Abstract
Background:
Major depression is associated with several alterations, including reduced neuronal plasticity and impaired synaptic function, which represent an important target of pharmacological intervention.
Methods:
In the present study, we have investigated the ability of the antipsychotic drug lurasidone to modulate behavioral and neuroplastic alterations in the chronic mild stress model of depression.
Results:
Rats that show reduced sucrose consumption after 2 weeks of chronic mild stress have reduced expression of the pool of Bdnf transcripts with the long 3′ untranslated region (3′-UTR) that may be targeted to the synaptic compartment, suggesting the contribution of the neurotrophin to the behavioral dysfunction produced by chronic mild stress. The downregulation of Bdnf expression persisted also after 7 weeks of chronic mild stress, whereas chronic lurasidone treatment improved anhedonia in chronic mild stress rats and restored Bdnf mRNA levels in the prefrontal cortex. Moreover, chronic lurasidone treatment was able to normalize chronic mild stress-induced defects of Psd95 and Gfap as well as changes in molecular regulators of protein translation at the synapse, including mTOR and eEF2.
Conclusions:
These results demonstrate that lurasidone shows antidepressant properties in the chronic mild stress model through the modulation of synaptic and neuroplastic proteins. Such changes may contribute to the amelioration of functional capacities, which are deteriorated in patients with major depression and stress-related disorders.
Keywords: lurasidone, chronic mild stress, BDNF, synaptic mechanisms, glutamate
Introduction
Depression is a complex and heterogeneous disorder that represents a major cause of disability in the world. It is characterized by different neurobiological alterations as well as structural and functional changes in brain structures, such as the prefrontal cortex (PFC) and the hippocampus, which play a crucial role in emotion, mood, and cognition (Rajkowska et al., 1999; Gass and Riva, 2007; Dantzer et al., 2008; Pariante and Lightman, 2008; Krishnan and Nestler, 2010; Duman and Aghajanian, 2012; Kang et al., 2012). It has been recently postulated that synaptic mechanisms may be crucial mediators of the dysfunctions associated with mood disorders (Radley et al., 2006; Duman and Aghajanian, 2012; Kavalali and Monteggia, 2012). Indeed, major depression is characterized by impaired ability to respond or cope with environmental challenges, possibly because of defects in neuronal circuitries as well as impairment in the mechanisms that are responsible for synaptic control of protein translation and activation (Duman and Aghajanian, 2012; Monteggia et al., 2013). This possibility is sustained by consistent data demonstrating that exposure to prolonged stress, a major susceptibility player for depression, produces dendritic atrophy and reduced spine number that may eventually contribute to the inability of selected neuronal populations to function within the context of brain circuitries (Radley et al., 2006; Fumagalli et al., 2007).
Rodent studies have provided valuable information on the mechanisms that may lead to structural alterations of neurons following stress experience (de Kloet et al., 2005; McEwen, 2007; Elizalde et al., 2010; Banasr et al., 2011). Although different studies have investigated synaptic alterations as a consequence of stress exposure, limited information exists with respect to how such alterations may develop as stress continues and the impact of a pharmacological intervention on these synaptic changes.
To address some of these questions, we employed the chronic mild stress (CMS) model of depression, which has been extensively used to investigate stress-related mechanisms (Willner, 2005; Hill et al., 2012; Pochwat et al., 2014). First of all, we used a sucrose consumption test as a measure of anhedonia to identify the animals that were susceptible or resilient to stress in order to investigate genes that may contribute to the behavioral dysfunction during the initial phase of the stress exposure. Sucrose consumption has been extensively validated as end point in CMS (Willner, 2005), and it has been also successfully used in other rat models of depression, such as learned helplessness (Vollmayr et al., 2004). Next, we established if and how synaptic mechanisms may change over time in stress-responsive rats and, ultimately, whether the pharmacological intervention with the multi-receptor modulator lurasidone would be able to normalize the anhedonic phenotype as well as stress-induced deterioration of key proteins and systems.
To establish the integrity of synaptic mechanisms, we investigated different structural and functional markers that have been previously associated with depression. Specifically, we investigated the neurotrophin BDNF, which plays a role in depression and is also critically involved in drug responsiveness (Kozisek et al., 2008; Calabrese et al., 2010 2011; Krishnan and Nestler, 2010; Autry and Monteggia, 2012). In this respect, we specifically analyzed the neurotrophin transcripts that localize to the synaptic compartment and may serve a specific function at this level (An et al., 2008; Lau et al., 2010). Markers of pre- and postsynaptic neuronal integrity as well as of glial function were also investigated (Banasr et al., 2010; Li et al., 2010). Lastly, to characterize functional defects that may contribute to synaptic dysfunction, we analyzed the impact of CMS and pharmacological intervention on mammalian target of rapamycin (mTOR) as well as eukaryotic elongation factor 2 (eEF2) and its kinase, eEF2K, that play a role in protein translation at the synapse and have recently been associated with rapid antidepressant response (Hoeffer and Klann, 2010; Li et al., 2010; Monteggia et al., 2013).
Methods
General reagents were purchased from Sigma-Aldrich (Milan, Italy) and molecular biology reagents were obtained from Bio-Rad Laboratories S.r.l. (Segrate, Italy), Eurofins MWG-Operon (Ebersberg, Germany), Life Technologies (Monza, Italy), PerkinElmer (Milan, Italy), and Roche (Monza, Italy). Lurasidone was kindly provided by Sumitomo Dainippon Pharma Co. Ltd (Japan).
Animals
Adult male Wistar rats (Charles River, Germany) were brought into the laboratory 1 month before the start of the experiment. The animals were singly housed with food and water available ad libitum and were maintained on a 12-h–light/–dark cycle in constant temperature (22±2oC) and humidity (50±5%) conditions. All experiments were performed in male animals to compare the effects of lurasidone with those obtained in other animal models (Fumagalli et al., 2012; Luoni et al., 2013 2014). However, it must be kept in mind that BDNF may be differently regulated in male and female rodents (Chourbaji et al., 2008 2012). All procedures used in this study were conducted according to the rules and principles of the 2010/63/EU Directive, were approved by the Local Bioethical Committee at the Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland and are adherent to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and reduce the total number of animals used while maintaining statistically valid group numbers.
Stress Procedure and Pharmacological Treatment
After a period of adaptation to laboratory and housing conditions, the animals were trained to consume a palatable sucrose solution (1%). Training consisted of nine 1 hour-baseline tests in which the sucrose solution was presented to the rats in their home cage after 14 hours of food and water deprivation. Sucrose intake was measured as differences in bottle weight before and after each test. Subsequently, sucrose consumption was monitored, under similar conditions, at weekly intervals throughout the whole experiment.
Together with the sucrose consumption monitoring, one group of animals was subjected to a well-established CMS procedure (Willner, 2005; Pochwat et al., 2014), while control animals (CTRL) were housed in separate rooms and had no contact with the stressed animals (CMS). Each week of the stress regime consisted of 2 periods of food or water deprivation, 2 periods of 45-degree cage tilt, 2 periods of intermittent illumination (lights on and off every 2 hours), 2 periods of soiled cage (250mL water in sawdust bedding), 1 period of paired housing, 2 periods of low-intensity stroboscopic illumination (150 flashes/min), and 3 periods of no stress. All stressors lasted 10 to 14 hours and were applied individually and continuously, day and night. All the animals were deprived of food and water for 14 hours preceding each sucrose test, but otherwise food and water were freely available in the home cage.
