Abstract
Background:
Olfactory bulbectomized rats generally manifest many of the neurochemical, physiological, and behavioral features of major depressive disorder in humans. Another interesting feature of this model is that it responds to chronic but not acute antidepressant treatments, including selective serotonin reuptake inhibitors. The purpose of the present study was first to characterize the firing activity of dorsal raphe serotonin neurons in olfactory bulbectomized rats and then examine the effects of 2 antidepressants, bupropion and paroxetine.
Methods:
Olfactory bulbectomy was performed by aspirating olfactory bulbs in anesthetized rats. Vehicle and drugs were delivered for 2 and 14 days via subcutaneously implanted minipumps. In vivo electrophysiological recordings were carried out in male anesthetized Sprague-Dawley rats.
Results:
Following ablation of olfactory bulbs, the firing rate of serotonin neurons was decreased by 36%, leaving those of norepinephrine and dopamine neurons unchanged. In olfactory bulbectomized rats, bupropion (30mg/kg/d) restored the firing rate of serotonin neurons to the control level following 2- and 14-day administration and also induced an increase in the tonic activation of serotonin1A receptors; paroxetine (10mg/kg/d) did not result in a return to normal of the attenuated firing of serotonin neurons in olfactory bulbectomized rats. In the hippocampus, although at a higher dose of WAY 100635 than that required in bupropion-treated animals, paroxetine administration also resulted in an increase in the tonic activation of serotonin1A receptors.
Conclusions:
The present results indicate that unlike paroxetine, bupropion administration normalized serotonin neuronal activity and increased tonic activation of the serotonin1A receptors in hippocampus.
Keywords: electrophysiology, serotonin, bupropion, paroxetine, olfactory bulbectomy
Introduction
Several behavioral, physiological, and neurochemical adaptations taking place following bilateral olfactory bulbectomy (OBX) in rats can be found in patients with major depressive disorder (Jesberger and Richardson, 1986; Song and Leonard, 2005). These effects of OBX include hyperactivity to mild stress (Baumbach and Sieck, 1977; Kelly et al., 1997), cognitive impairment (van Rijzingen et al., 1995; Yamamoto et al., 1997), circadian rhythm and sleep disruptions (Lumia et al., 1992), perturbations of eating habits (Meguid et al., 1993), and enhanced levels of corticosteroids (Cairncross et al., 1979; Jesberger and Richardson, 1986).
The most replicated behavioral characteristic of this model is the increase in locomotor activity seen, in the open field, following olfactory bulb ablation (van Riezen et al., 1977; Cryan et al., 1999; Breuer et al., 2008). It is also well established that chronic but not acute administration of various drugs, such as the selective serotonin (5-HT) reuptake inhibitors (SSRIs) fluoxetine (Mato et al., 2010), paroxetine (Cryan et al., 1999), and escitalopram (for review, see Song and Leonard, 2005; Breuer et al., 2007), can reverse behavioral hyperactivity observed in OBX rats in the open field. Furthermore, in the latter study, reversal of hyperactivity was no longer present 10 weeks after cessation of drug administration (Breuer et al., 2007).
Removal of the olfactory bulbs in rats also results in alterations of 5-HT, norepinephrine (NE), and dopamine (DA) systems (Tonnaer et al., 1980; Masini et al., 2004; Slotkin et al., 2005; Song and Leonard, 2005). Particularly, changes in 5-HT synthesis rate, 5-HT levels, 5-HT transporters (5-HTTs), and 5-HT receptor densities are reported in different brain structures following OBX (Zhou et al., 1998; Watanabe et al., 2003; Hasegawa et al., 2005; van der Stelt et al., 2005; Saitoh et al., 2007; Sato et al., 2010).
Preclinical studies show that antidepressant treatments increase 5-HT neurotransmission with a time course that is consistent with their delayed therapeutic effect. This enhancement would, however, be mediated through different mechanisms. Whereas bupropion increases discharge frequency of 5-HT neurons after 2 and 14 days of administration in naïve rats (El Mansari et al., 2008; Ghanbari et al., 2010), SSRIs, including paroxetine, decrease 5-HT neuronal activity after 2 days of administration that recovers to the control level only after 2 to 3 weeks of administration (Pineyro and Blier, 1999; Czachura and Rasmussen 2000; El Mansari et al., 2005). Hence, long-term administration of SSRIs increases 5-HT levels in the projecting area following recovery of firing of 5-HT neurons to control level and desensitization of the terminal 5-HT1B autoreceptor (Pineyro and Blier, 1999). An increase of 5-HT transmission was also obtained by short- and long-term administration of bupropion but also in a time-dependent fashion (Ghanbari et al., 2010, 2011). In contrast, sustained bupropion administration (2 days) increases the firing activity of 5-HT neurons above normal, partly because it desensitizes the 5-HT1A autoreceptor through an indirect noradrenergic mechanism. In addition, prolonged administration (14 days) of bupropion desensitizes the α2-adrenergic heteroreceptors on 5-HT terminals (Ghanbari et al., 2011), thereby delineating their primary site of action on the NE system.
