Abstract
Background:
Melancholic depression, described also as endogenous depression, is a mood disorder with distinctive specific psychopathological features and biological homogeneity, including anhedonia, circadian variation of mood, psychomotor activation, weight loss, diurnal cortisol changes, and sleep disturbances. Although several hypotheses have been proposed, the etiology of this disorder is still unknown.
Methods:
Behavioral, electrophysiological and biochemical approaches were used to characterize the emotional phenotype, serotonergic and noradrenergic electrical activity, and corticosterone in melatonin MT1 receptor knockout mice and their wild type counterparts, during both light and dark phases.
Results:
Melatonin MT1 receptor knockout mice have decreased mobility in the forced swim and tail suspension tests as well as decreased sucrose consumption, mostly during the dark/inactive phase. These mood variations are reversed by chronic treatment with the tricyclic antidepressant desipramine. In addition, MT1 receptor knockout mice exhibit psychomotor disturbances, higher serum levels of corticosterone the dark phase, and a blunted circadian variation of corticosterone levels. In vivo electrophysiological recordings show a decreased burst-firing activity of locus coeruleus norepinephrine neurons during the dark phase. The circadian physiological variation in the spontaneous firing activity of high-firing neuronal subpopulations of both norepinephrine neurons and dorsal raphe serotonin neurons are abolished in MT1 knockout mice.
Conclusions:
These data demonstrate that melatonin MT1 receptor knockout mice recapitulate several behavioral and neurobiological circadian changes of human melancholic depression and, for the first time, suggest that the MT1 receptor may be implicated in the pathogenesis of melancholic depression and is a potential pharmacological target for this mental condition.
Keywords: corticosterone, daily mood variations, melancholic depression, monoamines, MT1 receptors, norepinephrine, serotonin, circadian rhythm
Introduction
Melancholia, also described as endogenous, endogenomorphic, autonomous, type A, psychotic, and typical depression (Parker et al., 2010), is a mood disorder with specific psychopathological symptoms, including disturbances in affect, diurnal variation with mood generally worse in the morning, anhedonia, psychomotor retardation or agitation, cognitive impairment, and neurobiological/somatic impairments manifested as loss of weight, hypercortisolemia, and sleep disturbances, mainly at the level of rapid eye movement sleep (REMS; Rush and Weissenburger, 1994; Armitage, 2007). Melancholic patients respond better to broad-action tricyclic antidepressants than to narrow-action antidepressants (Perry, 1996), and their response to placebo, psychotherapy, or even social intervention is very poor (Brown, 2007).
Few animal models of melancholic depression have been proposed (Hill and Gorzalka, 2005; Kato et al., 2008; Shen et al., 2010), but their attempts to reproduce the whole complex symptomatology of the disease, in particular the diurnal variations of mood, failed.
Diurnal mood variations are indeed the hallmark of melancholic depression (Parker et al., 2010), and differentiate melancholic versus non-melancholic depression (Duncan, 1996). They have been linked to a dysfunction in circadian rhythms that are driven by the suprachiasmatic nucleus (SCN). The activity of the SCN is regulated by the neurohormone melatonin (MLT), which acts through its two G protein–coupled receptors, MT1 and MT2 (Liu et al., 1997; Jin et al., 2003; Dubocovich, 2007). Previous literature has suggested a link between MLT and melancholic depression. Brown et al. (1985) reported lower nocturnal MLT levels in melancholic patients than in controls, and Fountoulakis et al. (2001) found that melancholic patients had lower 23.00h MLT blood levels in comparison to atypical, somatic syndrome, or “undifferentiated” depressed patients. Melatonin MT1 receptor knockout (MT1 -/-) mice showed altered REMS and sleep architecture (Comai et al., 2013), increased immobility in the forced swim test, and deficits in sensorimotor gating (Weil et al., 2006). Altogether, these findings led us to extensively investigate the psychobiological phenotype of MT1 -/- mice as a possible model of melancholic depression using multiple behavioral and biological tests and in vivo electrophysiology recording of serotonin (5-HT) and norepinephrine (NE) neurons, whose neurotransmission was impaired in melancholic depression (Pier et al., 2004; Malhi et al., 2005). Nonetheless, we tried to reverse their depressive-like phenotype using the tricyclic antidepressant desipramine. To detect diurnal changes observed in melancholic patients, all experiments were performed during both the light and dark phases.
