Abstract
Background:
It is known that neurogenesis occurs throughout the life mostly in the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricle. We investigated whether neurogenesis occurred in the amygdala and its function in fear memory formation.
Methods:
For detection of newborn neurons, mice were injected intraperitoneally with 5-bromo-2’-deoxyuridine (BrdU) 2h before receiving 15 tone–footshock pairings, and newborn neurons were analyzed 14 and 42 days after training. To determine the relationship between neurogenesis and memory formation, mice were given a proliferation inhibitor methylazoxymethanol (MAM) or a DNA synthesis inhibitor cytosine arabinoside (Ara-C). To test whether sonic hedgehog (Shh) signaling was required for neurogenesis, Shh-small hairpin–interfering RNA (shRNA) was inserted into a retroviral vector (Retro-Shh-shRNA).
Results:
The number of BrdU+/Neuronal nuclei (NeuN)+ cells was significantly higher in the conditioned mice, suggesting that association of tone with footshock induced neurogenesis. MAM and Ara-C markedly reduced neurogenesis and impaired fear memory formation. Shh, its receptor patched 1 (Ptc1), and transcription factor Gli1 protein levels increased at 1 day and returned to baseline at 7 days after fear conditioning. Retro-Shh-shRNA, which knocked down Shh specifically in the mitotic neurons, reduced the number of BrdU+/NeuN+ cells and decreased freezing responses.
Conclusions:
These results suggest that fear learning induces Shh signaling activation in the amygdala, which promotes neurogenesis and fear memory formation.
Keywords: amygdala, fear memory, neurogenesis, sonic hedgehog
Introduction
Conventionally, memory is classified into short-term memory, lasting minutes to hours, and long-term memory, lasting several hours to days; the latter requires new protein synthesis (McGaugh, 2000; Abraham and Williams, 2008; Alberini, 2008; Gold, 2008; Rudy, 2008). In addition, 10–12h after a one-trial inhibitory avoidance task and light-footshock association, there is a novel protein synthesis and brain-derived neurotrophic factor (BDNF)-dependent phase of memory persistence lasting more than 7 days (Bekinschtein et al., 2007 2008; Ou et al., 2010). It is thought persistence of memory requires recurrent activation of neuronal activity (Wittenberg et al., 2002), which results in molecular changes that can reset a population of neurons to participate in future episodes of synaptic plasticity by reinforcing the efficacy of synaptic transmission and growth of dendrites and axons (Tolwani et al., 2002; Lamprecht and LeDoux, 2004). However, a single molecular cascade consisting of receptor activation, changes in protein phosphorylation, and synthesis of new proteins may not be sufficient for the persistence of long-term memory. Particularly, the basolateral amygdala (BLA) is necessary for the establishment and/or maintenance of remote fear memory (Poulos et al., 2009). We tested the hypothesis that neurogenesis may be a candidate mechanism contributing to long-term memory formation in the amygdala.
Neurogenesis occurs throughout life predominantly in the subgranular zone of the hippocampus and the subventricular zone of the lateral ventricle (Ming and Song, 2005; Balu and Lucki, 2009). Newly-generated neurons in the subventricular zone migrate to the olfactory bulb and play key roles in olfactory memory (Lledo et al., 2006). In the subgranular zone, new cells are differentiated into both neurons and glial cells, and the newborn neurons incorporate to the granule cell layer of the dentate gyrus (Lledo et al., 2006). A growing body of evidence supports the contribution of hippocampal newborn neurons to hippocampus-dependent memories (Shors et al., 2001; Saxe et al., 2006; Imayoshi et al., 2008). It was reported that neurogenesis in the adult hippocampus is required for trace eye blink conditioning (Shors et al., 2001). However, conflicting results also existed, as ablation of hippocampal neurogenesis had no effect on learning, even in hippocampus-dependent memory tasks such as the contextual fear memory task and the Morris water maze task (Shors et al., 2002; Snyder et al., 2005; Zhang et al., 2008). Recently, a unique functional role for newly-born neurons in exerting an antidepressant-like effect has been demonstrated (Snyder et al., 2011).
Sonic hedgehog (Shh) is a morphogen which plays a key role in regulating vertebrate organogenesis and numerous other processes in the developing nervous system, including midbrain and ventral forebrain neuronal differentiation and neuronal precursor proliferation (Ericson et al., 1995; Hynes et al., 1995; Chiang et al., 1996; Wechsler-Reya and Scott, 1999; Britto et al., 2002; Ruiz i Altaba et al., 2002). In the present study, we investigated the effect of fear conditioning on the neurogenesis in the amygdala. We found that activation of Shh signaling contributed to amygdala neurogenesis and the formation of fear memory.
Materials and methods
Animal Care
All procedures were approved by the Institutional Animal Care and Use Committee of the College of Medicine, National Cheng-Kung University. Four to five mice were housed in each cage, in a temperature-controlled (22 °C) animal colony, and food and water was available ad libitum. They were maintained on a 12 hour light-dark cycle with lights turned on at 07: 00 hours.
Pavlovian Fear Conditioning
Male C57BL/6J mice at 2 months of age were used in this study. The procedure of fear conditioning was described previously (Hsiao et al., 2011). After a 120 s acclimation period, mice were presented with a tone (20 s, 80 dB, 5kHz) that co-terminated with a foot shock (1 s, 0.7 mA). This tone–foot shock pairing was repeated 15 times with an inter-trial interval of 2min. Between training sessions, 75% ethanol was used for cleaning. Cue-dependent fear memory was assessed at 1, 14, and 42 days after training. Between each test session, 1% acetic acid was used for cleaning. The freezing behavior was defined as the absence of any movement except for respiration, which was recorded by camera and analyzed by Freeze Scan software (Med Associates).
Open Field Test
The mice were placed in the open field apparatus, made of a Plexiglas box (40×40×30cm), and allowed to explore freely for 15min. The total distance, velocity, and percentage of duration and distance in the central zone were measured by video tracking software (Ethovision).
Ara-C Infusion
Mice were anesthetized with chloral hydrate (400mg/kg, i.p.) and subsequently were mounted on a stereotaxic apparatus. The 26-gauge stainless steel tubing cannulae were implanted bilaterally into the amygdala (anteroposterior, -1.6mm; mediolateral, ±3.5mm; dorsoventral, -4.7mm). After the mice were allowed 7 days to recover, cytosine arabinoside (Ara-C, 1mM/1μl/per side; Colon-Cesario et al., 2006) dissolved in saline was infused bilaterally into the amygdala.
