Abstract
Background:
The adolescent brain is sensitive to experience-dependent plasticity and might be more vulnerable than the adult brain to the effects of some drugs of abuse. The factors that contribute to these differences are not fully identified. We have examined the ability of cannabinoids to induce a form of synaptic plasticity, long-term depression, in the nucleus accumbens and dorsolateral striatum of adolescent and adult mice.
Methods:
We measured field excitatory postsynaptic potentials/population spikes in brain slices.
Results:
We found that the cannabinoid receptor agonist WIN 55,212-2 (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) induced long-term depression in the nucleus accumbens of adolescent but not adult mice and failed to induce long-term depression in the dorsolateral striatum of adolescent or adult mice. Similar results were obtained with the group I metabotropic glutamate receptor agonist (S)-3,5- dihydroxyphenylglycine, which has previously been shown to promote the release of endocannabinoids. These age-related differences were associated with reduced protein levels of the cannabinoid type 1 receptor and metabotropic glutamate receptor 1 in adult nucleus accumbens and dorsolateral striatum and with an increased tone of endocannabinoids in the dorsolateral striatum of adult mice. We also found that N-methyl-d-aspartate receptor-dependent long-term depression, which was induced in the nucleus accumbens of adolescent mice, was blunted in adult mice, possibly because of decreased levels of GluN1, the obligatory subunit of N-methyl-d-aspartate receptors.
Conclusions:
This study identifies region- and age-specific differences in the ability of endogenous and exogenous cannabinoids, and of N-methyl-d-aspartate receptors, to induce long-term depression in the striatal complex. These observations might contribute to a better understanding of the increased sensitivity of the adolescent brain to drug induced-plasticity.
Keywords: age, nucleus accumbens, striatum, endocannabinoids, long-term depression
Introduction
The brain of adolescents is subject to extensive synaptic and neurochemical remodeling in areas involved in emotions, learning, decision-making, and reward-motivated behaviors. These changes may explain why adolescents are more vulnerable than adults to the effects produced by exposure to drugs of abuse (Spear, 2002; Clark et al., 2008; Doremus-Fitzwater et al., 2010; Gulley and Juraska, 2013). A number of studies have demonstrated that drugs of abuse modulate long-term changes in synaptic strength in brain regions involved in motivation, reward, and goal-oriented behaviors as well as in habit formation (Hoffman et al., 2003; Fourgeaud et al., 2004; Mato et al., 2004; Yin et al., 2007; Jeanes et al., 2011; Hoffman and Lupica, 2013). Thus, it is believed that alteration of excitatory neurotransmission and plasticity in the ventral striatum, or nucleus accumbens (NAc), and dorsal striatum is involved in the reinforcing effects of addictive substances (Hyman et al., 2006; Vengeliene et al., 2008). A well-described form of long-term depression (LTD) of glutamatergic synaptic transmission in the NAc and striatum involves endocannabinoids (eCBs), that is, anandamine and 2-arachidonoylglycerol. eCBs are produced and released from postsynaptic neurons following the activation of, for example, metabotropic glutamate receptors (mGluRs) or strong depolarization of postsynaptic neurons. eCBs inhibit neurotransmitter release through an action on cannabinoid type 1 (CB1) receptors located on presynaptic terminals (Robbe et al., 2001, 2002; Gerdeman et al., 2002; Kreitzer and Malenka, 2005; Ronesi and Lovinger, 2005; Ohno-Shosaku and Kano, 2014). Another form of LTD identified in the NAc was shown to be triggered following the activation of N-methyl-d-aspartate (NMDA) receptors (NMDA-LTD) (Thomas et al., 2000; Jeanes et al., 2011).
The possible age-related differences in the ability of eCBs or NMDA receptor activation to trigger LTD have not been thoroughly addressed. Indeed, most electrophysiological experiments are performed in the brains of adolescent rodents. Given that the adolescent brain is sensitive to experience-dependent plasticity, the first aim of our study was to determine whether age-related differences exist in the induction of 2 different forms of LTD, that is, one that is mediated by cannabinoids and a second form that is mediated by NMDA receptors. In an attempt to identify possible underlying mechanisms for the observed age-related differences, we also examined the protein levels of the receptors involved in LTD induction. The second aim of this study was to determine if the activation of cannabinoid receptors leads to similar LTD in the NAc, a brain region associated with reward mechanisms, and in the dorsolateral striatum (DLS), a brain region shown to support habitual instrumental performance (ie, mediates habit/stimulus-response learning). Our results demonstrate that the NAc of adolescent mice is more susceptible to long-lasting changes in synaptic strength induced by cannabinoids and NMDA receptor activation than the NAc of adult mice or the DLS.
Methods
Animals and Brain Slice Preparation
Experiments were approved by our local ethical committee (Stockholms norra djurförsöksetiska nämnd) and were performed as previously described (Schotanus et al., 2006; Chergui, 2011; Feng et al., 2014). All efforts were made to minimize animal suffering. We used male C57BL/6 mice (Harlan Laboratories, The Netherlands) aged 22 to 30 days (adolescent) and 5 to 8 months (adult). Mice were maintained on a 12-:12-h–light/–dark cycle and had free access to food and water. Mice underwent cervical dislocation followed by decapitation. Their brains were rapidly removed, and coronal brain slices (400 μm thick) containing the NAc, striatum, and overlying cortex were prepared with a microslicer (VT 1000S, Leica Microsystem, Heppenheim, Germany). Slices were incubated for at least 1 hour at 32°C in oxygenated (95% O2 + 5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 10 glucose, and 26 NaHCO3, pH 7.4. Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT; recording chamber from Scientifica Ltd., Uckfield, UK) mounted on an upright microscope (Olympus, Solna, Sweden and Scientifica Ltd) and were continuously perfused with oxygenated aCSF at 28°C.
