Abstract
Background:
Recently, Silexan, a patented active substance comprised of an essential oil produced from Lavandula angustifolia flowers, has been authorized in Germany as a medicinal product for the treatment of states of restlessness related to anxious mood. Its efficacy has been shown in several forms of anxiety disorders. Findings from preclinical and clinical studies attribute a major role to the serotonin-1A receptor in the pathogenesis and treatment of anxiety.
Methods:
To elucidate the effect of Silexan on serotonin-1A receptor binding, 17 healthy men underwent 2 positron emission tomography measurements using the radioligand [carbonyl-11C]WAY-100635 following the daily intake of 160mg Silexan or placebo for a minimum of 8 weeks (randomized, double-blind, cross-over design). Additionally, structural magnetic resonance imaging and voxel-based morphometry analysis was performed to determine potential effects on gray matter microstructure.
Results:
Serotonin-1A receptor binding potential was shown to be significantly reduced following the intake of Silexan compared with placebo in 2 large clusters encompassing the temporal gyrus, the fusiform gyrus and the hippocampus on one hand as well as the insula and anterior cingulate cortex on the other hand. No effects of Silexan on gray matter volume could be detected in this investigation.
Conclusion:
This positron emission tomography study proposes an involvement of the serotonin-1A receptor in the anxiolytic effects of Silexan.
The study was registered in the International Standard Randomized Controlled Trial Number Register as ISRCTN30885829 (http://www.controlled-trials.com/isrctn/).
Keywords: serotonin-1A receptor, positron emission tomography, lavender oil, Silexan, gray matter volume, structural magnetic resonance imaging
Introduction
Silexan 1 is a patented active substance with an essential oil produced from Lavandula angustifolia flowers by steam distillation. Silexan is formulated in a soft gelatin capsule containing 80mg of lavender oil, and it has been authorized in Germany as a medicinal product for the treatment of states of restlessness related to anxious mood. In a double-blind, placebo-controlled, randomized, clinical trial, Silexan showed superiority over placebo in 221 adults suffering from subsyndromal anxiety disorder (Kasper et al., 2010). Similarly, 80mg of Silexan administered for 6 weeks was shown to be as effective as 0.5mg of lorazepam in 77 patients suffering from generalized anxiety disorder (Woelk and Schlafke, 2010). Additionally, a better tolerability of Silexan compared to paroxetine has been confirmed in a trial including 539 generalized anxiety disorder patients (Kasper et al., 2014). Moreover, the effectiveness of Silexan has been demonstrated in patients with neurasthenia, posttraumatic stress disorder, and somatization disorder regarding the efficiency of sleep and mood improvement (Uehleke et al., 2012).
The mechanisms underlying the anxiolytic effects of the herbal drug remain unknown. However, some authors have suggested a mechanism of action through mediation of gamma-aminobutyric acid (GABA) (Aoshima and Hamamoto, 1999; Cavanagh and Wilkinson, 2002). Schuwald et al. (2013) demonstrated that Silexan inhibited voltage-dependent calcium channels (VOCCs) in synaptosomes, primary hippocampal neurons, and stably overexpressing cell lines, but did not interact with the a2d subunit of VOCCs. Silexan nonselectively reduced the calcium influx through several different types of VOCCs, such as the N type, P/Q type, and T type. In rats, an inhibitory effect of linalool on glutamate binding in the cerebral cortex has been reported, suggesting that this neurochemical effect might be underlying the mode of action of lavender oil (Elisabetsky et al., 1995).
Within the context of these findings, the investigation of essential oils as anxiolytic agents is well justified, especially when considering the wider acceptance of herbal drugs in the general population. Additionally, recent data showed prevalence rates of 14% for anxiety disorders in Europe, which thus represent the most frequent among mental illnesses (Wittchen et al., 2011). Besides the commonly used benzodiazepines, antidepressant compounds are the first-line treatment of anxiety disorders, acting via blocking of serotonin reuptake and including selective serotonin reuptake inhibitors (SSRIs) and serotonin-noradrenaline reuptake inhibitors (Bandelow et al., 2008). This, in turn, is indicative of the fact that alterations within the serotonergic neurotransmitter system represent a neural correlate of anxiety and might even reflect anxiolytic effects in the human brain. In fact, the inhibitory serotonin-1A receptor (5-HT1A), one major modulator of serotonergic neurotransmission, has been shown using in vivo neuroimaging techniques to be substantially involved in the neurobiology of anxiety, with lower levels in affected patients compared with healthy subjects (Neumeister et al., 2004; Lanzenberger et al., 2007; Nash et al., 2008; Akimova et al., 2009). In regard to brain structure, using voxel-based morphometry (VBM) in healthy subjects, an inverse correlation was detected between anxiety measures assessed with psychometric scales and cortical volume in regions of the limbic system and the prefrontal cortex (Spampinato et al., 2009), suggesting that anxiety might also be mirrored in morphological alterations of the brain. Moreover, SSRIs (Kraus et al., 2014) and sex hormones (Witte et al., 2010) have been shown to alter gray matter volumes.
The aim of the present study was to investigate the neurobiological correlates of the anxiolytic effects of Silexan. Based on the findings described above, we hypothesized that the administration of Silexan might have a significant impact on both 5-HT1A receptor binding and gray matter volume, assessed using positron emission tomography (PET) and structural magnetic resonance imaging (MRI), respectively. Regarding the 5-HT1A receptor binding, we expected a reduction after prolonged administration of Silexan compared with placebo analogical to the mode of action described for escitalopram (Spindelegger et al., 2009).
