tests or Wilcoxon's matched-pairs tests were used for comparison of two variables. Spearman's correlation test was used to identify association among study variables.
Results
Elevated plasmatic sCD40L in ART-naive HIV-infected patients is associated with CD4 T cell depletion but not with HIV viral load
It has been shown previously that HIV-infected patients have a higher plasma level of sCD40L compared to healthy subjects 14,28,29. Accordingly, our team reported recently that ART-naive HIV-infected patients displayed significantly elevated plasmatic sCD40L when compared to ST and HS, whereas, following ART in ST patients, plasmatic sCD40L levels became identical to EC and HS 7 and Table 1. For the ART-naive group, sCD40L was found to be correlated negatively with CD4 T cell counts (Fig. 1a) and CD4 : CD8 T cell ratio (Fig. 1a), but not correlated with viral load (Fig. 1c). Positive correlation was observed between sCD40L plasma levels and T cell immune activation defined by co-expression of CD38/HLA-DR on CD4 and CD8 T cells (Fig. 1d,e), as well as IDO-mRNA expression in ART-naive patients (7 and Fig. 1f). However, no correlation was observed between sCD40L and the expression of two other Trp catabolizing enzymes, IDO-2 and TDO mRNA (Spearman's P > 0·05, data not shown). In line with a previous study 28, we also observed a significant increase in the CD40L expression by CD4 T cells in ART-naive patients (Fig. 1g). As CD40L is highly expressed by platelets, which could be a major contributor of circulating sCD40L 32–34, we compared platelet counts in the study groups and no significant difference was observed (Kruskal–Wallis test P > 0·05, Table 1).
Fig 1.
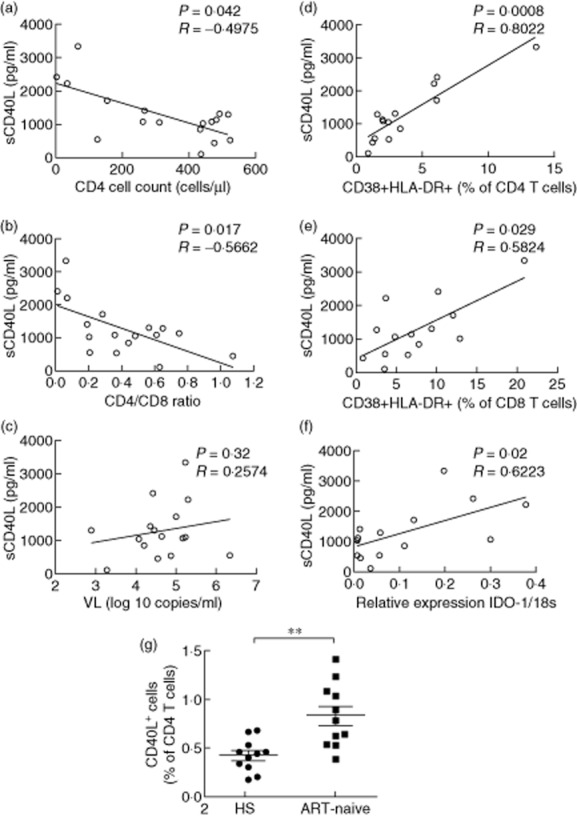
Plasma sCD40L is associated with CD4 T cell counts and indoleamine 2,3-dioxygenase (IDO)-1 mRNA expression but not HIV viral load in anti-retroviral therapy (ART)-naive patients. Association of plasma sCD40L levels are shown with (a) CD4 T cell count, (b) CD4:CD8 T cell ratio (c) HIV viral load (VL), (d) CD4 and (e) CD8 T cell activation as well as (f) IDO-1 mRNA expression. (g) Increased CD40L expression by CD4 T cells in ART-naive patients. Significant values determined by either Spearman's correlation or Mann–Whitney U-tests; **P < 0·01.
