Abstract
Background:
Despite the widespread use of antipsychotics, little is known of the molecular bases behind the action of antipsychotic drugs. A genome-wide study is needed to characterize the genes that affect the clinical response and their adverse effects.
Methods:
Here we show the analysis of the blood transcriptome of 22 schizophrenia patients before and after medication with atypical antipsychotics by next-generation sequencing.
Results:
We found that 17 genes, among the 21 495 genes analyzed, have significantly-altered expression after medication (p-value adjusted [Padj] <0.05). Six genes (ADAMTS2, CD177, CNTNAP3, ENTPD2, RFX2, and UNC45B) out of the 17 are among the 200 genes that we characterized with differential expression in a previous study between antipsychotic-naïve schizophrenia patients and controls (Sainz et al., 2013). This number of schizophrenia-altered expression genes is significantly higher than expected by chance (Chi-test, Padj 1.19E-50), suggesting that at least part of the antipsychotic beneficial effects is exerted by modulating the expression of these genes. Interestingly, all six of these genes were overexpressed in patients and reverted to control levels of expression after treatment. We also found a significant enrichment of genes related to obesity and diabetes, known adverse affects of antipsychotics.
Conclusions:
These results may facilitate understanding of unknown molecular mechanisms behind schizophrenia symptoms and the molecular mechanisms of antipsychotic drugs.
Keywords: antipsychotics, gene differential expression, next generation sequencing, RNA profile, schizophrenia
Introduction
Schizophrenia is a severe and chronic mental disorder whose onset typically occurs in late adolescence to young adulthood. The lifetime risk of schizophrenia ranges from 0.3% to 0.66% worldwide, and up to 2.3% if other psychotic disorders are considered. The standard of schizophrenia treatment is antipsychotic medications (Insel, 2010). The mechanism of action of these medications tends to block receptors in the brain’s dopamine D2 pathways (Jones and Pilowsky, 2002). Antipsychotics only achieve clinical improvement in a percentage of patients and also are highly associated with adverse effects (van Os and Kapur, 2009). Lifetime antipsychotic treatment is frequently required to prevent illness relapses and to maintain functionality. The use of antipsychotics also represents a huge economic burden to society. Studies of clinical response variability have established the importance of functional mutations detected in CYP metabolic enzymes (Nebert and Dieter, 2000). Other studies focused in genes related to neurotransmitter (dopamine and serotonin) pathways traditionally involved with schizophrenia have not clarified the molecular basis of the clinical response.
Alterations in gene expression could provide relevant information on the molecular basis of the disease and the mechanisms of action of the antipsychotics (Chana et al., 2013). Post-mortem studies of gene expression obtained from brains are affected by the period of time from death to sample collection, by the change in mRNA levels caused by all possible medication given to the individuals, and by the number of genes altered by the agony of death. However, a viable alternative is the study of mRNA levels in lymphocytes from living individuals, which are free of the variability observed in post-mortem studies (Czermak et al., 2004). It has been reported that the expression level of genes encoding neurotransmitter receptors and other proteins is similar in peripheral blood lymphocytes and in the central nervous system (Glatt et al., 2005).
To identify genes targeted by atypical antipsychotics, we investigated the expression profiles altered by these drugs. Here we report the first study, to our knowledge, of gene differential expression in blood between drug-naïve schizophrenia patients and the same patients after treatment with atypical antipsychotics using next-generation sequencing data. We hypothesize that this information could be used to identify biomarkers for the disease, to monitor the effect of the antipsychotics, and for drug repositioning and development. We sequenced total mRNA of a cohort of 22 individuals (Table 1) before treatment and the same individuals 3 months after treatment started. All patients showed improved symptoms after treatment (Table 2). These 22 patients are part of a larger cohort used for a previous case/control study (Sainz et al., 2013).
Table 1.
Sociodemographic and Clinical Characteristics of Study Individuals
| Characteristics | Total | |
|---|---|---|
| (n = 22) | ||
| Mean | SD | |
| Age at admission (years) | 32.1 | 10.6 |
| Age at psychosis onset (years) | 31.7 | 10.5 |
| Duration of untreated psychosis (months) | 4.5 | 8.0 |
| Duration of untreated illness (months) | 7.8 | 10.2 |
| n | % | |
| Sex | ||
| Male | 8 | 36.4 |
| Female | 14 | 63.6 |
| Race | ||
| Caucasians | 22 | 100 |
| Family history of psychosis (yes) | 7 | 31.8 |
| Tobacco use at intake (yes) | 13 | 59.1 |
| Cannabis use at intake (yes) | 7 | 31.8 |
| Alcohol use at intake (yes) | 11 | 50.0 |
Table 2.
