Abstract
Activated CD4+ T cells undergo blastogenesis and proliferation and they express several surface receptors, including β2-microglobulin-free human leucocyte antigen (HLA) heavy chains (open conformers). Intravenous immunoglobulin (IVIg) suppresses activated T cells, but the mechanism is unclear. IVIg reacts with HLA-Ia/Ib antigens but its reactivity is lost when the anti-HLA-E Ab is adsorbed out. Anti-HLA-E antibodies may bind to the peptides shared by HLA-E and the HLA-I alleles. These shared peptides are cryptic in intact HLA, but exposed in open conformers. The hypothesis that anti-HLA-E monoclonal antibodies (mAbs) that mimic HLA-I reactivity of IVIg may suppress activated T cells by binding to the shared peptides of the open conformers on the T cell surface was tested by examining the relative binding affinity of those mAbs for open conformers coated on regular beads and for intact HLA coated on iBeads, and by comparing the effects on the suppression of phytohaemagglutinin (PHA)-activated T cells of three entities: IVIg, anti-HLA-E mAbs that mimic IVIg [Terasaki Foundation Laboratory (TFL)-006 and (TFL)-007]; and anti-HLA-E antibodies that do not mimic IVIg (TFL-033 and TFL-037). Suppression of blastogenesis and proliferation of those T cells by both IVIg and the anti-HLA-E mAbs was dose-dependent, the dose required with mAbs 50–150-fold lower than with IVIg. TFL-006 and TFL-007 significantly suppressed blastogenesis and proliferation of activated CD4+ T cells, but neither the non-IVIg-mimicking mAbs nor control antibodies did so. The suppression may be mediated by Fab-binding of TFL-006/TFL-007 to the exposed shared peptides. The mAb binding to the open conformer may signal T cell deactivation because the open conformers have an elongated cytoplasmic tail with phosphorylation sites (tryosine320/serine335).
Keywords: activated T cells, blastogenesis, intravenous immunoglobulin (IVIg), open conformers, shared peptides
Introduction
Therapeutic preparations of intravenous immunoglobulin (IVIg) are known to suppress activated CD4+ T lymphocytes, and their production of proinflammatory cytokines, when the cells are activated by various stimuli in vitro 1–11. The mechanism of suppression remains speculative and has not been elucidated because IVIg is purified from plasma pooled from thousands of donors and contains several undefined antibodies. Nevertheless, IVIg is administered post-transplantation to reduce T cell activation and proliferation (blastogenesis) in allograft recipients to suppress antigen-presenting T cells involved in the production of antibodies targeting allograft antigens, biopsy-proven T cell-mediated allograft rejection and T lymphoproliferative disorders emanating after transplantation.
Recently, we showed that different therapeutic preparations of IVIg contained IgG antibodies reacting to a wide array of human leucocyte antigen (HLA) class Ia molecules (HLA-A, HLA-B and HLA-Cw) and HLA class Ib molecules (HLA-E, HLA-F and HLA-G) 12. Most importantly, the therapeutic preparations of IVIg differed in their reactivity to two different kinds of beads: iBeads™ coated with HLA heterodimers, i.e. HLA associated with β2-microglobulin (β2m), known as ‘intact HLA’ and regular beads coated with intact HLA, but also with more of the β2m-free heavy chain (HC) of HLA, known as ‘open conformers’ 13. All preparations of IVIg showed greatly reduced reactivity for iBeads, suggesting that they recognize the open conformers of all HLA class I alleles much better than they recognize intact HLA.
There was a further consideration, however. The HLA reactivity of IVIg was lost when the HLA-E antibody reactivity of IVIg was adsorbed out 12, suggesting that IVIg may bind to the peptide sequences shared between HLA-E and other HLA alleles (HLA-A, -B,-Cw, -F and -G). This hypothesis was verified when the HLA-E and HLA-Ia reactivity of anti-HLA-E polyreactive monoclonal antibodies (mAbs) was inhibited by a series of shared peptide sequences on HLA-E (e.g. 117AYDGKDY123 and 126LNEDLRSWTA135), but not by HLA-E-specific peptides 14–18. These shared peptide sequences not only inhibited binding of the anti-HLA-E mAbs to HLA-E, but also inhibited the entire HLA-Ia reactivity, confirming that they are indeed peptide sequences shared by all HLA class I alleles. However, the shared peptide sequences remain cryptic in their native conformation due to the presence of β2m, as was well illustrated in a previous report [Fig. 4 in (12)]. Until the late 1980s it was believed that HLA class I molecules occurred on cell surfaces only as heterodimers, but it is now recognized that the HCs of HLA-I molecules occur on cell surfaces – like those of activated T cells – as open conformers 13.
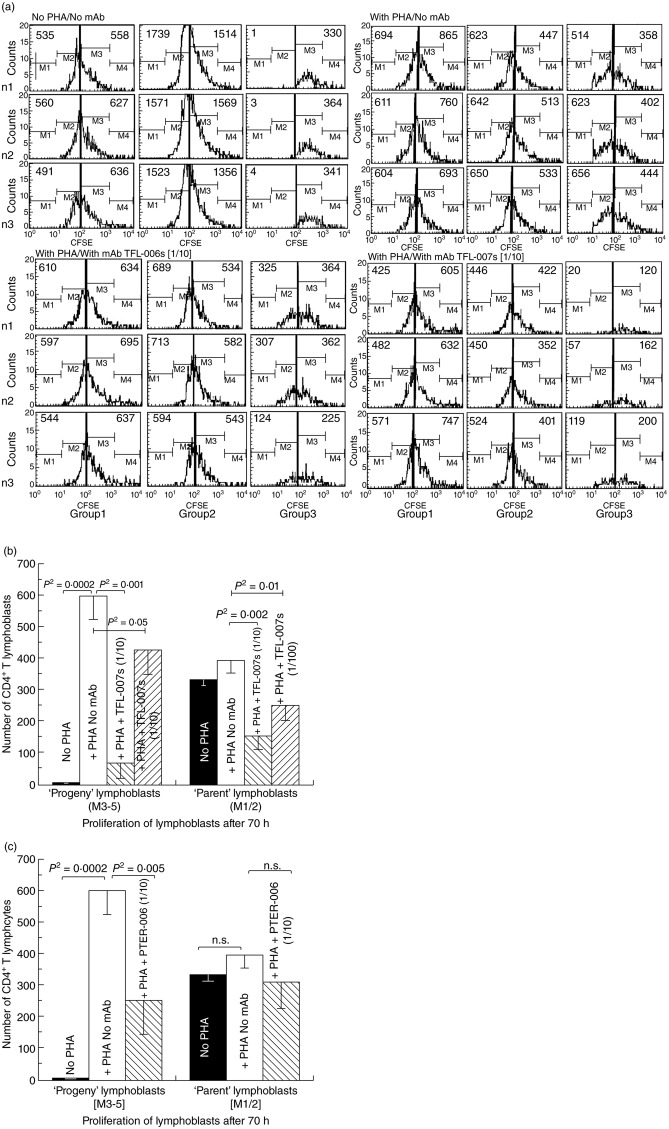
Fig 4.
Suppression of in-vitro proliferation of phytohaemagglutinin (PHA)-activated CD4+ and CD8+ T lymphocytes by anti-human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) mimicking human leucocyte antigen (HLA)-I reactivity of intravenous immunoglobulin (IVIg). The carboxyfluorescein succinimidyl ester (CFSE)-labelled lymphocytes were cultured with or without PHA or with PHA and mAb Terasaki Foundation Laboratory (TFL)-006s or PHA and mAb TFL-007s, both mAbs at 1/10 dilution. Three days after culture, cells were labelled with fluorescent dye-conjugated anti-CD4+ or anti-CD8+ antibodies before analysis. CFSE labelling allowed us to gauge and show cell proliferation: when the CFSE-labelled cell population undergoes mitosis, after 72 h it has migrated from the right to the left side of each rectangular box in the figure depending on the number of mitoses. The distance moved shows the number of cell divisions. (a) Effect of anti-HLA-E mAb TFL-006s and TFL-007s on proliferation of CD4+/CFSE+ T lymphocytes. After incubating cells with CFSE, the cells were treated as noted. Each box in the figure is divided by a vertical line into two sub-boxes, the right for mitoses 1 and 2 (M1/2) (parent lymphocytes) and the left for mitosis 3 to 5 (M3–5) (the progeny). The number of cells after each treatment (including ‘no PHA’) of each T lymphocyte population was counted and compared, the number shown in each sub-box. Note that with no PHA the number of cells in the M3–5 sub-box is very meagre for all groups of CD4+ T lymphocytes. With PHA-only treatment, the very high number of cells for M3–5 indicates that proliferation has occurred in all three groups. The impact of treatment with PHA and TFL-007s or PHA and TFL-006s is unmistakable: the number of cells in the M3–5 sub-box is reduced in all groups, indicating suppression of proliferation. No such decrease was observed with resting or naive T lymphocytes. (b) Effect of TFL-007s on proliferation of CD4+/CFSE+ T lymphoblasts after incorporation of CFSE. The values represent the mean of triplicate analysis, with treatment as indicated in the bars. Two-tailed P-values are indicated by a horizontal line connecting the two groups. (c) Effect of TFL-006s on proliferation of CD4+/CFSE+ T lymphoblasts after incorporation of CFSE. As with (c), the values represent the mean of triplicate analysis, with treatment as indicated in the bars. Two-tailed P-values are indicated by a horizontal line connecting the two groups.
Interestingly, there have been a few reports that, as with IVIg, mAbs raised against HLA-Ia alleles also suppressed T cell proliferation 19–22, T cell activation 20, interleukin (IL)-2 and IL-2R synthesis 22, and were capable of inducing apoptosis 23. These reports did not identify the specific epitopes or amino acid sequences recognized by the anti-HLA-I mAbs. However, we hypothesized that some anti-HLA-E mAbs are not only capable of replicating the HLA class I reactivity of IVIg, but may also recognize the shared peptides on the open conformers specifically over-expressed on the cell surface of activated CD4+ T lymphocytes 24–32.
To test this hypothesis, mAbs directed against the exposed shared epitopes of open conformers common to all HLA-Ia and -Ib molecules were generated by immunizing mice with the open conformers of HLA-E. After ascertaining the reactivity of these mAbs to HLA-Ia and -Ib alleles and confirming their reactivity to regular beads and iBeads, they were added to culture wells containing T cells activated by PHA-P (phytohaemagglutin-Phaseolus). In this comparative study, the effects of different preparations of IVIg and different kinds of anti-HLA-E mAbs on PHA-P-activated T cells were examined to elucidate the possible mechanism underlying the suppression of activated CD4+ T cells.
Materials and methods
Therapeutic preparations of IVIg
The therapeutic IVIg preparations from three sources were used. They included GamaSTAN™ S/D (15–18 protein %; Talecris Biotherapeutics, Inc., Research Triangle Park, NC, USA), Octagam® (6 gr%, lot A913A8431; Octapharma Pharmazeutika, Lachen, Switzerland) and IVIGlob EX (5 gr%; VHB Life Sciences Limited, Mumbai, India). All IVIg preparations were serially diluted with phosphate-buffered saline (PBS) (pH 7·2); the protein concentrations after dilution may vary depending upon the original protein concentration of therapeutic preparations of IVIg.
Monoclonal antibodies used for in-vitro suppression of activated T cells
These mAbs were produced by immunization with β2m-free heavy chains (open conformers) of two different HLA-E alleles (HLA-ER107 and HLA-EG107). The recombinant peptide heavy chains [10 mg/ml in 2-(N-morpholino)ethanesulphonic acid (MES) buffer] were obtained from the Immune Monitoring Laboratory (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). Each antigen was immunized in two different mice, as detailed elsewhere 12. The monoclonal antibodies, called ‘TFL’ mAbs in this study, were formerly called the ‘PTER’ series 12. Three different kinds of anti-HLA-E mAbs were used. As shown in Table 1a, eight types of anti-HLA-E mAbs with differing reactivity for different HLA class Ia alleles (HLA-A, -B and -Cw) and HLA class Ib alleles (HLA-E, -F and -G) were generated. Of these, we used three different types: the one comprising TFL-033 (type 1), which is monospecific for HLA-E (the peptide-binding domain of this mAb is identified by inhibiting the mAb by HLA-E-restricted peptide sequences located on the α1 and α2 helices 65RSARDTA71 and 143SEQKSNDASE152) 33; one comprising TFL-037 (type 5), which reacts with HLA-E, but not with HLA-F or HLA-G, and also with the classical HLA class Ia alleles; and one comprised of TFL-006 and TFL-007 (type 8) which, like IVIg, reacts with all the classical HLA class Ia and non-classical HLA class Ib alleles (the peptide binding domain of this group's mAbs is identified by the inhibition of the mAb by peptide sequences of HLA-E shared with several HLA class Ia alleles, e.g. 117AYDGKDY123 and 126LNEDLRSWTA135), but not by other peptide sequences 14–16. These earlier reports show that the polyreactivity is not targeted at other motifs. Figure 1a,b shows that the shared peptide sequences are masked by β2m.
Table 1.