Plasma levels of sCD40L are associated with altered Th17/Treg balance, microbial translocation and systemic inflammation in HIV-infected patients
It has been proved previously that sCD40L is involved in induction of IDO expression and activity 20. In line with this, we showed recently that plasma sCD40L levels are associated with a higher IDO enzyme activity (Trp/Kyn) ratio in HIV-infected patients 7. Indeed, in the patients studied here, a strong positive correlation was observed between levels of plasma sCD40L and IDO enzyme activity when evaluated in all HIV-infected patients (Spearman's P = 0·005, r = 0·4317, Fig. 2a). We and others have shown that higher IDO-induced Trp catabolism is involved in the imbalance of Th17/Treg in favour of Treg by stimulation of Treg differentiation in HIV infection 3,7. Recently, it has been shown in cancer that sCD40L can induce Treg expansion 31. We therefore evaluated the possible association between sCD40L, Treg frequency and the Th17/Treg ratio. ART-naive patients had the highest Treg absolute number with lowest Th17 frequencies when compared to ST, and the proportion of Treg as well as the Th17/Treg ratio was normalized following ART (data not shown). Interestingly, plasma sCD40L levels were correlated with increased Treg frequency (P = 0·01, r = 0·4001) (Fig. 2b) and a low Th17/Treg ratio in all HIV-infected patients (P = 0·008, r = –0·4210) (Fig. 2c). Such a correlation was not observed with Th17 cell frequency (P = 0·37, data not shown). Altogether, these results suggest the involvement of sCD40L in Treg induction via IDO enzyme activity. Furthermore, a low Th17/Treg ratio is harmful to gut mucosal immunity, resulting in microbial translocation in HIV infection 35–37. Importantly, elevated levels of the monocyte activation marker sCD14, a reliable marker of microbial translocation in HIV infection 37, was also correlated positively with plasma levels of sCD40L when evaluated in all HIV-infected patients (Fig. 2d).
Fig 2.
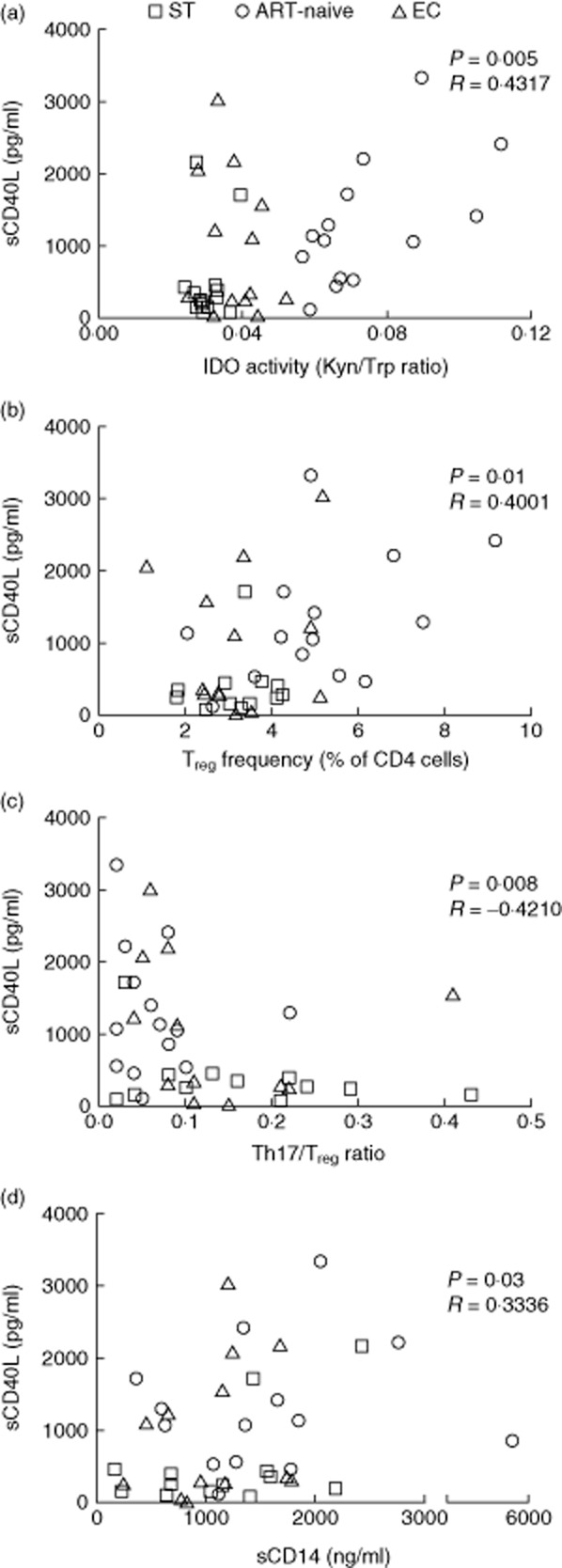
Plasma sCD40L levels correlates with indoleamine 2,3-dioxygenase (IDO) enzyme activity, T helper type 17 (Th17)/regulatory T cell (Treg) imbalance and microbial translocation in HIV-infected patients. Correlations are shown between plasma sCD40L levels and (a) IDO enzyme activity, (b) Treg frequency, (c) Th17/Treg ratio and (d) marker of microbial translocation sCD14 in HIV-infected patients. P-values determined by Spearman's test. EC = elite controllers; ST = anti-retroviral therapy (ART) treated successfully.
