Abstract
Background:
Galanin (GAL) plays a role in mood regulation. In this study we analyzed the action of the active N-terminal fragment [GAL(1–15)] in anxiety- and depression-related behavioral tests in rats.
Methods:
The effect of GAL(1–15) was analyzed in the forced swimming test, tail suspension test, open field test, and light/dark test. The proximity of GAL1 and GAL2 receptors was examined with the proximity ligation assay (PLA). We tested the GAL receptors involved in GAL(1–15) effects with the GAL2 receptor antagonist M871 and with an in vivo model of siRNA GAL2 receptor knockdown or siRNA GAL1 receptor knockdown rats. The effects of GAL(1–15) were also studied in the cell line RN33B.
Results:
GAL(1–15) induced strong depression-like and anxiogenic-like effects in all the tests. These effects were stronger than the ones induced by GAL. The involvement of the GAL2 receptor was demonstrated with M871 and with the siRNA GAL2 receptor knockdown rats. The PLA indicated the possible existence of GAL1 and GAL2 heteroreceptor complexes in the dorsal hippocampus and especially in the dorsal raphe nucleus. In the siRNA GAL1 receptor knockdown rats the behavioral actions of GAL(1–15) disappeared, and in the siRNA GAL2 receptor knockdown rats the reductions of the behavioral actions of GAL(1–15) was linked to a disappearance of PLA. In the cell line RN33B, GAL(1–15) decreased 5-HT immunoreactivity more strongly than GAL.
Conclusions:
Our results indicate that GAL(1–15) exerts strong depression-related and anxiogenic-like effects and may give the basis for the development of drugs targeting GAL1 and GAL2 heteroreceptor complexes in the raphe-limbic system for the treatment of depression and anxiety.
Keywords: anxiety, depression, galanin
Introduction
Mood disorders, including depression and anxiety, are among the most prevalent mental illnesses, with high socioeconomic impacts (Gelenberg, 2010; Wittchen et al., 2011). According to the World Health Organization, between 10% and 15% of the general population will experience a clinical depressive episode in their lifetime (Wittchen et al., 2011). Although the underlying mechanisms have not yet been clearly defined in the last decade, the importance of the role of neuropeptides, including galanin, and/or their receptors in the treatment of stress-related mood disorders is becoming increasingly apparent (Kormos and Gaszner, 2013).
Galanin (GAL) is a neuropeptide (Tatemoto et al., 1983) widely distributed in neurons within the central nervous system (CNS; Jacobowitz et al., 2004). Three GAL receptor (GAL1–3 receptors) subtypes with high affinities for GAL have been cloned (Branchek et al., 2000; Mitsukawa et al., 2008). GAL1 and GAL2 receptors are found in many regions of the CNS, as demonstrated with in situ hybridization, radioligand binding, and immunohistochemical studies (Jacobowitz et al., 2004). GAL1 and GAL3 receptors mainly activate inhibitory G proteins Gi/Go, while the GAL2 receptor mainly couples to Gq/G11 to mediate excitatory signaling (Wang et al., 1997; Branchek et al., 2000)
The GAL1–3 receptors are involved in a number of central functions modulating neuroendocrine levels, pain control, cardiovascular functions, addiction, and food intake (Mitsukawa et al., 2008; Diaz-Cabiale et al., 2010; Picciotto, 2010). GAL is also involved in mood regulation, including depression-related and anxiety-like behaviors (Juhasz et al., 2014). GAL-overexpressing mice and rodents in which GAL was infused either intraventricularly (i.c.v) or into the ventral tegmental area showed an increase in immobility during the forced swimming test (FST), indicative of depression-like behavior (Weiss et al., 1998; Kuteeva et al., 2005, 2007). Also, in a genetic rat model of depression, the Flinders Sensitive Line, an up-regulation of the GAL receptor binding sites, is found in the dorsal raphe nucleus and linked to high immobility in the FST (Bellido et al., 2002). However, intraperitoneal (i.p.) injections of GAL2 receptor agonists exhibit anti-depressant–like effects, and two non-selective GALR agonists, galnon and galmic, decreased immobility time in the FST (Bartfai et al., 2004; Lu et al., 2005). These discrepancies may be attributed to the different physiological roles of the GAL receptor subtypes. Intracerebroventricular (i.c.v.) infusion of a GAL receptor ligand agonist with higher specificity for GAL1 receptor (M617) or a GAL2 receptor antagonist (M871) elevated immobility time, while the GAL2/GAL3 receptors agonist (AR-M1896) produced an antidepressant-like effect in the rat FST (Kuteeva et al., 2008). Furthermore, administration of GAL3 receptor-selective antagonists to rats and mice produced antidepressant-like effects in both FST and the tail suspension test (TST; Swanson et al., 2005; Barr et al., 2006).
In view of these results, it has been proposed that activation of GAL1 and GAL3 receptors results in a depression-like behavior while stimulation of the GAL2 receptor leads to anti-depressant–like effects.
The role of GAL in anxiety-like behaviors depends on the route and site of drug administration, and also on the intensity of stress conditions (Holmes et al., 2003; Holmes and Picciotto, 2006). I.c.v GAL produced an anxiolytic-like effect in rats in the Vogel conflict test (Bing et al., 1993), whereas intra-amygdala microinjection induced the opposite effect on the same task (Moller et al., 1999). The varying effects of GAL on anxiety-related behaviors may also depend on the differentially-distributed GAL receptor subtypes (Holmes and Picciotto, 2006; Bailey et al., 2007; Brunner et al., 2014). GAL1 receptor knockout mice exhibit increased anxiety in the elevated plus-maze (Holmes et al., 2003), while GAL2 receptor knockout mice show anxiety-like behavior or no effect, depending on the genetic background of the mutants (Bailey et al., 2007; Lu et al., 2008). In contrast, an antagonist of GAL3 receptor produces anxiolytic-like effects in several behavioral tests (Swanson et al., 2005).
Not only GAL but also the galanin N-terminal fragments like GAL(1–15) are active in the CNS. Both GAL and GAL(1–15) molecules have specific roles in cardiovascular regulation and interact differently with other neuropeptides (Diaz-Cabiale et al., 2005, 2007). The three cloned receptors show a higher affinity for GAL than for GAL(1–15) (Branchek et al., 2000), but the demonstration of specific GAL(1–15) binding sites in the rat brain emphasizes the powerful role of GAL fragments in GAL communication, especially in the dorsal hippocampus, neocortex, and striatum (Hedlund et al., 1992). Only GAL(1–15), but not GAL, can antagonistically modulate the serotonin 5-HT1A receptors in the dorsal hippocampus, and this effect was blocked by the GAL receptor antagonist M35 (Hedlund et al., 1994). In the ventral limbic cortex, N-terminal GAL fragments can more strongly and more potently reduce postjunctional 5-HT1A receptor recognition than GAL, where high-affinity GAL receptors also exist (Diaz-Cabiale et al., 2000).
The purpose of the present study was to assess the role of GAL(1–15) in mood regulation using anxiety- and depression-related behavioral test in rats. Additionally, we used the proximity ligation assay (Trifilieff et al., 2011; Borroto-Escuela et al., 2013) to analyze the proximity of GAL1 and GAL2 receptors in the dorsal hippocampus and dorsal raphe nuclei showing specific GAL(1–15) binding (Hedlund et al., 1992) since GAL1 and GAL2 heteroreceptor complexes may exist in areas selectively enriched in GAL(1–15) binding sites (Fuxe et al., 2008, 2012). Therefore, we tested the involvement of the GAL2 receptor in the GAL(1–15) effect with the selective GAL2 receptor antagonist M871 and with an in vivo model of siRNA GAL2 receptor knockdown rats. The role of the GAL1 receptor in the behavioral actions of GAL(1–15) were also analyzed in siRNA GAL1 receptor knockdown rats. The effects of GAL(1–15) versus GAL were analyzed on 5-HT immunoreactivity (IR) in the rat medullary raphe-derived cell line RN33B to compare the actions of these two GAL peptides on 5-HT mechanisms on the RN33B cells, which may be of relevance for their actions on the dorsal raphe 5-HT nerve cells.