After 2 weeks of stress, the animals underwent the sucrose consumption test and, based on the results, were divided into 2 different groups: stress-reactive (SR) and non-SR (NSR). For the first part of the study (Experiment 1), a total of 30 rats (10 CTRL, 10 SR, and 10 NSR) were then killed by decapitation in a semirandomized order 24 hours after the end of the stress session, and the PFC was rapidly dissected, frozen on dry ice/isopentane, and stored at −700C. Dissections were performed according to the atlas of Paxinos and Watson (1996). In detail, the PFC was dissected from 2-mm–thick slices (PFC defined as Cg1, Cg3, and IL subregions corresponding to the plates 6–9).
For the second part of the study (Experiment 2), SR animals continued the CMS procedure for 5 more weeks, while CTRL rats were kept undisturbed. Moreover, both SR and CTRL groups were randomized into matched subgroups (n=10) to receive vehicle or lurasidone (Figure 3). In detail, lurasidone was prepared by suspending the drug at a concentration of 3mg/mL in a 1% hydroxyethylcellulose solution. Lurasidone and vehicle were administered via oral gavage (per os) in the amount of 1mL/kg body weight. We selected this dose and route of delivery based on previous studies demonstrating the efficacy of lurasidone as an antidepressant (Ishiyama et al., 2007 2010; Tarazi and Riva, 2013). Hence, animals received vehicle or lurasidone for 5 weeks. At 24 hours after the last drug administration, rats were killed by decapitation in a semirandomized order and the PFC was rapidly dissected, as described above.
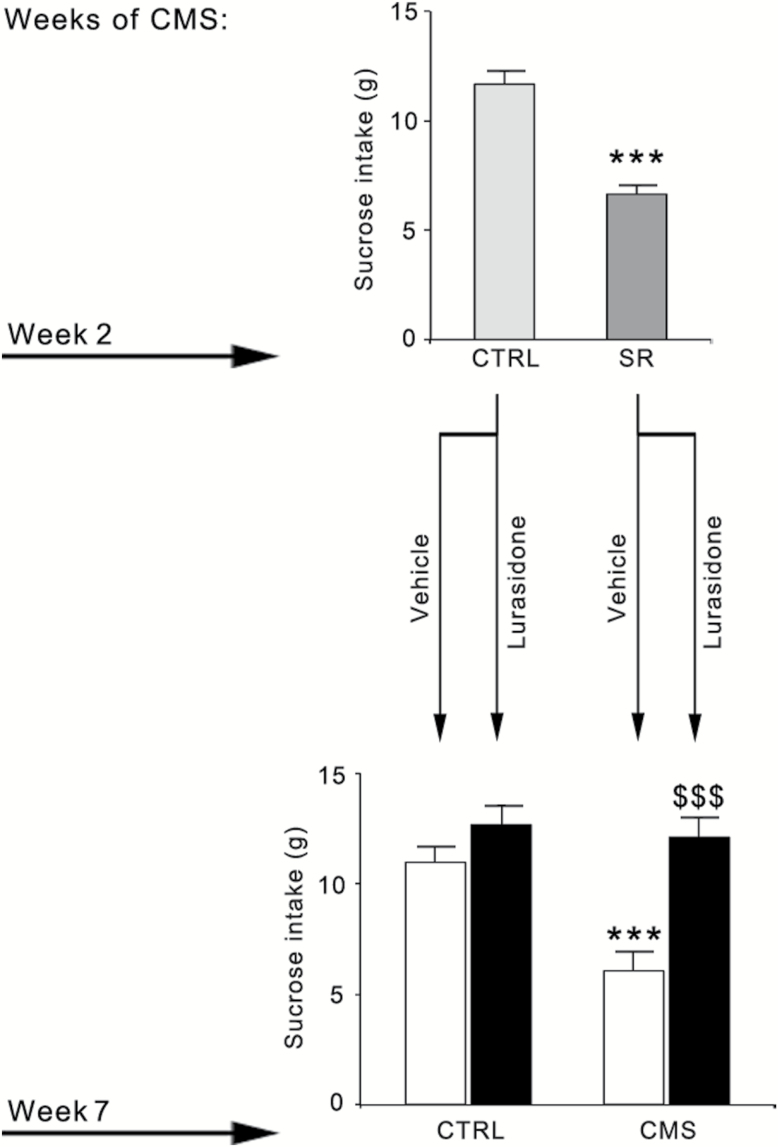
Figure 3.
Effect of chronic lurasidone treatment on sucrose consumption in control (CTRL) or chronic mild stress (CMS) rats. After 2 weeks of CMS exposure, a group of SR rats (n=20) were identified and randomized to receive vehicle or lurasidone for 5 more weeks, concurrently with CMS. A group of CTRL rats (n=20) was also randomized to receive vehicle or lurasidone for 5 more weeks. The data, expressed as grams (g) of sucrose intake, are the mean ± SEM of at least 9 independent determinations. Upper panel: *** P<.001 vs CTRL (Student’s t test). Lower panel: *** P<.001 vs CTRL/vehicle; $$$ P<.001 vs CMS/vehicle (2-way analysis of variance followed by Fisher’s LSD posthoc comparison).
RNA Preparation for qRT-PCR Analysis of mRNA Levels
For gene expression analyses, total RNA was isolated from the PFC by single-step guanidinium isothiocyanate/phenol extraction using PureZol RNA isolation reagent (Bio-Rad Laboratories S.r.l., Segrate, Italy) according to the manufacturer’s instructions and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for reverse-transcriptase real-time polymerase chain reaction (qRT-PCR) to assess mRNA levels. An aliquot of each sample was treated with DNase to avoid DNA contamination.
RNA was analyzed by TaqMan qRT-PCR instrument (CFX384 real time system, Bio-Rad Laboratories) using the iScriptTM one-step RT-PCR kit for probes (Bio-Rad Laboratories). Samples were run in 384-well formats in triplicate as multiplexed reactions with a normalizing internal control (36b4).
Probe and primer sequences of long 3′-UTR (untranslated region) Bdnf (assay ID: Rn02531967_s1) were purchased from Life Technologies (Monza, Italy) and are available on request, while the other TaqMan gene expression assays were purchased from Eurofins MWG-Operon (Germany) and are summarized in Table 1.
Table 1.
