Abstract
Angioedema (AE) is a clinical syndrome characterized by localised swelling lasting several hours. The swelling is often recurring and can be lethal if it is located in the laryngeal region. Much progress has been made recently in the treatment of acute episodes, but no consensus has been reached on maintenance treatment. We have performed a national retrospective observational study to assess the use of tranexamic acid (TA) as maintenance treatment for non-histaminergic AE [hereditary AE (HAE) or idiopathic non-histaminergic AE]. Records for 64 cases were collected from 1 October 2012 to 31 August 2013; 37 of these were included (12 HAE with C1-inhibitor deficiency, six with HAE with normal C1-inhibitor and 19 idiopathic non-histaminergic AE). When treated with TA over six months, the number of attacks was reduced by 75% in 17 patients, 10 patients showed a lower level of reduction and 10 had the same number of attacks. In no instances were symptoms increased. No thromboembolic events were observed, and the main side effects were digestive in nature. Thus, TA, which is well tolerated and inexpensive, appears to be an effective maintenance treatment for some patients with HAE or idiopathic non-histaminergic AE.
Keywords: angioedema, bradykinin, hereditary angioedema, tranexamic acid
Introduction
Angioedema (AE) is a clinical syndrome characterized by swelling of subcutaneous or submucosal tissues. The swelling is contained, does not itch, and can last from a few hours to several days. Upon regression it leaves no after-effects, but it can recur. AE is dangerous, as swelling of the tongue or larynx can lead to asphyxia if it is not rapidly diagnosed and treated 1–3. In addition to histaminergic aetiologies (in these cases AE is associated frequently with urticaria), there are several types of AE which are linked to bradykinin. Thus, we can distinguish hereditary angioedema (HAE) with or without C1-inhibitor deficiency, acquired AE with C1-inhibitor deficiency, AE linked to angiotensin-converting enzyme inhibitors (ACEi) and idiopathic non-histaminergic AE, in which bradykinins are thought to be involved 4. In recent years, much therapeutic progress has been made for HAE with C1-inhibitor deficiency, mainly for acute treatment of attacks 5–7. Long-term prophylactic treatment remains problematic: androgen derivatives have side effects which can be significant, and C1-inhibitor concentrate is expensive and must be administered parenterally 6–8. Anti-fibrinolytics, in particular tranexamic acid (TA), were proposed as a maintenance treatment for HAE with C1-inhibitor deficiency several decades ago 9–12. Since then, despite their frequent use in day-to-day clinical practice 13, little has been reported on their efficacy for this indication 14. Due to this lack of data, the recent recommendations for HAE management do not mention TA as a maintenance treatment 6,7. Because of this apparent discrepancy between clinical practice and general recommendations, and to contribute to the literature on the efficacy of TA as a treatment for AE, we reviewed the long-term use of TA in France as part of the management of non-histaminergic AE (HAE with or without C1-inhibitor deficiency, idiopathic non-histaminergic AE).
Patients and methods
This is a retrospective, multi-centre, French study. From 1 October 2012 to 31 August 2013, all the doctors participating in the National Reference Centre for Angioedema (CREAK), the national network for AE diagnosis and treatment, were asked to report on patients taking or having taken TA as a maintenance treatment for non-histaminergic AE (HAE or idiopathic non-histaminergic AE). The inclusion criteria were as follows, patients must: (i) have HAE with or without C1-inhibitor deficiency or idiopathic non-histaminergic AE; (ii) take or have taken TA as a maintenance treatment for at least 6 months; and (iii) be at least 16 years of age. Exclusion criteria were: (i) concomitant maintenance treatment which could affect the number and severity of AE attacks, including treatment with C1-inhibitor concentrate or danazol, or any progestin for whatever reason (oral contraception or progestin-releasing intrauterine device, chlormadinone acetate, hormone replacement therapy); (ii) uncertain diagnosis of HAE or idiopathic non-histaminergic AE; (iii) treatment with TA for less than 6 months; and (iv) aged less than 16 years. Taking specific treatments during an attack or for prophylaxis in a high-risk situation (dental or surgical intervention, etc.) was accepted.
The diagnosis of hereditary AE with normal C1 inhibitor was made when a patient had recurrent AE without urticaria, with a duration of more than 24 h and with familial history of AE. In all these patients, factor XII mutation was investigated and was not found.