Materials and methods
Animals
Male Sprague Dawley rats (body weight 225–250g, age 8 weeks) were obtained from Criffa and maintained in a humidity- and temperature-controlled (20–22ºC) room under a 12:12h light/dark cycle (lights on 08:00 and off 20:00h). The animals had free access to food pellets and tap water. All animal experimentation was conducted in accordance with the University of Málaga Guidelines for the Care and Use of Laboratory Animals.
Intracerebroventricular Injections
This protocol has been used previously (Parrado et al., 2007; Diaz-Cabiale et al., 2011). Briefly, the rats were anesthetized intraperitoneally with Equitesin (3.3mL/kg body weight), and stereotaxically implanted with a unilateral chronic 22-gauge stainless-steel guide cannula into the right lateral cerebral ventricle using the following coordinates: 1.4mm lateral and 1mm posterior to the bregma, and 3.6mm below the surface of the skull (Paxinos, 1986). After surgery, animals were individually housed and allowed a recovery period of 7 days. The injections in the lateral ventricle were performed using a 26-gauge stainless-steel injection cannula connected via a PE-10 tubing to a Hamilton syringe. The total volume was 5 μl per injection and the infusion time was 1min.
Solutions were prepared freshly and the peptides were dissolved in artificial cerebrospinal fluid (composition is 120nM NaCl, 20nM NaH2CO3, 2nM KCl, 0.5nM KH2PO4, 1.2nM CaCl2, 1.8nM MgCl2, 0.5nM Na2SO4, and 5.8nM D-glucose, pH 7.4). GAL was obtained from NeoMPS; GAL(1–15) and the GAL2 receptor antagonist M871 were obtained from Tocris Bioscience.
Behavioral Assessment
Groups of rats were assessed in two behavioral tests: the open field and the forced swim test. Rats were adapted to handling and were taken into the experimental room (80–90 lux) to habituate for at least 1 hour before the peptides administration. All tests were performed between 08:00 and 14:00h.
Three sets of experiments were conducted. In the first set of experiments, a dose-response curve of GAL(1–15) was performed. For this, groups of rats received i.c.v GAL(1–15) 1 nmol, 3 nmol, 6 nmol, or vehicle 15min before the test. In the second set of experiments, the effects in the behavioral test of GAL and GAL(1–15) were compared; for this, groups of rats received i.c.v GAL 3 nmol, GAL(1–15) 3 nmol, or vehicle 15min before the test. In the last set of experiments, the role of the GAL2 receptor was studied; for this, groups of rats received i.c.v GAL(1–15) 3 nmol combined with GAL2 receptor antagonist M871 3 nmol 15min before the test.
In a second group of experiments, GAL(1–15) at the effective dose obtained in the experiments described above was assessed in two more behavioral tests: the light/dark test and the tail suspension test.
Forced Swimming Test
Animals were individually placed in a vertical glass cylinder (50cm height, 20cm diameter) containing water (25ºC) to a height of 30cm. Two swimming sessions were conducted: a 15min pre-test followed 24h later by a 5min test (Kuteeva et al., 2007). The total duration of immobility behavior and climbing were recorded during the second 5min. Immobility was defined as floating passively in an upright position in water with only small movements necessary to keep the head above the water surface. Climbing was defined as forepaw movements directed toward the walls of the cylinder.
Open Field Test
Rats were individually placed and allowed to freely explore; their behavior was recorded over a 5min period by a ceiling-mounted video camera. Their activity was analyzed using the video-tracking software EthovisionXT. After each trial, all surfaces were cleaned with a paper towel and 70% ethanol solution. For the open-field (100 x 100 x 50cm), total time spent in and entries into the inner square were recorded (Grivas et al., 2013; Narvaez et al., 2014).
Light/Dark Test
The light–dark box apparatus consisted of two chambers (26 x 25 x 27cm) joined together lengthwise. One chamber was black and the other was white. The white chamber was illuminated by a 40W light bulb. Animals were individually placed in the center of the light box and were allowed to freely explore during 5 minutes. The latency to enter (with all four paws) the dark compartment, time spent in the light chamber, and the latency time for re-entering the light box were recorded (Grivas et al., 2013; Desikan et al., 2014).
Tail Suspension Test
In this test (Hinojosa et al., 2006), the rat was hung upside down using an adhesive tape to fix its tail to a rope through an eyebolt at 60cm. The animal was considered immobile when it was not making any movements of struggling, attempting to catch the adhesive tape, body torsions, or jerks (Hinojosa et al., 2006; Yan et al., 2014).
Proximity Ligation In Situ Assay (Duolink)
Proximity ligation assay (PLA) and quantification were carried out as described previously using a Duolink in situ PLA detection kit (Borroto-Escuela et al., 2012, 2013). The primary antibodies of different species directed to the GAL1 receptor (goat polyclonal, Santa Cruz Biotechnology Inc., EEUU, 1:250) and GAL2 receptor (rabbit polyclonal, Alomone Lab, 1/250) were used. Sections were mounted on slides with fluorescent mounting medium containing 4’, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), staining the nuclei with blue color. The in situ PLA positive signals were visualized using a Leica SP5 confocal microscope and the Duolink Image Tool software.
For in situ PLA in raphe-derived RN33B cells used the same primary antibodies and the same procedure.
Double Immunofluorescence
The procedures have been previously used (Narváez et al., 2014) . Primary antibody rabbit anti-GAL2 receptor (Alomone Lab, 1/250) was incubated for 12 hours at 4ºC and detected with the red secondary antibody mouse anti-rabbit DyLight 549 (Jackson inmunoResearch Laboratories, 1/100). Goat anti-GAL1 receptor (Santa Cruz Biotechnology INC, EEUU, 1:250) was incubated 12 hours at 4ºC and detected with the secondary antibody rabbit biotynilated anti-goat (Vector Labs Inc.) and Alexa Fluor 488-conjugated Streptavidin (Jackson Laboratories InmunoResearch, 1:1000). Sections were mounted on slides with fluorescent mounting medium (Dako) and visualized using a Leica TCS-SL confocal microscope. The double immunolabeling was performed in the tissue of rats without treatment (Figure S1) or in knockdown rats of GAL2 receptor (Figure S4).
Knockdown Rats of GAL2 Receptor and GAL1 Receptor Animals and Treatment
For knockdown rats, animals were operated with the same procedure as previously described (Nakajima et al., 2012). When the cannula was implanted, rats were intracerebroventricularly injected with 5 μg (0.35 nmol) of Accell Smart pool siRNA GAL2 receptor or siRNA GAL1 receptor (Dharmacon) or 5 μl of vehicle (Acell siRNA Delivery Media). Also, some animals were injected with siRNA-Control (Acell non-targeting pool, Dharmacon) to verify that the Acell system has no effect on animals. Previous to injection, the siRNAs were previously resuspended in 1X siRNA buffer (Dharmacon B-002000-UB-100), aliquotted, and stored in -80º, following the manufacturer’s instructions. For the administration of siRNAs an aliquot was diluted in Acell siRNA Delivery Media (Dharmacon B-005000-100) at a concentration of 1 μg/μl (0.07 nmol/μl).
Time-course curve was performed and GAL2 receptor protein expression was examined via immunohistochemical analysis (Figure S2) and the GAL2 receptor and GAL1 receptor mRNA reduction was examined with real-time quantitative polymerase chain reaction (RT-PCR; Figures S3 and S5).
These knockdown rats were used in the open field test (7 days after the Accell Smart pool siRNA GAL2 receptor injection or siRNA GAL1 receptor injection) and in the forced swimming test (9 days after the Accell Smart pool siRNA GAL2 receptor injection or siRNA GAL1 receptor injection) either alone or after receiving i.c.v. GAL(1–15) 3 nmol or vehicle 15min before the test. The brain tissues of the knockdown rats of GAL2 receptor were used for in situ PLA and double inmunofluorecence.
Immunohistochemical
At 4, 6, 8, 10, 12, and 14 days after injection of siRNA GAL2 receptor, two rats per day were perfused transcardially. The brains were then dissected and serial, coronal free-floating sections (30 μm thick) were collected. The control group received an injection of Accel siRNA Delivery Media (vehicle). The sections were incubated with a primary antibody rabbit anti-GAL2 receptor (Alomone Lab, 1/250) overnight at 4ºC. An hour incubation with biotinylated, specific secondary antibodies (1:200; Vector Labs Inc) was performed at room temperature. The chromogen used was 0.03% 3-30-diaminobenzidine tetrahydrochloride (Sigma).