Sequences of Forward and Reverse Primers and Probes Used in qRT-PCR Analyses
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Total Bdnf | AAGTCTGCATTACATTCCTCGA | GTTTTCTGAAAGAGGGACAGTTTAT | TGTGGTTTGTTGCCGTTGCCAAG |
| Psd95 | CAAGAAATACCGCTACCAAGATG | CCCTCTGTTCCATTCACCTG | TCAACACGGACACCCTAGAAGCC |
| Syn1 | ATGGCACGTAATGGAGACTAC | TCTTGTGTAGTCGAACCATCTG | ATTGGGCTGCAGTATGCTGGGA |
| Gfap | GACTTTCTCCAACCTCCAGATC | CTCCTGCTTCGACTCCTTAATG | CCGCATCTCCACCGTCTTTACCA |
| Vglut1 | ACTGCCTCACCTTGTCATG | GTAGCTTCCATCCCGAAACC | CTTTCGCACATTGGTCGTGGACATT |
| Glt1 | TTGCTGGCATTTTCCAAGC | TTAATGGTTGCTCCGACTGG | CAAGCGTGTGACCAGATTCGTCCT |
| Eaac1 | CTCCCCGATTCCTCACAAAC | GAACCAAGACTCCTACCACG | CAGCAGCCAGTGATTCCGCAG |
| 36b4 | TTCCCACTGGCTGAAAAGGT | CGCAGCCGCAAATGC | AAGGCCTTCCTGGCCGATCCATC |
Abbreviations: qRT-PCR, reverse-transcriptase real-time polymerase chain reaction.
Purchased from Eurofins MWG Operon (Germany).
Thermal cycling was initiated with an incubation at 50°C for 10 minutes (RNA retrotranscription) and then at 95°C for 5 minutes (TaqMan polymerase activation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95°C for 10 seconds to enable the melting process and then for 30 seconds at 60°C for the annealing and extension reaction. Relative target gene expression was calculated according to the 2(-DeltaDeltaC(T)) method.
Preparation of Subcellular Fractions for Western-Blot Analysis
PFC from different experimental groups were manually homogenized in a glass-glass potter in ice-cold 0.32M sucrose buffer (pH 7.4) containing 1mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 0.1mM ethylene glycol tetraacetic acid (EGTA) and 0.1mM phenylmethylsulfonyl fluoride in the presence of commercial cocktails of protease (Roche, Monza, Italy) and phosphatase (Sigma-Aldrich, Milan, Italy) inhibitors. The homogenate was clarified at 1000g for 10 minutes. The resulting supernatant was then centrifuged at 13000g for 15 minutes. The supernatant was discarded while the pellet (P2), corresponding to the crude membrane fraction, was resuspended in a buffer (20mM HEPES, 0.1mM dithiothreitol, 0.1mM EGTA) supplemented with protease and phosphatase inhibitors. This fraction is enriched in synaptic proteins as previously demonstrated (Fumagalli et al., 2008). Total protein content was measured according to the Bradford Protein Assay procedure (Bio-Rad Laboratories) using bovine serum albumin as the calibration standard.
Equal amounts of protein (10 μg) were run under reducing conditions on Sodium Dodecyl Sulphate-polyacrylamide gels (AnyKd Criterion TGX precast gels, Bio-rad Laboratories) for BDNF and 8% SDS-PAGE (PolyAcrylamide Gel Electrophoresis) for the other proteins examined and then electrophoretically transferred onto polyvinylidene fluoride membranes. Unspecific binding sites were blocked for 1 hour in 10% nonfat dry milk in Tris-buffered saline (2 hour for BDNF), and membranes were then incubated overnight with primary antibodies at 4°C in blocking solution and then with secondary antibodies for 1 hour at room temperature (Table 2). Immunocomplexes were visualized by chemiluminescence using the Western Lightning Plus ECL (PerkinElmer) and the Chemidoc MP imaging system (Bio-Rad Laboratories). Results were normalized using β-actin as an internal standard, because its expression is not regulated by the experimental paradigm used.
Table 2.
List of Antibodies Used in the Western-Blot Analyses and Their Terms of Incubation
| Target Protein | Primary Antibody | Secondary Antibody |
|---|---|---|
| mBDNF (14kDa) |
Polyclonal Ab 1:500, Santa Cruz Biotechnology | HRP conjugated anti-rabbit IgG 1:1000 |
| proBDNF (32kDa) |
Monoclonal Ab 1:2000, GeneTex | HRP conjugated anti-rabbit IgG 1:5000 |
| eEF2 (95kDa) |
Polyclonal Ab 1:1000, Cell Signaling | HRP conjugated anti-rabbit IgG 1:1000 |
| eEF2K (105kDa) |
Polyclonal Ab 1:1000, Cell Signaling | HRP conjugated anti-rabbit IgG 1:1000 |
| phospho-mTOR (Ser2448) (279kDa) |
Polyclonal Ab 1:1000, Cell Signaling | HRP conjugated anti-rabbit IgG 1:1000 |
| mTOR (279kDa) |
Polyclonal Ab 1:1000, Cell Signaling | HRP conjugated anti-rabbit IgG 1:1000 |
| β-actin (43kDa) |
Polyclonal Ab 1:10000, Sigma-Aldrich | HRP conjugated anti-mouse IgG 1:10000 |
Abbreviations: Ig, immunoglobulin; HRP, horseradish peroxidase; mTOR, mammalian target of rapamycin.
Statistical Analyses
All the analyses were carried out in individual animals (independent determinations) by using different statistical tests according to the effect examined. Specifically, for Experiment 1, the data were analyzed by 1-way analysis of variance. Conversely, the effects of prolonged CMS and pharmacological treatment (Experiment 2) were evaluated by 2-way analysis of variance, with stress (CMS vs CTRL) and treatment (vehicle vs lurasidone) as independent factors. When appropriate, further differences were analyzed by Fisher’s Least Significant Difference postoc comparison. For graphic clarity, data are presented as means±standard error of the mean (SEM). Pearson product-moment correlations (r) between levels of sucrose consumption (after 2 or 7 weeks of CMS) and mRNA levels of total Bdnf, Bdnf long 3’-UTR, Psd95, Syn1, or Gfap were performed to evaluate the correlation between the expression levels of these genes in single animals and their anhedonic phenotype. Significance for all tests was assumed for P<.05.
Results
Experiment 1: Identification of CMS Susceptible Rats and Analysis of the Expression Profile for Synaptic/Neuroplastic Genes
Consistent with what we have previously observed (Pochwat et al., 2014), exposure to CMS for 2 weeks produced a significant effect on the consumption of 1% sucrose solution. Indeed, while at baseline we found that all animals drank approximately the same amount of sucrose solution (data not shown), the intake remained at similar level in CTRL-non stressed animals, whereas following 2 weeks of CMS, approximately 70% of the animals showed a reduction in sucrose consumption (−4.6g, P<.001). We defined these animals as reactive (SR) to distinguish them from those who did not show an anhedonic phenotype following CMS exposure, which we termed as nonreactive (SNR) (data not shown).
