Abstract
Background:
Antidepressants (ADs) are known to have the potential to cause various cardiovascular adverse drug reactions (ADRs). The tricyclic antidepressants (TCAs) were first revealed to be a possible source of cardiovascular ADRs. In recent years, newer classes of ADs were also suggested to have a higher risk of cardiovascular adverse effects. In particular, the selective serotonin reuptake inhibitors (SSRIs) were suspected to have the potential to induce QTc interval prolongation, and therefore increase the risk of ventricular arrhythmia. This descriptive study is based on the continuous pharmacovigilance program of German-speaking countries (Austria, Germany, and Switzerland), the Arzneimittelsicherheit in der Psychiatrie (AMSP), which assesses severe ADRs occurring in clinical routine situations.
Methods:
Of 169 278 psychiatric inpatients treated with ADs between 1993 and 2010, 198 cases of cardiovascular ADRs (0.12%) were analyzed.
Results:
Our study showed that the incidence rates of cardiovascular ADRs were highest during treatment with monoamine oxidase inhibitors (0.27%), TCAs (0.15%), and serotonin noradrenaline reuptake inhibitors (0.14%); the risk of occurring during treatment with SSRIs (0.08%) was significantly lower. The noradrenergic and specific serotonergic AD mirtazapine (0.07%) had a significantly lower risk of cardiovascular ADRs than all other ADs. Severe hypotension was the most frequent ADR, followed by hypertension, arrhythmia, and in some rare cases heart failure.
Conclusions:
Despite certain limitations due to the AMSP study design, our observations on cardiovascular ADRs can contribute to a better knowledge of the cardiovascular risk profiles of antidepressants in the clinical routine setting. However, prospective studies are needed to verify our findings.
Keywords: Antidepressant, cardiovascular side effects, QT prolongation, SSRI, tricyclic antidepressant
Introduction
Psychotropic drugs, like all other compounds used medically, are known to have the potential to cause cardiovascular adverse drug reactions (ADRs; Mackin, 2008). Besides antipsychotics (Kasper et al., 2010), the tricyclic antidepressants (TCAs) were first revealed to be a possible source of life-threatening arrhythmia, including QTc prolongation, by blocking cardiovascular ion channels (Davison, 1985; Lentini et al., 2001; Pacher and Kecskemeti, 2004; van Noord et al., 2009). Furthermore, they were consistently shown to cause orthostatic hypotension at therapeutic dosages (Glassman et al., 1979; Glassman and Bigger, 1981). Compared to TCAs, selective serotonin reuptake inhibitors (SSRIs) and newer classes of antidepressants (ADs) have been suggested to have safer cardiovascular profiles (Grimsley and Jann, 1992; Leonard, 1993; Kasper et al., 1996; Pacher et al., 1999; Ray et al., 2004; Fernandez et al., 2007; Zemrak and Kenna, 2008). However, an increasing number of case reports (Friberg et al., 2006; Winkler et al., 2006; Kozian and Syrbe, 2010; Deshmukh et al., 2012) and pharmacovigilance studies (Sadanaga et al., 2004; Wilting et al., 2006; Astrom-Lilja et al., 2008; Martinez et al., 2010) have revealed arrhythmias (Leonard et al., 2011), QT interval prolongation (Girardin et al., 2013; for review see Vieweg et al., 2009), and orthostatic hypotension during treatment with the new ADs. This has led to the ongoing discussion about their cardiovascular safety profiles (Pacher and Kecskemeti, 2004).
Recently, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) got involved in this discussion. Following postmarketing reports of a dose-dependent QTc interval prolongation during treatment with citalopram, the FDA announced that the compound could be safely prescribed (FDA, 2011). However, the FDA, as well as the EMA (EMA PWPP, 2011) stipulated that doses exceeding 40mg citalopram per day should no longer be used due to possible changes in the electrical activity of the heart. Further recommendations were made for its active S-isomer, escitalopram. With reference to the FDA statements, a cross-sectional study by Castro et al. (2013) reported a modest QTc interval prolongation during use of citalopram, escitalopram, and amitriptyline. In contrast to their findings, Thase et al. (2013) did not find a clinically meaningful effect of escitalopram treatment on electrocardiographic measurement (ECG) values; only placebo-level incidence rates of cardiac-associated adverse events were observed.
Serotonin noradrenaline reuptake inhibitors (SNRIs; e.g. venlafaxine), have also been reported to cause severe cardiovascular ADRs, in particular QTc interval prolongation (Letsas et al., 2006) or hypertension (Feighner, 1995). In contrast, Zhang et al. (2007) found no changes in ventricular repolarization for duloxetine, another SNRI, when administered in daily dosages higher than recommended.
In view of the existing disparity of the data on safety profiles of the newer ADs, findings on severe cardiovascular ADRs during AD treatment in a natural setting are of particular interest. Therefore, this descriptive analysis draws on the data of an ongoing pharmacovigilance program for its analysis of the incidence rates of severe cardiovascular ADRs and risk factors observed during treatment with different classes of ADs.
Methods
This analysis of severe cardiovascular ADRs during AD treatment was based on data obtained from the drug safety AMSP program (Arzneimittelsicherheit in der Psychiatrie, www.amsp.de). This ongoing pharmacovigilance program assesses severe ADRs in psychiatric inpatients under natural conditions (Grohmann et al., 2004; Letmaier et al., 2011; Konstantinidis et al., 2012). Up to 93 hospitals, including university, state, and municipal psychiatric hospitals or departments, participated in the AMSP program from 1993 until 2010. Methods of assessment and evaluation of severe ADRs in the AMSP program were previously published by Grohmann et al. (2004). In brief, drug monitors at the local psychiatric departments regularly monitored severe ADRs on their wards. The cases are documented by means of a standardized anonymized questionnaire. Information on potential risk factors, course and alternative explanations for the ADRs, measures taken, and prior exposure to the drug are also documented. An evaluation of possible pharmacodynamic and pharmacokinetic causes was given for each case and each individually used medication. Following the ADR assessment, a re-examination took place by senior psychiatrists, and cases were evaluated at regional and central case conferences attended by drug monitors from all participating hospitals, representatives of the national drug-regulating authorities, and drug safety experts of the pharmaceutical industry.
Adverse Drug Reactions
ADRs assessed in the AMSP program are defined as adverse events of psychotropic drugs at adequate doses for therapeutic or prophylactic treatment (adverse events occurring due to intoxication or inefficiency are not included in the AMSP database). Only severe ADRs, defined as (potentially) life-threatening or seriously endangering the patient’s health, considerably impairing everyday functioning, or requiring the patient’s transfer to another department or ward providing more intensive care are included in this AMSP analysis.
Probability Rating
Probability ratings for ADRs were performed on the basis of the proposals of Hurwitz and Wade (1969), as well as Seidl et al. (1965) and the AMSP study guidelines (Grohmann et al., 2004). An ADR is rated as possible if the ADR is not known for the drug in question, the time course or dosage of the drug is unusual, or alternative explanations are more probable. An ADR is rated as probable if the ADR is known for the given drug, time course and dosage are in accordance with previous experience, and alternative explanations are less likely. ADRs are rated as definite when the criteria necessary for “probable” are fulfilled and a re-appearance of the ADR occurs after re-exposure to the drug in question.
