Abstract
Background:
Withdrawal from chronic ethanol facilitates the formation of contextual fear memory and delays the onset to extinction, with its retrieval promoting an increase in ethanol consumption. Consequently, manipulations aimed to reduce these aversive memories, may be beneficial in the treatment of alcohol discontinuation symptoms. Related to this, pharmacological memory reconsolidation blockade has received greater attention due to its therapeutic potential.
Methods:
Here, we examined the effect of post-reactivation amnestic treatments such as Midazolam (MDZ, 3 mg/kg i.p) and Propranolol (PROP, 5 mg/kg i.p) on contextual fear memory reconsolidation in ethanol- withdrawn (ETOH) rats. Next, we examined whether the activation of N-methyl-D-aspartate (NMDA) receptors induced by d-cycloserine (DCS, 5 mg/kg i.p., a NMDA partial agonist) before memory reactivation can facilitate the disruptive effect of PROP and MDZ on fear memory in ETOH rats.
Results:
We observed a resistance to the disruptive effect of both MDZ and PROP following memory reactivation. Although intra-basolateral amygdala (BLA; 1.25 ug/side) and systemic PROP administration attenuated fear memory in DCS pre-treated ETOH rats, DCS/MDZ treatment did not affect memory in these animals. Finally, a decrease of both total and surface protein expression of the α1 GABAA receptor (GABAA-R) subunit in BLA was found in the ETOH rats.
Conclusions:
Ethanol withdrawal facilitated the formation of fear memory resistant to labilization post-reactivation. DCS administration promoted the disruptive effect of PROP on memory reconsolidation in ETOH rats. The resistance to MDZ’s disruptive effect on fear memory reconsolidation may be, at least in part, associated with changes in the GABAA-R composition induced by chronic ethanol administration/withdrawal.
Keywords: ethanol withdrawal, fear memory reconsolidation, GABA-A receptor, midazolam, propranolol
Introduction
Ethanol withdrawal syndrome is characterized by the presence of somatic signs and the emergence of negative affective states, including increased anxiety, dysphoria, and anhedonia, among others (Markou et al., 1998). Disturbances in the affective states in withdrawn subjects can persist for protracted periods of time, thus playing an important role in relapse and, in turn, the maintenance of addiction (Koob and LeMoal, 2001; Heilig et al., 2010). In animal studies, an increased anxiety-like behavior, a heightened response to stressful stimuli, and increased ethanol consumption have also been documented during both the early and protracted phases of withdrawal (Heilig, 2010). In addition, ethanol withdrawal alters learning and memory processes (Tipps et al., 2013). Related to this, we have reported that withdrawal from chronic ethanol administration facilitates the formation of contextual fear memory, which is resistant to extinction (Bertotto et al., 2006). Furthermore, we have shown that the retrieval of fear memory increased ethanol consumption in ethanol-withdrawn rats (Bertotto et al., 2010). Therefore, interventions to reduce aversive memories could be beneficial for the treatment of those symptoms and disturbances associated with alcohol withdrawal.
A growing amount of evidence has shown that consolidated fear memories can enter a transient labile phase upon retrieval. Following this phase, memories undergo a re-stabilization period dependent on new protein synthesis, referred to as reconsolidation (Nader et al., 2000; Alberini, 2005; Dudai, 2006). The administration of pharmacological agents during this unstable phase can affect the memory trace, with the disruption of memory reconsolidation having been suggested as a potential treatment for anxiety-related disorders and drug addiction (Tronson and Taylor, 2013). For instance, it has been shown that the post-reactivation administration of propranolol (β-adrenoceptor antagonist, PROP) or benzodiazepines (BZDs, positive allosteric modulators of GABA-A receptors [GABAA-Rs]) disrupts reconsolidation of fear memories (Debiec and LeDoux, 2004; Bustos et al., 2006). However, much less is known about the neural and behavioral basis of fear memory reconsolidation following drug withdrawal. Therefore, the first goal of the current study was to examine the effect of benzodiazepine midazolam (MDZ) and PROP administration on contextual fear memory reconsolidation in ethanol-withdrawn animals.
Diverse studies have suggested that there are certain conditions that place constraints on the onset of the recall-induced fragility and, therefore, the reconsolidation process (Tronson and Taylor, 2007). Thus, a robust fear memory induced either following intensive training or after experiencing stressful stimuli remains unaffected by BZDs or anisomycin upon retrieval (Wang et al., 2009; Bustos et al., 2010). However, resistant memories can become susceptible to disruption by the activation of NMDA receptors prior to recall. For example, Bustos et al. (2010) demonstrated that D-cycloserine (DCS), a partial agonist of the glycine recognition site of the NMDA receptor, promoted vulnerability to MDZ′s disruptive effects in resistant fear memory prior to reactivation. Given that withdrawal from ethanol facilitates the formation of a robust and persistent fear memory, we can predict a resistance to the reconsolidation-blocking effects of both MDZ and PROP. Therefore, it seems relevant to evaluate the influence of pre-retrieval DCS administration on MDZ′s and PROP′s disruptive effects on fear memory reconsolidation in withdrawn rats. As the basolateral amygdala (BLA) complex is one of the main structures involved in fear memory reconsolidation, we investigated the effect of PROP infused into the BLA in ethanol-withdrawn rats (Debiec and LeDoux, 2004).
GABA-A receptors are presumed to be heteropentamers composed of two α, two β, and one γ subunit, with the α1β2γ2 subunit combination being highly expressed in the amygdala (McDonald and Mascagni, 2004). It is well known that GABAA-Rs are involved in the learning and memory processes (Makkar et al., 2010) and that α1-containing receptors have a critical role in fear learning and plasticity in the amygdala (Heldt and Ressler, 2007; Wiltgen et al., 2009). Furthermore, the amnestic effects of BZDs in aversive motivated tasks have been attributed to α1-containing receptors in this structure (Mohler et al., 2002), with it being reported that ethanol withdrawal decreases the expression of the α-1 GABAA-R subunit in the amygdala (Papadeas et al., 2001; Floyd et al., 2004; Diaz et al., 2011).Therefore, an attenuated response to the amnestic effect of MDZ would be expected in ethanol-withdrawn rats. In the present study, we also examined the influence of ethanol withdrawal on the total and surface expression of the α1 subunit in the BLA.
Methods
Animals
Male Wistar rats from our breeding stock, weighing 240–260g, were housed in groups of 3 per cage (Bertotto et al., 2006), with food and water ad libitum except when detailed otherwise in the protocol. Animals were maintained in a 12h light-dark cycle (lights on at 0700h) at a room temperature of 21–22°C. The protocols used were approved by the Animal Care Committee of the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, and are consistent with the NIH Guide for the Care and Use of Laboratory animals.
Chronic Ethanol Administration
Ethanol was administered via a nutritionally complete liquid diet (Abbott Laboratories B.V.), as previously described (Bertotto et al., 2006, 2010). Briefly, rats were randomly allocated into two groups, one receiving a non-ethanol-containing liquid diet (CON) and the other one exposed to a diet containing 6% (v/v) ethanol for 14 days (ETOH). All rats received the diet without ethanol for an initial 3-day period, with water available ad libitum throughout the treatment. Dextrose was isocalorically substituted for ethanol in the CON group. These diets were removed at 0700h on the day of withdrawal, and the animals were fed with laboratory chow for the rest of the experiments. This treatment resulted in blood ethanol levels from 87 to 146mg/dl at the end of the treatment.