Monoclonal anti- human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) (n > 150) generated after immunizing the mice with open conformer of HLA-E
| (a) Based on the differences in their HLA class Ia and Ib reactivity, the anti-HLA-E mAbs are categorized into eight types. Type 1 [Terasaki Foundation Laboratory (TFL)-033] reacted only with HLA-E and not with any other HLA class I alleles; type 5 (TFL-037) did not recognize HLA-F and HLA-G; and type 8 (TFL-006 and TFL-007) reacted with all HLA class Ia and Ib alleles. The common shared peptide sequences are exposed in the open conformer in the absence of β2-microglobulin 15 | |||||||
|---|---|---|---|---|---|---|---|
| Types of anti-HLA-E mAbs | Non-classical HLA-Ib | Classical HLA-Ia | Names of mAbs | ||||
| HLA-E | HLA-F | HLA-G | HLA-A | HLA-B | HLA-Cw | ||
| Type 1 | + | − | − | − | − | − | TFL-033 |
| Type 2 | + | + | − | − | − | − | |
| Type 3 | + | − | + | − | − | − | |
| Type 4 | + | + | + | − | − | − | |
| Type 5 | + | − | − | + | + | + | TFL-037 |
| Type 6 | + | + | − | + | + | + | |
| Type 7 | + | − | + | + | + | + | |
| Type 8 | + | + | + | + | + | + | TFL-006 & TFL-007 |
| (b) Reactivity (determined by mean fluorescent intensity, MFI) of the undiluted culture supernatants of the mAbs on different human leucocyte antigen (HLA) class I alleles. Note that TFL-033 and TFL-037 did not recognize the heavy chains of HLA-F and HLA-G. The differences in MFI among the mAbs are critical to an understanding of the possible binding site of the mAbs on the cell surface of activated T lymphocytes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isotype | TFL-033 | TFL-037 | TFL-006 | TFL–007 | TFL-033 | TFL-037 | TFL-006 | TFL–007 | TFL-033 | TFL-037 | TFL-006 | TFL-007 | ||
| IgG1 | IgG2b | IgG2a | IgG2a | IgG1 | IgG2b | IgG2a | IgG2a | IgG1 | IgG2b | IgG2a | IgG2a | |||
| Neg | 3 | 8 | 15 | 7 | B*0702(B7) | 1 331 | 841 | CW*0102(Cw1) | 674 | 7 242 | 3 268 | |||
| Pos | 71 | 103 | 88 | 85 | B*0801(B8) | 2 092 | 1 033 | CW*0202(Cw2) [J] | 2 662 | 10 690 | 6 084 | |||
| HLA-E | 24 411 | 13 025 | 22 522 | 21 618 | B*1301(B13) | 3 684 | 5 654 | 3 979 | CW*0302(Cw10) | 1 321 | 5 917 | 3 062 | ||
| HLA-F | 12 650 | 11 035 | B*1302(B13) | 1 568 | 2 237 | 1 426 | CW*0303(Cw9) | 2 212 | 7 114 | 4 250 | ||||
| HLA-G | 7 193 | 2 670 | B*1401(B64) | 9 268 | 11 319 | 8 767 | CW*0304(Cw10) | 1 846 | 6 584 | 3 891 | ||||
| A*0101(A1) | 2 395 | 1 037 | B*1402(B65) | 1 492 | 4 414 | 2 558 | CW*0401(Cw4) | 2 843 | 1 272 | |||||
| A*0201(A2) | 856 | B*1501(B62) [R] | 1 097 | CW*0501(Cw5) | 10 015 | 16 131 | 13 096 | |||||||
| A*0203(A2) | 1 095 | B*1502(B75) | 5 150 | 6 256 | 4 497 | CW*0602(Cw6) | 1 060 | 9 396 | 4 274 | |||||
| A*0206(A2) | 1 494 | 843 | B*1503(B72) | 1 016 | 2 831 | 1 926 | CW*0702(Cw7) | 753 | 12 251 | 6 919 | ||||
| A*0301(A3) | 818 | B*1510(B71) | 2 616 | 1 470 | CW*0801(Cw8) | 7 709 | 13 456 | 10 733 | ||||||
| A*1101(A11) [J,R] | 7 062 | 10 190 | 8 476 | B*1511 (B75) | 5 715 | 9 041 | 5 902 | CW*1203(Cw12) | 5 055 | 2 102 | ||||
| A*1102(A11) | 860 | B*1512(B76) | 956 | 1 624 | 996 | CW*1402(Cw14) | 2 129 | 8 727 | 4 936 | |||||
| A*2301(A23) | 614 | B*1513(B77) | 4 650 | 5 326 | 3 365 | CW*1502(Cw15) [R] | 1 574 | 6 030 | 3 225 | |||||
| A*2402(A24) [J] | 1 287 | 3 133 | 2 011 | B*1516(B63) | 3 591 | 5 614 | 3 443 | CW*1601(Cw16) | 1 109 | 8 462 | 4 364 | |||
| A*2403(A24) | 1 005 | 3 151 | 1 967 | B*1801(B18) | 4 185 | 6 990 | 4 890 | CW*1701(Cw17) | 1 433 | 13 521 | 9 069 | |||
| A*2501(A25) | 1 230 | 692 | B*2705(B27) | 702 | 2 591 | 1 576 | CW*1802(Cw18) | 15 389 | 17 918 | 15 207 | ||||
| A*2601(A26) | 3 368 | 1 638 | B*2708(B27) | 1 438 | 4 437 | 2 671 | Cw* alleles | 0 | 14 | 16 | 16 | |||
| A*2901(A29) | 1 465 | 3 194 | 2 256 | B*3501(B35) | 8 235 | 10 205 | 8 594 | |||||||
| A*2902(A29) | 2 235 | 1 136 | B*3701(B37) | 3 039 | 6 472 | 4 338 | ||||||||
| A*3001(A30) | 2 229 | 1 237 | B*3801(B38) | 3 844 | 1 820 | |||||||||
| A*3002(A30) | 994 | 3 353 | 2 211 | B*3901(B39) | 4 068 | 7 093 | 5 304 | |||||||
| A*3101(A31) | 858 | B*4001(B60) | 2 518 | 5 743 | 3 758 | |||||||||
| A*3201(A32) | 1 053 | 2 237 | 1 508 | B*4002(B61) | 4 032 | 6 118 | 4 675 | |||||||
| A*3301(A33) [R] | 928 | 2 791 | 1 627 | B*4006(B61) | 14 399 | 15 643 | 13 758 | |||||||
| A*3303(A33) | 2 215 | 4 212 | 2 961 | B*4101(B41) | 3 721 | 7 191 | 5 277 | |||||||
| A*3401(A34) | 1 643 | 6 268 | 3 968 | B*4201(B42) | 636 | |||||||||
| A*3402(A34) | 1 399 | 893 | B*4402(B44) | 1 342 | 7 062 | 4 059 | ||||||||
| A*3601(A36) | 1 694 | 5 806 | 3 826 | B*4403(B44) | 4 459 | 7 256 | 5 638 | |||||||
| A*4301(A43) | 789 | 4 420 | 2 364 | B*4501(B45) | 5 725 | 9 535 | 7 646 | |||||||
| A*6601(A66) | 3 644 | 1 526 | B*4601(B46) | 3 924 | 6 491 | 4 130 | ||||||||
| A*6602(A66) | 1 395 | 789 | B*4701(B47) | 2 152 | 6 528 | 3 895 | ||||||||
| A*6801(A68) | 1 314 | 859 | B*4801(B48) | 1 254 | 4 365 | 2 716 | ||||||||
| A*6802(A68) | 2 078 | 1 276 | B*4901(B49) | |||||||||||
| A*6901(A69) | 1 964 | 917 | B*5001(B50) | 741 | ||||||||||
| A*7401(A74) | 723 | B*5101(B51) | 1 634 | 6 205 | 3 724 | |||||||||
| A*8001(A80) | 2 841 | 1 430 | B*5102(B51) | 2 082 | 5 251 | 3 579 | ||||||||
| A* alleles | 0 | 11 | 32 | 24 | B*5201(B52) | 1 538 | 4 524 | 2 728 | ||||||
| B*5301(B53) | 6 270 | 8 807 | 7 323 | |||||||||||
| B*5401(B54) | 3 122 | 5 556 | 4 153 | |||||||||||
| B*5501(B55) | 1 326 | 2 829 | 1 887 | |||||||||||
| B*5601(B56) | 1 386 | 777 | ||||||||||||
| B*5701(B57) | ||||||||||||||
| B*5703(B57) | 1 229 | 600 | ||||||||||||
| B*5801(B58) [R] | 7 525 | 10 160 | 8 047 | |||||||||||
| B*5901(B59) | 1 211 | 5 646 | 3 001 | |||||||||||
| B*6701(B67) | 675 | |||||||||||||
| B*7301(B73) | 1 271 | 3 347 | 2 171 | |||||||||||
| B*7801(B78) | 3 373 | 6 089 | 4 597 | |||||||||||
| B*8101(B81) | 1 352 | 729 | ||||||||||||
| B*8201(B82) | 2 195 | 4 367 | 3 069 | |||||||||||
| B* alleles | 0 | 37 | 48 | 44 | ||||||||||
| Letters J and R after HLA class I alleles refers to the typing of the donors who have provided T cells for this study. | ||||||||||||||
| (c) The number of HLA-A, HLA-B, HLA-Cw alleles recognized by type 8 mAbs TFL-006 and TFL-007 compared with the number of HLA alleles recognized by different therapeutic preparations of intravenous immunoglobulin (IVIg) | ||||||
|---|---|---|---|---|---|---|
| IVIgs versus TFL mAbs | Reactivity of different HLA class I antigens | |||||
| Classical HLA-Ia alleles | Non-classical HLA-Ib | |||||
| HLA-A | HLA-B | HLA-Cw | HLA-E | HLA-F | HLA-G | |
| IVIg (GamaStan, USA) | 31 | 49 | 16 | + | + | + |
| IVIg (Octagam, Mexico) | 30 | 47 | 16 | + | + | + |
| IVIg (GlobEx, India) | 20 | 39 | 16 | + | + | + |
| TFL-006 (formerly PTER006) | 31 | 48 | 16 | + | + | + |
| TFL-007 (formerly PTER007) | 26 | 44 | 16 | + | + | + |
| TFL-037 (formerly PTER037) | 11 | 37 | 14 | + | 0 | 0 |
| TFL-033 (formerly PTER033) | 0 | 0 | 0 | + | 0 | 0 |
Fig 1.
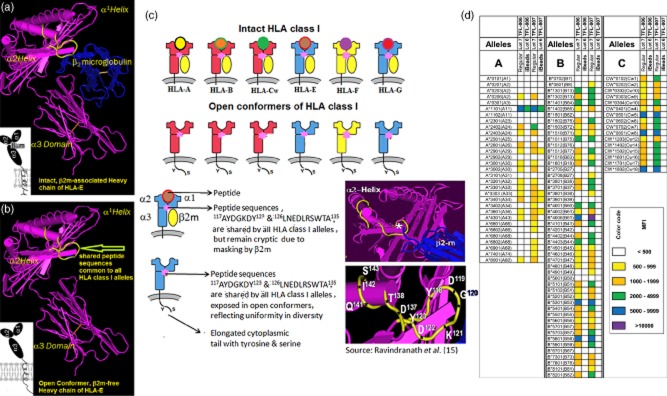
Dual expression of human leucocyte antigen (HLA) class I molecules on the cell surface of activated T lymphocytes. (a) An intact HLA class I occurs as HLA heavy chain (HC) polypeptides associated with β2-microglobulin (β2m). (b) On the cell surface of activated lymphocytes, HLA class I molecules also occur as open conformers or β2m-free HC. All HLA class I alleles share certain amino acid sequences, notably 117AYDGKDY123 and 126LNEDLRSWTA135. Although the shared peptides are present in the HC (shown in yellow), they are cryptic in native conformation due to masking by β2m. In open conformers, the shared peptide sequences are exposed to interact with other cell surface ligands and antibodies. (c) Diagrammatic comparison of intact and open conformers of different HLA class Ia (classical) and Ib (non-classical) alleles. (d) Anti-HLA-E monoclonal antibodies (mAbs) Terasaki Foundation Laboratory (TFL)-006s and TFL-007s recognized [as determined by mean fluorescence intensity (MFI)] regular HLA beads coated with both β2m-associated and β2m-free HCs of HLA (open conformers) but not iBeads, on which β2m-associated intact HLA predominated. These mAbs did not recognize intact HLA (as shown in Fig. 1a,c), but only β2m-free open conformers. The only HLA allele recognized by the mAbs on the iBeads was A*1101. We ascribe that recognition to the presence of more open conformers of HLA on the regular Beads than β2m-associated HLA. Intravenous immunoglobulin (IVIg) also recognized regular beads more readily than it did iBeads, but different therapeutic preparations differed in their MFI intensity 12. As negative control, we used culture media that was negative for both regular beads and iBeads.
Two preparations of each mAb were used to suppress activated T cells. The first consisted of culture supernatants from the clones cultured in a medium containing RPMI-1640 w/L-glutamine and sodium bicarbonate (cat. no. R8758; Sigma-Aldrich, St Louis, MO, USA), 15% fetal calf serum (FCS) 0·29 mg/ml L-glutamine/Penn-Strept (cat. no. 400-110; Gemini-Bio, MedSupply Partners, Atlanta, GA, USA) and 1 mM sodium pyruvate (cat. no. S8636; Sigma). Five hundred μl aliquots of the supernatants stored at −20°C were used in the investigation. Isotypes of all the mAbs were characterized, and no IgM antibodies were detected. The second preparation consisted of ascitic fluid; the ascites were produced in Balb/c mice. Four million cells were injected per mouse into the peritoneal cavity of each mouse, with the ascites recovered after 10 days. Approximately 5–6 ml of ascites were collected, centrifuged and stored at −20°C as 500 μl aliquots.
The IgG in the culture supernatant (400 μl) and ascites (150 μl mixed with 150 μl of PBS pH 7·0) were purified by passing each aliquot of supernatant or ascites through a Protein G Spin Kit (0·2 ml, cat. no. 89949; Thermo Scientific, Waltham, MA, USA). The TFL IgG mAbs were purified with both commercial and in-house buffers. The commercial buffer contained 0·02% sodium azide, so the mAbs were either used after extensive dilution, as recommended 34–36 in the initial experiments, or the interference of sodium azide was avoided completely by using in-house buffers (binding buffer, 50 mM sodium acetate, pH 7; elution buffer, 100 mM glycine, pH 2·8; and neutralizing buffer, 1 M Tris-HCl, pH 8·5) without any NaN3.
The purified antibodies were lyophilized overnight at 37°C, using Speedvac (Thermo Scientific). The purified culture supernatants were tested for HLA reactivity. The protein concentrations of the mAbs were measured with a BioPhotometer (Eppendorf, Hauppauge, NY, USA). In particular, the purified mAbs were diluted with AIM-V® medium (cat. no. 12055-083; Life Technologies-Gibco, Grand Island, NY, USA) with 1% HEPES, with an optimal pH range between 7·2 and 7·5. Sodium azide-free purified and lyophilized ascites and purified culture supernatants were used to study the immunomodulation of T cells.
Assessment of HLA-reactivity of anti-HLA-E mAbs
Multiplex Luminex®-based immunoassay was used 12,14–18 to measure the mean fluorescent intensity (MFI) of culture supernatants, ascites, IVIg and purified anti-HLA-E mAbs (the latter two at different dilutions). Using dual-laser flow cytometry (Luminex xMAP® multiplex technology), the single antigen assays were carried out for data acquisition and analysis of anti-HLA-Ia and anti-HLA-E antibodies. The LABScreen® Single Antigen assay (One Lambda, Inc., Canoga Park, CA, USA) consists of a panel of colour-coded microspheres (single antigen beads), coated with HLA antigens to identify antibody specificities. The single recombinant HLA-Ia antigens in the lot used contained 31 HLA-A, 50 HLA-B and 16 HLA-Cw allelic molecules. The array of HLA alleles on the beads is listed in the product information at http://www.onelambda.com. The HLA-I microbeads have built-in control beads: positive control beads coated with human IgG and negative control beads coated with serum albumin. Mean and standard deviation (s.d.) of MFI for each allele were recorded as.csv files.
Distinguishing β2m-associated intact HLA from β2m-free open conformers
The beads supplied by the manufacturer have two categories of proteins attached to them: β2m-free HLA heavy-chain polypeptide and heavy-chain polypeptide in association with β2m. Realizing the heterogeneity of proteins, the manufacturer has recently developed iBeads (provided to us as Felix beads for in-house experimental use), which are regular HLA-Ia antigen-coated microbeads subjected to proprietary enzymatic treatment to remove or reduce the amount of open conformers, referred to by the manufacturer as ‘denatured antigens’. The MFI values in Fig. 1d reflect the correction of the experiments' trimmed mean values against negative control after normalization.
