Abstract
Background:
Δ9-Tetrahydrocannabinol has been shown to modulate anxiety and facilitate the extinction of fear by inhibiting amygdala reactivity. Since functional coupling between the amygdala and prefrontal cortex is implicated in affective processes, it is possible that Δ9-tetrahydrocannabinol affects amygdala-prefrontal cortex functional connectivity in ways that differ across amygdala subregions: basolateral, centromedial, and superficial.
Methods:
The aim of the study was to examine the effects of Δ9-tetrahydrocannabinol on functional connectivity between amygdala subregions and the prefrontal cortex during socio-emotional threat in healthy adults using a double-blind, placebo-controlled, within-subjects design. Sixteen subjects completed a functional magnetic resonance imaging task designed to probe amygdala responses to social threat. Amygdala subregion-prefrontal cortex functional connectivity was compared between Δ9-tetrahydrocannabinol and placebo using generalized psychophysiological interaction analyses.
Results:
Findings indicated that Δ9-tetrahydrocannabinol enhanced basolateral and superficial amygdala connectivity to the rostral anterior cingulate/medial prefrontal cortex.
Conclusion:
These effects, including Δ9-tetrahydrocannabinol’s potential ability to reduce threat perception or enhance socio-emotional regulation, may help understand the neurocircuitry of affect.
Keywords: Δ9-tetrahydrocannabinol, cannabis, social threat, functional connectivity
Introduction
Cannabis sativa (or cannabis) is the most widely used drug in the world (United Nations Office on Drugs and Crime, 2012). The primary psychoactive ingredient in cannabis, Δ9-tetrahydrocannabinol (THC), binds to CB1 receptors in the brain and produces a variety of acute effects, including subjective feelings of sedation and euphoria (Johns, 2001). Consistent with the role of the endogenous cannabinoid system in the regulation of anxiety and fear learning (Chhatwal and Ressler, 2007), THC and other CB1 agonists have also been shown to modulate subjective anxiety (Wachtel et al., 2002) and facilitate the extinction of fear responses (Rabinak et al., 2013).
The effects of THC on anxiety and fear may be due to changes in amygdala reactivity. CB1 receptors are expressed at high levels in the amygdala, and activation of CB1 receptors can attenuate anxiety responses and amygdala activation to aversive stimuli (Rubino et al., 2007). Additionally, in humans, acute low-dose administration of THC (Phan et al., 2008) and higher levels of daily cannabis use (Cornelius et al., 2010) are associated with decreased threat-related amygdala reactivity. Frequent cannabis users also exhibit reduced amygdala activation to anger stimuli relative to healthy controls (Gruber et al., 2009). Hariri et al. (2009) further found that increased endocannabinoid signaling is associated with decreased threat-related amygdala activation. Taken together, THC may inhibit amygdala reactivity to threatening stimuli.
Given that the brain is organized into interconnected networks (van den Heuvel and Hulshoff Pol, 2010), it is possible that THC also impacts the functional coupling between the amygdala and other regions of the brain, specifically, the prefrontal cortex (PFC). The amygdala has strong reciprocal connections with the PFC (Ghashghaei et al. 2007), and dynamic interactions between these regions are necessary for the recognition and modulation of affective states, including fear and anxiety (Banks et al., 2007; Prater et al., 2013). Prior studies have demonstrated that exposure to threat (Gold et al., 2014) and administration of anxiolytic substances, such as alcohol and oxytocin (Gorka et al., 2013; Sripada et al., 2013), modulate amygdala-PFC functional coupling. It has also been demonstrated that acute THC administration increases PFC perfusion at baseline (van Hell et al., 2011), which could subsequently impact the way fear stimuli are processed and how the PFC and amygdala functionally interact.
To date, few studies have investigated the acute effects of THC on amygdala functional connectivity. In one, Lee and colleagues (2013) reported that THC reduced functional connectivity between the amygdala and the primary sensorimotor areas during aversive pain states. In a separate study, Fusar-Poli et al. (2010) found that cannabidiol, another cannabis derivative, but not THC, disrupted connectivity between the amygdala and the anterior cingulate cortex (ACC) during the viewing of threatening faces.