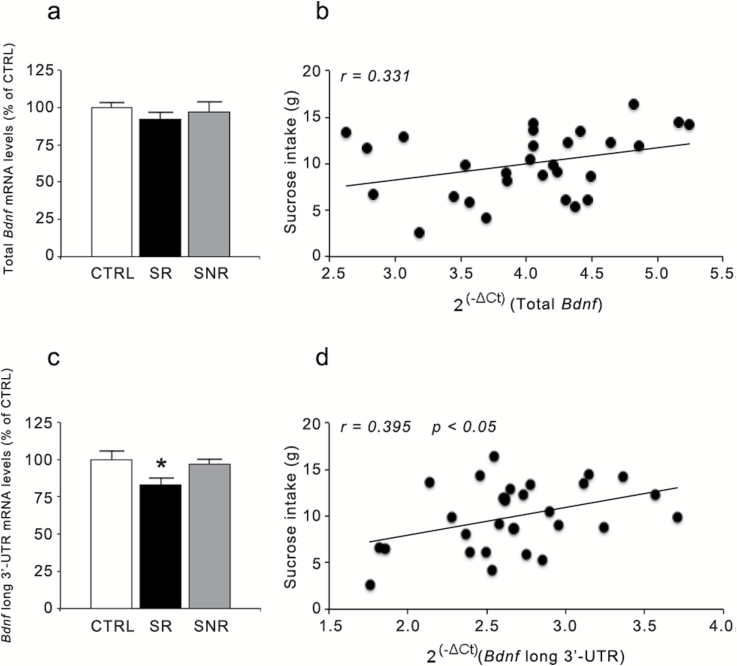
We next investigated if stress vulnerability was associated with significant changes in the PFC expression of neuroplastic-synaptic markers previously associated with mood disturbance (Kozisek et al., 2008; Calabrese et al., 2010; Autry and Monteggia, 2012). As shown in Figure 1, whereas total Bdnf expression was not altered in the PFC of CMS rats (F(2,29)=0.572, P=.571), we found a significant effect of stress on the mRNA levels encoding for the pool of transcripts with the long 3’-UTR (F(2,30)=3.619, P<.05), which were significantly reduced only in the animals that develop anhedonia (−17% vs CTRL, P<.05). Interestingly, we found a significant correlation between the levels of long 3’-UTR Bdnf and sucrose consumption (r=0.395, P<.05), suggesting that the development of anhedonia at an early stage of stress exposure associates with reduced neurotrophin expression (Figure 1d).
Figure 1.
Effect of 2 weeks of chronic mild stress (CMS) exposure on Bdnf mRNA levels in the rat prefrontal cortex (PFC). The mRNA levels of total (a) and long 3’-UTR (c) Bdnf were measured in the PFC of CMS rats (reactive or nonreactive) and are expressed as a percentage of unstressed animals (control [CTRL]) animals (set at 100%). The data are the mean of at least 9 independent determinations. Error bars in a and c represent SEM. * P<.05 vs CTRL (1-way analysis of variance followed by Fisher’s LSD posthoc comparison). The correlation between mRNA levels of total (b) and long 3’-UTR (d) Bdnf (expressed as 2(-ΔCt), where ΔCt is the difference between the threshold cycle of the target gene and the housekeeping gene) and the sucrose intake (expressed as grams) was analyzed by Pearson product–moment correlation (r).
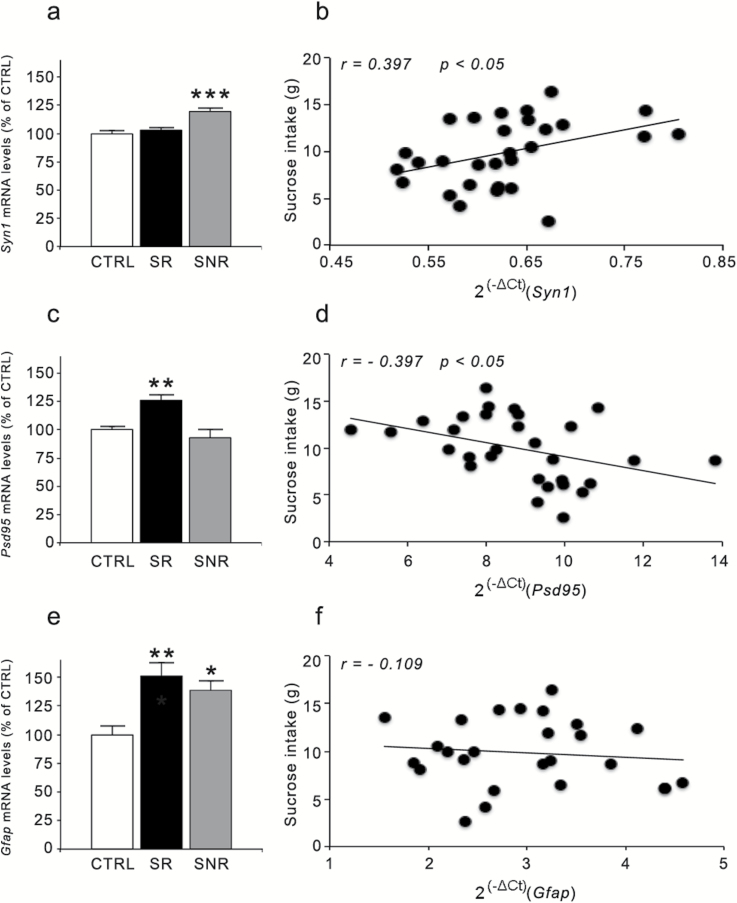
Considering the reduction of the pool of Bdnf transcripts with long 3’-UTR, which undergo dendritic targeting and may contribute to the synaptic function of the neurotrophin (An et al., 2008), we investigated if the expression of representative pre- and postsynaptic markers was also altered in CMS rats that develop anhedonia. As shown in Figure 2, we found significant effects for both the presynaptic marker synapsin-1 (Syn1) (F(2,29)=14.508, P<.001) and the postsynaptic marker Psd95 (F(2,30)=10.050, P<.01). Indeed, the mRNA levels of Syn1 were significantly upregulated only in SNR (+19%, P<.001 vs CTRL rats), whereas the expression of Psd95 was upregulated in CMS animals that develop anhedonia (+26%, P<.01 vs CTRL rats). Moreover, whereas the expression of Syn1 showed a positive correlation with sucrose consumption (r=0.397, P<.05), an opposite correlation was found for Psd95 (r=−0.397, P<.05). We also investigated the expression of glial fibrillary acidic protein (Gfap), a glial marker that is decreased in the PFC of depressed subjects (Rajkowska et al., 1999). Interestingly, its expression was modulated by CMS (F(2,26)=7.268, P<.01), with a significant increase of Gfap mRNA levels in both SR and SNR animals compared with CTRL animals (SR: +51%, P<.01; SNR: +38%, P<.05) (Figure 2e). However, there was no correlation between sucrose consumption and Gfap expression (Figure 2f), suggesting that these modifications were unrelated to the anhedonic phenotype (r=−0.109, P>.05).
Figure 2.
Effect of 2 weeks of chronic mild stress (CMS) exposure on synaptic and structural markers in the rat prefrontal cortex (PFC). The mRNA levels of Syn1 (a), Psd95 (c), and Gfap (e) were measured in the PFC of CMS rats (reactive or nonreactive) and are expressed as a percentage of unstressed (control [CTRL]) animals (set at 100%). The data are the mean of at least 8 independent determinations. Error bars in a, c, and e represent SEM. * P<.05, ** P<.01, and *** P<.001 vs CTRL (1-way analysis of variance followed by Fisher’s LSD posthoc comparison). The correlation between mRNA levels of Syn1 (b), Psd95 (d), and Gfap (f) (expressed as 2(-ΔCt), where ΔCt is the difference between the threshold cycle of the target gene and the housekeeping gene) and the sucrose intake (expressed as grams) were analyzed by Pearson product–moment correlation (r).