Electrophysiology in Brain Slices
Extracellular field potentials were recorded using a glass micropipette filled with aCSF positioned on the slice surface. These synaptic responses were evoked by stimulation pulses applied every 15 seconds to the brain slice through a concentric bipolar stimulating electrode (FHC, Bowdoinham, ME) placed near the recording electrode on the surface of the slice. Single stimuli (0.1-millisecond duration) were applied at an intensity yielding 50% to 60% maximal response as assessed by a stimulus/response curve established for each slice at the beginning of the recording session by measuring the amplitude of the field excitatory postsynaptic potentials/population spikes (fEPSP/PSs) evoked by increasing stimulation intensities. Signals were amplified 500 or 1000 times via an Axopatch 200B or a GeneClamp 500B amplifier (Axon Instruments, Foster City, CA) acquired at 10kHz and filtered at 2kHz.
Whole-cell patch clamp recordings of medium spiny neurons in the NAc were made with the help of infrared-differential interference contrast video microscopy. Patch electrodes were filled with a solution containing (in mM): 120 d-gluconic acid, 20 KCl, 2 MgCl2, 1 CaCl2, 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), 10 ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, 2 MgATP, and 0.3 Na3GTP, pH adjusted to 7.3 with KOH. Whole-cell membrane currents and potentials were recorded with a MultiClamp 700B (Axon Instruments) acquired at 10kHz and filtered at 2kHz. Excitatory postsynaptic currents (EPSCs) were evoked every 15 seconds by electrical stimulation of the slice using a patch electrode filled with aCSF positioned on the slice surface in the vicinity of the recorded neuron. The membrane potential was held at −80 mV, and EPSCs were recorded in the presence of bicuculline methiodide (10 µM) to inhibit γ-aminobutyric acid (GABAA) inhibitory postsynaptic currents.
Data Acquisition and Analysis of Electrophysiological Measurements
Data were acquired and analyzed with the pClamp 10 software (Axon Instruments). Numerical values are shown as means with SEM, with n indicating the number of slices or neurons tested. Data are expressed as percentage of the baseline response measured for each slice or neuron during the 5 to 10 minutes preceding the start of perfusion with a compound or 5-Hz stimulation. Statistical significance of the results was assessed by using the Student’s t test for paired observations (comparisons with baseline within single groups) and unpaired (comparisons between adolescents and adults).
Chemicals and Drugs
Chemicals and drugs were purchased from Sigma-Aldrich (Stockholm, Sweden), Tocris Bioscience (Bristol, UK), and Abcam Biochemicals (Cambridge, UK). All compounds were prepared in stock solutions, diluted in aCSF to the desired final concentration, and applied in the perfusion solution. The following compounds were used (final concentrations in μM): AM251 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide) (2); DL-AP5 (dl-2-amino-5-phosphonopentanoic acid) (50); bicuculline methiodide (10); (S)-3,5-DHPG ((S)-3,5- dihydroxyphenylglycine) (100); LY 2183240 (5-[(1,1’-Biphenyl]-4-yl)methyl]-N,N-dimethyl-1H-tetrazole-1-carboxamide) (0.5–1); and WIN 55,212-2 (WIN; (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) (1).
Western Blotting
Western blots were performed to analyze the levels of the receptors known to be involved in LTD induction. The DLS and NAc were visually identified in brain slices and were dissected with the help of a scalpel blade and tweezers. The tissue was frozen and stored at −20°C until processed. The samples were sonicated in 1% sodium dodecyl sulfate and boiled for 10 minutes. Protein concentration was determined in each sample with a bicinchoninic acid protein assay (BCA-kit, Pierce, Rockford, IL). Equal amounts of protein (30 μg) were resuspended in sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 10% running gel and transferred to an Immobilon-P (polyvinylidene difluoride) transfer membrane (Sigma-Aldrich). The membranes were incubated for 1 hour at room temperature with 5% (wt/vol) dry milk in Tris Buffered Saline (TBS)-Tween20. Immunoblotting was carried out with antibodies against CB1 (Sigma-Aldrich, 1:500 dilution), mGluR1 (Cell Signaling Technology, Danvers, MA, 1:500), mGluR5 (Upstate Biotechnology Inc., Waltham, MA, 1:500), GluN1 (Upstate Biotechnology Inc., 1:500), and β-actin (Sigma-Aldrich, 1:4000) in 5% dry milk dissolved in TBS-Tween 20. The membranes were washed 3 times with TBS-Tween20 and incubated with secondary horseradish peroxidase-linked anti-rabbit immunoglobulin G (H+L) (Thermo Scientific; Göteborg, Sweden, 1:6000 dilution) for 1 hour at room temperature. At the end of the incubation, the membranes were washed 6 times with TBS-Tween 20 and immunoreactive bands were detected by enhanced chemiluminescence (Perkin Elmer, Waltham, MA). The autoradiograms were scanned and quantified with the NIH Image 1.63 software. The levels of protein were normalized for the value of β-actin. Data were analyzed with the unpaired Student’s t test to evaluate statistical differences between protein levels in adolescent and adult NAc and striatum.