Psychopathology at Baseline and 3 Months, and Magnitude of the Change
| Variable | Total (n = 22) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 3-month change from baseline | % | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| CGI total score | 6.0 | 0.8 | 2.3 | 1.1 | -3.6 | 1.6 | n/a |
| BPRS total score | 53.0 | 9.1 | 28.7 | 3.9 | -24.3 | 10.2 | -45.9 |
| SANS total score | 5.8 | 5.7 | 4.0 | 3.7 | -1.8 | 6.9 | -30.7 |
| SAPS total score | 12.0 | 3.6 | 0.4 | 1.0 | -11.7 | 3.9 | -97.0 |
| CDSS total score | 2.0 | 3.1 | 1.4 | 3.6 | -0.6 | 5.3 | -30.2 |
| YMRS total score | 10.9 | 6.7 | 0.4 | 1.0 | -10.5 | 6.9 | -96.3 |
BPRS: Brief Psychiatric Rating Scale; CDSS: Calgary Depression Rating Scale for Schizophrenia; CGI: Clinical Global Impression; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; SD: standard deviation; YMRS: Young Mania Rating Scale.
Methods
Study Setting
The cohort analyzed in this study (Table 1) was obtained from an ongoing epidemiological and three-year longitudinal intervention program of first-episode psychosis (PAFIP) conducted at the outpatient clinic and the inpatient unit at the University Hospital Marques de Valdecilla, Cantabria, Spain (Pelayo-Teran et al., 2008). Conforming to international standards for research ethics, the research in this article was approved by the Cantabria Ethics Institutional Review Board. Patients meeting inclusion criteria and their families provided written informed consent to be included in the PAFIP. The biological samples of patients included in the study were provided by the Valdecilla biobank.
Subjects
All referrals to PAFIP were screened for patients who met the following criteria: (1) 15–60 years; (2) living in the catchment area (Cantabria); (3) experiencing their first episode of psychosis; (4) no prior treatment with antipsychotic medication or, if previously treated, a total lifetime of adequate antipsychotic treatment of less than 6 weeks; and (5) DSM-IV criteria for schizophrenia, schizophreniform disorder, schizoaffective disorder, or brief psychotic disorder. Patients were excluded for any of the following reasons: (1) meeting DSM-IV criteria for drug dependence; (2) meeting DSM-IV criteria for mental retardation; or (3) having a history of neurological disease or head injury. The diagnoses were confirmed using the Structured Clinical Interview for DSM-IV, carried out by an experienced psychiatrist 6 months after the baseline visit. Our operational definition for a first episode of psychosis included individuals with a non-affective psychosis (meeting the inclusion criteria defined above) who have not previously received antipsychotic treatment regardless of the duration of psychosis. Twenty-two individuals, who gave written consent to their participation in the program, fulfilled inclusion criteria at 6 months, and had mRNA samples at baseline and at 3 months, were included in our analyses.
Premorbid and Sociodemographic Variables
Premorbid and sociodemographic information was recorded from patients (Table 1), relatives, and medical records. Age of onset of psychosis was defined as the age when the first continuous (present most of the time) psychotic symptom emerged. Duration of untreated illness was defined as the time from the first unspecific symptoms related to psychosis (for such a symptom to be considered, there could be no return to the previous, stable level of functioning) to initiation of adequate antipsychotic drug treatment. Duration of untreated psychosis was defined as the time from the first continuous (present most of the time) psychotic symptom to initiation of adequate antipsychotic drug treatment. Information about alcohol, cannabis, and tobacco consumption was converted into binary variables and coded for either the presence or absence of use.
Study Design
This is a prospective, randomized, flexible-dose, open-label study. We used a simple randomization procedure. At study intake, all patients were antipsychotic-naïve and were randomized to aripiprazole (n = 13), risperidone (n = 3), olanzapine (n = 1), quetiapine (n = 3), or ziprasidone (n = 2). Dose ranges were 5–30mg/day aripiprazole, 2–6mg/day risperidone, 5–20mg/day olanzapine, 40–160mg/day ziprasidone, and 100–600mg/day quetiapine. A rapid titration schedule (5 days) was used until the optimal dose was reached, unless severe side effects occurred. At the treating physician′s discretion, the dose and type of antipsychotic medication could be changed based on clinical efficacy and the profile of side effects during the follow-up period. Antimuscarinic medication, lormetazepam, and clonazepam were permitted for clinical reasons. No antimuscarinic agents were administered prophylactically. Antidepressants and mood stabilizers were permitted if clinically needed. At the 3-month follow-up, patients were on: aripiprazole (n = 9), risperidone (n = 5), olanzapine (n = 4), quetiapine (n = 1), ziprasidone (n = 2), and risperidone depot (n = 1). The mean daily dose of antipsychotics during follow-up was 181.72 (93.97) mg/day of chlorpormazine-equivalent doses. The mean daily dose of antipsychotics at 3 months was 163.64 (86.47) mg/day of chlorpormazine-equivalent doses. A more detailed description of medication intake during the follow-up period is available upon request.