Experiment 2: Behavioral and Molecular Characterization of the Effects Produced by Lurasidone Treatment After 7 Weeks of CMS Exposure
We next examined the impact of chronic pharmacological intervention with lurasidone in rats susceptible to CMS. To this purpose, a separate group of rats that developed anhedonia (SR) after 2 weeks of CMS (−5.1g, P<.001 vs CTRL, Student’s t test) (Figure 3, upper panel) were randomized to receive vehicle or lurasidone for 5 weeks while continuing stress exposure. CTRL (nonstressed) rats were also randomized to receive vehicle or lurasidone. As shown in Figure 3 (lower panel), at the end of the 7-week period, sucrose consumption was significantly affected by CMS (F(1,38)=9.936, P<.01) and by lurasidone treatment (F(1,38)=19.757, P<.001), with a significant CMS × lurasidone interaction (F(1,38)=6.152, P<.05). Indeed, although CMS rats treated with vehicle showed a significant decrease in sucrose consumption (−5g, P<.001 vs CTRL/vehicle), the anhedonic phenotype was normalized in the group of rats receiving lurasidone during CMS exposure (P<.001 vs CMS/vehicle rats), whereas the drug had no effect on sucrose consumption when given to CTRL rats.
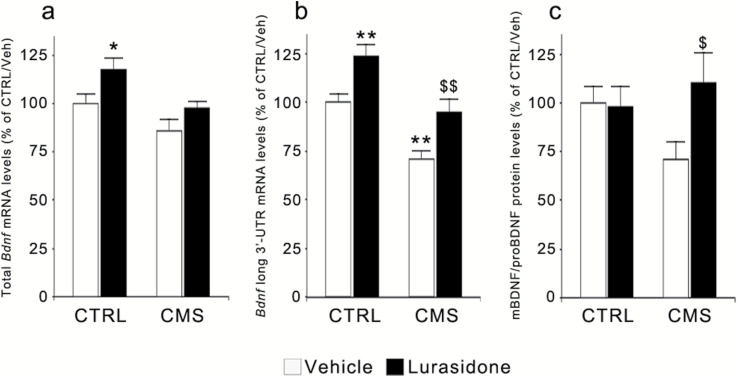
When investigating the expression of total Bdnf in the PFC (Figure 4a), we found a significant effect of 7 weeks of CMS exposure (F(1,35)=9.071, P<.01) and of lurasidone treatment (F(1,35)=6.893, P<.05). Prolonged CMS produced a trend toward a reduction in vehicle-treated rats, whereas lurasidone increased its mRNA levels in CTRL as well as in CMS rats. The expression of the pool of Bdnf transcripts with the long 3’-UTR was also significantly modulated by CMS (F(1,39)=24.194, P<.001) and by lurasidone treatment (F(1,39)=16.519, P<.001). Indeed, the reduction of its mRNA levels observed in CMS reactive rats at 2 weeks (Figure 1c) persisted at 7 weeks (−29%, P<.01 vs CTRL/vehicle), whereas chronic lurasidone treatment upregulated the expression of long 3’-UTR Bdnf in CTRL rats (+24%, P<.01 vs CTRL/vehicle) and normalized its mRNA levels when administered to CMS animals (P<.01 vs CMS rats treated with vehicle) (Figure 4b). Moreover, at the end of the 7-week period, we found a statistically significant correlation between Bdnf expression and sucrose consumption (total Bdnf: r=0.347, n=34, P<.05; long 3’-UTR Bdnf: r=0.395, n=38, P<.05), with lower neurotrophin expression corresponding to a more severe anhedonic phenotype (supplementary Figure S1a-b).
Figure 4.
Modulation of BDNF mRNA and protein levels following chronic mild stress (CMS) and/or lurasidone treatment in the rat prefrontal cortex (PFC). The mRNA levels of total (a) and long 3’-UTR (b) Bdnf as well as the ratio of the protein levels between its mature and precursor form (mBDNF/proBDNF) (c) were analyzed in rats exposed to CMS and/or to lurasidone treatment (for 7 and 5 weeks, respectively). The data, expressed as a percentage of unstressed rats treated with vehicle (control [CTRL]/vehicle, set at 100%), are the mean ± SEM of at least 5 independent determinations. * P<.05 and ** P<.01 vs CTRL/vehicle; $ P<.05 and $$ P<.01 vs CMS/vehicle (2-way analysis of variance followed by Fisher’s LSD posthoc comparison).
The changes of Bdnf mRNA levels were paralleled by significant modifications of BDNF protein levels in the crude membrane fraction. Indeed, exposure to CMS leads to a trend toward a decrease of mature/pro BDNF ratio (−29%, P<.05 vs CTRL/vehicle), which was normalized by chronic lurasidone administration (Figure 4c). These changes were less evident in the total homogenate (data not shown), suggesting that CMS and lurasidone treatment can primarily affect synaptic mechanisms.
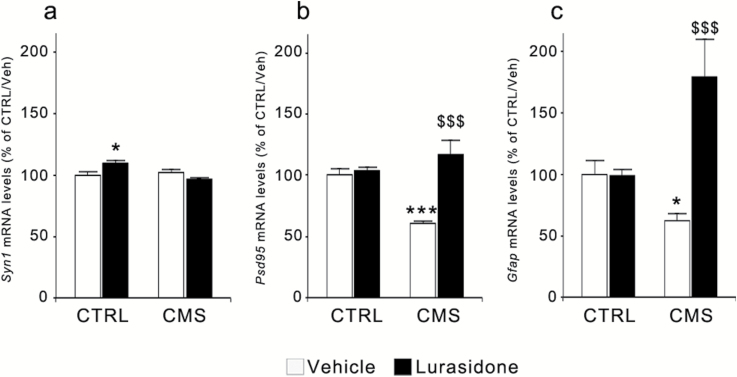
To explore this possibility, we reevaluated Syn1 and Psd95 expression at the end of the 7-week CMS exposure. As shown in Figure 5a, with respect to Syn1 mRNA levels, we found a significant effect of CMS exposure (F(1,36)=4.767, P<.05) and a significant CMS × treatment interaction (F(1,36)=7.748, P<.01). Posthoc analysis indicates only a small but significant elevation of Syn1 mRNA expression in CTRL animals treated with lurasidone (+10%, P<.05 vs CTRL/vehicle).
Figure 5.
Modulation of the mRNA levels for synaptic and structural markers following chronic mild stress (CMS) and/or lurasidone treatment in the rat prefrontal cortex (PFC). The mRNA levels of Syn1 (a), Psd95 (b), and Gfap (c) were analyzed in rats exposed to CMS and/or to lurasidone treatment (for 7 and 5 weeks, respectively). The data, expressed as a percentage of unstressed rats treated with vehicle (control [CTRL]/vehicle, set at 100%), are the mean ± SEM of at least 6 independent determinations. * P<.05 and *** P<.001 vs CTRL/vehicle; $$$ P<.001 vs CMS/vehicle (2-way analysis of variance followed by Fisher’s LSD posthoc comparison).
The expression of Psd95 (Figure 5b) was also significantly modulated by CMS (F(1,37)=4.147, P = 0.05) and by lurasidone treatment (F(1,37)=22.600, P<.001), with a significant CMS × treatment interaction (F(1,37)=18.164, P<.001). Although the expression of the postsynaptic marker was significantly downregulated after 7 weeks of CMS (−40%, P<.001 vs CTRL/vehicle), prolonged administration of lurasidone completely normalized such alteration (P<.001 vs CMS/vehicle) without affecting its expression in CTRL rats.