Materials and Methods
Subjects
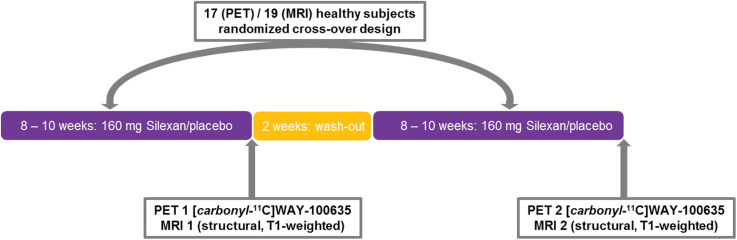
A total of 25 healthy subjects were included in this monocentric, double-blind, randomized, placebo-controlled, cross-over trial at the Medical University of Vienna, Department of Psychiatry and Psychotherapy, after giving written informed consent at the screening visit. For a detailed scheme of the study design, see Figure 1. Subjects were between 20 and 34 years of age (mean age±standard deviation=25.6±3.7). Subjects were deemed mentally healthy by an experienced psychiatrist using the Structured Clinical Interview for DSM-IV Axis I Disorders. Any psychiatric disorder, including drug abuse, neurological illness, severe allergies or history of such, was considered an exclusion criterion. Therefore, to detect relevant abnormalities in regard to physical health, each participant underwent a medical examination, including general physical and neurological status, routine laboratory measurements, urine test strips as well as an electrocardiogram. All subjects were naïve to psycho- pharmacological drugs and had never undergone psychotherapy. Additionally, subjects with a regular daily consumption of more than the equivalent quantity of approximately 20g of alcohol or more than 10 cigarettes were excluded of the trial. The study was approved by the Ethics Committee of the Medical University of Vienna and the General Hospital of Vienna.
Figure 1.
Scheme of the study design.
Administration of the Test Drug Silexan
The test drug was available in soft gelatin capsules containing 80mg of essential oil. The placebo capsules were identical in color, size and shape. The test drug and the packaging including coding of the containers following randomization procedure were provided by Dr. Willmar Schwabe, GmbH & Co. KG, to guarantee that both subjects and clinical investigators were blind to the treatment.
The study was conducted following a cross-over design with randomized treatment allocation separated by a wash-out phase. Subjects were advised to take 1x2 capsules of the investigational product [80mg Silexan or placebo] per day in the morning, preferably after breakfast, which corresponds to a daily dosage of 160mg. The minimum duration of medication intake was set at 8 weeks (56 days) for both treatment periods, either Silexan – placebo or placebo – Silexan (see Figure 1). Therefore, each subject received twice during the study 140 capsules (medication for 56 days plus for the reserve of 14 days) packed in blister cards. All unused drugs and containers had to be returned for drug accountability, and subjects were encouraged to keep a “drug intake diary” for monitoring purposes. The medication intake started the day following drug dispensation, specifically baseline 1 and baseline 2, and was discontinued as soon as both measurements, PET1/MRI1 and PET2/MRI2, were successfully completed. PET and MRI measurements were, when possible, performed on the same day. If the schedule did not allow the performance of both measurements on the same day, then a minimum drug intake of 56 and maximum of 70 days was maintained. Therefore, both measurements took place within a period of 14 days. Additionally, at least 14 days of wash-out between both treatment periods were respected (see Figure 1).
PET and Radiochemistry
Each subject underwent 2 PET scans (PET1 and PET2), which were performed at the Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, Medical University of Vienna, Austria, using a GE Advance PET scanner (General Electric Medical Systems, Milwaukee, Wisconsin) at the end of the respective treatment period, as previously described (Lanzenberger et al., 2007). Briefly, to ensure the covering of the cerebellum in the field of view, the subjects’ heads were placed in the scanner parallel to the orbitomeatal line by means of a laser beam system. A polyurethane molded cushion and straps around the forehead of the subjects were used to steady the head to minimize movements. Additionally, subjects were instructed not to speak or move during the measurements. For correction of tissue attenuation, a 5-minute transmission scan was carried out in 2-dimensional mode by retractable 68Ge rod sources. Subsequently, dynamic PET scans were acquired in 3-dimensional mode, and the measurements started simultaneously with bolus injection of [carbonyl-11C]WAY-100635 diluted in phosphate-buffered saline in a cubital vein. The radioligand was prepared at the Cyclotron Unit of the PET Center according to previously published methods (Wadsak et al., 2007). The ave- rage administered dose of the tracer was 254.9±49.00 MBq with a specific activity of 27.8±27.5 at PET1 and 238.9±36.0 MBq with a specific activity of 58.1±66.6 at PET2. Total acquisition time was 90 minutes. The emission data were scatter and attenuation corrected, and the final images comprised a spatial resolution of 4.36mm full-width at half-maximum 1cm next to the center of the field of view (matrix 128x128, 35 slices). Because of motion artifacts during the measurement, one subject was excluded from the analysis of the PET data.
PET Data Preprocessing and 5-HT1A Receptor Quantification
MRI
At the end of both treatment periods, structural magnetic resonance (MRI1 and MRI2) scans were acquired from each subject using a 3-Tesla Archieva MR-scanner (Philips Medical Systems) at the Diagnosezentrum Urania, Vienna, Austria. Each scan session included a high-resolution T1-weighted image sequence (TR=11ms, TE=4.6ms, voxel size=0.47x0.47mm, sagittal slice thickness=0.88mm).
VBM
The obtained images were segmented into gray matter, white matter and cerebrospinal fluid compartments using the VBM8 toolbox for SPM8 (statistical parametric mapping, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London, London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm/software/spm8), applying the Diffeomorphic Anatomical Registration using Exponentiated Lie algebra algorithm (Ashburner, 2007). Hence, gray matter maps were spatially normalized to a gray matter template representing the stereotaxic standardized Montreal Neurological Institute space at a voxel size of 1.5x1.5x1.5mm by this procedure (Kraus et al., 2013). To adjust for effects of nonlinear spatial normalization, maps were converted to gray matter volume by multiplication with the Jacobian determinants obtained by the spatial normalization algorithm (Ashburner and Friston, 2009). The images were smoothed with a Gaussian filter of 8x8x8mm full-width at half-maximum.