Drug Administration
Midazolam (GobbiNovag S.A.) was diluted in sterile saline (SAL, 0.9% w/v) to a concentration of 3mg/ml and given intraperitoneally (i.p; Bustos et al., 2010).
DL-Propranolol hydrochloride and D-cycloserine (Sigma-Aldrich) were both dissolved in SAL at concentrations of 10mg/ml and 5mg/ml, respectively, for i.p. injection (Debiec and LeDoux, 2004; Bertotto et al., 2006; Muravieva et al., 2010). The total volume of drug or an equivalent amount of SAL was 1.0ml/kg in all cases.
For intra-BLA infusions, PROP was dissolved in SAL to a final dose of 5 ug/ul and the amount infused was 1.25 ug/side (Debiec and LeDoux, 2004).
Crosslinking BS3
To examine surface expression of the α1 GABAA-R subunit, we used the membrane-impermeable cross-linker bis(sulfosuccinimidyl)suberate (BS3; Thermo Fisher Scientific), as previously described (Boudreau et al., 2012). Animals were sacrificed and the bilateral BLA was dissected from coronal brain slices of 2mm using an acrylic brain matrix (Stoelting CO.) on ice, according to the BLA boundaries defined by Paxinos and Watson (2009). These samples were prepared from pools of 2 animals. Next, the BLA tissue was chopped and the sample was immediately divided into two equal portions, of which one was incubated with BS3 in artificial cerebrospinal fluid (ACSF) and the other was incubated in ACSF only (untreated portion), for 30min at 4°C. Then, both samples were treated as described by Boudreau et al. (2012). BS3 does not cross the cell membranes, thereby enabling it to selectively cross-link surface-expressed proteins with sulphide bonds and form high molecular weight aggregates, while the intracellular proteins remained unmodified. This reaction allows surface and intracellular pools of protein to be distinguished based on molecular weight for Western blot analysis, with the difference between the intracellular fraction and the untreated portion (total protein) representing the surface pool (Diaz et al., 2011; Suryanarayanan et al., 2011).
Western Blot
Protein samples (15 μg) were separated on 7.5% sodium dodecyl sulfate -polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes as previously described (Bertotto et al., 2011). The protein blots were incubated with a rabbit polyclonal primary antibody GABAA-R α1-subunit (1:750; Millipore), followed by incubation with horse radish peroxidase-conjugated anti-rabbit secondary antibody (1:2500, Cell Signaling Technology). The resulting film samples were scanned and analyzed with an image analysis program (Gelpro31). Actin (Sigma) was used as a loading control.
Surgery
Intra-BLA cannulae implantation and histological procedures were described in Giachero et al. (2013). The coordinates relative to bregma used were: anterior, -3mm; lateral, ±5.0mm; ventral, -6.1mm (Paxinos and Watson, 2009), with only those animals with adequate injection sites being considered for statistical analysis.
Intra-BLA Infusions
The procedure for intra-BLA infusion was described in Giachero et al. (2013). Each rat was bilaterally infused with PROP or SAL at a flow rate of 0.25 μl/min. After completion of this volume injection, the infusion cannulas were kept in place for an additional period of 60 s to allow diffusion of the drug.
Contextual Fear Conditioning
Apparatus
The conditioning chamber was constructed of gray acrylic (20×23×20cm) with a transparent lid and connected to a scrambled shocker (Ugo Basile Biological Research Apparatus). The grid floor consisted of 10 parallel stainless-steel grid bars, each measuring 4mm in diameter and spaced 1.5cm apart (center to center). The chamber was placed in a room illuminated by a white fluorescent tube located on the ceiling, which was cleaned before and after each session, with background noise being supplied by ventilation fans and shock scramblers. The training and test sessions were conducted between 0900 and 1300h.
Fear Conditioning
On the day of conditioning, rats were moved from their housing room and individually placed in the conditioning chamber. Animals were then left undisturbed for a 3min acclimatization period (pre-shock period), followed by 3 unsignaled scrambled foot shocks (0.5 mA, 3 s duration, and 30 s intershock interval). Animals remained in the chamber for an additional 50 s (post-shock period), before being immediately brought back to their home cages and returned to the colony room. Cannulated rats were trained with 3 foot shocks of 0.65 mA in order to induce levels of conditioning similar to those exhibited by non-cannulated rats, because chronic cannulation of the BLA tends to attenuate the expression of conditioned freezing (Fendt, 2001). The fear training protocol employed induced a similar freezing response in both control and ETOH groups. Therefore, we discard the possibility that the differences obtained following the pharmacological treatments evaluated in the present study are dependent on the expression of freezing.
Re-Exposure Session (Reactivation Session)
One day following training, rats were re-exposed to the training context, without shock delivery, for 3 or 5min depending on the experiment performed.
Test Sessions
One (Test 1) and eight (Test 2) days after the reactivation session, animals were reintroduced into the training context without shock delivery for 10min.
The freezing response of each rat was scored during the pre-shock and post-shock periods and also in the reactivation and testing sessions. The total time spent freezing in each period was quantified (in seconds) using a stopwatch. Freezing, a commonly used index of fear in rats (Blanchard and Blanchard, 1969), was defined as the total absence of body and head movement except that associated with breathing. Freezing was scored by a person who was unaware of the experimental conditions of each animal.
Experimental Design
Experiment 1
This experiment was designed to test whether fear memory was vulnerable to MDZ′s and PROP′s disruptive effect in ETOH rats.
Animals assigned to CON or ETOH groups received the contextual fear training on the third day of withdrawal (Bertotto et.al, 2006). The following day, rats were subjected to the reactivation session for 3 or 5min. Immediately after, rats were injected with MDZ (3mg/kg, i.p.), PROP (10mg/kg, i.p), or SAL and tested 24h later for memory retention (Test 1). Seven days after, all animals were re-tested (Test 2).
Separate groups of rats from the CON and ETOH groups, which were trained at the same time, received a MDZ, PROP, or SAL injection in the home cage and served as non-reactivated controls. The retention tests were conducted as previously described.
Experiment 2
The goal of this experiment was to evaluate the influence of pre-reactivation DCS administration on MDZ′s and PROP′s disruptive effects on fear memory reconsolidation in ethanol-withdrawn rats.
Animals from the CON and ETOH groups were fear conditioned as described in Experiment 1. One day after training, rats received DCS (5mg/kg; i.p) or SAL 30min before a 5min reactivation session. Immediately after, rats were injected with MDZ (3mg/kg; i.p), PROP (10mg/Kg, i.p), or SAL. Memory retention was evaluated the following day (Test 1) and one week later (Test 2).
Separate groups of rats from the CON and ETOH groups served as non-reactivated controls: they all had been trained at the same time, received DCS or SAL administration the next day, and received a MDZ, PROP, or SAL injection 30min later in the home cage. The retention tests were conducted as previously described.
Experiment 3
In this experiment, we evaluated the effect of intra-BLA infusion of PROP on memory reconsolidation in DCS pre-treated rats.