Isolation and processing of T lymphocytes
On the day of the experiments, 60 ml of whole blood from a healthy normal male donor was collected after obtaining the donor's consent and Institutional Review Committee approval. Human T lymphocytes were isolated from the whole blood, using Ficoll™-Hypaque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) to recover peripheral blood mononuclear cells (PBMCs), and using Lympho-Kwick® (One Lambda) to recover lymphocytes. The isolated lymphocytes were separated into two lots, one treated with phytohaemagglutinin (PHA) (cat no. L-1800-100; EY Laboratories Inc., San Mateo, CA, USA) at a final concentration of 2·25 μl/ml, the other with no PHA, and both cultured in 96-well tissue culture plates containing AIM-V medium with 1% HEPES, optimal range of pH between 7·2 and 7·5. IVIg or mAbs were added to cells in culture within 2 h of adding PHA (the total volume was adjusted to 200 μl), based on an earlier report that evaluated the effects of time difference in the addition of IVIg and other toxins after adding PHA 5. The timing of adding PHA is important, as a kinetic and stereological study on activation of human T lymphocytes established that [3H]-leucine uptake started as early as 12 h after culture initiation 37. Furthermore, the uptake is associated with protein synthesis and accumulation, a process that occurs during the second phase of T cell activation after the change in membrane lipid and cytoskeleton of T cells 38–40.
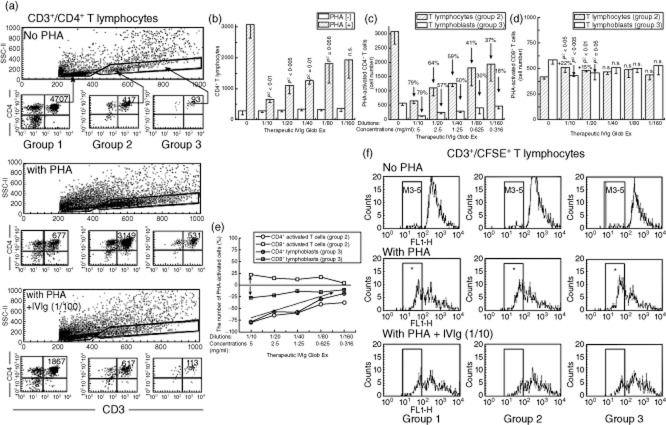
The number of cells after PHA activation or exposure to IVIg or mAbs was measured by flow cytometry after staining the cells with PE-labelled anti-CD4 and peridinin chlorophyll (PerCP)-labelled anti-CD3 and anti-CD8 mAbs. Fig. 2a presents a typical profile of CD3+ T lymphocyte categories and numbers in cultures after 72 h of exposure to PHA-P or only AIM-V medium (1% HEPES); this shows the population of activated CD4+ T lymphocytes. Both lots were exposed to IVIg or anti-HLA-E mAbs.
Fig 2.
Dose-dependent inhibition of phytohaemagglutinin (PHA)-activated CD3+/CD4+ T cells in vitro by a therapeutic preparation of intravenous immunoglobulin (IVIg) (GlobEx). (a) Flow cytometric profile of CD3+ T cells and gating of CD4+ T cells from a normal non-alloimmunized male (donor R). The lymphocytes were untreated as control, and treated as noted. The three sets of one large panel with three smaller panels show the profiles of CD3+/CD4+ T lymphocytes 72 h after culture without PHA (top set), after treatment with PHA (middle set) and after exposure to a therapeutic preparation of IVIg (GlobEx) and PHA (lower set). In each set, the large rectangular box shows the overall distribution patterns of CD3+/CD4+ T lymphocytes, which are divided into three groups based on CD4+ staining and size of cells to illustrate the differences in the CD4+ T cell population and number of events. Groups 1 and 2 are resting populations of CD4+ lymphocytes, whereas group 3 comprises lymphoblasts based on size and granularity. Note that the number of cells increased in both groups 2 and 3 after PHA treatment, reflecting their activation by PHA treatment. The bottom set of panels illustrates the impact of IVIg treatment on PHA-activated T cells. A comparison of the number of cells in groups 2 and 3 – between treatment with only PHA and with both PHA and IVIg – shows a marked decline in the number of CD4+ T cells after IVIg treatment. (b) Dose-dependent suppression of the activated total population of CD3+/CD4+ T lymphocytes by a therapeutic preparation of IVIg (GlobEx) in cultures with and without PHA. Examination was in triplicate after 3 days in the presence or absence of IVIg at different dilutions. The number of PHA-activated CD4+ T lymphocytes – but not the number of those control cells unexposed to PHA – decreased significantly (P-values above the bars) with the IVIg dilutions shown, except that the decrease with 1/160 dilution was not significant. Little change was observed in the control cells treated similarly with IVIg. (c) Dose-dependent suppression of both PHA-activated CD4+ T lymphocytes (group 2) and CD4+ T lymphoblasts (group 3) by the same therapeutic preparation of IVIg. (d) The therapeutic preparation of IVIg suppressed PHA-activated CD8+ T lymphoblasts (group 3) significantly (P-values indicated) only at dilutions of 1/10 and 1/20 but did not affect group 2 T lymphocytes. The population of CD8+ T cell cultures with and without PHA was examined in triplicate after 3 days in the presence or absence of IVIg at different dilutions. (e) A comparison of changes in the number of PHA-activated CD4+ and CD8+ cells among the T lymphocytes and T lymphoblasts showed that the effect of IVIg on CD4+ was more striking than on CD8+ T cells. (f) The same therapeutic preparation of IVIg suppressed proliferation of PHA-activated CD3+ T lymphocytes. Proliferation of carboxyfluorescein succinimidyl ester (CFSE)-labelled cells was examined in triplicate 3 days after treatments. The three rows provide a representative profile of the changes that occurred after incubating cells with CFSE when the cells were treated as noted. The IVIg was GlobEx. CFSE permeates the cells and is retained for more than 72 h. The left side in each box is indicated by a column marked in the upper row as ‘M3/4’ for mitoses 3 and 4, with mitosis 5 to its left; the right side of each box represents mitoses 1 and 2 (M1/2). The number of cells in each column was measured and compared for each treatment and group of T lymphocytes. Group 1 comprises resting T cells; group 2, T lymphocytes; and group 3, T lymphoblasts. In the top row (no PHA), the number of cells in the M3/4 column is very meagre in all three controls. The middle row (the PHA-treated groups) shows a quite opposite picture: cell numbers in in all three M3/4 columns are high, suggesting that cell proliferation occurred in all three T cell populations. The bottom row shows the impact of adding IVIg: the number of cells in the M3/4 column is highly reduced compared with that in the corresponding PHA-only columns, suggesting suppression of proliferation by IVIg. Decrease in the number of cells number in the right, M1/2, side of the boxes is a consequence of proliferation of CFSE-labelled cells; therefore, their migration to the left represents cells that underwent mitosis. All told, the data in this figure show that IVIg at the tested dilutions suppresses cell proliferation of PHA-activated CD3+ T lymphocytes and lymphoblasts.
Measurement of blastogenesis and proliferation of PHA-activated T cells
Blastogenesis of PHA-activated T cells was determined by counting the lymphoblasts after culturing purified lymphocytes from donors for 72 h with or (as control) without PHA. Lymphoblasts were recognized by flow cytometry based on size (side-scatter) and granularity (forward-scatter). In the tables and figures of flow cytometric profiles, three groups of T cells were identified for CD4+ and CD8+ T lymphocytes: groups 1 and 2, which comprise subsets of resting lymphocytes, and group 3, which comprises lymphoblasts.
The proliferation assay is based on labelling the purified lymphocytes during PHA activation with the intracellular fluorescent dye carboxyfluorescein succinimidyl ester (CFSE: C25H15NO9; mol. mass: 473·39 g/mol) and, using flow cytometry, measuring mitotic activity by the successive twofold reductions in fluorescent intensity of the T cells placed in culture for 72 h 38. CFSE is cell-permeable, and is retained for long periods within cells by covalently coupling by means of its succinimidyl group to intracellular molecules. Due to this stable linkage, once incorporated within cells, CFSE is not transferred to adjacent cells, but remains in the cell even after several mitotic divisions. After 72 h, the labelling of the cells was measured: PHA-treated T cells undergo, on average, four to six divisions. Most importantly, the suppressive effects of the agents used can be visualized by cessation of progression of mitotic activity, as measured by the successive twofold reductions in the fluorescent intensity after 72 h of treatment.
HLA molecular typing
DNA from the blood cells of donors was isolated by using the QIAamp DNA Mini Kit (Qiagen, Inc., Valencia, CA, USA). Extracted DNA was then PCR-amplified and typed using a LABType® SSO Typing Test Kit (One Lambda). Table 1b details the HLA typing of the T lymphocyte donors. The purpose of typing was to determine whether IVIg or anti-HLA-E mAbs (types 5 or 8) could recognize these typed alleles that occur as open conformers on activated T cells. Table 1b shows the donor HLA types that were recognized by TFL-037, TFL-006 and TFL-007 in square brackets ([ ]) after the alleles.
Results
Parallel diversity in HLA-Ia/-Ib reactivity of IVIg and anti-HLA-E mAbs TFL-006 and TFL-007
The HLA-Ia and -Ib reactivity of IVIg and two different anti-HLA-E mAbs were presented in our earlier report 12. A comparison of the HLA reactivity of different therapeutic preparations of IVIg with that of mAbs TFL-006 and TFL-007 and other anti-HLA-E mAbs used in the present study are presented in Tables 1a,b. Table 1a provides a classification of mouse mAbs generated after immunizing the mice with β2m-free heavy chains (open conformers) of two different HLA-E alleles (HLA-ER107 and HLA-EG107). Table 1b compares the number of HLA alleles recognized by the different preparations of IVIg and by the anti-HLA-E mAbs TFL-006, -007, -033 and -037; specific alleles were coated individually on microbeads.
In Table 1b, type 1 comprises monospecific mAbs, of which TFL-033 was studied in detail to show that it recognized HLA-E-restricted peptide sequences 33. Neither the purified culture supernatants nor purified ascites of TFL-033 reacted with HLA-A,-B or -Cw or with HLA-F or -G at any of the dilutions tested. TFL-033 was inhibited by two HLA-E-restricted peptides, 65RSARDTA71 and 143SEQKSNDASE152, but 143SEQKSNDASE152 showed dose-dependent inhibition of TFL-033s binding to the HLA-E coated on the microbeads 33. These two HLA-E-restricted peptide sequences were found on the surface of α1 (65RSARDTA71) and α2 (143SEQKSNDASE152) helices on the HC of intact HLA-E (Fig. 1a).
In striking contrast, type 8, which comprised the purified culture supernatants and purified ascites of mAbs TFL-006 and TFL-007, reacted with HLA-A, -B, -Cw, -E, -F and -G, suggesting that the mAbs recognized peptide sequences shared between HLA-E and HLA-A, -B, -Cw, -F and -G, as seen in the β2m-free HC of HLA-E (shown in yellow, Fig. 1b and as pink star in Fig. 1c). We reported earlier the inhibition of polyspecific HLA-E mAbs by these shared peptide sequences. The peptide binding domain of this group's mAbs was identified by inhibiting the mAb by peptide sequences of HLA-E shared with several HLA class Ia alleles, e.g. 117AYDGKDY123 and 126LNEDLRSWTA135 (14–16; see insert in Fig. 1c). In the present investigation, we compared the relative affinity of the TFL mAbs that mimic IVIg for regular beads and for iBeads. The HLA-Ia alleles on regular beads occur both as intact HLA (with β2m) and as β2m-free HCs, i.e. open conformers. Intact HLA predominates on iBeads, and they are considered to be restricted to intact HLA, the presence of open conformers so scant it can essentially be ignored. Figure 1d shows that both TFL-006 and TFL-007 had distinctly different reactivity to regular beads and iBeads. Strikingly, both of the anti-HLA-E mAbs showed reactivity to the regular beads and absence of reactivity to iBeads, except for reduced reactivity to HLA-A*1101, establishing the affinity of both TFL-006 and TFL-007 for the open conformers of all HLA-Ia.
The mAb TFL-037 is in a different category that, while also polyreactive, did not react with HLA-F or HLA-G, and therefore may not bind to the same shared peptide sequence as do TFL-006 and TFL-007. The peptide sequence of mAb TFL-037 remains to be defined.
Table 1c compares the HLA reactivity of the four mAbs with that of different therapeutic preparations of IVIg. Two different preparations of IVIg (GlobEx and Octagam) and the four different mAbs were used in this investigation to suppress proliferation of PHA-activated CD4+ T lymphocytes from two normal non-alloimmunized human males, donors R and J, R being about 30 years older than donor J.
IVIg suppressed blastic transformation of PHA-activated T cells
The efficacy of IVIg (GlobeEx) in suppressing PHA-activated CD4+ T lymphocytes is shown in Fig. 2a–f. Three major subpopulations of CD4+ T cells can be seen in fluorescence activated cell sorter (FACS) analysis (Fig. 2a), based on the forward- and side-scatters of the lymphocytes, which essentially distinguish the size of the cell population. The three subpopulations are designated group 1 (resting), group 2 (resting and possibly naive T lymphocytes) and group 3 (T lymphoblasts). In all groups, the density of cell population changed markedly depending on treatment. The number of group 2 T lymphocytes and T lymphoblasts increased after PHA treatment, the number of CD4+ T lymphoblasts increasing five- to sixfold over those that were not treated with PHA, and decreasing fivefold after treatment with IVIg. The number of activated T lymphocytes, but not of those unexposed to PHA, decreased significantly in a dose-dependent manner when treated with IVIg (Fig. 2b). The significant dose-dependent decrease is seen most distinctly in group 2 (CD4+ T lymphocytes) and group 3 (CD4+ T lymphoblasts) (Fig. 2c). Interestingly, no such dose-dependent decrease is seen with the same groups of CD8+ T cells (Fig. 2d) except for the CD8+ T lymphoblasts of group 3, which showed a decrease only at higher concentrations of IVIg (dilutions of 1/10 and 1/20). The differences in the dosimetric effects of IVIg on CD4+ and CD8+ in group 2 (T lymphocytes) and group 3 (T lymphoblasts) are summarized in Fig. 2e. It is noteworthy that only PHA-activated CD4+ T cells were suppressed significantly in a dose-dependent manner by IVIg.