Materials and Methods
Animals
Adult (2–4 month-old) male C3H/HeN mice were purchased from Charles River. C3H/HeN MT1 -/- mice (Liu et al., 1997) were purchased from David Weaver (University of Massachusetts Medical School), and age-matched mice born in our facility were also used. Mice were kept under standard laboratory conditions (12h light/dark cycle, lights on at 07:30h; temperature at 20±2°C) with food and water ad libitum, and were used and handled in accordance with the guidelines of McGill University and the Canadian Institutes of Health Research for animal care and scientific use. Light phase experiments were conducted between 14:00 and 17:00h, whereas dark phase experiments between 2:00 and 5:00h.
Treatment
Desipramine (10mg/kg, Sigma-Aldrich) was dissolved in 0.9% NaCl and injected intraperitoneally (0.1ml) once daily for 20 days.
Behavior
We used 7–11 mice per genotype and phase of the day. Given the high number of behavioral tests to be performed, we employed three different groups of animals per genotype and phase of the day: one group underwent the open field, forced swim, and tail suspension tests; a second group was used in the elevated plus maze and novelty suppressed feeding tests; and the third group was evaluated in the sucrose preference test only. For animals of groups 1 and 2, a 7-day interim period was interlaced between tests to minimize the stress and mood effects of one experiment carrying over to the next. For habituation, mice were placed in the behavioral room 1h prior to the experimental session. The apparatus was cleaned before each session using a 70% alcohol solution and paper towel. Behaviors were recorded, stored, and analyzed using an automated behavioral tracking system (Videotrack, View Point Life Science) equipped with infrared light-sensitive CCD cameras. Light phase experiments were conducted using standard room lighting (350lx) and a white lamp (100W), and dark phase experiments using infrared light-emitting diodes and a lamp with a red light bulb (8 lux).
Open Field Test
Mice were singly placed at the corner of a white-painted open field arena (40×40×30cm) and their behavior was recorded for 20min. Frequency and total duration of central zone (20×20cm) visits and total locomotor activity were analyzed (Bambico et al., 2010).
Novelty-Suppressed Feeding Test
Mice were food-deprived for 48h and then individually placed in the corner of an open arena (40×40×30cm) containing 3 pellets of lab chow in the middle. The latency to initiate feeding was measured. Animals were then returned to the home cage, in which 3 pellets of food where placed in the center. The home cage feeding latency was noted (Bambico et al., 2010).
Elevated Plus Maze Test
The plus maze (50cm off the floor) was made of white Plexiglass with two open arms (30×5cm) and two arms of the same size enclosed by walls (15cm) which converge perpendicularly into a central platform (5×5cm). Mice were singly placed in the central platform facing the open arm, and their behavior was recorded for 5min. The following measures were collected: time spent in the open and closed arms, frequency of open and closed arm entries, and frequency and total duration of head dips beyond the borders of the open arms (Bambico et al., 2010).
Forced Swim Test
The test was conducted according to Porsolt et al. (1977). Mice were individually placed in a Plexiglas cylinder (20cm diameter, 50cm high) containing 20cm water (25°C), from which they could not escape. The experiment lasted 6min and the duration and frequency of immobility during the last 4min were analyzed.
Tail Suspension Test
Mice were individually suspended from a lever by adhesive tape placed approximately 1cm from the tip of the tail. Duration and frequency of immobility were determined for 6min (Bambico et al., 2010).
