Abstract
Periodontitis is an inflammatory infectious disease that destroys the tooth-supporting tissues. It is caused by multi-species subgingival biofilms that colonize the tooth surface. Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia (i.e. ‘red complex’ bacteria) are characteristic subgingival biofilm species. The triggering receptor expressed on myeloid cells 1 (TREM-1) is a cell surface receptor of the immunoglobulin superfamily, with a role in the amplification of proinflammatory cytokine production during infection. This study aimed to investigate TREM-1 mRNA expression in gingival tissues from patients with chronic periodontitis, generalized aggressive periodontitis and healthy subjects and its correlation with the levels of periodontal pathogens in the tissue. A further aim was to investigate the regulation of TREM-1 in human monocytic cells (MM6) challenged with an in-vitro subgingival biofilm model. Gingival tissue TREM-1 expression was increased in both chronic and aggressive periodontitis, compared to health, and correlated with the levels of the ‘red complex’ species in the tissue. No significant differences were detected between the two forms of periodontitis. Biofilm-challenged MM6 cells exhibited higher TREM-1 expression and secretion compared to controls, with partial involvement of the ‘red complex’. Engagement or inhibition of TREM-1 affected the capacity of the biofilms to stimulate interleukin (IL)-1β, but not IL-8, secretion by the cells. In conclusion, this study reveals that TREM-1 tissue expression is enhanced in periodontal disease, and correlates with the level of periodontal pathogens. It also provides a mechanistic insight into the regulation of TREM-1 expression and the associated IL-1β production in biofilm-challenged monocytes.
Keywords: gingival tissue, inflammation, monocytes, periodontal pathogens, periodontitis, TREM-1
Introduction
Periodontitis is an inflammatory oral disease affecting the tooth-supporting (periodontal) tissues, including the gingiva, periodontal ligament and alveolar bone, leading to their progressive destruction. The most common forms of periodontal disease are chronic and aggressive periodontitis 1,2. Both forms are caused by the inflammatory host reaction to the accumulation of bacterial biofilm at the gingival margin, as well as subgingivally in periodontal pockets. Chronic periodontitis leads to the progressive destruction of the tooth supporting structures at a slower rate, whereas aggressive periodontitis is characterized by a faster destruction, often progressing in exacerbations.
The bacteria associated most commonly with chronic periodontitis are classified into the ‘red complex’ species, namely Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola, whereas Aggregatibacter actinomycetemcomitans is found predominantly in aggressive periodontitis 3,4. These Gram-negative anaerobes reside in the subgingival biofilm and have been shown to not only colonize the periodontal pockets, but also adhere to, and subsequently invade, host cells and tissues 5–8.
Triggering receptor expressed on myeloid cells 1 (TREM-1) is a transmembrane receptor of the immunoglobulin superfamily known to be expressed in monocytes and neutrophils 9, cells which are crucial regulators of the innate immune response against bacterial infection, including the establishment of subgingival biofilm microbiota in periodontitis. TREM-1 is considered to be an important regulator of the magnitude, rather than the initiation, of the immune response to bacterial challenge and the consequent production of proinflammatory cytokines 10. It is highly produced and released during systemic infections and postulated to play a role in systemic sepsis, as well as other inflammatory diseases 11–17.
Neutrophils and monocytes can be found in great numbers in the inflamed tissues around periodontal pockets and contribute to periodontal tissue destruction by their immunoreactions against invading bacteria, or their secreted virulence factors 18. TREM-1 may therefore play an important role in the regulation of the severity of host reactions that lead to periodontal tissue destruction. It has been shown previously that P. gingivalis, a member of the ‘red complex’, can stimulate the production of TREM-1 in monocytes and neutrophils, leading to an increased release of proinflammatory cytokines in vitro 19,20. It has also been demonstrated in clinical studies that the soluble form of TREM-1 (sTREM-1) can be found in increased amounts in the saliva and serum of patients with periodontitis, when compared to healthy individuals 21. Furthermore, it has been shown that the amount of sTREM-1 in gingival cervical fluid from sites affected by chronic or aggressive periodontitis is correlated with the presence of the ‘red complex’ bacteria, as well as the clinical measurements of the disease 22. It is not yet clear if these correlations of TREM-1 with clinical and microbiological parameters can also be found at the gene expression level, within the inflamed periodontal tissues.
Hence, the aim of the present study was to assess the expression of TREM-1 at the mRNA level in gingival tissue from sites affected by chronic and aggressive periodontitis, as well as healthy sites, and to investigate its correlation with clinical periodontal measurements and the levels of the ‘red complex’ bacteria and A. actinomycetemcomitans within these tissues. Furthermore, the in-vitro branch of this study aimed to investigate the regulation of TREM-1 as an innate immune response of monocytic cells, upon challenge with culture supernatants from a multi-species subgingival biofilm model. The effect of inhibition or engagement of TREM-1 on the production of proinflammatory cytokines by these cells was also assessed.
The hypothesis is that TREM-1 gene expression is increased in periodontal tissues affected by periodontitis and correlates positively with site-specific clinical and microbiological parameters. Furthermore, it is hypothesized that modulation of TREM-1 affects the levels of proinflammatory cytokines produced by monocytes challenged with oral biofilm-secreted products.
Materials and methods
Study population and clinical examination
Forty-five subjects recruited from November 2011 until March 2012 at the clinics of the Department of Periodontology, School of Dentistry, Ege University, Izmir, Turkey were included into this study. All participants gave their informed consent and the use of human participants met all requirements of the Ege University Institutional Review board (ethics approval number: 11–12·1/11). The study population included cases diagnosed with chronic periodontitis (n = 15), aggressive periodontitis (n = 15) and non-periodontitis healthy controls (n = 15). Diagnosis of the form of periodontitis was in accordance with the criteria of the 1999 International World Workshop for a Classification of Periodontal Disease and Conditions 1. This study population comprises part of a population that has been analysed previously. Hence, more detailed information on recruitment diagnostic procedures can be found elsewhere 21,22.