Results
LTD Induced by the Cannabinoid Receptor Agonist WIN 55,212-2
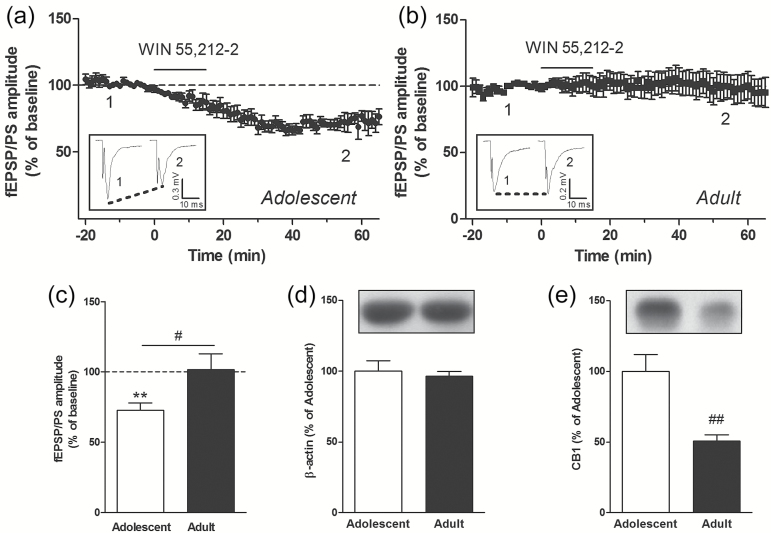
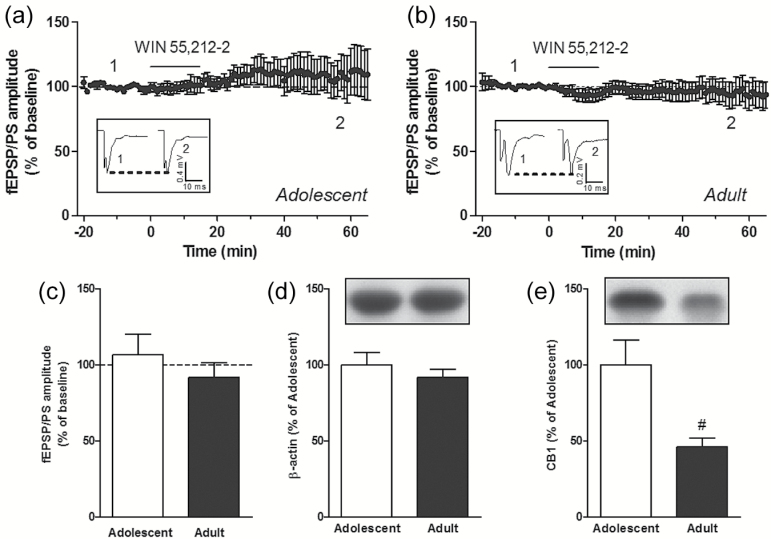
We used the cannabinoid receptor agonist WIN 55,212-2 (WIN) to examine age- and region-specific differences in the ability of an exogenous cannabinoid to trigger LTD of glutamatergic neurotransmission in the striatal complex. We have examined glutamatergic synaptic transmission in the core region of the NAc and in the DLS by recording extracellular fEPSP/PSs evoked by brief, single-pulse, electrical stimulation of glutamatergic fibers present in the brain slice, as previously described (Schotanus et al., 2006; Schotanus and Chergui, 2008). After a stable baseline fEPSP/PS was recorded for 20 minutes, we applied WIN (1 µM) in the perfusion solution for 15 minutes. We found that the amplitude of the fEPSP/PS, measured 55 to 60 minutes after the start of the perfusion with WIN, was decreased to 72.9±5.0% of baseline amplitude in the NAc of adolescent mice (P<.01 compared with baseline; n=8 slices) (Figure 1a, c). However, in the NAc of adult mice, the amplitude of the fEPSP/PS remained unchanged 1 hour after perfusion with WIN (1 µM) (percent of baseline: 101.7±11.3, P>.05, n=9 slices) (Figure 1b, c). In addition, WIN did not induce LTD in the DLS of adolescent (percent of baseline: 106.7±12.5, P>.05, n=9 slices) (Figure 2a, c) and adult (percent of baseline: 91.9±9.8, P>.05, n=11 slices) (Figure 2b, c) mice. We examined the protein levels of CB1 receptors given that these receptors were shown to be localized to glutamatergic terminals in the striatal complex and that they mediate presynaptic inhibition (Robbe et al., 2001). We first found that the levels of β-actin were similar in adolescent and adult NAc as well as DLS, indicating a stable level of this reference protein (Figures 1d and 2d). The levels of CB1 were, however, lower in the adult NAc compared with adolescent NAc (Figure 1e). The same observation was made for the DLS (Figure 2e). These results demonstrate that the synthetic cannabinoid receptor agonist WIN triggers LTD in the NAc of adolescent mice but not in adult mice. This age-related difference is associated with a reduced level of CB1 in the NAc of adult mice. In addition, WIN fails to trigger LTD in the DLS of adolescent and adult mice.
Figure 1.