We evaluated which inflammatory soluble factors were most associated with sCD40L levels among HIV-infected patients. Results presented in Table 2 showed that sCD40L was correlated positively with multiple inflammatory soluble factors, including markers of HIV disease progression such as IL-6 and IP-10 2,38,39.
Table 2.
Correlations between plasma sCD40L levels and other inflammatory soluble markers in all HIV-infected patients. Spearman's test was used for statistical analysis
| IL-1RA | IL-1β | IL-6 | IL-7 | IL-8 | IL-10 | IP-10 | IFN-γ | TNF-α | TNF-β | MIP-1α | MIP-1β | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-value | <0·0001 | <0·0001 | 0·04 | <0·0001 | 0·0003 | 0·004 | 0·05 | 0·017 | 0·038 | 0·0003 | 0·03 | <0·0001 |
| Spearman's r | 0·5737 | 0·5820 | 0·3121 | 0·7269 | 0·4734 | 0·4389 | 0·3038 | 0·3701 | 0·3254 | 0·5363 | 0·3352 | 0·4872 |
IL = interleukin; IFN = interferon; TNF = tumour necrosis factor; IP = IFN gamma-induced protein 10; MIP = macrophage inflammatory protein.
In-vitro sCD40L stimulation induces Treg expansion and differentiation
It has been shown that sCD40L plays an immunosuppressive role in cancer via induction of Tregs 31. To investigate the direct role of sCD40L on Treg expression, a 5-day in-vitro sCD40L stimulation of PBMCs from healthy donors (n = 10) was performed in cell culture. Treg subsets were characterized according to the expression of CD45RA and CD27 (Fig. 3a). Results presented in Fig. 3b show a significant increase of Treg frequency following sCD40L stimulation compared to non-stimulated PBMCs (1·67 ± 0·57 versus 1·37 ± 0·6%, Wilcoxon's matched-pairs P = 0·037). To evaluate the potential impact of sCD40L on Treg differentiation, we further assessed the changes in Treg subsets and phenotypes following in-vitro sCD40L stimulation. Interestingly, sCD40L favoured Treg differentiation by reducing central memory Tregs (44·41 ± 18·45 versus 51·53 ± 17·24%, Wilcoxon's matched-pairs P = 0·019; Fig. 3c) and increasing terminal effector Tregs (6 ± 3·6 versus 1·2 ± 1·33%, Wilcoxon's matched-pairs P = 0·004; Fig. 3d). To test the hypothesis that sCD40L can directly impact Tregs, we evaluated the expression of CD40 on Tregs. The expression of CD40 on Tregs was significantly higher compared to non-Treg conventional CD4 T cells (4·4 ± 1 versus 2·6 ± 08, Wilcoxon's matched-pairs P = 0·001, Fig. 4a). Interestingly, we observed the highest expression of CD40 on the terminal effector (TE) subset of Tregs, suggesting a potential direct impact of sCD40L on Tregs (Kruskal–Wallis P = 0·0015, Fig. 4b). To assess whether the generation of Tregs in this model was induced by T cell activation, we evaluated the potential impact of sCD40L stimulation on the co-expression of activation markers CD38 and HLA-DR on CD4 and CD8 T cells. Results showed that sCD40L increased both CD4 and CD8 T cell activation in our in-vitro model (2·55 ± 2 versus 3·36 ± 2·6, P = 0·01 and 4·75 ± 5·1 versus 6·32 ± 5·5, P = 0·05, respectively, Fig. 5a,b). To evaluate if sCD40L was able to change the milieu of common secreted cytokines between Tregs and monocytes, we quantified the production of IL-10 in our model. Therefore, either total PBMCs or monocyte-depleted PBMCs (depletion performed by cell sorting) were stimulated with sCD40L over 5 days, as described in the Methods section. At day 5, supernatants were removed and the IL-10 production was quantified by ELISA. Our results showed no significant difference in IL-10 production by total PBMCs between non-stimulated and sCD40L-stimulated conditions (28·3 ± 33·4 versus 32 ± 34·9 pg/ml, respectively, Wilcoxon's matched-pairs P = 0·31, data not shown). As expected, the IL-10 production by monocyte-depleted PBMCs was lower, but no significant difference was observed between non-stimulated and sCD40L-stimulated conditions (2·23 ± 2·78 versus 6·8 ± 11·72 pg/ml, respectively, Wilcoxon's matched-pairs P = 0·5, data not shown). Our results are in line with previously published articles, which indicated that neither IL-2 40 nor sCD40L 28,31 are strong stimuli capable of inducing large amounts of IL-10.