Notably, however, the amygdala is not a single, uniform structure. The human amygdala can be separated into at least 3 structurally and functionally distinct subregions: basolateral (AMYG-BL), centromedial (AMYG-CM), and superficial (AMYG-SF) (Amunts et al., 2005). These subregions have different patterns of functional connectivity (Amunts et al., 2005) and are part of distinct socio-emotional networks: aversion (AMYG-BL), affiliation (AMYG-CM), and perception (AMYG-SF) (Bickart et al., 2012). Broadly, the AMYG-BL and AMYG-SF are implicated in fear detection and perception, whereas the AMYG-CM is responsible for behavioral and autonomic outputs of fear expression (Bzdok et al., 2013). Given these differences, it is critical to examine the potentially unique and shared THC-inducted patterns of functional connectivity across subregions.
The aim of the current study was to conduct a more nuanced analysis of existing functional magnetic resonance imaging (fMRI) data on the neural effects of THC in healthy adults (Phan et al., 2008). The study was a 2-session (placebo vs THC), double-blind, within-subjects design. Our prior analyses indicated that THC attenuated amygdala reactivity to threatening faces (ie, angry and fearful). In the present study, we employed a generalized form of context-dependent psychophysiological interaction analyses (gPPIs; McLaren et al., 2012), using each amygdala subregion as the seed region of interest, to determine the effects of THC on amygdala-PFC functional connectivity during the viewing of angry and fearful faces. Given that our task (ie, viewing threatening faces) probed fear perception, we hypothesized that THC would modulate functioning coupling between the AMYG-BL and AMYG-SF and the PFC, as these two amygdala subregions are most relevant for detecting threat and extracting information from social stimuli.
Methods
Participants
Sixteen right-handed healthy volunteers (50% male; ages 18–28; M age=20.8 years [SD=2.6]) were included in the study (see Phan et al., 2008 for complete description). All participants had used marijuana at least 10 times in their lives, but none were daily marijuana users or had a lifetime substance abuse/dependence or any other neurological, psychiatric, or medical illness as confirmed by medical examination and structured clinical interview. Participants drank approximately 5.4±3.1 alcoholic beverages and smoked 12.0±21.3 cigarettes per week. On scanning days, all subjects had a negative urine drug toxicology (including cannabinoids) and alcohol breathalyzer screen. All participants provided written informed consent after explanation of the protocol, as approved by the University of Chicago Institutional Review Board.
Procedure
The study was a within-subjects, double-blind, placebo-controlled design. Each participant completed 2 fMRI scan sessions (order randomized) in which they ingested an opaque gelatin capsule (size 00) with dextrose filler. The capsule contained either Δ9 -THC (Marinol; 7.5mg; Solvay Pharmaceuticals, Marietta, GA) or placebo (PBO; containing only dextrose). The 2 sessions occurred at least 7 days apart. Prior to scanning, participants were instructed to abstain from food for 2 hours and all drugs for 24 hours; participants were instructed to abstain from any drug for 12 hours postscanning.
Approximately 120 minutes after ingestion of the capsule, the fMRI session began. Timing of the experiment was designed to match the expected window of peak subjective effects and plasma levels of THC (Wachtel et al., 2002). Self-report affect and drug effects were measured 30 minutes before and 30, 90, 210, and 270 minutes post-capsule ingestion using the Drug Effects Questionnaire and visual-analog scales. Participants rated the extent to which they felt the drug, liked the drug, wanted more, and felt “high.” They also rated how “stimulated,” “high,” “anxious,” and “hungry” they felt. As reported previously (Phan et al., 2008), THC significantly increased ratings of “feel” drug and feeling “high” at 90, 210, and 270 minutes postingestion, corresponding to the expected timing of peak subjective THC effects.