The cannabinoid receptor agonist WIN 55,212-2 (WIN) ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) induces long-term depression (LTD) in the nucleus accumbens (NAc) of adolescent but not adult mice. Graphs show the time course of the effect of WIN (1 µM), applied for 15 minutes in the perfusion solution at the time indicated by the black bar, on the mean (±SEM) amplitude of the field excitatory postsynaptic potentials/population spike (fEPSP/PS) measured in the NAc (a-b). Data is expressed as a percentage of baseline fEPSP/PS in n=8 slices from adolescent mice (a) and n=9 slices from adult mice (b). Insets show records of fEPSP/PSs measured in 2 slices at the time points indicated on the graphs, that is, before (1) and 50 to 60 minutes after (2) perfusion with WIN. Average magnitude of WIN-induced inhibition measured 55 to 60 minutes after the start of the perfusion of WIN in slices from adolescent (white bar) and adult (black bar) mice (c). ** P<.01 compared with baseline (paired Student’s t test); # P<.05 (unpaired Student’s t test). Protein levels of β-actin (d) and cannabinoid type 1 receptor (CB1) (e) in the NAc of adolescent (n=12) and adult (n=8) mice expressed as a percentage of the levels measured in adolescent mice. ## P<.01 (unpaired Student’s t test).
Figure 2.
WIN ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) fails to induce long-term depression (LTD) in the dorsolateral striatum (DLS) of adolescent and adult mice. Time course of the effect of WIN (1 µM for 15 minutes) on the mean (± SEM) amplitude of the field excitatory postsynaptic potentials/population spikes (fEPSP/PS) measured in the DLS, expressed as a percentage of baseline, in n=9 slices from adolescent mice (a) and n=11 slices from adult mice (b). Insets show records of fEPSP/PSs measured in 2 slices at the time points indicated on the graphs. Averaged fEPSP/PS amplitude measured 55 to 60 minutes after the start of the perfusion of WIN in slices from adolescent and adult mice (c). Protein levels of β-actin (d) and cannabinoid type 1 receptor (CB1) (e) in the DLS of adolescent (n=11) and adult (n=8) mice. #P<.05 (unpaired Student’s t test).
Effect of eCBs
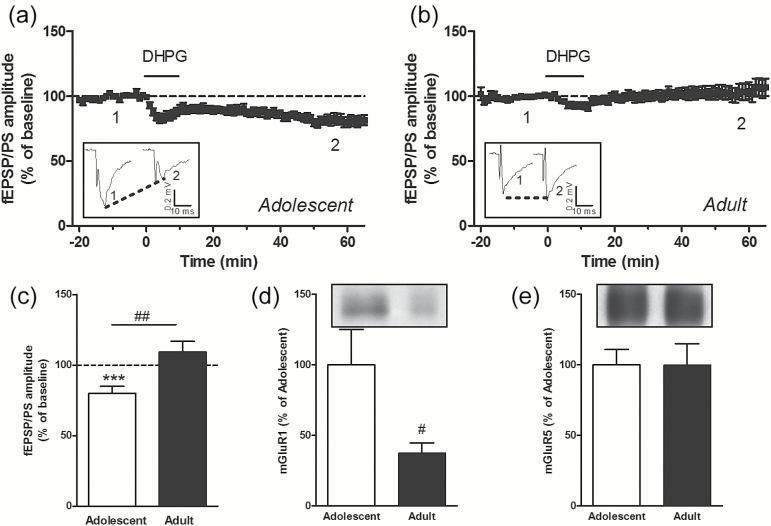
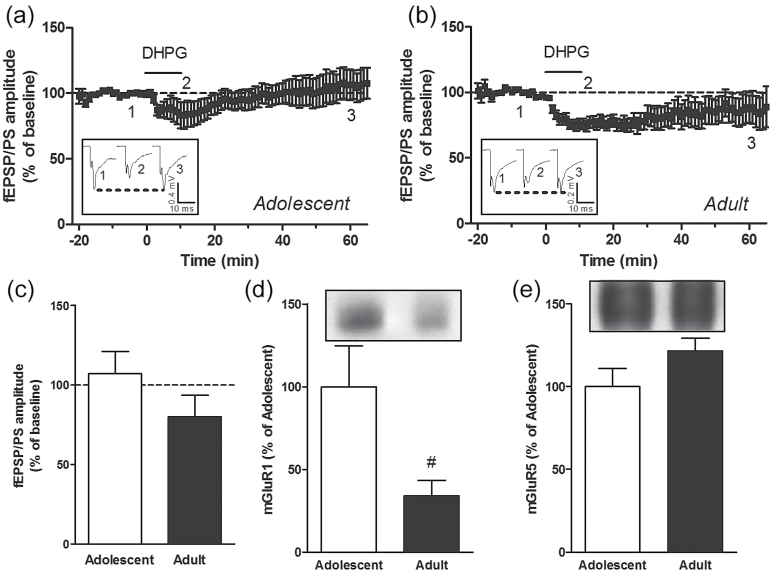
eCBs, released following various stimulation paradigms, were also shown to trigger LTD in the striatal complex (Gerdeman et al., 2002; Robbe et al., 2002; Adermark and Lovinger, 2007). For example, the group I mGluR agonist DHPG induces LTD in the NAc via the release of eCBs acting on presynaptic CB1 receptors in young rodents (Robbe et al., 2002, 2003; Grueter et al., 2010; Huang et al., 2011). We examined whether age- and region-specific differences occurred in LTD induced by eCBs. We first confirmed that under our experimental conditions, DHPG (100 µM, applied for 10 minutes in the perfusion solution) triggered LTD in the NAc of adolescent mice (percent of baseline: 79.9±4.9, P<.001, n=14 slices) (Figure 3a, c). However, this treatment did not result in LTD in the NAc of adult mice (percent of baseline: 109.1±7.6, P>.05, n=16 slices) (Figure 3b, c). In the DLS, DHPG induced an initial depression of the fEPSP/PS in adolescent and adult mice, but no LTD was observed 1 hour following perfusion with this compound (percent of baseline in adolescent DLS: 107.1±14.1, P>.05, n=10 slices [Figure 4a, c]; percent of baseline in adult DLS: 79.9±13.7, P>.05, n=9 slices [Figure 4b, c]). Thus, DHPG induces LTD in the NAc of adolescent mice but fails to trigger LTD in the NAc of adult mice and in the DLS of both adolescent and adult mice.