Collection of gingival tissue samples
Gingival tissue samples, including both epithelium and connective tissue, were taken from the proximal sites of single-rooted teeth prior to non-surgical periodontal therapy in the instance of periodontally diseased subjects, and during tooth extractions for orthodontic reasons or crown-lengthening procedures in non-periodontitis subjects, as described previously 23. One tissue sample from each subject was obtained and submerged immediately in a sterile tube containing RNAlater solution (Ambion Inc., Austin, TX, USA), and stored at −80°C until further laboratory analysis.
Extraction of DNA and RNA from gingival tissue samples
The total DNA and RNA were extracted from the gingival tissue samples using the AllPrep Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. The obtained DNA was used for the determination of the bacterial levels in the tissue, whereas the RNA was used for the determination of TREM-1 gene expression in the tissue. The concentration of both nucleic acids was measured using a NanoDrop 1000 spectophotometer. One μg of total RNA was further reverse-transcribed into cDNA using 0·5 μg oligo-dT primers (Promega, Madison, WI, USA). Prior to adding the reverse transcription master mix, the RNA and primers in 15 μl of nuclease-free water were heated to 70°C for 5 min and then cooled to 6°C and centrifuged. The master mix contained 5 μl Moloney murine leukaemia virus reverse transcriptase (M-MLV RT) buffer, 1·25 μl of 10 mM deoxynucleotide triphosphates (dNTPs), 1 μl M-MLV RT (all from Promega) and 3·75 μl nuclease-free water. For the RT reaction, the samples were heated at 40°C for 60 min and then at 70°C for 15 min, and were finally cooled to 6°C. The resulting cDNA samples were stored at −20°C, until further use.
Analysis of TREM-1 mRNA expression in gingival tissue samples by TaqMan quantitative real-time polymerase chain reaction (qPCR)
Relative expression of human TREM-1, as well as of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the gingival tissue samples, were analysed by qPCR (TaqMan Gene Expression; Applied Biosystems, Carlsbad, CA, USA). The Applied Biosystems assay IDs were for TREM-1 Hs00218624_m1 and for GAPDH Hs99999905_m1. The cDNA amplification and detection was performed in a real-time PCR system (Step One Plus; Applied Biosystems) using TaqMan Gene Expression Master Mix (Applied Biosystems). Each reaction was carried out in a total volume of 16 μl. Thereafter, the relative expression of TREM-1 was calculated using the 2–ΔCT method.
Bacterial quantification by qPCR
Quantification of the ‘red complex’ bacteria (namely, T. forsythia, T. denticola, P. gingivalis) and A. actinomycetemcomitans was performed by qPCR, using species-specific oligonucleotide primers targeting the 16S rRNA gene. The primers were designed and used as described previously in 24, and their sequences are provided in Table 1. DNA amplification and detection was performed in a real-time PCR system (Step One Plus; Applied Biosystems) using SYBR Green Master Mix for the amplification reaction (Applied Biosystems). Each reaction was performed in a total volume of 12 μl, including the primer solution, master mix and 50 ng of the total DNA extracted from the tissue samples. Standard curves generated from extracted DNA of known concentrations from pure cultures of all four species tested were used to calculate the estimated amount of bacteria in the tissue samples, as described previously in 22.
Table 1.
Primer sequences for bacterial quantification by quantitative polymerase chain reaction (qPCR)
| Primer | Sequence |
|---|---|
| Tannerella forsythia forward | CGATGATACGCGAGGAACCTTACCC |
| T. forsythia reverse | CCGAAGGGAAGAAAGCTCTCACTCT |
| Treponema denticola forward | TAAGGGACAGCTTGCTCACCCCTA |
| T. denticola reverse | CACCCACGCGTTACTCACCAGTC |
| Porphyromonas gingivalis forward | GCGAGAGCCTGAACCAGCCA |
| P. gingivalis reverse | ACTCGTATCGCCCGTTATTCCCGTA |
| A. actinomycetemcomitans forward | GTGGGGAGCAAACAGGATTAG |
| Aggregatibacter actinomycetemcomitans reverse | CCTAAGGCACAAACCCATCTC |
Human monocytic cell culture
The human monocytic cell line Mono Mac 6 (MM6), obtained from the German Collection of Microorganisms and Cell Cultures (Mascheroder, Braunschweig, Germany), was used in this study. Cells frozen in liquid nitrogen were defrosted quickly and transferred into complete medium containing 87% RPMI-glutamax, 10% fetal bovine serum, 1% non-essential amino acids, 1% sodium pyruvate, 0·02% fungizone solution (all from Gibco BRL Life Technologies, Paisley, UK), 1% penicillin/streptomycin solution and 0·1% recombinant human insulin solution (both from Sigma-Aldrich, St Louis, MO, USA). The supernatant was removed by centrifugation, while the resulting cell pellet was resuspended in freshly reconstituted complete media. For the experiments, the cells were then counted using a haemocytometer and Trypan blue staining, and were seeded at a concentration of 106 cells/ml.
Biofilm supernatants
To challenge the cells, culture supernatants from a 10-species ‘subgingival’ biofilm model were used, or its seven-species variant excluding the ‘red complex’, as described elsewhere 25–27. In brief, these multi-species biofilms were grown on hydroxyapatite discs, in medium consisting of 60% of processed whole unstimulated pooled saliva, 30% modified fluid universal medium (mFUM) and 10% heat-inactivated human serum under anaerobic conditions at 37°C, for a total of 64·5 h. The 10 species composing the biofilm were Streptococcus oralis SK248 (OMZ 607), Streptococcus anginosus ATCC 9895 (OMZ 871), Actinomyces oris (OMZ 745; formerly Actinomyces naeslundii), Fusobacterium nucleatum subsp. nucleatum OMZ 598, Veillonella dispar ATCC 17748T (OMZ 493), Campylobacter rectus OMZ 698, Prevotella intermedia ATCC 25611T (OMZ 278), P. gingivalis ATCC 33277T (OMZ 925), T. forsythia OMZ 1047 and T. denticola ATCC 35405T (OMZ 661). This 10-species biofilm composition was designated as ‘BF’. The relative involvement of the ‘red complex’ species was evaluated growing a biofilm variant whereby T. denticola, P. gingivalis and T. forsythia had been omitted from the composition (designated as ‘BF-RC’). After a total 64·5 h incubation, at an advanced stage of biofilm maturation, the culture supernatants were collected, filtered and stored at −80°C. The bacterial protein concentration in these biofilm culture supernatants was determined by the bicinchoninic acid (BCA) Protein Assay (Pierce, Rockford, IL, USA), as described previously 25. For the experiments, these biofilm supernatant preparations were diluted into the final cell culture medium in order to challenge the MM6 cells.