PET Data Preprocessing and 5-HT1A Receptor Quantification
PET scans were motion-corrected and spatially normalized to a 5-HT1A receptor distribution template in Montreal Neurological Institute space using SPM8. Spatial normalization of the PET data was carried out using the respective T1-weighted MR images acquired from each subject (Meyer et al., 1999). First PET scans were summed across time and co-registered to the corres- ponding gray matter segment (PET1 to MRI1 and PET2 to MRI2). The transformation matrix obtained from spatial normalization of the MR images (see VBM section) was then applied to the (summed and dynamic) co-registered PET images (Kranz et al., 2012). To prevent unnecessary interpolation, reslicing of dynamic PET images was done only once after application of the nonlinear transformation matrix (while linear transformations were stored in the NIfTI-header). Whole-brain 5-HT1A binding potential maps (BPND; Innis et al., 2007) were computed in PMOD 3.3 (PMOD Technologies, Ltd., Zurich, Switzerland) as previously described (Hahn et al., 2010; Baldinger et al., 2013) with the noninvasive Logan plot (Logan et al., 1996). The Logan plot displays 2 main advantages: specifically, that it makes no assumption about compartmental configuration and that it shows robustness in presence of noise (Logan et al., 1996), which makes it particularly useful for voxel-wise analyses. Here, the clearance rate of the radiotracer from the reference region to plasma (k2’) was calculated from the insula (receptor-rich region) and cerebellar gray matter (receptor-poor region) (Ichise et al., 2003; Varnas et al., 2004) using the simplified reference tissue model 2 (Wu and Carson, 2002). These regions of interest were taken from an automated anatomical labeling-based atlas (Tzourio-Mazoyer et al., 2002; Savli et al., 2012), whereas the cerebellar gray matter (excluding vermis and sagittal sinus) served as reference region because of negligible specific receptor binding in this area as previously described (Hall et al., 1997; Hahn et al., 2010). The BPND maps were smoothed with a Gaussian filter of 8x8x8mm FWHM.
Statistical Analysis
To compare 5-HT1A receptor binding potential and gray matter volume after Silexan intake versus placebo, a repeated-measures analysis of variance (ANOVA) was computed in SPM8 including 17 (5-HT1A BPND) and 19 subjects (gray matter volume), respectively. To exclude a potential influence of the sequence (Silexan − placebo versus placebo − Silexan), this factor was included as factor of no interest in the statistical analysis. Statistical tests were evaluated at a significance level of α=0.05 corrected for multiple comparisons with the family-wise error rate (FWE) at cluster level following α=0.005 uncorrected voxel level (Friston, 2007). In other words, a cluster represents an ensemble of voxels, which are significant and connected in 3-dimensional space. These clusters were then tested for significance of their spatial extent. Regions of no interest (eg, skull, cerebral blood vessels) were excluded from the statistical analysis.
Results
A total of 25 subjects was included in the study and randomized for the sequence of investigational products. Because of sche- duling interferences and technical problems with the PET scanner, 6 healthy subjects dropped out (absence of available scan time within the predetermined time frame). Overall, 19 subjects terminated study participation regularly. Two of these subjects were excluded from the analysis of PET data due to deficient radioligand synthesis (low specific activity of [carbonyl-11C]WAY-100635). Specification of protocol violations and definition of analysis sets were fixed before breaking the blind. The durations of the treatment periods 1 and 2 were 65.6±6.5 (minimum 57 and maximum 80 days) and 65.4±4.3 days (minimum 58 and maximum 72 days), respectively (n=17 subjects with valid PET data). The wash-out period corresponded to a duration of 16.6±7.8 days (minimum 14 and maximum 47 days).
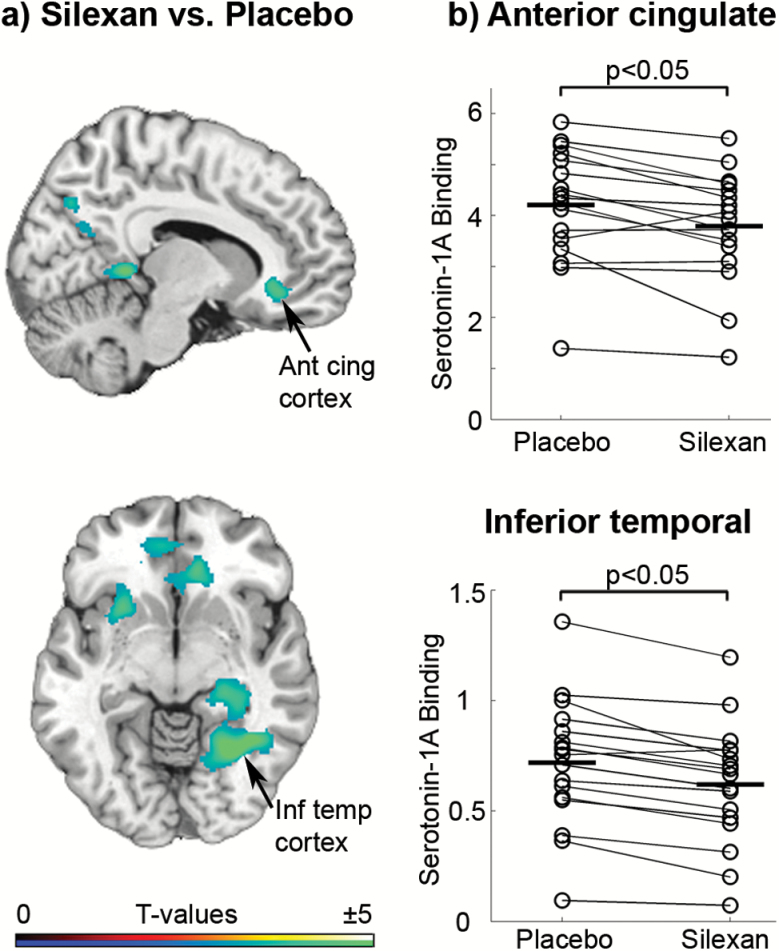
Regarding the PET data, repeated-measures ANOVA revealed a significant difference of 5-HT1A receptor binding potential following 8 weeks of treatment with Silexan as compared with placebo intake (n=17) (Figures 2 and 3a). Significant differences were detected in 2 clusters displaying a reduced 5-HT1A BPND after Silexan intake compared with placebo (Figure 3a): one cluster encompassed the left inferior temporal gyrus, left fusiform gyrus, left lingual gyrus, left calcarine gyrus, left precuneus, and left hippocampus (peak t value=6.64, k=5334, P<.05, FWE corrected at cluster-level) (Figure 3b), and the second cluster included the right insula, right inferior orbitofrontal gyrus, right anterior cingulate cortex, right medial superior frontal gyrus, right putamen, and right caudate (peak t value=6.08, k=4812, P<.05, FWE corrected at cluster-level) (Figure 3b). Results are summarized in Table 1. There was no region showing higher 5-HT1A BPND after intake of Silexan compared with placebo (Figure 3a).
