Abstract
Background:
In Alzheimer’s disease, growing evidence has shown that uncontrolled glial activation and neuroinflammation may contribute independently to neurodegeneration. Antiinflammatory strategies might provide benefits for this devastating disease. The aims of the present study are to address the issue of whether glial activation and proinflammatory cytokine increases could be modulated by quetiapine in vivo and in vitro and to explore the underlying mechanism.
Methods:
Four-month–old amyloid precursor protein (APP) and presenilin 1 (PS1) transgenic and nontransgenic mice were treated with quetiapine (5mg/kg/d) in drinking water for 8 months. Animal behaviors, total Aβ levels, and glial activation were evaluated by behavioral tests, enzyme-linked immunosorbent assay, immunohistochemistry, and Western blot accordingly. Inflammatory cytokines and the nuclear factor kappa B pathway were analyzed in vivo and in vitro.
Results:
Quetiapine improves behavioral performance, marginally affects total Aβ40 and Aβ42 levels, attenuates glial activation, and reduces proinflammatory cytokines in APP/PS1 mice. Quetiapine suppresses Aβ1-42-induced activation of primary microglia by decresing proinflammatory cytokines. Quetiapine inhibits the activation of nuclear factor kappa B p65 pathway in both transgenic mice and primary microglia stimulated by Aβ1–42.
Conclusions:
The antiinflammatory effects of quetiapine in Alzheimer’s disease may be involved in the nuclear factor kappa B pathway. Quetiapine may be an efficacious and promising treatment for Alzheimer’s disease targeting on neuroinflammation.
Keywords: quetiapine, neuroinflammation, microglia, astrocyte, NF-κB
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder leading to dementia. Extracellular β-amyloid (Aβ) plaques, intracellular neurofibrillary tangles, and massive neuronal cell and synapse loss represent the main pathological hallmarks in AD brains (Storey and Cappai, 1999; Storey et al., 1999; Selkoe, 2002). Apart from these classic hallmarks, increasing evidence has demonstrated uncontrolled glial activation and neuroinflammation in AD brain may contribute independently to neural dysfunction and cell death (Akiyama et al., 2000; Wyss-Coray and Mucke, 2002). Robust activation of microglia has been found in and around the area of amyloid plaques in the AD brain, and reactive astrocytes have been shown to form a halo surrounding the amyloid plaques (Itagaki et al., 1989; Ho et al., 2005). Additionally, numerous proinflammatory factors have been reported to be elevated in both patients with AD and transgenic animal models of AD (Griffin et al., 1989; Akiyama et al., 2000; Ruan et al., 2009). Whether alleviation of neuroinflammation will offer therapeutic benefit for AD remains unclear. Epidemiological studies show a possible association between suppression of inflammation and reduced risk for AD (in t’ Veld et al., 2001; Vlad et al., 2008). Therefore, drugs targeting neuroinflammation might provide benefits for the prevention and treatment of this devastating disease.
In the central nervous system, microglia and astrocytes are the major type of glial cells, and activation of these cells has been involved in all neurodegenerative diseases (Wyss-Coray and Mucke, 2002). Nevertheless, the diverse physiological functions of glial activation might complicate the interpretation of experimental investigations and clinical observations related to AD pathology. For example, glial phagocytosis of Aβ is considered to be one key mechanism of the initial defense of the brain against the toxic accumulation of Aβ (Wyss-Coray et al., 2003; Zhang et al., 2014). As the disease progresses, continuous glial activation by Aβ releases excessive multiple cytokines and chemokines such as tumor necrosis factor α (TNFα) and interleukin 1β (IL-1β), monocyte chemotactic protein-1, and nitric oxide, which leads to a vicious cycle of further glial activation and neurotoxic damage through generating chronic self-sustaining inflammatory reactions (Paradisi et al., 2004; Perry et al., 2010). This process may stimulate and even accelerate the progression of AD.
The nuclear factor kappa B (NF-κB) is a transcription factor that is involved in regulating immune and inflammatory responses (Li and Verma, 2002; Kucharczak et al., 2003). The mammalian NF-κB family consists of RelA/p65, RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2) (Zheng et al., 2011). These proteins can form homo- or heterodimers, which often are held captive in cytoplasm, remaining inactive. The activated NF-κB translocates to the nucleus, which leads to expression of a number of inflammatory genes, including cyclooxygenase (COX), IL-1β, and TNFα (Zhang et al., 2009). NF-κB signaling has been proven to be involved in AD. Enhanced immunoreactivity was observed in neurons surrounding amyloid plaques in the brains of AD patients (Kaltschmidt et al., 1997). In addition, activated NF-κB has been found in microglia of patents with AD (Mattson and Camandola, 2001). In in vitro studies, NF-κB can be activated by Aβ in both neuronal and microglial cells (Huang et al., 2012). Together, it suggested that activation of NF-κB plays an important role in mediating neuroinflammation in AD.
Quetiapine (Seroquel) is a novel atypical antipsychotic drug that was approved for the treatment of patients with schizophrenia (Purdon et al., 2001; Velligan et al., 2002). Clinically, quetiapine is also used to treat psychosis in AD as well as cognition in Parkinson’s disease (Juncos et al., 2004; Madhusoodanan et al., 2007). In animal studies, quetiapine decreases the accumulation of activated astrocytes and microglia in demyelinated sites followed by cuprizone administration (Zhang et al., 2008), modulates immune responses in an experimental autoimmune encephalomyelitis model of multiple sclerosis (Mei et al., 2012), and inhibits NF-κB p65/p50 expression in ischemic mice (Bi et al., 2009). In vitro studies have shown that quetiapine inhibits nitric oxide generation and TNFα release from activated microglia (Bian et al., 2008). Although quetiapine has some beneficial effects on cognition in AD mice (Zhu et al., 2013), there are no data published with respect to its effect on glial activation and neuroinflammation in AD mice. In the present study, we wanted to address the issue of whether glial activation and proinflammatory cytokine increases could be modulated by quetiapine through regulating the NF-κB pathway in an amyloid precursor protein (APP) and presenilin 1 (PS1) humanized knockin mouse model of AD.
Materials and methods
Animals and Treatments
APP/PS1 double transgenic and nontransgenic mice were generated from mating between single transgenic mice expressing human mutant APPK670N/M671L (Hsiao et al., 1996) and mutant PS1M146L (Duff et al., 1996) and chosen by the genotyping results of polymerase chain reaction. The age- and sex-matched wild-type mice were used as the controls. All mice had free access to food and water under controlled laboratory conditions. All procedures with animals were performed in accordance with the guidelines established by the Canadian Council on Animal Care and were approved by the Animal Care Committee of the University of Manitoba.
Quetiapine was obtained from AstraZeneca Pharmaceuticals (Macclesfield, UK). The drug was dissolved in sterile water and delivered to mice at the dose 5mg/kg/d for 8 months, starting from the age of 4 months. The doses chosen referred to our previous report (He et al., 2009). APP/PS1 double transgenic mice and wild-type littermates were randomly assigned to 4 groups: nontransgenic + water (control age- and sex-matched wild-type mice), nontransgenic + quetiapine 5mg/(kg d), transgenic + water, and transgenic + quetiapine 5mg/(kg d).