Cardiovascular Adverse Drug Reactions
Severe cardiovascular ADRs were defined according to the AMSP study guidelines (Grohmann et al., 2004) as follows: cardiac failure; collapse; severe hypotension (symptomatic and systolic blood pressure less than 90 mmHg); hypertension (systolic blood pressure more than 180 mmHg or diastolic blood pressure more than 120 mmHg); arrhythmias, including bradycardia (heart rate less than 40 bpm); tachycardia (heart rate more than 120 bpm); atrial flutter; AV-block II° or III°; prolongation of heart rate–corrected QT interval (QTc; QTc more than 500ms or an increase of more than 60ms); or ventricular arrhythmia of at least Lown III. In addition, all cases of death following use of ADs—alone or in combination therapy—were reviewed to identify cases of a possible cardiac death with AD involvement.
Statistical Analysis
All ADs imputed to cause defined severe cardiovascular ADRs and rated as possible, probable, or definite were included in this AMSP analysis. Since drug combination therapy is very common in psychiatry and ADR profiles of co-prescribed drugs are often similar, each co-prescribed drug was given a causality rating in cases in which pharmacodynamic interactions were suspected to be responsible for the ADR. In a first step, the incidence rates of cardiovascular ADRs caused by AD treatment were calculated. This included cases in which ADs were imputed in combination with other drug groups or alone (all cases). Additionally, the incidence rates of cardiovascular ADRs in cases in which the ADs in question were only imputed alone (imputed alone) were calculated. Due to the heterogeneous group of co-administered psychotropic and non-psychotropic medication to antidepressant treatment, a statistical analysis of the influence of individual co-prescribed compounds on incidence rates of cardiovascular ADRs was not performed. Furthermore, dosage of ADs imputed to cause severe cardiovascular ADRs is stated as median dosage per day, whereas individual dose was not included in the statistical analyses. The total number of assessed cases differed from the sum of numbers for single ADs, due to antidepressant drug combinations and multiple imputations of different antidepressants.
Cardiovascular ADRs were divided into the following four groups: hypotension and collapse; hypertension; heart failure; and arrhythmias (including bradycardia, tachycardia, atrial flutter, AV-block II° or III°, QTc interval prolongation, and ventricular arrhythmias). ADs were grouped into the following classes: all tricyclic ADs and tetracyclic maprotiline were grouped as TCAs; all licensed SSRIs as one group; venlafaxine and duloxetine as SNRIs; tranylcypromine and moclobemide as monoamine oxidase inhibitors (MAOIs); mirtazapine and mianserin as noradrenergic and specific serotonergic AD (NaSSAs); and all others form the heterogeneous group of “other Ads,” consisting of reboxetine, trazodone, bupropion, agomelatine, and nefazodone.
The incidence rates of cardiovascular ADRs were calculated as percentage of patients exposed to a given compound, drug class, or subclass between 1993 and 2010. The incidence rates are presented together with their 95% confidence intervals (CIs). Since the actual ADR incidence rates were low and the number of individual patients exposed was high, CIs were calculated according to the exact method and not approximately (Vollset, 1993). Furthermore, demographic variables of age and gender are stated. As a guide to the interpretation of the CIs, Fisher’s exact tests were applied to compare ADR incidence rates during administration of a single drug or drug group with ADR incidence rates during administration of all other drugs. These comparisons were not independent of each other and were interpreted in a descriptive sense.
Ethical Section
The AMSP pharmacovigilance program was approved by the leading boards of each participating institute prior to implementation, and the Ethics Committee of the University of Munich formally approved evaluations based on the AMSP databank.
Results
During the observation period (1993 to 2010), 362 577 psychiatric inpatients were monitored in the AMSP program. Among the reported classes of severe ADRs (e.g. neurological, psychiatric, urological, cutaneous, haematological, and hepatic ADRs; toxic delirium; hormone/electrolyte disturbances; and impaired sexual function) cardiovascular ADRs constituted almost 6% of all ADRs monitored.
Of a total of 169 278 patients treated with ADs (46.7% of all monitored subjects), 198 cases of severe cardiovascular ADRs were recorded (all cases). This yielded a relative frequency of 0.12% for cardiovascular ADRs during antidepressant treatment. A combination of multiple drug groups (e.g. antipsychotics, tranquilizers, or other drug classes) was imputed in 136 cases (68.7% of all cardiovascular ADR cases). The highest proportion of combined compounds during treatment with ADs constituted antipsychotics (93 cases). Non-psychotropic drugs, mostly antihypertensive and cardiac drugs, were “co-imputed” in 43 cases. Two ADs were involved in 34 cases. Only 63 cases (incidence rate of 0.04%) were identified in which no other treatment besides a given AD drug was imputed for a severe cardiovascular ADR. Socio-demographic data are given in Table 1. The risk for cardiovascular ADRs (incidence rate 0.184%) in patients 65 years old or older was almost double that in younger patients (incidence rate 0.095%; p < 0.001).
Table 1.
Socio-Demographic Data of Cardiovascular Adverse Drug Reactions During Antidepressant Treatment Between 1993 and 2010.
| All patients monitored treated with AD | Cardiovascular ADRs | ||
|---|---|---|---|
| n (%) | n (%) | p value | |
| Total | 169,278 (100%) | 198 (100%) | |
| Age (yr) | |||
| <65 | 127,944 (75.6.%) | 122 (61.6%) | p< 0.001* |
| ≥65 | 41,334 (24.4%) | 76 (38.4%) | |
| Sex | |||
| Female | 106,890 (63.1%) | 130 (65.7%) | p > 0.05 |
| Male | 62,388 (36.9%) | 68 (34.3%) | |
AD, antidepressant; ADRs, adverse drug reactions.
*Significantly higher risk of cardiovascular ADRs in patients older than 65 years (Fisher Exact Significance Test).