Analysis of GAL2 receptor IR was performed under light microscopy (Nikon Optiphot-2) in the neuro-anatomical area of interest. Four sections of two animals per day were examined and captured by a video camera (Olimpus UC30) linked to a PC computer, and GAL2 receptor IR was quantified by optical density using the computer software ImageJ (National Institutes of Health).
RT-PCR
At 4, 6, 8, and 14 days after injection of siRNA GAL2 receptor or siRNA GAL1 receptor, 4 animals treated with siRNA GAL2 receptor or siRNA GAL1 receptor were killed by decapitation. Four animals injected with vehicle followed the same process as controls.
The hippocampi of all animals were quickly extracted and frozen until use. Total RNA was isolated from the dorsal hippocampus using RNeasy Lipid Tissue Kit (Qiagen), followed by treatment to remove genomic DNA with Recombinant DNase I (RNase-free) (Takara biotechnology Co., TLD). cDNA was obtained using a Reverse Transcriptase Core kit (Eurogentec). These three steps were performed according to the manufacturer’s instructions.
All PCR were conducted in triplicate using Power SYBR Green PCR Master Mix (Applied Biosystems) in a 7500 RT-PCR system (Applied Biosystems). The primer sequences used in this study are: GAPDH-Forward: 5′-GCTCTCTGCTCCTCCCTGTTC; GAPDH-Reverse: 5′-GAGGCTGGCACTGCACAA; GAL2 RECEPTOR-Forward: 5′-AACAGGAATCCACAGACC; GAL2 RECEPTOR-Reverse: 5′-CCCTTTGGTCCTTTAACAAG; GAL1 RECEPTOR-Forward: 5′-AAAACTGGACAAAACTTAGCC; and GAL1 RECEPTOR-Reverse: 5′-GGATACCTTTGTCTTTGCTC. The data were analyzed using the comparative Ct method and normalized to measures of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Cell Culture and Immunocytochemistry
RN33B cells (a CNS raphe-derived neuronal precursor cell line; Lundberg et al., 2002; American Type Culture Collection) were grown in DMEM/F12 supplemented with 2mM L-glutamine, 100U/ml penicillin/streptomycin, and 10% (v/v) fetal bovine serum at 37°C in an atmosphere of 5% CO2. RN33B cells were treated for 1h with GAL 100nM, GAL(1–15) 100nM, or control, followed for 1h by recuperation. Cells were stained with rabbit anti-5-HT monoclonal antibody (1/5000; Sigma-Aldrich). The secondary antibody used was Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:2000; Invitrogen). 5-HT immunoreactivity semiquantification is expressed as the 5-HT immunoreactivity of 30 positive cells per condition for each experiment; two independent experiments were performed. The application settings were adjusted at the beginning of analysis and kept the same for all images in the experiment.
Statistical Analysis
Data are presented as the means ± standard error of the mean and sample numbers (n) are indicated in figure legends. All data were analyzed using GraphPad PRISM 4.0 (GraphPad Software).
For comparing two experimental conditions, student’s unpaired t-test statistical analyses were performed. Otherwise, one-way analyses of variance (ANOVAs) followed by Newman-Keuls comparison post-tests were performed. Differences were considered significant at p < 0.05 (*p < 0.05, ** p < 0.01, *** p < 0.001).
Results
Behavioral Studies
GAL(1–15) Induced a Depression-Related and Anxiogenic-Like Effect in the Behavioral Tests
In the FST, rats were pre-exposed to water for 15 minutes. Twenty-four hours later, their immobility and climbing during a second 5min exposure to water were analyzed as signs of depression-like behavior.
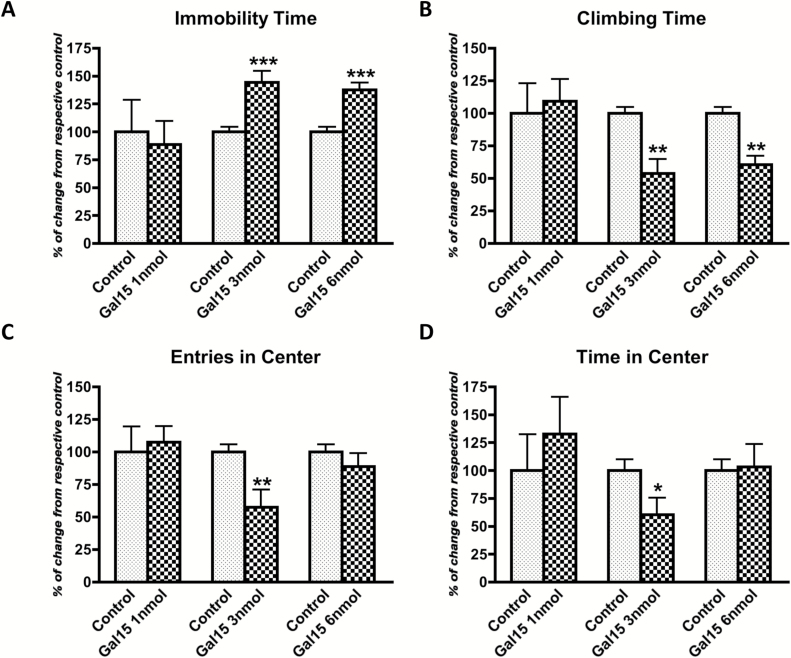
GAL(1–15) at 3 nmol significantly increased the immobility (t = 4.071, p < 0.001, df = 13) and decreased the climbing (t = 3.857, p < 0.01, df = 12) behavior by 44% and 46%, respectively (Figure 1A and B). These results indicate that GAL(1–15) evokes a strong depression-like behavior. Similar results were obtained at the dose of 6 nmol: GAL(1–15) also significantly increased the immobility (t = 4.97, p < 0.001, df = 13) and decreased the climbing (t = 4.815, p < 0.01, df = 12) behavior (Figure 1A and B).
Figure 1.
Dose-response curve of galanin(1–15) [GAL(1–15)] in the forced swimming test (A and B) and open field test (C and D) in rats. GAL(1–15) (at 1, 3, or 6 nmol/rat) was administered intracerebroventricularly 15min before the tests. Cerebrospinal fluid–injected rats were used as the control group. Vertical bars represent mean ± standard error of the mean of the percentage of change from the respective controls in the time of immobility (A) or climbing (B) and the number of entries (C) or time in the central square (D) during a 5min test period (6–12 animals per group). *p < 0.05; **p < 0.01, and ***p < 0.001 versus respective control according to student’s t-test.
In the tail suspension test, GAL(1–15) at the dose of 3 nmol significantly increased the immobility behavior recorded during the 6min of testing ((t = 2.02, p < 0.05, df = 12; Table 1)
Table 1.
Effect of Gal(1–15) at 3 nmol in the Light/Dark Test and Tail Suspension Test.
| Light/Dark Test | Tail Suspension Test | |||
|---|---|---|---|---|
| Parameters Measured | Latency to enter in dark chamber (s) | Time spent in light chamber (s) | Latency time for re-entering to light (s) | Immobility (s) |
| Control | 32.6±7.8 | 92±6.9 | 24.9±5.9 | 185.8±11.03 |
| Gal(1–15) 3nmol | 11.5±3.3* | 44.1±15.9* | 138.3±40.9* | 215±7.7* |
Group of animals (n = 5–8) were injected intracerebroventricularly with Gal(1–15) or cerebrospinal fluid as the vehicle 15min before the test. Values are indicated as mean ± standard error of the mean. *p < 0.05 vs control according to student′s t-test.
To assess the role of GAL(1–15) in the regulation of anxiety-related behaviors, the time spent in and numbers of entries into the central square were examined in the open field.
GAL(1–15) at the dose of 3 nmol induced an anxiogenic-like effect, since it significantly decreased the entries (t = 3.379, p < 0.01, df = 16; Figure 1C) and the time spent (t = 2.318, p < 0.05, df = 13; Figure 1D) in the central square in the open field by 42% and 39%, respectively. GAL(1–15) lacked effects at the doses of 1 nmol or 6 nmol (Figure 1C and D).
These anxiogenic-like effects were independent of the locomotor activity, since the total distance reached and speed were equivalent between all the groups (Supplementary Table 1).