Challenging MM6 cells with biofilm supernatants
For the experiments, 250 μl of MM6 cell suspension (106 cells/ml) were cultured in complete media without antibiotics, in the presence or absence of the biofilm supernatants. In some experiments, prior to being challenged with the biofilm, the MM6 cells were pretreated either by engaging or inhibiting TREM-1, as described in the next sections. The cells were challenged for up to 24 h with 100 μg/ml protein concentration of either biofilm supernatant (BF or BF-RC), while corresponding MM6 cultures in the absence of biofilm supernatant were used as controls. Upon completion, the cells were pelleted by centrifugation, and 5 μl of protease inhibitor [ethylenediamine tetraacetic acid (ETDA)-free Protease Inhibitor Cocktail Tablets; Roche, Basel, Switzerland] were added to the resulting culture supernatants. The supernatants were then processed for further analysis by enzyme-linked immunosorbent assay (ELISA), as described in the next sections. The cell pellets were washed once in sterile phosphate-buffered saline (PBS), and stored at −20°C until further use.
TREM-1 engagement
To enhance the activity of the TREM-1 pathway in MM6 cells upon challenge with biofilm supernatants, TREM-1 was engaged by 10 μg/ml anti-human TREM-1 antigen affinity-purified polyclonal goat immunoglobulin (Ig)G, whereas goat IgG was used as an isotype control (both from R&D Systems, Abingdon, UK). The culture plates were precoated with 100 μl of either IgG suspension and left overnight at +4°C. Before use, they were placed at room temperature for 1 h, and the wells were washed once with sterile PBS. Thereafter, the cells were incubated for 24 h in these precoated wells in the presence or absence of biofilm supernatants, and the samples harvested as described above.
TREM-1 inhibition
To reduce the activity of the TREM-1 pathway in MM6 cells upon challenge with biofilm supernatants, TREM-1 was inhibited by its synthetic antagonist LP17 (Pepscan Presto B.V., Lelystad, the Netherlands). For the experiments, LP17 was added to the cell suspension at a concentration of 100 ng/ml and then further incubated at 37°C for 20 min. Thereafter, the cell suspension was centrifuged and the cells were resuspended in complete medium in the absence of antibiotics, followed by further addition of LP17 (100 ng/ml) in the presence or absence of biofilm supernatants for 6 h and 24 h, as described above.
ELISA
Human interleukin-1β (IL-1β), IL-8 and TREM-1 ELISA kits (DuoSet; R&D Systems) were used to determine the amounts of these molecules in the MM6 culture supernatants, following biofilm challenge, according to the manufacturer's instructions. Upon completion of the experiments, the cell-free culture supernatants were transferred to 96-well ELISA plates and analysed spectophotometrically, using an EPOCH microplate reader (BioTek, Lucerne, Switzerland) by measuring the absorbance at 450 nm and subtracting background readings at 570 nm. Standard curves were calculated by four-parameter polynomial regression using recombinant human (rh)IL-1β, rhIL-8 and recombinant human soluble (rhs)TREM-1 standards provided in the ELISA kits, and the concentrations of these molecules in the experimental samples were calculated based on these curves.
RNA isolation from MM6 cells
The RNeasy Mini Kit (Qiagen) was used to isolate total RNA from MM6 cells. The cells were harvested from the culture and washed with sterile PBS. Thereafter, 350 μl of lysis buffer RLT, provided in the kit, were added to the cell pellet and the cells lysed by pipetting. Total RNA was then extracted and isolated, following the manufacturer's protocol for purification of total RNA from animal cells. The concentration of the extracted total RNA was measured with a NanoDrop 1000 Spectrophotometer and the RNA samples stored at −20°C until further use.
Analysis of TREM-1 mRNA expression in MM6 cells by qPCR
One μg of RNA isolated from MM6 cells was reverse-transcribed to cDNA using the oligo-dT method, and the relative expression of human TREM-1 was then analysed by qPCR as described above, calibrated against the expression of the β2-microglobulin (B2M) (Applied Biosystems Gene Expression Assay ID: Hs99999907_m1) that was used as internal housekeeping gene control.
Cytotoxicity assay
The potential cytotoxicity elicited by the two biofilm supernatants on the MM6 cultures was evaluated by measuring the extracellularly released cytosolic lactate dehydrogenase (LDH), using the CytoTox96® Non-Radioactive Cytotoxicity Assay (Promega), according to the manufacturer's instructions. After completion of the experiments, the cell culture supernatants were collected, centrifuged and transferred into an optically clear 96-well plate, followed by addition of reaction solution and incubated for 30 min in the dark. The reaction was then stopped and the absorbance was measured spectophotometrically at 490 nm in and EPOCH microplate reader (Biotek), subtracting background values from all samples.
Statistical analysis
Clinical and microbiological findings were analysed statistically using the IBM spss statistics program version 21 (International Business Machines Corporation, Armonk, NY, USA). Mann–Whitney U-tests were performed to calculate significance levels. To analyse correlations between TREM-1 expression and clinical and microbiological parameters, Spearman's rank correlation coefficients were calculated. The data of the in-vitro experiments were analysed by GraphPad Prism software version 6·02 (GraphPad Software, Inc., La Jolla, CA, USA). Unpaired t-tests were used to calculate significance levels.