On the second day of withdrawal, CON and ETOH rats were bilaterally cannulated at the BLA; they were subjected to fear training 5 days later. One day after training, rats received DCS (5mg/kg; i.p) or SAL 30min before a 5min reactivation session, and PROP (1.25 ug/side) or SAL was infused into the BLA after the end of this phase. The memory retention was evaluated on the following day and also one week later (Tests 1 and 2).
Additional groups of rats from the CON and ETOH groups that underwent the same conditioning and drug treatments in their home cages served as non-reactivated controls.
A longer interval between ethanol discontinuation and fear training was used, because cannulated rats need to have a recovery period of at least 5 days. Moreover, previous findings from our laboratory have shown that the influence of ethanol withdrawal on fear memory formation persists for at least up to two weeks following discontinuation (Bertotto et al., 2006, 2011).
Experiment 4
The purpose of this experiment was to examine the total and surface expression of the α1 GABAA-R subunit in the BLA following ethanol discontinuation. Animals were sacrificed for the crosslinking assay on the third day of withdrawal and then the expression of the α1 subunit was analysed by Western blot.
Statistical Analyses
Results were expressed as the means ± standard error of the mean. The data were analyzed by the Student’s t-test or analyses of variance (ANOVAs) followed by Tukey honestly-significant- difference post hoc (p < 0.05 was regarded as significant).
Results
Experiment 1: Ethanol Withdrawal Enhanced the Resistance of Memory Trace to MDZ′s and PROP′s Disruptive Effects on Fear Memory Reconsolidation
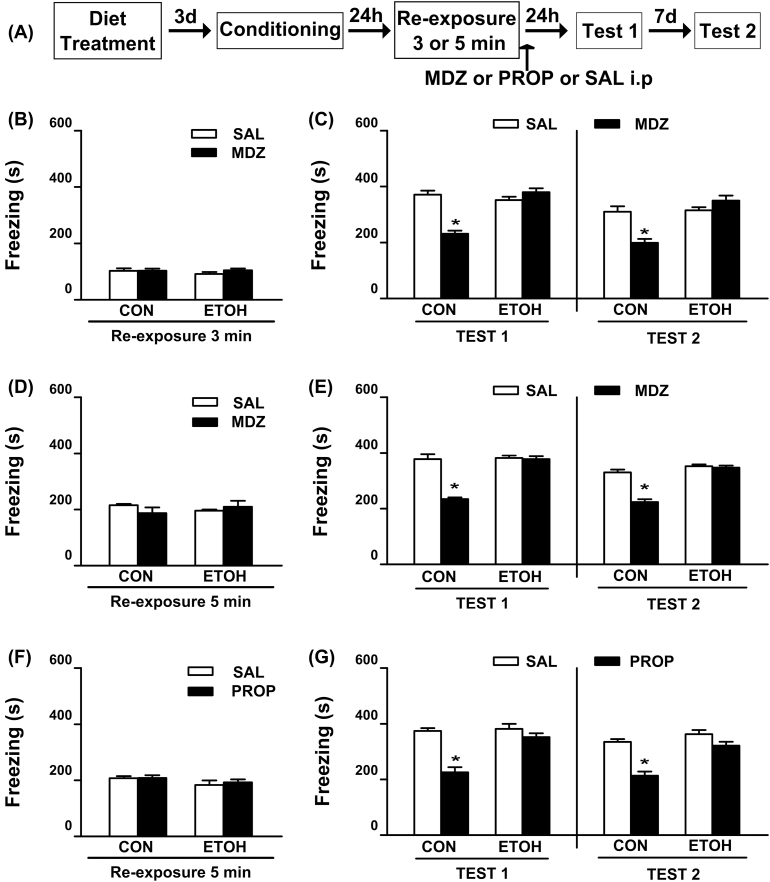
As can be observed in Figure 1B, all animals displayed equivalent levels of freezing during the 3min reactivation session [F(1, 31) = 1.93, p = 0.17]. The effect of MDZ injection after 3min reactivation is shown in Figure 1C, where during Test 1 a significant reduction of freezing was only detected in MDZ treated rats from the CON group. This effect lasted for up to one week (Test 2). In contrast, MDZ did not affect the fear response in ETOH rats. ANOVA showed significant diet treatment x drug treatment interactions for Test 1 [F(1, 31) = 42.63; p < 0.05] and Test 2 [F(1,31) = 21.19; p < 0.05]. In both tests, the post hoc analysis revealed that CON-MDZ rats exhibited significantly less freezing than the remaining groups.
Figure 1.
Effect of systemic administration of MDZ and PROP following retrieval on contextual fear memory reconsolidation in ethanol-withdrawn rats.
(A) Timeline for Experiment 1. (B) All groups exhibited comparable levels of freezing during the 3min reactivation session. (C) Post-retrieval MDZ (3mg/kg, i.p.) attenuated the freezing behavior on Test 1 and Test 2 in CON, but not in ETOH rats. CON-SAL (n = 9), CON-MDZ (n = 10), ETOH-SAL (n = 8), and ETOH-MDZ (n = 8). (D) All groups assigned to test the disruptive effect of MDZ on memory reconsolidation exhibited comparable levels of freezing during the 5min reactivation session. (E) Post-retrieval MDZ (3mg/kg, i.p.) attenuated the freezing behavior on Test 1 and Test 2 in CON, but not in ETOH rats. CON-SAL (n = 6), CON-MDZ (n = 6), ETOH-SAL (n = 6), and ETOH-MDZ (n = 6). (F) All groups assigned to test the disruptive effect of PROP on memory reconsolidation exhibited comparable levels of freezing during the 5min reactivation session. (G) Post-retrieval PROP (10mg/kg, i.p.) attenuated the freezing behavior on Tests 1 and 2 in CON, but not in ETOH rats. CON-SAL (n = 8), CON-PROP (n = 11), ETOH-SAL (n = 8), and ETOH-PROP (n = 9). Data are the mean ± standard error of the mean of time spent freezing during reactivation session, Test 1, and Test 2. *Significantly different from the remaining groups (p < 0.05). CON, control rats; ETOH, ethanol-withdrawn rats; MDZ, midazolam; PROP, propranolol; SAL, sterile saline.
Because stronger memories may require longer retrieval durations to become sensitive to interference (Suzuki et al., 2004), we investigated whether increasing the duration of the reactivation session would favor MDZ′s disruptive effect on fear memory reconsolidation in ETOH rats. As depicted in Figure 1D, all animals assigned to test the disruptive effects of MDZ exhibited comparable levels of freezing during the 5min reactivation session [F(1, 20) = 1.92, p = 0.18]. As shown in Figure 1E, MDZ attenuated the freezing response in the CON group on Test 1, with this effect lasting for up to one week. In contrast, MDZ did not affect the freezing levels in the ETOH group. ANOVA revealed significant diet treatment x drug treatment interactions for Test 1 [F(1,20) = 33.32; p < 0.05] and Test 2 [F(1,20) = 33.05; p < 0.05]. In both tests, the post hoc analysis revealed that CON-MDZ rats exhibited significantly less freezing than the remaining groups.
To increase the probability of memory interference, the duration of the reactivation session used in subsequent experiments was maintained at 5min.