The aim of this study was to determine how 2 antidepressant drugs with different mechanisms of action, the SSRIs paroxetine and bupropion, act on in vivo characteristics of the 5-HT system in an OBX rat model, where several 5-HT alterations have taken place. The choice of these 2 antidepressant drugs stemmed mainly from their different mechanism of action on 5-HT neurons.
Methods
Animals
The in vivo electrophysiological experiments were carried out in male Sprague-Dawley rats (Charles River, St. Constant, QC, Canada) weighing between 300 and 500g at the time of recordings. Upon arrival, the rats were allowed to acclimatize to their new environment for 1 week prior to start of any drug administration or experiment. The animals were kept, 2 per cage, under standard laboratory conditions (12:12-hour–light/–dark cycle with access to food and water ad libitum). Following surgery, control and OBX rats were kept individually in cages. All the experiments were approved by the local Animal Care Committee (Institute of Mental Health Research) and conducted in accordance with the Canadian Council on Animal Care) for the care and use of laboratory animals.
OBX Surgery
Bilateral OBX was performed in rats anesthetised with equitezine (Marcilhac et al., 1999). For olfactory bulb ablation, rats were mounted in a stereotaxic frame. A medline incision was made extending at least 1mm to bregma. A burr hole was drilled at 7mm anterior to bregma and 2.5mm either side of the midline at a point corresponding to the posterior margin of the orbit of the eye. After a localized electrical coagulation of the dura and sagittal sinus, olfactory bulbs were removed by suction with a vacuum pump attached to a Pasteur pipette. The burr hole was filled with haemostatic sponges and the wound was closed with sterile sutures. Sham-operated rats were treated similarly, with the exception that olfactory bulbs were not removed. After surgery, animals were injected 5mL intraperitoneally (i.p.) with 5% glucose and temgesic for postsurgical analgesia. The animals were allowed to recover for 14 days following surgery, at which time behavioral changes occur (Lucas et al., 2007). On the 14th to 18th days following surgery, the rats were recorded or implanted with minipumps for drug administration. Only rats that showed complete ablation of the olfactory bulbs and no damage to the frontal cortex were included for data analysis.
Assessment of Hyperlocomotion in OBX Rats
The open field chamber consisted of a square plastic box (85×85×70cm). Since conditions of very high brightness are crucial to observe the hyperactivity in OBX rats (Mar et al., 2000; Song and Leonard, 2005), the inner faces of the walls were covered with aluminium foil and illumination was provided by a 100-watt bulb. Each rat was placed into the center of the open field, and exploratory activity was monitored by a video camera fixed above the arena and relayed to a computer system that calculates the walked distance during the first 5 minutes (Viewpoint Life Sciences, Lyon, France). All experiments were performed between 8:30 and 10:30 am.
Minipump Implantation and Electrophysiological Experiments
The rats were anesthetized with isoflurane to implant, subcutaneously, the osmotic Alzet minipumps (Alza, Palo Alto, CA) to ensure slow and steady release of bupropion (30mg/kg/d; Dong and Blier, 2001), paroxetine (10mg/kg/d; Besson et al., 2000), or vehicle (water for bupropion, 50% water+50% ethanol for paroxetine) for 2, 14, and 28 days. The electrophysiological experiments were carried out with the minipump in place. Prior to the electrophysiological recordings, rats were anesthetized with chloral hydrate (400mg/kg, i.p.) and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Supplemental doses of the anesthetic (100mg/kg, i.p.) were given to maintain constant anesthesia and prevent any nociceptive reaction to pinching of the hind paws. Body temperature was maintained at 37°C throughout the experiment with a heating pad. Prior to the electrophysiological experiments, a catheter was inserted in a lateral tail vein for systemic intravenous injection of pharmacological agents.
Electrophysiological Recordings of Dorsal Raphe Nucleus (DRN) 5-HT Neurons
In vivo extracellular recordings of presumptive 5-HT neurons were obtained using single-barrel glass micropipettes. The impedance of the electrodes was between 4 and 6 MΩ. The microelectrodes were positioned 0.9 to 1.2mm anterior to lambda on the midline and lowered into the DRN. The 5-HT neurons were encountered over a distance of 1mm starting immediately below the ventral border of the Sylvius aqueduct. These neurons were identified by their slow (0.5–2.5 Hz), regular firing rate and a long duration (0.8–1.2ms) positive action potential (VanderMaelen and Aghajanian, 1983).
Electrophysiological Recordings of Locus Coeruleus NE Neurons
NE neurons were reliably recorded at the following coordinates (in millimeters from lambda): anterior-posterior (AP), -1.0 to -1.2; median-lateral (ML), 1.0 to 1.3; dorsal-ventral (DV), 5.0 to 7.0. They were identified by their regular firing rate (1–5 Hz) and their positive action-potential duration, which is between 0.8 and 1.2 milliseconds. They can also be identified by a brief neuronal excitation to a nociceptive pinch of the contralateral hind paw followed by a short period of inhibition (see Szabo et al., 2000).