All patients with a diagnosis of idiopathic non-histaminergic AE had recurrent AE without urticaria, with a duration of more than 24 h, without familial history, without efficacy of anti-histaminic treatment (even at doses four times higher than recommended) and without abnormality of complement exploration (C4 and C1-inhibitor).
All patients were followed within the CREAK network. They had a physical examination every 6 months, with systematic collection of the number and the severity of attacks.
Data were gathered using a questionnaire relating to the diagnosis of AE, how TA and other treatments were administered and the number of attacks over the 6 months prior to and the 6 months following the start of treatment with TA. Questionnaires were completed by the doctor (including the patient), and responses were checked by a doctor specializing in AE.
Severity ranking
Any attack affecting the ear–nose–throat or facial region and abdominal attacks with a score greater than 5 out of 10 on the visual analogue scale was considered a severe attack.
Data collection and treatment
Data were collated in an Excel table (Microsoft). The figures were produced using the same software.
Results
Population studied
From 1 October 2012 to 31 August 2013, medical records were collected for 64 cases. These corresponded to 63 individual patients. Twenty-seven of these dossiers were excluded: eight patients were taking another prophylactic maintenance treatment for AE (seven danazol, one C1-inhibitor concentrate); 12 patients were taking progestins (eight chlormadinone, two desogestrel, one progestin-releasing intrauterine device and one hormone replacement therapy); three were aged less than 16 years; for two patients, diagnosis of HAE or idiopathic non-histaminergic AE was uncertain (one had urticaria, and a Münchausen syndrome was suspected for the other); one had taken TA for only 8 days; and one report was duplicated. Of the 37 patients included, 12 had HAE with C1-inhibitor deficiency, six had HAE without C1-inhibitor deficiency and 19 had idiopathic non-histaminergic AE 1). There were 24 women versus 13 men, median age 50 (range: 17–73) years. Three women had continued to take TA during pregnancy. The median duration of treatment with TA at the date of inclusion of patients was 30 months (range: 6–138), and the daily dose of TA varied between 1 and 3 g per day. The characteristics of the patients included, for each AE type, are summarized in Table 1.
Table 1.
Characteristics of the study population
| Hereditary angioedema with C1-inhibitor deficiency | Hereditary angioedema with normal C1-inhibitor | Idiopathic non-histaminergic AE | Total | |
|---|---|---|---|---|
| Number | 12 | 6 | 19 | 37 |
| Gender (male/female) | 2/10 | 3/3 | 8/11 | 13/24 |
| Pregnancy (n) | 2 | 1 | 3 | |
| Median age (years) | 34 | 60 | 52 | 50 |
| Median dose of TA (g per day) | 3 | 2·5 | 2 | 2·5 |
| Median duration of treatment (months) | 34 | 30 | 29 | 30 |
AE = angioedema; TA = tranexamic acid.
Efficacy of TA
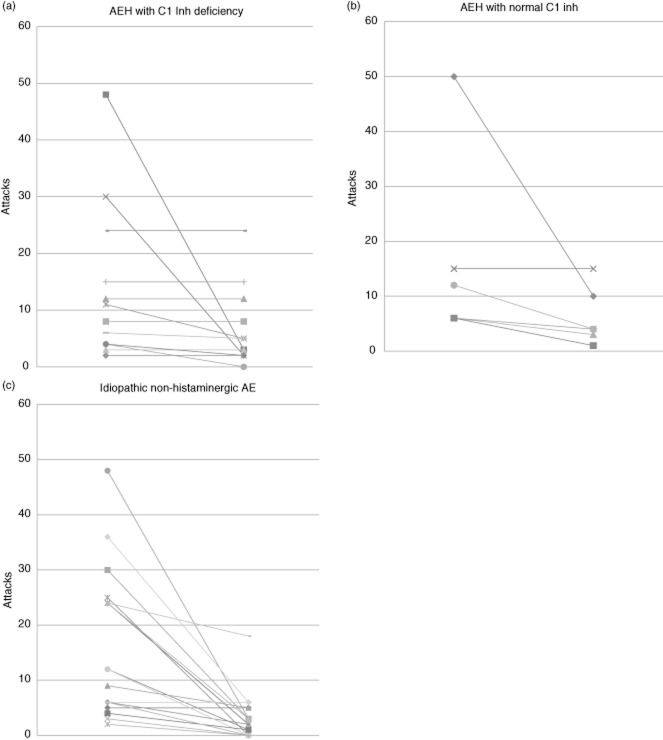
During the 6 months preceding the introduction of maintenance treatment with TA, the 12 patients with HAE with C1-inhibitor deficiency had an average of 14 AE attacks (range: three to 48), of which three (range: one to six) were severe. During the 6 months following the introduction of this treatment, attacks were reduced to an average of seven (range: none to 24), of which one (range: none to three) was severe (Table 2). The change in the number of attacks is detailed for each patient in Fig. 1a. This shows that, for three patients, the number of attacks was reduced by more than 75% following the introduction of TA; for three others the reduction was more moderate; and for six patients the total number of attacks was unchanged. No aggravation of symptoms was noted for this patient group after the introduction of TA (Fig. 2a).