The effects of GAL(1–15) at 3 nmol on performance in the light/dark test are described in Table 1. GAL(1–15) significantly decreased the total time spent in the light compartment (t = 2.56, p < 0.05, df = 9) and the latency to enter in the dark chamber compared with controls (t = 2.66, p < 0.05, df = 9; Table 1). Moreover, the latency time for reentering to light was significantly increased after GAL(1–15) treatment (t = 2.74, p < 0.05, df = 8; Table 1).
Comparison Between GAL and GAL(1–15)
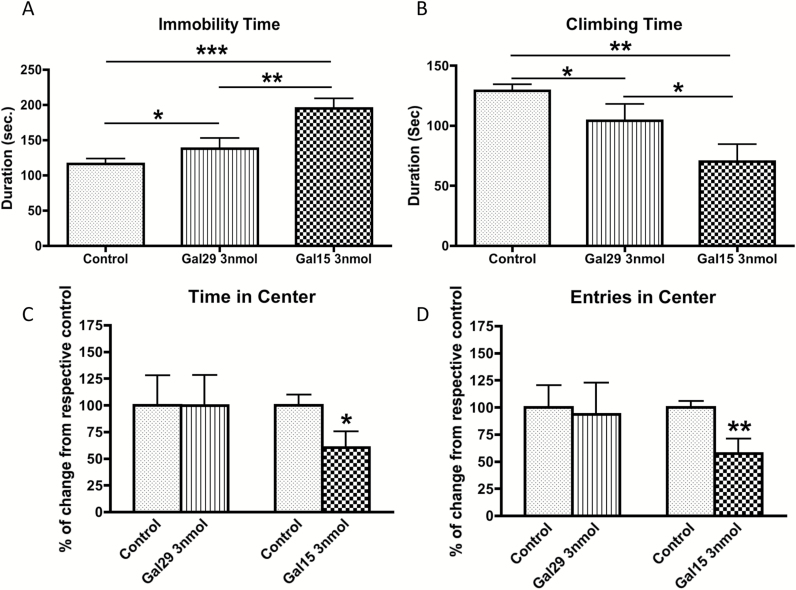
In the FST, the overall one-way ANOVA revealed a significant effect of treatment for immobility (one-way ANOVA, F2,26 = 12.8, p < 0.001). The increase in the immobility induced by GAL(1–15) was significantly higher than the one induced by GAL (Newman-Keuls post-hoc test: p < 0.01; Figure 2A). In the climbing behavior we observed the same pattern of response (one-way ANOVA, F2,26 = 7.6, p < 0.01): GAL(1–15) induced a significantly stronger decrease in climbing response compared with GAL (Newman-Keuls post-hoc test: p < 0.05; Figure 2B).
Figure 2.
Behavioral effect of administration of galanin (GAL29) and galanin(1–15) [GAL(1–15)] in the Forced Swimming Test (A,B) and Open Field Test (C,D) in rats. GAL29 (3 nmol/rat) and GAL(1–15) (3 nmol/rat) were administered intracerebroventricularly 15min before the test. Cerebrospinal fluid–injected rats were used as the control group. Values, indicated as mean ± standard error of the mean, represent time of immobility (A) and climbing (B) in the forced swimming test and percentage of change from respective controls in entries (D) and time in the center in the open field test (C) during a 5min test period (6–15 animals per group). *p < 0.05, **p < 0.01, and ***p < 0.001, according to (A and B) one-way analyses of variance followed by Newman Keuls Multiple Comparison Tests and (C and D) versus respective controls according to student’s t-tests.
In the open field, in agreement with other authors (Narvaez et al., 2014) the dose of 3 nmol of GAL did not affect the time spent in and number of entries into the central square in the open field (Figure 2C and D). In view of the fact that GAL(1–15) at 3 nmol significantly decreased the time (t = 2.318, p < 0.05, df = 13) and number (t = 3.379, p < 0.01, df = 16) of entries in the open field, our results indicate than only GAL(1–15) and not GAL modifies the anxiety parameters in the present model.
GAL2Receptor Antagonist M871 Induced Blockade of the Behavioral Effects of GAL(1–15)
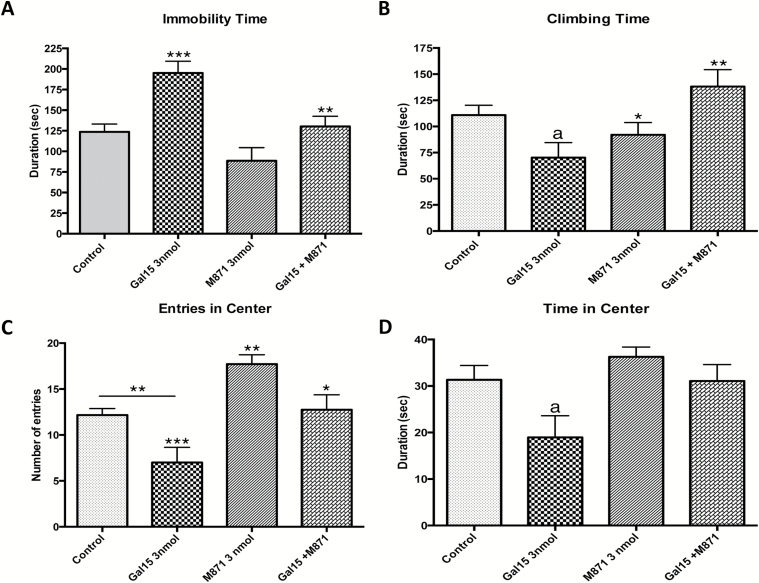
In the FST, GAL2 receptor antagonist M871 significantly blocked the increased the immobility (one-way ANOVA, F3,32 = 10.38, p < 0.001, Newman-Keuls post-hoc test: p < 0.01; Figure 3A) and decreased the climbing (one-way ANOVA, F3,32 = 4.535, p < 0.01, Newman-Keuls post-hoc test: p < 0.05) induced by GAL(1–15) (Figure 3B).
Figure 3.
Behavioral effects of the co-administration of the GAL2 receptor antagonist M871 (3 nmol/rat) and an effective dose of galanin(1–15) [GAL(1–15)] (3 nmol/rat) in the forced swimming test (FST; A and B) and open field test (C and D). Treatments were injected intracerebroventricularly 15min prior the tests. Cerebrospinal fluid–injected rats were used as a control group. Data represents mean ± standard error of the mean of immobility (A) and climbing time (B) in the FST and entries (C) and time in the center in the open field test (D) during the 5min test period (6–14 rats per group). (A,B) *p < 0.05 versus Gal15+M871; **p < 0.01 versus Gal15 group; ***p < 0.001 versus Control and M871 group; a p < 0.05 versus Control group. (C,D) *p < 0.05 versus Gal15; **p < 0.01 versus Control and versus Gal15+M871; ***p < 0.001 versus M871; a p < 0.05 versus rest of the groups according to one-way ANOVA followed by Newman Keuls Multiple Comparison Test.
The GAL2 receptor antagonist M871 alone in the dose of 3 nmol lacked effects with respect to immobility or climbing time (Figure 3A and B).
In the open field the same type of change was observed. GAL2 receptor participates in the effect induced by GAL(1–15), since the GAL2 receptor antagonist M871 significantly decreased the number of entries (one-way ANOVA, F3,29 = 10.6, p < 0.001, Newman-Keuls post-hoc test: p < 0.05; Figure 3C) and time spent in the central square (one-way ANOVA, F3,26 = 4.03, p < 0.05, Newman-Keuls post-hoc test: p < 0.05) mediated by GAL(1–15) (Figure 3D). In this test, M871 significantly increased the number of entries (Newman-Keuls post-hoc test: p < 0.01) but not the time spent in the central square of the open field (Figure 3C).
Neurochemical Studies
GAL1 and GAL2Receptors in the Dorsal Raphe Nucleus and Dorsal Hippocampus
Positive results were obtained with the in situ PLA indicating the formation of GAL1 and GAL2 heteroreceptor complexes. This was supported by studies using double immunolabeling, which demonstrated colocation of GAL1 and GAL2 receptors IR in the nerve cells of the dorsal raphe nucleus and of the dorsal hippocampus (Supplementary Figure S1A and B).