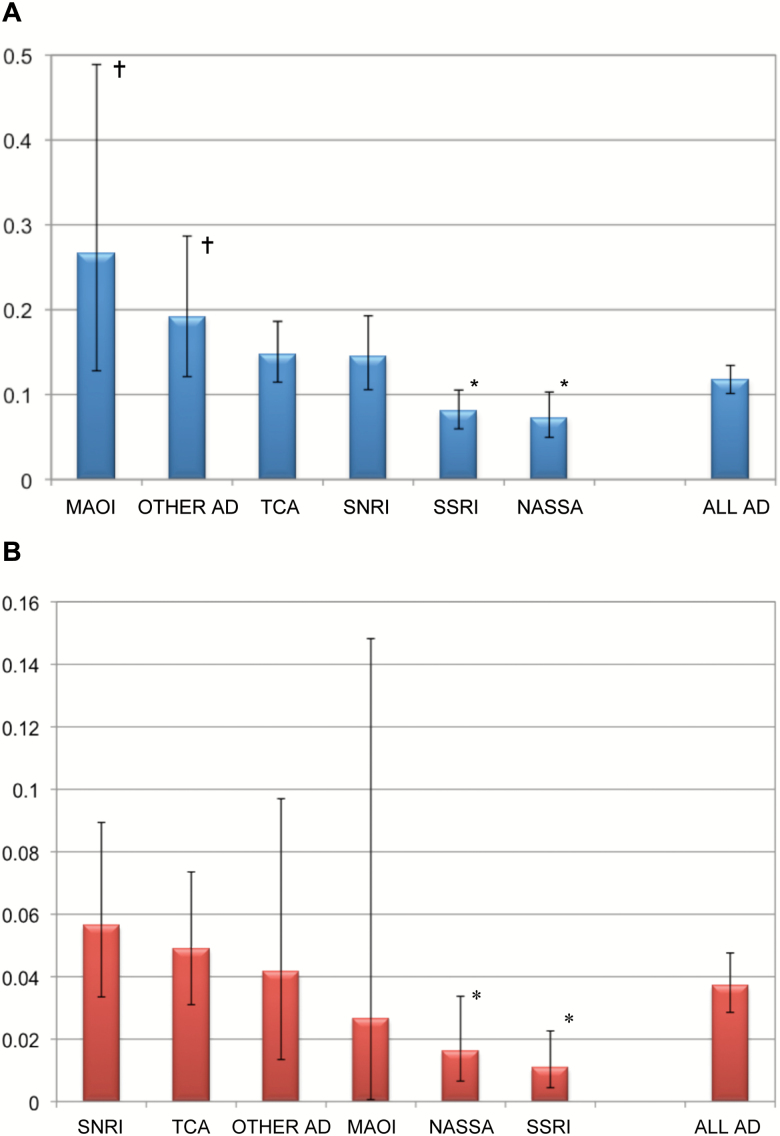
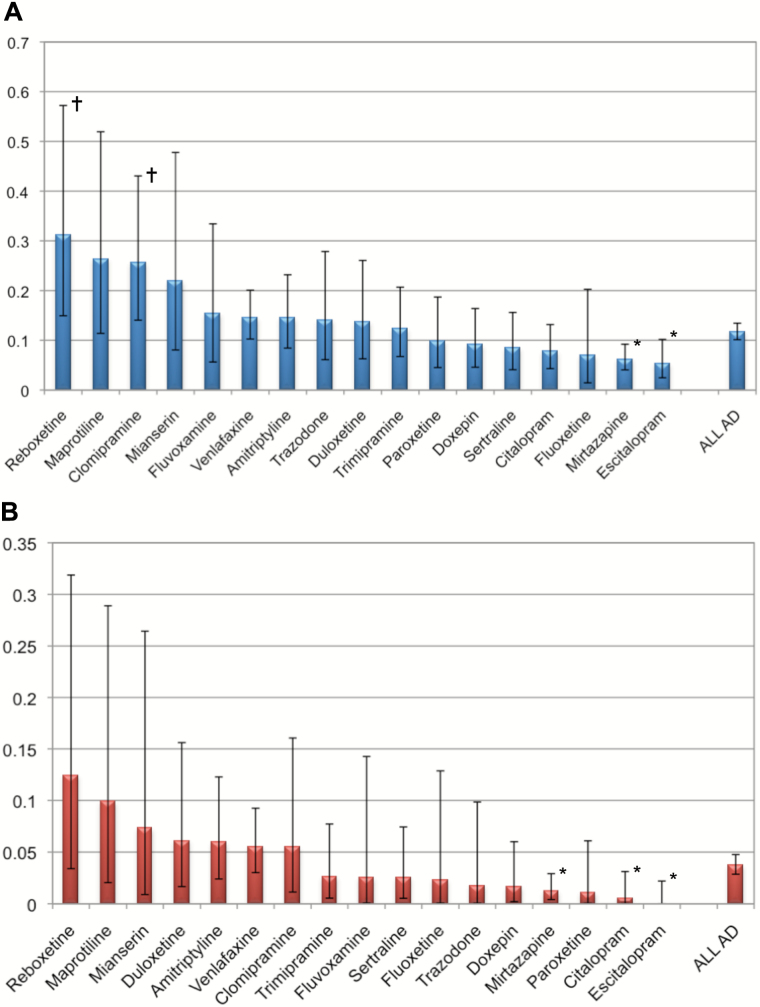
Figure 1 shows the incidence rates of severe cardiovascular ADRs during AD treatments with the various classes of ADs, either when imputed at all (i.e. alone or in combination with other drugs; Figure 1A) or when imputed alone (Figure 1B). The numbers themselves and the results of descriptive significance tests are given in Table 2. Of all imputations (198 cases, Figure 1A), MAOIs and the heterogeneous group of “other ADs” carried an enhanced risk for cardiovascular ADRs (0.27 and 0.19, respectively), whereas the risks with SSRIs (0.08) and the NaSSAs (0.07) were reduced. In those cases in which only one drug was imputed (63 cases only, Figure 1B), the risk was again reduced with NASSAs and SSRIs. Figure 2 shows the same information for all individual drugs given to more than 2 000 patients. As a single compound, the selective noradrenaline reuptake inhibitor reboxetine was imputed in a total of 10 cases of 3 210 prescriptions (incidence rates 0.31). This is significantly above the mean incidence rates for cardiovascular ADRs during AD treatment (0.12). Five cases of cardiovascular adverse drug reactions were found where agomelatine (one case), bupropion (two cases), and nefazodone (two cases) have been imputed in combination with other compounds to cause the adverse reaction. No case of cardiovascular adverse drug reaction was detected when agomelatine, bupropion, or nefazodone was imputed alone.
Fig. 1.
Incidence rates (%) of severe cardiovascular adverse drug reactions during antidepressant treatment.
(A) ADs imputed alone and in combination (total cases 198), (B) ADs imputed alone (total cases 63). Incidence rates are given with their 95% confidence intervals. †Risk for cardiovascular adverse drug reactions enhanced compared to all other ADs. *Risk for cardiovascular ADRs reduced compared to all other ADs. AD, antidepressant; MAOI monoamine oxidase inhibitors; NaSSA noradrenergic and specific serotonergic antidepressant; SNRI, serotonin noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Table 2.