Table 2.
Efficacy and tolerance of tranexamic acid (TA) in the 37 patients included
| Hereditary angioedema with C1-inhibitor deficiency | Hereditary angioedema with normal C1-inhibitor | Idiopathic non-histaminergic AE | Total | |
|---|---|---|---|---|
| TA efficacy | n = 12 patients | n = 6 patients | n = 19 patients | n = 37 |
| Attacks in the 6 months prior to TA: mean (range) | 14 (3, 48) | 16 (6, 50) | 15 (2, 48) | 15 (2, 50) |
| Of which severe attacks | 3† (1, 6) | 6‡ (3, 10) | 5‡ (0, 28) | 5 (0, 12) |
| Attacks in the 6 months after starting TA: mean (range) | 7 (0, 24) | 6 (4, 15) | 3 (0, 18) | 5 (0, 24) |
| Of which severe attacks | 1† (0, 3) | 2‡ (1, 3) | 1‡ (0, 5) | 1 (0, 5) |
| TA tolerance | ||||
| UE: number (patients) | 6 (4) | 3 (3) | 6 (4) | 15 (11) |
| Type (number) | Digestive (3), migraine (1), limb pains (1), weakness 1) | Digestive (3) | Digestive (4), dizziness (2) | |
| Reduction in dose due to UE | 2 | 1 | 1 | 4 |
| Discontinuation due to UE | 0 | 0 | 1 | 1 |
†Two cases with missing data; ‡one case with missing data. AE = angioedema; UE = undesirable event.
Fig 1.
Patient-by-patient breakdown of the number of attacks over the 6 months preceding and the 6 months following the introduction of maintenance treatment based on tranexamic acid. AE = angioedema; HAE = hereditary angioedema; C1 Inh = C1-inhibitor.
Fig 2.
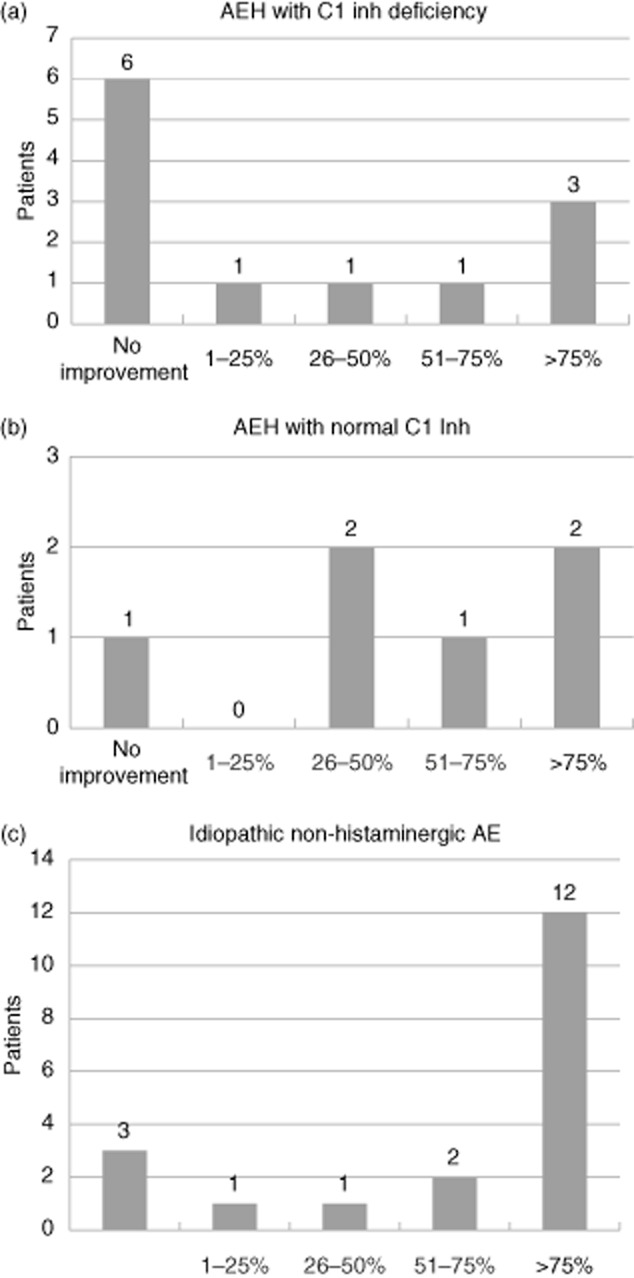
Improvements in the number of attacks after initiating treatment with tranexamic acid (comparison between the number of attacks over the 6 months preceding treatment and the number of attacks over the 6 months following initiation of treatment). AE = angioedema; HAE = hereditary angioedema; C1 inh = C1-inhibitor.