Figure 3.
The group I metabotropic glutamate receptor (mGluR) agonist ((S)-3,5- dihydroxyphenylglycine) (DHPG) induces long-term depression (LTD) in the nucleus accumbens (NAc) of adolescent but not adult mice. Time course of the effect of DHPG (100 µM for 10 minutes) on the mean (± SEM) amplitude of the field excitatory postsynaptic potentials/population spike (fEPSP/PS) measured in the NAc, expressed as a percentage of baseline, in n=14 slices from adolescent mice (a) and n=16 slices from adult mice (b). Insets show records of fEPSP/PSs measured in 2 slices at the time points indicated on the graphs. Average magnitude of DHPG-induced inhibition measured 55 to 60 minutes after the start of the perfusion of DHPG in slices from adolescent and adult mice (c). ***P<.001 compared with baseline (paired Student’s t test); ##P<.01 (unpaired Student’s t test). Protein levels of mGluR1 (d) and mGluR5 (e) in the NAc of adolescent (n=12) and adult (n=8) mice. #P<.05 (unpaired Student’s t test).
Figure 4.
(S)-3,5-Dihydroxyphenylglycine (DHPG) does not induce long-term depression (LTD) in the dorsolateral striatum (DLS) of adolescent and adult mice. Time course of the effect of DHPG (100 µM for 10 minutes) on the mean (± SEM) amplitude of the field excitatory postsynaptic potentials/population spike (fEPSP/PS) measured in the DLS, expressed as a percentage of baseline, in n=10 slices from adolescent mice (a) and n=9 slices from adult mice (b). Insets show records of fEPSP/PSs measured in 2 slices at the time points indicated on the graphs. Averaged fEPSP/PS amplitude measured 55 to 60 minutes after the start of the perfusion of DHPG in slices from adolescent and adult mice (c). Protein levels of metabotropic glutamate receptor (mGluR)1 (d) and mGluR5 (e) in the DLS of adolescent (n=12) and adult (n=8) mice. #P<.05 (unpaired Student’s t test).
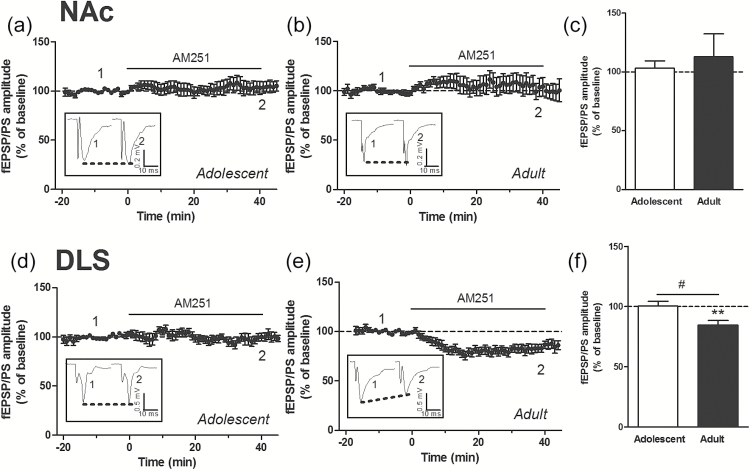
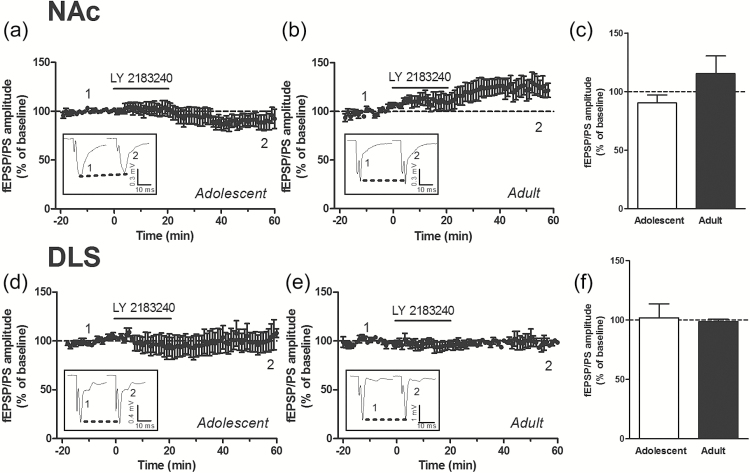
Group I mGluRs include mGluR1 and mGluR5 receptors. We found that the levels of mGluR1 were lower in the NAc and DLS of adult mice compared with adolescent mice (Figures 3d and 4d). However, the levels of mGluR5 were similar in both the NAc and DLS of adult mice compared with adolescent mice (Figures 3e and 4e). Differences in the protein levels of CB1 and mGlu receptors might not fully explain the age- and region-related differences in the ability of endogenous and exogenous cannabinoids to induce LTD in the striatal complex. We therefore attempted to identify an additional mechanism that could contribute to these differences. We hypothesized that activation of CB1 receptors by a tone of eCBs might differ between adolescent and adult mice in a region-dependent manner. To test this hypothesis, we used the CB1 receptor antagonist/inverse agonist AM251 to reveal the effect of a tone of eCBs on glutamatergic synaptic transmission. We found that AM251 (2 µM) had no significant effect on the fEPSP/PS amplitude in the NAc of adolescent and adult mice (Figure 5a-c) and in the DLS of adolescent mice (Figure 5d, f). However, AM251 produced a significant decrease in the fEPSP/PS amplitude measured in the DLS of adult mice (percent of baseline: 84.5±3.9, P<.01, n=7 slices) (Figure 5e, f). We also tested the hypothesis that increasing the concentration of eCBs through blocking eCB uptake might contribute to impairment of LTD. In an attempt to increase the brain concentration of eCBs, we used the eCB uptake inhibitor LY 2183240, which also inhibits fatty acid amide hydrolase, the enzyme that degrades anandamine. LY 2183240 applied in the perfusion solution did not significantly affect the fEPSP/PS amplitude in the NAc or DLS of adolescent and adult mice (percent of baseline in NAc: 90.2±6.9, n=9 slices from adolescent mice [Figure 6a, c]; 115.3±15.1, n=7 slices from adult mice [Figure 6b, c]; percent of baseline in DLS: 101.7±11.9, n=5 slices from adolescent mice [Figure 6d, f]; 98.7±2.0, n=6 slices from adult mice [Figure 6e, f]). These results suggest that the tone of eCBs cannot be further increased without concurrent stimulation to induce the production and release of eCBs.