Cardiovascular Adverse Drug Reactions During Antidepressant Treatment Between 1993 and 2010.
| Patients monitored | Cardiovascular ADRs | Hypotension | Hypertension | Arrhythmia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (AD imputed alone and in combination) | (AD imputed alone) | (AD imputed alone) | (AD imputed alone) | (AD imputed alone) | ||||||||||||
| n (%) | p | n (%) | p | n (%) | p | n (%) | p | n (%) | p | |||||||
| AD total | 169,278 | 198 | (0.12) | 63 | (0.04) | 12 | (0.01) | 22 | (0.01) | 27 | (0.02) | |||||
| TCA | 46,915 | 69 | (0.15) | n.s. | 23 | (0.05) | n.s. | 6 | (0.01) | n.s. | 2 | (0.004) | 0.015* | 14 | (0.03) | 0.014 |
| Amitriptyline | 11,733 | 17 | (0.14) | n.s. | 7 | (0.06) | n.s. | 3 | (0.03) | n.s. | 0 | (0.00) | n.s. | 4 | (0.03) | n.s. |
| Clomipramine | 5,451 | 14 | (0.26) | 0.010 | 3 | (0.06) | n.s. | 1 | (0.02) | n.s. | 0 | (0.00) | n.s. | 2 | (0.04) | n.s. |
| Doxepin | 12,010 | 11 | (0.09) | n.s. | 2 | (0.02) | n.s. | 1 | (0.01) | n.s. | 1 | (0.01) | n.s. | 0 | (0.00) | n.s. |
| Maprotiline | 3,031 | 8 | (0.26) | n.s. | 3 | (0.10) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 3 | (0.10) | 0.024† |
| Trimipramine | 11,350 | 14 | (0.12) | n.s. | 3 | (0.03) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 3 | (0.03) | n.s. |
| MAOI | 3,756 | 10 | (0.27) | 0.027† | 1 | (0.03) | n.s. | 1 | (0.03) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. |
| NaSSA | 42,727 | 31 | (0.07) | 0.002* | 7 | (0.02) | 0.009* | 2 | (0.005) | n.s. | 1 | (0.002) | 0.028* | 4 | (0.01) | n.s. |
| Mirtazapine | 40,022 | 25 | (0.06) | <0.001* | 5 | (0.01) | 0.002* | 2 | (0.005) | n.s. | 0 | (0.00) | 0.005* | 3 | (0.01) | n.s. |
| Mianserin | 2,730 | 6 | (0.22) | n.s. | 2 | (0.07) | n.s. | 0 | (0.00) | n.s. | 1 | (0.04) | n.s. | 1 | (0.04) | n.s. |
| SNRI | 31,817 | 46 | (0.14) | n.s. | 18 | (0.06) | n.s. | 1 | (0.003) | n.s. | 15 | (0.05) | <0.001† | 2 | (0.01) | n.s. |
| Duloxetine | 6,551 | 9 | (0.14) | n.s. | 4 | (0.06) | n.s. | 0 | (0.00) | n.s. | 2 | (0.03) | n.s. | 2 | (0.03) | n.s. |
| Venlafaxine | 25,397 | 37 | (0.15) | n.s. | 14 | (0.06) | n.s. | 1 | (0.004) | n.s. | 13 | (0.05) | <0.001† | 0 | (0.00) | 0.025* |
| SSRI | 63,651 | 51 | (0.08) | <0.001* | 7 | (0.01) | <0.001* | 0 | (0.00) | 0.007* | 1 | (0.001) | <0.001* | 6 | (0.01) | n.s. |
| Citalopram | 17,844 | 14 | (0.08) | n.s. | 1 | (0.01) | 0.015* | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 1 | (0.01) | n.s. |
| Escitalopram | 16,776 | 9 | (0.05) | 0.009* | 0 | (0.00) | 0.003* | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. |
| Fluoxetine | 4,326 | 3 | (0.07) | n.s. | 1 | (0.02) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 1 | (0.02) | n.s. |
| Fluvoxamine | 3,903 | 6 | (0.15) | n.s. | 1 | (0.03) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 1 | (0.03) | n.s. |
| Paroxetine | 9,134 | 9 | (0.10) | n.s. | 1 | (0.01) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 1 | (0.01) | n.s. |
| Sertraline | 11,782 | 10 | (0.08) | n.s. | 3 | (0.03) | n.s. | 0 | (0.00) | n.s. | 1 | (0.01) | n.s. | 2 | (0.02) | n.s. |
| Other AD** | 12,026 | 23 | (0.19) | 0.028† | 5 | (0.04) | n.s. | 2 | (0.02) | n.s. | 3 | (0.02) | n.s. | 1 | (0.01) | n.s. |
| Reboxetine | 3,210 | 10 | (0.31) | 0.010† | 4 | (0.12) | n.s. | 2 | (0.06) | 0.042† | 1 | (0.03) | n.s. | 1 | (0.03) | n.s. |
| Trazodone | 5,655 | 8 | (0.14) | n.s. | 1 | (0.02) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. | 0 | (0.00) | n.s. |
Total prescriptions (only ADs with more than 2 000 prescriptions are depicted) of different antidepressants (ADs) and incidence rates of severe cardiovascular adverse drug reactions (ADRs) during AD treatment.
†Significantly higher risk for cardiovascular ADRs compared to the risk of all ADs. *Significantly lower risk for cardiovascular ADRs compared to the risk of all ADs. **Including also agomelatine, bupropion, and nefazodone, which had less than 2 000 prescriptions in this time span. Numbers of assessed cases differ from the sum of numbers for single ADs, due to antidepressant drug combinations and multiple imputations of different antidepressants. AD, antidepressant; ADR, adverse drug reaction; MAOI monoamine oxidase inhibitors; NaSSA noradrenergic and specific serotonergic antidepressant; SNRI, serotonin noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant. Significance level was set to p = 0.05 (two-tailed).
Fig. 2.
Incidence rates (%) of severe cardiovascular adverse drug reactions during different antidepressant compounds. (A) AD compounds imputed alone and in combination (total cases 198), (B) AD compounds imputed alone (total cases 63). Only ADs with more than 2000 prescriptions are depicted. Incidence rates are given with their 95% confidence intervals. †Risk for cardiovascular adverse drug reactions enhanced compared to all other ADs. *Risk for cardiovascular ADRs reduced compared to all other ADs. AD, antidepressant; ADR,
Clomipramine had enhanced risks within the general imputations, but the risks were not significantly enhanced within the single imputations. Reduced risks for cardiovascular ADRs were seen when the AD treatment consisted of mirtazapine, citalopram, or escitalopram (imputed alone).
Fatal Cardiovascular ADRs
Five fatal (incidence rate 0.0029%) cardiovascular ADRs during AD treatment were recorded during the observation period. The death of the first patient (male, 76 years, hypertension as pre-existing risk factor) was classified as a probable consequence of an orthostatic collapse during nortriptyline therapy (see Grohmann et al., 1999). The second patient (female, 52 years, hypertension and prior asymptomatic myocardial infarction as pre-existing cardiac risk factors) experienced syncope and hypotension 2 days after mirtazapine was added to a longstanding regime of several antihypertensives and two antipsychotics (fluphenazine and risperidone). She was found dead the following morning. The suspected cause of death was insufficient cardiac perfusion due to drug-induced hypotension. The combination of antihypertensive medication and antipsychotics, as well as mirtazapine, was imputed. The third patient (female, 84 years, hypertension as risk factor) had been on long-term treatment with doxepin, had already suffered from several syncopes, and had had a left bundle branch block on admission. Doxepin dosage was lowered and citalopram was introduced and increased to 20mg per day. Two weeks later the patient experienced another syncope and death was instantaneous. In addition to the left bundle branch block, a QTc prolongation (>500ms) had been documented the day before. A fatal conduction defect due to the combined antidepressive medication was suspected, whereas non-drug related acute myocardial infarction or embolism were possible alternative explanations. The fourth patient (female, 60 years, no pre-existing cardiac illness or risk factors) was found comatose one morning following three weeks of uneventful treatment with trimipramine and clomipramine (TCAs) in combination with olanzapine. An atrioventricular block III (AV-block III) as well as a diffusely-impaired myocardial contractility had been detected in the intensive care unit. The patient died within 24 hours in spite of intensive attempts at reanimation. The AV-block was suspected to be the cause of myocardial ischemia, which led to death. This ADR was judged a possible ADR due to the combination of two TCAs and olanzapine. The fifth patient (male, 85 years, hypertension and coronary arterial disease as risk factors) experienced severe hypotension and collapse following an increase in dosage of the recently introduced tranylcypromine (MAOI) therapy. Due to treatment-resistant depression, tranylcypromine had been added to two low-potency antipsychotics (prothipendyl and melperone) and a longstanding antihypertensive treatment. The patient suffered a cardiac arrest shortly after collapsing and died. Again, severe hypotension due to the drug combination was suspected as crucial for the fatal outcome.