Emotional Face Matching Task
The emotional face matching task is described in detail elsewhere (Phan et al., 2008) and has been used in previous pharmaco-fMRI studies to probe amygdala-frontal connectivity (Gorka et al., 2013). Briefly, using a block design, participants viewed a trio of faces and were asked to select from 2 faces at the bottom of the screen one that matched a target face at the top of the screen. Target faces and congruent probes displayed 1 of 3 expressions (angry, fearful, or happy), while the incongruent probe displayed a neutral expression. Face-matching trials were interspersed with a control task in which simple geometric shapes (ie, circles, rectangles, or triangles) were similarly matched. The paradigm consisted of eighteen 20-second blocks: 9 matching emotional faces (3 blocks of each target expression) plus 9 blocks matching shapes, for a total task time of 6 minutes. Expression order was counterbalanced between 2 runs, and participants responded via right-handed button press to indicate match selections. As reported in Phan et al. (2008), there were no main effects of THC or THC × facial expression interactions on task accuracy or response times.
Brain Imaging
fMRI was performed on a 3T GE magnetic scanner using T2*-sensitive gradient-echo reverse spiral acquisition images (30 axial slices, 5-mm thickness, 2-second repetition time (TR), 25-millisecond echo time (TE), 77° flip angle, 240-mm field of view (FOV), 64×64 matrix) optimized to minimize susceptibility artifacts in the amygdala.
Data Analysis
Imaging data previously processed using Statistical Parametric Mapping software SPM2 (Wellcome Trust Centre for Neuroimaging, London, UK) were reprocessed and reanalyzed using updated SPM8 software. Images were slice-time corrected, spatially realigned, warped to standardized Montreal Neurological Institute space using the participant’s mean functional image, resampled to 2-mm3 voxels, and smoothed with an 8-mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was sapplied to the time series and convolved with the canonical hemodynamic response function and with a 128-second high-pass filter. Condition effects were modeled separately with box-car regressors representing the occurrence of each block type (angry, fearful, happy faces, and shapes). Effects were estimated at each voxel and for each subject. Realignment parameters (ie, movement) were included in the model to account for motion-related effects. Individual contrast maps (statistical parametric maps) combining threatening stimuli (angry and fearful faces) > shapes were created for each participant, separately for each session, and analyzed at the second level using a paired-samples t test. We first confirmed using a whole-brain analysis that, consistent with Phan et al. (2008), there was an effect of THC on left amygdala reactivity to threat (angry and fearful faces > shapes; peak Montreal Neurological Institute coordinates: (−22, 2, −16), Z=2.50, k =65, P<.05, small volume corrected) such that amygdala activity during threat matching was greater during PBO relative to THC.
To examine functional connectivity, we used a generalized form of context-dependent psychophysiological interaction analyses (gPPI; http://brainmap.wisc.edu/PPI, McLaren et al., 2012). Anatomical masks for left and right AMYG-BL, AMYG-CM, and AMYG-SF subregions were created using cytoarchitectonic boundaries defined by Amunts et al. (2005) and implemented in SPM’s Anatomy toolbox (see Bzdok et al., 2013). Deconvolved time series from each mask was extracted for each subject to create the physiological variable. Condition onset times for fearful faces, angry faces, happy faces, and shapes were separately convolved with the canonical hemodynamic response function for each condition, creating psychological regressors. Interaction terms (PPIs) were computed by multiplying extracted time series from the psychological regressors with the physiological variable. Activity within each subregion was regressed on a voxel-wise basis against the interaction, with the physiological and psychological variables serving as regressors of interest. Individual contrast images for threat vs shapes were then entered into separate second-level paired-samples t tests.
To determine our significance threshold, we applied an anatomically derived (Automated Anatomical Labeling [AAL] atlas) partial brain mask of the entire PFC to our data (search volume =451840mm3, encompassing 56480 voxels). Cluster-based significance thresholding was used to adjust for multiple comparisons within the search volume. Based on simulations (10000 iterations) performed with 3DClustSim, an adaptation of AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html), a family wise error correction at α<.05 was achieved for voxel threshold of P<.005 with minimum cluster size of 156 contiguous voxels. We extracted parameter estimates/β weights representing connectivity strength (arbitrary units) from 5-mm-radius spheres surrounding peak connectivity clusters from each subject in order to conduct posthoc paired samples t tests to determine the direction of effects.