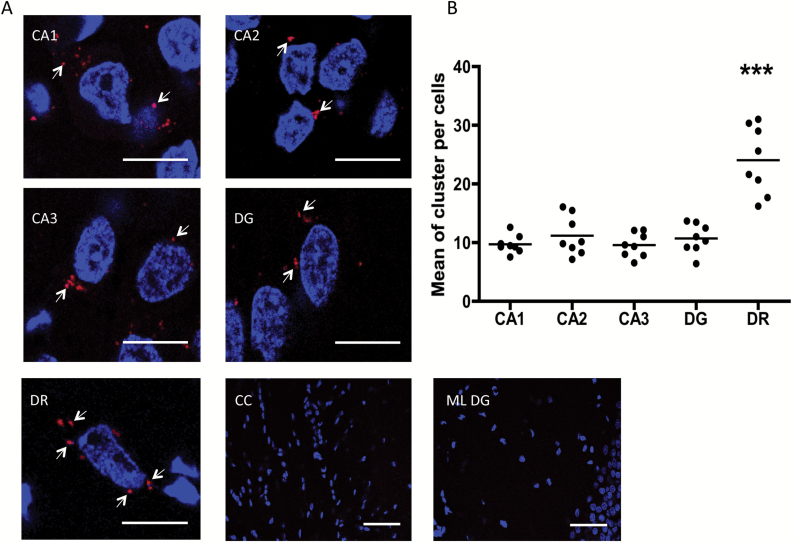
In Figure 4A, large numbers of the Ammon′s horn 1, 2, and 3 (CA1, CA2, and CA3) and the dentate gyrus of the dorsal hippocampus and the dorsal raphe show a significant number of red clusters (blobs) representing positive PLA signals. The quantification of PLA in these areas demonstrate that the highest number of PLA clusters are present in the cytoplasm of nerve cells of the dorsal raphe (one-way ANOVA, F4,35 = 26.57, p < 0.001, Newman-Keuls post-hoc test: p < 0.001; Figure 4B).
Figure 4.
Close proximity between GAL1 and GAL2 receptors is detected by in situ proximity ligation assay (PLA; seen as red clusters indicated by arrows) in dorsal rat hippocampi and dorsal raphe nuclei. In situ PLA was performed using primary antibodies of different species directed to the GAL1 receptor and GAL2 receptor, followed by PLA reagents. (A) Representative photographs for positive PLA regions. (B) Quantification of red clusters/6-diamidino-2-phenylindole–positive nuclei. Quantification was made in 10–20 cells per photo and performed 8 photos per brain zone. Thus, each point in the scatter plot represents the mean between 10–20 cells (80–160 cells per zone). The line in each group represents the mean value. ***p < 0.001 versus the rest of the groups according to one-way analyses of variance followed by Newman Keuls Multiple Comparison Tests. Nuclei are shown in blue (6-diamidino-2-phenylindole). Scale bars are 10 μm and 50 μm in the CC and ML DG photographs. CA1, CA2 and CA3: Ammon’s horn 1, 2, and 3; CC: corpus callosum; DG: dentate gyrus; DR: dorsal raphe nucleus; ML DG: molecular layer of dentate gyrus.
The specificity was demonstrated by the fact that no PLA clusters were observed in the lateral corpus callosum (Figure 4A), an area that seems to lack the GAL2 receptor (O’Donnell et al., 1999). The fact that we did not observe a PLA positive signal in the molecular layer of the dentate gyrus confirms the specificity of the signal (Figure 4A). The results obtained in the dorsal raphe were validated in PLA experiments on raphe RN33B cells (see below).
siRNA GAL2 and GAL1 Receptor Knockdown Validates the Involvement of Heteroreceptor Complexes
To verify the Acell system employed, we generated siRNA-Control rats (Acell Non-targeting pool) and vehicle rats (Acell siRNA Delivery Media). We did not observe any differences between these groups compared to artificial cerebrospinal fluid normal rats in any behavioral tests (data not shown).
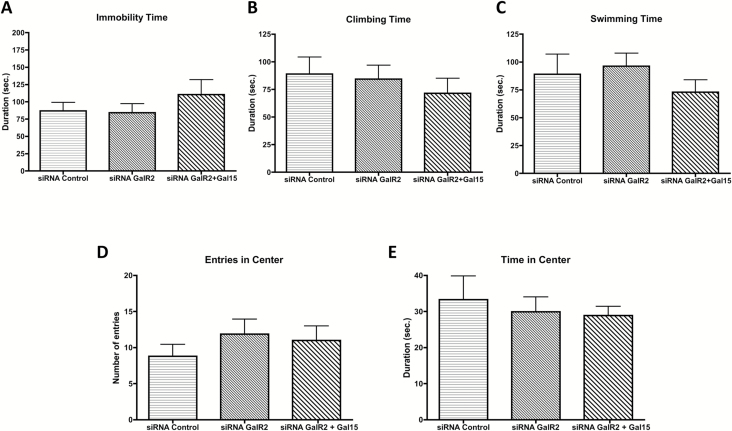
Down-regulation of the GAL2 receptor by siRNA did not affect any parameter of the behavioral tests, neither in the FST nor in the open field (Figure 5A–E). However, the decrease in the GAL2 receptor was sufficient to block the effect of GAL(1–15) in the siRNA GAL2 receptor knockdown rats (Figure 5A–E). Thus, GAL(1–15) at the dose of 3 nmol lacked effect on the immobility (one-way ANOVA, F2,18 = 0.743, p = 0.48), climbing (one-way ANOVA, F2,18 = 0.426, p = 0.65), and swimming time (one-way ANOVA, F2,17 = 0.923, p = 0.41) in the FST (Figure 5A–C). The same effect was observed in the number of entries (one-way ANOVA, F2,17 = 0.517, p = 0.6) and time spent in the central square (one-way ANOVA, F2,16 = 0.253, p = 0.77) in the open field test (Figure 5D and E).
Figure 5.
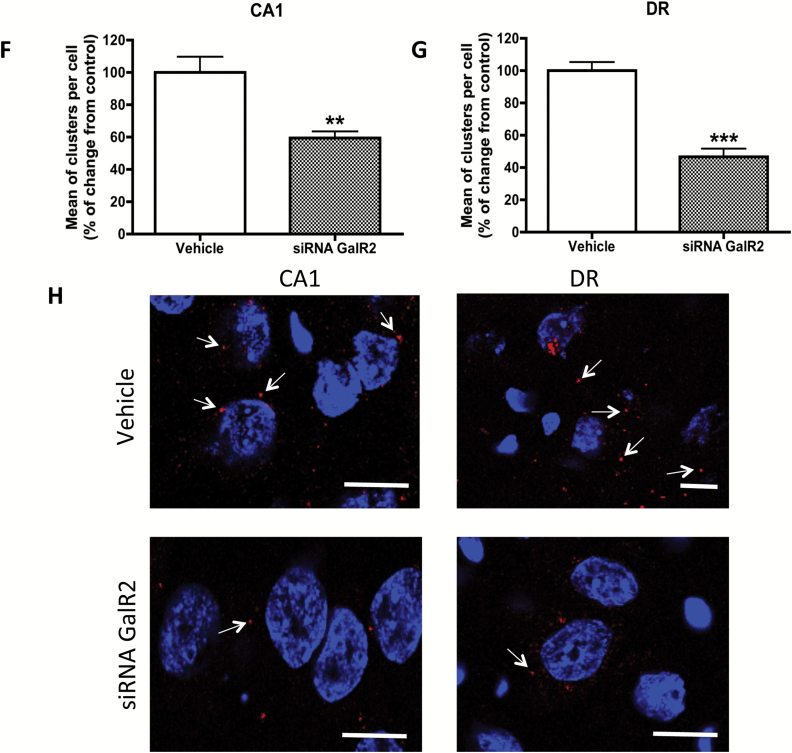
Analysis of galanin(1–15) [GAL(1–15)] in GAL2 receptor knockdown rats in the forced swimming test (FST) and open field test or in the detection of close proximity between GAL1 and GAL2 receptors in rat brains. (A–E) Behavioral effects of an effective dose of GAL(1–15) (3 nmol/rat) in the GAL2 receptor knockdown in FST and open field test. Treatments were injected intracerebroventricularly 15min prior to the tests. siRNA control–injected rats were used as the control group. Data represents mean ± standard error of the mean of immobility (A), climbing (B), and swimming time (C) in the FST and entries (D) and time (E) in the center in the open field test during the 5min test period (6–14 rats per group). No differences were found according to one-way analyses of variance. (F–H) Detection of close proximity between GAL1 and GAL2 receptors in the dorsal hippocampus and dorsal raphe in GAL2 receptor knockdown rats by in situ proximity ligation assay (PLA). Quantification of red clusters/6-diamidino-2-phenylindole–positive nuclei in the dorsal hippocampus (CA1; F) and dorsal raphe (G) 9 days after a single intracerebroventricular injection of siRNA GAL2 receptor or vehicle. Vertical bars represent mean ± standard error of the mean of percentage of change from respective controls. **p < 0.01 and ***p < 0.001 versus respective control according to student’s t-test. (H) Representative photographs for decrease of detection of cytoplasmatic GAL1 and GAL2 receptors via in situ PLA (seen as red clusters indicated by arrows) in the dorsal hippocampus (CA1) and dorsal raphe in GAL2 receptor knockdown rats. Nuclei are shown in blue (6-diamidino-2-phenylindole). Scale bar, 10 μm. CA1: Ammon’s horn 1; DR: dorsal raphe nucleus.