During the 6 months preceding the introduction of treatment with TA, the six patients with HAE without C1-inhibitor deficiency had an average of 16 (range: six to 50) attacks, of which six (range: three to 10) were severe. With treatment by TA, the mean number of attacks was six (range: four to 15), of which two (range: one to three) were severe (Table 2). The change in the number of attacks is detailed for each patient in Fig. 1b, revealing that the number of attacks was reduced by more than 75% following the introduction of TA for two patients; three others experienced a more moderate reduction; and one patient saw no change in the total number of attacks. No increase in the number of attacks was noted after the introduction of TA (Fig. 2b).
For the 19 patients with idiopathic non-histaminergic AE, during the 6 months preceding the introduction of treatment with TA, an average of 15 (range: two to 48) attacks were reported, of which five (range: none to 28) were severe. With maintenance treatment by TA, the mean number of attacks was three (range: none to 18), of which one (range: none to five) was severe (Table 2). The change in the number of attacks is detailed for each patient in Fig. 1c. For 12 patients, the number of attacks was reduced by more than 75% following the introduction of TA; for four others, the reduction was more moderate; and for three patients the total number of attacks was unchanged. Once again, no increase in the number of attacks was noted for this group of patients after the introduction of TA (Fig. 2c).
TA tolerance
In total, for the 37 patients included in our study, 15 undesirable events were reported for 11 patients: 10 of these were abdominal pains; the other events observed were dizziness (two), weakness (one), pain in the lower limbs (one) and migraine headaches (one). None of these events was serious, and no thromboembolic episodes were reported. The daily dose of TA was reduced to diminish undesirable events in four patients, and in one case the treatment was discontinued after reporting an undesirable event (Table 2). Two other patients also elected to discontinue TA as a maintenance treatment: one because they preferred to use TA sporadically when prodromes occurred and the other because they were being treated for melanoma.
Discussion
Anti-fibrinolytics were shown originally to be useful in managing HAE in the 1970s by two placebo-controlled prospective studies 9,10. One of these examined the efficacy of TA in 18 patients 9, while the other studied the efficacy of aminocaproic acid in five patients 10. Since then, TA, which has a better tolerance profile than aminocaproic acid, has been used regularly in patients with non-histaminergic AE, either as an acute treatment during attacks or as a long-term preventive treatment. TA has also been proposed as a test therapy for patients presenting other forms of recurrent localized cutaneous or mucosal swelling 1,15. A recent literature review of maintenance treatments for HAE 16 listed anti-fibrinolytic treatments (with androgen derivatives and C1-inhibitor concentrate) among the three therapeutic classes known to be effective in reducing the number of AE attacks based on controlled prospective trials. However, due to the lack of published data relating to TA since the 1970s, long-term treatment with TA is no longer recommended in the most recent guidelines 6,7,17,18. Despite this, TA remains in frequent use as a maintenance treatment for HAE, particularly in France, especially when androgen derivatives are contraindicated or poorly tolerated 8,13. Dispenza et al. 13 undertook an international survey of practices in 2011. Their report reveals that 18% of the 201 practitioners specializing in HAE management questioned used TA preferentially as a long-term prophylactic treatment, even though the treatment was no longer recommended. They also showed that practices vary widely between different countries. In corroboration of this, an Italian prospective observational study carried out from 2004 to 2008 14 reported a lower frequency of TA use as a maintenance treatment, with only six of 103 (5·8%) patients being prescribed this product long term. We therefore performed a further observational study to determine how TA is used as a maintenance treatment for non-histaminergic AE, hereditary or not (idiopathic non-histaminergic AE) in France.