MAM Injection
MAM (7mg/kg; Dupret et al., 2005) was injected intraperitoneally once per day for 7 consecutive days, and then fear conditioning was conducted.
BrdU Labeling
BrdU (300mg/kg; Sigma Aldrich) was injected intraperitoneally into the mice 2h before fear conditioning (Cameron and McKay, 2001; Taupin, 2007; Magavi and Macklis, 2008). Mice were sacrificed at 14 or 42 days after fear conditioning to measure the newborn neurons (Bischofberger, 2007).
Immunofluorecent Staining
Brains were removed after perfusion with saline and 4% paraformaldehyde in phosphate buffered saline (PBS). Sections were pretreated with a saline-sodium citrate buffer at 85°C for 15min, incubated in 2 N HCl at 37°C for 30min, and then rinsed in 0.1M boric acid (pH 8.5) at room temperature for 10min. They were incubated in a blocking solution (3% bovine serum in PBS) at room temperature for at least 1h. The sections were incubated with primary antibodies in PBST plus 3% bovine serum at 4°C overnight. After being washed in PBS and Tween 20 (PBST) three times, the mixture of the secondary antibodies was applied to the sections. The sections were placed at room temperature for 2h, followed by washing three additional times with PBST. Fluorescence signals were detected with a Leica DM 2500 microscope.
Quantitation
The total numbers of BrdU-positive cells per section were counted in the amygdala, central amygdala nuclei, and medial amygdala nuclei. BrdU labeling was performed on every second section of 20 μm (microtome setting) in thickness, at least 560 µμm apart, from each mouse. Cells numbers were done by a MetaMorph image analysis system. Quantitative analysis of BrdU-immune fluoresccent-stained sections was done on a Leica DM 2500 microscope equipped with a video camera. One-way analysis of variance (ANOVA) and Newman–Keuls post hoc tests were performed to analyze the multiple comparisons. For double labeling, the colocalization of both BrdU and the markers NeuN, doublecortin (DCX), or Glial fibrillary acidic protein (GFAP) were confirmed on the slices using confocal laser scanning microscopy (Olmypus IX81, FV10-ASW). Triple labeling was performed for further verification of the BrdU positive cell phenotype. 4ʹ,6-diamidino-2-phenylindole (DAPI) nuclear labeling was performed on every second section, and the total number of Shh-positive cells was counted in the amygdala using a Leica DM 2500 microscope. To determine whether fear memory was specific to pairing groups, coronal sections of the amygdala from naive and unpaired groups were processed for Shh with DAPI labeling.
Antibodies for Staining
The following primary antibodies were used: mouse monoclonal antibodies for BrdU (1:200, Millipore), NeuN (1:1 000, Millipore), and GFAP (1:1 000, eBioscience); rat monoclonal antibody for BrdU (1:100, Abcam) and Shh (1:100, R&D systems); rabbit polyclonal antibody for Shh (1:500, Millipore) and enhanced GFP (1:1000, Thermo Fisher Scientific); and rabbit monoclonal antibody for doublecortin (1:200, Cell Signaling Technology). The following secondary antibodies were used: Alexa Fluor®594-conjugated Goat-anti-rabbit IgG, Alexa Fluor®594-conjugated Donkey-anti-rat IgG, Alexa Fluor®488-conjugated Sheep-anti-mouse IgG, Alexa Fluor®488-conjugated Goat-anti-rabbit IgG, Alexa Fluor®488-conjugated Donkey-anti-rat IgG, DyLightTM 405-conjugated Sheep-anti-mouse IgG, and DyLightTM 405-conjugated Donkey-anti-rat IgG (all 1:200, Jackson ImmunoResearch). DAPI (4’,6-diamidino-2-phenylindole; 1:1 000, Sigma-Aldrich) dye was used for staining of nuclei.
Western Blot Analysis
Basolateral amygdala tissues were lysed by homogenization buffer (50mM Tris-HCl, pH 7.5, 0.3M sucrose, 5mM EDTA), with protease/phosphatase inhibitor cocktail (Roche). Lysates were centrifuged at 12 000rpm for 30min. Supernatants were collected, and then protein concentration was measured by a Bradford assay. Proteins (150 μg) were taken and denatured at 95°C for 5 minutes. After electrophoresis via SDS-polyacrylamide gels (6.5–12.5%), proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk for 1h at room temperature. Western blotting analysis used rabbit anti-Shh (1:3 000, Millipore), rabbit anti-Ptc1 (1:500, Proteintech Group), and rat anti-Gli1 (1:15 000, R&D systems) antibodies overnight at 4℃, and then incubated with HRP-conjugated secondary antibodies for 1h at room temperature. Signals were detected using Hyperfilm-ECL (Amersham Biosciences). The intensity was measured using Image-J.
Shh-shRNA Retrovirus Production
To specifically knock down Shh in newborn cells, Shh-shRNA or scrambled shRNA was inserted into a pSIREN-RetroQ-ZsGreen1 retroviral shRNA expression vector (Clontech Laboratories, Inc.). The fluorescent ZsGreen1 protein was used as a tag to indicate transfection efficiency. Retroviruses were produced by the GP2-293 packaging cell line that cotransfected with the pSIREN-RetroQ-ZsGreen1 retroviral shRNA expression vector and pVSV-G using Lipofectamine® LTX Reagent (Life Technologies). Retroviruses were harvested at 48h after transfection, concentrated by Retro-X™ Concentrator (Clontech Laboratories), and purified to yield 1 × 106 transducing units/ml, which were stored at -80 ℃ until use. Viruses were stereotaxically injected into the amygdalas of the mice at the age of 2 months (anteroposterior, -1.6mm; mediolateral, ±3.5mm; dorsoventral, -4.7mm). The target sequence of Shh-shRNA and scrambled shRNA were described as follows:
Shh-shRNA, 5×-CCCGAC ATCA TATTTAAGGAT-3×; and
scrambled shRNA, 5×-GCTGTTGGACAG CGAGACCAT-3×.
Statistical Analysis
All values in the text were mean ± standard error of the mean. Two-way ANOVA was used to analyze the differences in freezing responses tested at 1, 14, and 42 days after post-training between MAM- and Vehicle-, Ara-C- and Vehicle-, and retroviral vector (Retro-Shh-shRNA)- and Retro-scramble-shRNA-treated mice. One-way ANOVA and Newman–Keuls post hoc comparisons were used to analyze the differences in BrdU+/NeuN+ cells and protein levels among naïve, unpaired, and paired mice. Unpaired t-tests were used to analyze differences of BrdU+/NeuN+ cells between Shh-shRNA-treated and scrambled control mice, and between MAM-treated and vehicle control mice. The level of significance was p < 0.05.