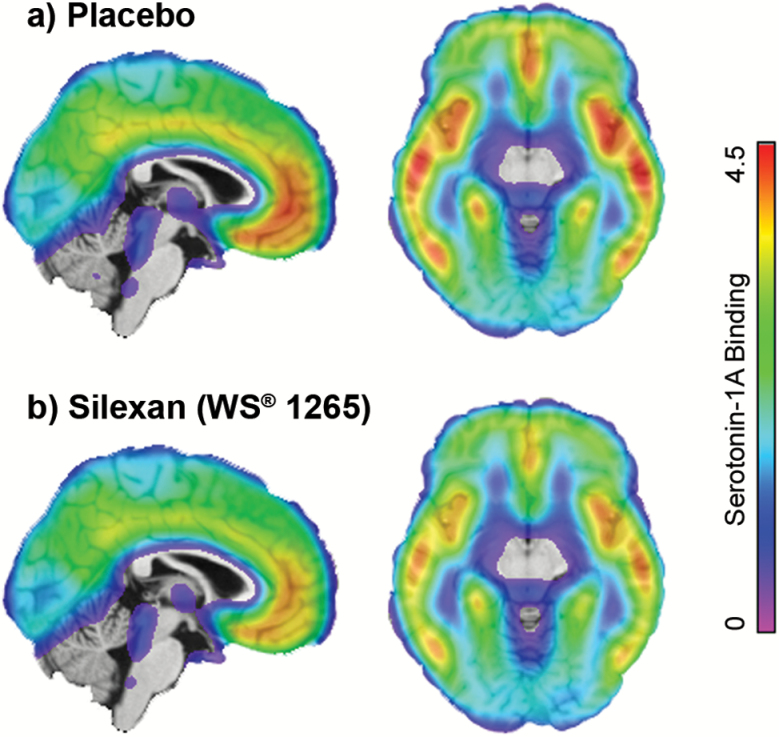
Figure 2.
Average serotonin-1A receptor (5-HT1A) binding potential of 17 healthy men after chronic administration of (a) placebo and (b) Silexan. Positron emission tomography (PET) data are superimposed on a sagittal and axial view of a structural magnetic resonance imaging (MRI) template. The color table indicates the 5-HT1A receptor binding potential. A reduced 5-HT1A receptor binding after the intake of Silexan can be observed in several brain regions.
Figure 3.
(a) Differences in serotonin-1A receptor (5-HT1A) binding potential following the administration of Silexan vs placebo for a minimum of 8 weeks, presented on a sagittal and axial view, superimposed on a structural magnetic resonance imaging (MRI) template. Shown are t values of the analysis of variance (ANOVA) (voxel level: P<.005 uncorrected for multiple comparisons; cluster level: P<.05, family-wise error rate [FWE] corrected) displaying significant diffe rences in 2 interconnected clusters (encompassing the inferior temporal gyrus, fusiform gyrus, lingual gyrus, precuneus, and left hippocampus on the one hand and the right insula, orbitofrontal gyrus, and anterior cingulate cortex on the other hand). Peak t values can be observed in the insula, fusiform gyrus, and anterior cingulate cortex. The color table indicates the t values. (b) Scatter plots displaying regional differences of 5-HT1A receptor binding after the intake of Si lexan vs placebo in the anterior cingulate cortex and inferior temporal lobe. In both regions, a significant reduction of 5-HT1A receptor binding potential after the intake of Silexan as compared with placebo can be observed (P<.05 FWE-corrected cluster level). Ant cing cortex, anterior cingulate cortex; Inf temp cortex, inferior temporal cortex.
Table 1.
Repeated-Measures ANOVA Comparing 5-HT1A Receptor Binding After Intake of Silexan vs Placebo
| Anatomical Region (AAL) | MNI Coordinates | Statistics | ||||
|---|---|---|---|---|---|---|
| x | Y | z | T | Cluster size | Difference, % | |
| Inferior temporal gyrus_L | −38 | −52 | −6 | −6.64 | 5334* | −15.5±10.4 |
| Fusiform gyrus_L | −30 | −57 | −6 | −6.42 | 5334* | −16.8±11.2 |
| Insula_R | 34 | 0 | 20 | −6.08 | 4812* | −18.1±13.6 |
| Lingual gyrus_L | −12 | −42 | 2 | −5.48 | 5334* | −14.1±11.0 |
| Precuneus_R | 9 | −52 | 16 | −5.16 | 1881 | −12.7±11.3 |
| Middle cingulum_L | −10 | −27 | 51 | −4.69 | 1211 | −15.1±12.3 |
| Lingual gyrus_R | 14 | −90 | −8 | −4.51 | 933 | −11.8±12.1 |
| Calcarine gyrus_L | −26 | −57 | 8 | −4.33 | 5334* | −16.8±18.9 |
| Hippocampus_L | −26 | −28 | −8 | −4.26 | 5334* | −14.4±16.3 |
| Putamen_L | −24 | 6 | −6 | −4.17 | 449 | −12.6±12.4 |
| Cerebellum_R | 12 | −66 | −50 | −4.02 | 368 | −32.7±39.1 |
| Putamen_R | 24 | −6 | 8 | −5.23 | 4812* | −30.2±24.4 |
| Anterior cingulum_R | 4 | 45 | −4 | −3.88 | 4812* | −9.8±11.9 |
| Middle temporal gyrus_L | −66 | −24 | −8 | −3.71 | 445 | −12.4±14.9 |
| Medial superior frontal gyrus_R | 8 | 56 | 2 | −3.44 | 4812* | −9.9±13.3 |
| Middle temporal gyrus_R | 50 | −4 | −18 | −3.43 | 605 | −7.7±12.3 |
| Precuneus_L | −9 | −64 | 26 | −3.16 | 5334* | −9.3±14.2 |
| Caudate_R | 8 | 21 | 9 | −3.14 | 4812* | −9.9±43.5 |
| Inferior orbitofrontal gyrus_R | 20 | 27 | −20 | −2.97 | 4812* | −9.5±15.3 |
Abbreviations: AAL, automated anatomical labeling; L, left; MNI, Montreal Neurologic Institute; R, right.