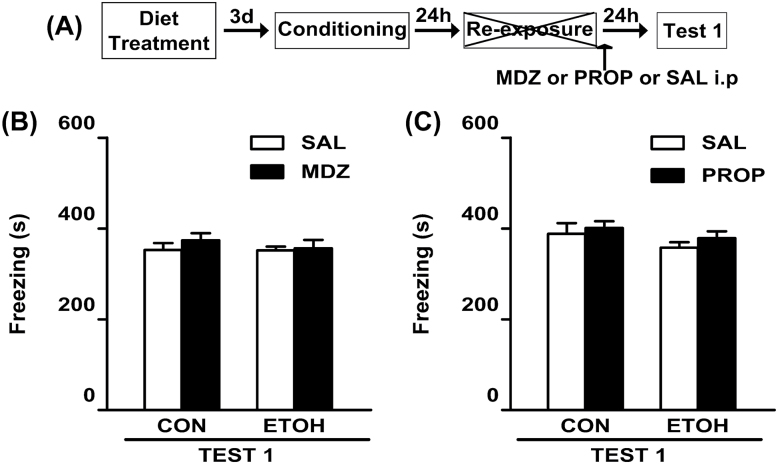
As depicted in Figure 1F, all animals assigned to test the disruptive effects of PROP exhibited comparable levels of freezing during the reactivation session [F(1, 32) = 0.15, p = 0.70]. The CON group that received post-reactivation PROP exhibited a reduced freezing on Test 1, which lasted for up to one week (Figure 1G). However, PROP did not affect the fear response in ETOH rats. ANOVA showed significant diet treatment x drug treatment interactions for Test 1 [F(1,32) = 13.34; p < 0.05] and Test 2 [F(1,32) = 8.32; p < 0.05]. A post hoc analysis revealed that CON rats injected with PROP exhibited significantly less freezing than the remaining groups during both Test 1 and Test 2. All non-reactivated groups displayed similar levels of freezing, which did not differ from those shown by the reactivated groups during Test 1 [F(1,25) = 0.28; p = 0.60 and F(1,33) = 0.05; p = 0.82; Figure 2B and C, respectively].
Figure 2.
Effect of systemic administration of MDZ and PROP on memory reconsolidation in animals without memory reactivation.
(A) Timeline for non-reactivated rats from Experiment 1. (B and C) In the absence of the re-exposure session, neither MDZ nor PROP, respectively, reduced the freezing response on Test 1. (B) CON-SAL (n = 7), CON-MDZ (n = 7), ETOH-SAL (n = 7), and ETOH-MDZ (n = 8). (C) CON-SAL (n = 9), CON-PROP (n = 8), ETOH-SAL (n = 9), and ETOH-PROP (n = 11). Data are the mean ± standard error of the mean of time spent freezing during Test 1. CON, control rats; ETOH, ethanol-withdrawn rats; MDZ, midazolam; PROP, propranolol; SAL, sterile saline.
In summary, and regardless of the duration of the reactivation session, neither MDZ nor PROP affected the freezing response during the retention tests in the ETOH groups.
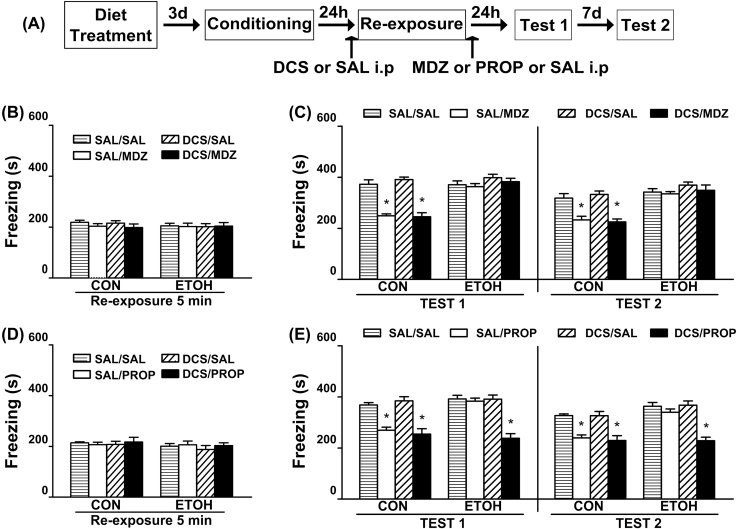
Experiment 2: The Disruptive Effects of PROP But Not of MDZ on Memory Reconsolidation were Facilitated by Pre-Reactivation DCS Administration in Ethanol-Withdrawn Rats
All rats showed comparable levels of freezing during the 5min reactivation session [F(1,61) = 0.04; p = 0.84 and F(1,54) = 0.04; p = 0.84; Figure 3B and D, respectively]. The effect of pre-reactivation DCS administration on MDZ′s disruptive consequences on memory reconsolidation is shown in Figure 3C. Regardless of the drug injected before the reactivation session, a significant decrease in fear response was detected in CON groups treated with MDZ. These effects lasted up to one week. However, none of the treatments affected the freezing response in ETOH rats. ANOVA revealed no significant diet treatment x pre-reactivation drug x post-reactivation drug interaction for Test 1 [F(1,61) = 0.12; p = 0.72] or Test 2 [F(1,61) = 0.05; p = 0.83], but significant diet treatment x post-reactivation drug interactions for Test 1 [F(1,61) = 40.24; p < 0.05] and Test 2 [F(1,61) = 15.47; p < 0.05]. In both tests, the post hoc analysis of significant diet treatment x post-reactivation drug interaction revealed that the freezing levels of the MDZ-CON groups were significantly lower than the remaining groups.
Figure 3.
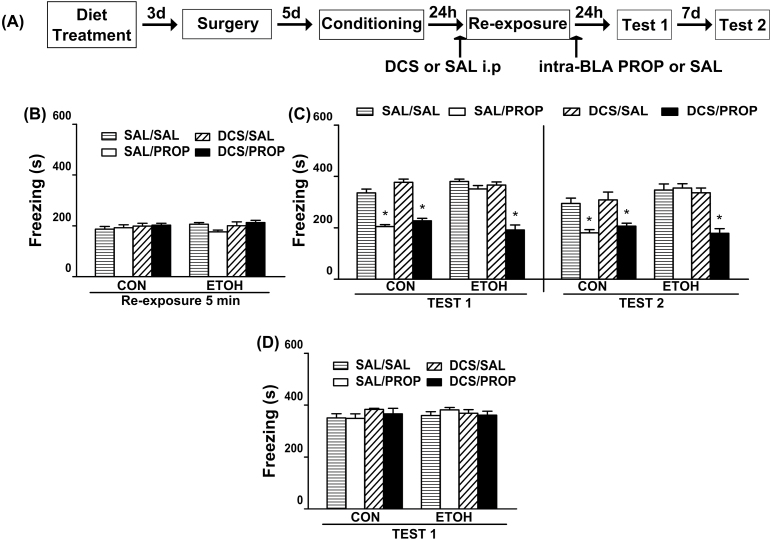
Influence of pre-retrieval DCS administration on MDZ’s and PROP’s disruptive effects on the reconsolidation of contextual fear memory in ethanol-withdrawn rats.