In addition, 13 cases of life-threatening cardiovascular ADRs (during treatment with ADs mostly in combination with antipsychotics) were detected in the observation period between 1993 and 2010. Seven cases of life-threatening arrhythmia, three cases of severe hypotension, and three cases of heart failure were monitored in this group.
For a more detailed statistical analysis, the cardiovascular ADRs were divided into the following four groups: hypotension and collapse, hypertension, heart failure and arrhythmias (see Methods).
Hypotension and Collapse
Between 1993 and 2010, 89 cases of hypotension or collapse (incidence rate 0.053%, all cases) during AD treatment were recorded in the AMSP program. Only 12 cases of hypotension or collapse were found in which ADs were imputed alone (incidence rate 0.01%). Hypotension or collapse made up almost half (45%) of the cardiovascular ADRs in this study. In about one third (30%) of hypotension or collapse cases, TCAs (incidence rate 0.057%, not significant) were imputed to have caused the ADR alone or in combination with other drug groups. Mirtazapine caused 22% of cases of severe hypotension (incidence rate 0.056%). However, there was no significant difference in the risk of this compound compared to all other ADs. MAOIs, SSRIs, SNRIs, or other ADs accounted for 10–15% each of cases of hypotension or collapse. SSRIs showed a significantly lower risk than all other ADs (p = 0.007, imputed alone). Reboxetine (median dosage 2.5mg/day) was revealed to have a higher risk (p = 0.042, imputed alone) for this ADR than all other ADs. Incidence rates and significance levels are given in Table 2 (for ADs imputed alone).
While 64% of the patients developing hypotension during antidepressant treatment were female, the ADR was distributed almost equally between the age groups <65 and ≥65 years (i.e. there was no significant difference in risk between sex or age groups). The analysis of risk factors revealed that 67.5% of the patients with hypotension or collapse during antidepressant treatment showed some risk factors for developing this ADR. The cardiovascular system was commonly affected (52.1%): in 25 cases there had been a pre-existing cardiac disease (25 cases, 28.1%); hypertension treated with antihypertensive drugs was present as a risk factor in 23 out of the 89 cases (25.8%), and the antihypertensives were co-imputed in these cases due to possible additive pharmacodynamic effects; and pre-existing hypotension was documented in 15 cases (16.9%).
Hypertension
Twenty-five cases of hypertension were detected during AD treatment (incidence rate 0.01%, all cases) in the observation period (13% of all reported cardiovascular ADRs in this study). ADs were imputed alone in most of these cases (22 of 25 cases). In 68% SNRIs were imputed to cause the ADR (all cases) and showed a significantly higher risk of this ADR (p < 0.001) in cases in which SNRIs had been imputed alone. Especially venlafaxine (incidence rate 0.05%, median dosage 150mg/day) was revealed to have a significantly higher risk of hypertension (p < 0.001) than all other ADs. The risk for hypertension during the administration of SSRIs was significantly lower (p < 0.001, imputed alone) than that of all other ADs (see Table 2). Only one case of hypertension was recorded during SSRI treatment (sertraline median dosage, 100mg/day).
Risk factors were identified in 52% of the cases. A pre-existing hypertension was documented in 48% of the patients. The proportion of females (72%) affected by hypertension due to antidepressant treatment was higher; this was consistent with the other cardiovascular ADRs reported in this study and reflected the sex distribution of the total study population. Analysis of the age distribution in this group of ADRs showed that 68% of the patients were below the age of 65.
Heart Failure
Heart failure comprised the smallest group of the cardiovascular ADRs (2%) with only five cases recorded. TCAs were imputed in three of these cases, namely clomipramine (one case, daily dosage of 150mg) and nortriptyline (two cases, daily dosages 25mg and 150mg). Another two cases of heart failure were assessed during trazodone treatment (incidence rate 0.04%, daily dosage of 100mg). In two cases the AD treatment was imputed alone for the heart failure (one case of nortriptyline and one of trazodone). The combination of AD and antipsychotic treatment was imputed for the ADR in the other three cases.
All patients with heart failure were female (four patients over 65 years), and each showed at least one pre-existing risk factor (cardiac insufficiency, right ventricular load, mild AV-block, renal insufficiency, and diabetes).
Arrhythmias
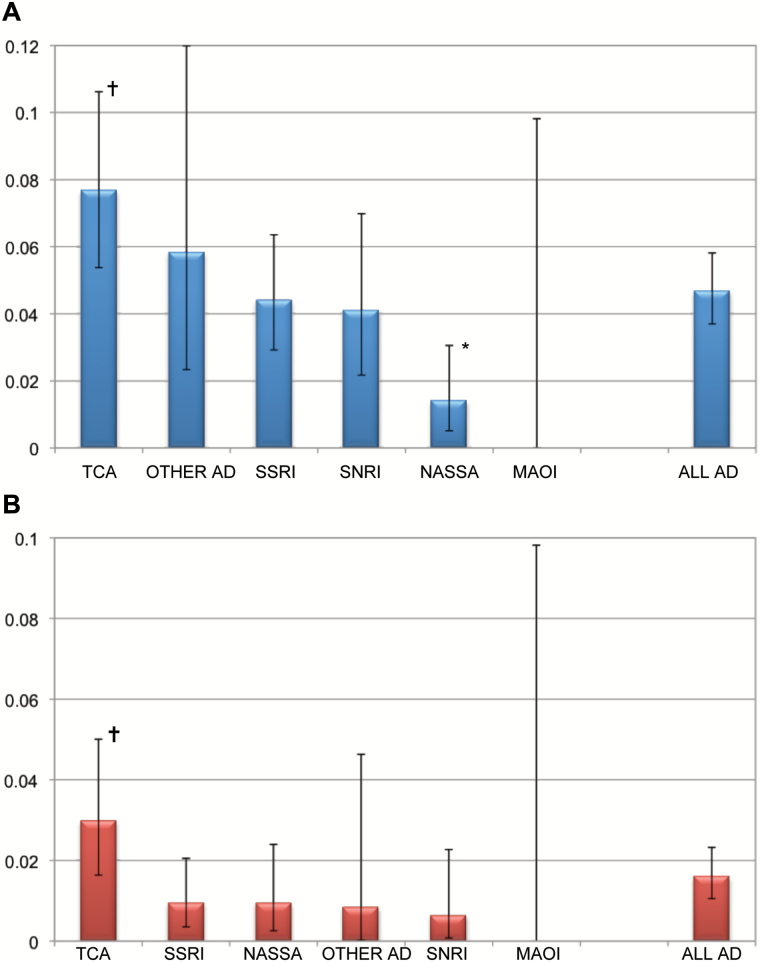
A total of 79 cases of arrhythmia (41% of all cardiovascular ADRs, incidence rate 0.05%) during treatment with ADs (all cases) were documented (see Figure 3). Nine cases of bradycardia, 23 cases of tachycardia, 17 cases of multiple extrasystoles, 1 case of ventricular fibrillation, 6 cases of atrial fibrillation, 4 cases of heart blocks, and 19 cases of prolonged QTc interval were recorded. Antidepressant treatment was imputed alone for the ADR arrhythmia in only 27 (0.02%) cases. The different types of arrhythmia showed incidence rates between 0.002% (heart blocks) and 0.014% (tachycardia). Overall, the treatment with TCAs was connected with a higher risk for arrhythmia (0.08%, all cases, p = 0.02; see Figure 3A). Especially, maprotiline (0.1%, p = 0.024, imputed alone) had a higher risk for this ADR than all other ADs. In contrast, mirtazapine showed a significantly lower risk for arrhythmia than all other ADs (6%, p < 0.001, all cases; see Table 2 and Figure 3A). No case of severe arrhythmia was detected during treatment with the SNRI venlafaxine (imputed alone). Incidence rates for arrhythmia during treatment of ADs are given in Table 2 and Figure 3.