Results
Learning Induces Amygdalar Neurogenesis
Mice were conditioned with 15 tone–footshock pairings and were tested for retention of conditioned fear, using freezing behavior during tone presentation as a measure of the strength of cue-dependent memory. We found that 15 pairs elicited reliable retention of freezing responses for at least 42 days after conditioning (baseline: 2.8 ± 0.84%; 42 days: 71.0 ± 5.1%; F(4,105) = 92.55, p < 0.001, n = 22; Figure 1A). Therefore, we used 15 conditioned pairs in the following experiments.
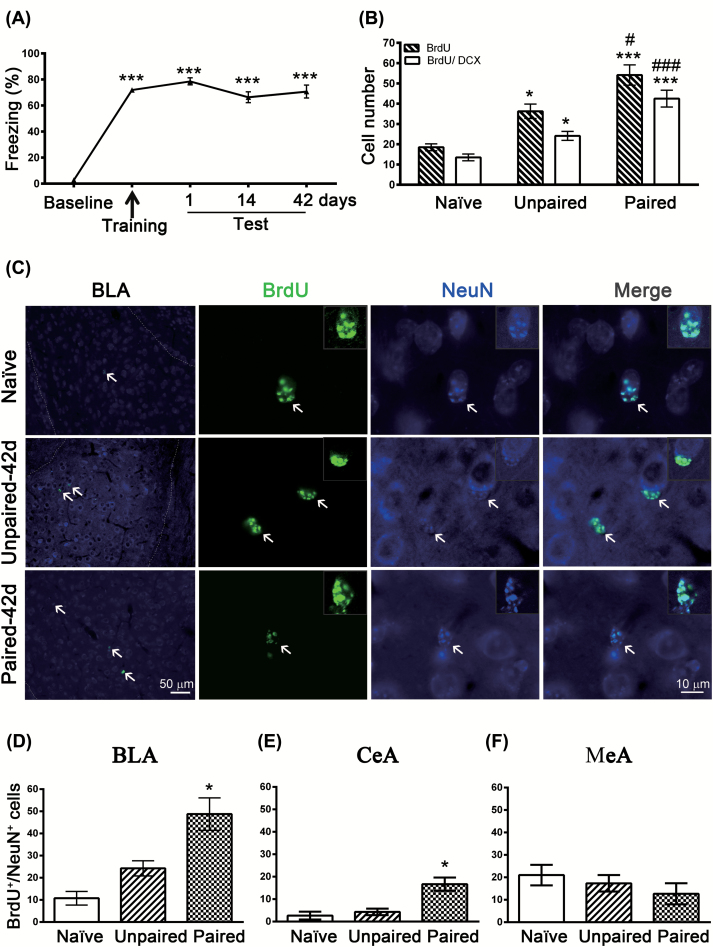
Figure 1.
Fear conditioning induces amygdalar neurogenesis. (A) Mice were given 15 tone-shock pairings and were tested for retention of conditioned fear at 1, 14, and 42 days after training, using freezing behavior during tone presentation as a measure of the strength of cue-dependent memory. **p < 0.001 vs. baseline (n = 22 mice/group). (B) Mice were intraperitoneally injected with 5-bromo-2’-deoxyuridine (BrdU; 300mg/kg) 2h before fear conditioning and BrdU+ and BrdU+/doublecortin+ cells were analyzed 14 days after training. *p < 0.05, **p < 0.001 vs. naïve (n = 6–8 mice/group); #p < 0.05, ##p < 0.001 vs. unpaired (n = 8 mice/group). (C) Mice were intraperitoneally injected with BrdU (300mg/kg) 2h before fear conditioning and BrdU+/Neuronal nuclei (NeuN)+ cells were analyzed 42 days after training. (D) Quantification of BrdU+/NeuN+ cells in the basolateral amygdala (BLA). *p < 0.05 vs. unpaired (n = 4 mice/group). (E) Quantification of BrdU+/NeuN+ cells in the central amygdala nuclei (CeA). *p < 0.05 vs. unpaired (n = 3 mice/group). (F) Quantification of BrdU+/NeuN+ cells in the medial amygdala nuclei (MeA). (n = 3 mice/group).
Mice were intraperitoneally injected with BrdU (300mg/kg) 2h before fear conditioning and BrdU+ cells were analyzed 14 days after training. The unpaired control mice were exposed to the conditioned stimulus (CS) and unconditioned stimulus (US) in an unpaired, pseudorandom fashion. One-way ANOVA showed that conditioning increased BrdU+ cells (F(2,19) = 18.87, p < 0.001, n = 6–8). Unexpectedly, the number of BrdU+ cells was significantly higher in unpaired than in naïve mice (t(19) = 3.049, p = 0.0198, n = 6–8), suggesting that footshock itself may increase neurogenesis (Figure 1B). This result was in contrast with the hippocampus, where stress is a potent inhibitor of adult neurogenesis (Gould et al., 1998; Malberg and Duman, 2003). Notably, the number of BrdU+ cells was significantly higher in paired than in unpaired mice (t(19) = 3.316, p = 0.0109, n = 8), suggesting that the association of tone with footshock did enhance neurogenesis. To determine whether fear conditioning had any effect on differentiation of BrdU+ cells, we performed a double-labeling immunohistochemistry experiment with antibodies against BrdU and immature neuronal marker DCX. We found 73% of BrdU+ cells were also positive for DCX. One-way ANOVA showed that conditioning increased BrdU+/DCX+ cells (F(2,19) = 21.88, p < 0.001, n = 6–8). Post hoc tests revealed that the number of BrdU+/DCX+ cells were significantly higher in the paired than in the unpaired mice (t(19) = 4.392, p = 0.0009, n = 8). Furthermore, double-labeling of BrdU and the neuronal marker NeuN (Figure 1C) showed a significant increase 42 days after fear conditioning (F(2,9) = 14.91, p = 0.0014, n = 4; Figure 1D). We also determined whether fear conditioning induced neurogenesis in other amygdala nuclei. As shown in Figure 1E and F, fear conditioning evoked neurogenesis in the central amygdala nuclei (F(2,6) = 12.53, p = 0.0072, n = 3), but not in the medial amygdala nuclei (F(2,6) = 0.9253, p = 0.4464, n = 3). Since the BLA is necessary for the establishment and/or maintenance of remote fear memory (Poulos et al., 2009), in the present study we focused our study on the BLA.