Regional peak t values are shown following repeated-measures analysis of variance (ANOVA) comparing serotonin-1A receptor (5-HT1A) receptor binding potential after prolonged intake of Silexan to placebo. t values are given for comparisons with P<.005 uncorrected for multiple comparisons, voxel level; *P<.005 family-wise error rate (FWE) corrected, cluster level. Only clusters with >284 voxels are shown as given by statistical parametric mapping (SPM8) expected voxels per cluster. Same cluster size indicates that regions are interconnected within one cluster. Percentage difference (mean± SD) is computed as nondisplaceable binding potential (BPND _Silexan – BPND_Placebo) / BPND_Placebo * 100.
Repeated-measures ANOVA based on MRI data (n=19) revealed inconsistent and very limited findings regarding the effect of medication intake, Silexan versus placebo (Table 2). Whereas gray matter volume was found to be reduced in the fusiform gyrus, gray matter volume was heightened in the pre- central and middle frontal gyri as well as the precuneus and superior temporal pole after ingestion of Silexan compared with placebo (all P<.005 uncorrected on voxel-level, none withstan ding FWE-correction) (Table 2).
Table 2.
Repeated-Measures ANOVA Comparing Gray Matter Volume After Intake of Silexan vs Placebo
| Anatomical Region (AAL) | MNI Coordinates | Statistics | ||||
|---|---|---|---|---|---|---|
| x | y | z | T | Cluster size | Difference, % | |
| Fusiform gyrus_R | 32 | −52 | −4 | −4.21 | 208 | −2.1±2.3 |
| Precentral gyrus_L | −54 | 15 | 39 | 5.05 | 378 | 2.8±2.7 |
| Precuneus L | −3 | −54 | 12 | 4.07 | 88 | 1.2±1.4 |
| Middle frontal gyrus R | 28 | 34 | 39 | 4.04 | 86 | 1.1±1.5 |
| Middle frontal gyrus L | −22 | 56 | 32 | 3.55 | 102 | 1.9±2.5 |
| Paracentral lobe R | 9 | −28 | 64 | 3.96 | 87 | 3.0±3.7 |
| Superior temporal pole_R | 56 | 16 | −9 | 3.10 | 227 | 6.6±7.6 |
Abbreviations: AAL, automated anatomical labeling; L, left; MNI, Montreal Neurologic Institute; R, right.
Regional peak t values are shown following repeated-measures ANOVA comparing gray matter volume after chronic intake of Silexan compared with placebo. t Values are given for comparisons with P<.005 uncorrected for multiple comparisons, voxel level. Only cluster with an extent of k>85 voxels are shown as given by SPM8’s expected voxels per cluster. None of the results withstand correction for multiple comparisons at α=0.05 FWE-corrected cluster level. Percentage difference (mean±SD) is computed as gray matter volume (GMVSilexan – GMVPlacebo) / GMVPlacebo * 100
Discussion
Evidence from preclinical and clinical research clearly demonstrates a major role of the serotonergic neurotransmitter system, particularly the 5-HT1A receptor, in the neurobiology of anxiety (Akimova et al., 2009). 5-HT1A receptor knockout mice were shown to be more fearful in several behavioral tests compared with wild-type mice (Heisler et al., 1998; Parks et al., 1998). More specifically, Gross et al. (2002) suggested that the posts y naptic 5-HT1A receptor, located on GABAergic and glutamatergic neurons in the forebrain, rather than autoinhibitory 5-HT1A receptors in the raphe nuclei, were able to restore normal levels of anxiety as observed in wild-type mice. This indicates that 5-HT1A hetero-receptors are essential to a “healthy” expression of anxiety in rodents.
The anxiolytic effect of lavender (Lavandula angustifolia) essential oil, which has been in use in folk medicine for the treatment of anxiety since ancient times, was brought in relation with the 5-HT1A receptor in a recently published study performed in mice (Chioca et al., 2013). Chioca et al. demonstrated that the admi nistration of lavender essential oil via inhalation leads to reduced anxiety-related behavior as determined using the marble-burying test, which is similar to systemic admi nistration of 8-OH-DPAT, a 5-HT1A receptor agonist. Moreover, WAY100635, a 5-HT1A receptor antagonist, was able to reverse the anxiolytic-like effect of both lavender essential oil and 8-OH-DPAT in this behavioral model of anxiety (Chioca et al., 2013). Interestingly, lavender oil did not alter [3H]flunitrazepam binding to the benzodiazepine site on the GABAA receptor. These findings provide strong evidence that the anxiolytic effect of la vender essential oil in rodents is mediated via the serotonergic neurotransmitter system, particularly the 5-HT1A receptor, and not through a GABAergic mechanism. This is in accordance with results discussed in our PET study showing variations in 5-HT1A receptor binding after intake of Silexan, which contains lavender oil, in healthy men.