We observed the same pattern of response with the down-regulation of the GAL1 receptor by siRNA (Figure S5). The decrease in the GAL1 receptor blocked the effect of GAL(1–15) in the siRNA GAL1 receptor knockdown rats in all the parameters analyzed in the FST: immobility (one-way ANOVA, F2,20 = 0.737, p = 0.49), climbing (one-way ANOVA, F2,20 = 0.568, p = 0.57), and swimming time (one-way ANOVA, F2,20 = 0.026, p = 0.97; Figure 5S A–C). It also blocked the effects in the open field: the number of the entries (one-way ANOVA, F2,17 = 0.209, p = 0.81) and time spent in the central square (one-way ANOVA, F2,19 = 0.114, p = 0.89; Figure 5S D–E).
PLAs
In the siRNA GAL2 receptor–treated animals, PLA-positive red clusters (blobs) were still observed in the dorsal hippocampus and dorsal raphe (Figure 5F–H). However, the quantification of PLA shows a reduction of around 40% (t = 3.174, p < 0.01, df = 19) in nerve cells of the CA1 area and 60% (t = 7.325, p < 0.001, df = 14) of the dorsal raphe compared with the vehicle group.
These results confirm that the PLA signal obtained is specifically due to the GAL1 and GAL2 receptors complex.
In agreement with these results, the colocalization of GAL1 and GAL2 receptor immunoreactivities was reduced in the siRNA GAL2 receptor in the dorsal raphe and also in the dorsal hippocampus (Supplementary Figure 4).
GAL(1–15) Decreased the 5-HT Immunoreactivity in Raphe RN33B Cells
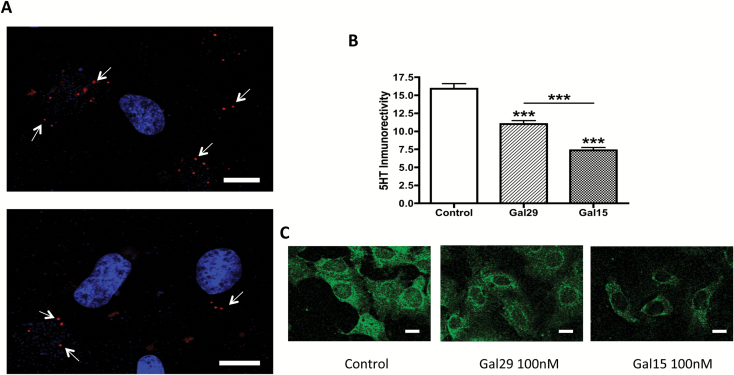
The presence of GAL1 and GAL2 receptors in raphe RN33B cells was established with immunohistochemical techniques (data not shown). Moreover, PLA-positive clusters were detected within them, which supports the presence of GAL1 and GAL2 heteroreceptor complexes in these cells and the validity of these cells as a target for GAL(1–15) (Figure 6A).
Figure 6.
Close proximity between GAL1 and GAL2 receptors and their effects in 5-HT synthesis and storage in RN33B cells. (A) Detection of close proximity between GAL1 and GAL2 receptors (seen as red clusters indicated by arrows) in RN33B cells by in situ proximity ligation assay; nuclei are shown in blue (6-diamidino-2-phenylindole). (B) Quantification of 5-HT immunoreactivity (green color) was used as a marker for 5-HT synthesis and storage in RN33B cell cultures after incubation with GAL29 and GAL(1–15). RN33B brain-derived immortalized cells were treated for 1h with GAL29 (100nM), GAL(1–15) (100nM), or control, followed for 1h by recuperation. 5-HT immunoreactivity was determined after immunofluorescent labeling. The data are presented as mean ± standard error of the mean, n = 60 cells for two independent experiments. ***p < 0.001 vs. control according to one-way analyses of variance followed by Newman Keuls Multiple Comparison Tests. (C) Representative images of 5-HT stained RN33B cells under different conditions are presented. Scale bars, 10 μm.
In the semiquantitative image analysis, the one-way ANOVA showed a significant effect in 5-HT immunoreactivity after GAL and GAL(1–15) treatment (one-way ANOVA, F2,147 = 63.85, p < 0.001). The incubation with GAL caused a significant (Newman-Keuls post-hoc test: p < 0.001) decrease in the 5-HT immunoreactivity in RN33B cells (Figure 6B and C). GAL(1–15) also significantly decreased the 5-HT immunoreactivity in these cells (Newman-Keuls post-hoc test: p < 0.001). This reduction was significantly stronger than the one induced by GAL (Newman-Keuls post-hoc test: p < 0.001; Figure 6B and C).
Discussion
In the current study we describe for the first time how GAL(1–15) induces strong depression-related and anxiogenic-like effects in the FST, the tail suspension test, the open field test, and the light/dark test. Importantly, these effects were significantly stronger than the ones induced by GAL. PLA indications were also obtained for the existence of GAL1 and GAL2 heteroreceptor complexes in the dorsal hippocampus and especially in the dorsal raphe nerve cells, areas rich in GAL-fragment binding sites (Hedlund et al., 1992). Indications were also obtained for the involvement of this heteroreceptor complex in the actions of GAL(1–15), based on the use of the specific GAL2 receptor antagonist M871 and i.c.v. injections of GAL1 receptor siRNA or GAL2 receptor siRNA, producing a reduction of the GAL1 receptor or GAL2 receptor, respectively. In the latter experiment, the reduction of the behavioral actions of GAL(1–15) was linked to a substantial disappearance of PLA-positive blobs in the nerve cells of the hippocampus and the dorsal raphe, and thus to a reduction in the GAL1 and GAL2 heteroreceptor complexes. Thus, the current study supports the concept that GAL-fragment binding sites mainly exist on GAL1 and GAL2 heteroreceptor complexes which preferentially bind GAL(1–15) (Fuxe et al., 2008, 2012). The relevance of the dorsal raphe as a target for GAL(1–15) was supported by studies in rat medullary raphe-derived cell line RN33B, where GAL(1–15) decreased 5-HT IR more strongly than GAL.
In the behavioral tests we observed that GAL induced the same pattern of response as in previous studies, validating the conditions of our behavioral models. In the FST GAL infused either i.c.v or into the ventral tegmental area increased immobility (Weiss et al., 1998; Kuteeva et al., 2005, 2007). This effect was reproduced with i.c.v. GAL at 3 nmol in this test. Moreover, in the open field GAL at 3 nmol lacked behavioral effects (Narvaez et al., 2014). This indicates the absence of stress conditions in our model, since GAL produced anxiolytic-like effects only in animals tested under heightened stress conditions (Morilak et al., 2003; Barrera et al., 2005).
GAL(1–15) induced a strong depression- and anxiogenic-like effect in all the tests, indicating a potential role of GAL(1–15) in mood disorders. The anxiogenic-like effect of GAL(1–15) at 3 nmol in the open field test is not present at 6 nmol. This rapid disappearance of the action at the higher concentration may reflect the depletion of a factor necessary for the formation of the GAL1 and GAL2 heteroreceptor complexes or the rapid development of a desensitisation mechanism at the GAL receptors. Similar biphasic concentration-response curves have also been observed for the GAL in its interaction with α2-adrenoreceptor (Diaz-Cabiale et al., 2005) and with the neuropeptide Y (Diaz-Cabiale et al., 2011).