Open Field Test
The open field test was performed in a bare square box (36×36 inches) made of compressed wood and painted in grey. The open field box was divided into outer and inner zones. Mice were placed in a particular corner of the arena and tracked using ANY-Maze Video Tracking Software (Stoelting, Wood Dale, IL) with a digital camera. Mice were allowed to explore the maze for 5 minutes, after which they were returned to their home cage. The maze was cleaned with 75% ethanol wipes before commencing testing with the next mouse. The time in the center and total ambulation (in meters) were taken as measures of anxiety.
Object Recognition Test
Nonspatial memory of mice was measured using the object recognition test as previously described (Clark et al., 2000). Mice were placed into a 40cm (width) × 40cm (width) × 23cm (height) Plexiglas square box. It consisted of 3 sessions: habituation, training, and retention (He et al., 2006). During the training session, mice were individually placed in the activity box for 10 minutes of free exploration, in which 2 identical objects (objects A1 and A2) were positioned in 2 adjacent corners. During the retention session for the short-term memory test, mice were placed back into the same box 1 hour later containing 1 of the previous objects (A1 or A2) and a novel object (B) for a 5-minute testing session. During the retention session for the long-term memory test, animals were subsequently placed back into the same box 23 hours after the short-term memory test (24 hours after the training session) for 5 minutes of free exploration, where object B was replaced by a novel object C. During the retention session, the time spent exploring the novel object (B or C) was used to measure memory function. Object exploration was considered as a mouse’s nose touching the object or was facing and within 2cm to the object (Oh et al., 2010). Exploratory activity of each object was recorded for both training and testing sessions using ANY-Maze Video Tracking Software (Stoelting) and analyzed off-line with the experimenter blinded to treatment and genotypes.
Tissue Processing
After the above behavioral tests, animals were anesthetized and perfused with phosphate-buffered saline (PBS; pH 7.4). The hemispheres were separated by cutting at the midline. The cortex and hippocampus from the right hemisphere were separated and used for biochemistry analyses. The left hemisphere was post-fixed in 4% paraformaldehyde in PBS and then cryoprotected in 30% sucrose in PBS (Qing et al., 2008; He et al., 2009). Finally, the left hemisphere was cut into 30-μm thick coronal sections.
Immunohistochemistry
Six free-floating sections from each animal were first incubated with 0.3% H2O2 in 0.01M PBS for 30 minutes at room temperature to quench endogenous peroxidase activity, then blocked with 5% goat serum and 0.3% Triton X-100 in PBS for 1 hour, and then incubated overnight at 4°C with anti-glial fibrillary acidic protein (GFAP) mouse mAb (1:1000; Sigma, St. Louis, MO) and anti-ionized calcium binding adapter molecule 1 (Iba1) rabbit pAb (1:500; Wako Chemicals, Richmond, VA). After rinsing, the sections were incubated with appropriate biotinylated secondary antibody (1:500; Vector Laboratories, Burlingame, CA) at room temperature for 1 hour. Staining was achieved with the avidin biotin complex kit (Vector Laboratories, Burlingame, CA) and visualized with 3,3-diaminobenzidine chromogen (ThermoFisher Scientific, Waltham, MA). Slides were viewed with an Axio-Imager M2 and Zen software for image acquisition (Carl Zeiss, Jena, Germany). The immunohistochemical controls were performed as above, but with the omission of the primary antibodies. No positive immunostaining was found in any of the controls.
Western Blotting
Protein samples were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis mini-gels under reducing conditions. They were then electrophoretically transferred onto nitrocellulose membranes. Membranes were blocked with 5% (wt/vol) nonfat dried milk in Tris-buffered saline with Tween 20 buffer and were probed at 4°C overnight with the following antibodies: a rabbit polyclonal anti-C-terminal APP (1:3000) antibody (Sigma, St. Louis, MO), a rabbit polyclonal anti-PS1 (1:1000) antibody (Cell Signaling Technology, Danvers, MA), a mouse monoclonal anti-GFAP (1:1000) antibody (Sigma), a mouse monoclonal anti-NF-κB p65 (1:500) antibody (Santa Cruz Biotechnology, CA), a mouse monoclonal anti-β-actin (1:5000) antibody (Santa Cruz), and a mouse monoclonal anti-glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (1:1000) antibody (Abcam, Cambridge, MA). Blots were then incubated at room temperature for 2 hours with corresponding peroxidise-conjugated secondary antibodies. Proteins were detected by enhanced chemiluminescence (Amersham Biosciences, NJ). Band densities were quantified using the Bio-Rad Laboratories Quantity One Software (Hercules, CA). All target proteins were normalized to β-actin or GAPDH and then standardized to the corresponding control group.
Mouse Primary Microglia Culture and Treatment
Microglial cultures were prepared from mixed glial cultures as previously described (Kauppinen et al., 2008). Briefly, cortices were dissected from 1-day-old mice in Hanks’ Balanced Salt Solution (Invitrogen). Cells were dissociated by mincing, followed by incubation in trypsin for 25 minutes at 37°C with agitation. After centrifugation for 5 minutes at 1000rpm, the cells were resuspended with Dulbecco’s Modified Eagle Medium (Invitrogen) with 10% fetal bovine serum. Cells were plated on 75-cm2 flasks at a density of 1.5×107 cells per flask and maintained in a 37°C, 5% CO2 incubator. The medium was changed every 3 to 4 days. After 2 weeks in vitro, microglia were harvested by shaking the flasks at 200rpm on a rotary shaker for 4 hours at 37°C. Cells were collected, washed, and seeded at a density of 1×106 cells/mL. The purity of the microglial cultures was tested by immunocytochemical staining for Iba1, a microglia marker, and for GFAP, an astrocyte marker. The purity of microglial cultures was found to be ∼95%. Also, the cellular morphology was carefully investigated under phase contrast microscope.
Synthetic Aβ1–42 peptide (American Peptide Company, Sunnyvale, CA) was dissolved in distilled water and incubated for 1 week at 37°C before use to induce fibril formation. Primary microglia were pretreated with quetiapine (0, 10 µM) for 1 hour and then they were exposed to 0 or 25 µM Aβ in the presence of the same concentrations of quetiapine for 6 hours.
ELISA
The levels of total Aβ40 and Aβ42 were measured using the Human Aβ ELISA Kits following the manufacturer’s protocol (Invitrogen-Biosource, Camarillo, CA). Each sample was assayed in duplicate at appropriate dilutions, so that relative luminescent units fell within the range of standard curves.
The proinflammatory cytokines IL-1β and TNFα in both brain and the supernatant of cultured microglia were measured using commercial ELISA kits (Invitrogen). Assays were performed according to the manufacturer’s instructions. The levels of IL-1β and TNFα in brain were corrected for total protein of tissue and dilution factor, and the final value in each group was standardized to the control group. The levels of IL-1β and TNFα in cultured microglia were expressed in picograms per milliliter.
Immunocytochemistry
Primary microglia were plated on culture slides (BD Science, Franklin Lakes, NJ). After treatment, cultured microglia were washed twice with PBS and fixed with 4% paraformaldehyde for 30 minutes. After washing twice with PBS, the cells were permeabilized with 0.2% Triton X-100 for 10 minutes and then incubated overnight with anti-NF-κB p65 (1:100) antibody (Santa Cruz) at 4°C. After washing, the cells were incubated with Alexa Fluor 594-conjugated secondary antibody (1:200, Invitrogen). Then, the cells were incubated with Alexa Fluor 488-conjugated phalloidin (Invitrogen) at room temperature for 50 minutes. Finally, the cells were stained with Hoechst 33342 (Calbiochem, Billerica, MA) for 5 minutes at room temperature. Images were taken with a fluorescence microscope (Olympus).