Sucrose Preference Test
Mice were individually housed 3 days before the beginning of the test. They were then trained for 3 days to consume water from two bottles. During these 3 days, the two bottles containing water were replaced for 1h a day with two bottles filled with a 2% (w/v) sucrose solution. Next, mice were subjected to a 48h procedure during which they were allowed to discriminate and select between 2 drinking bottles, one containing water and the other the sucrose solution. To avoid conditioned place preference learning, the position of the bottles was switched at the middle of the light and dark phases. Bottles were weighed at the onset of the light and dark phases in order to measure separately the sucrose preference for each phase of the day. The sucrose preference (%) was determined as follows: sucrose solution intake (g)/total fluid intake (g) × 100.
Electrophysiology
In vivo single-unit extracellular recordings of dorsal raphe nucleus (DRN) 5-HT and locus coeruleus (LC) NE neurons were performed following well-validated procedures (Gobbi et al., 2005; Bambico et al., 2010; Bambico, 2010) in our lab and are detailed in Supplementary Figure 1. Briefly, mice were anesthetized and placed in a stereotaxic frame. The stereotaxic brain coordinates of the DRN and LC were in agreement with the Paxinos and Franklin (2001) atlas. Spontaneous electrical activity of single cells was recorded using single-barreled glass micropipettes. The analog signal was converted into a digital signal via a 1401 Plus interface (CED) and analyzed off-line using Spike2 version 5.20 (CED). The recording site was marked for later histological verification.
Determination of Corticosterone Serum Levels
All animals were sacrificed by decapitation at the same time behavioral and electrophysiological experiments were conducted (light phase, 14:00h; dark phase, 02:00h). Trunk blood was collected within 30 s after the animal’s removal from the cage. Corticosterone serum levels were analyzed using the DetectX Corticosterone Enzyme Immunoassay kit (K-014-H1, Arbor Assays).
Statistical Analysis
Data are reported as mean ± standard error of the mean. Data analysis was performed using SigmaPlot 11 and SPSS 17. When normality and variance homogeneity were satisfied, two-way analyses of variance (ANOVA) or two-way ANOVA for repeated measures followed by Student-Newman-Keuls post hoc comparisons were used, employing the factors genotype and phase of the day. Three-way ANOVA was used to assess the effects of desipramine. Student’s t-test was used to compare weight differences between genotypes. Differences between subgroups of 5-HT and NE firing neurons were measured using the Kruskal–Wallis ANOVA on ranks followed by Dunn’s method. Genotype differences in the open field test (OFT) parameters were analyzed using a regression analysis controlling for the factor time. Fisher’s exact test was employed to determine genotype differences between bursting and non-bursting LC-NE neurons. A p-value < 0.05 was considered significant.