Fig. 3.
Incidence rates (%) of the adverse drug reactions arrhythmia during antidepressant treatment. Incidence rates are given with their 95% confidence intervals, (A) AD imputed alone and in combination (total cases of arrhythmia 79), (B) ADs imputed alone (total cases of arrhythmia 27); †Risk for arrhythmia enhanced compared to all other Ads; *risk for arrhythmia reduced compared to all other ADs. AD, antidepressant; MAOI monoamine oxidase inhibitors; NaSSA noradrenergic and specific serotonergic antidepressant; SNRI, serotonin noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Analysis of sex and age distribution in the arrhythmia group revealed that 62% of those affected were female, and 72% of the patients were below the age of 65 years. Risk factors like pre-existing cardiac problems were documented in 38% of all arrhythmia cases.
Subanalysis: QTc Interval Prolongation
The QTc interval prolongation was monitored in this AMSP observation in 19 cases (incidence 0.01%, AD imputed alone and in combination). A significant QTc interval prolongation was detected in only one case without an increase of the QTc interval above 500ms (prolongation from 300ms to 460ms). All other cases showed a QTc interval prolongation above 500ms (maximum found QTc interval 621ms) during treatment with ADs. TCAs were imputed for more than half of these cases (incidence rate 0.02%). Due to the small number of events, statistical significance cannot be shown. Alternative explanations for the QTc interval prolongation or pre-existing risk factors were suspected in 11 cases. In two cases a hypopotassemia was detected. Five patients showed pre-existing heart damage or hypothyroidism. In one case, hepatitis resulting in a possible liver insufficiency was suspected to have led to higher levels of imputed drugs (no drug levels were measured).
Discussion
Cardiovascular ADRs are severe and potentially life-threatening side effects that are known to occur during AD treatment (Pacher and Kecskemeti, 2004; Vieweg et al., 2009). The ongoing discussion about the possibility of non-beneficial cardiovascular safety profiles for the newer classes of ADs has suggested that the incidence rates of cardiovascular ADRs for these drugs are higher than for TCAs (Coupland et al., 2011).
Our descriptive study identified 198 cases of severe cardiovascular ADRs in a natural setting (169 278 inpatients were exposed to antidepressants and ADRs were monitored). The analysis revealed that the cardiovascular ADR incidence rates and the risk of cardiovascular ADRs were highest for the AD classes of MAOIs and “other Ads” (like reboxetine; all cases). MAOIs were imputed only for the ADR hypotension or collapse, which is in line with previous reported findings (Volz and Gleiter, 1998). However, MAOIs did not show a significantly higher risk for this ADR when imputed alone. Compared to all other ADs, SSRIs and mirtazapine were revealed to have a significantly lower incidence rate of cardiovascular ADRs in this study. Especially in the case of arrhythmia, the class of TCAs showed higher incidence rates than other classes of ADs.
The risk of cardiovascular ADRs like orthostatic hypotension and tachycardia has been reported to have increased during administration of citalopram, which has incidence rates of about 1% (Fernandez et al., 2007). In contrast, our analysis showed a lower incidence rate of citalopram for orthostatic hypotension and tachycardia. Furthermore, this compound was suggested to have an increased risk for QTc interval prolongation, which can lead to life-threatening arrhythmias like torsades de pointes (FDA, 2011; Castro et al., 2013). Only two cases of QTc interval prolongation were reported during treatment with citalopram (17 844 patients exposed) in this study. In these cases, citalopram was co-administered with TCA (trimipramine) and other drug classes (antipsychotics and anticonvulsants). Not a single case of QTc interval prolongation was detected for citalopram, when imputed alone for this ADR between 1993 and 2010. However, in 2013, QTc prolongation >500ms was observed in the AMSP project in a 49-year-old male patient without cardiovascular risk factors during monotherapy with citalopram at a dosage of 60mg (R Grohmann, personal communication). Like citalopram, its active S-isomer escitalopram was imputed for QTc interval prolongation in two cases when administered in combination with antipsychotics. One case was detected in combination with the two antipsychotics amisulpride and quetiapine; the second case was described during a combination of escitalopram and prothipendy. The probability rating for escitalopram was stated as possible in both cases. In our study, escitalopram had an overall significantly lower risk for cardiovascular ADRs than all other ADs. However, this observation generally revealed that the incidence rates of cardiovascular ADRs during different SSRI treatments were lower than previously reported (Hyttel, 1994; Fernandez et al., 2007). According to a recent paper of Girardin et al. (2013), citalopram (including escitalopram) appears to carry a higher risk for QTc prolongation than other ADs.
In agreement with previously reported findings (Feighner, 1995; Thase, 1998), SNRIs showed the highest incidence rates for hypertension than all other ADs. The relative frequency of hypertension was found to be higher during venlafaxine than during duloxetine administration. Our observation is consistent with previous findings (Stahl et al., 2005), which showed a more beneficial cardiovascular safety profile for duloxetine than suspected.
An increased risk of sudden death and ventricular arrhythmia has been reported for the NaSSA mirtazapine compared to SSRI treatment with paroxetine (Leonard et al., 2011). The incidence rates of cardiovascular ADRs during mirtazapine were comparable to those of SSRIs in the present AMSP observation. Mirtazapine had a significantly lower risk of cardiovascular ADRs than all other ADs in the AMSP program.
Socio-demographic variables like age (Coupland et al., 2011) and gender (Vieweg et al., 2009), as well as pre-existing risk factors (Glassman et al., 1993; Schmid et al., 2004), have been suggested to influence the development of cardiovascular ADRs. The findings of this study support this suggestion by showing a high percentage of pre-existing cardiovascular risk factors. For example, patients above age 65 years were observed to have a significantly higher risk of most cardiovascular ADRs.