(A) Timeline for Experiment 2. (B) Regardless of the pre-retrieval drug treatment, all groups of animals assigned to test the disruptive effect of MDZ on memory reconsolidation exhibited comparable levels of freezing during the 5min reactivation session. (C) Pre-reactivation DCS (5mg/kg, i.p.) did not facilitate the disruptive effect of MDZ (3mg/kg, i.p.) on memory reconsolidation in ETOH rats. CON-SAL/SAL (n = 8), CON-SAL/MDZ (n = 8), CON-DCS/SAL (n = 8), CON-DCS/MDZ (n = 10), ETOH-SAL/SAL (n = 8), ETOH-SAL/MDZ (n = 8), ETOH-DCS/SAL (n = 9), and ETOH-DCS/MDZ (n = 10). (D) Regardless of the pre-retrieval drug treatment, all group of animals assigned to test the disruptive effect of PROP on memory reconsolidation exhibited comparable levels of freezing during the 5min reactivation session. (E) Pre-reactivation DCS (5mg/kg, i.p.) facilitated the disruptive effect of PROP (10mg/kg, i.p.) on memory reconsolidation in ETOH rats. CON-SAL/SAL (n = 7), CON-SAL/PROP (n = 7), CON-DCS/SAL (n = 7), CON-DCS/PROP (n = 8), ETOH-SAL/SAL (n = 8), ETOH-SAL/PROP (n = 8), ETOH-DCS/SAL (n = 8), and ETOH-DCS/PROP (n = 9). Data are expressed as the mean ± standard error of the mean of time spent freezing during the reactivation session, Tests 1 and 2. *Significantly different from the remaining groups (p < 0.05). CON, control rats; DCS, d-cycloserine; ETOH, ethanol-withdrawn rats; MDZ, midazolam; PROP, propranolol; SAL, sterile saline.
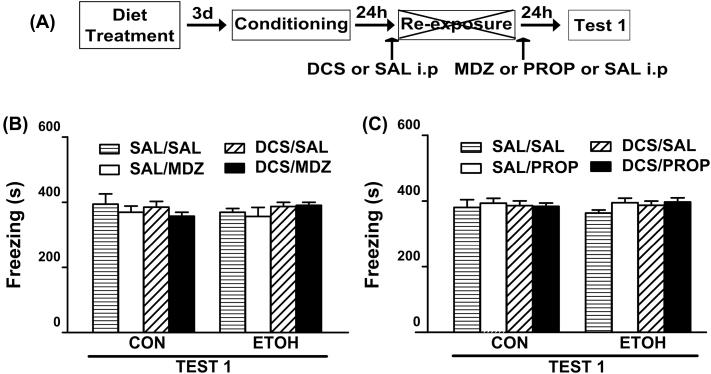
The effect of pre-reactivation DCS administration on PROP′s disruptive effect on memory reconsolidation is shown in Figure 3E. Regardless of the treatment before the reactivation session, CON animals injected with PROP showed reduced levels of freezing. In the ETOH group, the DSC-PROP treatment elicited a decrease in the freezing levels that did not differ from those shown by the CON-PROP groups and that lasted up to one week. ANOVA revealed significant diet treatment x pre-reactivation drug x post-reactivation drug interactions for Test 1 [F(1,54) = 6.38; p < 0.05] and Test 2 [F(1,54) = 6.44; p < 0.05]. For both tests, the freezing levels of DCS-PROP and SAL-PROP from the CON group and DCS-PROP from the ETOH group did not differ from each other and were significantly lower than the remaining groups. All non-reactivated groups displayed similar levels of freezing which did not vary from those shown by the reactivated groups during Test 1 [F(1,32) = 0.13; p = 0.72 and F(1,41) = 0.02; p = 0.90; Figure 4B and C, respectively].
Figure 4.
Influence of DCS administration on MDZ’s and PROP’s effect on memory reconsolidation in animals without memory reactivation.
(A) Timeline for non-reactivated rats from Experiment 2. (B and C) In the absence of a re-exposure session, none of the drug treatments reduced the freezing response on Test 1. (B) CON-SAL/SAL (n = 5), CON-SAL/MDZ (n = 5), CON-DCS/SAL (n = 5), CON-DCS/MDZ (n = 5), ETOH-SAL/SAL (n = 5), ETOH-SAL/MDZ (n = 5), ETOH-DCS/SAL (n = 5) and ETOH-DCS/MDZ (n = 5). (C) CON-SAL/SAL (n = 7), CON-SAL/PROP (n = 7), CON-DCS/SAL (n = 6), CON-DCS/PROP (n = 6), ETOH-SAL/SAL (n = 7), ETOH-SAL/PROP (n = 6), ETOH-DCS/SAL (n = 5), and ETOH-DCS/PROP (n = 5). Data are the mean ± standard error of the mean of time spent freezing during Test 1. CON, control rats; DCS, d-cycloserine; ETOH, ethanol-withdrawn rats; MDZ, midazolam; PROP, propranolol; SAL, sterile saline.
Taken together, these results suggest that DCS/PROP treatment was effective in reducing the freezing response in ETOH group, whereas the DCS/MDZ treatment was ineffective.
Experiment 3: Intra-BLA PROP Infusion Disrupted Memory Reconsolidation in Ethanol-Withdrawn Rats Treated with DCS Prior to Reactivation
As shown in previous experiments, all rats showed comparable levels of freezing during the reactivation session [F(1,45) = 2.47; p = 0.12; Figure 5B]. The effect of intra-BLA PROP on Test 1 and Test 2 is shown in Figure 5C. Regardless of the drug treatment before the reactivation session, the CON group infused with PROP revealed reduced levels of freezing. Intra-BLA PROP decreased the levels of freezing in DCS-ETOH rats, with the PROP effects lasting up to one week in both groups. ANOVA revealed significant diet treatment x pre-reactivation drug x post-reactivation drug interactions for Test 1 [F(1,45) = 11.84; p < 0.05] and Test 2 [F(1,45) = 9.45; p < 0.05]. In both tests, the levels of freezing for DCS-PROP and SAL-PROP from the CON group and DCS-PROP from the ETOH group did not differ from each other and were significantly lower than the remaining groups. All non-reactivated groups displayed similar levels of freezing, which did not vary from those shown by the reactivated groups during Test 1 [F(1,33) = 0.10; p = 0.75; Figure 5D]. The cannula placements are shown in Figure 6.
Figure 5.
Effect of intra-BLA PROP on contextual fear memory reconsolidation in ethanol-withdrawn rats and the influence of DCS.
(A) Timeline for Experiment 3. (B) Regardless of the pre-retrieval drug treatment, all groups of animals exhibited comparable levels of freezing during the 5min reactivation session. (C) Pre-reactivation DCS (5mg/kg, i.p.) facilitated the disruptive effect of intra-BLA PROP (1.25 ug/side) on memory reconsolidation in ETOH rats. CON-SAL/SAL (n = 7), CON-SAL/PROP (n = 6), CON-DCS/SAL (n = 6), CON-DCS/PROP (n = 7), ETOH-SAL/SAL (n = 7), ETOH-SAL/PROP (n = 6), ETOH-DCS/SAL (n = 7), and ETOH-DCS/PROP (n = 7). (D) In the absence of a reactivation session none of the drug treatments reduced the freezing response on Test 1. CON-SAL/SAL (n = 6), CON-SAL/PROP (n = 5), CON-DCS/SAL (n = 5), CON-DCS/PROP (n = 5), ETOH-SAL/SAL (n = 5), ETOH-SAL/PROP (n = 5), ETOH-DCS/SAL (n = 5), and ETOH–DCS/PROP (n = 5). Data are the mean ± standard error of the mean of time spent freezing during reactivation session, Test 1, and Test 2. *Significantly different from the remaining groups (p < 0.05). BLA, basolateral amygdala; CON, control rats; DCS, d-cycloserine; ETOH, ethanol-withdrawn rats; PROP, propranolol; SAL, sterile saline.