Clinical Assessment
The severity of the Clinical Global Impression (CGI; 3) scale, the Brief Psychiatric Rating Scale (BPRS; expanded version of 24 items), the Scale for the Assessment of Positive symptoms (SAPS), the Scale for the Assessment of Negative symptoms (SANS), the Calgary Depression Scale for Schizophrenia (CDSS) and the Young Mania Rating Scale (YMRS) were used to evaluate clinical symptomatology. The same trained psychiatrist (Dr Crespo-Facorro) completed all clinical assessments. The severity of symptoms at baseline and at 3 months and the percentage of change at 3 months are reported in Table 2 (a reduction in total scores means clinical improvement).
The analysis of clinical efficacy reveals that there was a significant improvement of general psychopathology (assessed by the BPRS total score change at 3 months) and positive symptoms (hallucinations, delusions, positive formal thought disorders, bizarre behavior; assessed by SAPS total score changes at 3 months; Table 2). The patients were defined as responders to the optimum dose of antipsychotic at 3 months if there was a >50% reduction of the BPRS total score compared to intake. The rate of responders at 3 months was 95.55%.
Laboratory Assessments
Blood samples were assessed for biochemical and hematological parameters. To minimize the effects of diet and technique, blood samples were obtained from fasting subjects from 08:00 to 10:00 hours by the same personnel and in the same setting. A detailed description of methodology followed to assess hematological and biochemical variables (data available upon request). No patient had a chronic inflammation or infection, or was taking medication that might influence the results of blood tests. Smoking status and alcohol use were recorded at baseline. Anthropometric, hematological, and metabolic variables at baseline and 3 months, and changes at 3 months, are described in Table 3.
Table 3.
Blood and Metabolic Parameters of the 22 Patients at Baseline and at 3 Months
| Variable | Total (N=22) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 3-month change from baseline | % | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| BMI (kg/m2) | 22.5 | 2.7 | 24.0 | 2.5 | 1.4 | 2.2 | 6.4 |
| Weight (kg) | 62.9 | 11.0 | 66.9 | 10.1 | 4.0 | 5.9 | 6.3 |
| Leukocytes (10^3µ/L) | 7.6 | 1.8 | 6.6 | 1.3 | -1.0 | 2.2 | -13.4 |
| Hemoglobin (g/dl) | 14.1 | 1.0 | 14.0 | 1.0 | -0.1 | 1.2 | -0.5 |
| Glucose (mg/dl) | 86.1 | 12.9 | 84.4 | 7.2 | -1.7 | 14.3 | -2.0 |
| GGT (U/l) | 14.4 | 7.4 | 15.8 | 9.0 | 1.4 | 7.7 | 9.5 |
| Cholesterol (mg/dl) | 176.3 | 30.2 | 185.6 | 33.7 | 9.3 | 30.2 | 5.3 |
| Triglycerides (mg/dl) | 73.4 | 26.0 | 88.9 | 51.0 | 15.6 | 53.7 | 21.2 |
| HDL cholesterol (mg/dl) | 59.4 | 17.9 | 58.0 | 11.8 | -1.4 | 14.9 | -2.4 |
| CRP (mg/dl) | 0.5 | 1.1 | 0.1 | 0.2 | -0.3 | 1.1 | -75.4 |
BMI: body mass index; CRP: C-reactive protein; GGT: gamma-glutamytransferease; HDL: high density lipoprotein; SD: standard deviation.
RNA Extraction
Total RNA was extracted from blood using the Tempus™ Blood RNA Tube and Tempus™ Spin RNA Isolation Kit (Applied Biosystems) using the manufacturer’s protocols. To define expression profiles, a key factor is that the RNA is intact. To select only RNA with good quality, the RNA Integrity Number (RIN) was characterized with a Bioanalyzer (Agilent Technologies) and samples with a RIN of at least 7.2 were selected. The selected samples have RINs that range from 7.2 to 9.8 with an average of 8.5. Overall, the rate of samples from patients with a RIN equal to or higher than 7.2 was slightly higher than 89%.
RNA Next Generation Sequencing
The mRNA obtained from blood was sequenced using a Genome Analyzer IIx from Illumina. Total RNA was extracted from peripheral blood of each individual. The mRNA was isolated from the total RNA and, once transformed into cDNA, was fragmented by sonication. Fragments of 300bp on average were selected to construct the libraries for sequencing. The libraries to sequence were constructed using the reagents according to the indications of the manufacturer (Illumina). Single-end sequences of 35 nucleotides were produced using a Genome Analyzer IIx (Illumina). Alignment of the reads was performed in an Simple Linux Utility for Resource Management High Performance Computer server running Tophat 2.0.6 with default options (Trapnell et al., 2009). Tophat aligns RNA-Seq reads to genomes using the Bowtie 2.0.2 alignment program (Langmead et al., 2009), and then analyzes the mapping results to identify splice junctions between exons.