Results
Clinical and microbiological findings
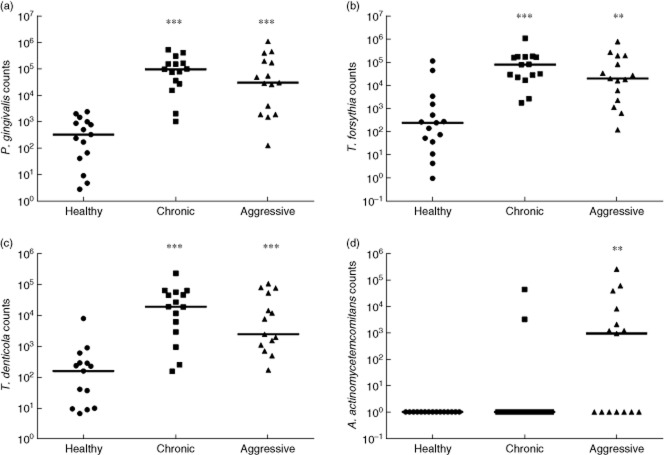
Full mouth clinical measurements are provided in Table 2, whereas site-specific clinical parameters are provided in Table 3. Full mouth clinical parameters were significantly higher in cases of chronic and aggressive periodontitis compared to healthy controls (Table 2). Site-specific clinical parameters were also significantly higher in both forms of periodontitis compared to healthy sites (P < 0·0001) (Table 3). Matching the clinical measurements, the levels of all three ‘red complex’ bacteria were significantly higher in chronic, as well as aggressive, periodontitis than in healthy controls. P. gingivalis numbers were increased by almost 3-log in chronic and aggressive periodontitis (P < 0·0001) (Fig 1a). T. forsythia numbers were increased by ≥1-log in chronic (P < 0·0001) and aggressive periodontitis (P < 0·01) (Fig. 1b). T. denticola numbers were increased by 1·7-log in chronic periodontitis and 1·5-log in aggressive periodontitis, compared to healthy controls (P < 0·0001) (Fig. 1c). Nevertheless, A. actinomycetemcomitans numbers showed no significant increase in chronic periodontitis compared to healthy controls, whereas in aggressive periodontitis the numbers were increased significantly by a factor of 4·4-log (P < 0·01) (Fig 1d). Overall, with the exception of A. actinomycetemcomitans, which was increased significantly in aggressive periodontitis when compared to chronic periodontitis (P = 0·034), no other differences were observed between the two forms of periodontitis in terms of clinical parameters or bacterial numbers.
Table 2.
Full mouth clinical periodontal measurements of the study groups
| Clinical parameters | Controls (healthy) (n = 15) | Chronic periodontitis (n = 15) | Aggressive periodontitis (n = 15) |
|---|---|---|---|
| Age (years) | 36·2 ± 13·25 | 45·2 ± 7·91* | 34·93 ± 5·85 |
| PD (mm) | 1·91 ± 0·21 | 4·95 ± 0·4*** | 5·55 ± 0·79*** |
| CAL (mm) | 1·94 ± 0·22 | 5·69 ± 0·75*** | 6·41 ± 1·05*** |
| PI | 0·78 ± 0·38 | 2·42 ± 0·5*** | 2·06 ± 0·5*** |
| BOP (%) | 25·6 ± 15 | 78·3 ± 49·5*** | 60 ± 22·9*** |
Significance of differences compared to the control group (***P < 0·0001; **P < 0·01; *P < 0·05). Values represent means ± standard deviations. PD = pocket depth; CAL = clinical attachment loss; PI = plaque index; BOP = bleeding on probing.
Table 3.
Site-specific clinical measurements
| Measurements | Control (healthy site) (n = 15) | Chronic periodontitis (n = 15) | Generalized aggressive periodontitis (n = 15) |
|---|---|---|---|
| PD (mm) | 1·27 ± 0·45 | 6·8 ± 1·65*** | 7·07 ± 1·48*** |
| CAL (mm) | 1·27 ± 0·45 | 8·4 ± 2·35*** | 8·07 ± 1·79*** |
| PI | 0·07 ± 0·25 | 2·53 ± 0·64*** | 2·07 ± 0·79*** |
| BOP (%) | 0 ± 0 | 93·3 ± 25·8*** | 86·7 ± 35·2*** |
Significance of differences compared to the control group (***P < 0·0001; **P < 0·01; *P < 0·05). Values represent means ± standard deviations. PD = pocket depth; CAL = clinical attachment loss; PI = plaque index; BOP = bleeding on probing.
Fig 1.
(a) Site-specific counts of Porphyromonas gingivalis, (b) Tannerella forsythia, (c) Treponema denticola and (d) Aggregatibacter actinomycetemcomitans per mg of tissue sample. The horizontal line depicts the median value (***P < 0·0001 and **P < 0·01).
TREM-1 mRNA expression in gingival biopsies
TREM-1 relative mRNA expression in gingival biopsies from sites with chronic and aggressive periodontitis, as well as from healthy sites, was analysed by qPCR, using GAPDH as an internal housekeeping gene control and the relative expression of TREM-1 calculated by the 2–ΔCT method. TREM-1 expression was increased significantly in both forms of periodontitis when compared to healthy sites (P < 0·05) (Fig. 2). In chronic periodontitis, TREM-1 expression was increased by threefold, whereas in aggressive periodontitis by 13-fold, compared to healthy controls. Nevertheless, this difference between chronic and aggressive periodontitis did not prove to be significant.
Fig 2.
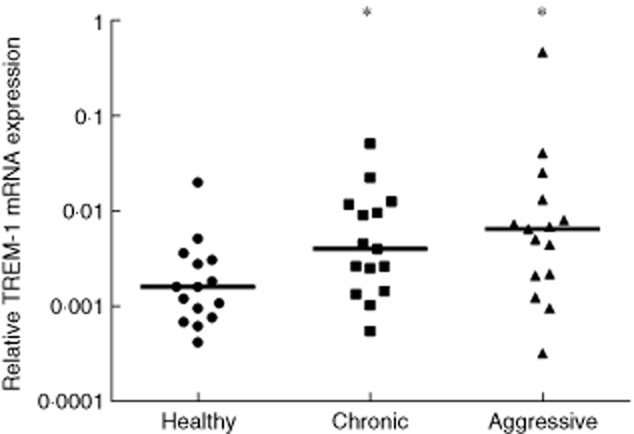
Relative expression of triggering receptor expressed on myeloid cells 1 (TREM-1) mRNA in gingival tissue samples from healthy sites as well as sites affected by chronic and aggressive periodontitis. The horizontal line depicts the median value (*P < 0·05).