Limitations
In contrast to prospective, placebo-controlled studies conducted with a randomized design and healthy controls, the data obtained in this naturalistic study have several limitations. First of all, this study is based on data obtained from the drug safety AMSP program that only records severe adverse drug reactions in hospitalized patients, whereas the majority of antidepressants are prescribed to outpatients. However, since hospitalized patients can be viewed as more vulnerable for cardiovascular adverse reactions, due to the severity, complexity, and comorbidity of the disorder, we do not think that our sample is biased towards detection of low rates of cardiovascular side effects. Secondly, an individual bias in detecting severe cardiovascular ADRs cannot be ruled out. The AMSP group is aware of this possible bias, and in order to address this point, reported ADR cases are discussed and evaluated in a systematic way at regional as well as central case conferences. Furthermore, underreporting must be taken into account, and thus the incidence rates of ADRs may be underestimated. This is probably particularly relevant in the case of asymptomatic ADRs such as QTc interval prolongation. These ADRs are difficult to detect without periodic examination of ECGs. However, ECGs were probably not regularly performed as part of the established clinical routine, in particular during SSRI therapy alone, since these drugs have been considered safe for many years as regards their cardiac effects. In addition, differences in surveillance procedures (e.g. rare ECGs under treatment with antidepressants vs. periodic ECG measurements) across hospitals participating in the AMSP program may contribute to an underestimation of specific cardiovascular ADRs. Due to the low number of cardiovascular ADR cases and the fact that the comparisons between different antidepressants were not independent of each other, statistical analyses are interpreted only in a descriptive sense.
In conclusion, the present findings suggest that the cardiovascular safety profiles of newer ADs, especially SSRIs and mirtazapine (but not SNRIs), are more favorable than that of TCAs and MAOIs. Although SSRIs were suspected to have an increased risk of cardiovascular ADRs, we found overall a significantly lower risk for this class compared to all other ADs in the present study. However, the limitations of our observational study have to be considered. Our data show that pre-existing cardiovascular risk factors as well as additive effects with other drug groups like antipsychotics and even long-established cardiac drug regimens should be taken into account in the decision process when prescribing an AD drug to prevent life-threatening cardiovascular ADRs. Furthermore, the cardiovascular safety profile of an AD drug should also be considered in this decision. Further prospective and placebo-controlled investigations are needed to supplement our naturalistic observations.
Statement of Interest
The AMSP Drug Safety Program is based on non-profit associations in the German-speaking countries of Germany, Austria, and Switzerland. During the last decades, financial support was contributed by almost all pharmaceutical companies involved in CNS research. Since 1993 educational and research grants have been given by the following pharmaceutical companies to the three local non-profit associations of the AMSP: (1) Austrian companies: AESCA Pharma GmbH, AstraZeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol–Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform, Servier Austria GmbH, and Wyeth Lederle Pharma GmbH; (2) German companies: Abbott GmbH & Co. KG, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE-O/R/N, Bayer Vital GmbH & Co. KG, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Novartis Pharma GmbH, Nordmark Arzneimittel GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Rhone-Poulenc Rohrer, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH, and Wyeth-Pharma GmbH; and (3) Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol–Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Mepha Schweiz AG/Teva, MSD Merck Sharp & Dohme AG, Organon AG, Pfizer AG, Pharmacia, Sandoz Pharmaceuticals AG, Sanofi-Aventis (Suisse) S.A., Sanofi-Synthe′labo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Vifor SA, Wyeth AHP (Suisse) AG, and Wyeth Pharmaceuticals AG.
Dr Spindelegger received a travel grant from Lundbeck and speaker’s fees from Bristol-Myers Squibb. Dr Papageorgiou received honoraria from RB Pharmaceuticals and Bristol–Myers Squibb. Dr Konstantinidis received honoraria from Affiris, AstraZeneca, Novartis, Pfizer, and Servier, served as a consultant for AstraZeneca, and was a speaker for AstraZeneca, Bristol–Myers Squib, and Janssen. Drs Grohmann and Toto are involved in the project management of AMSP. Dr Engel declared that he had no conflicts of interest. Dr Agelink has received speaker’s fees and conference subsidies from Merz and Eli Lilly. Dr Bleich has received speaker’s fees from Lundbeck and Servier. Dr Greil has been a member of an advisory board for Lundbeck and has received speaker’s fees from AstraZeneca, Lundbeck, and Lundbeck Institute. Dr Ruether recieved Grants from Lundbeck, BMS, Otsouka, Astra Zeneca, and Eli Lilly, and was an advisor for Merz and Servier. Dr Kasper received grant/research support from Bristol–Myers Squibb, Eli Lilly, GlaxoSmithKline, Lundbeck, Organon, Sepracor, and Servier; has served as a consultant or on advisory boards for AOP Orphan Pharmaceuticals AG, AstraZeneca, Bristol–Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Merck Sharp and Dome (MSD), Novartis, Organon, Pfizer, Schwabe, Sepracor, and Servier; and has served on speakers’ bureaus for Angelini, AstraZeneca, Bristol–Myers Squibb, Eli Lilly, Janssen, Lundbeck, Pfizer, Pierre Fabre, Schwabe, Sepracor, and Servier.
Acknowledgments
None.
References
- Astrom-Lilja C, Odeberg JM, Ekman E, Hagg S. (2008). Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf 17:587–592. [DOI] [PubMed] [Google Scholar]
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH. (2013). QT interval and antidepressant use: a cross sectional study of electronic health records. The BMJ 346:f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. (2011). Antidepressant use and risk of adverse outcomes in older people: population based cohort study. The BMJ 343:d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison ET. (1985). Amitriptyline-induced Torsade de Pointes. Successful therapy with atrial pacing. J Electrocardiol 18:299–301. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Ulveling K, Alla V, Abuissa H, Airey K. (2012). Prolonged QTc interval and torsades de pointes induced by citalopram. Tex Heart Inst J 39:68–70. [PMC free article] [PubMed] [Google Scholar]
- EMA PWPP (2011). Pharmacovigilance Working Party (PhVWP). EMA/CHMP/PhVWP/845939/2011. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/10/WC500117061.pdf.
- FDA (2011). FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide).http://www.fda.gov/Drugs/DrugSafety/ucm269086.htm.