Differential Expression Statistical Analyses
Bedtools 2.17.0 (multicov option; Quinlan and Hall, 2010) was used to count the amount of reads mapped to each gene. The Reference Sequence gene coordinates were defined using the RefFlat file from the UCSC Genome Bioinformatics Site (retrieved February 28, 2014). DESeq 1.10.1 package (Anders and Huber, 2010), setting up fit-only as fitting method, was used to test for differential expression using gene-count data.
Validation of Expression Data Using quantitative reverse transcription PCR
To validate the expression data obtained by RNA-Seq, we performed quantitative reverse transcription PCR amplification of 10 of the genes that were found to have differential expression between cases and controls (Sainz et al., 2013) on 30 randomly selected samples (15 cases before medication and 15 controls). We used pre-designed Taqman probes (Life technologies) using the manufacturer’s instructions for LAMP3 (Hs00180880_m1), RFX2 (Hs01100925_m1), CTAG2 (Hs00535628_m1), GPR128 (Hs00262184_m1), IFI27 (Hs01086373_g1), ADAMTS2 (Hs01029111_m1), BRS3 (Hs00179951_m1), MSLN (Hs00245879_m1), RSAD2 (Hs00369813_m1), and IFI144L (Hs00119115_m1) using GADPH (Hs02758991_g1) as the control. Overall, we obtained a correlation ratio of 87.15% between the two technologies, with gene-individual correlation ratios varying from 75.0% (CTAG2) to 99.9% (MSLN).
Results
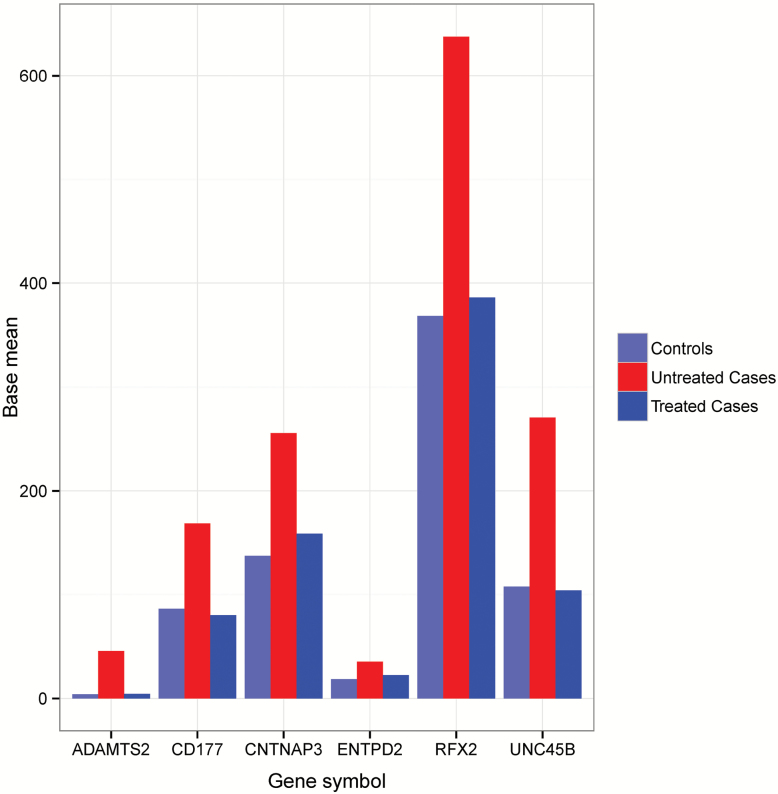
We found 17 genes with significant differential expression (Table 4) after adjusting for multiple testing (Padj Value <0.05) in the 21 495 human genes analyzed (Supplementary Table 1). In the previous study (Sainz et al., 2013) we found 200 genes with differential expression between schizophrenia patients and controls (36 schizophrenic, drug-naïve patients and 40 controls). Six of the differentially-expressed genes between untreated/treated patients (ADAMTS2, CD177, CNTNAP3, ENTPD2, RFX2, and UNC45B) also have differential expression between drug-naïve patients/controls. The 6 schizophrenia-related genes that we found in our previous study case/control (Sainz et al., 2013) represent a large fraction (35.29%) of the 17 genes that we found with differential expression after medication with antipsychotics. This fraction is significantly higher (approximately 39-fold) than the one expected by chance (Chi test, p-value = 1.19E-50). All six genes that we previously implicated with schizophrenia have their expression levels significantly higher in patients than in controls and, after medication with atypical antipsychotics, their expression reverted to control levels. Normalized data that reflects this reversion is depicted in Figure 1. These results support the theory that the expression of these six genes could be modulated by the drugs and the possibility that they are implicated in the positive symptoms of the disease (hallucinations, delusions, positive formal thought disorders, bizarre behavior) that are improved by the antipsychotics.
Table 4.