Figure 5.
Effect of blocking cannabinoid type 1 (CB1) receptors on the field excitatory postsynaptic potentials/population spike (fEPSP/PS). Time course (a, b, d, e) and average magnitude (c, f) of the effect of the CB1 receptor antagonist AM251 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide) (2 µM) on the amplitude of the fEPSP/PS measured in the nucleus accumbens (NAc) (a-c) and dorsolateral striatum (DLS) (d-f). Insets show records of fEPSP/PSs measured in 4 slices at the time points indicated on the graphs. n=7 to 12 slices in each condition. **P<.01 compared with baseline (paired Student’s t test); #P<.05 (unpaired Student’s t test).
Figure 6.
Effect of increasing the tone of endocannabinoids (eCBs) on the field excitatory postsynaptic potentials/population spike (fEPSP/PS). Time course (a, b, d, e) and average magnitude (c, f) of the effect of the eCB uptake inhibitor LY 2183240 (5-[(1,1’-Biphenyl]-4-yl)methyl]-N,N-dimethyl-1H-tetrazole-1-carboxamide) (0.5–1 µM) on the amplitude of the fEPSP/PS measured in the nucleus accumbens (NAc) (a-c) and DLS (b-f). n=5 to 9 slices in each condition. Insets show records of fEPSP/PSs measured in 4 slices at the time points indicated on the graphs.
NMDA Receptor-Dependent LTD in the NAc
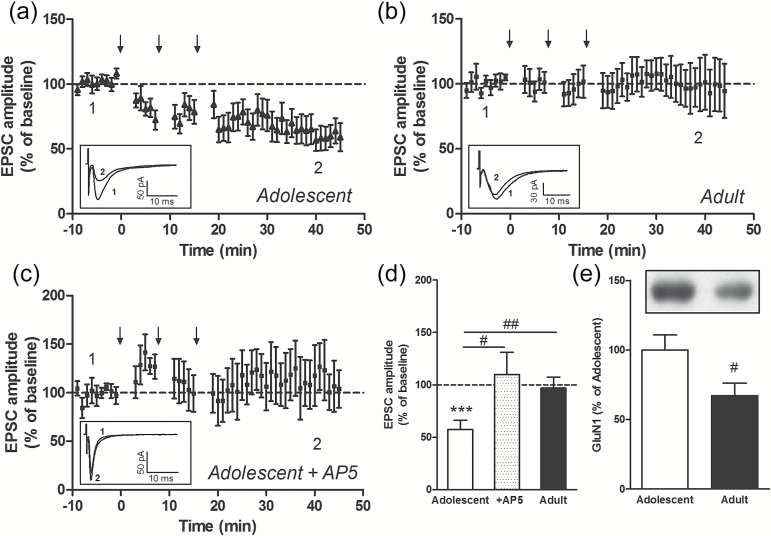
We tested whether the age-related differences in the ability of exogenous and endogenous cannabinoids to induce LTD were specific for this type of synaptic plasticity or if similar differences existed in the induction of another form of LTD, that is, NMDA-LTD. In the NAc, a NMDA-LTD has been described using whole-cell patch-clamp recordings from medium spiny projection neurons (MSNs) (Thomas et al., 2000, 2001; Jeanes et al., 2011). In the dorsal striatum, however, LTD is independent of NMDA receptors (Calabresi et al., 2000; Lovinger et al., 2003). We therefore measured, in individual MSNs in the NAc core, EPSCs evoked by local stimulations as previously described (Chergui, 2011). To induce NMDA-LTD, we used a moderate frequency stimulation protocol that consisted of 3 bouts at 5 Hz, 3-minute duration, 5-minute inter-train intervals with the same stimulation intensity as for evoking EPSCs, paired with a membrane depolarization to −50 mV during the bouts. This stimulation protocol induced a long-lasting decrease in the amplitude of the EPSC in the NAc of adolescent mice (percent of baseline: 57.4±8.6, P<.001, n=15 MSNs) (Figure 7a, d). In the presence of the NMDA receptor antagonist dl-2-amino-5-phosphonopentanoic acid (50 µM), a 5-Hz stimulation did not induce LTD (percent of baseline: 109.8±21.2, P>.05, n=5 MSNs) (Figure 7c-d), demonstrating that this form of LTD was dependent on NMDA receptors. In the NAc of adult mice, the same 5-Hz stimulation did not induce LTD (percent of baseline: 96.9±10.4, P>.05, n=11 MSNs) (Figure 7b, d). We analyzed the levels of GluN1, the obligatory subunit of NMDA receptors, and found that GluN1 was downregulated in the NAc of adult mice compared with the levels measured in the NAc of adolescent mice (Figure 7e). Thus, the reduced ability of a 5-Hz stimulation protocol to induce LTD in the NAc of adult mice is associated with a reduced level of GluN1.