Statistical Analysis
All results are expressed as means±SEM. Analyses were performed using a 2-way analysis of variance (ANOVA) followed by Newman-Keuls posthoc test for multiple comparisons. A 2-tailed t test for independent samples was used for 2-group comparisons. Differences were considered significant at P<.05.
Results
Quetiapine Improves Behavioral Performance of APP/PS1 Mice
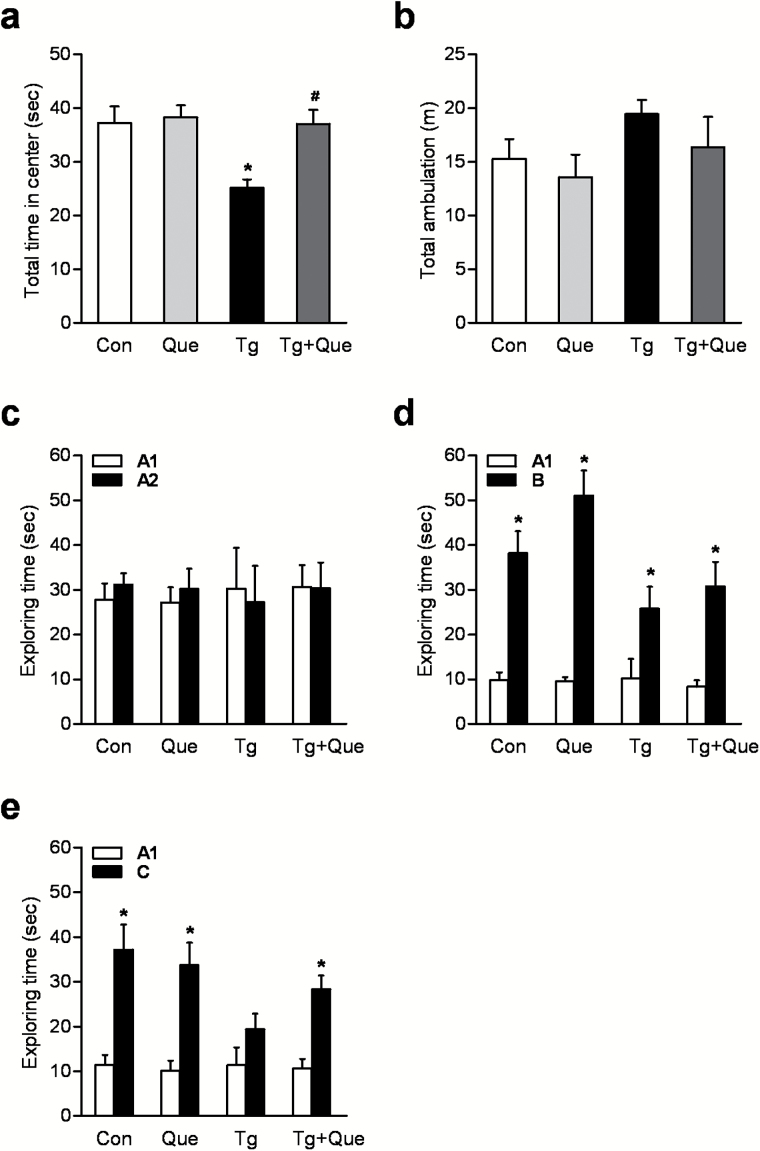
The open field test was used to measure locomotion, exploration, and anxiety-like behavior. Two-way ANOVA analysis conducted on the data for total time spent in the central area of the field showed that genotype [F(1, 28)=6.13, P<.05], and quetiapine [F(1, 28)=5.80, P<.05] produced a significant change on the time spent in the center (Figure 1a) and there was an interaction between genotype and quetiapine [F(1, 28)=4.10, P=.0526]. A posthoc analysis indicated that the time spent in the center in transgenic mice was less than that in control mice, which demonstrated an anxiety-like phenotype that developed in AD mice at 12 months of age. Quetiapine treatment significantly improved the decreased interaction with the center zone in transgenic mice (Figure 1a). To evaluate whether quetiapine or genotype significantly influenced results, general locomotor activity was examined by looking at total distance travelled in the open field test. There was no difference in the total distance travelled among all the groups (Figure 1b).
Figure 1.
Quetiapine improves behavioral performance in amyloid precursor protein (APP)/presenilin 1 (PS1) mice. Total time spent in the center (a) and the total distance travelled in an open field test (b). c, The exploration time of mice on identical objects (A1 and A2) in the object recognition test. A t test showed that all groups of mice demonstrated equal total exploration time for each of the identical objects in the training session. d, The exploration time of mice on a familiar object (A1) and a novel object (B) in a retention trial 1 hour after training. A t test showed that all mice spent more time exploring the novel objective. e, The exploration time of mice on a familiar object (A1) and a novel object (C) in a retention trial 24 hours after training. A t test showed that transgenic mice exhibited an impaired ability to discriminate between the familiar object and a novel object C, whereas transgenic mice treated with quetiapine spent more time exploring the novel object C. Data are expressed as means±SEM, n=7 to 10 mice per group. *P<.05 vs Con; # P<.05 vs transgenic + water.
The object recognition task measures nonspatial visual-discrimination memory in rodents and takes advantage of the mouse’s unprompted nature to prefer exploring novel objects in its surroundings (Kamei et al., 2006). In the training session, mice spent equal amounts of time on each of the 2 identical objects (Figure 1c), indicating that the 2 objects were equally preferred. In addition, the total amount of time spent exploring the objects (A1 + A2) was similar in all mice, suggesting that genotype and quetiapine had no effect on the levels of attention and motivation of these mice for the objects. During the 1-hour retention session, all mice spent more time exploring the novel object B (Figure 1d), indicating that transgenic mice exhibited no defects in memory for novel objects measured 1 hour after training. During the 24-hour retention test, nontransgenic mice treated with water or quetiapine were still able to discriminate between the familiar object and a novel object C (Figure 1E), exploring the latter for a significantly longer time. As expected, transgenic mice had no memory for the novel object C (Figure 1E), showing an impaired long-term memory. In contrast, transgenic mice treated with quetiapine spent more time exploring the novel object C (Figure 1E), implying quetiapine treatment significantly improved this long-term memory impairment in transgenic mice.