IVIg suppressed proliferation of PHA-activated T cells
PHA activation involves proliferation of all subpopulations of CD3+ T lymphocytes as seen in the shift of CFSE+ cells from M1/2 to M3/5 (Fig. 2f). More than 95% of the CFSE+ cells not treated with PHA remained in M1/2 and did not migrate to the M3/4 zone, whereas PHA treatment resulted in their migration (see the M3/4-zone boxes marked with asterisks in Fig. 2f), indicating that proliferation is the outcome of PHA activation. IVIg inhibited proliferation, as shown by the fewer number of cells in the M3/5 zone (Fig. 2f). At the same time, and in contradistinction for subpopulations not treated with PHA, the number of undivided cells (M1/2) did not remain high but declined sharply, suggesting that the cells had undergone cell death.
To validate these observations, we used another therapeutic preparation of IVIg (Octagam) to examine in triplicate analyses whether the proliferation of CD4+ and CD8+ T lymphoblasts was suppressed by IVIg in a dose-dependent manner (Table 2). Indeed, significant dose-dependent suppression of the number of PHA-activated CD4+ T lymphoblasts occurred. There was also suppression of PHA-activated CD8+ T lymphoblasts at a higher concentration of IVIg, but not dose-dependent suppression. PHA treatment resulted in significant migration of CFSE+ CD4+ and C8+ T lymphoblasts to the M3/4 zone. Indeed, IVIg significantly inhibited both PHA-activated CD4+ and CD8+ T lymphoblasts.
Table 2.
Dose-dependent inhibition of phytohaemagglutinin (PHA)-activated CD4+ and CD8+ T lymphoblasts in vitro by a therapeutic preparation of intravenous immunoglobulin (IVIg) (Octagam)
| Octagam-IVIg | T lymphoblasts | |||||||
|---|---|---|---|---|---|---|---|---|
| Blastogenesis | Proliferation | |||||||
| M1/2 | M3–5 | |||||||
| Mean | s.d. | P2 | Mean | s.d. | Mean | s.d. | P2 | |
| CD4 | ||||||||
| No PHA | 46 | 8 | 73 | 17 | 1 | 1 | ||
| With PHA only | 1685 | 89 | <0·0001 | 338 | 15 | 1486 | 95 | <0·0001 |
| PHA + IVIg (1/10) | 945 | 87 | 0·0005 | 405 | 5 | 675 | 68 | 0·0003 |
| PHA + IVIg (1/20) | 1365 | 100 | 0·019 | 432 | 23 | 1105 | 71 | 0·005 |
| PHA + IVIg (1/40) | 1796 | 81 | n.s. | 464 | 31 | 1540 | 43 | n.s. |
| CD8 | ||||||||
| No PHA | 53 | 13 | 80 | 20 | 4 | 7 | ||
| With PHA only | 1951 | 171 | <0·0001 | 329 | 15 | 1751 | 193 | <0·0001 |
| PHA + IVIg (1/10) | 1134 | 13 | 0·001 | 431 | 115 | 893 | 9 | 0·002 |
| PHA + IVIg (1/20) | 1717 | 198 | n.s. | 380 | 17 | 1596 | 46 | 0·005 |
| PHA + IVIg (1/40) | 2280 | 127 | n.s. | 419 | 61 | 2036 | 137 | n.s. |
Significant P-values are indicated in bold.
The lymphocytes were from a normal non-alloimmunized male (donor J). The values represent the mean of triplicate analyses ± standard deviation (s.d.) and two-tailed P-values. The table shows the flow cytometric profile of the number of CD4+ and CD8+ T lymphoblasts when untreated (as control), and when treated as noted. The ‘Blastogenesis’ column shows the number of CD4+ or CD8+ T lymphoblasts 72 h after culture. Note that IVIg significantly suppressed PHA-activated CD4+ T lymphoblasts in a dose-dependent manner, whereas it suppressed PHA-activated CD8+ T lymphoblasts only at a 1/10 dilution. The ‘Proliferation’ column shows the M1/2 and M3–5 numbers of CD4+/carboxyfluorescein succinimidyl ester (CFSE)+ and CD8+/CFSE+ T lymphoblasts with the same treatment pattern as above after exposure for 72 h. Note that IVIg significantly suppressed proliferation (as seen by the M3–5 numbers) of both PHA-activated CD4+ and CD8+ T lymphoblasts in a dose-dependent manner; n.s. = not significant.
Anti-HLA-E mAbs (TFL-006 and TFL-007) suppressed the blastic transformation of PHA-activated T lymphocytes
These mAbs that mimic the HLA-I reactivity of IVIg suppressed both PHA-activated CD4+ and CD8+ T lymphocytes. Suppression by TFL-007 is evident from a comparison in Fig. 3a–d of the flow cytometric profiles of CD4+ and CD8+ T lymphocytes treated only with PHA, and treated with PHA and TFL-007s at 1/10 and 1/100 dilutions. Note that TFL-007s at both dilutions significantly suppressed the number of both CD4+ and CD8+ T lymphoblasts (see the statistical profile on the sides of Fig. 3a), while no such decrease in cell population was observed for the resting cells. Importantly, the CD8+ T lymphocytes showed significant dose-dependent decrease (Fig. 3d). Although the group 2 T lymphocytes also decreased, the decrease was significant only at 1/10.
Fig 3.
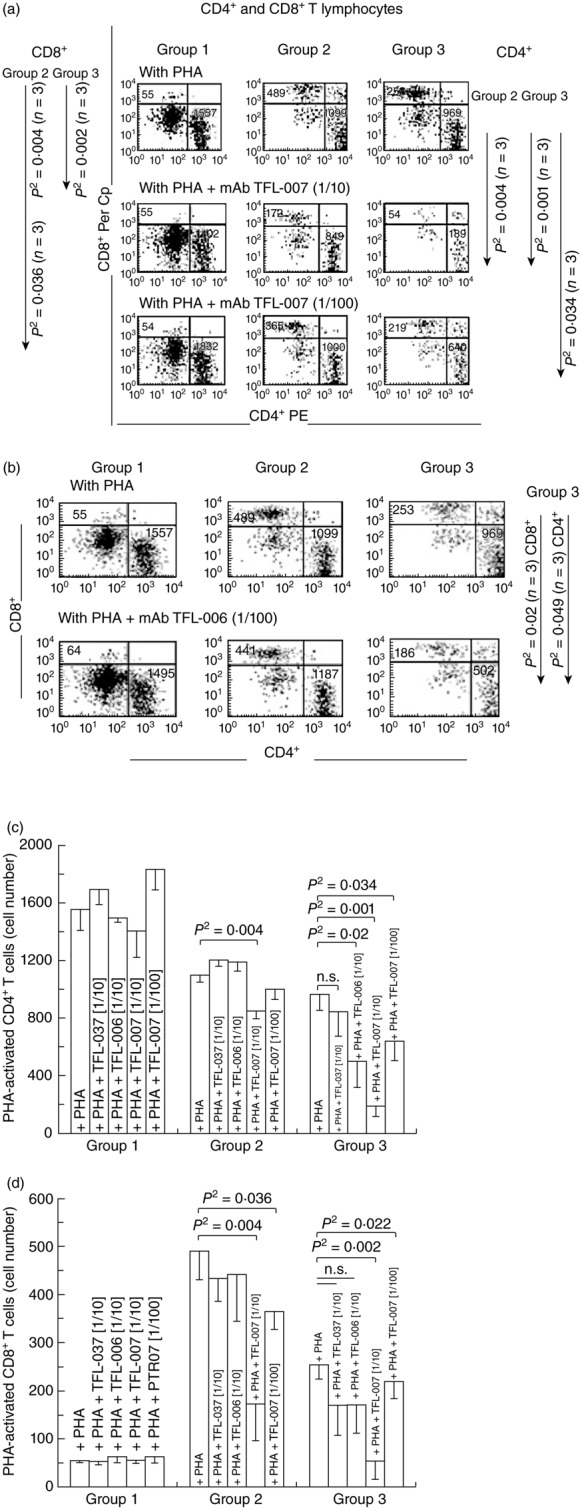
Dose-dependent inhibition of phytohaemagglutinin (PHA)-activated CD4+/CD8+ T cells in vitro with two human leucocyte antigen (HLA) class I polyreactive anti-HLA-E monoclonal antibodies (mAbs), Terasaki Foundation Laboratory (TFL)-007s and TFL-006s; ‘s’ = culture supernatants. The T cells were stained with phycoerythrin (PE)-labelled anti-CD4 mAbs (x-axis) and peridinin chlorophyll (PerCP)-labelled anti-CD8 mAbs (y-axis). The profile is divided into three groups based on staining and size of cells to illustrate the differences in the CD4+ and CD8+ T cell populations and number of events. Group 1 comprises resting CD4+ and CD8+ lymphocytes, group 2 resting CD4+ and CD8+ lymphocytes and group 3 CD4+ and CD8+ lymphoblasts. (a) Flow cytometric profiles of PHA-treated CD4+ T cells (lower right of the boxes) and CD8+ T cells (upper left) from a normal non-alloimmunized donor (R) after treatment with mAb TFL-007s. Comparison of groups 1, 2 and 3 in the top row (treated only with PHA) shows that the number of CD4+ T cells in all three groups and the CD8+ T cells in groups 2 and 3 were high. The middle row (PHA and mAb TFL-007s at 1/10 dilution) shows that the number of both CD4+ and CD8+ T cells have decreased significantly in groups 2 and 3 (note the statistical profile on the right side). In comparison, the bottom row with the same treatment, but at 1/100 dilution, shows a dose-dependent decrease in the number of PHA-activated CD4+ and CD8+ T lymphocytes. Figure represents one analysis. (b) Flow cytometric profiles of PHA-treated CD4+ T cells (lower right of the boxes) and CD8+ T cells (upper left) from the same donor after treatment with mAb TFL-006s. The top row (only PHA) shows that the number of CD4+ T cells and CD8+ T cells in groups 2 and 3 were higher than when the cells were treated with PHA and mAb TFL-006s at 1/100 dilution. Indeed, with that treatment, the number of both CD4+ and CD8+ T cells in group 3 decreased significantly (see the statistical profile). Figure represents one analysis. (c) The density of the population of CD4+ T cell cultures with and without PHA, and after adding TFL-037s at 1/10 dilution, TFL-006s (1/10) and TFL-007s (1/10 and 1/100) to the PHA-treated CD4+ T cells. The values are expressed as the mean of triplicate analyses ± standard deviation (s.d.) and two-tailed P-values. The two-tailed P-values, if significant, are indicated by a horizontal line connecting the two groups. Groups 1 and 2 comprise resting CD4+ lymphocytes, and group 3 CD4+ lymphoblasts. Note that all groups of the PHA-activated CD4+ T lymphocytes remained unaffected by treatment with mAb TFL-037s, and that only group 3 decreased because of treatment with mAb TFL-006s. Both PHA-treated groups 2 and 3 decreased significantly after treatment with mAb TFL-007s at 1/10, but only PHA-treated group 3 showed significant decrease after treatment with mAb TFL-007s at 1/100 dilution. Clearly, both anti-HLA-E mAbs TFL-006s and TFL-007s significantly suppress PHA-activated CD4+ T lymphoblasts, although the dosimetric suppression byTFL-007s is more obvious. (d) The density of the population of CD8+ T cell cultures with and without PHA, and after adding TFL-037s at 1/10 dilution, TFL-006s (1/10) and TFL-007s (1/10 and 1/100). The values are expressed as in (c) (triplicate analyses), with division into the same three groups, but of CD8+ cells. Note that all groups of the PHA-activated CD8+ T lymphocytes remained unaffected by treatment with mAb TFL-037s or mAb TFL-006s, but that the cell populations of both groups 2 and 3 decreased significantly after by treatment with mAb TFL-007s at both 1/10 and 1/100. The anti-HLA-E mAbs TFL-007s showed significant dose-dependent suppression of PHA-activated CD8+ T lymphoblasts.
The effect of anti-HLA-E mAb TFL-006s on PHA-treated T lymphocytes from the same donor was somewhat similar to that of TFL-007s (Fig. 3b–d). TFL-006s at 1/10 (Fig. 3c) and at 1/100 (Fig. 3b) dilution decreased the number of PHA-activated CD4+ and CD8+ T lymphoblasts. Further examination compared the effect of TFL-006s with that of TFL-006a, and found that both significantly suppressed both PHA-activated CD4+ and CD8+ T lymphoblasts in a dose-dependent manner (Table 3).
Table 3.
Dose-dependent inhibition of phytohaemagglutinin (PHA)-activated CD4+ and CD8+ T lymphoblasts in vitro by anti-human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) Terasaki Foundation Laboratory (TFL)-006s and TFL-006a
| CD4+ lymphoblasts | CD8+ lymphoblasts | ||||||
|---|---|---|---|---|---|---|---|
| TFL mAbs | Concentration | Mean | s.d. | P2 | Mean | s.d. | P2 |
| Culture supernatant | |||||||
| No PHA | 192 | 14 | 78 | 11 | |||
| PHA only | 1190 | 91 | 0·002 | 364 | 59 | 0·01 | |
| PHA + serum free AVIM medium | 1075 | 94 | n.s. | 376 | 27 | n.s. | |
| PHA + murine IgG (1/100) | 1033 | 92 | n.s. | 332 | 64 | n.s. | |
| PHA + TFL-006s (1/10) | 231 | 59 | 0·0003 | 70 | 25 | 0·007 | |
| PHA + TFL-006s (1/20) | 320 | 79 | 0·003 | 117 | 32 | 0·008 | |
| PHA + TFL-006s (1/40) | 575 | 63 | 0·004 | 204 | 20 | 0·03 | |
| PHA + TFL-006s (1/80) | 894 | 73 | 0·02 | 298 | 26 | n.s. | |
| PHA + TFL-006s (1/160) | 904 | 91 | 0·02 | 275 | 29 | n.s. | |
| Ascites | |||||||
| No PHA | 190 | 3 | 70 | 3 | |||
| PHA only | 1243 | 106 | 0·003 | 403 | 31 | 0·003 | |
| PHA + murine IgG (1/100) | 1330 | 166 | n.s. | 422 | 37 | n.s. | |
| PHA + TFL-006a (1/100) | 8·870 µg/ml | 478 | 193 | 0·008 | 176 | 75 | 0·02 |
| PHA + TFL-006a (1/200) | 4·435 µg/ml | 568 | 173 | 0·008 | 191 | 72 | 0·02 |
| PHA + TFL-006a (1/400) | 2·218 µg/ml | 588 | 195 | 0·01 | 207 | 67 | 0·02 |
| PHA + TFL-006a (1/800) | 1·109 µg/ml | 786 | 127 | 0·009 | 248 | 16 | 0·005 |
| PHA + TFL-006a (1/1600) | 0·555 µg/ml | 1499 | 158 | n.s. | 477 | 52 | n.s. |
The lymphocytes were from another normal non-alloimmunized male (donor R). The top set shows results with anti-HLA-E mAb TFL-006s in culture supernatant without purifying immunoglobulin (IgG). The bottom set shows results with a fresh batch of ascites without purifying IgG. The top two rows of cell numbers in both sets are with and without adding PHA, the following rows with PHA and various dilutions of TFL-006s/a. Note that the ‘s’ dilutions are from 1/10 to 1/160 and the ‘a’ dilutions from 1/100 to 1/1600. In the experiments involving ascites, the concentration of the stock solution of mAb TFL-006a was 887 µg/ml, which was appropriately diluted in 200 μl of medium in the culture wells. The concentrations at different dilutions show that the optimal concentration needed for significant suppression is about 1 μg/ml. All experiments were performed in triplicate. The significant values are expressed as mean ± standard deviation (s.d.) with two-tailed P-values. In both experiments, the mAb-treated PHA-activated CD4+ or CD8+ lymphoblasts decreased significantly in a dose-dependent manner. Clearly, both the anti-HLA-E mAbs TFL-006s and TFL-006a significantly suppress PHA-activated CD4+ and CD8+ T lymphoblasts dosimetrically. Purified mAb were diluted in 200 μl of culture media per well; n.s. = not significant.