Fig 3.
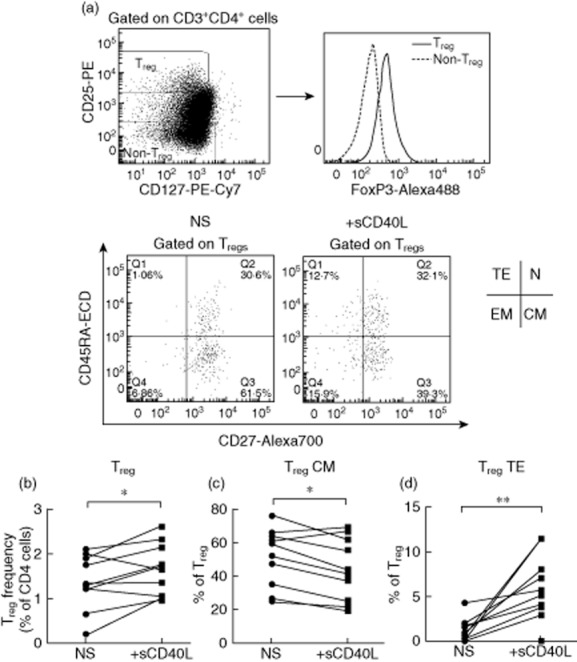
In-vitro sCD40L stimulation causes regulatory T cell (Treg) expansion and differentiation. (a) Gating strategy used for characterization of Tregs and Treg subsets including naive (N), central memory (CM), effector memory (EM) and terminal effector (TF). (b) Impact of in-vitro stimulation by sCD40L on (b) total Tregs, (c) central memory Treg and (d) terminal effector Treg. n = 10; P-values determined by Wilcoxon's matched-pairs test; *P < 0·05; **P < 0·01.
Fig 4.
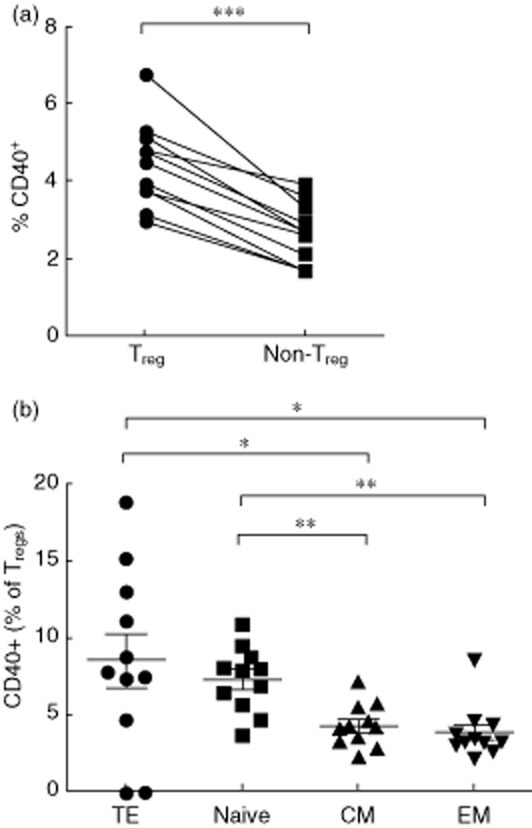
Regulatory T cells (Tregs) express higher levels of extracellular CD40. (a) Tregs express higher levels of extracellular CD40 compared to non-Treg CD4 T cells. Wilcoxon's matched-pairs test was used for statistical analysis. (b) Ex-vivo expression of CD40 on Treg subsets, including naive (N), central memory (CM), effector memory (EM) and terminal effector (TF), Kruskal–Wallis and Mann–Whitney U-tests were used for statistical analysis. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig 5.