Role of Amygdalar Neurogenesis in Fear Conditioning
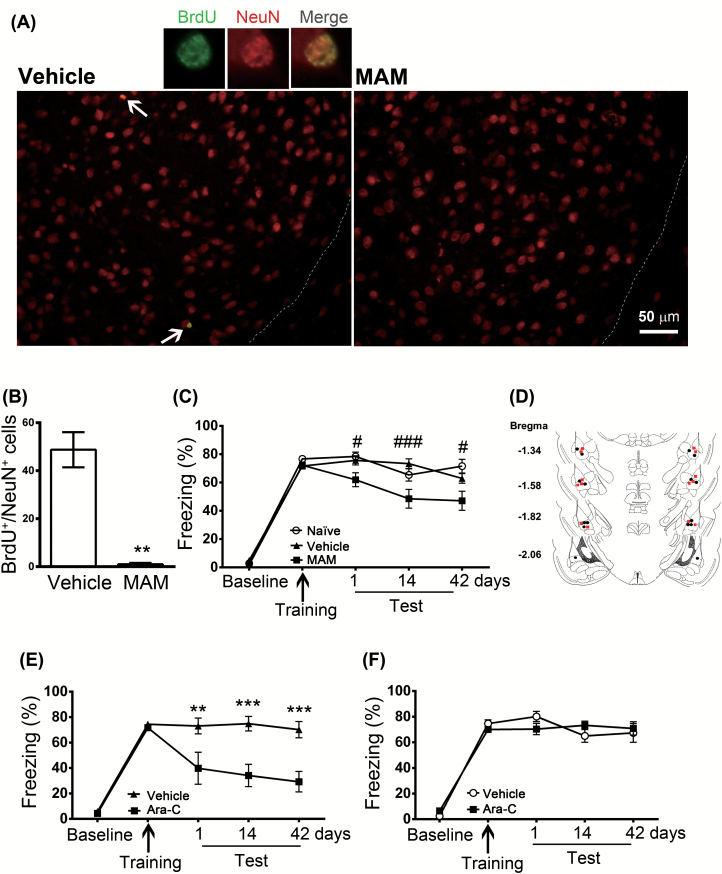
We determined the relationship between neurogenesis and memory formation by using the cell proliferation inhibitor, MAM. Mice received an intraperitoneal injection of MAM (7mg/kg) or vehicle once per day for 7 days, and fear conditioning was performed after the last injection. BrdU+/NeuN+ cells were analyzed 42 days after training. Treatment with MAM almost completely abolished BrdU+/NeuN+ cells (t(5) = 5.5142, p = 0.0027, n = 3–4; Figure 2A and B). Retention of memory was tested at 1, 14, and 42 days after conditioning. Figure 2C shows that both MAM and vehicle-treated mice were able to acquire fear. However, MAM decreased freezing responses to 60.9 ± 3.9%, 48.5 ± 6.6%, and 50.3 ± 5.9% when tested on 1, 14, and 42 days after training, respectively. A two-way ANOVA revealed a main effect of treatment (F(2,36) = 20.26, p < 0.001, n = 19), interaction (F(8,144) = 2.724, p = 0.008, n = 19), and post-training tests (F(4,72) = 178, p < 0.001, n = 19). Freezing responses in the MAM group were significantly lower at 1 (t(144) = 2.524, p = 0.0381, n = 19), 14 (t(144) = 4.576, p < 0.001, n = 19), and 42 (t(144) = 2.919, p = 0.0122, n = 19) days after training (Figure 2C). At the dose used (7mg/kg, i.p.), MAM slightly retarded weight gain. The body weights were 104.4 ± 2.9% and 92.7 ± 2.5% (t(24) = 3.16664, p = 0.0042, n = 13) of the final weight in vehicle- and MAM-treated mice, respectively. We examined the effect of MAM on open-field behaviors. There were no significant differences among saline and MAM treatment in the total distance moved (t(14) = 1.3707, = 0.1921, n = 8), percent of time spent in inner area (t(14) = 0.66299, p = 0.5181, n = 8), distance moved in inner area (t(14) = 1.3460, p = 0.1997, n = 8), and moving velocity (t(14) = 1.3315, p = 0.2043, n = 8).
Figure 2.
Effects of methylazoxymethanol acetate and cytosine arabinoside on freezing responses. Mice received an intraperitoneal injection of methylazoxymethanol (MAM) (7mg/kg) or vehicle once per day for 7 days. Two hours after the last injection, the mice were given 15 tone-shock pairings. (A) Mice were intraperitoneally injected with 5-bromo-2’-deoxyuridine (BrdU; 300mg/kg) 2h before fear conditioning and BrdU+/NeuN+ cells were analyzed 42 days after training. (B) Quantification of BrdU+/NeuN+ cells in the basolateral amygdala. *p < 0.01 vs. vehicle (n = 3–4 mice/group). (C) Retention of memory was tested 1, 14, and 42 days after conditioning. #p < 0.05, ##p < 0.001 vs. MAM (n = 19 mice/group). (D) Schematic representations of the cannula implanted with vehicle (●) or arabinoside (Ara-C; ■). (E) Ara-C (1mM/1 μl) was infused bilaterally into the amygdala 1h after the conditioning, and retention of memory was tested 1, 14, and 42 days after conditioning. *p < 0.01, **p < 0.001 vs. Ara-C (n = 8 mice/group). (F) Freezing response was unaltered when Ara-C (1mM/ 1μl) was infused bilaterally into the hippocampus (n = 12 mice/group).
Cytosine arabinoside (Ara-C) is rapidly converted into cytosine arabinoside triphosphate, which interferes with DNA synthesis. Ara-C (1mM/1 μl) was infused bilaterally into the amygdala 1h after the conditioning, decreasing freezing responses to 42.6 ± 7.9%, 40.6 ± 6.5%, and 28.4 ± 5.7% when tested on 1, 14, and 42 days after training, respectively (Figure 2E). A two-way ANOVA revealed significance for the interaction effect (F(4,28) = 4.661, p = 0.0025, n = 8), main effect of treatment (F(4,28) = 32.97, p = 0.001, n = 8), and post-training tests (F(1,7) = 24.42, p < 0.0017, n = 8). As a control, bilateral infusion of Ara-C into the hippocampal CA1 area did not impair formation of cue-dependent fear memory (F(4,44) = 1.457, p = 0.2317, n = 12; Figure 2F). Schematic representations of the cannula implanted with vehicle or Ara-C are shown in Figure 2D. We examined the effect of Ara-C on open-field behaviors. There were no significant differences among naïve, saline, and Ara-C treatments in the total distance moved (F(2,15) = 1.297, p = 0.3023, n = 6), percent of time spent in inner area (F(2,15) = 1.553, p = 0.2437, n = 6), distance moved in inner area (F(2,15) = 1.199, p = 0.3287, n = 6), and moving velocity (F(2,15) = 0.03509, p = 0.9656, n = 6). These results indicate that animals have normal exploratory activity and do not exhibit anxiety after treatment with Ara-C.