To validate these observations, we replicated the above experiments in triplicate with lymphocytes from another normal non-alloimmunized male (donor J) to study the effect on CD4+ and CD8+ T lymphoblasts by TFL-006a and TFL-007a after purifying IgG from the ascites (Table 4) As with donor R's T lymphocytes, the number of CD4+ T lymphoblasts increased after PHA treatment, and when the PHA-activated CD4+ T lymphoblasts were exposed to the TFL mAbs at the dilutions listed in the table, a significant dose-dependent suppression was observed. Again, no such suppression was evident for group 1. Note that this donor's group 2 subpopulation did not respond to PHA.
Table 4.
Dose-dependent suppression of both blastogenesis and proliferation of CD4+ T lymphoblasts by anti- human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) Terasaki Foundation Laboratory (TFL)-006a and TFL-007a, as compared with Groups 2 and 3. The lymphocytes were obtained from donor J
| Resting (group 1) | Naive (group 2) | Lymphoblasts (group 3) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blastogenesis | Proliferation | Blastogenesis | Proliferation | Blastogenesis | Proliferation | ||||||||||||||||
| Mean | ± s.d. | M1/2 | M3–5 | Mean | ± s.d. | M1/2 | M3–5 | Mean | ± s.d. | M1/2 | M3–5 | ||||||||||
| CD4 | Concentration | Mean | ± s.d. | Mean | ± s.d. | Mean | ± s.d. | Mean | ± s.d. | **P2 | Mean | ± s.d. | Mean | ± s.d. | **P2 | ||||||
| TFL-006a | (stock: 887 µg/ml) | ||||||||||||||||||||
| No PHA | 3457 | 38 | 3432 | 22 | 89 | 18 | 3429 | 36 | 3480 | 37 | 6 | 6 | 39 | 6 | 53 | 13 | 1 | 1 | |||
| With PHA | 2584 | 108 | 1044 | 124 | 1685 | 38 | 783 | 138 | 656 | 132 | 147 | 10 | 1977 | 62 | <0·0001 | 320 | 3 | 1850 | 64 | <0·0001 | |
| 1/100 | 8·870 µg/ml | 2745 | 33 | 2371 | 24 | 486 | 38 | 1894 | 33 | 1869 | 34 | 57 | 14 | 934 | 69 | <0·0001 | 506 | 30 | 576 | 51 | <0·0001 |
| 1/200 | 4·435 µg/ml | 2583 | 55 | 1873 | 46 | 823 | 93 | 1440 | 55 | 1366 | 52 | 98 | 9 | 1259 | 105 | <0·0001 | 470 | 22 | 954 | 118 | 0·0003 |
| 1/400 | 2·218 µg/ml | 2476 | 123 | 1592 | 181 | 1017 | 56 | 1194 | 139 | 1110 | 142 | 104 | 12 | 1437 | 30 | 0·0002 | 485 | 27 | 1162 | 57 | 0·0002 |
| 1/800 | 1·109 µg/ml | 2493 | 5 | 1310 | 73 | 1327 | 68 | 1003 | 102 | 896 | 91 | 129 | 18 | 1660 | 110 | 0·01 | 404 | 20 | 1450 | 120 | 0·007 |
| 1/1600 | 0·555 µg/ml | 2542 | 50 | 1187 | 31 | 1494 | 86 | 880 | 49 | 773 | 27 | 131 | 20 | 1851 | 72 | n.s. | 390 | 21 | 1669 | 75 | 0·03 |
| TFL-007a | (stock: 627 µg/ml) | ||||||||||||||||||||
| No PHA | 3752 | 21 | 3686 | 25 | 129 | 10 | 3726 | 18 | 3767 | 15 | 7 | 3 | 34 | 5 | 40 | 6 | 3 | 3 | |||
| With PHA | 2856 | 133 | 1150 | 71 | 1814 | 169 | 850 | 118 | 750 | 102 | 122 | 29 | 2198 | 224 | <0·0001 | 333 | 15 | 2021 | 221 | <0·0001 | |
| 1/100 | 6·270 µg/ml | 2984 | 74 | 2524 | 101 | 550 | 47 | 2006 | 138 | 1975 | 137 | 56 | 11 | 1050 | 67 | 0·001 | 462 | 27 | 714 | 60 | 0·0006 |
| 1/200 | 3·135 µg/ml | 2886 | 68 | 2111 | 64 | 876 | 5 | 1587 | 87 | 1520 | 71 | 92 | 14 | 1372 | 106 | 0·0050 | 473 | 35 | 1072 | 38 | 0·0020 |
| 1/400 | 1·568 µg/ml | 2820 | 108 | 1735 | 29 | 1194 | 115 | 1164 | 36 | 1101 | 42 | 82 | 6 | 1789 | 132 | 0·05 | 521 | 71 | 1471 | 142 | 0·02 |
**The P-values were obtained for comparison of two parameters. First values obtained after phytohaemagglutinin (PHA) only is compared with values obtained with no PHA; secondly, the values obtained after TFl-006a or TFL-007a treatment were compared with values obtained with PHA only; purified mAb were diluted in 200 ml of culture media per well. The table shows the results after treatment with anti-HLA-E mAbs TFL-006a, then TFL-007a, both obtained from fresh batches of ascites after purifying IgG. The results shown are after treatment of cultures with and without PHA, and then after the PHA-treated cells were exposed to TFL mAbs at the listed dilutions. Note the change in number of cells in each group after PHA activation and the changes from M1/2 to M3–5. The increase in the M3–5 phase only after PHA-activation indicates that activation induces proliferation; these groups were examined for the effect of TFL mAbs in suppressing proliferation, with all experiments in triplicate. The numbers are expressed as mean ± standard deviation (s.d.) two-tailed P-values. Both TFL-006a and TFL-007a significantly decreased the number of PHA-activated CD+ lymphocytes, suppressing proliferation in a dose-dependent manner. No such suppression during either blastogenesis or proliferation was observed for the other groups. As in Table 3, the concentration of the stock solution of mAb TFL-006a was 887 µg/ml and that of TFL-007a was 627 µg/ml, both appropriately diluted in 200 μl of medium in the culture wells. The concentrations at different dilutions show that the optimal concentration needed for significant suppression by TFL-006a is 1–1·5 μg/ml, and that of TFL-007a is 5 μg/ml.
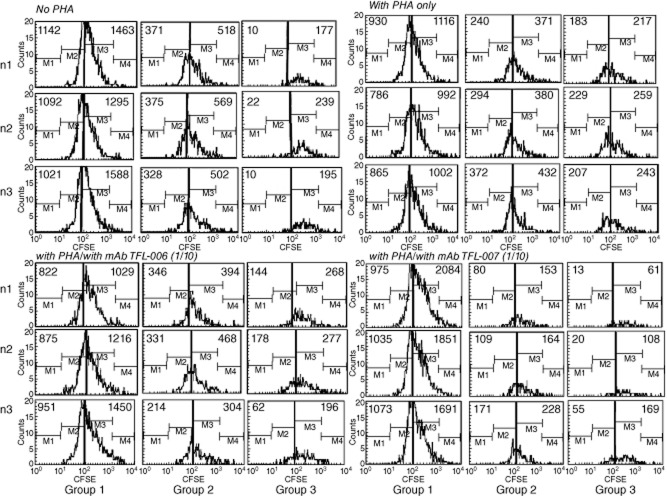
Anti-HLA-E mAbs (TFL-006 and TFL-007) suppressed proliferation of PHA-activated T cells
In Fig. 4a, CFSE-labelled lymphocytes cultured for 3 days without PHA were compared with those treated with PHA, those treated with PHA and mAb TFL-006s, and those treated with PHA and TFL-007s. Analysis was in triplicate. Proliferation was inferred when the number of cells decreased for mitoses 1 and 2 (M1/2) with a concomitant increase for mitoses 3–5 (M3–5). While the number of cells after M3–5 is very meagre when untreated with PHA, it is very high for M3–5 in PHA-treated groups, indicating that PHA significantly promoted T cell proliferation in all groups of CD4+ T lymphocytes. In fact, treatment with PHA and TFL-007s, or with PHA and TFL-006s, reduced the number of CD4+ T lymphoblasts in M3–5, indicating suppression of PHA-induced proliferation of CD4+ T lymphoblasts. No such decrease was observed for groups 1 and 2 T lymphocytes. A decrease in the CD4+ M1/2 cell population after treatment with TFL-007s or TFL-006s suggests possible cell death of T lymphoblasts after the addition of mAb. The suppression of CD4+ T lymphoblast proliferation caused by mAb TFL-007s was significant and dose-dependent, as shown in Fig. 4b, which compares the number of CD4+ T lymphoblasts that were not treated with PHA with their number when treated with PHA only, and when treated with PHA and TFL-007s at 1/10 and at 1/100 dilution. The mAb TFL-006s also suppressed proliferation of CD4+ T lymphoblasts significantly (Fig. 4c) and in a dose-dependent manner (data not shown). This suppression of proliferation was observed for group 2 T lymphocytes after treatment with TFL-007s but not with TFL-006s. Anti-HLA-E mAb TFL-006s and TFL-007s also suppressed proliferation of PHA-activated CD8+ T lymphoblasts (Fig. 5).
Fig 5.
Effect of Terasaki Foundation Laboratory (TFL)-006s and TFL-007s on proliferation of CD8+ T lymphocytes. The details are the same as in Fig. 4a, but for CD8+ T lymphocytes.
These experiments with T lymphocytes from another donor confirmed that proliferation of CD4+ T lymphocytes and T lymphoblasts was indeed affected by anti-HLA-E mAbs TFL-006 and TFL-007. The increase observed in M3–5 after PHA activation shows that PHA activation involves proliferation. Examination of the effects of TFL-006a and TFL-007a confirmed that these mAbs suppressed proliferation of PHA-activated CD4+ T lymphocytes and T lymphoblasts. Two-tailed P-values confirmed that both mAbs decreased the number of PHA-activated CD4+ lymphoblasts, thereby suppressing proliferation significantly and in a dose-dependent manner.
HLA-E or HLA-I mAbs that do not mimic HLA-reactivities of IVIg did not suppress blastic transformation and proliferation of PHA-activated CD4+ T lymphocytes
The anti-HLA-E mAbs TFL-006 and TFL-007 bound to several HLA-A, -B, -Cw, -E, -F and -G alleles in a manner strikingly similar to that of different therapeutic preparations of IVIg (Table 1c). In marked contrast, another anti-HLA-E mAb (TFL-033) proved monospecific, binding only to HLA-E and not to any other HLA alleles. TFL-033 recognized a peptide sequence different from those recognized by TFL-006 and TFL-007. Yet another anti-HLA-E mAb, TFL-037, bound to HLA-E and HLA-A, -B and -Cw but not to HLA-F or -G, suggesting that this mAb did not recognize the peptide sequences recognized by TFL-006 and TFL-007. These findings, involving different kinds of HLA-E mAbs, helped to determine whether suppression of proliferation of activated CD4+ T lymphocytes mediated by TFL-006 and TFL-007 is due to the binding of these mAbs to shared peptide sequences of HLA-I. In Table 5, the suppressive effects of HLA-E monospecific mAb TFL-033 and of anti-HLA-E mAb TFL-037, which is non-reactive to HLA-F and -G, are compared with those of IVIg-mimetic polyreactive anti-HLA-E mAbs TFL-006 and TFL-007, as well as control antibodies – human IgG1 isotype control mouse antibody and anti-HLA class Ia allelic mAbs ×2124, ×9123 and ×9133. While both TFL-006a and TFL-007a decreased the number of PHA-activated CD4+ T lymphoblasts significantly, neither the control mAbs nor TFL-033 nor TFL-037 suppressed those cells (Table 5, Figs 3d and 6a,b).
Table 5.
Comparison of the effect of control antibodies [mouse “anti-human IgG” antibody (isotype control)], anti-human leucocyte antigen (HLA) class Ia allele Abs, anti-HLA-E monospecific mAb Terasaki Foundation Laboratory (TFL)-033, and anti-HLA-E mAb TFL-037 (HLA-F- and G-non-reactive) versus intravenous immunoglobulin (IVIg)-mimetic anti-HLA-E monoclonal antibodies (mAbs) TFL-006s and TFL-007s on suppression of blastogenesis
| CD4 | Mean | SD | P2 value |
|---|---|---|---|
| Negative control mAbs (n = 3) | |||
| No PHA | |||
| Naive | 3454 | 106 | |
| T lymphoblasts | 263 | 14 | |
| With PHA | |||
| Naive | 1099 | 48 | <0·0004 |
| T lymphoblasts | 969 | 117 | 0·0005 |
| mouse “anti-human IgG” antibody (isotype control) | |||
| Naive | 1024 | 131 | n.s. |
| T lymphoblasts | 1758 | 84 | n.s. |
| Iλ HLA-Ia (A11, A43) mAb ×2124 (source: Nadim/TFL) | |||
| Naive | 924 | 11 | 0·02 |
| T lymphoblasts | 1758 | 84 | n.s. |
| Iλ HLA-Ia mAb ×9123 (source: Nadim/TFL) | |||
| Naive | 950 | 117 | n.s. |
| T lymphoblasts | 1435 | 276 | n.s. |
| Iλ HLA-Ia mAb ×9133 (source: Nadim/TFL) | |||
| Naive | 1032 | 54 | n.s. |
| T lymphoblasts | 1511 | 162 | n.s. |
| TFL HLA-E mAbs (n = 3) | |||
| No PHA | |||
| Naive | 3055 | 195 | |
| T lymphoblasts | 254 | 18 | |
| With PHA | |||
| Naive | 1099 | 48 | 0·0001 |
| T lymphoblasts | 969 | 117 | 0·0005 |
| PHA +/TFL-007 (1/100) | |||
| Naive | 1000 | 65 | n.s. |
| T lymphoblasts | 640 | 137 | 0·03 |
| PHA +/TFL-006 (1/100) | |||
| Naive | 1187 | 65 | n.s. |
| T lymphoblasts | 502 | 184 | 0·02 |
| PHA +/TFL-037 (1/100) | |||
| Naive | 1169 | 72 | n.s. |
| T lymphoblasts | 911 | 54 | n.s. |
| PHA +/TFL-033 (HLA-E monospecific mAb) (1/100) | |||
| Naive | 401 | 38 | n.s. |
| T lymphoblasts | 509 | 78 | n.s. |
All antibodies were obtained from culture supernatants, the CD4+ T lymphocytes from donor R. Treatment of cultures was with and without adding phytohaemagglutinin (PHA) (first two rows). The number of cells is shown for group 2 (T lymphocytes) and group 3 (T lymphoblasts) after exposing PHA-treated cells to the various control antibodies, and then to the TFL mAbs at 1/100 dilution. The values are expressed as mean ± standard deviation (s.d.) (n = 3) with two-tailed P-values for treatment with only PHA compared with no PHA, and with PHA plus mAb dilutions compared with only PHA. Note that both TFL-006a and TFL-007a decreased the number of PHA-activated T cells significantly while neither the control mAbs nor TFL-033, an HLA-E monospecific mAb, suppressed the blastogenesis. The data establish the importance of the Fab portion of the antibody in binding to the shared peptide sequences exposed on the open conformers of HLA class Ia and Ib alleles. The Fc portions of these and other mAbs did not suppress proliferation. These data also establish that open conformers of HLA class I may act as inhibitory ligands for activated T cells. See Table Ib for HLA-reactivities of the TFL-mAbs. 1λ refers to the original source of mAb as One Lambda, Inc.