When considering the expression of Gfap (Figure 5c), we found a significant effect of chronic lurasidone administration (F(1,33)=18.599, P<.001) and a significant CMS × treatment interaction (F(1,33)=19.505, P<.001). CMS reduced Gfap mRNA levels (−36%, P<.05 vs CTRL/vehicle), whereas lurasidone treatment produced a significant upregulation of its expression when given to CMS rats (+80% vs CTRL/vehicle, P<.001 vs CMS/vehicle). We also found a positive, highly significant correlation between sucrose intake and Psd95 expression (r=0.587, P<.001), whereas no correlations were found for Syn1 (r=0.252, P<.05) or Gfap (r=0.312, P<.05) mRNA levels (supplementary Figure S1c, d, e).
After observing the changes brought about by CMS exposure on Psd95 and Gfap expression and considering the role of glutamate in stress-induced depressive phenotype (Sanacora et al., 2012), we decided to investigate the expression of glutamatergic markers, namely the vesicular glutamate transporter Vglut1 and two major glutamate membrane transporters, the glial transporter Glt1 and the neuronal transporter Eaac1, which are involved in the clearance of synaptic glutamate in astrocytes and neurons, respectively. As shown in Table 3, the expression of Vglut1 was not significantly modulated by CMS or lurasidone treatment (F(1,35)=1.954, P>.05 and F(1,35)=0.566, P>.05, respectively). Conversely, Glt1 mRNA levels were significantly modulated by stress exposure (F(1,39)=15.625, P<.001) but not by lurasidone treatment (F(1,39)=2.989, P>.05), since CMS rats show a significant upregulation of its expression independently from pharmacological treatment. On the other hand, we found a significant CMS × treatment interaction in the regulation of Eaac1 mRNA levels (F(1,37)=7.650, P<.01) that were significantly reduced in CMS rats treated with vehicle (−26% vs CTRL/vehicle, P<.01), an effect that was completely normalized by lurasidone treatment (P<.01 vs CMS/vehicle rats).
Table 3.
Modulation of the mRNA Levels for Glutamatergic Markers Following CMS and Lurasidone Treatment in the Rat PFC
| CTRL/Vehicle | CTRL/ Lurasidone | CMS/Vehicle | CMS/ Lurasidone | |
|---|---|---|---|---|
| Vglut1 | 100±6 | 102±6 | 88±5 | 96±7 |
| Glt1 | 100±4 | 94±4 | 120±4 ** | 110±7 # |
| Eaac1 | 100±4 | 94±6 | 74±8 ** | 100±4 $$ |
Abbreviations: CMS, chronic mild stress; CTRL, control; PFC, prefrontal cortex.
The mRNA levels of Vglut1, Glt1, and Eaac1 were analyzed in rats exposed to CMS and/or to lurasidone treatment (for 7 and 5 weeks, respectively). The data, expressed as a percentage of unstressed rats treated with vehicle (CTRL/vehicle, set at 100%), are the mean ± SEM of at least 6 independent determinations. ** P<.01 vs CTRL/vehicle; $$ P<.01 vs CMS/vehicle; # P<.05 vs CTRL/lurasidone (2-way analysis of variance followed by Fisher’s LSD posthoc comparison).
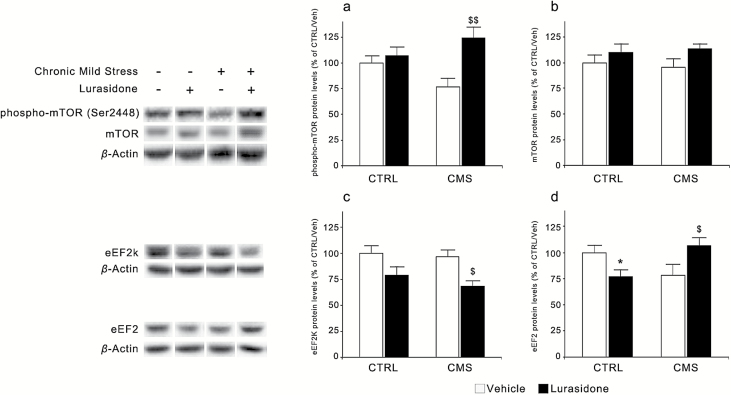
These data suggest that CMS may produce significant effects in synaptic proteins and that such alterations can be modulated by pharmacological intervention. It has been recently shown that the rapid antidepressant effect of ketamine is also associated with modifications of synaptic function, through the modulation of pathways involved in protein translation, including mTOR and eEF2 (Hoeffer and Klann, 2010; Li et al., 2010; Monteggia et al., 2013). We found that the levels of phospho-mTOR (Ser2448) were modulated by lurasidone treatment (F(1,37)=8.961, P<.01), with a significant CMS × treatment interaction (F(1,37)=5.146, P<.05). In fact, while its levels show a trend toward a reduction in CMS rats (−24%, P = 0.078 vs CTRL/vehicle), chronic treatment with lurasidone produced a significant increment of phospho-mTOR levels in CMS rats (+63%, P<.01 vs CMS/vehicle rats) without affecting them in CTRL rats. Conversely, total levels of mTOR were not significantly modulated by CMS or lurasidone treatment (Fig. 6 a, b).
Figure 6.
Modulation of mammalian target of rapamycin (mTOR) and eukaryotic elongation factor 2 (eEF2) protein levels following chronic mild stress (CMS) and/or lurasidone treatment in the rat prefrontal cortex (PFC). The protein levels of phospho-mTOR (Ser2448) (a), mTOR (b), eEF2 kinase (eEF2K) (c), and eEF2 (d) were analyzed in rats exposed to CMS and/or to lurasidone treatment (for 7 and 5 weeks, respectively). The data, expressed as a percentage of unstressed rats treated with vehicle (control [CTRL]/vehicle, set at 100%), are the mean ± SEM of at least 7 independent determinations. * P<.05 vs CTRL/vehicle; $ P<.05 and $$ P<.01 vs CMS/vehicle (2-way analysis of variance followed by Fisher’s LSD posthoc comparison).
A significant effect of chronic lurasidone treatment was also found on eEF2K (F(1,32)=10.974, P<.001), since prolonged drug administration appears to reduce the expression of the kinase in both CTRL and CMS rats. Moreover, we found a significant CMS × treatment interaction in the modulation of eEF2 (F(1,37)=9.458, P<.01). Indeed, whereas a nonsignificant decrease of eEF2 levels was seen in CMS rats (−21%, P>.05), lurasidone treatment increased its expression in CMS rats (+34%, P < .05 vs CMS/vehicle rats) while reducing eEF2 levels in CTRL rats (−23%, P < .05 vs CTRL/vehicle rats) (Figure 6 c, d).
Discussion
In the present study, we demonstrate that exposure to CMS is associated with early and late synaptic changes in the PFC that may contribute to the development as well as to the maintenance of the anhedonic phenotype. Moreover, we show that chronic treatment with lurasidone is able to normalize the behavioral and molecular alterations elicited by the stressful condition.