Figure 7.
N-methyl-d-aspartate (NMDA) receptor-dependent long-term depression (LTD) in the nucleus accumbens (NAc). Moderate stimulation (3 bouts of 5 Hz, 3 minutes each, paired with membrane depolarization to −50 mV, applied at the times indicated by the arrows) induces LTD of the excitatory postsynaptic currents (EPSC) in NAc projection neurons of adolescent mice (n=15 medium spiny projection neurons [MSNs]) (a). The same stimulation protocol does not induce LTD in adult mice (n=11 MSNs) (b) and in adolescent mice in the presence of dl-2-amino-5-phosphonopentanoic acid (n=5 MSNs) (c). Insets show examples of EPSC recordings in 3 MSNs at the time points indicated on the graphs, that is, before (1) and 35 to 40 minutes after (2) the first 5-Hz bout. Average magnitude of stimulation-induced LTD in adolescent (control MSNs and MSNs recorded in the presence of AP5) and adult NAc (d). ***P<.001 compared with baseline (paired Student’s t test); #P<.05, ##P<.01 (unpaired Student’s t test). Protein levels of GluN1 in the NAc of adolescent (n=12) and adult (n=9) mice (d). #P<.05 (unpaired Student’s t test).
Discussion
Previous studies have identified and characterized forms of LTD in the striatal complex that are dependent on eCBs and NMDA receptors (Thomas et al., 2000; Robbe et al., 2001; Gerdeman et al., 2002; Kreitzer and Malenka, 2005). These studies were, however, performed with brains from adolescent mice, and the potential age-related differences in the modulation of synaptic strength have not been thoroughly investigated. We have examined whether the ability of cannabinoids and NMDA receptors to induce LTD differed between adolescent and adult mice and between the NAc and the DLS. Our results demonstrate that cannabinoid-mediated LTD is induced in the NAc, but not in the DLS, of adolescent mice and is not induced in adult mice. In addition, NMDA-LTD in the NAc is observed in adolescent but not adult mice. These differences might result from decreased protein levels of the receptors involved in LTD induction as well as a tone of eCBs in the adult brain.
LTD Is Induced in the NAc of Adolescent but Not Adult Mice
We found that, under the same experimental condition, endogenous or exogenous cannabinoids reliably induced LTD in the NAc of adolescent mice. Cannabinoid-LTD was shown to result from the CB1 receptor-mediated inhibition of glutamate release but also from the internalization of postsynaptic AMPA receptors (Grueter et al., 2010; Ohno-Shosaku and Kano, 2014). Thus, it is likely that the LTD induced by WIN and also by DHPG in the NAc of adolescent mice was mediated by CB1 receptors in our study. LTD induced by WIN and DHPG in the NAc is age dependent, that is, it is not induced in adult mice. This age-related difference in the ability of eCBs to induce LTD might be due to downregulation of CB1 receptors and mGluR1. However, eCB-LTD induced by DHPG in the NAc was shown to involve mGluR5 and not mGluR1 (Robbe et al., 2003; Grueter et al., 2010). Because the levels of mGluR5 were similar in the NAc of adult and adolescent mice, other mechanisms, which remain to be identified, likely underlie the blunted LTD in the NAc of adult mice. It is unlikely that a tone of eCBs in the NAc of adult mice occludes LTD induction, because blocking CB1 receptors or eCB reuptake/degradation does not result in increased fEPSP/PS amplitude. We also found that NMDA-LTD in the NAc is age dependent and that the blunted NMDA-LTD in adult mice is associated with a reduced level of GluN1. Taken together, our results suggest that the NAc of adolescent mice is more sensitive to not only eCB-induced plasticity but also to other forms of long-term changes in synaptic strength.