5-HT1A receptors were suggested to modulate the SSRI-induced increase of synaptic serotonin levels, as the latter were found to be heightened after administration of fluoxetine in 5-HT1A receptor knockout mice (Parsons et al., 2001). Similarly, in humans, 5-HT1A receptor antagonists, for example, pindolol, have been shown to accelerate the therapeutic efficacy of SSRIs in depression by counteracting the 5-HT1A auto-receptor–induced reduction of cell firing that can be observed early in treatment (Artigas et al., 1994). In vivo neuroimaging findings investigating the role of the 5-HT1A receptor in anxiety support the assumption of reduced 5-HT1A receptor densities in anxiety disorders. In 19 healthy subjects, an inverse relationship of 5-HT1A receptor binding and anxiety traits was reported in 4 cortical brain areas (Tauscher et al., 2001), which is in line with earlier findings described in knockout mice. Neumeister et al. (2004) found lower 5-HT1A receptor binding distributions in the anterior cingulate cortex, the posterior cingulate cortex and the raphe nuclei in patients with panic disorder with or without comorbid depression compared with healthy control subjects. In accordance with this, our group previously reported decreased 5-HT1A receptor binding potentials in the amygdala, insula, and anterior cingulate cortex in 12 patients suffering from social anxiety disorder as compared with healthy subjects using PET and the radio tracer [carbonyl-11C]WAY-100635. Additionally, cortisol plasma levels were shown to negatively correlate with 5-HT1A receptor binding in the amygdala, hippocampus, and retrosplenial cortex in social phobia (Lanzenberger et al., 2010). These findings clearly indicate that low 5-HT1A receptor binding might mirror a surrogate of anxiety in the human brain, although this property might not be anxiety specific. Reduced 5-HT1A receptor binding might play a role in both affective and anxiety disorders, which is supported by the high comorbidity rates between these disorders and the fact that SSRIs are effective in both (Savitz et al., 2009). Nonetheless, considering the lack of evidence regarding an effect of lavender oil in major depression, the variations of 5-HT1A receptor binding shown in this study were attributed to a potential anxiolytic mechanism of action of Silexan.
The main finding of this investigation is the detection of a widespread reduction of the 5-HT1A receptor binding potential following prolonged administration of the herbal drug Silexan compared with placebo in 17 healthy men. This is in concert with our initial assumption that the anxiolytic effect of lavender oil might be reflected in variations of the 5-HT1A receptor, a major player in the neurobiology of anxiety. In fact, prolonged intake of Silexan is accompanied by a reduction in 5-HT1A receptors in the hippocampus and the anterior cingulate cortex, which is similar to the effects of escitalopram (Spindelegger et al., 2009). Interestingly, in anxiety disorders, response to SSRIs was not shown to be associated with elevated 5-HT1A receptor levels as one might intuitively assume. In fact, 5-HT1A receptor binding potential was shown to be reduced after 12 weeks of escitalopram intake in patients suffering from anxiety disorders compared with baseline in the subgenual cortex, hippocampus and posterior cingulate cortex (Spindelegger et al., 2009). This is in accordance with investigations in depressed patients similarly exhibiting reduced 5-HT1A receptor densities compared with healthy subjects (Drevets et al., 1999). Just as shown for SSRIs in anxiety disorders (Spindelegger et al., 2009), electroconvulsive therapy was accompanied by a downregulation of 5-HT1A receptor binding in depressed patients (Lanzenberger et al., 2013). Additionally, Gray et al. (2013) determined a decrease of 5-HT1A receptor binding in depressed patients following short-term treatment with SSRIs. This might be explained by the fact that blockage of the serotonin transporter by SSRIs in the raphe region leads to an increase of extracellular serotonin levels, as has been shown in rodents. This in turn causes a desensitization of somatodendritic 5-HT1A auto-receptors (Le Poul et al., 1995), which results in a disinhibition of serotonergic cell firing (Bundgaard et al., 2006). In the periphery, enhancement of serotonin levels by chronic SSRI administration is made responsible for a desensitization of 5-HT1A heteroreceptors in the hypothalamus, amygdala and orbitofrontal cortex (Li et al., 1996 1997; Bosker et al., 2001; El Mansari and Blier, 2005). However, this mechanism of action is not directly applicable to Silexan, since this substance did not reveal any affinity to SERT (Schuwald et al., 2013). Essential oils, including lavender oil, seem to have no specific target because of their great number of constituents; due to their lipophilicity, they penetrate the cell wall and cytoplasmic membrane, thereby disrupting the structure of the different layers of fatty acids, polysaccharides, and phospholi pids that in turn might affect the activity of ion channels and protein-coupled receptors (Bakkali et al., 2008). This might be the first step in a complex chain of molecular effects, ultimately altering the density of 5-HT1A receptors (Chioca et al., 2013).
The regions displaying the most prominent variations in 5-HT1A receptor binding in this study, such as the hippocampus and anterior cingulate cortex, represent areas of the limbic system known to be involved in emotion regulation, including the expression of fear, and were repeatedly shown to exhibit altered 5-HT1A receptor binding (Liotti et al., 2000; Tauscher et al., 2001; Neumeister et al., 2004). Our findings are evident in 2 prominent clusters encompassing the temporal gyrus, fusiform gyrus, and hippocampus on one hand and the insula and anterior cingulate on the other hand. This is in line with earlier functional MRI studies reporting changes of brain activity in similar brain areas (Liotti et al., 2000). The fact that we did not detect any brain region exhibiting elevated 5-HT1A receptor binding after prolonged intake of Silexan underlines the conclusiveness of our findings. Interestingly, we were not able to show variations of 5-HT1A receptor binding in the midbrain, as described by other authors after the intake of SSRIs in major depression and panic disorder (Sargent et al., 2000; Neumeister et al., 2004). However, our finding is in line with results revealed by the study performed by Spindelegger et al. (2009).
Many studies investigating morphometric brain changes in various manifestations of anxiety disorders have been conducted (Radua et al., 2010). Alterations have been detected, among other regions, in the amygdala, hippocampus and para hippocampal area, and anterior cingulate cortex (Massana et al., 2003; Yamasue et al., 2003; Hayano et al., 2009). In healthy subjects, an inverse correlation between anxiety measures and cortical volumes of limbic brain areas and the prefrontal cortex has been described (Spampinato et al., 2009). Studies investigating the effects of anxiolytics on brain morphology, however, remain scarce. Kraus et al. (2013) postulated significant gray matter increases in the posterior cingulate cortex and ventral cuneus after 10 days of SSRI intake. Within this context, it seems remarkable that we were not able to show consistent alterations of gray matter after prolonged intake of Silexan. Additionally, using multimodal imaging combining PET and structural MRI, regional gray matter volume was shown to be positively associated with 5-HT1A heteroreceptor binding in the temporal cortex and hippocampus on both hemispheres (Kraus et al., 2012). Furthermore, autoreceptor binding in the midbrain raphe was positively associated with gray matter volume of forebrain projection sites (Kraus et al., 2012). These findings clearly suggest that one would expect altered gray matter volume following the intake of Silexan, as we detected significant effects of lavender oil on 5-HT1A receptor binding potential in several brain regions. This inconsistency might be the result of a too-low sample size in regard to structural MRI studies to detect effects of Silexan on brain morphology (Radua et al., 2010).