Genes with Significant Differential Expression in Schizophrenia Patients Before and After Treatment with Antipsychotics
| Gene ID | Gene symbol | Base mean | Base mean untreated | Base mean treated | Fold change | Log2 fold change | Pval | Padj |
|---|---|---|---|---|---|---|---|---|
| 9509 | ADAMTS2 | 23.86 | 43.58 | 4.14 | 0.10 | -3.39 | 1.93E-45 | 3.77E-41 |
| 57126 | CD177 | 125.21 | 182.64 | 67.79 | 0.37 | -1.43 | 4.16E-15 | 4.06E-11 |
| 146862 | UNC45B | 228.32 | 355.59 | 101.05 | 0.28 | -1.82 | 9.36E-13 | 6.09E-09 |
| 10321 | CRISP3 | 115.80 | 67.59 | 164.01 | 2.43 | 1.28 | 4.87E-09 | 2.38E-05 |
| 85495 | RPPH1 | 26.05 | 39.65 | 12.45 | 0.31 | -1.67 | 3.21E-07 | 0.00125 |
| 249 | ALPL | 4431.75 | 5880.32 | 2983.17 | 0.51 | -0.98 | 1.49E-06 | 0.00345 |
| 4317 | MMP8 | 183.67 | 123.53 | 243.80 | 1.97 | 0.98 | 1.80E-06 | 0.00345 |
| 4973 | OLR1 | 33.13 | 20.86 | 45.40 | 2.18 | 1.12 | 1.41E-06 | 0.00345 |
| 10562 | OLFM4 | 137.50 | 93.08 | 181.92 | 1.95 | 0.97 | 1.94E-06 | 0.00345 |
| 1088 | CEACAM8 | 187.21 | 127.35 | 247.08 | 1.94 | 0.96 | 1.60E-06 | 0.00345 |
| 154664 | ABCA13 | 92.18 | 61.89 | 122.47 | 1.98 | 0.98 | 1.37E-06 | 0.00345 |
| 5990 | RFX2 | 520.36 | 681.17 | 359.54 | 0.53 | -0.92 | 7.00E-06 | 0.01051 |
| 4057 | LTF | 1632.35 | 1170.32 | 2094.38 | 1.79 | 0.84 | 6.51E-06 | 0.01051 |
| 2852 | GPER1 | 28.34 | 36.77 | 19.90 | 0.54 | -0.89 | 1.01E-05 | 0.01408 |
| 79937 | CNTNAP3 | 195.23 | 249.03 | 141.42 | 0.57 | -0.82 | 1.68E-05 | 0.02189 |
| 954 | ENTPD2 | 29.04 | 36.51 | 21.57 | 0.59 | -0.76 | 2.52E-05 | 0.03069 |
| 387755 | INSC | 31.74 | 41.60 | 21.87 | 0.53 | -0.93 | 3.83E-05 | 0.04395 |
Base mean: mean normalized counts, averaged over all samples from both conditions; base mean untreated: mean normalized counts from condition A; base mean treated: mean normalized counts from condition B; fold change: fold change from condition A to B; log2 fold change: the logarithm, to basis 2, of the fold change; Pval: p-value for the statistical significance of this change; Padj: p-value adjusted for multiple testing with the Benjamini-Hochberg procedure, which controls false discovery rate.
Figure 1.
Schizophrenia differential expression genes reverted by antipsychotic medications. Base mean: mean of normalized counts, averaged over all samples for each condition; controls: normalized expression level of controls; untreated cases: normalized expression level of untreated patients; treated cases: normalized expression level from the patients, obtained after 3 months of treatment.
To determine if the 17 genes with altered expression by antipsychotics were enriched for certain biological processes, molecular functions, or cellular localizations, data from the Gene Ontology Repository was analyzed using the tool FatiGO (http://babelomics.bioinfo.cipf.es/). We found enrichment for the cellular components—extracellular matrix and proteinaceous extracellular matrix—which are involved in many functions, such as segregating tissues from one another and regulating intercellular communication. The fraction of genes modulated by antipsychotics in both categories represents 23.53%, while expected fractions are 1.49% and 1.59% respectively (Padj value = 0.015 for both categories). The four genes included in these categories are ENTPD2, MMP8, ADAMTS2, and CRISP3. Gene Set Enrichment Analysis did not show any pathway enriched for the 17 genes with differential expression.
Eight genes out of the 17 (LTF, OLFM4, ADAMTS2, RFX2, ALPL, MMP8, ABCA13, and INSC) appear to associate, either as reported or as mapped gene, with 24 diseases/traits in the Genome Wide Association Studies Catalog (GWAS; retrieved February 28, 2014). The association with diseases includes schizophrenia and bipolar disorder (RFX2; Wang et al., 2010), time to onset of attention deficit hyperactivity disorder (ADAMTS2; Lasky-Su et al., 2008), response to amphetamine (ABCA13; Hart et al., 2012), AIDS progression (LTF; Troyer et al., 2011), metabolic traits (ALPL; Suhre et al., 2011), obesity (OLFM4; Bradfield et al., 2012), obesity-related traits (INSC; Comuzzie et al., 2012), and visceral adipose tissue adjusted for body mass index (ADAMTS2; Fox et al., 2012), among other disease-traits. The three genes associated with obesity represent a fraction (3 out of 8 or 37.5%) larger than the fraction of genes present in the complete GWAS Catalog (15.5%).