Fig 6.
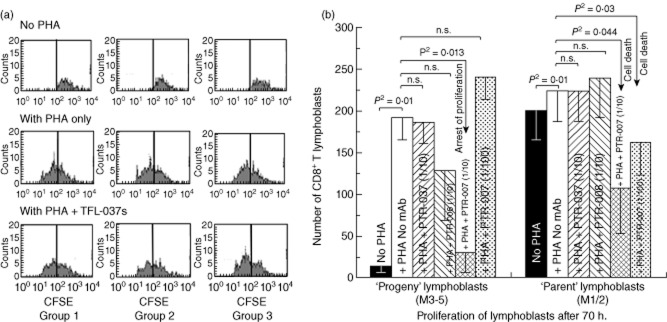
Anti-human leucocyte antigen (HLA)-E mAb Terasaki Foundation Laboratory (TFL)-037s, which did not mimic the HLA reactivity of intravenous immunoglobulin (IVIg) in that it did not recognize HLA-F and HLA-G, although it recognized the peptide sequences not shared by HLA-F and HLA-G, did not suppress phytohaemagglutinin (PHA)-induced proliferation of T lymphocytes. (a) TFL-037s did not affect PHA-induced proliferation of CD4+ subpopulations of T lymphocytes. After incubating cells with carboxyfluorescein succinimidyl ester (CFSE), treatment was as indicated above each row. The three groups of T cells are the same as described in Fig. 2. Note that in the upper row (no PHA) all cells are present in the right side of the sub-box and there are no lymphocytes in left sub-box (M3–5). With PHA only (second row), there is a very high number of CD4+ T lymphocytes in the M3–5 sub-boxes, indicating that proliferation had occurred in all groups. Addition of anti-HLA-E mAb TFL-037s did not affect the number of CD4+ T cells, suggesting that TFL-037s does not affect their proliferation. (b) Neither TFL-037 nor TFL-006 suppressed PHA-activated CD8+ T lymphoblasts, whereas TFL-007s reduced proliferation significantly in M3–5 (progeny lymphoblasts) and M1/2 (parent lymphoblasts).
The fact that the anti-HLA-E mAb TFL-037 did not suppress proliferation of CD4+ T lymphocytes or lymphoblasts was evident after CFSE-labelling (Fig. 6a,b).
Anti-HLA-E mAbs TFL-006 and TFL-007 had no effect on non-activated T lymphocytes
Every set of experiments on PHA-activated T lymphocytes included, as controls, purified T lymphocytes that were not exposed to PHA. Table 6 provides a typical profile of the effect of TFL-006 and TFL-007 on a donor's non-activated T cell population. The T lymphoblasts remained very few in number and were unaffected by the anti-HLA-E mAbs or by isotype control antibodies. It is interesting that anti-HLA-E antibodies, isotype control and, notably, AIM-V with 1% HEPES media control, per se, increased the number of CD8+ T cells in all three groups. Therefore, the increase observed in culture with different dilutions of mAb TFL-007 was due to AIM-V media rather than the mAb itself.
Table 6.
Effect of anti- human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) Terasaki Foundation Laboratory (TFL)-006s and TFL-007s, and of media and mouse isotype control antibodies on CD4+ and CD8+ T lymphocytes not activated by phytohaemagglutinin (PHA)
| Group 1, resting | Group 2, naive | Group 3, lymphoblasts | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 (n = 3) | CD8 (n = 3) | CD4 (n = 3) | CD8 (n = 3) | CD4 (n = 3) | CD8 (n = 3) | |||||||||||||
| Mean | s.d. | P2 | Mean | s.d. | P2 | Mean | s.d. | P2 | Mean | s.d. | P2 | Mean | s.d. | P2 | Mean | s.d. | P2 | |
| No PHA | 3669 | 110 | 739 | 18 | 3487 | 99 | 669 | 19 | 192 | 12 | 78 | 9 | ||||||
| Experimental | ||||||||||||||||||
| TFL-006s 1/10 | 3280 | 61 | 0·012 | 1071 | 48 | 0·001 | 3068 | 51 | 0·006 | 961 | 41 | 0·001 | 235 | 23 | n.s. | 117 | 7 | 0·008 |
| TFL-006s 1/20 | 3258 | 218 | n.s. | 944 | 86 | 0·03 | 3033 | 182 | 0·036 | 861 | 87 | 0·037 | 241 | 40 | n.s. | 89 | 12 | n.s. |
| TFL-006s 1/40 | 3394 | 225 | n.s. | 1012 | 36 | 0·001 | 3175 | 244 | n.s. | 933 | 33 | 0·001 | 236 | 20 | n.s. | 88 | 8 | n.s. |
| TFL-006s 1/80 | 3415 | 199 | n.s. | 990 | 49 | 0·002 | 3243 | 174 | n.s. | 927 | 43 | 0·002 | 187 | 30 | n.s. | 67 | 9 | n.s. |
| TFL-006s 1/160 | 3637 | 30 | n.s. | 982 | 32 | 0·001 | 3464 | 22 | n.s. | 924 | 27 | 0·0004 | 188 | 16 | n.s. | 62 | 10 | n.s. |
| TFL-007s 1/10 | 2881 | 47 | 0·001 | 858 | 20 | 0·004 | 2723 | 33 | 0·0005 | 792 | 32 | 0·01 | 176 | 16 | n.s. | 71 | 10 | n.s. |
| Controls | ||||||||||||||||||
| Media control | 3621 | 12 | n.s. | 1181 | 67 | 0·0004 | 3325 | 13 | n.s. | 1040 | 50 | 0·0003 | 315 | 10 | 0·0003 | 152 | 15 | 0·002 |
| Mouse IgG control 1/100 | 3294 | 132 | 0·03 | 914 | 59 | 0·01 | 3111 | 122 | 0·02 | 829 | 49 | 0·007 | 198 | 14 | n.s. | 205 | 14 | n.s. |
Significant P-values are indicated in bold.
T lymphoblasts were not affected by the mAbs at any dilution, whereas the CD8+ T cell populations of groups 1 and 2 showed marked increase after mAb treatment as well as with media and isotype controls. AIM-V culture medium with 1% HEPES may be responsible for the increase in media control as well as in isotype and mAbs that were diluted in AIM-V culture medium with 1% HEPES. At higher concentrations of TFL mAbs (1/10 and 1/20), a marginal decrease in CD4+ lymphocytes was observed in groups 1 and 2 and also in the isotype controls.
Discussion
The results of this investigation show the differential effects on the suppression of PHA-activated T lymphocytes by IVIg, by anti-HLA-E mAbs that mimic the HLA class Ia and Ib reactivity of IVIg (TFL-006 and TFL-007), and by anti-HLA-E mAbs that do not mimic that reactivity (TFL-033 and TFL-037). The mAbs TFL-006 and TFL-007 proved to be more potent suppressors of blastogenesis and proliferation of activated CD4+ T lymphocytes than IVIg. The concentration of anti-HLA-E mAbs required for this suppression was as much as 150-fold lower than the required concentration of IVIg. Neither IVIg nor the mAbs TFL-006 and TFL-007 affected the non-activated CD4+ T cell population (Fig. 2b, Table 6).
T cell activation occurs in three successive steps 39. The first involves changes in the cell's lipids (metabolism of arachidonic acid) and cytoskeleton. These changes modify the physical properties of the T cell membranes to modulate the activity and expression of membrane proteins 40. The second step involves initiation of transcription leading to the production of lymphokines with an increase in RNA and protein content (mitotic phase G1). Consequently, cytoplasmic volume increases and the nuclear/cytoplasmic ratio decreases. The final step involves DNA replication (phase S–G2) leading to mitosis and proliferation. Strikingly, the entire event occurs within 12 h and reaches maximum activation by 48 h 38.
Both blastic transformation and proliferation result in transitory cell-surface expression of several molecules. These include: IL-2R 41–43; Fc receptors for IgG (FcγRI/CD64, FcγRII/ CD32 and FcγRIII/CD16 44; IgE (FcεRII)/CD23) 45; insulin receptors; insulin-like growth factor 1R and IL-2R 46; alpha-fetoprotein and transferrin receptors 47; a non-disulphide-linked heterodimer of polypeptide chains 33 kDa and 38 kDa called ‘Me14/D12’ 48; MICA 49; HLA class II antigens HLA-DR, -DP and -DQ 50–52; and, most importantly, the over-expression of β2m-free heavy chains of HLA class I, called ‘open conformers’ 19–21,25.
Our observations of IVIg dose-dependent suppression of PHA-activated human CD4+ T lymphocytes accord with previous reports. Such suppression was observed in separate in-vitro cell cultures after activation by PHA, by anti-CD3 antibody, by tetanus toxoid pokeweed mitogen or by allogenic mixed cells 3,53. Similar IVIg-mediated suppression of proliferation of activated T cells was observed in vivo 8,54. Although suppression of proliferation of activated T cells was noticed in patients with multiple sclerosis, IVIg did not cause cell death as determined by caspase activation, DNA fragmentation or CD95 blockade or Bcl-2 8. This leads to the inference that the therapeutic preparations of IVIg down-regulated activated T cells through suppression of blastogenesis and proliferation rather than by modulation of cell death.
Our earlier report 12, that IVIg HLA-reactivity was lost when the HLA-E Ab reactivity of IVIg was adsorbed out, led us to develop mAbs against HLA-E that might mimic the HLA-Ia and HLA-Ib reactivity of IVIg. Of the many anti-HLA-E mAbs developed, some (e.g. TFL-006 and TFL-007) indeed mimicked the HLA-I reactivity of IVIg; others were either monospecific, in that they recognized only HLA-E (e.g. TFL-033), or they recognized only a few HLA class I alleles (e.g. TFL-037). Inhibition of the binding of the anti-HLA-E mAbs TFL-006 and TFL-007 to HLA-E-coated beads by the most common and accessible shared peptide sequences1 located on the α2 helix – HLA-E (117AYDGKDY123 and 126LNEDLRSWTA135) 14–16,18 (Fig. 1c; Table 7) – confirmed that these anti-HLA-E mAbs recognized the shared amino acid sequences or epitopes on other HLA class I molecules. The ability of TFL-006 and TFL-007 to bind to regular beads but not to iBeads, which are coated mainly with intact HLA-Ia, and the suppression of blastogenesis and proliferation by TFL-006 and TFL-007, but not by mAbs TFL-033 or TFL-037, which do not bind to shared peptide sequences of open conformers, perhaps because they do not recognize the shared peptide sequences recognized by TFL-006 and TFL-007, further confirmed that TFL-006 and TFL-007 bind to the shared peptide domains on open conformers. Furthermore, failure of IVIg (Fig. 2b) or TFL-006 and TFL-007 (Table 6) to influence the number of non-activated CD4+ T cells, which do not over-express open conformers 19–21,25, lends support to the hypothesis that IVIg and TFL-006 and TFL-007 suppress activated T cells by binding to the open conformers. Figure 1a–c illustrates that the shared peptide sequences of HLA class Ia and Ib (shown by the yellow line in the structure, Fig. 1a,b, and by the pink star on all HLA alleles in Fig. 1c) are masked by β2-microglobulin in intact HLA but are exposed on the open conformers of HLA class I. These figures are key to understanding the binding site of anti-HLA-E mAbs that mimic IVIg. Previous studies reported that T cells, upon activation, over-express open conformers on the cell surface 19–21,25. The HLA class I open conformers generated on the surface of activated T lymphocytes carry the binding site for IVIg as well as for anti-HLA-E mAbs, i.e. the peptide sequences shared by HLA-A, -B, -Cw, -E, -F and -G (Fig. 1c).
Table 7.
Amino acid sequences of human leucocyte antigen (HLA)-E that are E-restricted and those shared by one or more HLA-Ia and Ib alleles (extracted and modified from references 14,16,70)
| Peptide sequences of two HLA-E alleles | Number of HLA alleles | ||||||
|---|---|---|---|---|---|---|---|
| Classical class Ia | Non-classical class Ib | ||||||
| HLA-E107G & HLA-E107R | No. of amino acids | A | B | Cw | F | G | Specificity |
| 65RSARDTA71 | 7 | 0 | 0 | 0 | 0 | 0 | E-restricted |
| 90AGSHTLQW97 | 8 | 1 | 10 | 48 | 0 | 0 | Polyspecific |
| 102ELGPDR(G)RF109 | 8 | 0 | 0 | 0 | 0 | 0 | E-restricted |
| 115QFAYDGKDY123 | 9 | 1 | 104 | 75 | 0 | 0 | Polyspecific |
| 117AYDGKDY123 | 7 | 491 | 831 | 271 | 21 | 30 | Polyspecific |
| 126LNEDLRSWTA135 | 10 | 239 | 219 | 261 | 21 | 30 | Polyspecific |
| 137DTAAQI142 | 6 | 0 | 824 | 248 | 0 | 30 | Polyspecific |
| 137DTAAQIS143 | 7 | 0 | 52 | 4 | 0 | 30 | Polyspecific |
| 143SEQKSNDASE152 | 10 | 0 | 0 | 0 | 0 | 0 | E-restricted |
| 163TCVEWL168 | 6 | 282 | 206 | 200 | 0 | 30 | Polyspecific |
Position 107 may have either [R] or [G]; only peptides with six or more amino acids are shown in the Table.