Results
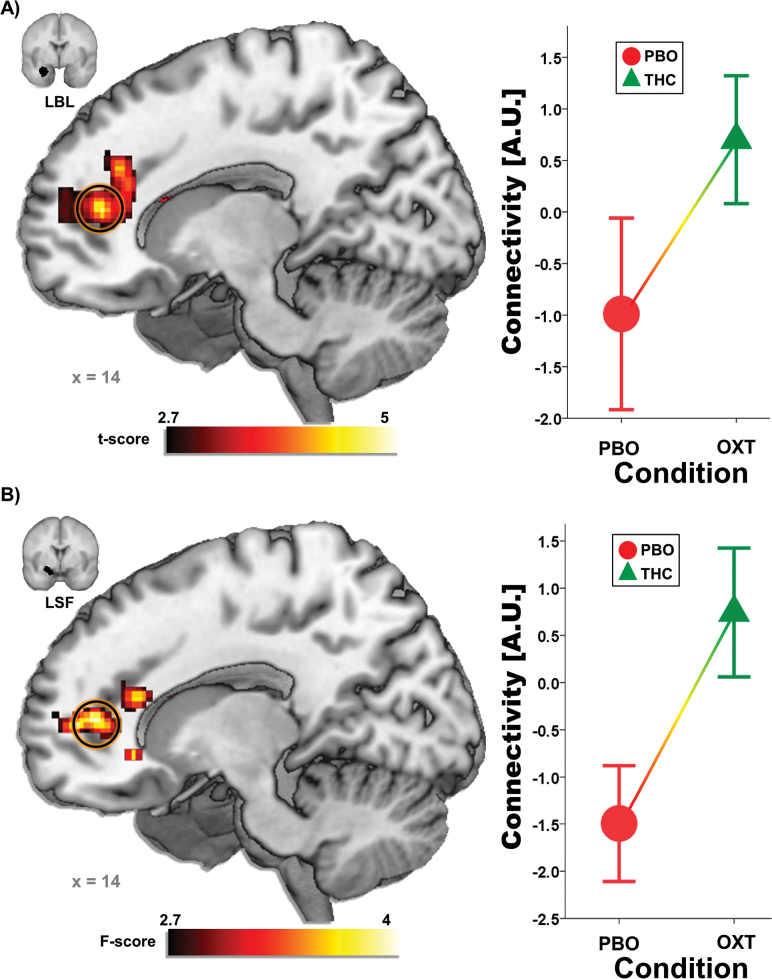
THC altered functional coupling between left AMYG-BL and rostral ACC (rACC)/medial PFC (mPFC) [(14, 42, 12); Z=3.65, k=398, P<.05, corrected] and left AMYG-SF and rACC/mPFC [(14, 52, 6); Z=3.71, k=760, P<.05, corrected]. Functional connectivity in both cases was greater during THC compared with PBO (Figure 1). As expected, given the lack of THC effects on right amygdala reactivity, there were no effects of THC on functional connectivity with any of the right amygdala seeds. For completeness, all other whole-brain results are presented in Table 1.
Figure 1.
(A) The anatomical left basolateral amygdala (AMYG-BL) seed and a voxel-wise statistical t map on a canonical brain showing enhanced left AMYG-BL – rostral anterior cingulate cortex (rACC)/medial prefrontal cortex (mPFC) functional connectivity during Δ9-tetrahydrocannabinol (THC) compared with placebo (PBO). Line graph illustrating extracted AMYG-BL-rACC/mPFC connectivity parameter estimates during the THC and PBO conditions. (B) The anatomical left superficial amygdala (AMYG-SF) seed and a voxel-wise statistical t-map on a canonical brain showing enhanced left AMYG-SF – rACC/mPFC functional connectivity during THC compared with PBO; Line graph illustrating extracted AMYG-SF-rACC/mPFC connectivity parameter estimates during the THC and PBO conditions; LBL,left basolateral subregion; LSF,left superficial subregion.