Results
MT1 -/- Mice Display a Depressive–Like Phenotype and Anhedonia
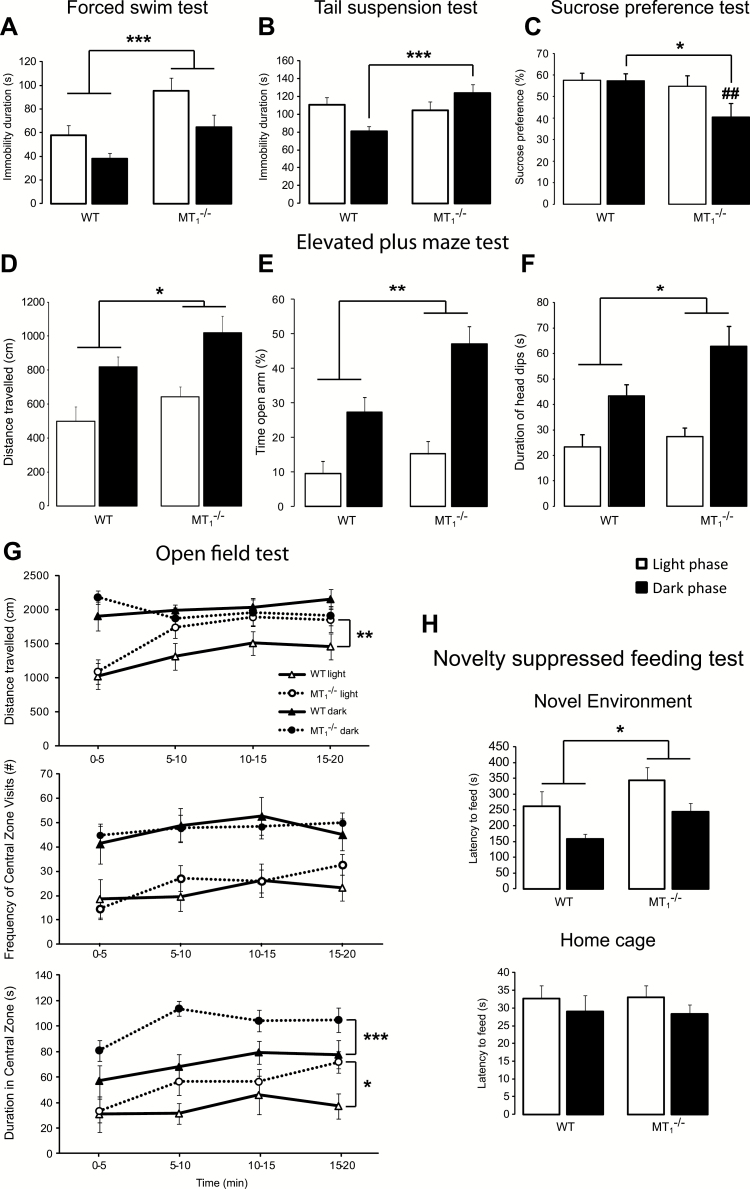
In the forced swim test (FST) and tail suspension test (TST; Figure 1), MT1 -/- mice showed a depressive-like phenotype when compared to wild-type controls (WT). In the FST (Figure 1A), MT1 -/- mice spent more time immobile than WT (effect of genotype: F1,38 = 12.46, p = 0.001; phase of the day: F1,38 = 7.74, p = 0.008; no interaction genotype x phase of the day). In the TST (Figure 1B), the duration of immobility was longer in MT1 -/- than in WT mice during the dark phase only (p = 0.006; interaction: F1,38 = 5.36, p = 0.026). The sucrose preference (Figure 1C), a measure of anhedonia, was reduced in MT1 -/- compared to WT mice during the dark phase only (p = 0.017, interaction: F1,38 = 6.37, p = 0.021). Interestingly, while no effect due to the phase of the day was observed in WT, in MT1 -/- mice the sucrose preference was lower during the dark than during the light phase (p < 0.002). In the novelty-suppressed feeding test (NSFT; Figure 1H), the latency to eat in a new environment was longer in MT1 -/- than in WT mice (genotype: F1,38 = 6.07, p = 0.018; phase of the day: F1,38 = 8.95, p = 0.005; no interaction). No differences were observed for the latency to eat in the home cage.
Figure 1.
MT1 -/- mice displayed depressive-like behavior and psychomotor disturbances. MT1 -/- mice showed increased immobility time in the forced swim test (A) and in the tail suspension test (B). (C) The sucrose preference was decreased during the dark phase and was also affected by the phase of the day in MT1 -/- mice. MT1 -/- mice exhibited increased locomotion (D), greater % of time spent in the open arm (E), and longer time spent head dipping (F) in the elevated plus maze test. (G) Locomotor activity in the open field test is increased in MT1 -/- mice during the light phase. The time spent in the center of the open field is higher in MT1 -/- mice during both the light and the dark phases. (H) MT1 -/- mice showed increased latency to eat in a new environment but not in the home cage in the novelty suppressed feeding test. Results are given as mean ± standard error of the mean. n = 10 per genotype in the light phase and n = 11 per genotype in the dark phase. *p < 0.05 and ***p < 0.001 MT1 -/- vs. WT mice; ##p < 0.01 light vs. dark phase, two-way ANOVA, followed by Student-Newman-Keuls post hoc test. In the open field test (G), *p < 0.05, **p < 0.01, ***p < 0.001 MT1 -/- vs. WT mice, with regression analysis controlling for the factor time.