Figure 6.
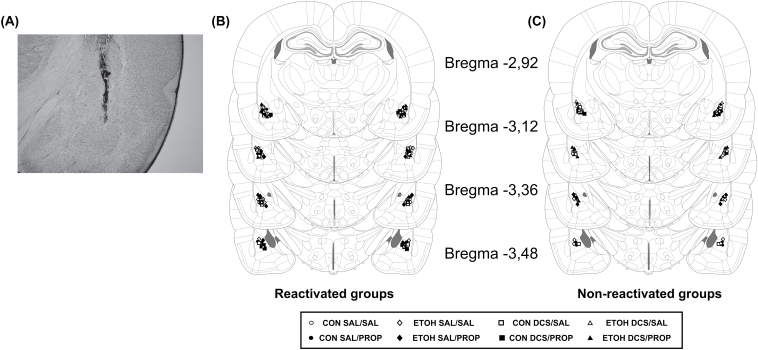
Placement of infusion cannulas.
(A) Photomicrograph of a coronal brain section showing the location of the infusion site in the BLA. Magnification: 25x. Schematic representation of coronal sections of the rat brain showing the cannula tip placements for reactivated groups (B) and non-reactivated groups (C) in the BLA for Experiment 3 (adapted from Paxinos and Watson, 2009). BLA, basolateral amygdala; CON, control rats; DCS, d-cycloserine; ETOH, ethanol-withdrawn rats; PROP, propranolol; SAL, sterile saline.
These results suggest that intra-BLA PROP infusion was effective in reducing the freezing response in ETOH rats that were treated with DCS.
Experiment 4: Ethanol Withdrawal Reduced Both the Total and Surface Expression of α 1 GABA-A Receptor Subunit in the BLA
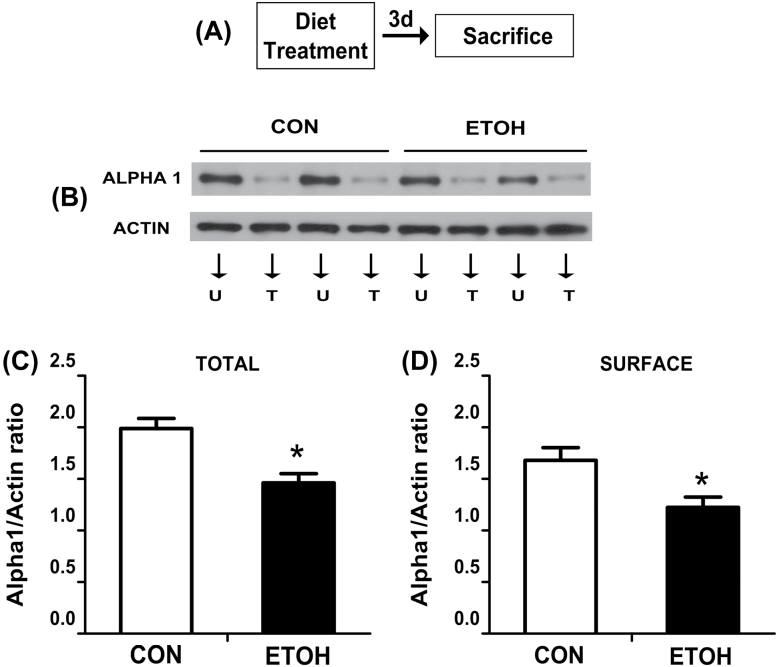
In this experiment, we examined whether the lack of an amnesic effect of MDZ in ETOH rats could be attributed to changes in the total and/or surface expression of the α1 GABAA-R subunit in the BLA. Western blot analysis revealed a decreased total [t = 3.93; p < 0.05] and surface expression [t = 2.86; p < 0.05] of the GABAA-R α1 subunit at day 3 of withdrawal (Figure 7C and D).
Figure 7.
Effect of ethanol withdrawal on total and surface protein expression of α1 GABAA-R subunit in the BLA.
(A) Timeline for Experiment 4. (B) Representative immunoreactive bands of total and surface α1 GABAA-R subunit and actin in the BLA from the CON and ETOH groups. (C) Bars represent the total expression of α1 in the BLA (n = 8/group). (D) Bars represent the surface expression of α1 in the BLA (n = 8/group). U, untreated portion; T, treated portion with bis(sulfosuccinimidyl)suberate. *Significantly different from the CON group (p < 0.05). BLA, basolateral amygdala; CON, control rats; ETOH, ethanol-withdrawn rats.
Discussion
As expected, and in agreement with previous findings, the post-retrieval systemic administration of either MDZ or PROP or PROP intra-BLA administration reduced freezing on subsequent memory tests in CON rats. This disruptive effect of MDZ and PROP was dependent on memory reactivation, since no amnestic effect was observed in CON rats receiving these drugs in the absence of the reactivation session. It could be argued that the reduction of freezing observed following such pharmacological treatments is due to facilitated extinction rather than disrupting reconsolidation. However, this possibility seems unlikely. In fact, the attenuation of freezing was maintained during the second test performed 1 week later, thus indicating the absence of fear recovery.
Also, it is well known that the activation of GABAA-Rs interferes with the acquisition and consolidation of extinction memory (Makkar et al., 2010). Moreover, it is clear that PROP does not facilitate the onset of extinction memory (Cain et al., 2004; Rodriguez-Romaguera et al., 2009). Collectively, our results have replicated previous findings showing that MDZ or PROP given systemically, or PROP directly infused into the BLA following retrieval, results in the disruption of contextual fear memory reconsolidation in CON rats (Debiec and LeDoux, 2004; Bustos et al., 2006; Zhang and Cranney, 2008; Muravieva et al., 2010). Interestingly, Debiec and LeDoux (2004) reported that the intra-amygdala administration of PROP impaired auditory fear memory reconsolidation. Taken together, these findings suggest that the β-adrenoreceptors (β-ARs) located in the BLA are also involved in contextual fear memory reconsolidation.
In contrast to the effects observed in CON rats, neither MDZ nor PROP (after either systemic or intra-BLA administration) attenuated the subsequent freezing response in ETOH rats (Figures 1 and 5). This lack of induced amnesia in these rats cannot be attributed to low drug dosage because MDZ (3mg/kg) and PROP (at 10mg/kg; 1.25 ug/side) had been the highest doses that were reported to be effective in studies involving memory reconsolidation blockade (Debiec and LeDoux, 2004; Bustos et al. 2010; Muravieva et al., 2010). Taken together, our results suggest that a previous history of ethanol dependence/withdrawal leads to the generation of a fear memory trace that is less vulnerable to disruption after recall.