Table 1.
Whole-Brain Results for the Paired-Sample t Tests of gPPI Functional Connectivity for Each Amygdalasubregion
| Seed | Direction | Region | MNI Coordinates | Voxels | Z-Score | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left BL | PBO > THC | R cerebelum | 38 | −42 | −32 | 82 | 3.02 |
| Left BL | THC > PBO | R Rostral anterior cingulate/mPFC | 14 | 42 | 12 | 398 | 3.65 |
| L superior frontal gyrus | −18 | 56 | 4 | 104 | 3.59 | ||
| Right BL | PBO > THC | R parietal lobe | 30 | −52 | 70 | 111 | 2.95 |
| Right BL | THC > PBO | Middle cingulate gyrus | −2 | 26 | −10 | 55 | 3.31 |
| L inferior frontal lobe | −46 | 16 | 18 | 84 | 3.15 | ||
| Left CM | PBO > THC | R rectus | 8 | 32 | −26 | 114 | 3.55 |
| Left CM | THC > PBO | L inferior frontal lobe | −42 | 22 | 24 | 180 | 3.21 |
| Right CM | PBO > THC | L precentral gyrus | −50 | −14 | 58 | 91 | 3.63 |
| R parietal lobe | 20 | −56 | 78 | 66 | 3.01 | ||
| Right CM | THC > PBO | None | |||||
| Left SF | PBO > THC | R precentral gyrus | 60 | 10 | 46 | 117 | 5.19 |
| L lingual gyrus | −8 | −96 | −12 | 131 | 4.17 | ||
| R superior frontal gyrus | 30 | 58 | 30 | 57 | 2.87 | ||
| Left SF | THC > PBO | R posterior frontal lobe | 28 | −38 | 36 | 456 | 3.90 |
| R rostral anterior cingulate/mPFC | 14 | 52 | 6 | 760 | 3.71 | ||
| Middle cingulate gyrus | 0 | −44 | 42 | 121 | 3.23 | ||
| L cingulate gyrus | −16 | −28 | 30 | 67 | 3.10 | ||
| L middle frontal lobe | −20 | 18 | 46 | 212 | 3.08 | ||
| Right SF | PBO > THC | R superior frontal gyrus | 20 | 48 | 22 | 144 | 3.60 |
| R middle temporal gyrus | 54 | −56 | −12 | 312 | 3.32 | ||
| L cerebelum | −48 | −54 | −28 | 63 | 3.31 | ||
| L fusiform gyrus | −26 | −94 | −24 | 74 | 3.28 | ||
| R angular gyrus | 40 | −58 | 30 | 164 | 3.23 | ||
| L middle frontal gyrus | −34 | 58 | 14 | 186 | 3.19 | ||
| L inferior parietal lobe | −42 | −64 | 46 | 74 | 2.87 | ||
| Right SF | THC > PBO | None | |||||
Reporting of all clusters exhibiting significance threshold at P<.005(uncorrected) with a cluster extent threshold of k (number of contiguous voxels) >50. *Bold italics represent a priori areas of interest for significant, corrected for multiple comparisons. gPPI, generalized form of context-dependent psychophysiological interaction analyses; L, left; MNI,Montreal Neurologic Institute; mPFC, medial prefrontal cortex; PBO, placebo; R,right; SF, superficial; THC, Δ9-tetrahydrocannabinol.