Our results provide further support to the notion that synaptic changes may contribute to stress-related conditions and depressive disorders (Duman and Aghajanian, 2012; Kang et al., 2012). Accordingly, we showed that Bdnf defects primarily affect the pool of neurotrophin transcripts with the long 3’-UTR, which localize to the dendritic compartment (An et al., 2008) and may contribute to rapid activity-dependent translation of the neurotrophin (Lau et al., 2010). Moreover, we found a significant reduction of the ratio mBDNF/proBDNF protein in the membrane compartment, which can be suggestive of impaired BDNF processing. These changes resemble what we have recently observed in environmental or genetic models of depression susceptibility, including serotonin transporter knockout rats (Luoni et al., 2013) and rats exposed to gestational stress (Luoni et al., 2014), suggesting that depressive-related dysfunction may be characterized by alterations of the mechanisms controlling the synaptic production of BDNF. Neurotrophin changes may therefore be relevant for the vulnerability to the stressful manipulation (Taliaz et al., 2011) as well as for its maintenance. Long-term changes of Bdnf expression are associated with a significant downregulation of Psd-95, a scaffolding protein that plays a key role in anchoring glutamate postsynaptic receptors (Xu, 2011). Dysfunctions of excitatory synapses have been associated with psychiatric conditions, including major depression (Sanacora et al., 2012). In particular, significant reductions in the expression of prominent postsynaptic proteins involved in glutamate transmission, including PSD95, have been found in the PFC from depressed subjects (Feyissa et al., 2009), although opposite changes may be observed in other brain regions (Karolewicz et al., 2009). A similar reduction has been shown to occur in animal models of depression (Luo et al., 2013). Our results provide support to these changes but suggest that synaptic alterations may represent a late consequence of prolonged stress exposure while, seen the upregulation after 2 weeks of CMS, they do not contribute to the development of the anhedonic phenotype. Moreover, we demonstrated that CMS exposure primarily affects the postsynaptic compartment, since the expression of 2 presynaptic markers, namely synapsin-1 and the vescicular glutamate transporter VGlut1, were marginally altered by CMS.
We also showed that long-term exposure to CMS leads to a significant downregulation of Gfap expression, which is suggestive of glial pathology. These data are in line with previous studies demonstrating that stress-based models of depression are associated with reduced GFAP expression (Liu et al., 2009; Banasr et al., 2010). Reduced number and altered morphology of glial cells were also shown in the PFC from depressed subjects (Rajkowska et al., 1999). Alterations of astrocyte activity have important consequences for synaptic function, considering that glial cells represent the major system buffering excitatory neurotransmitters, such as glutamate (Hertz and Zielke, 2004). Hence, loss or reduced activity of glial cells may eventually lead to increased glutamatergic activation, particularly at extra-synaptic receptors that may promote neurotoxic-like effects (Hardingham and Bading, 2010). As discussed for Psd95, the reduction of Gfap expression in CMS-exposed rats appears to be a late consequence of stress exposure and does not correlate with the development of the anhedonic phenotype. In fact, 2 weeks of CMS exposure leads to an upregulation of Gfap mRNA levels in animals that develop anhedonia as well as in rats that are resilient to the stressful manipulation. These changes may be indicative of glial activation, a mechanism that usually occurs under conditions that imply prolonged neuronal activation, such as during epileptic seizures (Riva et al., 1994). Hence, dynamic changes may occur on glial function during CMS exposure with a shift from an attempt to preserve the system (initial upregulation) toward a glial dysfunction (long-term downregulation) that may contribute to an overall deterioration of synaptic mechanisms in the PFC of stressed animals.
Our results demonstrate that lurasidone treatment, starting from the end of the second week of CMS exposure, is able to normalize CMS-induced anhedonia. Lurasidone is a multi-receptor modulator, with the highest affinity for 5-HT7 receptors, followed by D2, 5-HT2A, and 5-HT1A receptors. Behavioral studies indicate that lurasidone is active in different animal models predictive of antipsychotic and antidepressant activity (Ishibashi et al., 2010; Meltzer et al., 2011; Tarazi and Riva, 2013). For example, lurasidone was shown to possess antidepressant properties in the forced swim test, an effect that appears to be at least in part due to its antagonistic activity toward 5-HT7 receptors (Cates et al., 2013). It also displays procognitive effects in several animal models of memory, cognitive, and executive functions both in rats and primates (Horisawa et al., 2013; Tarazi and Riva, 2013).
The behavioral effects of lurasidone are associated with a significant modulation of CMS-induced molecular changes in the PFC. In fact, pharmacological treatment in CMS rats normalized the expression of long 3’-UTR Bdnf transcripts while also upregulating its expression in nonstressed animals, in agreement with previous results from our laboratory (Fumagalli et al., 2012; Luoni et al., 2013 2014). Similarly, lurasidone treatment normalized the reduced ratio of mBDNF/proBDNF protein in the membrane fraction of CMS rats, providing support for the ability of the drug to improve defective BDNF function in the synaptic compartment.
The synaptic activity of chronic lurasidone treatment is further supported by its ability to regulate other dysfunctions associated with CMS, in particular the expression of Psd95 and Gfap. Indeed, treatment of CMS rats with this multi-receptor modulator completely restored the deficits of Psd95 expression. In a previous work, Lin and coworkers (2010) showed that the rapid antidepressant activity of acute ketamine is associated with an upregulation of PSD95, although chronic treatment of normal animals with imipramine or fluoxetine had no effect on the expression of the scaffolding protein. This is in line with our data, since we do not observe changes in the expression of the scaffolding protein following lurasidone administration in nonstressed animals, but only in CMS rats, suggesting that the ability of pharmacological intervention to improve postsynaptic mechanisms may be limited to the ongoing pathological condition. In a recent study, neotrofin, a purine hypoxanthine derivative neurotrophin agonist, was able to counteract CMS-induced downregulation of PSD95 in the amygdala (Luo et al., 2013), suggesting that pharmacological intervention may act in an anatomically selective manner to normalize functions that are altered in stressed animals.
Chronic lurasidone was also able to normalize Gfap expression in CMS rats, leading to an actual upregulation of its expression over control levels. Once more, this effect is restricted to the subgroup of rats exposed to CMS, since the expression of the glial marker was not affected by chronic lurasidone in nonstressed animals. Previous studies have shown that some antidepressants may normalize the changes of hippocampal GFAP expression in rats exposed to CMS (Liu et al., 2009; Araya-Callis et al., 2012). Similarly, CMS-related glial pathology in the PFC can be normalized by subchronic treatment with the glutamate-modulating drug riluzole (Banasr et al., 2010). Moreover, it has been shown that CMS-induced decrease of GFAP expression in the rat hippocampus is partially rescued by BDNF infusion (Ye et al., 2011), suggesting a potential link between the ability of lurasidone in regulating neurotrophin expression and its restorative properties on glial function.
To strengthen the relevance of synaptic mechanisms in the effects of lurasidone, we also demonstrated its modulatory activity on 2 intracellular mechanisms that are important for synaptic homeostasis and protein translation, namely mTOR and eEF2 (Hoeffer and Klann, 2010; Heise et al., 2014). Interestingly, we found that the levels of eEF2 are downregulated in CMS rats, which may be suggestive of an impairment in local protein translation. These changes are normalized by lurasidone treatment that, per se, is also able to reduce the expression of eEF2 kinase in CMS rats. Together, these 2 effects may lead to reduced eEF2 phosphorylation that will eventually desuppress the translation of BDNF during ongoing neuronal activity, an effect that has been shown to occur in response to acute injection of ketamine (Autry et al., 2011).