A limitation of our study is that no psychological assessments were performed to evaluate measures of anxiety of the participants in order to determine a potential correlation between 5-HT1A receptor binding and Silexan-induced changes in anxiety-related traits. Therefore, we can only indirectly conclude that the alteration of 5-HT1A receptor binding reported in our study represents a mechanism underlying the anxiolytic effects of Silexan. In fact, the objective of this study was not to determine a clinical effect of Silexan, as this has already been shown in various indications (Kasper et al., 2010; Woelk and Schlafke, 2010), but rather to investigate the biological mechanism underlying this effect. Moreover, as the study population was a group of physically and mentally healthy men (as determined by Structured Clinical Interview for DSM-IV Axis I Disorders), the performance of psychometric scales to record anxiety symptoms does not seem appropriate, as we must assume very low scores that cannot be included in a meaningful statistical analysis.
Moreover, we did not include female subjects in this PET study in order to minimize the case number, as 5-HT1A receptor binding was shown to display gender differences (Parsey et al., 2002). Further investigations in females are therefore required, especially as the latter are more susceptible to developing anxiety disorders than men (Wittchen et al., 2011). The sample size can be considered as quite small; however, when reviewing the literature for imaging studies, our sample size of 17/19, for PET/MRI respectively, seems appropriate and comparable with other investigations. Naturally, a power calculation was performed prior to the study on the basis of another PET study in anxiety disorder patients showing significant differences in 5-HT1A bi nding when 12 patients and 18 healthy subjects were contrasted (Lanzenberger et al., 2007). Regarding the nonsignif icant differences in the VBM analysis, it is possible that the low sample size impeded detection of subtle changes in gray matter volume. Still, our data indicate that changes in gray matter are less pronounced than those in 5-HT1A binding.
Conclusions
This is the first study investigating the effects of Silexan using cutting-edge neuroreceptor imaging techniques in vivo. To sum up, this PET investigation shows a reduced 5-HT1A receptor binding in healthy subjects following the daily administration of 160mg of Silexan for a minimum of 8 weeks compared with placebo. This is in agreement with SSRI- or ECT-induced reduction in 5-HT1A binding and might be a general mechanism in therapeutic action of anxiolytic or antidepressant treatment. The lack of structural volumetric changes as demonstrated with MRI indicates that this reduction is mainly based on changes in receptor expression or affinity. Based on these findings, we postulate that changes of the serotonergic system, more specifically of the 5-HT1A receptor, represent a mechanism underlying the anxiolytic effect of Silexan.
Statement of Interest
Without any relevance to this work, S. Kasper declares that he has received grant/research support from Eli Lilly, Lundbeck A/S, Bristol-Myers Squibb, Servier, Sepracor, GlaxoSmithKline, Organon, and Dr. Willmar Schwabe GmbH & Co. KG and has served as a consultant or on advisory boards for AstraZeneca, Austrian Sick Found, Bristol-Myers Squibb, German Research Foundation (DFG), GlaxoSmithKline, Eli Lily, Lundbeck A/S, Pfizer, Organon, Sepracor, Janssen, and Novartis, and has served on speakers’ bureaus for AstraZeneca, Eli Lilly, Lundbeck A/S, Servier, Sepracor, and Janssen. R. Lanzenberger received travel grants or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH & Co. KG and Roche Austria GmbH. M. Mitterhauser and W. Wadsak received speaker honoraria from Bayer. P. Baldinger received travel grants from Roche Austria GmbH and AOP Orphan Pharmaceuticals AG.
Acknowledgments
This research was supported by Dr. Willmar Schwabe GmbH & Co. KG. We thank the PET team at the Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, for technical support, especially J. Ungersboeck for initial radio tracer preparation, and the staff at the Department of Psychiatry for medical support. We thank S. Klement, A. Dienel, and S. Schläfke for revising the manuscript and S. Pichler for clinical support.
Silexan is an active substance manufactured by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.