To focus more clearly on the effect of TA on AE attacks, patients who also took another maintenance treatment (androgen derivatives 19 or C1-inhibitor concentrate 20) and patients treated with progestins were excluded. This last was necessary, as a recent study 21 showed that these could be used effectively as a maintenance treatment, reducing the frequency of attacks. Of the 37 patients taking only TA as a maintenance treatment, for 17 the number of attacks was reduced by more than 75% after introducing the treatment; 10 had a more moderate improvement; and 10 reported no improvement. The number of attacks was never increased when patients were treated with TA. These overall results hide a large degree of interindividual variability, as reported in previous studies 16. In our series, TA appears more effective in the treatment of patients with idiopathic non-histaminergic AE than in patients with HAE. The efficacy of TA in patients with idiopathic non-histaminergic AE was still reported by Du-Than, with 12 complete responses and one decrease of intensity, frequency and duration of attacks in 23 patients 22. Nevertheless, more than half (11 of 18) the patients with HAE showed improvement when treated with TA. In patients with HAE with C1-inhibitor deficiency, symptoms for six of 12 patients were improved by treatment with TA. This appears to contradict the results of a recent Italian study 14, where six patients treated with TA had a comparable number of attacks to patients taking no maintenance treatment. Zanichelli et al. 14 therefore concluded that TA was ineffective in this indication. This apparent discrepancy could be due to methodological differences: the Italian study did not compare the number of attacks in the same patient before and after initiating the maintenance treatment, thus their data do not take into account interindividual differences in terms of disease severity.
Our results encourage us to test TA in patients for whom a maintenance treatment is appropriate. This treatment is well tolerated, inexpensive and appears to be safe; no thromboembolic events were described and the main undesirable event reported was digestive effects, which could be improved by reducing the dose. A single patient, in whom TA was not effective, stopped using the drug due to side effects (digestive discomfort and dizziness). In addition, TA has other indications for which it is well tolerated. In particular, it is used widely to treat haemostatic disorders and haemorrhagic syndromes, including in pregnant women 23,24. This contrasts with the side effects associated with androgen derivatives (virilization, weight gain, hepatotoxicity, increase in the risk of hepatocarcinoma 17), and with the high cost (around €2000 per week) and parenteral route of administration of C1-inhibitor concentrate.
To conclude definitively on the efficacy of TA as maintenance treatment for non-histaminergic AE, and to compare it to other maintenance treatments, comparative prospective studies would be necessary. However, these are difficult to perform with this rare disease, for which severity is variable between patients. We therefore chose to perform a purely descriptive and retrospective analysis based on a study population, which is partly biased due to the inclusion criteria used. With our study design, we cannot draw conclusions regarding AT efficacy, but our data suggest that AT could be helpful for patients with AE: this case-series shows that TA can significantly improve symptoms for some patients. These results, reflecting day-to-day clinical practice 13, should lead us to reconsider the place of TA as a maintenance treatment for HAE. It is also a potentially interesting treatment for idiopathic non-histaminergic AE, where it spectacularly reduces the number and severity of attacks.
Disclosure
None.