Electrophysiological Recordings of Ventral Tegmental Area (VTA) DA Neurons
DA neurons were reliably recorded at the following coordinates (in millimeters from lambda): AP, 3.2 to 3.6; ML, 0.9 to 1.1; DV, 7.0 to 9.0. They were identified by their established electrophysiological criteria (Ungless and Grace, 2012), including: (1) spontaneous firing rate between 2 and 90 spikes/s, exhibiting bursting activity or irregular firing; (2) biphasic or triphasic waveforms, with an initial positive deflection (usually notched followed by prominent negative phase; (3) long duration potential (2–4 milliseconds); and (4) low pitch sound when monitored by an audioamplifier. A duration >1.1 milliseconds from the start of the action potential to the center of the negative trough was also used to determine the DA neuron identity (Ungless and Grace, 2012).
Bursts Analysis
The firing patterns of the monoaminergic neurons were analyzed by interspike interval (ISI) burst analysis. The onset of a burst was signified by the occurrence of 2 spikes with ISI <0.08 seconds for NE and DA and ISI <0.01 seconds for 5-HT. The termination of a burst was defined as an ISI >0.16 seconds for NE and DA and ISI >0.01 seconds for 5-HT (Grace and Bunney, 1983; Hajos and Sharp, 1996; Dawe et al., 2001).
Extracellular Recording and Microiontophoresis of Dorsal Hippocampus CA3 Pyramidal Neurons
Extracellular recording and microiontophoresis of CA3 pyramidal neurons were performed with 5-barreled glass micropipettes. The impedance of the electrodes ranged from 2 to 4 MΩ. The central barrel used for the unitary recording was filled with a 2-M NaCl solution, and the 4 side barrels were filled with the following solutions: 5-HT creatinine sulfate (10mM in 200mM NaCl, pH 4), quisqualic acid (1.5mM in 200mM NaCl, pH 8), and a 2-M NaCl solution used for automatic current balancing. The electrodes were lowered into the dorsal CA3 region of the hippocampus using the following coordinates: 4mm anterior to lambda and 4.2mm lateral. Pyramidal neurons were encountered at a depth of 4.0±0.5mm below the surface of the brain. Because these neurons do not spontaneously discharge in chloral hydrate anesthetized rats, they were activated within their physiological firing range (10–15 Hz; Kandel and Spencer, 1961) by a small current of quisqualate in nanoamperes (nAs; using Harvard Apparatus module, Montreal, Canada). The CA3 pyramidal neurons were identified by their large amplitude (0.5–1.2 mV) and long-duration simple action potentials, alternating with complex spike discharges.
5-HT1A Receptor Sensitivity
Neuronal responsiveness of the CA3 pyramidal neurons after 5-HT1A receptors activation was determined by microiontophoretically applying 5-HT (in nA) for 50 seconds and measuring the number of spikes suppressed per nA. The more spikes are suppressed per nA, the more sensitive are the receptors.
Determination of 5-HT Reuptake Inhibition
To determine the degree to which the 5-HTTs were blocked by paroxetine, the 50% recovery time (RT50) was measured by determining the time (in seconds) following a 50-second ejection period of 5-HT to recover 50% of the initial firing rate (Pineyro et al., 1994).
Statistics
The data are presented as mean values ± SEM. Statistical comparisons were performed using the Mann-Whitney U test or Wilcoxon signed rank test when a parameter was studied in control and treated rats. For DRN experiments, statistical comparisons were carried out using a 1-way analysis of variance (ANOVA) on ranks followed by Dunn’s method for all pairwise multiple comparison procedures. For the tonic activation, statistical comparisons were carried out using a 2-way analysis of variance with repeated measures (treatment as the main factor) followed by the Tukey posthoc analysis. Statistical significance was taken as P<.05.
Drugs
Bupropion and paroxetine (Sequoia Research Products, Pangbourne, UK). WAY 100635, 5-HT creatinine sulfate, and chloral hydrate were purchased from Sigma (St. Louis, MO). Quisqualic acid was purchased from Tocris (Ellisville, MO).
Results
Open Field
Following ablation of their olfactory bulbs, rats were significantly more active compared with shams in the open field (total distance traveled: prebulbectomy, 1357±222cm [12 rats], postbulbectomy: 2809±406cm [12 rats]; P<.01; Mann-Whitney U test). Rats tested in the behavioral paradigm were not used for electrophysiology experiments.
DRN 5-HT Neurons Activity in OBX Rats
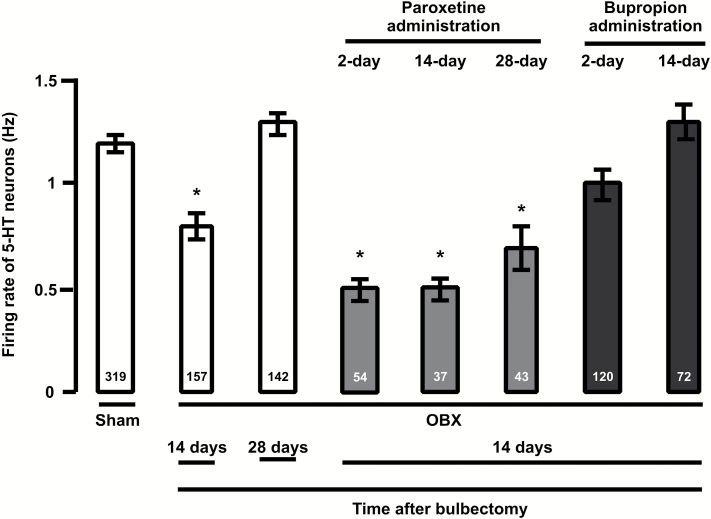
Sham rats from different treatment groups were pooled together, because there was no significant difference in their 5-HT neuron firing activity. Fourteen to 18 days following OBX, the average firing rate of 5-HT neurons was significantly decreased by 36% compared with control (Figure 1; P<.001, 1-way ANOVA). However, the average firing activity returned to control levels 4 weeks after the olfactory ablation (Figure 1; P>.05, 1-way ANOVA).