- Feighner JP. (1995). Cardiovascular safety in depressed patients: focus on venlafaxine. J Clin Psychiatry 56:574–579. [PubMed] [Google Scholar]
- Fernandez A, Bang SE, Srivathsan K, Vieweg WV. (2007). Cardiovascular side effects of newer antidepressants. Anadolu Kardiyol Derg 7:305–309. [PubMed] [Google Scholar]
- Friberg LE, Isbister GK, Duffull SB. (2006). Pharmacokinetic-pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 61:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin FR, Gex-Fabry M, Berney P, Shah D, Gaspoz JM, Dayer P. (2013). Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry study. Am J Psych 170:1468–1476. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT., Jr. (1981). Cardiovascular effects of therapeutic doses of tricyclic antidepressants. A review. Arch Gen Psychiatry 38:815–820. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Jr., Giardina EV, Kantor SJ, Perel JM, Davies M. (1979). Clinical characteristics of imipramine-induced orthostatic hypotension. Lancet 1:468–472. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Roose SP, Bigger JT., Jr. (1993). The safety of tricyclic antidepressants in cardiac patients. Risk-benefit reconsidered. JAMA 269:2673–2675. [PubMed] [Google Scholar]
- Grimsley SR, Jann MW. (1992). Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm 11:930–957. [PubMed] [Google Scholar]
- Grohmann R, Ruther E, Engel RR, Hippius H. (1999). Assessment of adverse drug reactions in psychiatric inpatients with the AMSP drug safety program: methods and first results for tricyclic antidepressants and SSRI. Pharmacopsychiatry 32:21–28. [DOI] [PubMed] [Google Scholar]
- Grohmann R, Engel RR, Ruther E, Hippius H. (2004). The AMSP drug safety program: methods and global results. Pharmacopsychiatry 37(Suppl 1):S4–11. [DOI] [PubMed] [Google Scholar]
- Hurwitz N, Wade OL. (1969). Intensive hospital monitoring of adverse reactions to drugs. The BMJ 1:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel J. (1994). Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clin Psychopharmacol 9(Suppl 1):19–26. [DOI] [PubMed] [Google Scholar]
- Kasper S, Pletan Y, Solles A, Tournoux A. (1996). Comparative studies with milnacipran and tricyclic antidepressants in the treatment of patients with major depression: a summary of clinical trial results. Int Clin Psychopharmacol 11(Suppl 4):35–39. [DOI] [PubMed] [Google Scholar]
- Kasper S, Moller HJ, Hale A. (2010). The European post-marketing observational sertindole study: an investigation of the safety of antipsychotic drug treatment. Eur Arch Psychiatry Clin Neurosci 260:59–68. [DOI] [PubMed] [Google Scholar]
- Konstantinidis A, Papageorgiou K, Grohmann R, Horvath A, Engel R, Kasper S. (2012). Increase of antipsychotic medication in depressive inpatients from 2000 to 2007: results from the AMSP International Pharmacovigilance Program. Int J Neuropsychop 15:449–457. [DOI] [PubMed] [Google Scholar]
- Kozian R, Syrbe G. (2010). QTc prolongation during treatment with agomelatine. Psychiatr Prax 37:405–407. [DOI] [PubMed] [Google Scholar]
- Lentini S, Rao ML, Schroder R, Luderitz B, Bauriedel G. (2001). QT prolongation and torsade de pointes tachycardia during therapy with maprotiline. Differential diagnostic and therapeutic aspects. Dtsch Med Wochenschr 126:1396–1400. [DOI] [PubMed] [Google Scholar]
- Leonard BE. (1993). The comparative pharmacology of new antidepressants. J Clin Psychiatry 54(Suppl 8):3–15. [PubMed] [Google Scholar]
- Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. (2011). Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 20:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letmaier M, Painold A, Holl AK, Vergin H, Engel R, Konstantinidis A, Kasper S, Grohmann R. (2011). Hyponatraemia during psychopharmacological treatment: results of a drug surveillance programme. Int J Neuropsychop 15:739–748. [DOI] [PubMed] [Google Scholar]
- Letsas K, Korantzopoulos P, Pappas L, Evangelou D, Efremidis M, Kardaras F. (2006). QT interval prolongation associated with venlafaxine administration. Int J Cardiol 109:116–117. [DOI] [PubMed] [Google Scholar]
- Mackin P. (2008). Cardiac side effects of psychiatric drugs. Hum Psychopharmacol 23(Suppl 1):3–14. [DOI] [PubMed] [Google Scholar]
- Martinez C, Assimes TL, Mines D, Dell’aniello S, Suissa S. (2010). Use of venlafaxine compared with other antidepressants and the risk of sudden cardiac death or near death: a nested case-control study. The BMJ 340:c249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Kecskemeti V. (2004). Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des 10:2463–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. (1999). Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem 6:469–480. [PubMed] [Google Scholar]
- Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. (2004). Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther 75:234–241. [DOI] [PubMed] [Google Scholar]
- Sadanaga T, Sadanaga F, Yao H, Fujishima M. (2004). Abnormal QT prolongation and psychotropic drug therapy in psychiatric patients: significance of bradycardia-dependent QT prolongation. J Electrocardiol 37:267–273. [DOI] [PubMed] [Google Scholar]
- Schmid C, Grohmann R, Engel RR, Ruther E, Kropp S. (2004). Cardiac adverse effects associated with psychotropic drugs. Pharmacopsychiatry 37(Suppl 1):S65–69. [DOI] [PubMed] [Google Scholar]
- Seidl LG, Thornton GF, Cluff LE. (1965). Epidemiological Studies of Adverse Drug Reactions. Am J Public Health Nations Health 55:1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Grady MM, Moret C, Briley M. (2005). SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr 10:732–747. [DOI] [PubMed] [Google Scholar]
- Thase ME. (1998). Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 59:502–508. [DOI] [PubMed] [Google Scholar]
- Thase ME, Larsen KG, Reines E, Kennedy SH. (2013). The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol 23:1391–1400. [DOI] [PubMed] [Google Scholar]
- van Noord C, Straus SM, Sturkenboom MC, Hofman A, Aarnoudse AJ, Bagnardi V, Kors JA, Newton-Cheh C, Witteman JC, Stricker BH. (2009). Psychotropic drugs associated with corrected QT interval prolongation. J Clin Psychopharmacol 29:9–15. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Wood MA, Fernandez A, Beatty-Brooks M, Hasnain M, Pandurangi AK. (2009). Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging 26:997–1012. [DOI] [PubMed] [Google Scholar]
- Vollset SE. (1993). Confidence intervals for a binomial proportion. Stat Med 12:809–824. [DOI] [PubMed] [Google Scholar]
- Volz HP, Gleiter CH. (1998). Monoamine oxidase inhibitors. A perspective on their use in the elderly. Drugs Aging 13:341–355. [DOI] [PubMed] [Google Scholar]
- Wilting I, Smals OM, Holwerda NJ, Meyboom RH, de Bruin ML, Egberts TC. (2006). QTc prolongation and torsades de pointes in an elderly woman taking fluoxetine. Am J Psych 163(2):325. [DOI] [PubMed] [Google Scholar]
- Winkler D, Ortner R, Pjrek E, Aschauer H, Kasper S. (2006). Trazodone-induced cardiac arrhythmias: a report of two cases. Hum Psychopharmacol 21:61–62. [DOI] [PubMed] [Google Scholar]
- Zemrak WR, Kenna GA. (2008). Association of antipsychotic and antidepressant drugs with Q-T interval prolongation. Am J Health Syst Pharm 65:1029–1038. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chappell J, Gonzales CR, Small D, Knadler MP, Callaghan JT, Francis JL, Desaiah D, Leibowitz M, Ereshefsky L, Hoelscher D, Leese PT, Derby M. (2007). QT effects of duloxetine at supratherapeutic doses: a placebo and positive controlled study. J Cardiovasc Pharmacol 49:146–153. [DOI] [PubMed] [Google Scholar]