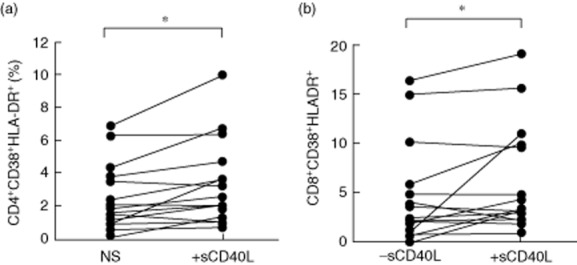
Increase of T cell activation following sCD40L stimulation. Increase of T cell immune activation defined by the co-expression of CD38 and human leucocyte antigen D-related (HLA-DR) on (a) CD4 and (b) CD8 T cells following in-vitro sCD40L stimulation. Wilcoxon's matched-pairs test was used for statistical analysis; *P < 0·05.
Discussion
The co-stimulatory molecule CD40L is expressed mainly on the surface of activated T cells 41, and to a lesser extent by B cells and platelets. CD40L plays a key role in T cell-dependent B cell responses and stimulating antigen-presenting cells 21,42. CD40L signalling has been implicated in diseases associated with immune activation such as HIV 43,44. Over-expression of CD40L on the surface of activated CD4 T cells promotes HIV replication, contributing to CD4 T cell depletion 43,44. The soluble form of CD40L (sCD40L) is cleaved and released from the cell surface, where it retains its ability to bind to the CD40 receptor 33. Elevated sCD40L in HIV-infected patients compared to healthy controls have been observed previously 14,28,29. In line with these findings, our results showed a higher level of plasma sCD40L in ART-naive chronically infected patients that became comparable to healthy subjects in ART-treated patients in association with CD4 T cell lymphopenia and the CD4 : CD8 T cell ratio, but not HIV viral load. It was not surprising that sCD40L was elevated in ART-naive subjects with low CD4 T cell counts, as CD40L is expressed on activated T cells, which are the main targets for HIV infection 15,41. Furthermore, it has been shown recently that the cell cycling CD4 memory T cells in chronically HIV-infected patients contains lower intracellular CD40L compared to healthy subjects 45. Surprisingly, however, in the study by Sipsas et al., despite the higher plasma sCD40L levels evaluated in 77 patients with a mean CD4 T cell count of 335 ± 248 cells/μl compared to healthy subjects, the correlation between CD4 T cell count and plasma sCD40L was seen only for patients having CD4 T cell count <130 cells/μl, and they observed an unexpected positive correlation between CD4 count and sCD40L 14. Moreover, platelets represent one of the main sources of plasmatic sCD40L released upon stimulation in a variety of inflammatory disorders 17. In the Sipsas et al. study, platelet count was not assessed and it may, in part, explain the different findings. We observed no differences in platelet counts between study groups, despite important differences in plasmatic sCD40L indicating that the activated T cells were most probably the source of elevated sCD40L in HIV infection 10,22. Indeed, we observed strong associations between sCD40L levels and T cell immune activation defined by co-expression of CD38/HLA-DR in ART-naive patients. Accordingly, we observed a higher membrane expression of CD40L in ART-naive patients compared to HS, which confirms a previous study 28.
The catabolism of Trp into Kyn via IDO enzyme expressed by DCs plays a detrimental immunosuppressive role in HIV infection by favouring Treg expansion 3,7. Of particular interest, IDO-1 activity has been observed through CD40L signalling after T cell interactions. CD40L may further heighten IDO responses, behaving as a second stimulus 10,13,20,46. Indeed, stimulation and maturation of immature DC by sCD40L induces IDO expression 10,13, resulting in inhibition of T cell proliferation 10. Furthermore, sCD40L as well as Cholera toxin increased the expression of IDO mRNA in mDC in association with a higher Kyn/Trp ratio in vitro 12. Accordingly, we observed a positive correlation between sCD40L levels and IDO-1 mRNA and not with the two other Trp catabolizing enzymes IDO-2 and TDO in ART-naive viraemic patients. In addition, our results showed that plasma sCD40L was associated positively with IDO enzyme activity as defined by the Kyn/Trp ratio in all HIV-infected patients. As emphasized earlier, IDO-induced Trp catabolism favours Treg expansion and has a harmful impact on the gut mucosal immunity by decreasing the Th17/Treg ratio 3,7,8,35. Our results showed a positive association between sCD40L levels and Tregs but not with Th17 frequency, resulting in a lower Th17/Treg ratio. An impaired Th17/Treg balance favours microbial translocation from gut to peripheral blood in HIV infection 1,8,37. In line with this, our results show a positive correlation between higher sCD40L and a reliable marker of microbial translocation, sCD14 37. In addition, a higher expression of CD40L has been observed in inflammatory bowel disease 25,26, where the CD40–CD40L pathway contributes to the proinflammatory function of intestinal epithelial cells 47.