Fear Conditioning Induces Activation of Sonic Hedgehog Signaling
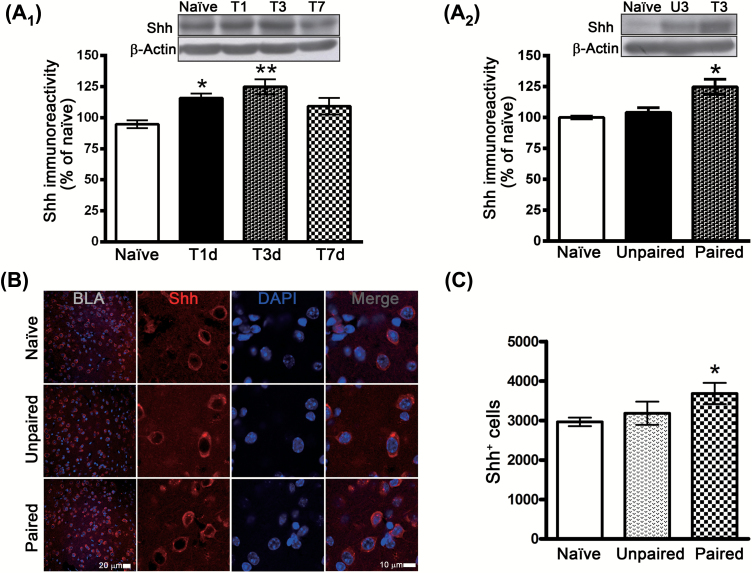
The Shh signaling pathway is important in the maintenance and proliferation of neural progenitor cells in adult rodent brains (Ahn and Joyner, 2005; Palma et al., 2005). We determined whether fear conditioning induced Shh activation. Mice were given 15 tone-footshock pairings, and the expression of Shh protein was determined by Western blotting analysis at 1, 3, and 7 days after conditioning. As illustrated in Figure 3A, Shh protein levels increased at 1 day (F(3,19) = 6.807, p = 0.0026, n = 5–6) and returned to baseline at 7 days after conditioning. The unpaired mice did not exhibit increased Shh protein (t(9) = 1.16554, p = 0.2738, n = 5–6), indicating that the increased expression is specific to the tone-footshock association (F(2,14) = 10.42, p < 0.0017, n = 5–6; Figure 3A). We confirmed the upregulation by immunohistochemistry (Figure 3B), and showed that Shh+ cells increased at 3 days after conditioning (F(2,10) = 5.975, p < 0.0196, n = 3–5; Figure 3C).
Figure 3.
Fear conditioning induces sonic hedgehog (Shh) expression in the basolateral amygdala (BLA). (A) Mice were given 15 tone-footshock pairings, and the expression of the Shh protein in the BLA was determined by Western blotting analysis at 1, 3, and 7 days after conditioning. *p < 0.05, **p < 0.01 vs. naïve (n = 5–6 mice/group). (B) Western blotting analysis of Shh protein levels in BLA of naïve, unpaired, and paired mice at 3 days after conditioning. *p < 0.05 vs. naïve (n = 5–6 mice/group). (C) Representative images of Shh-immunoreactive cells and 4’,6-diamidino-2-phenylindole (DAPI) nuclei in the BLAs of naïve unpaired and paired mice at 3 days after conditioning. (D) Quantification of Shh-positive cells in the BLAs of naïve, unpaired, and paired mice at 3 days after conditioning. *p < 0.05 vs. naïve and unpaired (n = 3–5 mice/group).
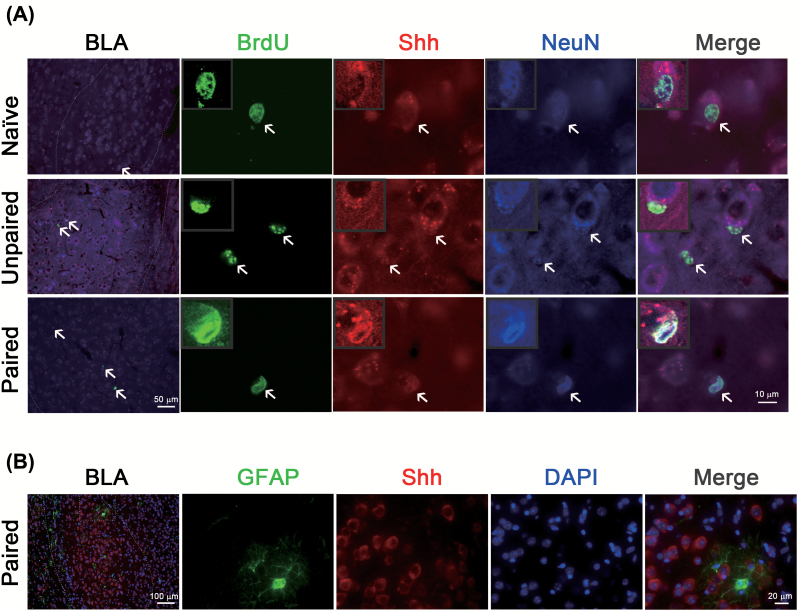
The basal level of Shh in the BLA neurons increased at 1 day and returned to baseline at 7 days after conditioning. In Figure 1, we have shown that fear conditioning increased BrdU+/NeuN+ cells at 42 days after training. We determined whether these upregulated proliferating and neuronal (BrdU+/NeuN+) cells co-localized with Shh. Figure 4A shows BrdU+/Shh+/NeuN+ cells increased significantly at 42 days after fear conditioning (F(2,9) = 15.22, p = 0.0013, n = 4) and that almost all BrdU+/NeuN+ cells were Shh+. By contrast, Shh was not co-localized with astrocyte biomarker GFAP (Figure 4B).
Figure 4.