Moreover, the levels of phospho-mTOR, which lies downstream from BDNF (Hoeffer and Klann, 2010; Duman and Aghajanian, 2012), are also reduced in CMS rats and normalized by lurasidone administration. Deficits in synaptic proteins and dysregulation of the mTOR signaling have been shown to occur in MDD patients (Jernigan et al., 2011), and the modulation of mTOR pathway appears to be crucial for the rapid antidepressant activity of ketamine (Li et al., 2010). All in all, our results provide compelling evidence for an integrated dysfunction of synaptic mechanisms in rats exposed to the CMS paradigm, which are improved by pharmacological intervention with lurasidone.
In summary, these results provide further support to the notion that synaptic deficits represent an important consequence of stress exposure and may contribute to anhedonia, a major feature of human depression. In this respect, the ability of lurasidone to normalize the behavioral and molecular defects that develop after CMS exposure may contribute to the amelioration of functional capacities closely associated with neuronal plasticity, which are deteriorated in patients with major depression and stress-related disorders.
Statement of Interest
M.A. Riva has received compensation as a speaker/consultant for Servier, Eli Lilly, Lundbeck, Sumitomo Dainippom Pharma Co. Ltd, and Sunovion. The other authors declare no financial interests or potential conflict of interests.
Acknowledgments
We are grateful to Dr. Juliet Richetto for language editing and to Sumitomo Dainippon Pharma Co. Ltd for the generous gift of lurasidone. This publication was made possible by grants from the Italian Ministry of University and Research to M.A.R. (PRIN 20107MSMA4) and from Sumitomo Dainippon Pharma Co. Ltd/Sunovion to M.A.R. All funding bodies had no role in designing the study.
References
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. (2008). Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Callis C, Hiemke C, Abumaria N, Flugge G. (2012). Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology 224:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. (2010). Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Dwyer JM, Duman RS. (2011). Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol 23:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, Gennarelli M, Ellenbroek BA, Riva MA. (2010). Long-term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol 77:846–853. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Riva MA. (2011). Antistress properties of antidepressant drugs and their clinical implications. Pharmacol Ther 132:39–56. [DOI] [PubMed] [Google Scholar]
- Cates LN, Roberts AJ, Huitron-Resendiz S, Hedlund PB. (2013). Effects of lurasidone in behavioral models of depression. Role of the 5-HT₇ receptor subtype. Neuropharmacology 70:211–217. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Hellweg R, Gass P. (2008). Nature vs. nurture: can enrichment rescue the behavioral phenotype of BDNF heterozygous mice? Behav Brain Res 192:254–258. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hörtnagl H, Molteni R, Riva MA, Gass P, Hellweg R. (2012). The impact of environmental enrichment on sex-specific neurochemical circuitries: effects on brain-derived neurotrophic factor and the serotonergic system. Neurosci 220:267–276. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde N, Pastor PM, Garcia-Garcia AL, Serres F, Venzala E, Huarte J, Ramirez MJ, Del Rio J, Sharp T, Tordera RM. (2010). Regulation of markers of synaptic function in mouse models of depression: chronic mild stress and decreased expression of VGLUT1. J Neurochem 114:1302–1314. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. (2009). Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. (2007). Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 81:197–217. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Frasca A, Racagni G, Riva MA. (2008). Dynamic regulation of glutamatergic postsynaptic activity in rat prefrontal cortex by repeated administration of antipsychotic drugs. Mol Pharmacol 73:1484–1490. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Calabrese F, Luoni A, Bolis F, Racagni G, Riva MA. (2012). Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int J Neuropsychopharmacol 15:235–246. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. (2007). CREB, neurogenesis and depression. BioEssays 29:957–961. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. (2010). Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C. (2014). Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front Cell Neurosci 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. (2004). Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci 27:735–743. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. (2012). Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisawa T, Nishikawa H, Toma S, Ikeda A, Horiguchi M, Ono M, Ishiyama T, Taiji M. (2013). The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav Brain Res 244:66–69. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M. (2010). Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181. [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. (2007). Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol 572:160–170. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. (2011). The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. (2012). Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18:1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. (2009). Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 12:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. (2012). Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169:1150–1156. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB. (2008). Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther 117:30–51. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. (2010). Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 167:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AG, Irier HA, Gu J, Tian D, Ku L, Liu G, Xia M, Fritsch B, Zheng JQ, Dingledine R, Xu B, Lu B, Feng Y. (2010). Distinct 3’UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc Natl Acad Sci U S A 107:15945–15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. (2009). Clomipramine treatment reversed the glial pathology in a chronic unpredictable stress-induced rat model of depression. Eur Neuropsychopharmacol 19:796–805. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang L, Ning N, Jiang H, Yu SY. (2013). Neotrofin reverses the effects of chronic unpredictable mild stress on behavior via regulating BDNF, PSD-95 and synaptophysin expression in rat. Behav Brain Res 253:48–53. [DOI] [PubMed] [Google Scholar]
- Luoni A, Hulsken S, Cazzaniga G, Racagni G, Homberg JR, Riva MA. (2013). Behavioural and neuroplastic properties of chronic lurasidone treatment in serotonin transporter knockout rats. Int J Neuropsychopharmacol 16:1319–1330. [DOI] [PubMed] [Google Scholar]
- Luoni A, Berry A, Calabrese F, Capoccia S, Bellisario V, Gass P, Cirulli F, Riva MA. (2014). Delayed BDNF alterations in the prefrontal cortex of rats exposed to prenatal stress: preventive effect of lurasidone treatment during adolescence. Eur Neuropsychopharmacol 24:986–995. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, Kalali AH, Schweizer E, Pikalov A, Loebel A. (2011). Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 168:957–967. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET. (2013). The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. (2008). The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1996). The rat brain in stereotaxis coordinates. New York: Academic Press. [Google Scholar]
- Pochwat B, Szewczyk B, Sowa-Kucma M, Siwek A, Doboszewska U, Piekoszewski W, Gruca P, Papp M, Nowak G. (2014). Antidepressant-like activity of magnesium in the chronic mild stress model in rats: alterations in the NMDA receptor subunits. Int J Neuropsychopharmacol 17:393–405. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16:313–320. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. (1999). Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098. [DOI] [PubMed] [Google Scholar]
- Riva MA, Donati E, Tascedda F, Zolli M, Racagni G. (1994). Short- and long-term induction of basic fibroblast growth factor gene expression in rat central nervous system following kainate injection. Neuroscience 59:55–65. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. (2011). Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci 31:4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Riva MA. (2013). The preclinical profile of lurasidone: clinical relevance for the treatment of schizophrenia. Expert Opin Drug Discov 8:1297–1307. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. (2004). Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav Brain Research 150:217–221. [DOI] [PubMed] [Google Scholar]
- Willner P. (2005). Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. [DOI] [PubMed] [Google Scholar]
- Xu W. (2011). PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol 21:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X. (2011). Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett 503:15–19. [DOI] [PubMed] [Google Scholar]