More recently, CD40L was shown to be immunosuppressive in cancer via the direct expansion of Tregs 31. To further explore its possible immunosuppressive role in HIV infection, we performed an in-vitro functional assay that demonstrated an expansion of Treg frequency upon sCD40L stimulation in HIV-negative subjects. Moreover, we showed that sCD40L not only induced an expansion of Tregs, but also favoured Treg differentiation by reducing central memory cells that are important for antigen-specific immune responses and increasing terminal effector cells. One of the potential impacts of sCD40L on Treg expansion and differentiation could be due to a direct effect of sCD40L on these cells, as we observed a higher expression of CD40 on Tregs versus non-Treg conventional CD4 T cells and the highest expression of CD40 by the TD subset of Tregs. In addition, we observed a higher T cell activation defined by the co-expression of CD38 and HLA-DR on CD4 and CD8 T cells following sCD40L stimulation. However, no significant change in IL-10 production was observed following sCD40L treatment, as sCD40L is not a strong stimulus for the induction of large amount of IL-10 28,31. During inflammatory conditions possibly influenced by elevated sCD40L, further signalling mediated by this co-stimulatory molecule may play an immunosuppressive role in HIV infection by favouring DC-induced IDO stimulation associated with Treg expansion and differentiation. Interestingly, it has been reported recently that Treg cells have the lowest intracellular CD40L among all CD4 T cell subsets to avoid compromising their suppressive activity 48.
Our results showed that the levels of sCD40L correlated with multiple inflammatory soluble factors, including HIV disease progression IL-6 2,38 and IP-10 38,39. We also observed a correlation between sCD40L and IL-1RA, which is strongly associated with T cell activation whose level is linked with the altered Th17/Treg balance 49. It has been shown recently by the Maggirwar S.B. team that CD40-CD40L signalling has been implicated in HIV-associated neuroinflammation, as the HIV Tat protein can increase blood–brain barrier (BBB) permeability in a CD40L-dependent manner 30,50. sCD40L has been implicated in increasing platelet–monocyte complexes, which are normally activated only during inflammatory conditions 50–52. In addition, Tat was shown to induce platelet activation in vivo, leading to an increase in plasma sCD40L concentration 30. Moreover, an increase in CD40L in both blood and cerebrospinal fluid of cognitively impaired HIV-infected patients was implicated in central nervous inflammation while impacting neuronal survival in alliance with the HIV-1 Tat protein 29. Indeed, CD40L, together with Tat, increased TNF-α release from primary monocytes and microglia in a nuclear factor kappa B (NF-κB)-dependent manner 29. CD40L may also stimulate IL-8 production in HIV-infected patients via NF-κB activation 53. Notably, we observed an association between sCD40L and IL-8 levels. sCD40L-induced IL-8 production was shown to also be involved in neuroinflammation in HIV-infected patients 54. Furthermore, previous studies have also reported that CD40L expressed by HIV-1 virions may augment virus transfer from CD40-expressing B cells, which are not HIV-reservoirs, to CD4 T cells upon entry into lymphoid tissues 23. Studies have also shown that sCD40L favours the HIV-induced dysfunction of humoral immunity by activation of B cells via CD40 signalling 22.
Together, our results indicate that elevated sCD40L induces immunosuppression in HIV infection by mediating IDO-induced Trp catabolism and Treg expansion. Therapeutic strategies which decrease sCD40L levels, such as valproic acid 50,55,56, or other compounds reversing inflammation could be helpful to overcome sCD40L release in the periphery in the context of HIV infection.
Acknowledgments
The authors are grateful to Professor Walid Mourad, Université de Montréal, for his helpful advice. The authors acknowledge Dr Dominique Gauchat PhD and Annie Gosselin MSc, from the flow cytometry core of the CHUM-Research Center, Montréal, QC, Canada, for technical assistance and Angie Massicotte, Anne Vassal, Mario Legault, Rhyan Pineda and Stephanie Matte for coordination and blood banking. This work was supported by the Canadian Institutes of Health Research (grant MOP no. 103230 and CTN no. 257), and Fonds de la Recherche Québec-Santé (FRQ-S): Thérapie cellulaire and Réseau SIDA/Maladies infectieuses, Québec, Canada. M. A. J. is supported by a CANFAR/CTN Postdoctoral Fellowship Award. J. P. R. is a holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University.