Fear conditioning induces Shh/BrdU/NeuN-positive cells in the basolateral amygdala (BLA). (A) Mice were intraperitoneally injected with BrdU (300mg/kg) 2h before fear conditioning and BrdU+/Shh+/NeuN+ cells were analyzed 42 days after training. (B) GFAP+/Shh+/DAPI+ cells were analyzed 3 days after training. BrdU, 5-bromo-2’-deoxyuridine; GFAP, Glial fibrillary acidic protein; Shh, sonic hedgehog.
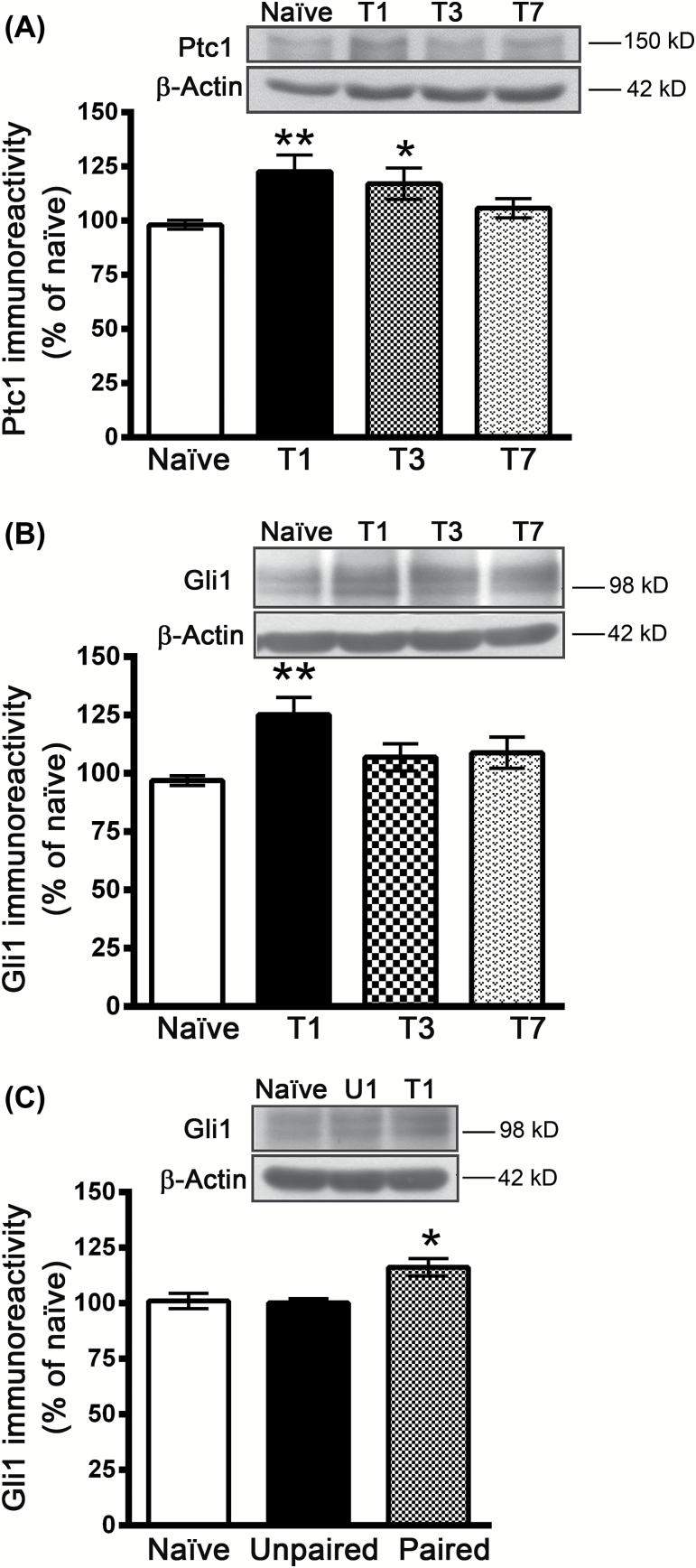
Binding of Shh to its receptor, patched 1 (Ptc1), blocks the inhibitory effect of Ptc1 on smoothened (Smo), which in turn activates the transcription factor Gli1 (Ruiz i Altaba et al., 2002). To determine whether fear conditioning induces Shh/Gli1 signaling activation, we examined the expression of Ptc1 and Gli1. Figure 5A shows that the protein level of Ptc1 increased at 1 day and returned to baseline at 7 days after conditioning (F(3,28) = 3.666, p = 0.024, n = 8). Similarly, the protein level of Gli1 increased at 1 day and returned to baseline at 7 days after conditioning (F(3, 15) = 4.306, p = 0.0222, n = 5–6; Figure 5B). The unpaired mice did not exhibit increased Gli1 proteins, indicating that the increased expression is specific to the tone-footshock association (F(2, 12) = 7.764, p = 0.0069, n = 5; Figure 5C).
Figure 5.
Fear conditioning induces expression of patched 1 (Ptc1) and Gli1. (A) Mice were given 15 tone-footshock pairings, and the expression of the Ptc1 protein in the basolateral amygdala was determined by Western blotting analysis at 1, 3, and 7 days after conditioning. *p < 0.05, **p < 0.01 vs. naïve (n = 8 mice/group). (B) The expression of the Gli1 protein in the BLA was determined by Western blotting analysis at 1, 3, and 7 days after conditioning. **p < 0.01 vs. naïve (n = 5–6 mice/group). (C) The expression of the Gli1 protein in the BLA was determined by Western blotting analysis at 1 day after conditioning in naïve, unpaired, and paired mice. *p < 0.05 vs. naïve and unpaired (n = 5 mice/group).