Correlations between site-specific clinical and microbiological findings and TREM-1 mRNA expression in gingival biopsies
Spearman's rank correlation coefficients were calculated for TREM-1 relative expression in gingival biopsies and site-specific clinical measurements as well as microbiological findings (Table 4). The pocket depth, clinical attachment loss and plaque index did not show a significant correlation with TREM-1 expression. However, as a clinical indicator of inflammation, bleeding on probing (BOP) demonstrated a significantly positive correlation with TREM-1 expression (r = 0·363, P = 0·014). Moreover, TREM-1 tissue expression showed a significant correlation with the numbers of all three ‘red complex’ species (P < 0·005), but not in the case of A. actinomycetemcomitans.
Table 4.
Correlations between site-specific clinical measurements, microbiological findings and relative tissue triggering receptor expressed on myeloid cells 1 (TREM-1) mRNA expression
| Measurements | Spearman's rho correlation coefficient (n = 45) |
|---|---|
| PD (mm) | 0·273 (P = 0·069) |
| CAL (mm) | 0·179 (P = 0·239) |
| PI | 0·229 (P = 0·130) |
| BOP | 0·363* (P = 0·014) |
| Porphyromonas gingivalis | 0·495** (P = 0·001) |
| Treponema denticola | 0·471** (P = 0·001) |
| Tannerella forsythia | 0·438** (P = 0·003) |
| Aggregatibacter actinomycetemcomitans | 0·206 (P = 0·174) |
Significance of correlation, *P < 0·05; **P < 0·005. PD = pocket depth; CAL = clinical attachment loss; PI = plaque index; BOP = bleeding on probing.
Regulation of TREM-1 in MM6 cells in response to biofilm challenge
The MM6 cells were challenged for 3 h with 100 μg/ml protein of biofilm culture supernatants, either BF or BF-RC. The sTREM-1 levels secreted in the cell culture were evaluated by ELISA, whereas TREM-1 mRNA expression was evaluated by qPCR. sTREM-1 secretion was induced significantly in both biofilm-challenged groups, whereas this was not detected in the control group that was not challenged by biofilm supernatants (Fig. 3a). The highest sTREM-1 secreted levels were observed in MM6 cells challenged with BF, which included the ‘red complex’, whereas BF-RC, lacking these species, resulted in 2·2-fold lower levels than BF. However, the difference between the two groups was at the borderline of being significant (P = 0·055). With regard to TREM-1 mRNA expression (Fig. 3b), this was significantly (P = 0·0256) higher by 1·35-fold in cells challenged with BF, compared to BF-RC. Both biofilm-challenged groups displayed significantly higher TREM-1 expression than the controls (P = 0·0033 and P = 0·0199, for BF and BF-RC, respectively).
Fig 3.
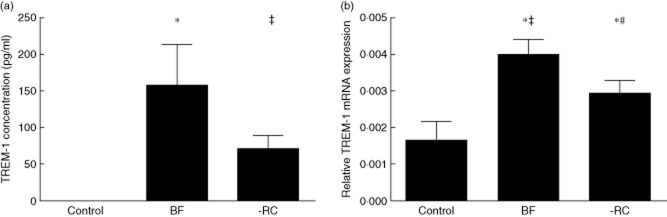
(a) Soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) secretion by human monocytic cells (MM6) cells after 3 h of challenge with 10 species biofilm supernatant, as well as supernatants of biofilms in which the three red complex species were excluded from the composition (*P = 0·0067; ‡P = 0·0023). (b) Relative TREM-1 mRNA expression after 3 h of challenge (*P = 0·0256; ‡P = 0·0033; #P = 0·0199). BF = 10-species biofilm; control = no biofilm; -RC = seven-species biofilm excluding the ‘red complex’.
The potential cytotoxic effects of either biofilm supernatants on the MM6 cells were analysed by measuring the extracellularly released LDH. After 24 h, no differences were found compared to the BF group, but the BF-RC group demonstrated a slight yet significant (P < 0·025) increase in extracellular LDH release (data not shown).
Cytokine release in response to biofilm challenge, with engagement or inhibition of TREM-1
To analyse the involvement of TREM-1 in cytokine release by MM6 cells upon biofilm challenge, the TREM-1 receptor was either engaged by goat anti-human TREM-1 IgG or inhibited by the synthetic LP17 peptide for a period of 24 h. The concentrations of IL-1β and IL-8 in the culture supernatant were measured thereafter by ELISA. In the absence of any modulation of TREM-1, the cells challenged with biofilm supernatants showed elevated IL-1β levels when compared to the controls (Fig. 4). However, the increase (1·43-fold) in IL-1β caused by BF was not significant (P = 0·08), whereas in the BF-RC group IL-1β was significantly (P = 0·017) increased by 1·37-fold. Nevertheless, when the effects of the two biofilms were compared, there were no significant differences between them.
Fig 4.
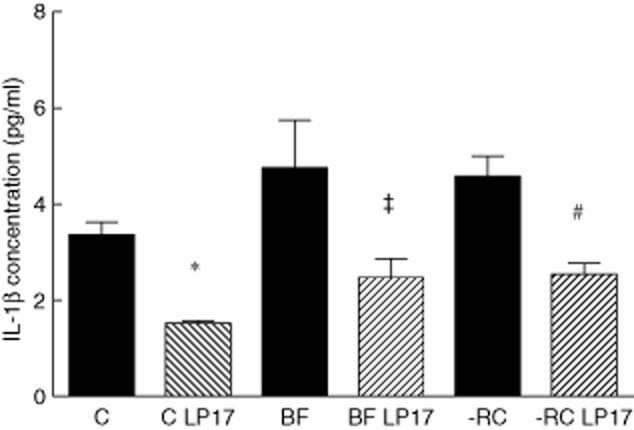
Effect of inhibition of triggering receptor expressed on myeloid cells 1 (TREM-1) by the LP17 peptide on interleukin (IL)-1β production (*P = 0·0006; ‡P = 0·0218; #P = 0·0024). BF = 10-species biofilm; control = no biofilm; -RC = seven-species biofilm excluding the ‘red complex’.