In a separate set of analyses, identical to those discussed above, we examined the effect of THC on amygdala subregion-PFC functional connectivity during threat faces vs happy faces. Since happy faces represent a social nonthreat condition, this additional comparison allows for a test of the specificity of the present findings to social threat. Results indicated that consistent with above, THC increased left AMYG-BL to rACC/mPFC functional connectivity relative to PBO [(12, 46, 12); Z=3.36, k=171, P<.05, corrected]. There was no effect of THC on left AMYG-SF or AMYG-CM on rACC/mPFC functional connectivity. There were also no effects of THC on any of the right amygdala subregions. Posthoc, we confirmed that there was no effect of THC on amygdala subregion-PFC functional connectivity during happy faces vs shapes. THC-induced changes in connectivity were not correlated with changes in self-reported affect and drug effects (all P<.05).
Discussion
The aim of the current study was to examine the effects of THC on functional connectivity between amygdala subregions and PFC during processing of social threat in healthy, recreational cannabis users. We found that THC enhanced functional connectivity between AMYG-BL and AMYG-SF subregions and rACC/mPFC, suggesting that, in the context of threat, THC modulates amygdala reactivity and functional connectivity. Notably, our findings indicated that THC had an effect on AMYG-BL and rACC/mPFC connectivity during threatening faces relative to shapes and happy faces. This implies that AMYG-BL to rACC/mPFC functional coupling may be particularly salient for social threat rather than just general threat or just to “social” signals/faces. Consistent with our hypotheses and the design of our threat task, THC affected PFC functional coupling with the 2 amygdala subregions that are most strongly implicated in the perception, rather than the expression, of threat: the AMYG-BL and AMYG-SF. As noted above, the AMYG-BL and AMYG-SF are involved in detecting innate threat and extracting the social value of stimuli, respectively (Bzdok et al., 2013). The direction of effects of THC on amygdala-PFC functional connectivity, relative to PBO, is noteworthy. Prior studies demonstrated that healthy individuals exhibit greater functional coupling between the amygdala and rACC/mPFC during threat (Prater et al., 2013) and that greater connectivity is associated with greater affect regulation efficiency (Banks et al., 2007). Thus, it is possible that enhanced functional connectivity during THC administration reflects increased rACC/mPFC regulatory influences on AMYG-BL and AMYG-SF, which could diminish threat perception and anxiety. In light of these possible relationships, future studies should explicitly test the direction of THC’s effects on amygdala subregion-PFC functional coupling.
It is also possible that THC’s impact on connectivity reflects broader changes in social-emotional functioning, which are independent of THC’s impact on amygdala reactivity. Functional coupling between the amygdala and rACC/mPFC is implicated in a wide range of social and emotional functions, including social cognition and decision-making (Kim et al., 2011). Cross-talk between these regions is necessary for determining the salience of incoming stimuli and integrating cognitive, emotional, and somatic information (Seeley et al., 2007). As such, increased connectivity between the AMYG-BL and AMYG-SF and the rACC/mPFC may indicate that during threat, THC enhances broad social-emotional capabilities, including extracting salience and organizing and guiding affective responses. Consistent with this explanation, a recent study found that administration of oxytocin, a neuropeptide with known prosocial effects including increasing trust and empathy (Kosfeld et al., 2005), also enhances amygdala-rACC/mPFC functional connectivity during resting-state in healthy individuals (Sripada et al., 2013). Meanwhile, individuals characterized by social deficits (eg, social anxiety disorder) reliably evidence abnormal functional connectivity between the amygdala and mPFC (Hahn et al., 2011). It is therefore possible that THC could have acute, prosocial effects, which could be potentially therapeutic for patients with social-emotional deficits.
It is important to highlight that THC impacted reactivity and functional connectivity of the left, but not the right, amygdala. Several prior studies have suggested that the left and right amygdala have separable roles in affective processes such that the right amygdala is responsible for rapid, automatic emotion detection, whereas the left amygdala is involved in sustained, conscious emotion evaluation and regulation (Dyck et al., 2011). Notably, this literature is consistent with the aforementioned proposed mechanisms, as affective regulation and sustained social-emotional processes are thought to be mediated by the left amygdala.