Figure 1.
Mean (±SEM) of the firing rate of dorsal raphe nucleus (DRN) serotonin (5-HT) neurons in control and olfactory bulbectomy (OBX) rats that received vehicle, paroxetine (10mg/kg/d) or bupropion (30mg/kg/d), for 2, 14, and 28 days. The number at the bottom indicates the total number of neurons recorded in each group. **P<.01 and *P<.05.
The mean number of neurons found per track was not changed in OBX rats compared with controls (control group: 4±0.2, N=17 rats; OBX group: 4.4±0.5, N=9 rats; P>.05; Mann-Whitney U test).
Locus Coeruleus NE Neurons Activity in OBX Rats
OBX resulted in no change in the neuronal firing rate of NE neurons. However, although the number of neurons displaying burst activities was increased (22% in control [90 neurons in 5 rats] vs 42% in OBX rats [115 neurons in 7 rats], Mann-Whitney U, P=.003), the percentage of spikes in bursts was not changed (control: 5±1 [20 neurons]; OBX: 7±0.9 [48 neurons], t test, P>.05).
VTA DA Neurons Activity in OBX Rats
There was no change in firing rate of VTA DA neurons following olfactory bulb ablation (sham: 5±0.2 Hz, 56 neurons in 5 rats; OBX: 5±0.3 Hz, 64 neurons in 6 rats). Moreover, there was no change in bursting activity of VTA DA neurons (percentage of spikes in bursts, control: 29±3 [in 5 rats]; OBX: 34±4 [in 6 rats]).
Effect of Paroxetine and Bupropion on the Firing Activity of 5-HT Neurons in OBX Rats
Because the firing activity of NE and DA neurons was largely unaffected by OBX, the effects of 2 antidepressants with distinct mechanisms of action were examined only on the 5-HT system.
After 14 to 18 days following OBX, rats were administered either bupropion (30mg/kg/d) or paroxetine (10mg/kg/d) for short and long periods. Two days of bupropion administration to OBX rats resulted in recovery to normal of the dampened firing activity of 5-HT neurons compared with OBX rats receiving vehicle (Figure 1; P < .01, 1-way ANOVA). Following a 14-day administration of bupropion, the firing rate of 5-HT neurons in OBX rats remained at control levels (Figure 1).
In OBX rats administered with paroxetine for 2, 14, and 28 days, the discharge activity of 5-HT neurons remained significantly inhibited by 58%, 58%, and 42%, respectively, compared with rats treated with vehicle (Figure 1; P < .01, 1-way ANOVA). The inhibition of 5-HT neurons firing seen in OBX rats that received paroxetine (for 2 and 14 days) was reversed to control discharge level after the injection of 100 µg of the selective 5-HT1A receptor antagonist WAY 100635 (control group: 1.2±0.1 Hz; 2-day treated group: 1.1±0.1 [23 neurons in 2 rats]; 14-day treated group: 1.1±0.1 [39 neurons in 2 rats]).
Effect of Bupropion and Paroxetine on the 5-HT Elements in the Hippocampus of OBX Rats
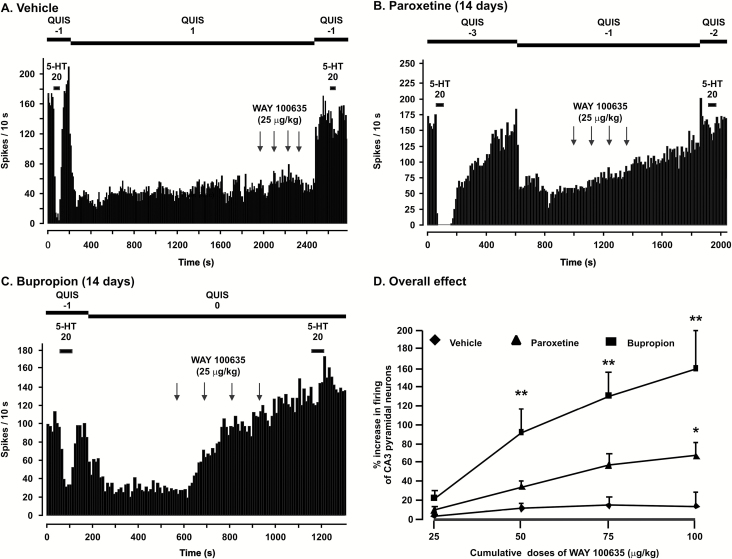
For all CA3 pyramidal neurons, iontophoretic application of 5-HT induced an inhibition of their firing activity (Figure 2). To determine the sensitivity of the 5-HT1A receptors located on pyramidal neurons, the number of action potentials suppressed by 5-HT ejection (nA) was calculated. Short- and long-term administration of bupropion did not modify the suppressant effect of 5-HT on firing activity of CA3 pyramidal neurons. The mean number of spikes suppressed per nA was not significantly different in OBX rats administered paroxetine and bupropion for 14 days compared with OBX rats treated with vehicle (spikes suppressed per nA; vehicle group: 8±1 [n=7]; paroxetine group: 9±1 [n=8] and 7±1 [n=6] in the bupropion group; P>.05; 1-way ANOVA).