Later, when TREM-1 was inhibited by the LP17 peptide, IL-1β levels were significantly (P < 0·025) reduced in both biofilm groups (1·9-fold and 1·8-fold decrease, BF and BF-RC, respectively) than in the same groups without inhibition of TREM-1 (Fig. 4). The same effect was observable in the unchallenged MM6 cell cultures. However, there was no significant difference between the two biofilm groups (P = 0·8).
Moreover, MM6 cells treated with goat anti-human TREM-1 IgG to engage TREM-1 activity, and challenged by either biofilm supernatant, demonstrated significantly higher levels of IL-1β secretion after 24 h compared to MM6 cells that had been treated with normal goat IgG isotype control (P < 0·02) (1·84-fold increase for BF and 1·4-fold for BF-RC, respectively) (Fig. 5). A similar increase in IL-1β production of the magnitude of 1·9-fold could be observed in MM6 cells engaged by goat anti-human TREM-1 IgG in the absence of any biofilm challenge. However, it was marginally non-significant (P = 0·051).
Fig 5.
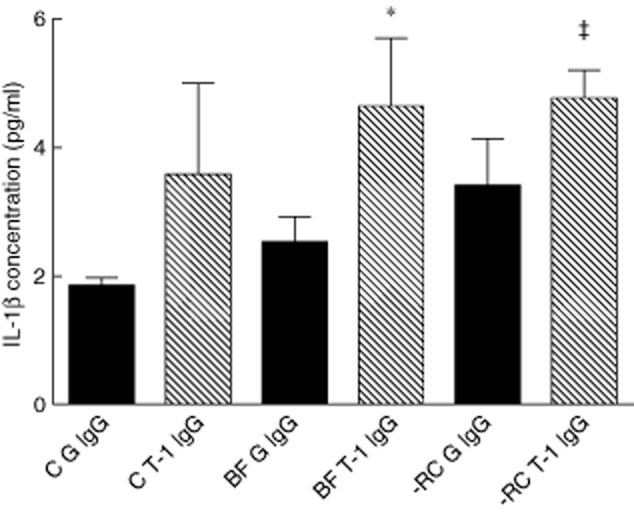
Effect of engagement of triggering receptor expressed on myeloid cells 1 (TREM-1) by anti-TREM-1 antibody on interleukin (IL)-1β production (*P = 0·0098; P = 0·0193). BF G IgG = 10-species biofilm, normal goat immunoglobulin (Ig)G; BF T-1 IgG = 10-species biofilm, goat anti-human TREM-1 IgG; C G IgG = control, normal goat IgG; C T-1 IgG = control, goat anti-human TREM-1 IgG; -RC G IgG = excluding red complex, normal goat IgG; -RC T-1 IgG = excluding red complex, goat anti-human TREM-1 IgG.
In the absence of any TREM-1 modulation, BF-RC caused significantly (P = 0·0021) higher secretion of IL-8 than the control by 24 h (2·1-fold increase). However, IL-8 secretion in response to BF was not significantly different than the control (P = 0·0983), despite a 1·8-fold increase (Fig. 6). Moreover, no statistically significant difference in IL-8 secretion was detected between the BF and BF-RC groups. Inhibition of TREM-1 by LP17 peptide did not affect IL-8 secretion levels after 24 h, irrespective of the biofilm challenge (Fig. 6). Engagement of TREM-1 by means of goat anti-human TREM-1 IgG did not affect the production of IL-8 (Fig. 7), as this was enhanced by both BF and BF-RC compared to the control, irrespective of treatment with anti-TREM-1 IgG or isotype-matched IgG (P < 0·0025). No significant difference was observed in IL-8 secretion between the two biofilm groups.
Fig 6.
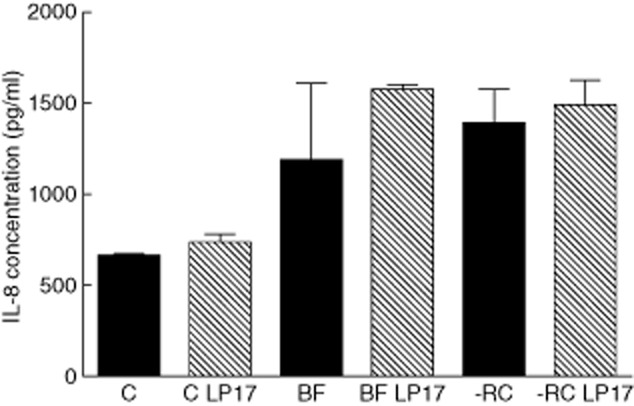
Effect of inhibition of triggering receptor expressed on myeloid cells 1 (TREM-1) by the LP17 peptide on interleukin (IL)-8 production. BF = 10-species biofilm; C = control (no biofilm); -RC = seven-species biofilm lacking ‘red complex’ species.
Fig 7.
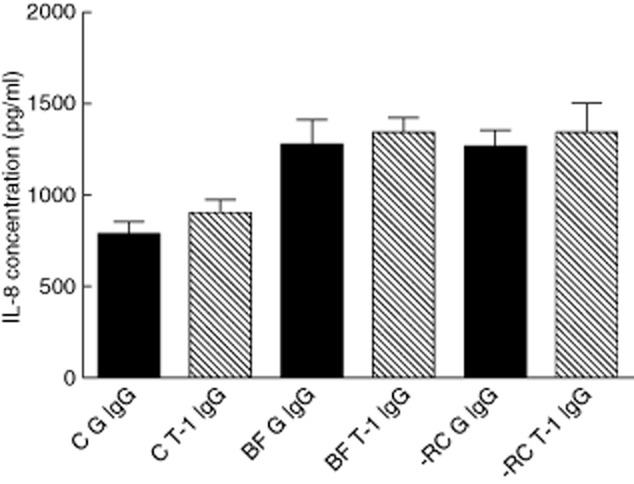
Effect of engagement of triggering receptor expressed on myeloid cells 1 (TREM-1) by anti-TREM-1 antibody on interleukin (IL)-8 production. BF G IgG = 10-species biofilm, normal goat IgG; BF T-1 IgG = 10-species biofilm, goat anti-human TREM-1 IgG; C G IgG = control, normal goat IgG; C T-1 IgG = control, goat anti-human TREM-1 IgG; -RC G IgG = excluding red complex, normal goat IgG; -RC T-1 IgG = excluding red complex, goat anti-human TREM-1 IgG.