According to the scientific literature deposited in the Gene Reference into Function (GeneRIF) repository at the National Center for Biotechnology Information (retrieved February 28, 2014), 15 out of the 17 differential expression genes are annotated for disease or functionality in 719 publications. Among these genes, ABCA13 has been implicated in schizophrenia, bipolar disorder, and depression (Knight et al., 2009). The 15 differential-expression genes annotated are significantly enriched for two known adverse effects caused by the atypical antipsychotics: obesity and diabetes. Five genes—GPER (Haas et al., 2009), LTF (Moreno-Navarrete et al., 2013), MMP8 (Belo et al., 2009), OLR1 (Brinkley et al., 2008), and OLFM4 (Bradfield et al., 2012)—have been implicated with obesity: these genes represent 33.33% of the differential-expression genes, while in the complete GeneRIF repository there are only 3.99% of the human genes annotated for obesity (Fisher; p = 0.0002). Four of the genes—ALPL (Kanazawa et al., 2009), LTF (Vengen et al., 2010), MMP8 (van der Zijl et al., 2010), and OLR1 (Tan et al., 2008)—have been implicated with diabetes: these genes represent 26.67% of the differential-expression genes, while 7.88% (Fisher; p = 0.026) is expected. Moreover, it is known that all antipsychotics tend to block receptors in the brain’s dopamine pathways, and that estrogen increases the concentration of neurotransmitters such as dopamine at neuronal synapses and their receptors, affecting their release, reuptake, and enzymatic inactivation (Mcewen and Alves, 1999). We found a significant enrichment of genes related to estrogen, including an estrogen receptor: GPER (Revankar et al., 2005), LTF (Teng et al., 2002), and OLFM4 (Dassen et al., 2010) represent a fraction of 20.00% of the total, while we expect to find 4.3% (Fisher; p = 0.026). Previously, we described an alteration of inflammatory genes in schizophrenia (Sainz et al., 2013): we also find in GeneRIF an enrichment of inflammatory genes among the genes with their expression modulated by the atypical antipsychotics. There are six genes—ALPL (Cheung et al., 2008), GPER (Chakrabarti and Davidge, 2012), LTF (Weinberg, 2001), MMP8 (Leppilahti et al., 2011), OLR1 (Takanabe-Mori et al., 2013) and CRISP3 (Plager et al., 2010)—related to inflammation, representing 40.00%, while only 10.97% is expected (Fisher; p = 0.005). These results are encouraging, though we recognize that it is really difficult to assess enrichment for such a small number of genes.
Discussion
We characterized 17 genes with differential expression in schizophrenia patients before and after medication. Previously, we characterized 200 genes with differential expression between non-medicated schizophrenia patients and controls. Six of the genes with expression modulated by the atypical antipsychotic medication also have altered expression in our case/control study of schizophrenia. The six genes with altered expression in both studies are overexpressed in schizophrenia and reverted to normal levels of expression after medication with atypical antipsychotics. The fact that the expression of these six genes is downregulated by antipsychotics and that they are overexpressed in schizophrenia is not proof of causality, but does make them candidates to have a relationship with some of the psychotic symptoms of the disease. It is known that the positive symptoms of the disease, such as hallucinations, delusions, and mood swings, are improved by the antipsychotics, resulting in a lower risk of suicide, better functional capacity, and an improved quality of life (Kapur and Remington, 2001). Given that more than 95% of the individuals had a good response to medication, we can hypothesize that the six genes reported are related functionally to these positive symptoms of psychosis. It is of note that nearly 95% of the patients were categorized as good responders to antipsychotic treatment. It is likely that the method of calculation we followed to assess the reduction of symptom severity may explain, at least in part, the high response. As has been proposed recently (Leucht et al., 2010; Obermeier et al., 2010), the calculation of responder rates based on percentage reduction of the BPRS total score from baseline has to be carried out after subtracting the 24 minimum points of the total score (24 points means no symptoms). Previous publications, in which the percentage reduction has not been correctly calculated, would have found lower numbers of responders. In addition, the fact that our patients were included in an intensive clinical program for treatment of first-episode individuals may have influenced the high rate of responders. All of the 17 genes characterized with expression significantly altered by the atypical antipsychotics are good candidates to be responsible for some of the adverse effects associated with the drugs: significant weight gain, increased risk of diabetes and stroke, blood clots, sexual dysfunction, and hyperlipidemia (Ucok and Gaebel, 2008). Our analysis of the results, with the caveat that it is based on a small number of genes, supports this hypothesis: according to the scientific literature, there is a significant enrichment of genes related to diabetes and obesity among the differential-expression genes. We also found an enrichment of genes associated to obesity or related traits according to the data deposited in the GWAS Catalog. This suggests that some of the genes with expressions altered by the atypical antipsychotics could be causative of obesity and diabetes. Additionally, the genes characterized with altered expressions are consistent with the known molecular mechanisms of the antipsychotic action: we find that there is a significant enrichment of genes related to estrogen that regulate the levels of dopamine receptors, and it is known that antipsychotics interact with receptors in the brain’s dopamine pathways (Seeman, 2002).