Figure 2.
Integrated firing rate histograms of dorsal hippocampus CA3 pyramidal neurons showing their responsiveness to the microiontophoretic application of serotonin (5-HT) and intravenous injection of WAY 100635 in vehicle-treated rats (A) and a rat administered with either paroxetine (B; 10mg/kg/d) or bupropion (C; 30mg/kg/d). Note the increase in firing activity of pyramidal neurons after the injection of WAY 100635. (D) Degree (percentage ± SEM) of increase of the firing activity of pyramidal neurons in control rats and those treated with paroxetine and bupropion for 14 days after the administration of WAY 100635. * P<.05, **P<.01.
It is established that 5-HT reuptake blockers increase RT50 values (Pineyro et al., 1994). Indeed, the mean RT50 value was significantly increased in OBX rats administered with paroxetine for 14 days (RT50; paroxetine group: 89±15 s [n=8] compared with vehicle group 24±2 s [n=7]; P<.001; Mann-Whitney U test).
As an indication that iontophoretic application of 5-HT was exerting its inhibitory effect via 5-HT1A receptors, the latter action was nearly abolished by WAY 100635 administration (Figure 2; P < .05, Wilcoxon signed-rank test).
Should there be a significant tonic activation of the 5-HT1A receptors, their blockade will result in significant enhancement of the firing activity of pyramidal neurons. In OBX rats that received vehicle, the overall effect of WAY 100635 was not significant on the firing activity of CA3 pyramidal neurons (Figure 2A, D). However, after 2 weeks administration of paroxetine, although the overall effect was not statistically significant, the tonic activation of 5-HT1A receptors was significantly increased when the dose of WAY 100635 reached 100 µg/kg (P<.05). However, in rats that received bupropion for 2 weeks, blockade of 5-HT1A receptors induced a significant enhancement in the mean firing activity of pyramidal neurons starting at 50 µg/kg (Figure 2; P < .001, F(2, 81) = 8.7, 2-way ANOVA for repeated measures, followed by Tukey post hoc test; vehicle group: n = 8, paroxetine group: n = 8, bupropion group: n = 6). This shows that the effectiveness of bupropion on tonic activation of 5-HT1A receptors is higher than bupropion in this model.
Discussion
An increase in locomotor activity in a novel environment such as the open field has been reported in many studies following olfactory bulb removal (Song and Leonard, 2005; van der Stelt et al., 2005). It is noteworthy that this alteration seen after ablation was reported not to be due to anosmia proper, as peripherally anosmic rats do perform as sham rats in the open field paradigm (van Riezen et al., 1977; Cairncross et al., 1979; for review, see Song and Leonard, 2005). In keeping with these previous findings (Cryan et al., 1999; Song and Leonard, 2005; Lucas et al., 2007; Breuer et al., 2008), OBX rats in the present study showed a significant increase in locomotion in the open field. Interestingly, the hyperactivity phenomenon was reported to be a durable characteristic of the OBX rats, as it is still present 20 weeks after surgery (van der Stelt et al., 2005). It has also been reported to be reversed by chronic but not acute administration with several antidepressants, including SSRIs (Cryan et al., 1998, 1999; Song and Leonard, 2005).
The present study showed that the firing activity of DRN 5-HT neurons was significantly decreased 2 weeks after OBX. Because the firing activity of NE and DA neurons was unchanged, as shown in the present study, these results cannot be explained by a diminution of the excitatory effect of NE and DA neurons through an action on α1-adrenoceptors and D2 receptors, respectively (Katz et al., 2010).
This decrease in firing of 5-HT neurons was unlikely to have been due to an overactivation of 5-HT1A autoreceptors, which would not have been possible in the presence of a significant decrease in 5-HT synthesis and a probable diminution of 5-HT concentrations in dorsal and medial raphe nuclei of OBX-rats (Barton and Hutson, 1999; Watanabe et al., 2003; Hasegawa et al., 2005). Even though the mechanism underlying this decrease in firing is not clear, data point to an alteration in 5-HT elements of DRN. Indeed, Saitoh et al. (2007) reported a significant decrease in tryptophan hydroxylase-positive cells in the rat DRN following OBX. However, in the present study, although 5-HT neurons firing was decreased, no reduction in the number of neurons encountered per electrode trajectory was observed, suggesting no change at least in the number of presumptive 5-HT neurons recorded in DRN of OBX rats. Consistently with the present results, it was reported that there was no general loss of neuronal function, because there was a similar increase in 5-HT accumulation following tryptophan loading in OBX rats compared with controls (van der Stelt et al., 2005).