From the finding that TFL-006 and TFL-007 suppressed the blastogenesis and proliferation of PHA-activated CD4+ T cells, but that TFL-033 and TFL-037 did not, we inferred that the suppression may involve binding of the Fab portion of those anti-HLA-E mAbs that mimic the HLA class I reactivity of IVIg or, of course, those present in IVIg itself, to the shared peptide epitopes exposed on the T cells' open conformers. The same suppressive mechanism would apply to IVIg, although previous investigators attributed the suppression of activated T cells by IVIg to the Fc portion of the antibodies binding to Fcγ receptors that are generated on the cell surface of T cells upon activation 54–57. The fact that neither the mAbs TFL-033 and TFL-037 nor the mouse isotype control IgG mixture of antibodies suppressed blastogenesis and proliferation of T lymphocytes does not support the contention that interaction between the Fc portion of Abs and Fcγ receptors contributes to the suppression of PHA-activated T cells. Another study cited ‘[i]nhibition of immunoglobulin production in vitro by F(ab′)2 fragments, but not by the Fc portion of a monoclonal antibody’ 58. Furthermore, while addressing the questions of whether IVIg-mediated inhibition is a result of purification processing of IgG, of donor pooling or of the intrinsic down-regulatory activity of IgG, MacMillan et al. compared the effect of the Fab fragment of IgG with that of IgG isolated from single-donor plasma on T cell proliferation by IVIg 9. The addition of Fab fragments of IgG to IgG-depleted plasma in culture reduced T cell proliferation significantly, establishing that the Fab region is sufficient to mediate this inhibition. It appears that the Fab portion of an antibody is capable of arresting different signalling pathways of lymphocytes. However, the investigators did not specify the Fab portion of any particular kind of IgG, such as that of Fab reacting to the shared peptide sequences of HLA class I alleles. In another study, the presence of IVIg's anti-CD4 activity was also implicated in the inhibition of T cell activation and functions 59.
Based on previous literature, the events leading to activation are illustrated by Fig. 7a–c. They show the transformation of resting T cells (Fig. 7a) to activated T cells (Fig. 7b) under the influence of PHA. PHA activation initiates phosphorylation of the cytoplasmic domain of CD3, resulting in signal transduction leading to activation of transcription factors 60, transcription and production of cell surface molecules such as IL-2Rα 41–43 and open conformers of HLA class I 19–21,25 (Fig. 7b,c). If the binding by mAbs TFL-006 and TFL-007 is responsible for the suppression of blastogenesis and proliferation, it may involve reversal of phases of activation of T lymphocytes, mediated possibly by signal transduction. In support of such an inference, several reports have clearly documented the elongation of the cytoplasmic tail of the HLA class I open conformers and the exposure of otherwise cryptic tyrosine320 61–64 and serine335 65 with a provision for phosphorylation (Fig. 7c). Although serine335 is generally considered the primary site of phosphorylation in this tail, phosphorylation of tyrosine320 has been indicated as that site by others 64,66. Either way, what is most important is that the open conformers in activated normal human T cells are associated with tyrosine phosphorylation and are capable of enabling cis interactions with cell surface receptors or other signalling molecules 65–69. The phosphorylation mediated by TFL mAbs may result in dephosphorylation of the cytoplasmic tails of CD3 molecules by activating phosphatases (Fig. 6c), leading to the arrest of transcription factors and synthesis of the proteins involved in blastogenesis and mitosis. These events, which are portrayed in Fig. 7 based on reports by other investigators, suggest that suppression of activation of T cells could be due to the binding of anti-HLA-E mAbs that mimic the HLA-reactivity of IVIg to the shared amino acid sequences exposed on the open conformers of HLA class I. This activation can involve any or all molecules of HLA class I open conformers expressed on the surface of T lymphocytes, as illustrated in Fig. 1c. If so, then the anti-HLA antibodies that recognize the shared peptides on the open conformers, and that occur naturally in the sera of normal, healthy and non-alloimmunized males 15,70, may play a novel role in regulating T lymphocytes that are activated in vivo during infection, inflammation, autoimmune diseases and cancer. The implications of our study also suggest a novel functional role for the natural anti-HLA antibodies occurring in healthy individuals that recognize the shared peptide sequences on the open conformers of HLA in regulating T lymphocytes activated in vivo.
Fig 7.
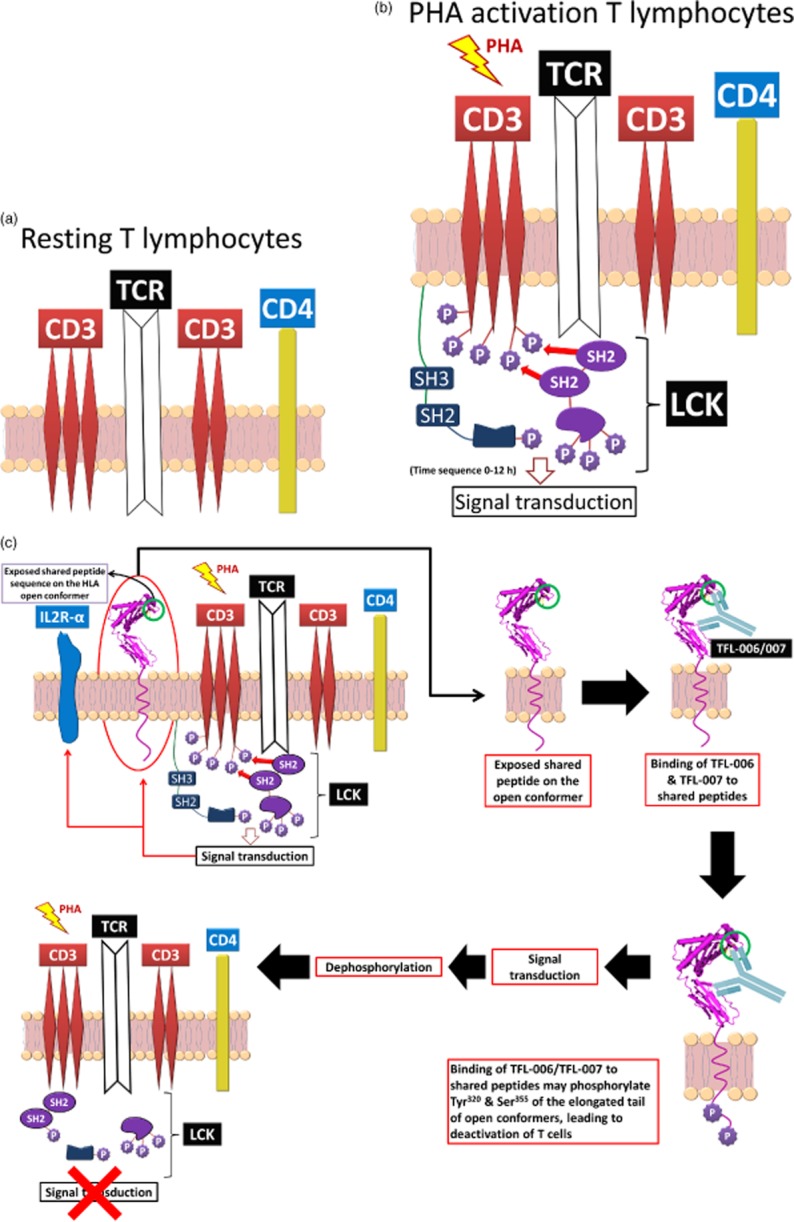
A model (developed based on Ref. 60) illustrating the possible mechanism underlying phytohaemagglutinin (PHA)-activation of T cells and the suppression of activated T cells mediated by polyspecific anti-human leucocyte antigen (HLA)-E monoclonal antibodies (mAbs) (Terasaki Foundation Laboratory (TFL)-006 and TLF-007) and possibly by intravenous immunoglobulin (IVIg). (a,b) Based on a model proposed by Mustelin, Vang and Bottini 60 for T cell activation. (a) Illustrates the structure of CD3/T cell receptor (TCR)/CD4 on the lipid raft (pink zone) of the bi-layered lipid membrane in the non-phosphorylated non-activated CD4+ T cells. Non-activated CD4+ T cells are least affected by either IVIg (Fig. 2b) or TFL-006 and TFL-007 (Table 6). (b) Protein tyrosine kinase exemplified by lymphocyte-specific protein tyrosine kinase (LCK)-induced phosphorylation of tyrosine-based activation in the cytoplasmic domain of CD3, which leads to activation of transcription factors and transcription of cell surface molecules such as interleukin (IL)-2Rα and open conformers of HLA class I. SH-1, SH-2 and SH-3 represent family members of Src homology; they are involved in mediating the cytoplasmic domain of CD3. Further activation of the tyrosyl-phosphorylated motifs then interact with SH-1 domains within the protein kinase LCK, leading to further signaling function 60,69. (c) Cell surface expression of IL-2Rα 43 and open conformers of HLA class I 13,24–32. Importantly, the exposure of shared amino acid sequences of all the HLA open conformers is indicated by a circle. It is this site that is recognized by TFL-006 and TFL-007. Possible interaction and consequences of recognition of the shared peptide sequences by the anti-HLA-E antibodies are illustrated in three steps: first, the exposure of the shared peptide sequence on the open conformer; secondly, recognition of the shared epitopes on the open conformer by anti-HLA-E mAbs; thirdly, possible phosphorylation of the elongated cytoplasmic tail of open conformers. That elongation results in exposure of cryptic tyrosine (Tyr320) 54–57 and serine (Ser355) 65 residues in the cytoplasmic tail. It may be the binding of anti-HLA-E mAbs to the shared peptide sequences that initiates the phosphorylation leading to signal transduction. A final step involves initiation of dephosphorylation of the cytoplasmic domain of CD3, resulting in arrest of activation or suppression. That seems plausible, as the phosphorylation is known to be reversible.
To sum up, anti-HLA-E mAbs TFL-006 and TFL-007, which mimic the HLA-I reactivity of IVIg, significantly suppressed both blastogenesis and proliferation of activated T lymphocytes in vitro in a dose-dependent manner. Furthermore, their suppressive ability was superior to that of IVIg because the mAbs required concentrations as much as 150-fold lower than the concentrations IVIg required for suppression. These findings, together with the recent report (71) that the anti-HLA-E mAb TFL-007 is also capable of suppressing allo-HLA IgG production in vitro much more efficiently than the therapeutic preparations of IVIg, strengthens the contention that the TFL anti-HLA-E mAbs can serve as IVIg mimetics.
It is most likely the monoclonality of the mAbs TFL-006 and TFL-007 and their F(ab′)2 binding to the shared peptide domains of the open conformers of HLA-I that account for their suppressing T cell proliferation better than IVIg polyclonal antibodies, which are admixed with several other antibodies, anti-idiotypic antibodies and antigens, and whose binding mechanism (hence suppressive mechanism) remains unclear.
In conclusion, suppression of antigen-specific activated T cells is highly desirable with autoimmune diseases, with transplant recipients and patients waiting for allografts. Thus, anti-HLA-E mAbs TFL-006 and TFL-007, humanized or chimerized, may prove a better suppressive agent than IVIg. Clinical trials can elucidate the question and help to establish that the anti-HLA-E mAbs reacting to HLA-I open conformers as a substitute for IVIg.
Acknowledgments
Octagam IVIg was provided by Dr Luis Eduardo Morales-Buenrostro, Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubiran, Mexico); IVIg-GlobEx was provided by Dr Miyuki Ozawa, One Lambda, Inc. The entire research project is supported by grants from a non-profit organization, the Terasaki Family Foundation, mediated through the Terasaki Foundation Laboratory. The Terasaki Foundation Laboratory has filed a patent, a part of which includes some of the data presented in this paper with funding support from the Terasaki Family Foundation.
Footnotes
Extensive search for the peptides at http://blast.ncbi.nlm.nih.gov/Blast.cgi?%20PROGRAM=blastp&%20PAGE_TYPE%20=BlastSearch&LINK%20LOC=blasthome revealed that the peptides are restricted only to MHC class I heavy chains.
Author contributions
M. H. R. designed the entire study, performed the experiments, collected and validated extensive data, developed and characterized the anti-HLA-E mAb TFL-007, analysed the HLA-reactivity of the sera of the subject involved, formulated the hypothesis, periodically monitored the experimental outcome, analysed the data and performed statistical analysis, prepared the figures, discussed and wrote the paper. P. I. T. was instrumental in undertaking this investigation, contributed to designing the study, examining the results and providing interpretations. T. P. assisted in carrying out the experiments and provided continuous support for data analyses and discussion. V. J. assisted in carrying out the experiments, was involved in data analysis and in discussion of the results. S. K. assisted in carrying out the experiments, was involved in the data analysis and in discussion of the results.
Disclosure
None of the authors have any conflicts of interest or financial interests.