We recognize that this study has the limitation of a small sample size and that a larger study is needed. But despite this limitation, we were able to obtain significant results, which could help to predict the effects of the atypical antipsychotics currently used in the clinical practice by monitoring the expression levels of the reported genes. We provide six candidate genes for schizophrenia that could be involved in the positive symptoms improved by the drugs, and a list of 17 genes that could be responsible for some of their adverse effects, such as obesity and diabetes. This information could help us not only to better understand schizophrenia, but also to develop new drugs and to improve the ones used today against psychosis.
Supplementary Material
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S00000000000000
Statement of Interest
The authors declare no conflicts of interest in connection with the article.
Acknowledgments
We would like to thank Noemi De la Fuente, Ana Berja, and Ines Santiuste for their help in the recruitment and preparation of the samples and the PAFIP crew for their help with data collection. We thank HUMV-IFIMAV biobank for providing the biological samples and associated data. We wish also to thank the participants and their families for enrolling in this study. Bioinformatics work was partially performed using the Altamira supercomputer (Spanish Supercomputing Network). This work was supported by the Spanish Ministry of Economy and Competitiveness (Proyects SAF2010-20840-C02-01 and SAF2010-20840-C02-02), Fundación Leonardo Torres Quevedo-Universidad de Cantabria (Exp.: FLTQ-2010), Foundation Ramón Areces, and Instituto de Investigación Marqués de Valdecilla (Exp.: AIP2011-02).
References
- Anders S, Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol 11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo VA, Souza-Costa DC, Lana CM, Caputo FL, Marcaccini AM, Gerlach RF, Bastos MG, Tanus-Santos JE. (2009). Assessment of matrix metalloproteinase (MMP)-2, MMP-8, MMP-9, and their inhibitors, the tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in obese children and adolescents. Clin Biochem 42:984–990. [DOI] [PubMed] [Google Scholar]
- Bradfield JP, et al. (2012). A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet 44:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. (2008). Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring) 16:1454–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, Butte NF. (2012). Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PlOS ONE 7:e51954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak C, Lehofer M, Renger H, Wagner EM, Lemonis L, Rohrhofer A, Schauenstein K, Liebmann PM. (2004). Dopamine receptor D3 mRNA expression in human lymphocytes is negatively correlated with the personality trait of persistence. J Neuroimmunol 150:145–149. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Davidge ST. (2012). G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PlOS ONE 7:e52357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Bousman CA, Money TT, Gibbons A, Gillett P, Dean B, Everall IP. (2013). Biomarker investigations related to pathophysiological pathways in schizophrenia and psychosis. Front Cell Neurosci 7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BM, Ong KL, Cheung RV, Wong LY, Wat NM, Tam S, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, Lam KS. (2008). Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med 46:523–527. [DOI] [PubMed] [Google Scholar]
- Dassen H, Punyadeera C, Delvoux B, Schulkens I, Marchetti C, Kamps R, Klomp J, Dijcks F, de Goeij A, D’Hooghe T, Kyama C, Ederveen A, Dunselman G, Groothuis P, Romano A. (2010). Olfactomedin-4 regulation by estrogen in the human endometrium requires epidermal growth factor signaling. Am J Pathol 177:2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, et al. (2012). Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLOS Genet 8:e1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sasik R, Khanlou N, Han M, Liew CC, Tsuang MT. (2005). Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci USA 102:15533–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. (2009). Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Engelhardt BE, Wardle MC, Sokoloff G, Stephens M, de Wit H, Palmer AA. (2012). Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13). PlOS ONE 7:e42646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. (2010). Rethinking schizophrenia. Nature 468:187–193. [DOI] [PubMed] [Google Scholar]
- Jones HM, Pilowsky LS. (2002). Dopamine and antipsychotic drug action revisited. Br J Psychiatry 181:271–275. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. (2009). Serum osteocalcin/bone-specific alkaline phosphatase ratio is a predictor for the presence of vertebral fractures in men with type 2 diabetes. Calcif Tissue Int 85:228–234. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. (2001). Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med 52:503–517. [DOI] [PubMed] [Google Scholar]