Unlike behavioral adaptations that lasted weeks after OBX, the diminished firing rate of 5-HT in OBX rats at 14-day postsurgery recovered to control levels after 28 days. It is worth noting that previous studies have shown that some adaptations, such as hyperactivity, found in OBX rats are durable whereas others are reversible. Indeed, although it was shown that the hyperactivity seen in OBX-rats lasted for 5 months postbulbectomy (van der Stelt et al., 2005), at least one study showed, for instance, that brain reward function, as measured by intra-cranial self-stimulation, was deficient for 8 days after OBX before returning to baseline levels (Slattery et al., 2007). The exact mechanisms underlying the presence or absence of such adaptations are unclear and need to be examined in future studies.
Despite recovery of the activity of 5-HT neurons after 1 month of ablation, it remained inhibited following paroxetine administration for 14 and 28 days, at which time neurons usually return to control levels in naïve rats under the same regimen (Besson et al., 2000). It is possible that the lack of recovery of 5-HT neurons firing stems from the inability of paroxetine to increase 5-HT levels in DRN, which consequently would have resulted in 5-HT1A autoreceptor desensitization, allowing a return to the control level of the firing. Indeed, 14-day administration of the SSRI citalopram in OBX rats did not reverse the decrease of DRN 5-HT synthesis and release (Barton and Hutson, 1999; Hasegawa et al., 2005). The lack of increase in 5-HT cannot be explained by an augmentation in 5-HTT density in OBX rats as was previously postulated, since there was no change in 5-HTT density in the DRN of these rats (Sato et al., 2010).
In the hippocampus, even though the density of 5-HT1A receptors was increased in OBX rats (Sato et al., 2008), this did not result in an enhancement of the responsiveness of these receptors after 14-day paroxetine administration (Figure 2). This indicates that 5-HT1A receptors remained normosensitive in the hippocampus, similarly to naïve rats that received the same regimen (Besson et al., 2000). However, an increase in the tonic activation of the 5-HT1A receptors was shown here, although only following injection of a high dose of WAY 100635 (100 μg/kg) in paroxetine-treated OBX rats but to a lesser extent than that obtained in naïve rats that received this SSRI (Besson et al., 2000). Accordingly, a dampened enhancement of extracellular levels of 5-HT in the hippocampus was already reported, since local application of the SSRI fluvoxamine significantly increased extracellular 5-HT in this region and to a greater extent in sham than OBX rats (Watanabe et al., 2003; Hasegawa et al., 2005; van der Stelt et al., 2005; Prins et al., 2011). It is not clear if the increase in 5-HTT density in OBX rat hippocampus is the cause for this dampening in 5-HT levels, as was proposed, since conflicting results were reported (no change vs an increase; Zhou et al., 1998; Licht et al., 2010; Sato et al., 2010). Furthermore, 5-HTT blockade as measured by the RT50 (a direct measure of 5-HTT reuptake; Pineyro et al., 1994) was shown in the current work to be increased to the same degree in paroxetine-treated OBX rats than in naïve rats (Besson et al., 2000). Altogether, these results showed that in the hippocampus of OBX rats, paroxetine induced a relatively modest increase in tonic activation of 5-HT1A receptor, 5-HT synthesis (Hasegawa et al., 2005), and 5-HT release (van der Stelt et al., 2005) compared with bupropion. It is worth noting, however, that paroxetine attenuated OBX-induced hyperactivity (Cryan et al., 1998, 1999).
An enhancement of tonic activation of the 5-HT1A receptors was obtained in the hippocampus of OBX rats following a 14-day regimen with bupropion using smaller doses of WAY 100635 than those used to induce it in rats that received paroxetine (Figure 2). This increase is similar to that obtained from previous studies using bupropion in naïve rats (Ghanbari et al., 2011). Altogether, these results indicate that inasmuch as 5-HT1A receptor sensitivity did not change following bupropion regimen, an increase was observed in its net effect of bupropion administration on 5-HT neurotransmission in OBX rats that was comparable with that obtained in naïve rats (Ghanbari et al., 2011). This increase likely resulted from attenuated function of α2-adrenoceptors located on 5-HT terminals (Ghanbari et al., 2011) combined with normalized firing of 5-HT neurons after 14-day administration of bupropion, as reported here. However, the fact that 5-HT neuron firing was not higher in OBX rats that received bupropion than that in naïve rats (El Mansari et al., 2008; Ghanbari et al., 2010) may stem from the fact that basal firing of 5-HT neurons was much lower in OBX rats compared with naïve animals.
Clinically, it is well known that certain patients with major depressive disorder may respond to some classes of antidepressants but not to others. The biological basis for this remains elusive. The present results showed that 5-HT transmission was not enhanced to the same degree using a SSRI in rats with a presynaptic anomaly of 5-HT neurons (ie, a diminished firing activity) than in rats receiving bupropion, an antidepressant with primary sites of action on catecholamine systems, which appeared to be largely unaltered in this model. The therapeutic implications of these findings are that patients with olfactory bulb atrophy may have a preferential response to bupropion than to a SSRI. Indeed, in patients with major depressive disorder, there is a correlation between the severity of depressive symptoms and atrophy of the olfactory bulbs (Negoias et al, 2010). It would thus be interesting to examine the antidepressant response using the 2 drugs studied herein in a clinical setting in relation to bulb atrophy.