Furthermore, the effects of GAL(1–15) in the FST and in the open field were significantly stronger than the corresponding behavioral effects induced by GAL. In previous work, N-terminal GAL fragments reduced post-junctional recognition of the 5-HT1A receptor, known to be involved in mood disorders, more strongly and potently than GAL (Diaz-Cabiale et al., 2000). This was true not only for behavioral functions, but also for cardiovascular functions. Thus, GAL(1–15) acts differentially from GAL. The N-terminal fragment GAL(1–15) antagonized the cardiovascular effects of GAL and GAL(1–15) but not GAL decreased baroreceptor reflex sensitivity (Diaz-Cabiale et al., 2005). GAL(1–15) also interacted differently from GAL with other neuropeptides, such as angiotensin or neuropeptide Y, involved in central cardiovascular functions (Diaz-Cabiale et al., 2005, 2010).
Our results at the behavioral level validate and extend the view of a specific role of GAL(1–15) in brain communication. The GAL2 receptor is involved in the GAL(1–15) effects, since the GAL2 receptor antagonist M871 blocked the GAL(1–15)-induced depression-like and anxiogenic effects.
The GAL2 receptor has been involved in neuronal mechanisms underlying antidepressant-like effects, but the specific role for the GAL2 receptor in anxiety is not well characterized. In fact, GAL2 receptor knockout mice showed either anxiety-like behavior or no effect depending on the genetic background of the mutants (Bailey et al., 2007; Lu et al., 2008). However, our results demonstrate the importance of the GAL2 receptor in the anxiogenic-like effect induced by GAL(1–15).
In previous work, a GAL2 and GAL3 receptor agonist (AR-M1896) administered i.c.v. to rats decreased immobility time in the FST, indicating antidepressant effects (Kuteeva et al., 2008). Also, transgenic mice overexpressing the GAL2 receptor demonstrated decreased immobility in the FST, indicative of antidepressant-like behavior (Le Maitre et al., 2011). Moreover, repeated fluoxetine treatment of rats increases the number of GAL2 receptor binding sites in the dorsal raphe nucleus, which could contribute to the antidepressant effects of fluoxetine via the GAL2 receptor in the dorsal raphe (Lu et al., 2005). These results indicate that the activation of the GAL2 receptor induces antidepressant-like effects. Instead, the current results showing that the GAL2 receptor antagonist blocks the depressant actions of the GAL fragment in the FST can be explained by our hypothesis that it targets the GAL1 and GAL2 heteroreceptor complex, which reduces the signaling from this complex, mediating the depression-like actions of GAL(1–15) (Fuxe et al., 2012).
In the current work, positive PLA blobs (puncta) formed from GAL1 and GAL2 receptors were, in fact, observed in the nerve cells of the dorsal hippocampus and dorsal raphe. This indicates that the distances between the two receptors are in a range compatible with the formation of GAL1 and GAL2 heteroreceptor complexes in these two brain regions (Trifilieff et al., 2011; Borroto-Escuela et al., 2012). The formation of GAL1 and GAL2 heteroreceptor complexes with high affinities for GAL(1–15) can help explain the fact that GAL(1–15) induces a stronger action than GAL at the behavioral level and in the reduction of 5-HT in RN33B cells. It is of interest that PLA blobs exist in higher number in the dorsal raphe nerve cells than in the dorsal hippocampal nerve cells, which indicates that the dorsal raphe nerve cells can be a special target for GAL(1–15). The meso-limbic 5-HT neurons are known to be dysfunctional in depression (Fuxe et al., 2008, 2012; Artigas, 2013). Therefore, the marked decrease in 5-HT IR induced by GAL(1–15) may indicate a mechanism contributing to the depression-like actions of GAL(1–15).
The GAL2 receptor may induce antidepressant actions via Gq/G11-mediated GAL2 receptor signaling. It seems possible that in the GAL1 and GAL2 heteroreceptor complex the GAL2 receptor protomer signals in a different way upon activation by its preferred ligand, GAL(1–15), leading to its strong depression-like action. Alternatively, the GAL(1–15) activation of the GAL1 receptor protomer may cause an allosteric receptor-receptor interaction that inhibits the Gq/G11-mediated signaling of the GAL2 receptor protomer and switches it towards Gi/o-mediated signaling. In this way, both GAL1 and GAL2 receptor protomers become coupled to Gi/o, which may lead to the strong depression-like actions observed with GAL(1–15). The formation of homodimers and heterodimers among neuropeptide receptors is known (AbdAlla et al., 2005). The GAL1 receptor can form homodimers (Wirz et al., 2005) and heterodimers with 5HT1A receptors (Borroto-Escuela et al., 2010; Fuxe et al., 2012) and likely with other G-protein coupled receptors.
GAL2 receptor siRNA was injected into the lateral ventricle of rats to reduce GAL2 receptor expression and validate the role of the GAL2 receptor in the formation of the PLA-positive blobs in the dorsal hippocampus and the dorsal raphe and in the GAL(1–15)-mediated behavioral actions. The time-course curve indicated a maximal reduction of GAL2 receptor protein expression 8 days after the injection, and this was the timepoint selected for the behavioral tests. Moreover, in these animals the quantification of PLA showed a maximal reduction of around 40% in the CA1 area and 60% in the dorsal raphe compared with the vehicle group. These results strongly indicate that the PLA signals obtained are specific and represent the GAL1 and GAL2 heteroreceptor complex. This reduction of the PLA signal was sufficient to block the depression- and anxiogenic-like effects of GAL(1–15), linking them to its actions at the GAL1 and GAL2 heteroreceptor complex. Moreover, we also blocked the behavioral effects of GAL(1–15) in siRNA GAL1 receptor knockdown rats, confirming that GAL(1–15) effects depend on the existence of GAL1 and GAL2 heteroreceptor complexes.
In conclusion, our results give strong support to the view that GAL(1–15) acts at GAL1 and GAL2 heteroreceptor complexes in the raphe-limbic system to exert its strong depression-like and anxiogenic effects. These results may give the basis for the development of novel therapeutic drugs specifically targeting the GAL1 and GAL2 heteroreceptor complexes for treatment of depression and anxiety.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the TV3-Marató 090130/31/32, Junta de Andalucia CVI-6476, and the Swedish Royal Academy of Sciences (Stiftelsen B von Beskows Fond and Stiftelsen Hierta-Retzius stipendiefond) and Karolinska Institutets Forskningsstiftelser 2012 and 2013 to Dr Borroto-Escuela, and by grants from the Swedish Medical Research Council (04X-715) and Hjärnfonden 2012 to Dr Fuxe. Dr Borroto-Escuela belongs to Academia de Biólogos Cubanos.