- Knight HM, Pickard BS, Maclean A, Malloy MP, Soares DC, McRae AF, Condie A, White A, Hawkins W, McGhee K, van Beck M, MacIntyre DJ, Starr JM, Deary IJ, Visscher PM, Porteous DJ, Cannon RE, St Clair D, Muir WJ, Blackwood DH. (2009). A cytogenetic abnormality and rare coding variants identify ABCA13 as a candidate gene in schizophrenia, bipolar disorder, and depression. Am J Hum Genet 85:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky-Su J, et al. (2008). Genome-wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 147B:1355–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppilahti JM, Ahonen MM, Hernandez M, Munjal S, Netuschil L, Uitto VJ, Sorsa T, Mantyla P. (2011). Oral rinse MMP-8 point-of-care immuno test identifies patients with strong periodontal inflammatory burden. Oral Dis 17:115–122. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. (2010). The PANSS should be rescaled. Schizophr Bull 36:461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. (1999). Estrogen actions in the central nervous system. Endocr Rev 20:279–307. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Serrano M, Sabater M, Ortega F, Serino M, Pueyo N, Luche E, Waget A, Rodriguez-Hermosa JI, Ricart W, Burcelin R, Fernandez-Real JM. (2013). Study of lactoferrin gene expression in human and mouse adipose tissue, human preadipocytes and mouse 3T3-L1 fibroblasts. Association with adipogenic and inflammatory markers. J Nutr Biochem 24:1266–1275. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dieter MZ. (2000). The evolution of drug metabolism. Pharmacology 61:124–135. [DOI] [PubMed] [Google Scholar]
- Obermeier M, Mayr A, Schennach-Wolff R, Seemuller F, Moller HJ, Riedel M. (2010). Should the PANSS be rescaled? Schizophr Bull 36:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo-Teran JM, Perez-Iglesias R, Ramirez-Bonilla M, Gonzalez-Blanch C, Martinez-Garcia O, Pardo-Garcia G, Rodriguez-Sanchez JM, Roiz-Santianez R, Tordesillas-Gutierrez D, Mata I, Vazquez-Barquero JL, Crespo-Facorro B. (2008). Epidemiological factors associated with treated incidence of first-episode non-affective psychosis in Cantabria: insights from the Clinical Programme on Early Phases of Psychosis. Early Interv Psychiatry 2:178–187. [DOI] [PubMed] [Google Scholar]
- Plager DA, Kahl JC, Asmann YW, Nilson AE, Pallanch JF, Friedman O, Kita H. (2010). Gene transcription changes in asthmatic chronic rhinosinusitis with nasal polyps and comparison to those in atopic dermatitis. PlOS ONE 5:e11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630. [DOI] [PubMed] [Google Scholar]
- Sainz J, Mata I, Barrera J, Perez-Iglesias R, Varela I, Arranz MJ, Rodriguez MC, Crespo-Facorro B. (2013). Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry 18:1056–1057. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2002). Atypical antipsychotics: mechanism of action. Can J Psychiatry 47:27–38. [PubMed] [Google Scholar]
- Suhre K, et al. (2011). Human metabolic individuality in biomedical and pharmaceutical research. Nature 477:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanabe-Mori R, Ono K, Wada H, Takaya T, Ura S, Yamakage H, Satoh-Asahara N, Shimatsu A, Takahashi Y, Fujita M, Fujita Y, Sawamura T, Hasegawa K. (2013). Lectin-like oxidized low-density lipoprotein receptor-1 plays an important role in vascular inflammation in current smokers. J Atheroscler Thromb 20:585–590. [DOI] [PubMed] [Google Scholar]
- Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. (2008). Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J Lipid Res 49:1438–1444. [DOI] [PubMed] [Google Scholar]
- Teng CT, Gladwell W, Beard C, Walmer D, Teng CS, Brenner R. (2002). Lactoferrin gene expression is estrogen responsive in human and rhesus monkey endometrium. Mol Hum Reprod 8:58–67. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer JL, et al. (2011). Genome-wide association study implicates PARD3B-based AIDS restriction. J Infect Dis 203:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucok A, Gaebel W. (2008). Side effects of atypical antipsychotics: a brief overview. World Psychiatry 7:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zijl NJ, Hanemaaijer R, Tushuizen ME, Schindhelm RK, Boerop J, Rustemeijer C, Bilo HJ, Verheijen JH, Diamant M. (2010). Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin Biochem 43:635–639. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. (2009). Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- Vengen IT, Dale AC, Wiseth R, Midthjell K, Videm V. (2010). Lactoferrin is a novel predictor of fatal ischemic heart disease in diabetes mellitus type 2: long-term follow-up of the HUNT 1 study. Atherosclerosis 212:614–620. [DOI] [PubMed] [Google Scholar]
- Wang KS, Liu XF, Aragam N. (2010). A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr Res 124:192–199. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. (2001). Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmacol 53:1303–1310. [DOI] [PubMed] [Google Scholar]