Statement of Interest
P. Blier has financial involvements with: Astra-Zeneca, Bristol Myers, Eli Lilly, Janssen, Labopharm, Lundbeck, Merck, Pfizer, Servier, Takeda, and Valeant. M. El Mansari, S. Manta, C. Oosterhof, K. El Iskandrani, F. Chenu, and S. Shim declare no conflicts of interest.
Acknowledgments
This work was financially supported by the Ontario Brain Institute. P. Blier has a Canada Research Chair and an endowed chair from the University Of Ottawa Institute of Mental Health Research. We thank N. Oosterhof for kindly providing BurstiDAtor for burst analysis.
References
- Barton CL, Hutson PH. (1999). Inhibition of hippocampal 5-HT synthesis by fluoxetine and paroxetine: evidence for the involvement of both 5-HT1A and 5-HT1B/D autoreceptors. Synapse 31:13–19. [DOI] [PubMed] [Google Scholar]
- Baumbach HD, Sieck MH. (1977). Temporal effects of discrete lesions in the olfactory and limbic systems on open-field behavior and dyadic encounters in male hooded rats. Physiol Behav 18:617–637. [DOI] [PubMed] [Google Scholar]
- Besson A, Haddjeri N, Blier P, de Montigny C. (2000). Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur Neuropsychopharmacol 10:177–188. [DOI] [PubMed] [Google Scholar]
- Breuer ME, Groenink L, Oosting RS, Westenberg HG, Olivier B. (2007). Long-term behavioral changes after cessation of chronic antidepressant treatment in olfactory bulbectomized rats. Biol Psychiatry 61:990–995. [DOI] [PubMed] [Google Scholar]
- Breuer ME, Chan JS, Oosting RS, Groenink L, Korte SM, Campbell U Schreiber R, Hanania T, Snoeren EM, Waldinger M, Olivier B. (2008). The triple monoaminergic reuptake inhibitor DOV 216,303 has antidepressant effects in the rat olfactory bulbectomy model and lacks sexual side effects. Eur Neuropsychopharmacol 18:908–916. [DOI] [PubMed] [Google Scholar]
- Cairncross KD, Wren A, Forster C, Cox B, Schnieden H. (1979). The effect of psychoactive drugs on plasma corticosterone levels and behavioural in the bulbectomised rat. Pharmacol Biochem Behav 10:355–359. [DOI] [PubMed] [Google Scholar]
- Cryan JF, McGrath C, Leonard BE, Norman TR. (1998). Combining pindolol and paroxetine in an animal model of chronic antidepressant action--can early onset of action be detected? Eur J Pharmacol 352:23–28. [DOI] [PubMed] [Google Scholar]
- Cryan JF, McGrath C, Leonard BE, Norman TR. (1999). Onset of the effects of the 5-HT1A antagonist, WAY-100635, alone, and in combination with paroxetine, on olfactory bulbectomy and 8-OH-DPAT-induced changes in the rat. Pharmacol Biochem Behav 63:333–338. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K. (2000). Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch Pharmacol 362:266–275. [DOI] [PubMed] [Google Scholar]
- Dawe GS, Huff KD, Vandergriff JL, Sharp T, O’Neill MJ, Rasmussen K. (2001). Olanzapine activates the rat locus coeruleus: in vivo electrophysiology and c-Fos immunoreactivity. Biol Psychiatry 50:510–20. [DOI] [PubMed] [Google Scholar]
- Dong J, Blier P. (2001). Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 155:52–57. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sánchez C, Chouvet G, Renaud B, Haddjeri N. (2005). Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology 30:1269–1277. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Ghanbari R, Janssen S, Blier P. (2008). Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacol 55:1191–1198. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, El Mansari M, Blier P. (2010). Electrophysiological effects of the co-administration of escitalopram and bupropion on rat serotonin and norepinephrine neurons. J Psychopharmacol 24:39–50. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, El Mansari M, Blier P. (2011). Enhancement of serotonergic and noradrenergic neurotransmission in the rat hippocampus by sustained administration of bupropion. Psychopharmacology (Berl) 217:61–73. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Hajos M, Sharp T. (1996). Burst-firing activity of presumed 5-HT neurones of the rat dorsal raphe nucleus: electrophysiological analysis by antidromic stimulation. Br Res 740:162–168. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Watanabe A, Nguyen KQ, Debonnel G, Diksic M. (2005). Chronic administration of citalopram in olfactory bulbectomy rats restores brain 5-HT synthesis rates: an autoradiographic study. Psychopharmacology (Berl) 179: 781–790. [DOI] [PubMed] [Google Scholar]
- Jesberger JA, Richardson JS. (1986). Effects of antidepressant drugs on the behavior of olfactory bulbectomized and sham-operated rats. Behav Neurosci 100:256–274. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA. (1961). Electrophysiology of hippocampal neurons. II. After potential and repetitive firing. J Neurophysiol 24:243–259. [DOI] [PubMed] [Google Scholar]
- Katz NS, Guiard BP, El Mansari M, Blier P. (2010). Effects of acute and sustained administration of the catecholamine reuptake inhibitor nomifensine on the firing activity of monoaminergic neurons. J Psychopharmacol 24:1223–1235. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. (1997). The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 74:299–316. [DOI] [PubMed] [Google Scholar]