Discussion
The present study investigated the expression of TREM-1 mRNA in healthy and periodontitis-affected human gingival tissue samples, as well as its correlation with site-specific clinical and microbiological parameters. Furthermore, to approach the mechanisms of TREM-1 regulation by periodontal pathogens, human monocytic cells were challenged with oral biofilm supernatants and were analysed for the expression of TREM-1 mRNA and sTREM-1 release, as well as the production of IL-1β and IL-8. As expected, levels of all three ‘red complex’ species were increased significantly in gingival tissue samples from both forms of periodontitis, whereas A. actinomycetemcomitans was only found to be elevated significantly in aggressive periodontitis. These microbiological findings are in concordance with the known occurrence of periodontal pathogens in the two types of periodontitis, with A. actinomycetemcomitans being most abundant in aggressive cases 3,4.
TREM-1 mRNA expression in gingival tissue was increased significantly in chronic and in aggressive periodontitis when compared to health, but the difference between the two forms of periodontitis was not statistically significant. Furthermore, TREM-1 mRNA expression was correlated positively with all three ‘red complex’ species, but not A. actinomycetemcomitans, as well as BOP, a clinical marker of inflammation. The role of TREM-1 is thought to be the propagation of inflammatory response, particularly following challenge of monocytes and neutrophils with bacteria or their released products 10,11,28. Hence, the observed correlation of TREM-1 expression with the increased levels of three major periodontal pathogens is well in line with the inflammatory nature of periodontitis. Other clinical parameters were not correlated significantly with TREM-1 mRNA expression, due possibly to these being associated with the severity, or past progression, of the disease, rather than active inflammation. These findings at the mRNA level are in agreement with previous observations showing that periodontitis-affected sites exhibit increased amounts of sTREM-1 protein in gingival cervical fluid (GCF). In a recent study in GCF, it was also shown that sTREM-1 levels correlated significantly with parameters of clinical severity of the disease 22, which was not the case in the present study investigating mRNA expression. Nevertheless, in both studies the magnitude of correlations with clinical parameters was similar, and not too large. The differences in statistical significance could well be attributed to more GCF samples (n = 62) being analysed in this earlier study than were tissue samples (n = 45) in the present study. Although the magnitude of difference in TREM-1 tissue expression between chronic and aggressive periodontitis was greater than what was found earlier in GCF, in neither case did it prove to be statistically significant. This may not be surprising, as no solid differences in the immunopathology of chronic and aggressive periodontitis have been established 29,30.
Beyond these clinical findings in TREM-1 tissue expression in periodontal health and disease, the present study also employed an in-vitro model in an attempt to investigate further the mechanisms of TREM-1 regulation in response to oral biofilms. The MM6 monocytic cell line was challenged with culture supernatants of an in-vitro-generated biofilm that consisted of species representative of the subgingival bacterial flora. While no sTREM-1 could be detected in the control group, challenge of the cells with a 10-species variant including, the ‘red complex’ species, resulted in a significant release sTREM-1 protein in the culture after 3 h. The seven-species biofilm variant lacking the ‘red complex’ caused a less pronounced increase of sTREM-1. In terms of mRNA expression levels, both biofilms enhanced TREM-1 expression compared to the control, while the difference between the two biofilm groups was also significant, with the lack of the ‘red complex’ causing a weaker effect. The increase in TREM-1 suggests that the bacterial products of the ‘red complex’ species may have a greater ability to activate the TREM-1 pathway than other oral microbes. This could result in increased inflammation, which suggests a possible mechanism of exerting their high virulence properties. These findings may well be in line with previous observations, demonstrating that live P. gingivalis stimulates TREM-1 mRNA expression in MM6 cells concomitantly with an enhanced secretion of sTREM-1 in the culture medium 19, an effect that can be diminished by administration of doxycycline 31. The process by which sTREM-1 is shed and secreted from the cell surface may involve bacterial proteinases, especially the P. gingivalis gingipains, as reported previously 20. This could further explain the ability of the ‘red complex’ species to enhance sTREM-1 secretion in the present experimental model, with consequences in the induction of periodontal inflammation.
The production of the proinflammatory cytokines IL-8 and IL-1β was also investigated in the present in-vitro experimental system. Although there was a tendency towards increased IL-1β and IL-8 secretion in response to either biofilm, a significant enhancement was only confirmed when the ‘red complex’ was absent, and there was no difference between the two biofilm groups. Hence, differences in the TREM-1 regulatory effects between the two biofilms did not directly match cytokine production. This could be either because of the existence of additional pathways other than TREM-1 responsible for cytokine regulation, or because of the high proteolytic activity of the ‘red complex’ species that can degrade proinflammatory cytokines, once secreted. Indeed, when the ‘red complex’ is present in the biofilm, it can cause reduction of IL-8 secretion by gingival epithelial cells 20.
When TREM-1 was engaged by anti-TREM-1, IL-1β was increased in all experimental groups, including the unchallenged controls. It has been reported previously that in co-culture of P. gingivalis with MM6 cells, TREM-1 engagement increases IL-1β production 19. Collectively, these results suggest that the TREM-1 signalling pathway is indeed involved in increased IL-1β secretion in response to live P. gingivalis, as well as bacterial products from biofilms. Accordingly, when TREM-1 was inhibited by the LP17 peptide, the opposite effect could be observed, also in line with earlier reporting 19. These findings corroborate further the role of TREM-1 in the production of IL-1β upon bacterial challenge.