References
- Bouillet L, Ponard D, Drouet C, Massot C. [Non-histaminic angioedema management: diagnostic and therapeutic interest of tranexamic acid] Rev Med Interne. 2004;25:924–926. doi: 10.1016/j.revmed.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Zuraw BL. Hereditary angiodema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann Allergy Asthma Immunol. 2008;100:S13–18. doi: 10.1016/s1081-1206(10)60581-9. [DOI] [PubMed] [Google Scholar]
- Bouillet L, Boccon-Gibod I, Massot C. Bradykinin mediated angioedema. Rev Med Interne. 2011;32:225–231. doi: 10.1016/j.revmed.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Zuraw BL, Bork K, Binkley KE, et al. [Hereditary angioedema with normal C1 inhibitor function: consensus of an international expert panel] Allergy Asthma Proc. 2012;33(Suppl. 1):S145–156. doi: 10.2500/aap.2012.33.3627. [DOI] [PubMed] [Google Scholar]
- Bouillet L. [Hereditary angioedema: a therapeutic revolution] Rev Med Interne. 2012;33:150–154. doi: 10.1016/j.revmed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Cicardi M, Bork K, Caballero T, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2011;67:147–157. doi: 10.1111/j.1398-9995.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- Craig T, Aygoren-Pursun E, Bork K, et al. WAO guideline for the management of hereditary angioedema. World Allergy Organ J. 2012;5:182–199. doi: 10.1097/WOX.0b013e318279affa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl M, Gower RG, Chrvala CA. Current medical management of hereditary angioedema: results from a large survey of US physicians. Ann Allergy Asthma Immunol. 2011;106:316–322. doi: 10.1016/j.anai.2010.12.012. e4. [DOI] [PubMed] [Google Scholar]
- Blohme G. Treatment of hereditary angioneurotic oedema with tranexamic acid. A random double-blind cross-over study. Acta Med Scand. 1972;192:293–298. doi: 10.1111/j.0954-6820.1972.tb04818.x. [DOI] [PubMed] [Google Scholar]
- Frank MM, Sergent JS, Kane MA, Alling DW. Epsilon aminocaproic acid therapy of hereditary angioneurotic edema. A double-blind study. N Engl J Med. 1972;286:808–812. doi: 10.1056/NEJM197204132861503. [DOI] [PubMed] [Google Scholar]
- Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med. 1972;287:452–454. doi: 10.1056/NEJM197208312870907. [DOI] [PubMed] [Google Scholar]
- Cugno M, Cicardi M, Agostoni A. Activation of the contact system and fibrinolysis in autoimmune acquired angioedema: a rationale for prophylactic use of tranexamic acid. J Allergy Clin Immunol. 1994;93:870–876. doi: 10.1016/0091-6749(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dispenza MC, Craig TJ. Discrepancies between guidelines and international practice in treatment of hereditary angioedema. Allergy Asthma Proc. 2012;33:241–248. doi: 10.2500/aap.2012.33.3566. [DOI] [PubMed] [Google Scholar]
- Zanichelli A, Vacchini R, Badini M, Penna V, Cicardi M. Standard care impact on angioedema because of hereditary C1 inhibitor deficiency: a 21-month prospective study in a cohort of 103 patients. Allergy. 2010;66:192–196. doi: 10.1111/j.1398-9995.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- Cicardi M, Bergamaschini L, Zingale LC, Gioffre D, Agostoni A. Idiopathic non histaminergic angioedema. Am J Med. 1999;106:650–654. doi: 10.1016/s0002-9343(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Costantino G, Casazza G, Bossi I, Duca P, Cicardi M. Long-term prophylaxis in hereditary angio-oedema: a systematic review. BMJ Open. 2012;2:e000524. doi: 10.1136/bmjopen-2011-000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temino VM, Peebles RS., Jr The spectrum and treatment of angioedema. Am J Med. 2008;121:282–286. doi: 10.1016/j.amjmed.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Bowen T, Brosz J, Brosz K, Hebert J, Ritchie B. Management of hereditary angioedema: 2010 Canadian approach. Allergy Asthma Clin Immunol. 2010;6:20–30. doi: 10.1186/1710-1492-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976;295:1444–1448. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- Tallroth GA. Long-term prophylaxis of hereditary angioedema with a pasteurized C1 inhibitor concentrate. Int Arch Allergy Immunol. 2011;154:356–359. doi: 10.1159/000321830. [DOI] [PubMed] [Google Scholar]
- Saule C, Boccon-Gibod I, Fain O, et al. Benefits of progestin contraception in non allergic angioedema. Clin Exp Allergy. 2013;43:475–482. doi: 10.1111/cea.12055. [DOI] [PubMed] [Google Scholar]
- Du-Thanh A, Raison-Peyron N, Drouet C, Guillot B. Efficacy of tranexamic acid in sporadic idiopathic bradykinin angioedema. Allergy. 2010;65:793–795. doi: 10.1111/j.1398-9995.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- Chi C, Kulkarni A, Lee CA, Kadir RA. The obstetric experience of women with factor XI deficiency. Acta Obstet Gynecol Scand. 2009;88:1095–1100. doi: 10.1080/00016340903144238. [DOI] [PubMed] [Google Scholar]
- Roberts I, Perel P, Prieto-Merino D, et al. Effect of tranexamic acid on mortality inpatients with traumatic bleeding: pre specified analysis of data from randomised controlled trial. BMJ. 2012;345:e5839. doi: 10.1136/bmj.e5839. [DOI] [PMC free article] [PubMed] [Google Scholar]