Quetiapine Marginally Affects Total Aβ40 and Aβ42 Levels in APP/PS1 Mice
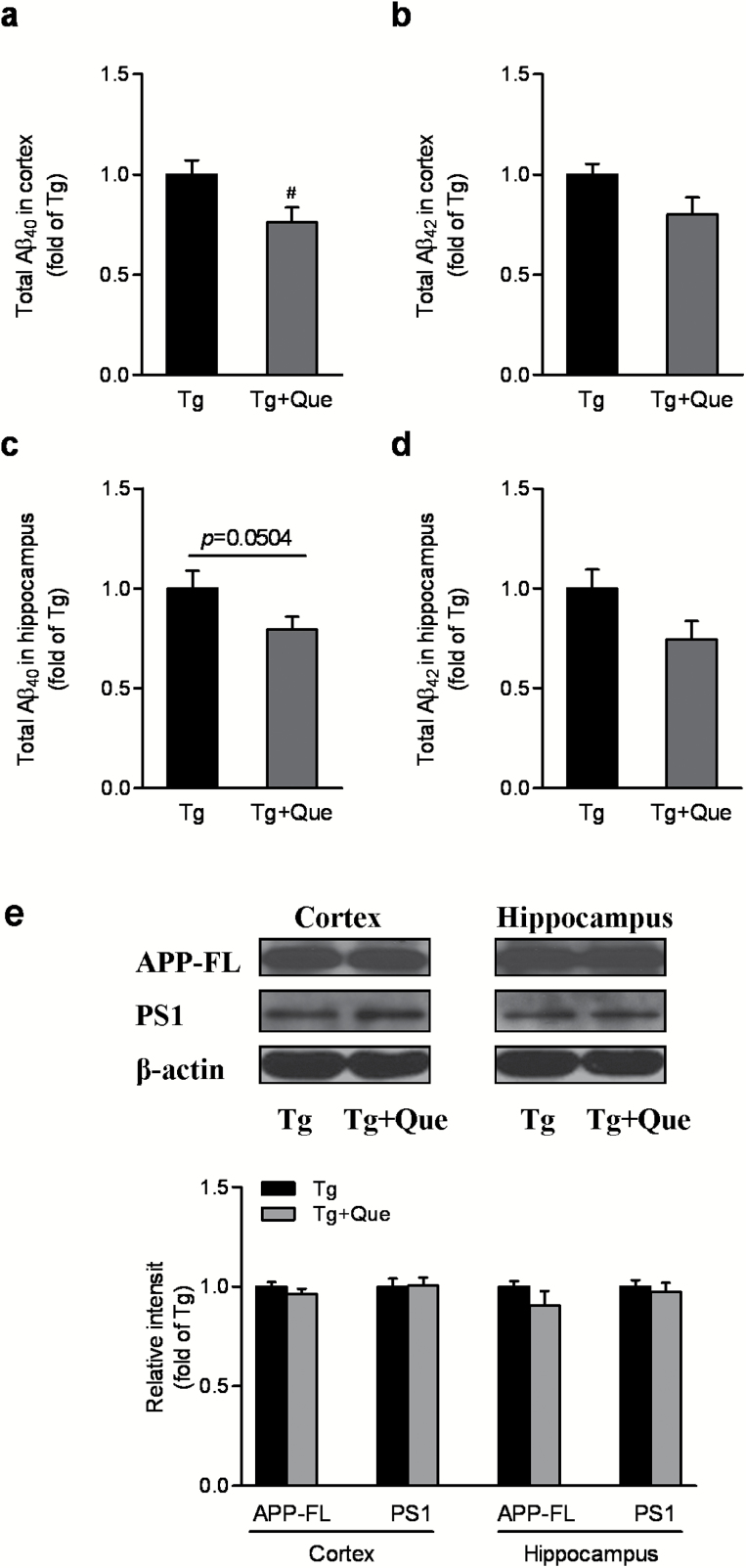
To understand why quetiapine improved the behavior of APP/PS1 mice, we next assessed the effects of quetiapine in aged APP/PS1 mice on total Aβ levels. Quantitative Aβ ELISA revealed a significant reduction of total Aβ40, but not Aβ42, in the cerebral cortex of quetiapine-treated APP/PS1 mice (P<.01) (Figure 2A-B). In the hippocampus of APP/PS1 mice, quetiapine also showed a tendency to decrease total Aβ40 and not Aβ42, but it was not statistically significant (P=.0504) (Figure 2C-D). Given the important role of APP and PS1 on Aβ production during APP processing, the expression of full-length APP and PS1 was determined by Western blot. As shown in Figure 2e, quetiapine had no influence on APP expression or processing, because the steady-state levels of full-length APP or PS1 were not altered by the treatment. These results suggest that quetiapine treatment may be capable of reducing certain Aβ species. However, this marginal effect of quetiapine on Aβ production cannot fully explain its beneficial effects in APP/PS1 mice on behavioral performance.
Figure 2.
Quetiapine marginally affects total β-amyloid (Aβ)40 and Aβ42 levels in APP/PS1 mice. Total Aβ40 (a) and total Aβ42 (b) in the cortex of transgenic mice. A t test showed a significant reduction of total Aβ40 but not Aβ42 in the cerebral cortex after quetiapine treatment. Total Aβ40 (c) and total Aβ42 (d) in the hippocampus of transgenic mice. e, Immunoblot analysis of APP and PS1 in both cortex and hippocampus following the treatment. Quantification of full-length APP and PS1 was shown in the graph. No statistcial significance was detected. Data are expressed as means±SEM, n=4 to 6 in each group. # P<.05 vs transgenic + water.
Quetiapine Attenuates Microglial Activation and Reduces Proinflammatory Cytokine Levels in APP/PS1 Mice
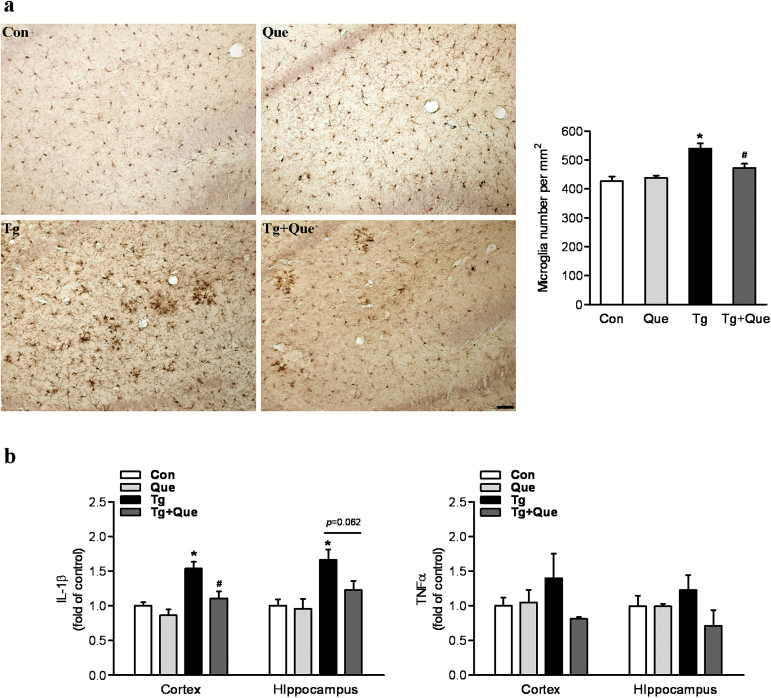
Neuroinflammation is reflected in AD and its transgenic models brain as elevated inflammatory cytokines and chemokines and the accumulation of activated microglia, particularly occurring around amyloid plaques (Matsuoka et al., 2001). We thus examined whether the activation of microglia was ameliorated by quetiapine treatment. The density of microglia was accessed by using the immunostaining of Iba1 antibody. Two-way ANOVA analysis showed that genotype [F(1, 25)=26.79, P<.0001] and quetiapine [F(1, 25)=3.96, P=.0577] produced significant changes on microglial cell density and that there was an interaction between genotype and quetiapine [F(1, 25)=7.61, P=.0107]. A posthoc analysis indicated that the Iba1-positive cells per mm2 were significantly increased in brains of APP/PS1 mice compared with those in brains of nontransgenic mice. Quetiapine treatment decreased microglia density in transgenic mouse brains (Figure 3a).