References
- AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. (2005). Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci 26:185–192. [DOI] [PubMed] [Google Scholar]
- Artigas F. (2013). Serotonin receptors involved in antidepressant effects. Pharmacol Ther 137:119–131. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. (2007). Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav 86:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Kinney JW, Hill MN, Lu X, Biros S, Rebek J, Jr., Bartfai T. (2006). A novel, systemically active, selective galanin receptor type-3 ligand exhibits antidepressant-like activity in preclinical tests. Neurosci Lett 405:111–115. [DOI] [PubMed] [Google Scholar]
- Barrera G, Echevarria DJ, Poulin JF, Laforest S, Drolet G, Morilak DA. (2005). One for all or one for one: does co-transmission unify the concept of a brain galanin “system” or clarify any consistent role in anxiety? Neuropeptides 39:289–292. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Lu X, Badie-Mahdavi H, Barr AM, Mazarati A, Hua XY, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, Somogyi L, Krock L, Rebek J., Jr. (2004). Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci USA 101:10470–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido I, Diaz-Cabiale Z, Jimenez-Vasquez PA, Andbjer B, Mathe AA, Fuxe K. (2002). Increased density of galanin binding sites in the dorsal raphe in a genetic rat model of depression. Neurosci Lett 317:101–105. [DOI] [PubMed] [Google Scholar]
- Bing O, Moller C, Engel JA, Soderpalm B, Heilig M. (1993). Anxiolytic-like action of centrally administered galanin. Neurosci Lett 164:17–20. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Narvaez M, Marcellino D, Parrado C, Narvaez JA, Tarakanov AO, Agnati LF, Diaz-Cabiale Z, Fuxe K. (2010). Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem Biophys Res Commun 393:767–772. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Mudo G, Perez-Alea M, Ciruela F, Tarakanov AO, Narvaez M, Di Liberto V, Agnati LF, Belluardo N, Fuxe K. (2012). Fibroblast growth factor receptor 1- 5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol Psychiatry 71:84–91. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K. (2013). G protein-coupled receptor heterodimerization in the brain. Methods Enzymol 521:281–294. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. (2000). Galanin receptor subtypes. Trends Pharmacol Sci 21:109–117. [DOI] [PubMed] [Google Scholar]
- Brunner SM, Farzi A, Locker F, Holub BS, Drexel M, Reichmann F, Lang AA, Mayr JA, Vilches JJ, Navarro X, Lang R, Sperk G, Holzer P, Kofler B. (2014). GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc Natl Acad Sci USA 111:7138–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan A, Wills DN, Ehlers CL. (2014). Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacol Biochem Behav 122:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Narvaez JA, Finnman UB, Bellido I, Ogren SO, Fuxe K. (2000). Galanin-(1–16) modulates 5-HT1A receptors in the ventral limbic cortex of the rat. Neuroreport 11:515–519. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Vela C, Razani H, Covenas R, Fuxe K, Narvaez JA. (2005). Role of galanin and galanin(1–15) on central cardiovascular control. Neuropeptides 39:185–190. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Fuxe K, Agnati L, Narvaez JA. (2007). Receptor-receptor interactions in central cardiovascular regulation. Focus on neuropeptide/alpha(2)-adrenoreceptor interactions in the nucleus tractus solitarius. J Neural Transm 114:115–125. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Narvaez M, Millon C, Puigcerver A, Fuxe K, Narvaez JA. (2010). Neurochemical modulation of central cardiovascular control: the integrative role of galanin. EXS 102:113–131. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Parrado C, Narvaez M, Puigcerver A, Millon C, Santin L, Fuxe K, Narvaez JA. (2011). Galanin receptor/Neuropeptide Y receptor interactions in the dorsal raphe nucleus of the rat. Neuropharmacology 61:80–86. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Rivera A, Diaz-Cabiale Z, Filip M, Gago B, Roberts DC, Langel U, Genedani S, Ferraro L, de la Calle A, Narvaez J, Tanganelli S, Woods A, Agnati LF. (2008). Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev 58:415–452. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Calvo F, Garriga P, Tena M, Narvaez M, Millon C, Parrado C, Ciruela F, Agnati LF, Narvaez JA, Diaz-Cabiale Z. (2012). On the existence and function of galanin receptor heteromers in the central nervous system. Front Endocrinol 3:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ. (2010). The prevalence and impact of depression. J Clin Psychiatry 71:e06. [DOI] [PubMed] [Google Scholar]
- Grivas V, Markou A, Pitsikas N. (2013). The metabotropic glutamate 2/3 receptor agonist LY379268 induces anxiety-like behavior at the highest dose tested in two rat models of anxiety. Eur J Pharmacol 715:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Yanaihara N, Fuxe K. (1992). Evidence for specific N-terminal galanin fragment binding sites in the rat brain. Eur J Pharmacol 224:203–205. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Finnman UB, Yanaihara N, Fuxe K. (1994). Galanin-(1–15), but not galanin-(1–29), modulates 5-HT1A receptors in the dorsal hippocampus of the rat brain: possible existence of galanin receptor subtypes. Brain Res 634:163–167. [DOI] [PubMed] [Google Scholar]
- Hinojosa FR, Spricigo L, Jr., Izidio GS, Bruske GR, Lopes DM, Ramos A. (2006). Evaluation of two genetic animal models in behavioral tests of anxiety and depression. Behav Brain Res 168:127–136. [DOI] [PubMed] [Google Scholar]
- Holmes A, Picciotto MR. (2006). Galanin: a novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets 5:225–232. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. (2003). Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28:1031–1044. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Kresse A, Skofitsch G. (2004). Galanin in the brain: chemoarchitectonics and brain cartography--a historical review. Peptides 25:433–464. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Hullam G, Eszlari N, Gonda X, Antal P, Anderson IM, Hokfelt TG, Deakin JF, Bagdy G. (2014). Brain galanin system genes interact with life stresses in depression-related phenotypes. Proc Natl Acad Sci USA 111:E1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormos V, Gaszner B. (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47:401–419. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Hokfelt T, Ogren SO. (2005). Behavioral characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides 39:299–304. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Hokfelt T, Ogren SO. (2007). Galanin enhances and a galanin antagonist attenuates depression-like behavior in the rat. Eur Neuropsychopharmacol 17:64–69. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundstrom L, Sollenberg U, Langel U, Hokfelt T, Ogren SO. (2008). Differential role of galanin receptors in the regulation of depression-like behavior and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology 33:2573–2585. [DOI] [PubMed] [Google Scholar]
- Le Maitre TW, Xia S, Le Maitre E, Dun XP, Lu J, Theodorsson E, Ogren SO, Hokfelt T, Xu ZQ. (2011). Galanin receptor 2 overexpressing mice display an antidepressive-like phenotype: possible involvement of the subiculum. Neuroscience 190:270–288. [DOI] [PubMed] [Google Scholar]
- Lu X, Barr AM, Kinney JW, Sanna P, Conti B, Behrens MM, Bartfai T. (2005). A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci USA 102:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ross B, Sanchez-Alavez M, Zorrilla EP, Bartfai T. (2008). Phenotypic analysis of GalR2 knockout mice in anxiety- and depression-related behavioral tests. Neuropeptides 42:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg C, Englund U, Trono D, Bjorklund A, Wictorin K. (2002). Differentiation of the RN33B cell line into forebrain projection neurons after transplantation into the neonatal rat brain. Exp Neurol 175:370–387. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. (2008). Galanin, galanin receptors and drug targets. Cell Mol Life Sci 65:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Sommer W, Thorsell A, Heilig M. (1999). Anxiogenic-like action of galanin after intra-amygdala administration in the rat. Neuropsychopharmacology 21:507–512. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. (2003). Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sci 73:715–726. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Kubo T, Semi Y, Itakura M, Kuwamura M, Izawa T, Azuma YT, Takeuchi T. (2012). A rapid, targeted, neuron-selective, in vivo knockdown following a single intracerebroventricular injection of a novel chemically modified siRNA in the adult rat brain. J Biotechnol 157:326–333. [DOI] [PubMed] [Google Scholar]
- Narvaez M, Millon C, Borroto-Escuela D, Flores-Burgess A, Santin L, Parrado C, Gago B, Puigcerver A, Fuxe K, Narvaez JA, Diaz-Cabiale Z. (2014). Galanin receptor 2-neuropeptide Y Y1 receptor interactions in the amygdala lead to increased anxiolytic actions. Brain Struct Funct. 10.1007/s00429-014-0788-7 [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. (1999). Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol 409:469–481. [PubMed] [Google Scholar]
- Parrado C, Diaz-Cabiale Z, Garcia-Coronel M, Agnati LF, Covenas R, Fuxe K, Narvaez JA. (2007). Region specific galanin receptor/neuropeptide Y Y1 receptor interactions in the tel- and diencephalon of the rat. Relevance for food consumption. Neuropharmacology 52:684–692. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. (1986). The rat brain in stereotaxic coordinates. New York: Academic Press. [Google Scholar]
- Picciotto MR. (2010). Galanin and addiction. EXS 102:195–208. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hokfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. (2005). Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci USA 102:17489–17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. (1983). Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett 164:124–128. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slattman M, Gullberg M, Javitch JA. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hashemi T, He C, Strader C, Bayne M. (1997). Molecular cloning and pharmacological characterization of a new galanin receptor subtype. Mol Pharmacol 52:337–343. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CH. (1998). Galanin: a significant role in depression? Ann NY Acad Sci 863:364–382. [DOI] [PubMed] [Google Scholar]
- Wirz SA, Davis CN, Lu X, Zal T, Bartfai T. (2005). Homodimerization and internalization of galanin type 1 receptor in living CHO cells. Neuropeptides 39:535–546. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:655–679. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wang YL, Su Z, Zhang Y, Guo SX, Liu AJ, Wang CH, Sun FJ, Yang J. (2014). Effect of oxytocin on the behavioral activity in the behavioral despair depression rat model. Neuropeptides 48:83–89. [DOI] [PubMed] [Google Scholar]