References
- Akimova E, Lanzenberger R, Kasper S. (2009). The serotonin-1A receptor in anxiety disorders. Biological Psychiatry 66:627–635. [DOI] [PubMed] [Google Scholar]
- Aoshima H, Hamamoto K. (1999). Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci Biotechnol Biochem 63:743–748. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. (1994). Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry 51:248–251. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. (2009). Computing average shaped tissue probability templates. Neuroimage 45:333–341. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. (2008). Biological effects of essential oils--a review. Food Chem Toxicol 46:446–475. [DOI] [PubMed] [Google Scholar]
- Baldinger P, Hahn A, Mitterhauser M, Kranz GS, Friedl M, Wadsak W, Kraus C, Ungersbock J, Hartmann A, Giegling I, Rujescu D, Kasper S, Lanzenberger R. (2013). Impact of COMT genotype on serotonin-1A receptor binding investigated with PET. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- Bandelow B, et al. (2008). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry 9:248–312. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. (2001). Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem 76:1645–1653. [DOI] [PubMed] [Google Scholar]
- Bundgaard C, Larsen F, Jorgensen M, Gabrielsson J. (2006). Mechanistic model of acute autoinhibitory feedback action after administration of SSRIs in rats: application to escitalopram-induced effects on brain serotonin levels. Eur J Pharm Sci 29:394–404. [DOI] [PubMed] [Google Scholar]
- Cavanagh HMA, Wilkinson JM. (2002). Biological activities of lavender essential oil. Phytotherapy Research 16:301–308. [DOI] [PubMed] [Google Scholar]
- Chioca LR, Ferro MM, Baretta IP, Oliveira SM, Silva CR, Ferreira J, Losso EM, Andreatini R. (2013). Anxiolytic-like effect of lavender essential oil inhalation in mice: participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J Ethnopharmacol 147:412–418. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. (1999). PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry 46:1375–1387. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Blier P. (2005). Responsiveness of 5-HT(1A) and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry Neurosci 30:268–274. [PMC free article] [PubMed] [Google Scholar]
- Elisabetsky E, Marschner J, Souza DO. (1995). Effects of Linalool on glutamatergic system in the rat cerebral cortex. Neurochem Res 20:461–465. [DOI] [PubMed] [Google Scholar]
- Friston K, ed (2007). Topological inference. London: Academic Press. [Google Scholar]
- Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang YY, Mann JJ, Parsey RV.(2013) Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol Psychiatry 74:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. (2002). Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416:396–400. [DOI] [PubMed] [Google Scholar]
- Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien LK, Mitterhauser M, Kasper S. (2010). Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci 30:14482–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. (1997). Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Research 745:96–108. [DOI] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y. (2009). Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci 63:266–276. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. (1998). Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A 95:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow J-S, Lu J-Q, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. (2003). Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112. [DOI] [PubMed] [Google Scholar]
- Innis RB, et al. (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Muller WE, Volz HP, Moller HJ, Dienel A, Schlafke S. (2010). Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: a randomized, double-blind, placebo controlled trial. Int Clin Psychopharmacol 25:277–287. [DOI] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Muller WE, Volz HP, Moller HJ, Schlafke S, Dienel A. (2014). Lavender oil preparation Silexan is effective in generalized anxiety disorder - a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol: 17:859–869. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Baldinger P, Haeusler D, Philippe C, Kaufmann U, Wadsak W, Savli M, Hoeflich A, Kraus C, Vanicek T, Mitterhauser M, Kasper S, Lanzenberger R. (2014). Cerebral serotonin transporter asymmetry in females, males and male-to-female transsexuals measured by PET in vivo. Brain Struct Funct. 219:171–183. [DOI] [PubMed] [Google Scholar]
- Kraus C, Hahn A, Savli M, Kranz GS, Baldinger P, Hoflich A, Spindelegger C, Ungersboeck J, Haeusler D, Mitterhauser M, Windischberger C, Wadsak W, Kasper S, Lanzenberger R. (2012). Serotonin-1A receptor binding is positively associated with gray matter volume: a multimodal neuroimaging study combining PET and structural MRI. Neuroimage 63:1091–1098. [DOI] [PubMed] [Google Scholar]
- Kraus C, Ganger S, Losak J, Hahn A, Savli M, Kranz GS, Baldinger P, Windischberger C, Kasper S, Lanzenberger R. (2014). Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. Neuroimage. 84:236–244. [DOI] [PubMed] [Google Scholar]
- Kraus C, Ganger S, Losak J, Hahn A, Savli M, Kranz GS, Baldinger P, Windischberger C, Kasper S, Lanzenberger R. (2014). Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. Neuroimage 84:236–244. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. (2007). Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry 61:1081–1089. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Wadsak W, Spindelegger C, Mitterhauser M, Akimova E, Mien LK, Fink M, Moser U, Savli M, Kranz GS, Hahn A, Kletter K, Kasper S. (2010). Cortisol plasma levels in social anxiety disorder patients correlate with serotonin-1A receptor binding in limbic brain regions. Int J Neuropsychopharmacol 13:1129–1143. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, Micskei Z, Stein P, Karanikas G, Wadsak W, Kasper S, Frey R. (2013). Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry 18:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. (1995). Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352:141–148. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, van de Kar LD. (1996). Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther 279:1035–1042. [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. (1997). A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther 282:1581–1590. [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. (2000). Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biological Psychiatry 48:30–42. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. (1996). Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840. [DOI] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gasto C, Junque C, Massana J, Mercader JM. (2003). Parahippocampal gray matter density in panic disorder: a voxel-based morphometric study. Am J Psychiatry 160:566–568. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Gunn RN, Myers R, Grasby PM. (1999). Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 9:545–553. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. (2008). Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Brit J Psychiatry 193:229–234. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. (2004). Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci 24:589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. (1998). Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A 95:10734–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. (2002). Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 954:173–182. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Kerr TM, Tecott LH. (2001). 5-HT(1A) receptor mutant mice exhibit enhanced tonic, stress-induced and fluoxetine-induced serotonergic neurotransmission. J Neurochem 77:607–617. [DOI] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. (2010). Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry 67:701–711. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. (2000). Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry 57:174–180. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. (2009). 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 88:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savli M, Bauer A, Mitterhauser M, Ding Y-S, Hahn A, Kroll T, Neumeister A, Haeusler D, Ungersboeck J, Henry S, Isfahani SA, Rattay F, Wadsak W, Kasper S, Lanzenberger R. (2012). Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage 63:447–459. [DOI] [PubMed] [Google Scholar]
- Schuwald AM, Noldner M, Wilmes T, Klugbauer N, Leuner K, Muller WE. (2013). Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PLoS One 8:e59998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, Wood JN, De Simone V, Grafman J. (2009). Neural correlates of anxiety in healthy volunteers: a voxel-based morphometry study. J Neuropsychiatry Clin Neurosci 21:199–205. [DOI] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, Moser U, Holik A, Pezawas L, Kletter K, Kasper S. (2009). Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry 14:1040–1050. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. (2001). Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry 158:1326–1328. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uehleke B, Schaper S, Dienel A, Schlaefke S, Stange R. (2012). Phase II trial on the effects of Silexan in patients with neurasthenia, post-traumatic stress disorder or somatization disorder. Phytomedicine 19:665–671. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. (2004). Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp 22:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsak W, Mien LK, Ettlinger DE, Eidherr H, Haeusler D, Sindelar KM, Keppler BK, Dudczak R, Kletter K, Mitterhauser M. (2007). 18F fluoroethylations: different strategies for the rapid translation of 11C-methylated radiotracers. Nucl Med Biol 34:1019–1028. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:655–679. [DOI] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. (2010). Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage 49:1205–1212. [DOI] [PubMed] [Google Scholar]
- Woelk H, Schlafke S. (2010). A multi-center, double-blind, randomised study of the lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 17:94–99. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. (2002). Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. (2003). Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 100:9039–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]