Figure 3.
Quetiapine attenuates microglial activation and reduces proinflammatory cytokines in APP/PS1 mice. a, Representative immunohistochemical staining with anti-ionized calcium binding adapter molecule 1 (Iba1) in hippocampus following the treatment. The scale bar represents 50 μm. Quantification of the number of Iba positive cells was shown in the graph. Two-way analysis of variance (ANOVA) showed microglial cell density was increased in transgenice mice and decreased following quetiapine treatment. (b) Enzyme-linked immunosorbent assay (ELISA) analysis of selected proinflammatory cytokines. Two-way ANOVA showed quetiapine treatment greatly attenuated the increase of interleukin 1β (IL-1β) in the cortex of transgenic mice. No statistcial significance was detected in the level of tumor necrosis factor α (TNFα). Data are expressed as means±SEM, n=5 to 8 mice per group. * P<.05 vs Con; # P<.05 vs transgenic + water.
To further confirm inhibitory inflammation of quetiapine in vivo, levels of proinflammatory cytokines IL-1β and TNFα in both cortex and hippocampus were determined. As shown in Figure 3b, 2-way ANOVA analysis conducted on the data for the level of IL-1β in cerebral cortex showed that genotype [F(1, 18)=21.56, P=.0002] and quetiapine [F(1, 18)=11.33, P=.0034] produced significant changes on the IL-1β level and that there was an interaction between genotype and quetiapine [F(1, 18)=3.03, P=.099]. A posthoc analysis indicated that IL-1β was significantly increased in the cerebral cortex of APP/PS1 mice compared with that in the cortex of nontransgenic mice. Quetiapine treatment greatly attenuated the increase of IL-1β in the cortex of transgenic mice (Figure 3b). Similar results were seen in the hippocampus. But the difference between transgenic mice and transgenic mice treated with quetiapine did not reach statistical significance (P=.062) (Figure 3B). However, there was no significant difference in the level of TNFα in both cerebral cortex and hippocampus among all the groups.
Quetiapine Inhibits Activation of Astrocytes in APP/PS1 Mice
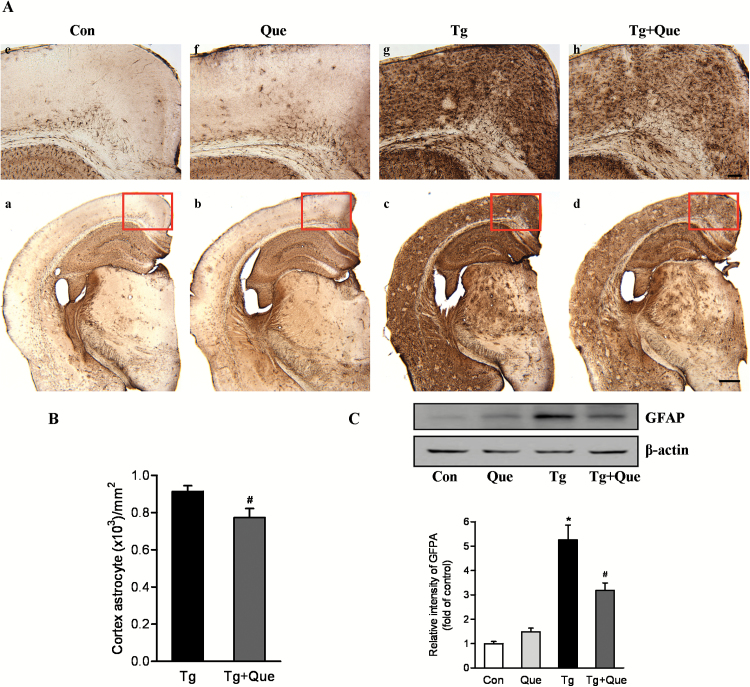
In brains of AD patients (Mancardi et al., 1983) and transgenic AD mice models (Wirths et al., 2010), activated astrocytes that are mainly cells that respond to the neuroinflammation process are often observed in and around the area of amyloid plaques (Itagaki et al., 1989; Matsuoka et al., 2001). Initially, we evaluated the reactivity of astrocytes in the transgenic mouse model of AD. There was a notable increase in activated astrocyte positive cells in the frontal cortex and hippocampus of APP/PS1 transgenic mice (Figure 4A, c) compared with age-matched nontransgenic counterparts (Figure 4A, a). In contrast, the GFAP immunoreactivity was remarkably decreased in the quetiapine-treated APP/PS1 transgenic mice (Figure 4A, d) compared with the nontreated counterparts (Figure 4A, c). This reduction was apparent both in the frontal cortex and hippocampus, indicating that astrogliosis was reduced after quetiapine treatment. More interestingly, whereas GFAP-positive cells in the frontal cortices of transgenic mice (Figure 4A, g) was largely diffuse, cortical GFAP staining of quetiapine-treated transgenic mice (Figure 4A, h) appeared to be focal, which may suggest that activated astrocytes within quetiapine-treated brains are confined to smaller areas than in the brains of nontreated transgenic animals. Quantification of cell number in the cerebral cortex showed the GFAP-positive cells were significantly greater in transgenic mice compared with transgenic mice treated with quetiapine (Figure 4B).
Figure 4.
Quetiapine inhibits activation of astrocytes in APP/PS1 mice. A, Representatvie immunohistochemical staining using anti-glial fibrillary acidic protein (GFAP) antibody indicated reduced astroglial cell densities in brain sections of treated transgenic mice compared with untreated transgenic mice. Upper panel shows the higher magnification of the field in red frame.The scale bars represent 100 μm (upper) and 500 μm (lower). B, Quantification of cell number in the cerebral cortex showed the GFAP-positive cells were significantly greater in APP/PS1 transgenic mice compared with APP/PS1 transgenic mice treated with quetiapine. C, Immunoblot analysis of GFAP in cerebral cortex. Quantification of GFAP was shown in the graph. Data are expressed as means±SEM, n=4 to 5 mice per group. * P<.05 vs Con; # P<.05 vs transgenic + water.
To confirm the immunohistochemistry results, Western blot was conducted to quantify the expression level of GFAP in cortical tissues. Two-way ANOVA analysis showed that genotype [F(1, 14)=80.41, P<.0001] and quetiapine [F(1, 14)=5.80, P=.0304] produced significant changes on the GFAP expression level and that there was an interaction between genotype and quetiapine [F(1, 14)=14.87, P=.0018]. A posthoc analysis indicated that the protein level of GFAP was significantly increased in the cortex of transgenic mice compared with that in the cortex of nontransgenic mice. Quetiapine treatment prevented the up-regulation of GFAP protein content in transgenic mouse brains (Figure 4C). Taken together, these observations confirm the finding that quetiapine treatment suppresses the prolonged astrocyte activation associated with AD progression.