Disclosures
No conflicts of interest to declare.
Author contributions
M. A. J. and J. P. R. designed the study; M. A. J., I. K., M. P., K. V., P. T. and C. T. performed the experiments; M. A. J., D. R., P. A., N. G. and J. P. R. analysed and discussed the data; D. R., R. L., C. T., P. A. and J. P. R. contributed reagents/materials/analysis tools; M. A. J. and J. P. R. wrote the paper.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Gating strategy used for characterization of T helper type 17 (Th17) cells.
References
- Douek DC. Immune activation, HIV persistence, and the cure. Top Antivir Med. 2013;21:128–132. [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–6991. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- Abumaree MH, Chamley LW, Badri M, El-Muzaini MF. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94:131–141. doi: 10.1016/j.jri.2012.03.488. [DOI] [PubMed] [Google Scholar]
- Jenabian MA, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLOS ONE. 2013;8:e78146. doi: 10.1371/journal.pone.0078146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- Hryniewicz A, Boasso A, Edghill-Smith Y, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavica L, Nurkkala-Karlsson M, Karlson T, Ingelsten M, Nystrom J, Eriksson K. Indoleamine 2,3-dioxygenase expression and functional activity in dendritic cells exposed to cholera toxin. Scand J Immunol. 2012;76:113–122. doi: 10.1111/j.1365-3083.2012.02713.x. [DOI] [PubMed] [Google Scholar]
- Von Bubnoff D, Scheler M, Wilms H, Fimmers R, Bieber T. Identification of IDO-positive and IDO-negative human dendritic cells after activation by various proinflammatory stimuli. J Immunol. 2011;186:6701–6709. doi: 10.4049/jimmunol.1003151. [DOI] [PubMed] [Google Scholar]
- Sipsas NV, Sfikakis PP, Kontos A, Kordossis T. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin Diagn Lab Immunol. 2002;9:558–561. doi: 10.1128/CDLI.9.3.558-561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Alaaeddine N, Hassan GS, Yacoub D, Mourad W. CD154: an immunoinflammatory mediator in systemic lupus erythematosus and rheumatoid arthritis. Clin Dev Immunol. 2012;2012:490148. doi: 10.1155/2012/490148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- Matthies KM, Newman JL, Hodzic A, Wingett DG. Differential regulation of soluble and membrane CD40L proteins in T cells. Cell Immunol. 2006;241:47–58. doi: 10.1016/j.cellimm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Farrington M, Hollenbaugh D, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Godin-Ethier J, Hanafi LA, Duvignaud JB, Leclerc D, Lapointe R. IDO expression by human B lymphocytes in response to T lymphocyte stimuli and TLR engagement is biologically inactive. Mol Immunol. 2011;49:253–259. doi: 10.1016/j.molimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Imbeault M, Ouellet M, Giguere K, et al. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J Virol. 2011;85:2189–2200. doi: 10.1128/JVI.01993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Roy J, Barat C, Ouellet M, Gilbert C, Tremblay MJ. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J Virol. 2007;81:5872–5881. doi: 10.1128/JVI.02542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggirwar S. 2013. Elevated levels of soluble CD40L causes increase in circulating platelet-monocyte complexes: possible role in HIV-associated neuro-inflammation. 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, Georgia,
- Vowinkel T, Anthoni C, Wood KC, et al. CD40–CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007;132:955–965. doi: 10.1053/j.gastro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Danese S, Scaldaferri F, Vetrano S, et al. Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease. Gut. 2007;56:1248–1256. doi: 10.1136/gut.2006.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis. 2003;18:142–147. doi: 10.1007/s00384-002-0425-4. [DOI] [PubMed] [Google Scholar]
- Donhauser N, Pritschet K, Helm M, et al. Chronic immune activation in HIV-1 infection contributes to reduced interferon alpha production via enhanced CD40:CD40 ligand interaction. PLOS ONE. 2012;7:e33925. doi: 10.1371/journal.pone.0033925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Sniderhan LF, Schifitto G, et al. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J Immunol. 2007;178:3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- Davidson DC, Hirschman MP, Sun A, Singh MV, Kasischke K, Maggirwar SB. Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders. PLOS ONE. 2012;7:e51793. doi: 10.1371/journal.pone.0051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Jochems C, Talaie T, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030–3038. doi: 10.1182/blood-2012-05-427799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard JF, Solanilla A, Gauthier B, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99:2612–2614. doi: 10.1182/blood.v99.7.2612. [DOI] [PubMed] [Google Scholar]