LTD Is Not Induced in the DLS of Adolescent or Adult Mice
In contrast to the effect of WIN and DHPG in the NAc of adolescent mice, we found that these compounds failed to induce LTD of the fEPSP/PS in the DLS of adolescent and adult mice. A similar lack of effect of DHPG and WIN in the striatum has been described in earlier studies, whereas other studies found that WIN induces LTD in the striatum (Huang et al., 2001; Kreitzer and Malenka, 2005, 2007; Sergeeva et al., 2007; Chepkova et al., 2009). The reasons for these discrepancies might lie in the experimental protocols used in the different studies to induce LTD. Several experimental factors such as the region of the striatum where glutamatergic transmission is measured (ie, DLS vs dorsomedial striatum and the type of animals used [species, strain, and age]) could have influenced the ability of WIN and DHPG to induce LTD in the striatum. The membrane potential of the recorded neurons is also a key determinant in the effects of eCBs. Indeed, DHPG failed to induce LTD in MSNs of the striatum when their membrane potential was clamped near their resting membrane potential (hyperpolarized) but induced LTD at a more depolarized membrane potential, close to the UP state measured in vivo (Kreitzer and Malenka, 2005). In the striatum, concurrent depolarization of the postsynaptic membrane via, for example, cortical activity, and stimulation of mGluRs is therefore required to induce LTD, whereas such a requirement does not seem necessary in the NAc. In addition, eCB-LTD is cell-type specific, that is, it is induced in MSNs of the indirect pathway (that express the dopamine D2 receptor) and not in MSNs of the direct pathway (that express the dopamine D1 receptor) (Kreitzer and Malenka, 2007).
In addition, GABAA receptor antagonists, which are often used in electrophysiological experiments, likely affect the ability of exogenous and endogenous cannabinoids to induce LTD. Indeed, low concentrations (50–300nM) of WIN were shown to increase the amplitude of the field potential measured in the striatum through inhibition of GABAergic neurotransmission, but 1 µM WIN inhibited the field potential in the presence of a GABAA receptor antagonist (Clarke and Adermark, 2010). The lack of LTD in the DLS of adolescent mice following WIN or DHPG might result from a combination of synaptic potentiation and inhibition in our experimental conditions, which reflects the involvement of GABAergic local circuits. In addition, we found that the CB1 receptor antagonist AM251 significantly decreased the amplitude of the fEPSP/PS in the DLS of adult mice. Together, these observations suggest that a tone of eCBs increases glutamate release from presynaptic terminals in the DLS of adult mice. Further modulation of neurotransmission by exogenous and endogenous cannabinoids might be occluded or prevented by this tone of eCBs. A reduced level of CB1 receptors might also contribute to the blunted LTD in the DLS of adult mice.
In conclusion, our results demonstrate that in adolescent mice, cannabinoids induce a form of synaptic plasticity in the NAc, a brain region that integrates motivational information, but not in the DLS, which is involved in habit formation. They also provide further evidence for an increased sensitivity of the NAc of adolescent mice to cannabinoid- and NMDA receptor-dependent synaptic plasticity than the NAc of adult mice. Our findings contribute to a better understanding of the increased vulnerability of adolescents to drug-induced plasticity.
Statement of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Swedish Research Council (grant 2011–2770), Svenska Läkaresällskapet, and the Karolinska Institute.
References
- Adermark L, Lovinger DM. (2007). Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci 27:6781–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G. (2000). Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol 61:231–265. [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Fleischer W, Kazmierczak T, Doreulee N, Haas HL, Sergeeva OA. (2009). Developmental alterations of DHPG-induced long-term depression of corticostriatal synaptic transmission: switch from NMDA receptor-dependent towards CB1 receptor-dependent plasticity. Pflugers Arch 459:131–141. [DOI] [PubMed] [Google Scholar]
- Chergui K. (2011). Dopamine induces a GluN2A-dependent form of long-term depression of NMDA synaptic responses in the nucleus accumbens. Neuropharmacology 60:975–981. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. (2008). Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res 32:375–385. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Adermark L. (2010). Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology 58:799–805. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. (2010). Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn 72:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZJ, Zhang X, Chergui K. (2014). Allosteric modulation of NMDA receptors alters neurotransmission in the striatum of a mouse model of Parkinson’s disease. Exp Neurol 255:154–160. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. (2004). A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci 24:6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. (2002). Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5:446–451. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. (2010). Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Juraska JM. (2013). The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience 249:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. (2013). Synaptic targets of Delta9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. (2003). Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci 23:4815–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. (2001). Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol 532:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. (2011). Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci 31:4194–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. (2011). In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J Pharmacol Exp Ther 336:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. (2005). Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25:10537–10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. (2007). Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445:643–647. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. (2003). Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. In: Glutamate and Disorders of Cognition and Motivation, pp 226–240. Annals of the New York Academy Of Sciences, New York Acad Sciences, New York, NY, USA. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. (2004). A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci 7:585–586. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Kano M. (2014). Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol 29:1–8. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. (2001). Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci 21:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. (2002). Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A 99:8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Manzoni OJ. (2003). Exogenous and endogenous cannabinoids control synaptic transmission in mice nucleus accumbens. Ann N Y Acad Sci 1003:212–225. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. (2005). Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol 562:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. (2008). Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors. Eur J Neurosci 27:1957–1964. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Fredholm BB, Chergui K. (2006). NMDA depresses glutamatergic synaptic transmission in the striatum through the activation of adenosine A1 receptors: evidence from knockout mice. Neuropharmacology 51:272–282. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Doreulee N, Chepkova AN, Kazmierczak T, Haas HL. (2007). Long-term depression of cortico-striatal synaptic transmission by DHPG depends on endocannabinoid release and nitric oxide synthesis. Eur J Neurosci 26:1889–1894. [DOI] [PubMed] [Google Scholar]
- Spear LP. (2002). The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl 14:71–81. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. (2000). Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci 20:5581–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. (2001). Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4:1217–1223. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. (2008). Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. (2007). Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 25:3226–3232. [DOI] [PubMed] [Google Scholar]