Quetiapine Reduces Proinflammatory Cytokine Levels in Aβ1-42-Treated Primary Microglia
To investigate the effect of quetiapine on the inflammatory response induced by Aβ1–42 in vitro, primary microglia were pretreated with quetiapine (10 µM) for 1 hour and then with Aβ1–42 (25 µM) for 6 hours. The amount of proinflammatory cytokine IL-1β and TNFα secreted into the culture medium from primary microglial cells was examined by ELISA. As shown in Figure 5a, 2-way ANOVA followed by Newman-Keuls posthoc test analysis revealed that exposure of microglia to Aβ increased the secreted IL-1β levels by about 5-fold, whereas quetiapine significantly attenuated Aβ-induced IL-1β secretion. A similar trend was seen in the results of TNFα (Figure 5b). The level of TNFα was significantly increased after Aβ treatment. Although this up-regulation tended to be decreased in the presence of quetiapine, this difference did not reach statistical significance.
Figure 5.
Quetiapine reduces proinflammatory cytokines in β-amyloid (Aβ)1-42-treated primary microglia. Primary microglia were pretreated with quetiapine (10 µM) for 1 hour and then with Aβ1–42 (25 µM) for 6 hours. a, Enzyme-linked immunosorbent assay (ELISA) analysis of interleukin 1β (IL-1β). Two-way analysis of variance (ANOVA) showed quetiapine significantly attenuated Aβ-induced IL-1β increase. b, ELISA analysis of tumor necrosis factor α (TNFα). Two-way ANOVA showed exposure of microglia to Aβ increased the secreted TNFα levels. No statistcial significance was detected after quetiapine treatment. Data are expressed as means±SEM, n=4. * P<.05 vs Con; # P<.05 vs Aβ.
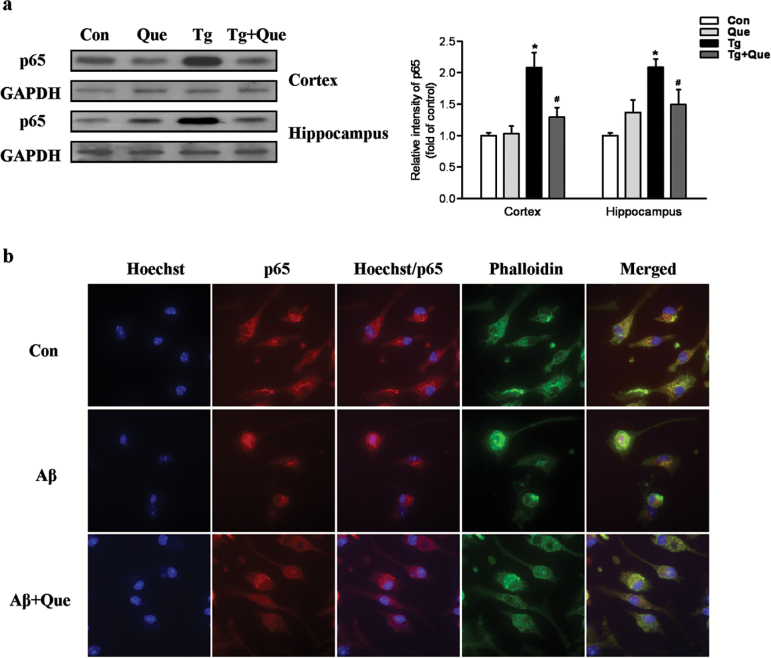
Quetiapine Suppresses the Activation of NF-κB p65 Pathway in vivo and in vitro
To elucidate the possible mechanism of quetiapine in suppression of inflammation, the NF-κB p65 signaling pathway, which has been implicated in microglial activation and neuroinflammation, was studied. The expression of p65 in both cortex and hippocampus was determined by Western-blot analysis. As shown in Figure 6a, 2-way ANOVA analysis showed that genotype [F(1, 18)=17.86, P<.001] and quetiapine [F(1, 18)=5.79, P<.05] produced significant changes on the p65 expression level in cerebral cortex and that there was an interaction between genotype and quetiapine [F(1, 18)=9.76, P<.01]. A posthoc analysis indicated that the protein level of p65 was significantly increased in the cortex of APP/PS1 transgenic mice compared with that in the cortex of nontransgenic mice. Quetiapine treatment significantly attenuated this increase in transgenic mice (Figure 6a). Similar results were also observed in the hippocampus. These results indicated that quetiapine treatment could inhibit the activation of NF-κB p65 in APP/PS1 transgenic mice.
Figure 6.
Quetiapine inhibits the activation of the nuclear factor kappa B (NF-κB) p65 pathway in vivo and in vitro. a, Immunoblot analysis of p65 in both cortex and hippocampus following the treatment. Quantification of p65 was shown in the graph. Two-way analysis of variance (ANOVA) showed quetiapine treatment significantly attenuated this increase of p65 in both cortex and hippocampus of transgenic mice. Data are expressed as means±SEM, n=6 to 9 mice per group. * P<.05 vs Con; # P<.05 vs transgenic + water. b, Representative immunocytochemistry showing the effect of quetiapine on β-amyloid (Aβ)1-42-induced NF-κB p65 nuclear translocation in primary microglia. Fluorescent images (100× magnification): blue, hoechst; red, p65; green, phalloidin.
It has been reported that Aβ could stimulate NF-κB activation by inducing nuclear translocation (Huang et al., 2012). The immunostaining of p65 in primary microglial cells showed that p65 was mainly located in the cytoplasm of untreated cells and Aβ1–42 treatment induced a translocation of p65 from the cytoplasm to the nucleus, whereas quetiapine significantly attenuated the p65 translocation induced by Aβ1–42 (Figure 6b). These findings suggest that quetiapine might regulate the inhibition of neuroinflammation via suppressing NF-κB p65 pathway.
Discussion
A chronic administration of quetiapine in APP/PS1 transgenic mice resulted in a marked change in microglial and astrocyte activation and proinflammatory cytokine levels and an improvement in behavioral performance. These beneficial effects of quetiapine occurred when there were only marginal changes in levels of total Aβ, suggesting that the antiinflammatory effect of quetiapine may account for the majority of cognitive improvement in APP/PS1 transgenic mice. Moreover, we confirmed that quetiapine significantly reduced Aβ1-42-induced secretion of proinflammatory cytokines in primary cultured microglia. Furthermore, both in vitro and in vivo experiments demonstrated that quetiapine ameliorated proinflammatory cytokine increases via suppression of the activation of NF-κB pathway.
The primary clinical presentation of AD is progressive cognitive decline. As AD progresses, a number of neuropsychiatric symptoms, including depression and anxiety, are exhibited (Garcia-Alberca et al., 2008). Twelve-month-old APP/PS1 transgenic mice showed higher anxiety levels than nontransgenic controls, as seen in decreased time spent in the center of the open field box. Quetiapine reduced heightened anxiety in transgenic mice with no significant effects on general locomotor activity. This suggests that quetiapine may have some level of anxiolytic effect. APP/PS1 mice showed nonspatial visual-discrimination memory deficits indicated by a lower exploration time of the novel object after 24-hour training in the object recognition test. This long-term retention memory deficit was significantly improved in APP/PS1 mice treated with quetiapine for 8 months, suggesting that this treatment paradigm was effective in improving the nonspatial memory.