Conversely, inhibition or engagement of TREM-1 did not affect IL-8 secretion, even though this was increased upon bacterial challenge by both biofilm supernatants. This is in contrast to the previous study demonstrating that IL-8 secretion in MM6 challenged with P. gingivalis is modulated by TREM-1 in the same manner 20. It is unclear why this discrepancy occurs between live P. gingivalis and subgingival biofilm supernatants. Potentially, TREM-1 may only modulate IL-8 production in response to live bacteria, whereas secreted bacterial products may be insufficient to induce this effect. Alternatively, the regulation of TREM-1 may be more complex in response to a mix of bacterial stimulants, where their different virulence properties may out-compete each other, resulting in the ablation of the effect. Further research is necessary to identify the TREM-1 ligand and the downstream mechanisms behind the selective involvement of TREM-1 in cytokine regulation.
In conclusion, the present study demonstrates that TREM-1 expression is increased in the gingival tissue affected by aggressive and chronic periodontitis, and is associated with the bacterially induced inflammation of the tissue. Furthermore, mechanistically it shows that a periodontitis-associated biofilm can enhance TREM-1 in monocytes, which may in turn selectively enhance IL-1β secretion by the cells. The three ‘red complex’ species have a particular role in the magnitude of these effects. TREM-1 exerts a role in the inflammation associated with periodontal disease, most probably via the propagation of proinflammatory cytokine production by cells of the immune system present in the tissues. These findings suggest that inhibition of TREM-1 may be a viable point of vantage to consider in future anti-inflammatory periodontal therapy, as has been shown in the case of doxycycline 31.
Acknowledgments
The authors would like to thank Professor Gülnur Emingil and Dr Özgen Öztürk (Ege University, Izmir, Turkey) for providing the clinical samples and Mrs Verena Osterwalder for her technical assistance. This study was supported by the authors’ Institute.
Disclosure
The authors declare no conflicts of interest.
References
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000. 2010;54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Zhang G. Streptococci dominate the diverse flora within buccal cells. J Dent Res. 2005;84:1165–1171. doi: 10.1177/154405910508401214. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Sedgewick GJ. Actinobacillus actinomycetemcomitansPorphyromonas gingivalis, and Tannerella forsythensis are components of a polymicrobial intracellular flora within human buccal cells. J Dent Res. 2005;84:59–63. doi: 10.1177/154405910508400110. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. [Pathogenic potential of Porphyromonas gingivalisTreponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis] Pathol Biol (Paris) 2007;55:154–162. doi: 10.1016/j.patbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Yilmaz O. In or out: the invasiveness of oral bacteria. Periodontol 2000. 2002;30:61–69. doi: 10.1034/j.1600-0757.2002.03006.x. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- Bleharski JR, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Collins CE, La DT, Yang HT, et al. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann Rheum Dis. 2009;68:1768–1774. doi: 10.1136/ard.2008.089557. [DOI] [PubMed] [Google Scholar]
- Gibot S, Kolopp-Sarda MN, Béné MC, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- Gibot S, Alauzet C, Massin F, et al. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194:975–983. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- Gibot S, Buonsanti C, Massin F, et al. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect Immun. 2006;74:2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia and severe sepsis. Semin Respir Crit Care Med. 2006;27:29–33. doi: 10.1055/s-2006-933671. [DOI] [PubMed] [Google Scholar]
- Zadeh HH, Nichols FC, Miyasaki KT. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol 2000. 1999;20:239–288. doi: 10.1111/j.1600-0757.1999.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Thurnheer T, Belibasakis GN. Involvement of the TREM-1/DAP12 pathway in the innate immune responses to Porphyromonas gingivalis. Mol Immunol. 2011;49:387–394. doi: 10.1016/j.molimm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Thurnheer T, Aduse-Opoku J, Curtis MA, Zinkernagel AS, Belibasakis GN. Porphyromonas gingivalis regulates TREM-1 in human polymorphonuclear neutrophils via its gingipains. PLOS ONE. 2013;8:e75784. doi: 10.1371/journal.pone.0075784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Oztürk V, Emingil G, Belibasakis GN. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in periodontitis. J Dent Res. 2013;92:161–165. doi: 10.1177/0022034512470691. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Oztürk VO, Emingil G, Soluble BN. Triggering receptor expressed on myeloid cells (sTREM)-1 in gingival crevicular fluid: association with clinical and microbiological parameters. J Periodontol. 2014;85:204–210. doi: 10.1902/jop.2013.130144. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Ilgenli T, Emingil G, et al. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J Periodont Res. 2007;42:287–293. doi: 10.1111/j.1600-0765.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- Ammann TW, Bostanci N, Belibasakis GN, Thurnheer T. Validation of a quantitative real-time PCR assay and comparison with fluorescence microscopy and selective agar plate counting for species-specific quantification of an in vitro subgingival biofilm model. J Periodont Res. 2013;48:517–526. doi: 10.1111/jre.12034. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Meier A, Guggenheim B, Bostanci N. The RANKL–OPG system is differentially regulated by supragingival and subgingival biofilm supernatants. Cytokine. 2011;55:98–103. doi: 10.1016/j.cyto.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Guggenheim B. Induction of prostaglandin E(2) and interleukin-6 in gingival fibroblasts by oral biofilms. FEMS Immunol Med Microbiol. 2011;63:381–386. doi: 10.1111/j.1574-695X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Guggenheim B, Bostanci N. Down-regulation of NLRP3 of NLRP3 inflammasome in gingival fibroblasts by subgingival biofilms: involvement of Porphyromonas gingivalis. Innate Immun. 2013;19:3–9. doi: 10.1177/1753425912444767. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- Ford PJ, Gamonal J, Seymour GJ. Immunological differences and similarities between chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:111–123. doi: 10.1111/j.1600-0757.2010.00349.x. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Behle JH, Wolf DL, et al. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 2008;79:2112–2124. doi: 10.1902/jop.2008.080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Belibasakis GN. Doxycycline inhibits TREM-1 induction by Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2012;66:37–44. doi: 10.1111/j.1574-695X.2012.00982.x. [DOI] [PubMed] [Google Scholar]