Role of Sonic Hedgehog Signaling in Amygdalar Neurogenesis and Fear Memory Formation
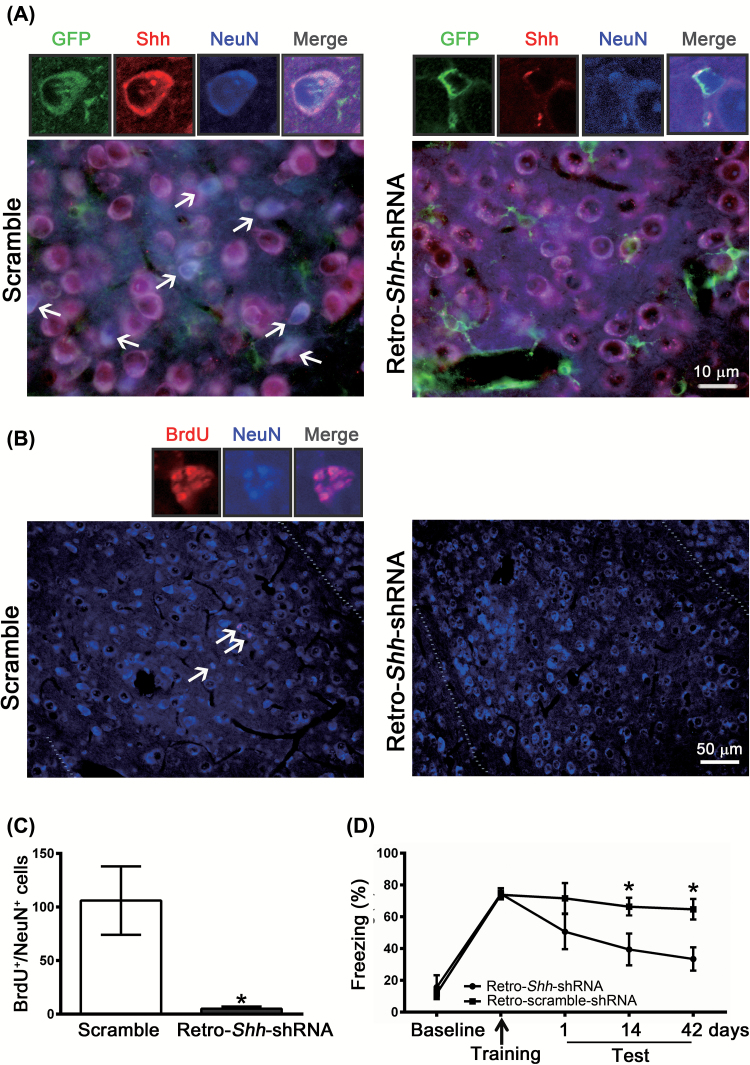
To test whether Shh signaling is required for fear conditioning–induced neurogenesis, we performed experiments in which Shh-shRNA was inserted into a retroviral vector (Retro-Shh-shRNA) tagged with GFP, which allowed us to knockdown Shh specifically in the mitotic neurons. Retention of memory was assessed at 1, 14, and 42 days after conditioning. Shh-shRNA or control-scrambled shRNA was infused bilaterally into the BLA 10 days before fear conditioning. Figure 6A is the representative micrographs of amygdala slices from the mice injected with Retro-Scramble or Retro-Shh-shRNA. Compared with scramble-treatment, treatment with Retro-Shh-shRNA reduced the number of BrdU+/NeuN+ cells by 90% (t(4) = 3.155, p = 0.0343, n = 3; Figure 6B and C). In parallel, the magnitude of freezing response in Retro-Shh-shRNA–treated mice was significantly lower than those of Retro-scramble-shRNA–treated mice (Figure 6D). Two-way ANOVAs revealed main effects of treatment (Retro-Shh-shRNA vs. scramble, F(1,11) = 7.004, p = 0.0227, n = 6–7), post-training tests (F(4,44) = 18.31, p < 0.001, n = 6–7), and interaction (F(4,44) = 2.239, p = 0.0801, n = 6–7). Together, these results indicate that Shh signaling in the newborn neurons contribute to conditioning-induced neurogenesis in the amygdala.
Figure 6.
Selective knockdown of sonic hedgehog (Shh) in mitotic neurons with small hairpin–interfering RNA (shRNA) by means of a retrovirus expression system reduces neurogenesis and impairs long-term memory formation. We generated retroviral vectors carrying either Shh-shRNA (Retro-Shh-shRNA) or scrambled shRNA. Retro-Shh-shRNA or scramble shRNA was infused bilaterally to the amygdala 10 days before fear conditioning. (A) Representative images of GFP+/Shh+/NeuN+ cells in the basolateral amygdala of Retro-Shh-shRNA– or scramble-shRNA–treated mice were detected 42 days after training. (B) Mice were intraperitoneally injected with BrdU (300mg/kg) 2h before fear conditioning and BrdU+/NeuN+ cells were analyzed 42 days after training. (C) Quantification of BrdU+/NeuN+ cells in BLA of scramble- or Retro-Shh-shRNA–treated mice. *p < 0.05 vs. scramble (n = 3 mice/group). (D) Freezing responses of scramble- and Retro-Shh-shRNA–treated mice were assessed at 1, 14, and 42 days after conditioning. *p < 0.05 vs. retro-Shh-shRNA (n = 6–7 mice/group).
Discussion
The primary findings of this study were: (1) pairing tone with footshock increased adult neurogenesis in the amygdala; (2) pretreatment with proliferation inhibitor MAM and post-training application of DNA synthesis inhibitor Ara-C impaired consolidation of fear memory; (3) Western blotting analysis showed that Shh protein level increased at 1 day and returned to baseline at 7 days after conditioning; (4) immunohistochemistry for BrdU, Shh, and NeuN revealed that Shh+ cells significantly increased after fear conditioning; (5) protein levels of Ptc1 and Gli1 increased at 1 day and returned to baseline at 7 days after conditioning; and (6) silencing Shh gene expression with shRNA by retrovirus expression system decreased freezing responses. These results suggest that cued fear conditioning can induce Shh signaling in the amygdala, which in turn promotes neurogenesis and long-term fear memory formation.
We have demonstrated that auditory fear conditioning increased neurogenesis in the amygdala. However, it was noted that neurogenesis also increased when mice received tone and footshock conditioning in a random, unpaired manner, implying that footshock itself also increased neurogenesis. This result was in conflict with previous reports in the hippocampus, where neurogenesis was inhibited by exposure to stressful events such as footshocks (Gould et al., 1998; Malberg and Duman, 2003). Indeed, the same behavioral stress could elicit opposite effects on the hippocampus and amygdala simultaneously. For example, repeated or chronic stress caused dendritic atrophy in hippocampal CA3 neurons but triggered dendritic growth and spinogenesis in BLA neurons (Vyas et al., 2002; Duman and Monteggia, 2006). Lakshminarasimhan and Chattarji (2012) demonstrated that both acute and chronic stress triggered opposite effects on BDNF levels in the hippocampal CA3 and BLA. Stress reduced BDNF levels in area CA3, but increased them in the BLA (Lakshminarasimhan and Chattarji, 2012). Because BDNF is known to regulate neurogenesis, it is likely that footshocks elicit BDNF release in the amygdala and subsequently increase neurogenesis.