The behavioral improvement following quetiapine treatment may be associated with its effects on Aβ pathology according to the amyloid hypothesis (Hardy and Selkoe, 2002). However, the effect of quetiapine on levels of total Aβ was unexpectedly marginal. Only certain species in certain brain regions, such as total Aβ40 in cerebral cortex, were significantly reduced following the treatment of quetiapine. This is inconsistent with our previous report with respect to the effect of quetiapine on Aβ pathology. He and colleagues (He et al., 2009) have reported that quetiapine treatment significantly decreased total Aβ40 and Aβ42 production. Various factors, such as age, therapeutic time window, and duration of treatment, could be involved in showing this difference. For example, in this present study, total Aβs were measured in relatively old (12 month old) APP/PS1 transgenic mice, whereas they were evaluated in much younger mice in our previous report. Additionally, we started quetiapine administration after the onset of overt amyloid pathology beginning at the age of 4 months as opposed to 2 months old in the previous study. Given that amyloid plaques account for the majority of total Aβs, growing evidence has shown that the severity of amyloid plaques in the brain does not correlate well with the degree of cognitive impairment in AD patients (Schmitz et al., 2004). Therefore, we reasoned that the capacity of quetiapine to improve behavioral performance may be related to the antiinflammatory effects of quetiapine.
Numerous studies show the presence of a number of markers of inflammation in the AD brain: accumulation of activated microglia occurring mainly around amyloid plaques accompanied by excessive or dysregulated release of proinflammatory cytokines and chemokines, which contributes to neuronal death and degeneration (Lucin and Wyss-Coray, 2009). It has been well known that higher inflammatory levels are related to higher risk of cognitive impairment (Rosano et al., 2012). In the present study, the activation of microglia observed in the hippocampus of APP/PS1 transgenic mice, as well as a strong increase of IL-1β but not TNFα compared with the nontransgenic mice, was greatly reduced following quetiapine treatment, suggesting that quetiapine could have antiinflammatory effects. To confirm, quetiapine’s antiinflammatory effects were tested on primary microglia culture, which was activated by Aβ. In agreement with our animal findings, quetiapine drastically decreased the release of both IL-1β and TNFα in microglial culture treated with Aβ1–42. Furthermore, our study has also shown that the doses of Aβ1–42 (25 µM) used in this study did not affect microglial cell viability (data not shown). As these proinflammatory mediators in turn further activate microglia creating a self-perpetuating vicious cycle by which inflammation induces further neuronal damage (Paradisi et al., 2004; Perry et al., 2010), blocking these cytokines by quetiapine possibly alleviates the chronic propagating inflammation associated with AD, which could help protect neurons and eventually attenuate behavioral impairment. Thus, quetiapine could ameliorate behavioral deficits through inhibiting brain inflammation in an APP/PS1mouse model of AD.
Apart from microglia, astrocytes are also recruited during the inflammation process. Astrogliosis process has already been considered as another feature of AD, and there are many studies showing that it is an important source of oxidative stress in AD patients (Wyss-Coray and Mucke, 2002; Paradisi et al., 2004). Quetiapine, on the other hand, has been well studied for decreasing the increase of reactive astrocytes in different animal models of global ischemia (Yan et al., 2007), cuprizon-induced schizophrenia (Zhang et al., 2008), and multiple sclerosis (Mei et al., 2012). To date, there has been no report on the effects of quetiapine on astrogliosis in APP/PS1 mice. In the present study, astrocyte numbers and GFAP expression in the cerebral cortex of APP/PS1 mice were significantly reduced by chronic administration of quetiapine. This effect seemed to be mainly due to the decrease of diffusely distributed astrocytes, since both transgenic and transgenic mice with quetiapine showed comparable numbers of astrocyte clusters.
NF-κB is known to be a critical regulator of inflammation by acting as an essential transcription factor for induction of COX2, inducible nitric oxide synthase (iNOS), IL-1β, and TNFα (Zhang et al., 2009). It has been shown that Aβ can directly stimulate microglia through the NF-κB signaling pathway, resulting in increased secretion of cytokines, chemokines, and adhesion molecules (Wyss-Coray and Rogers, 2012). In turn, some proinflammatory cytokines activate NF-κB and lead to a detrimental cycle of neuroinflammation and neurodegeneration. Moreover, studies have shown that NF-κB is activated in both glial cells and neurons in the brains of AD patients as well as in cultured neurons and glia following Aβ stimulation (Kaltschmidt et al., 1997; Mattson and Camandola, 2001; Huang et al., 2012). Suppression of NF-κB ameliorates astrogliosis in APP/PS1 transgenic mice (Zhang et al., 2009). More importantly, our previous study has shown that quetiapine decreased p50/p65 expression levels in mice subject to global cerebral ischemia (Bi et al., 2009). Therefore, to further understand the molecular mechanism of the effects of quetiapine on the expression of IL-1β and TNFα and subsequent glial activation, the expression of NF-κB subunit p65 was analyzed in brains using Western blots. Consistent with previous reports, the present study showed increased expression of NF-κB p65 subunit in both cortex and hippocampus of APP/PS1 transgenic mice. Quetiapine effectively ameliorated the activation of NF-κB in these mice, suggesting the effects of quetiapine against the increased levels of proinflammatory cytokines may be in part attributed to its ability of inhibition of NF-κB p65 expression. Moreover, the activation of NF-κB requires it to translocate from the cytosol to the nucleus and binds to its cognate DNA-binding sites, leading to expression of inflammatory mediators (Kucharczak et al., 2003). Our in vitro study has demonstrated that p65 are translocated into nucleus following Aβ1–42 stimulation, while quetiapine treatment can reverse this translocation.
Many inflammatory mediators such as IL-1β, TNFα, COX, and iNOS are believed to play a vital role in the inflammatory process of AD, because they have been reported to be elevated in the plasma, brains, and cerebrospinal fluid of patients with AD as well as transgenic animal models of AD (Griffin et al., 1989; Blum-Degen et al., 1995; Akiyama et al., 2000; Galimberti et al., 2006; Ruan et al., 2009). IL-1β and TNFα represent downstream targets that are regulated by the transcription factor NF-κB in the inflammatory cascade, which is an attractive candidate as a therapeutic target. Furthermore, NF-κB has also been directly implicated in APP processing. The activity of the β-secretase-1 (BACE1) promoter is controlled by a NF-κB–dependent pathway in the presence of excessive Aβ (Buggia-Prevot et al., 2008), whereas inhibition of NF-κB signaling pathway can enhance α-secretase activity, which is responsible for the benign, nonamyloidogenic processing of APP (Lee et al., 2009). Therefore, suppressing the NF-κB signaling pathway should not only effectively inhibit individual proinflammatory mediators such as IL-1β and TNFα in AD but also reduce Aβ production. Despite our finding that quetiapine showed only a minimal effect on total Aβ production in 12-month–old transgenic mice, levels of the soluble forms of Aβ40 and Aβ42 were significantly reduced following quetiapine treatment in another study (Zhu et al., 2013). The exact mechanism by which quetiapine specifically reduces only soluble Aβs is yet unknown, but its result is significant, since soluble Aβ specifically is believed to be the primary driver of AD-related pathogenesis, resulting in glial activation, synapse loss, and neuronal cell death (Hardy and Selkoe, 2002; Tanzi and Bertram, 2005). We believe that quetiapine may be an efficacious and promising treatment for AD because of its multiple effects, from suppressing the NF-κB pathway to reducing inflammation and soluble Aβs.