References
- Sbrana S, Ruocco L, Vanacore R, Azzarà A, Ambrogi F. In vitro effects of an immunoglobulin preparation for intravenous use (IVIG) on T-cells activation. Allerg Immunol (Paris) 1993;25:35–37. [PubMed] [Google Scholar]
- Hurez V, Kaveri SV, Mouhoub A, et al. Anti-CD4 activity of normal human immunoglobulin G for therapeutic use (intravenous immunoglobulin, IVIg) Ther Immunol. 1994;1:269–277. [PubMed] [Google Scholar]
- Amran D, Renz H, Lack G, Bradley K, Gelfand EW. Suppression of cytokine-dependent human T-cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol. 1994;73:180–186. doi: 10.1006/clin.1994.1186. [DOI] [PubMed] [Google Scholar]
- Kaveri S, Vassilev T, Hurez V, et al. Antibodies to a conserved region of HLA class I molecules, capable of modulating CD8 T cell-mediated function, are present in pooled normal immunoglobulin for therapeutic use. J Clin Invest. 1996;97:865–869. doi: 10.1172/JCI118488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Skansén-Saphir U, Sparrelid E, Andersson U. Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages. Clin Exp Immunol. 1996;104(Suppl 1):10–20. [PubMed] [Google Scholar]
- Viard I, Wehrli P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- Prasad NK, Papoff G, Zeuner A, et al. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998;161:3781–3790. [PubMed] [Google Scholar]
- Aktas O, Waiczies S, Grieger U, Wendling U, Zschenderlein R, Zipp F. Polyspecific immunoglobulins (IVIg) suppress proliferation of human (auto)antigen-specific T cells without inducing apoptosis. J Neuroimmunol. 2001;114:160–167. doi: 10.1016/s0165-5728(01)00243-0. [DOI] [PubMed] [Google Scholar]
- MacMillan HF, Lee T, Issekutz AC. Intravenous immunoglobulin G-mediated inhibition of T-cell proliferation reflects an endogenous mechanism by which IgG modulates T-cell activation. Clin Immunol. 2009;132:222–233. doi: 10.1016/j.clim.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Aubin E, Lemieux R, Bazin R. Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood. 2010;115:1727–1734. doi: 10.1182/blood-2009-06-225417. [DOI] [PubMed] [Google Scholar]
- Aubin É, Proulx DP, Trépanier P, Lemieux R, Bazin R. Prevention of T cell dependent native antigen presentation. Clin Immunol. 2011;141:273–283. doi: 10.1016/j.clim.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E Abs that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood. 2013;121:2013–2028. doi: 10.1182/blood-2012-08-447771. [DOI] [PubMed] [Google Scholar]
- Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–123. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Taniguichi M, Chen CW, et al. HLA-E monoclonal Abs recognize shared peptide sequences on classical HLA class Ia: relevance to human natural HLA Abs. Mol Immunol. 2010;47:1121–1131. doi: 10.1016/j.molimm.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Kaneku H, El-Awar N, Morales-Buenrostro LE, Terasaki PI. Abs to HLA-E in nonalloimmunized males: pattern of HLA-Ia reactivity of anti-HLA-E-positive sera. J Immunol. 2010;185:1935–1948. doi: 10.4049/jimmunol.1000424. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Pham T, El-Awar N, Kaneku H, Terasaki PI. Anti-HLA-E mAb 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: web-tools validate the immunogenic epitopes of HLA-E recognized by the Abs. Mol Immunol. 2011;48:423–430. doi: 10.1016/j.molimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Pham T, Ozawa M, Terasaki PI. Antibodies to HLA-E may account for the non-donor-specific anti-HLA class-Ia antibodies in renal and liver transplant recipients. Int Immunol. 2012;24:43–57. doi: 10.1093/intimm/dxr094. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Selvan SR, Terasaki PI. Augmentation of anti-HLA-E antibodies with concomitant HLA-Ia reactivity in IFNγ-treated autologous melanoma cell vaccine recipients. J Immunotoxicol. 2012;9:282–291. doi: 10.3109/1547691X.2011.645582. [DOI] [PubMed] [Google Scholar]
- Cavallini G, Pozzan T, Selvatici R, Baricordi R, Mazzilli C, Gandini E. Monoclonal antibodies against HLA class I antigens inhibit human lymphocyte proliferation but do not affect mitogen dependent second messenger generation. Biochem Biophys Res Commun. 1988;154:712–718. doi: 10.1016/0006-291x(88)90198-2. [DOI] [PubMed] [Google Scholar]
- De Felice M, Turco MC, Costanzo F, Corbo L, Ferrone S, Venuta S. Inhibition by anti-HLA class I mAb of IL-2 and IL-2 receptor synthesis in lymphocytes stimulated with PHA-P. Cell Immunol. 1990;126:420–427. doi: 10.1016/0008-8749(90)90333-m. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Sekimata M, Ferrone S, Takiguchi M. Structural and functional analysis of monomorphic determinants recognized by monoclonal antibodies reacting with the HLA class I alpha 3 domain. J Immunol. 1992;148:3202–3209. [PubMed] [Google Scholar]
- Smith DM, Bluestone JA, Jeyarajah DR, et al. Inhibition of T cell activation by a monoclonal antibody reactive against the alpha 3 domain of human MHC class I molecules. J Immunol. 1994;153:1054–1067. [PubMed] [Google Scholar]
- Woodle ES, Smith DM, Zhou N. Class I MHC mediates programmed cell death in human lymphoid cells. Transplantation. 1997;64:140–146. doi: 10.1097/00007890-199707150-00024. [DOI] [PubMed] [Google Scholar]
- Schnabl E, Stockinger H, Majdic O, et al. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J Exp Med. 1990;171:1431–1442. doi: 10.1084/jem.171.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JA, Belich MP, Benjamin RJ, et al. Molecular definition of a polymorphic antigen (LA45) of free HLA-A and -B heavy chains found on the surfaces of activated B and T cells. J Exp Med. 1991;174:1085–1095. doi: 10.1084/jem.174.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Schwab R, Bushkin Y. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell Immunol. 1992;142:103–113. doi: 10.1016/0008-8749(92)90272-q. [DOI] [PubMed] [Google Scholar]
- Santos SG, Powis SJ, Arosa FA. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J Biol Chem. 2004;279:53062–53070. doi: 10.1074/jbc.M408794200. [DOI] [PubMed] [Google Scholar]
- Hansen TH, Lybarger L, Yu L, Mitaksov V, Fremont DH. Recognition of open conformers of classical MHC by chaperones and monoclonal antibodies. Immunol Rev. 2005;207:100–111. doi: 10.1111/j.0105-2896.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- Zacharias M, Springer S. Conformational flexibility of the MHC class I α1–α2 domain in peptide bound and free states: a molecular dynamics simulation study. Biophys J. 2004;87:2203–2214. doi: 10.1529/biophysj.104.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matko J, Bushkin Y, Wei T, Edidin M. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J Immunol. 1994;152:3353–3360. [PubMed] [Google Scholar]
- Pickl WF, Holter W, Stöckl J, Majdic O, Knapp W. Expression of β2-microglobulin-free HLA class I α-chains on activated T cells requires internalization of HLA class I heterodimers. Immunology. 1996;88:104–109. doi: 10.1046/j.1365-2567.1996.d01-644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickl WF, Holter W, Stöckl J, Majdic O, Knapp W. Expression of LA45 reactive beta 2-microglobulin free HLA class I alpha-chains on activated T-cells is regulated by internalization, constitutive and protein kinase C inducible release. Tissue Antigens. 1996;48:15–21. doi: 10.1111/j.1399-0039.1996.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ravindranath MH, Terasaki PI, Freitas MC, Kawakita S, Jucaud V. Gastric cancer progression may involve a shift in HLA-E profile from an intact heterodimer to β2microglobulin-free monomer. Int J Cancer. 2014;134:1558–1570. doi: 10.1002/ijc.28484. [DOI] [PubMed] [Google Scholar]
- Wekerle H, Lonai P, Feldman M. Fractionation of antigen reactive cells on a cellular immunoadsorbent: factors determining recognition of antigens by T-lymphocytes. Proc Natl Acad Sci USA. 1972;69:1620–1624. doi: 10.1073/pnas.69.6.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman SP, Mertelsmann R, Venuta S, Andreeff M, Welte K, Moore MA. Sodium azide enhancement of interleukin-2 production. Blood. 1983;61:815–818. [PubMed] [Google Scholar]
- Daenke S, Cox KO. B-cells purified using azide give different responses in vitro to B-cells purified without azide. Immunol Lett. 1987;14:331–333. doi: 10.1016/0165-2478(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Petrzilka GE, Schroeder HE. Activation of human T-lymphocytes. A kinetic and stereological study. Cell Tissue Res. 1979;201:101–127. doi: 10.1007/BF00238051. [DOI] [PubMed] [Google Scholar]
- Angulo R, Fulcher DA. Measurement of Candida-specific blastogenesis: comparison of carboxyfluorescein succinimidyl ester labelling of T cells, thymidine incorporation, and CD69 expression. Cytometry. 1998;34:143–151. [PubMed] [Google Scholar]
- Mornex JF, Cordier G, Revillard JP. Criteria for T-lymphocyte activation in alveolitis. Rev Fr Mal Respir. 1983;11:787–812. [PubMed] [Google Scholar]
- Anel A, Naval J, González B, et al. Fatty acid metabolism in human lymphocytes. I. Time–course changes in fatty acid composition and membrane fluidity during blastic transformation of peripheral blood lymphocytes. Biochim Biophys Acta. 1990;1044:323–331. doi: 10.1016/0005-2760(90)90076-a. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, De Sanctis JB, Blasini AM, Leon-Ponte M, Abadi I. Human IFN-gamma up-regulates IL-2 receptors in mitogen-activated T lymphocytes. Immunology. 1990;69:554–557. [PMC free article] [PubMed] [Google Scholar]
- Modai D, Berman S, Sheleg Y, Cohn M, Weissgarten J, Averbukh Z. Interleukin-2 receptor is similarly expressed by activated lymphocytes from patients on chronic hemodialysis and healthy subjects. Clin Immunol Immunopathol. 1990;55:237–241. doi: 10.1016/0090-1229(90)90099-c. [DOI] [PubMed] [Google Scholar]
- Stentz FB, Kitabchi AE. Transcriptome and proteome expression in activated human CD4 and CD8 T-lymphocytes. Biochem Biophys Res Commun. 2004;324:692–696. doi: 10.1016/j.bbrc.2004.09.113. [DOI] [PubMed] [Google Scholar]
- Engelhardt W, Matzke J, Schmidt RE. Activation-dependent expression of low affinity IgG receptors Fc gamma RII(CD32) and Fc gamma RIII(CD16) in subpopulations of human T lymphocytes. Immunobiology. 1995;192:297–320. doi: 10.1016/s0171-2985(11)80172-5. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Maekawa N, Maeda Y, Hosoda M, Yodoi J. Induction of Fc epsilon RII/CD23 on phytohemagglutinin-activated human peripheral blood T lymphocytes. I. Enhancement by IL-2 and IL-4. J Immunol. 1991;147:548–553. [PubMed] [Google Scholar]
- Stentz FB, Kitabchi AE. Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun. 2005;335:491–495. doi: 10.1016/j.bbrc.2005.07.109. [DOI] [PubMed] [Google Scholar]
- Torres JM, Laborda J, Naval J, et al. Expression of alpha-fetoprotein receptors by human T-lymphocytes during blastic transformation. Mol Immunol. 1989;26:851–857. doi: 10.1016/0161-5890(89)90141-7. [DOI] [PubMed] [Google Scholar]
- Carrel S, Salvi S, Isler P, et al. gp33-38, an early human T cell activation antigen. J Immunol. 1990;144:2053–2062. [PubMed] [Google Scholar]
- Molinero LL, Fuertes MB, Rabinovich GA, Fainboim L, Zwirner MW. Activation-induced expression of MICA on T lymphocytes involves engagement of CD3 and CD28. J Leukoc Biol. 2002;71:791–797. [PubMed] [Google Scholar]
- Tate G, Katagiri M. Analysis of HLA-class II antigen mRNAs on T lymphoblasts. Hokkaido Igaku Zasshi. 1986;61:249–256. [PubMed] [Google Scholar]
- Moriya N, Sanjoh K, Yokoyama S, Hayashi T. Mechanisms of HLA-DR antigen expression in phytohemagglutinin-activated T cells in man. Requirement of T cell recognition of self HLA-DR antigen expressed on the surface of monocytes. J Immunol. 1987;139:3281–3286. [PubMed] [Google Scholar]
- Puppo F, Gurreri G, Pattarini R, Ranise A, Indiveri F. Proliferation in autologous mixed lymphocyte reactions, expression of HLA-class II antigen and serum immunomodulatory activity in patients with renal insufficiency on chronic dialysis. Allergol Immunopathol (Madr) 1991;19:167–173. [PubMed] [Google Scholar]
- Dibirdik I, Durak N, Kişlaoğlu E, Kutluay T, Aytemiz C. Effects of prophylactic intravenous immunoglobulin-G therapy on humoral and cellular immune components and their functions in burned patients. Burns. 1995;21:130–135. doi: 10.1016/0305-4179(95)92138-3. [DOI] [PubMed] [Google Scholar]
- van Schaik IN, Lundkvist I, Vermeulen M, Brand A. Polyvalent immunoglobulin for intravenous use interferes with cell proliferation in vitro. J Clin Immunol. 1992;12:325–334. doi: 10.1007/BF00920789. [DOI] [PubMed] [Google Scholar]
- Klaesson S, Ringdén O, Markling L, Remberger M, Lundkvist I. Immune modulatory effects of immunoglobulins on cell-mediated immune responses in vitro. Scand J Immunol. 1993;38:477–484. doi: 10.1111/j.1365-3083.1993.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Miyagi F, Horiuchi H, Nagata I, et al. Fc portion of intravenous immunoglobulins suppresses the induction of experimental allergic neuritis. J Neuroimmunol. 1997;78:127–131. doi: 10.1016/s0165-5728(97)00090-8. [DOI] [PubMed] [Google Scholar]
- Tawfik DS, Cowan KR, Walsh AM, Hamilton WS, Goldman FD. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol. 2012;23:88–95. doi: 10.1111/j.1399-3038.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- Klaesson S, Tammik L, Markling L, Lundkvist I, Ringdén O. Inhibition of immunoglobulin production in vitro by IgG and F(ab')2 fragments, but not by the Fc portion. Scand J Immunol. 1996;43:574–582. doi: 10.1046/j.1365-3083.1996.d01-72.x. [DOI] [PubMed] [Google Scholar]
- Hurez V, Kaveri SV, Mouhoub A, Dietrich G. Mani JC, Klatzmann D, Kazatchkine MD. Anti-CD4 activity of normal human immunoglobulin G for therapeutic use (intravenous immunoglobulin, IVIg) Ther Immunol. 1994;1:269–277. [PubMed] [Google Scholar]
- Mustelin T, Vang T, Bottini TN. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- Le Gall S, Erdtmann L, Benichou S, et al. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Santos SG, Antoniou AN, Sampaio P, Powis SJ, Arosa FA. Lack of tyrosine 320 impairs spontaneous endocytosis and enhances release of HLA-B27 molecules. J Immunol. 2006;176:2942–2949. doi: 10.4049/jimmunol.176.5.2942. [DOI] [PubMed] [Google Scholar]
- Guild BC, Strominger JL. Human and murine class I MHC antigens share conserved serine 335, the site of HLA phosphorylation in vivo. J Biol Chem. 1984;259:9235–9240. [PubMed] [Google Scholar]
- Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J Biol Chem. 1999;274:16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- Allen RL, Trowsdale J. Recognition of classical and heavy chain forms of HLA-B27 by leukocyte receptors. Curr Mol Med. 2004;4:59–65. doi: 10.2174/1566524043479329. [DOI] [PubMed] [Google Scholar]
- Jones DC, Kosmoliaptsis V, Apps R, et al. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol. 2011;186:2990–2997. doi: 10.4049/jimmunol.1003078. [DOI] [PubMed] [Google Scholar]
- Tamir I, Cambier JC. Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene. 1988;17:1353–1364. doi: 10.1038/sj.onc.1202187. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Terasaki PI, Kawakita S. Naturally occurring anti-HLA-E autoantibodies: evidences for HLA-Ia reactivity of anti-HLA-E antibodies. In: Shoenfeld Y, Meroni PL, Gershwin ME, editors. The autoantibodies. 3rd edn. Oxford, UK: Elsevier; 2013. pp. 285–303. . In:, eds. [Google Scholar]
- Zhu D, Ravindranath MH, Terasaki PI, Miyazaki T, Pham T, Jucaud V. Suppression of allo-HLA antibodies secreted by B memory cells in vitro: IVIg versus A monoclonal anti-HLA-E IgG that mimics HLA-I reactivities of IVIg. Clin Exp Immunol. 2014;177:464–477. doi: 10.1111/cei.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]