Whether adult neurogenesis contributes to the circuitry of memory formation is still in controversy. In the present study, we found that pre-conditioning administration of MAM did not influence the acquisition of a fear response but impaired memory 1, 14, and 42 days later. A similar result was observed with post-conditioning administration of Ara-C. The detrimental effects of MAM and Ara-C treatments on fear memory were not directly attributable to the effects of MAM on movement or induction of anxiety. All groups displayed similar levels of total distance moved, percent of time spent in inner area, distance moved in inner area, and moving velocity in the open field test. In addition, the effect of Ara-C was region-specific, since administration of Ara-C to the hippocampus was without effect. Thus, we conclude that learning induces neurogenesis in the amygdala, which in turn contributes to long-term memory formation.
How Shh signaling is activated by fear conditioning is not known. It was reported that electroconvulsive seizures upregulated Shh signaling pathways in the subgranular zone, suggesting that glutamate release during seizures may impact the expression of Shh and its receptors. It is thus conceivable that strong 15 CS-US pairings trigger a large glutamate release in the BLA, which activates Shh signaling and subsequently induces neurogenesis and long-term fear memory formation. Future study should elucidate the mechanism of glutamate-induced activation of Shh signaling in the BLA.
Shh exerts its biological functions through binding to Ptc1, associated with smoothened (Smo) a G protein-coupled receptor family member, leading to downstream activation of target genes such as the transcription factor Gli1 (Ruiz i Altaba et al., 2002). In situ hybridization experiments in the adult rat brain revealed the presence of Ptc1-positive cells, mostly distributed in the Purkinje cell layer of the cerebellum, the median eminence region, and the facial nucleus (Traiffort et al., 1998 1999). Moderate to strong signals were observed in numerous cells of medium size located in the basomedial and medial amygdala nuclei (Traiffort et al., 1999). Although previous in situ hybridization results showed that Shh transcripts were not present at detectable levels in the adult BLA (Traiffort et al., 1999), we have shown Shh expression in this brain region using immunohistochemistry. In addition, almost all Shh-positive cells were NeuN-positive and Shh-labeling was not co-localized with GFAP-labeling, suggesting that Shh was selectively expressed in the neuron. A possible explanation for this discrepancy is that the latter is a more sensitive technique. The upregulation of Shh, Ptc1, and Gli1 protein expression in the BLA of paired mice but not in unpaired mice suggests that the association of tone with footshocks activates Shh signaling. Furthermore, the inhibition of fear memory 42 days after conditioning by Shh-shRNA is consistent with a previous report that the BLA is necessary for the establishment and/or maintenance of remote fear memory (Poulos et al., 2009). The incomplete block of fear memory formation suggests that Shh signaling and neurogenesis in the BLA contribute in part to the fear memory formation. Persistent long-term memory also requires gene expression and the resultant synthesis of new proteins, as well as structural changes at postsynaptic sites on dendritic spines (Lamprecht and LeDoux, 2004). It was noted that fear conditioning also evoked neurogenesis in the central amygdala nuclei, but not in the medial amygdala nuclei. It is likely that neurogenesis in the central amygdala may participate in long-term memory formation. Further study is warranted.
In summary, the present study demonstrates an unexplored role for hedgehog signaling in the mediation of fear learning–induced neural progenitor proliferation and differentiation in the BLA. Our results suggest that blocking Shh signaling could interfere with amygdalar neurogenesis and the long-term fear memory. Thus, Shh signaling cascades may represent a new set of potential therapeutic targets for prevention of excessive fear or anxiety-induced post-traumatic stress disorder.
Statement of Interest
None.
Acknowledgment
This study was supported by grants NHRI-EX101-10117NI from the National Health Research Institute and NSC101-2321-B-006-025 from the National Science Council. We thank Dr Min-Der Lai for critical comments on the manuscript.
References
- Abraham WC, Williams JM. (2008). LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem 89:260–268. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. (2005). In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437:894–897. [DOI] [PubMed] [Google Scholar]
- Alberini CM. (2008). The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol Learn Mem 89:234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Lucki I. (2009). Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev 33:232–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. (2007). Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron 53:261–277. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. (2008). BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA 105:2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J. (2007). Young and excitable: new neurons in memory networks. Nat Neurosci 10:273–275. [DOI] [PubMed] [Google Scholar]
- Britto J, Tannahill D, Keynes R. (2002). A critical role for sonic hedgehog signaling in the early expansion of the developing brain. Nat Neurosci 5:103–110. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406–417. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413. [DOI] [PubMed] [Google Scholar]
- Colon-Cesario M, Wang J, Ramos X, Garcia HG, Davila JJ, Laguna J, Rosado C, Pena de Ortiz S. (2006). An inhibitor of DNA recombination blocks memory consolidation, but not reconsolidation, in context fear conditioning. J Neurosci 26:5524–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. (2006). A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dupret D, Montaron MF, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. (2005). Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur J Neurosci 22:778–783. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. (1995). Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell 81:747–756. [DOI] [PubMed] [Google Scholar]
- Gold PE. (2008). Protein synthesis inhibition and memory: formation vs amnesia. Neurobiol Learn Mem 89:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. (1998). Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 95:3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YH, Chen PS, Chen SH, Gean PW. (2011). The involvement of Cdk5 activator p35 in social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit in the transgenic mice. Neuropsychopharmacology 36:1848–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. (1995). Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 15:35–44. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11:1153–1161. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. (2012). Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLOS ONE 7:e30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. (2004). Structural plasticity and memory. Nat Rev Neurosci 5:45–54. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. (2006). Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. (2008). Identification of newborn cells by BrdU labeling and immunocytochemistry in vivo . Methods Mol Biol 438:335–343. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. (2003). Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2000). Memory—a century of consolidation. Science 287:248–251. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. [DOI] [PubMed] [Google Scholar]
- Ou LC, Yeh SH, Gean PW. (2010). Late expression of brain-derived neurotrophic factor in the amygdala is required for persistence of fear memory. Neurobiol Learn Mem 93:372–382. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. (2005). Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. (2009). Persistence of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci USA 106:11737–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. (2008). Is there a baby in the bathwater? Maybe: some methodological issues for the de novo protein synthesis hypothesis. Neurobiol Learn Mem 89:219–224. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Palma V, Dahmane N. (2002). Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci 3:24–33. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. (2007). BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 53:198–214. [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM. (2002). BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience 114:795–805. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk DA, Faure H, Ruat M. (1998). Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem 70:1327–1330. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. (1999). Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur J Neurosci 11:3199–3214. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22:103–114. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Sullivan MR, Tsien JZ. (2002). Synaptic reentry reinforcement based network model for long-term memory consolidation. Hippocampus 12:637–647. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. (2008). A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451:1004–1007. [DOI] [PubMed] [Google Scholar]