Overall, the findings reported here confirmed glial activation and proinflammatory cytokine overproduction as a common pathophysiologic mechanism and potential therapeutic target in AD. This study is the first description revealing that quetiapine improves behavioral performance while attenuating microglial and astrocyte activation in APP/PS1 transgenic mice and reduces proinflammatory cytokine levels in vivo and in vitro, which may be related to its inhibition of NF-κB activation.
Statement of Interest
X-M. Li has received research grants from AstraZeneca Canada, Inc. No conflict of interest exists for any of the other authors.
Acknowledgments
This work was supported by grants from the Research Manitoba and the Canadian Institutes of Health Research to X-M. Li. S. Zhu was supported by a doctoral award from the Alzheimer Society of Canada.
References
- Akiyama H, et al. (2000). Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Yan B, Fang S, Yang Y, He J, Li XM, Kong J. (2009). Quetiapine regulates neurogenesis in ischemic mice by inhibiting NF-kappaB p65/p50 expression. Neurol Res 31:159–166. [DOI] [PubMed] [Google Scholar]
- Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, Kanba S. (2008). The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry 32:42–48. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. (1995). Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20. [DOI] [PubMed] [Google Scholar]
- Buggia-Prevot V, Sevalle J, Rossner S, Checler F. (2008). NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem 283:10037–10047. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. (2000). Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20:8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. (1996). Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383:710–713. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. (2006). Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging 27:1763–1768. [DOI] [PubMed] [Google Scholar]
- Garcia-Alberca JM, Pablo Lara J, Gonzalez-Baron S, Barbancho MA, Porta D, Berthier M. (2008). [Prevalence and comorbidity of neuropsychiatric symptoms in Alzheimer’s disease]. Actas Esp Psiquiatr 36:265–270. [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. (1989). Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 86:7611–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. [DOI] [PubMed] [Google Scholar]
- He J, Yang Y, Yu Y, Li X, Li XM. (2006). The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav Brain Res 172:39–45. [DOI] [PubMed] [Google Scholar]
- He J, Luo H, Yan B, Yu Y, Wang H, Wei Z, Zhang Y, Xu H, Tempier A, Li X, Li XM. (2009). Beneficial effects of quetiapine in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 30:1205–1216. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. (2005). Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy 4:247–256. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen Y, Zhang H, Ma Q, Zhang YW, Xu H. (2012). Salubrinal attenuates beta-amyloid-induced neuronal death and microglial activation by inhibition of the NF-kappaB pathway. Neurobiol Aging 33:1007 e1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. (2001). Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 345:1515–1521. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. (1989). Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24:173–182. [DOI] [PubMed] [Google Scholar]
- Juncos JL, Roberts VJ, Evatt ML, Jewart RD, Wood CD, Potter LS, Jou HC, Yeung PP. (2004). Quetiapine improves psychotic symptoms and cognition in Parkinson’s disease. Mov Disord 19:29–35. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. (1997). Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci U S A 94:2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. (2006). Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry 59:75–84. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. (2008). Zinc triggers microglial activation. J Neurosci 28:5827–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczak J, Simmons MJ, Fan Y, Gelinas C. (2003). To be, or not to be: NF-kappaB is the answer: role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene 22:8961–8982. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, Oh KW, Hong JT. (2009). Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr 139:1987–1993. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. (2002). NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–734. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. (2009). Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusoodanan S, Shah P, Brenner R, Gupta S. (2007). Pharmacological treatment of the psychosis of Alzheimer’s disease: what is the best approach? CNS Drugs 21:101–115. [DOI] [PubMed] [Google Scholar]
- Mancardi GL, Liwnicz BH, Mandybur TI. (1983). Fibrous astrocytes in Alzheimer’s disease and senile dementia of Alzheimer’s type. Acta Neuropathol (Berl) 61:76–80. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI, O’Banion MK, Tenner AJ, Lemere CA, Duff K. (2001). Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am J Pathol 158:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. (2001). NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Guo S, He Y, Wang L, Wang H, Niu J, Kong J, Li X, Wu Y, Xiao L. (2012). Quetiapine, an atypical antipsychotic, is protective against autoimmune-mediated demyelination by inhibiting effector T cell proliferation. PLoS One 7:e42746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D, et al. (2010). Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins. J Neurosci 30:14134–14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F. (2004). Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia 46:252–260. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. (2010). Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Malla A, Labelle A, Lit W. (2001). Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. J Psychiatry Neurosci 26:137–149. [PMC free article] [PubMed] [Google Scholar]
- Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S, Sun X, Chen CH, Zhou W, Wang K, Song W. (2008). Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med 205:2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Marsland AL, Gianaros PJ. (2012). Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. Aging Dis 3:16–33. [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Kang Z, Pei G, Le Y. (2009). Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res 6:531–540. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rutten BP, Pielen A, Schafer S, Wirths O, Tremp G, Czech C, Blanchard V, Multhaup G, Rezaie P, Korr H, Steinbusch HW, Pradier L, Bayer TA. (2004). Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. Am J Pathol 164:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. (2002). Alzheimer’s disease is a synaptic failure. Science 298:789–791. [DOI] [PubMed] [Google Scholar]
- Storey E, Cappai R. (1999). The amyloid precursor protein of Alzheimer’s disease and the Abeta peptide. Neuropathol Appl Neurobiol 25:81–97. [DOI] [PubMed] [Google Scholar]
- Storey E, Katz M, Brickman Y, Beyreuther K, Masters CL. (1999). Amyloid precursor protein of Alzheimer’s disease: evidence for a stable, full-length, trans-membrane pool in primary neuronal cultures. Eur J Neurosci 11:1779–1788. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. (2005). Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120:545–555. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL. (2002). Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res 53:239–248. [DOI] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. (2008). Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70:1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Marcello A, Cotel MC, Bruck W, Bayer TA. (2010). Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiol Aging 31:747–757. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. (2002). Inflammation in neurodegenerative disease--a double-edged sword. Neuron 35:419–432. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. (2012). Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2:a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9:453–457. [DOI] [PubMed]
- Yan B, Bi X, He J, Zhang Y, Thakur S, Xu H, Gendron A, Kong J, Li XM. (2007). Quetiapine attenuates spatial memory impairment and hippocampal neurodegeneration induced by bilateral common carotid artery occlusion in mice. Life Sci 81:353–361. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu H, Jiang W, Xiao L, Yan B, He J, Wang Y, Bi X, Li X, Kong J, Li XM. (2008). Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr Res 106:182–191. [DOI] [PubMed] [Google Scholar]
- Zhang X, Luhrs KJ, Ryff KA, Malik WT, Driscoll MJ, Culver B (2009) Suppression of nuclear factor kappa B ameliorates astrogliosis but not amyloid burden in APPswe/PS1dE9 mice. Neuroscience 161:53–58. [DOI] [PubMed]
- Zhang H, Su YJ, Zhou WW, Wang SW, Xu PX, Yu XL, Liu RT (2014) Activated scavenger receptor a promotes glial internalization of abeta. PLoS One 9:e94197. [DOI] [PMC free article] [PubMed]
- Zheng C, Yin Q, Wu H. (2011). Structural studies of NF-kappaB signaling. Cell Res 21:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, He J, Zhang R, Kong L, Tempier A, Kong J, Li XM. (2013). Therapeutic effects of quetiapine on memory deficit and brain beta-amyloid plaque pathology in a transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res 10:270–278. [DOI] [PubMed] [Google Scholar]