- Licht CL, Kirkegaard L, Zueger M, Chourbaji S, Gass P, Aznar S, Knudsen GM. (2010). Changes in 5-HT4 receptor and 5-HT transporter binding in olfactory bulbectomized and glucocorticoid receptor heterozygous mice. Neurochem Int 56:603–610. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Piñeyro G, Sadikot AF, Debonnel G. (2007). Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55:712–725. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. (1992). Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res 587:181–185. [DOI] [PubMed] [Google Scholar]
- Mar A, Spreekmeester E, Rochford J. (2000). Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology 150: 52–60. [DOI] [PubMed] [Google Scholar]
- Marcilhac A, Faudon M, Anglade G, Hery F, Siaud P. (1999). An investigation of serotonergic involvement in the regulation of ACTH and corticosterone in the olfactory bulbectomized rat. Pharmacol Biochem Behav 63:599–605. [DOI] [PubMed] [Google Scholar]
- Masini CV, Holmes PV, Freeman KG, Maki AC, Edwards GL. (2004). Dopamine overflow is increased in olfactory bulbectomized rats: an in vivo microdialysis study. Physiol Behav 81:111–119. [DOI] [PubMed] [Google Scholar]
- Mato S, Vidal R, Castro E, Díaz A, Pazos A, Valdizán EM. (2010). Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol Pharmacol 77:424–434. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Gleason JR, Yang ZJ. (1993). Olfactory bulbectomy in rats modulates feeding pattern but not total food intake. Physiol Behav 54:471–475. [DOI] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. (2010). Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 169:415–421. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Blier P, Dennis T, de Montigny C. (1994). Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci 14:3036–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro G, Blier P. (1999). Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 51:533–591. [PubMed] [Google Scholar]
- Prins J, Westphal KGC, Korte-Bouws GAH, Quinton MS, Schreiber R, Olivier B, Groenink L, Olivier B, Korte SM. (2011). The potential and limitations of DOV 216,303 as a triple reuptake inhibitor for the treatment of major depression: a microdialyis study in olfactory bulbectomized rats. Pharmacol Biochem Behav 97:444–452. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yamaguchi K, Tatsumi Y, Murasawa H, Nakatani A, Hirose N. (2007). Effects of milnacipran and fluvoxamine on hyperemotional behaviors and the loss of tryptophan hydroxylase-positive cells in olfactory bulbectomized rats. Psychopharmacology (Berl) 191:857–865. [DOI] [PubMed] [Google Scholar]
- Sato H, Skelin I, Debonnel G, Diksic M. (2008). Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Res Bull 75:545–55. [DOI] [PubMed] [Google Scholar]
- Sato H, Skelin I, Diksic M. (2010). Chronic buspirone treatment decreases 5-HT1B receptor densities and the serotonin transporter but increases the density of 5-HT2A receptors in the bulbectomized rat model of depression: an autoradiographic study. Brain Res 1345:28–44. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Cryan JF. (2007). Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 190:555–568. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. (2005). Serotonergic cell signaling in an animal model of aging and depression: olfactory bulbectomy elicits different adaptations in brain regions of young adult vs aging rats. Neuropsychopharmacology 30:52–57. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. (2005). The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647. [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P. (2000). Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol 3:1–11. [DOI] [PubMed] [Google Scholar]
- Tonnaer JA, Rigter H, Versteeg DH, Nickolson VJ. (1980). Changes in rat brain norepinephrine levels and turnover after olfactory bulbectomy. Brain Res Bull 5:683–686. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. (2012). Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderMaelen CP, Aghajanian GK. (1983). Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res 289:109–119. [DOI] [PubMed] [Google Scholar]
- van der Stelt HM, Breuer ME, Olivier B, Westenberg HG. (2005). Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: an in vivo microdialysis study. Biol Psychiatry 57:1061–1067. [DOI] [PubMed] [Google Scholar]
- van Riezen H, Schnieden H, Wren AF. (1977). Olfactory bulb ablation in the rat: behavioural changes and their reversal by antidepressant drugs. Br J Pharmacol 60: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijzingen IM, Gispen WH, Spruijt BM. (1995). Olfactory bulbectomy temporarily impairs Morris maze performance: an ACTH(4–9) analog accellerates return of function. Physiol Behav 58:147–152. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Tohyama Y, Nguyen KQ, Hasegawa S, Debonnel G, Diksic M. (2003). Regional brain serotonin synthesis is increased in the olfactory bulbectomy rat model of depression: an autoradiographic study. J Neurochem 85:469–475. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Jin J, Watanabe S. (1997). Characteristics of memory dysfunction in olfactory bulbectomized rats and the effects of cholinergic drugs. Behav Brain Res 83: 57–62. [DOI] [PubMed] [Google Scholar]
- Zhou D, Grecksch G, Becker A, Frank C, Pilz J, Huether G. (1998). Serotonergic hyperinnervation of the frontal cortex in an animal model of depression, the bulbectomized rat. J Neurosci Res 54:109–116. [DOI] [PubMed] [Google Scholar]