Evidence from other studies involving fear conditioning in rodents has demonstrated that stronger memories are more resistant to reconsolidation blockade than weaker memories. For instance, robust memories induced by increasing the number of shocks during acquisition, or by exposing animals to a stressful experience prior to fear acquisition, were resistant to reconsolidation interference (Suzuki et al., 2004; Wang et al., 2009; Bustos et al., 2010). Interestingly, we have previously demonstrated that the withdrawal from chronic alcohol exposure facilitated the formation of a robust contextual fear memory accompanied by impaired extinction (Bertotto et al., 2006). In agreement with these findings, it was recently reported that withdrawal from chronic, intermittent ethanol administration also impaired auditory fear memory extinction (Holmes et al., 2012). Given that the fear memory formed under ethanol withdrawal is resistant to extinction and to pharmacological blockade following retrieval, our present results strongly support the notion that withdrawal from chronic ethanol, similar to previous stress exposure, strengthens fear memory formation.
Activation of NMDA receptors seems to play a major role in reactivation-induced fragility (Ben Mamou et al., 2006) and make these resistant memories, such as those formed under stress conditions, become more susceptible to disruption (Bustos et al., 2010). Here, we show that DCS administration before memory reactivation was effective in inducing instability following retrieval, and in turn, promoting vulnerability to the disruptive effect of PROP (after either systemic or intra-BLA administration) on fear memory reconsolidation in ETOH rats (Figures 3 and 5). This is based on four results: (1) PROP can induce memory interference in ETOH animals that received pre-reactivation DCS but not SAL; (2) the DCS/PROP treatment was ineffective in the absence of the re-exposure session, thus indicating that such interference is selectively dependent on memory reactivation; (3) DCS/PROP-treated ETOH rats displayed similar freezing levels to those exhibited by PROP-treated CON rats; and (4) DCS was ineffective in CON rats, because SAL/PROP and DCS/PROP rats exhibited similar freezing during Test 1 and Test 2, thus supporting the contention that memory destabilization induced by DCS is selectively promoted in those memories resistant to PROP′s disruptive effect. Consistent with previous findings (Bustos et al., 2010; Lee et al., 2006), DCS administration before the brief reactivation session did not influence freezing expression, because all rats showed comparable levels of freezing during this trial. Although other evidence indicated that DCS facilitates the formation of extinction memory (Walker et al., 2002; Bertotto et al., 2006), this did not occur under our experimental conditions, because the DCS/SAL group from the CON rats exhibited high levels of freezing during Test 1 and Test 2. The fact that the cannulated rats were fear trained 7 days after withdrawal, and that post-retrieval intra-BLA infusions of PROP did not impair subsequent memory in ETOH rats, provides additional evidence to suggest a relatively long-lasting effect of ethanol withdrawal that induces the formation of strong fear memories.
The present study is the first to propose that the DCS/PROP administration in combination with memory retrieval can be a potential treatment for interference on fear memory reconsolidation. Related to this, it should be noted that both DCS and PROP drugs are accepted for human use, and that the disruptive effect of PROP on the reconsolidation of fear and drug-related memories has been previously demonstrated in pre-clinical and clinical studies (Debiec and LeDoux, 2004; Kindt et al. 2009; Wouda et al., 2010; Saladin et al., 2013).
The mechanism by which DCS promotes retrieval-induced memory destabilization is still unknown. Mao et al. (2008) reported that the DCS-induced enhancement of memory extinction is prevented by a proteasome inhibitor infused into the amygdala, suggesting that the DCS effect is mediated by the activation of the ubiquitin/proteasome system. Moreover, recent evidence has demonstrated that protein degradation in the hippocampus and amygdala regulates fear memory destabilization after reactivation (Lee et al., 2008; Jarome et al., 2011). In these investigations, the proteasome inhibitor applied to the CA1 region of the hippocampus and amygdala following memory retrieval blocked the amnestic effect of anisomycin on memory reconsolidation. Jarome et al. (2011) demonstrated that ifenprodil (a NR2B-selective antagonist of the NMDA receptor) reduced the amount of polyubiquitination in the amygdala following retrieval, suggesting that the protein degradation involved in memory labilization depends on NMDA receptor activation. Therefore, the promoting influence of pre-reactivation DCS on memory destabilization in resistant memories might be caused by activation of the ubiquitin/proteasome system. Further studies are needed to clarify this topic.
Recent evidence has suggested a critical role of NMDA receptors on memories resistant to interference after retrieval. In agreement with this, Wang et al. (2009) reported that resistant fear memory induced by a strong conditioning protocol is associated with a down-regulation of the NR2B receptor subunit in the BLA. Moreover, as mentioned above, Bustos et al. (2010) indicated that NMDA activation by DCS facilitated vulnerability to disruption after retrieval in resistant memories of stressed animals. Our results have indicated that this assumption can be extended to the resistant memory induced by ethanol dependence. Furthermore, it is well known that ethanol dependence leads to neuroadaptive changes in the NMDA receptors from brain areas relevant for fear learning, such as the amygdala (Krystal et al., 2003; McCool et al., 2010). Therefore, it could be speculated that the resistance to retrieval-induced labilization in ETOH rats may be associated with an altered expression or change in the function of the NMDA receptors following ethanol dependence.
Central noradrenergic transmission is activated during ethanol withdrawal (Koob, 2008; Becker, 2012). Beta blockers such as PROP attenuate withdrawal symptoms and the increased self-administration of alcohol in dependent animals (Koob, 2008; Gilpin and Koob, 2010). Although ethanol withdrawal leads to increased β-ARs density in the whole brain (Banerjee et al.,1978; Kuriyama et al., 1981) and to an up-regulation of cortical β2-adrenoreceptor gene expression (Rimondini et al., 2002), the effect on these receptors in the BLA has not been reported. Regardless of the potential changes induced by ethanol withdrawal on β-ARs expression, the memory destabilization facilitated by DCS was a crucial requirement for PROP’s disruptive effect on reconsolidation in ETOH rats. Although a potential interaction between NMDA receptors and β-ARs on memory reconsolidation cannot be fully discarded, further experiments are necessary to elucidate this possibility. As predicted, we found that MDZ did not affect memory of ETOH animals that had received DCS previous to recall. This finding is opposite to that reported by Bustos et al. (2010), who showed that MDZ-induced memory impairment in stressed rats became evident with pre-reactivation DCS. Although stress exposure and ethanol withdrawal can induce the formation of fear memories resistant to pharmacological blockade after retrieval, it seems that the mechanisms involved in such resistance may be different.
At this point, the question that needs answering is: why did MDZ not affect memory reconsolidation in DCS-pretreated ETOH animals? Previously, it has been demonstrated that ethanol dependence can induce neuroadaptive changes in the expression of GABAA-Rs associated with alterations in the pharmacological responses to benzodiazepines agonists and inverse agonists (Papadeas et al., 2001; Cagetti et al., 2003; Diaz et al., 2011). Moreover, the sedative and amnestic effects of BZDs have been attributed to α1-containing GABAA-Rs (Mohler et al., 2002). Based on these data, the expression of the α1 GABAA-R subunit was evaluated in the BLA from the ETOH rats. As shown in Figure 7, both total and surface expressions of α1 were reduced in ETOH animals. These findings extend previous evidence by indicating that the reduction in the α1 GABAA-R subunit was evident up to the third day of ethanol withdrawal. Therefore, the resistance to MDZ’s disruptive effect on fear memory reconsolidation may have been due to, at least in part, the decrease of surface expression of α1 in the BLA, induced by chronic ethanol administration/withdrawal. Furthermore, previous findings from our laboratory demonstrated that a reduction of the inhibitory control mediated by GABAergic transmission in the BLA projection neurons leads to neuronal hyperexcitability and increased plasticity, which facilitates fear learning in ETOH rats (Isoardi et al., 2007). The neuroadaptive change in α1-containing GABAA-Rs observed in the present study complements these results, suggesting that GABAergic transmission in the BLA could have been altered by changes in the subunit composition of GABAA receptors or the associated chloride channel.