While these results significantly add to the literature on the acute neural effects of THC, there are a few limitations worth noting. First, although the study used a within-subjects design, the sample size was small, resulting in low statistical power and limited ability to detect associations between THC-inducted changes in functional connectivity and self-reported affect. Related to this point, because of the relative small sample, we did not examine moderators of the current findings, and future studies are needed to test whether individual differences could impact the pattern of results. Second, we used a relatively low dose of THC and since higher doses may have anxiogenic effects (Viveros et al., 2005), the findings may not generalize across doses. As such, dose-dependent THC studies are critically needed to extend this work. Similarly, all participants in the study were casual cannabis users and thus, future research is needed to determine whether the findings apply to those with less or more history of cannabis exposure. Lastly, gPPI analyses are correlational and as is noted above, studies are greatly needed to test the directionality of THC’s effects on amygdala-PFC functional connectivity. This could include studies that implement Granger causality analyses or dynamic causal modeling (eg, Zhang et al., 2013).
In sum, the present results suggest that THC dampens amygdala reactivity and enhances AMYG-BL and AMYG-SF functional connectivity with the rACC/mPFC during the processing of threat in recreational cannabis users. These neural effects may have important affective implications, including increasing affect regulation capabilities and/or broad social-emotional processing.
Statement of Interest
None.
Acknowledgments
This work was supported in part by the Brain Research Imaging Center at University of Chicago and National Institutes of Health grants MH076198 (K.L.P.), DA024197 (K.L.P), DA002812, and DA009133 (H.d.W.).
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: inter-subject variability and probability maps. Anat Embryol 210:343–352. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan K L. (2007). Amygdala–frontal connectivity during emotion regulation. Soc Cog Affect Neur 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. (2012). Intrinsic amygdala–cortical functional connectivity predicts social network size in humans. J Neurosci 32: 14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 34: 3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Ressler KJ. (2007). Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectrums 12:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. (2010). Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav 35:644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. (2011). Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. Neuroimage 54:2503–2513. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, Martin-Santos R, Seal ML, O’Carol C, Atakan Z, Zuardi AW, McGuire P. (2010). Modulation of effective connectivity during emotional processing by Δ9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychop 13:421–432. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Morey RA, McCarthy G. (2014). Amygdala–prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol Psychiatry e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, King AC, Phan KL. (2013). Alcohol attenuates amygdala–frontal connectivity during processing social signals in heavy social drinkers. Psychopharmacology 229:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. (2009). Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend 105:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. (2011). Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56:881–889. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder F, Ducci F, Ferrell RE, Goldman D, Manuck SB. (2009). Divergent effects of genetic variation in endocannabinoid signaling on human threat-and reward-related brain function Biol Psychiatry 66:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A. (2001). Psychiatric effects of cannabis. Br J Psychiatry 178:116–122. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. (2005). Oxytocin increases trust in humans. Nature 435:673–676. [DOI] [PubMed] [Google Scholar]
- Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, Menon DK, Tracey I. (2013). Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 154(1):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61(4):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenui I, Povpovska A, de Wit H. (2008). Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28:2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. (2013). Aberrant amygdale-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 00:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. (2013). Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 64:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Sala M, Vigano D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, Parolaro D. (2007). Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Δ9-tetrahydrocannabinol in rats. Neuropsychopharmacology 32:2036–2045. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG. (2013). Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacology 16:255–260. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report 2012. New York: United Nations; 2012. [Google Scholar]
- Van Den Heuvel MP, Hulshoff Pol HE. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharm 20:519–534. [DOI] [PubMed] [Google Scholar]
- Van Hell HH, Bossong MG, Jager G, Kristo G, van Osch MJ, Zelaya F, Kahn RS, Ramsey NF. (2011). Evidence for involvement of the insula in the psychotropic effects of THC in humans: a double-blind, randomized pharmacological MRI study. Int J Neuropsychoph 14(10):1377–1388. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Beh 81:331–342. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Elsohly MA, Ross SA, Ambre J, de Wit H. (2002). Comparison of the subjective effects of Delta (9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology 161:331–339. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CSR. (2014). Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc Cogn Affect Neurosci 9:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]