- Mazzei GJ, Edgerton MD, Losberger C, et al. Recombinant soluble trimeric CD40 ligand is biologically active. J Biol Chem. 1995;270:7025–7028. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Merhi Y, Mourad WM. CD154 and its receptors in inflammatory vascular pathologies. Trends Immunol. 2009;30:165–172. doi: 10.1016/j.it.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Jenabian MA, Ancuta P, Gilmore N, Routy JP. Regulatory T cells in HIV infection: can immunotherapy regulate the regulator? Clin Dev Immunol. 2012;2012:908314. doi: 10.1155/2012/908314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLOS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- Leeansyah E, Malone DF, Anthony DD, Sandberg JK. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS. 2013;8:117–124. doi: 10.1097/COH.0b013e32835c7134. [DOI] [PubMed] [Google Scholar]
- Liovat AS, Rey-Cuille MA, Lecuroux C, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLOS ONE. 2012;7:e46143. doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Majdic O, Knapp W, High-level HW. IL-10 production by monoclonal antibody-stimulated human T cells. Immunology. 1995;86:364–371. [PMC free article] [PubMed] [Google Scholar]
- Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth RS. The emerging role of CD40 ligand in HIV infection. J Leukoc Biol. 2000;68:373–382. [PubMed] [Google Scholar]
- Sousa AE, Chaves AF, Doroana M, Antunes F, Victorino RM. Early reduction of the over-expression of CD40L, OX40 and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin Exp Immunol. 1999;116:307–315. doi: 10.1046/j.1365-2249.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Younes SA, Funderburg NT, et al. Cycling memory CD4+ T cells in HIV disease have a diverse TCR repertoire and a phenotype consistent with bystander activation. J Virol. 2014;88(10):5369–5380. doi: 10.1128/JVI.00017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcherding F, Nitschke M, Hundorfean G, et al. The CD40–CD40L pathway contributes to the proinflammatory function of intestinal epithelial cells in inflammatory bowel disease. Am J Pathol. 2010;176:1816–1827. doi: 10.2353/ajpath.2010.090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koguchi Y, Buenafe AC, Thauland TJ, et al. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8– thymocytes and invariant NKT cells but not in Treg cells. PLOS ONE. 2012;7:e31296. doi: 10.1371/journal.pone.0031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Petitjean G, Dunyach-Remy C, et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLOS Pathog. 2013;9:e1003453. doi: 10.1371/journal.ppat.1003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DC, Jackson JW, Maggirwar SB. Targeting platelet-derived soluble CD40 ligand: a new treatment strategy for HIV-associated neuroinflammation? J Neuroinflammation. 2013;10:144. doi: 10.1186/1742-2094-10-144. . 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Davidson DC, Kiebala M, Maggirwar SB. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J Virol Methods. 2012;181:170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Davidson DC, Silva J, Ramirez SH, Maggirwar SB. 2013. Elevated levels of sCD40L cause increase in circulation platelet-monocyte complex: possible role in HIV-associated neuro-inflammation. 20th Conference on Retroviruses and Opportunistic Infections (CROI 2013), Atlanta, GA, USA,
- Maurais E, Cantin R, Tremblay MJ. Human immunodeficiency virus type 1-anchored CD40 ligand induces secretion of the chemokine interleukin-8 by human primary macrophages. Virology. 2009;385:227–232. doi: 10.1016/j.virol.2008.11.033. [DOI] [PubMed] [Google Scholar]
- D'Aversa TG, Eugenin EA, Berman JW. CD40-CD40 ligand interactions in human microglia induce CXCL8 (interleukin-8) secretion by a mechanism dependent on activation of ERK1/2 and nuclear translocation of nuclear factor-kappaB (NFkappaB) and activator protein-1 (AP-1) J Neurosci Res. 2008;86:630–639. doi: 10.1002/jnr.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DC, Schifitto G, Maggirwar SB. Valproic acid inhibits the release of soluble CD40L induced by non-nucleoside reverse transcriptase inhibitors in human immunodeficiency virus infected individuals. PLOS ONE. 2013;8:e59950. doi: 10.1371/journal.pone.0059950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DC, Hirschman MP, Spinelli SL, et al. Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals. J Immunol. 2011;186:584–591. doi: 10.4049/jimmunol.1001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gating strategy used for characterization of T helper type 17 (Th17) cells.