In conclusion, we have demonstrated that: (1) ethanol withdrawal facilitated the formation of a fear memory which is resistant to post-reactivation destabilization; (2) DCS pre-reactivation administration facilitated the onset of a PROP, but not MDZ, disruptive effect on memory reconsolidation in ETOH rats; (3) the effect of PROP was mediated, although not exclusively, by β-adrenoreceptors in the BLA; and (4) the lack of MDZ′s effect on memory reconsolidation may have been a consequence, at least in part, of a reduced α1 GABAA-R subunit expression in the BLA induced by chronic ETOH treatment and/or its withdrawal.
Considering the critical role of negative emotional experiences in the development and maintenance of psychiatric disorders and drug addition, our results suggest that the blockade of fear memory reconsolidation by DCS/PROP treatment, in conjunction with memory retrieval, provides a potential treatment for the attenuation of maladaptive memories involved in relapse following withdrawal.
Statement of Interest
None.
Acknowledgments
This work was supported by grants from MinCyT-Córdoba, Universidad Nacional de Córdoba, SECYT-UNC, CONICET, and Agencia Nacional de Promoción Científica y Tecnológica -FONCYT (Argentina) to Drs Molina and Martijena. We thank Estela Salde and Lorena Mercado for the technical assistance and Dr Paul Hobson, native speaker, for the revision of the manuscript. We thank Elsevier for granting us permission to adapt figures 57 to 62 from “The Rat Brain in Stereotaxic Coordinates” compact 6th edition by George Paxinos and Charles Watson, Academic Press, 2009, copyright © 2009.
References
- Alberini CM. (2005). Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 28(1):51–56. [DOI] [PubMed] [Google Scholar]
- Banerjee SP, Sharma VK, Khanna JM. (1978). Alterations in beta-adrenergic receptor binding during ethanol withdrawal. Nature 276(5686):407–409. [DOI] [PubMed] [Google Scholar]
- Becker HC. (2012). Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res 34(4):448–458. [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. (2006). NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9(10):1237–1239. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Bustos SG, Molina VA, Martijena ID. (2006). Influence of ethanol withdrawal on fear memory: effect of D-cycloserine. Neuroscience 142(4):979–990. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Bussolino DF, Molina VA, Martijena ID. (2010). Increased voluntary ethanol consumption and c-Fos expression in selected brain areas induced by fear memory retrieval in ethanol withdrawn rats. Eur Neuropsychopharmacol 20(8):568–581. [DOI] [PubMed] [Google Scholar]
- Bertotto ME, Maldonado NM, Bignante EA, Gorosito SV, Cambiasso MJ, Molina VA, Martijena ID. (2011). ERK activation in the amygdala and hippocampus induced by fear conditioning in ethanol withdrawn rats: modulation by MK-801. Eur Neuropsychopharmacol 21(12):892–904. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. (1969). Crouching as an index of fear. J Comp Physiol Psychol 67(3):370–375. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME. (2012). A protein cross-linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Curr Protoc Neurosci. Chapter 5: Unit 5 30 31–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. (2006). Midazolam disrupts fear memory reconsolidation. Neuroscience 139(3):831–842. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Giachero M, Maldonado H, Molina VA. (2010). Previous stress attenuates the susceptibility to Midazolam’s disruptive effect on fear memory reconsolidation: influence of pre-reactivation D-cycloserine administration. Neuropsychopharmacology 35(5):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. (2003). Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63(1):53–64. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. (2004). Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem 11(2):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129(2):267–272. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. (2011). Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharm Exp Ther 337(1):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. (2006). Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol 16(2):174–178. [DOI] [PubMed] [Google Scholar]
- Fendt M. (2001). Injections of the NMDA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. J Neurosci 21(11):4111–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. (2004). Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharm Exp Ther 311(3):1071–1079. [DOI] [PubMed] [Google Scholar]
- Giachero M, Bustos SG, Calfa G, Molina VA. (2013). A BDNF sensitive mechanism is involved in the fear memory resulting from the interaction between stress and the retrieval of an established trace. Learn Mem 20(5):245–255. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. (2010). Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 212(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15(2):169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. (2007). Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci 26(12):3631–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. (2012). Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci 15(10):1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoardi NA, Bertotto ME, Martijena ID, Molina VA, Carrer HF. (2007). Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci 26(4):1036–1044. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ. (2011). Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLOS ONE 6(9):e24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. (2009). Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12(3):256–258. [DOI] [PubMed] [Google Scholar]
- Koob GF. (2008). A role for brain stress systems in addiction. Neuron 59(1):11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2):97–129. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. (2003). N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther 99(1):79–94. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Muramatsu M, Aiso M, Ueno E. (1981). Alteration in beta-adrenergic receptor binding in brain, lung and heart during morphine and alcohol dependence and withdrawal. Neuropharmacology 20(7):659–666. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. (2006). Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26(39):10051–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Kim H, Kaang BK. (2008). Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319(5867):1253–1256. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. (2010. ) Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35(8):1625–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Lin HC, Gean PW. (2008). Augmentation of fear extinction by D-cycloserine is blocked by proteasome inhibitors. Neuropsychopharmacology 33(13):3085–3095. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. (1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18(3):135–174. [DOI] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. (2010). Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol 91:205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, AJ, Mascagni, F (2004) Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol 473(1):137–146. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. (2002). A new benzodiazepine pharmacology. J Pharm Exp Ther 300(1):2–8. [DOI] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. (2010). Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem 17(6):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406(6797):722–726. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. (2001). Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res 25(9):1270–1275. [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2009). The rat brain in stereotaxic coordinates: 6th edition. San Diego, CA: Academic Press. [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. (2002). Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J 16(1):27–35. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. (2009). Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry 65(10):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD, Baker NL, Hartwell KJ, Brady KT. (2013). A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 226(4):721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I. (2011). Subunit compensation and plasticity of synaptic GABA(A) receptors induced by ethanol in alpha4 subunit knockout mice. Front Neurosci 5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24(20):4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Lattal KM. (2013). Substance abuse, memory, and post-traumatic stress disorder. Neurobiol Learn Mem. Advance online publication. 10.1016/j.nlm.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. (2007). Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8(4):262–275. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. (2013). Addiction: a drug-induced disorder of memory reconsolidation. Curr Opin Neurobiol 23(4):573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. (2002). Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 22(6):2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. (2009). Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci 12(7):905–912. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Linn ML, Cook JM, Houser CR, O’Dell TJ, Homanics GE, Fanselow MS. (2009). The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ. (2010). Disruption of long-term alcohol-related memory reconsolidation: role of beta-adrenoceptors and NMDA receptors. Front Behav Neurosci 4:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cranney J. (2008). The role of GABA and anxiety in the reconsolidation of conditioned fear. Behav Neurosci 122(6):1295–1305. [DOI] [PubMed] [Google Scholar]