MT1 -/- Mice Exhibit Hyperactivity and Increased Time in the Center of OFT and in the Open Arm of EPMT
Regression analysis, testing for genotype and controlling for the factor time, showed that in the OFT (Figure 1G) MT1 -/- mice covered a longer distance than WT during the light phase (p = 0.005). While the number of entries in the center of the OF was not affected by genotype, the time spent in the center of the open field was higher in MT1 -/- than in WT mice during both light (p = 0.015) and dark (p < 0.001) phases. In the elevated plus maze test (EPMT; Figure 1D–F), the distance traveled during the 5min session by MT1 -/- mice was longer than that covered by WT (genotype: F1,38 = 419, p = 0.047; Figure 1D). The distance traveled was longer during the dark phase compared to the light phase (phase of the day: F1,38 = 23.50, p < 0.001). The percentage of time spent in the open arm was greater in MT1 -/- compared to WT mice (p = 0.006) and longer during the dark phase (genotype: F1,38 = 8.64, p = 0.006; phase of the day: F1,38 = 32.21, p < 0.001; no interaction). The total duration of head dips was increased in MT1 -/- compared to WT mice (genotype: F1,38 = 4.19, p = 0.047) and longer during the light than during the dark phase (phase of the day: F1,38 = 20.78, p < 0.001).
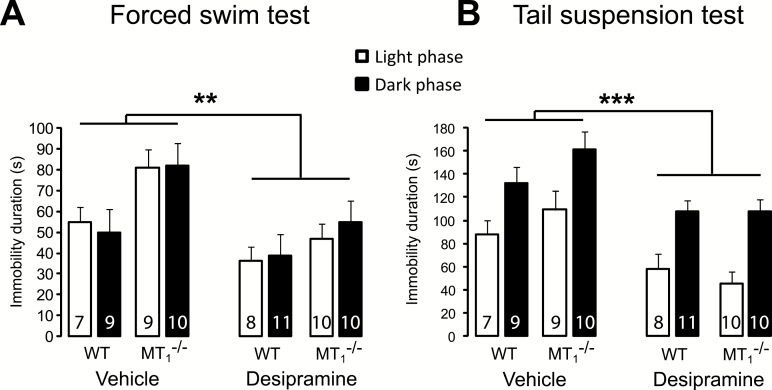
Chronic Treatment with Desipramine Reverses the Depressive-Like Phenotype of MT1 -/- Mice
After 20 days of treatment with desipramine, WT and MT1 -/- mice underwent the FST and the TST (Figure 2). In the FST (Figure 2A), desipramine reversed the differences of the duration of immobility between WT and MT1 -/- that were observed in animals receiving vehicle. Three-way ANOVA (genotype x treatment x phase of the day) indicated an effect of treatment (F1,66 = 10.36, p = 0.002) and an effect of genotype (F1,66 = 8.93, p = 0.004), with no interaction. Similarly, in the TST (Figure 2B) desipramine produced a significant decrease of immobility in both WT and MT1 -/- mice (treatment: F1,66 = 18.74, p < 0.001; phase of the day: F1,66 = 33.28, p < 0.001; no interaction), reinstating a normal behavioral phenotype in MT1 -/- mice. In order to rule out possible false-negative or false-positive results in the FST and TST due to the effect of desipramine on motor activity, mice were tested for 10min in the OFT prior to the FST (Figure S1 in Supplementary Material). Desipramine significantly reduced the locomotor activity of both WT and MT1 -/- mice, thus confirming its antidepressant-like properties (increased swimming, decreased immobility) in both behavioral paradigms of depression (treatment: F1,66 = 11.27, p < 0.001; phase of the day: F1,66 = 80.98, p < 0.001; no interactions).
Figure 2.
Chronic antidepressant treatment with desipramine reverses depressive-like behavior of MT1 -/- mice. (A and B) Desipramine reduced the immobility time in the forced swim test (A) and in the tail suspension test (B) in WT and MT1 -/- mice. Results are given as mean ± standard error of the mean. The number of animals per genotype and phase of the day is reported within the bar. *p < 0.01 and **p < 0.001 desipramine vs. vehicle, three-way ANOVA followed by Student-Newman-Keuls post hoc test.
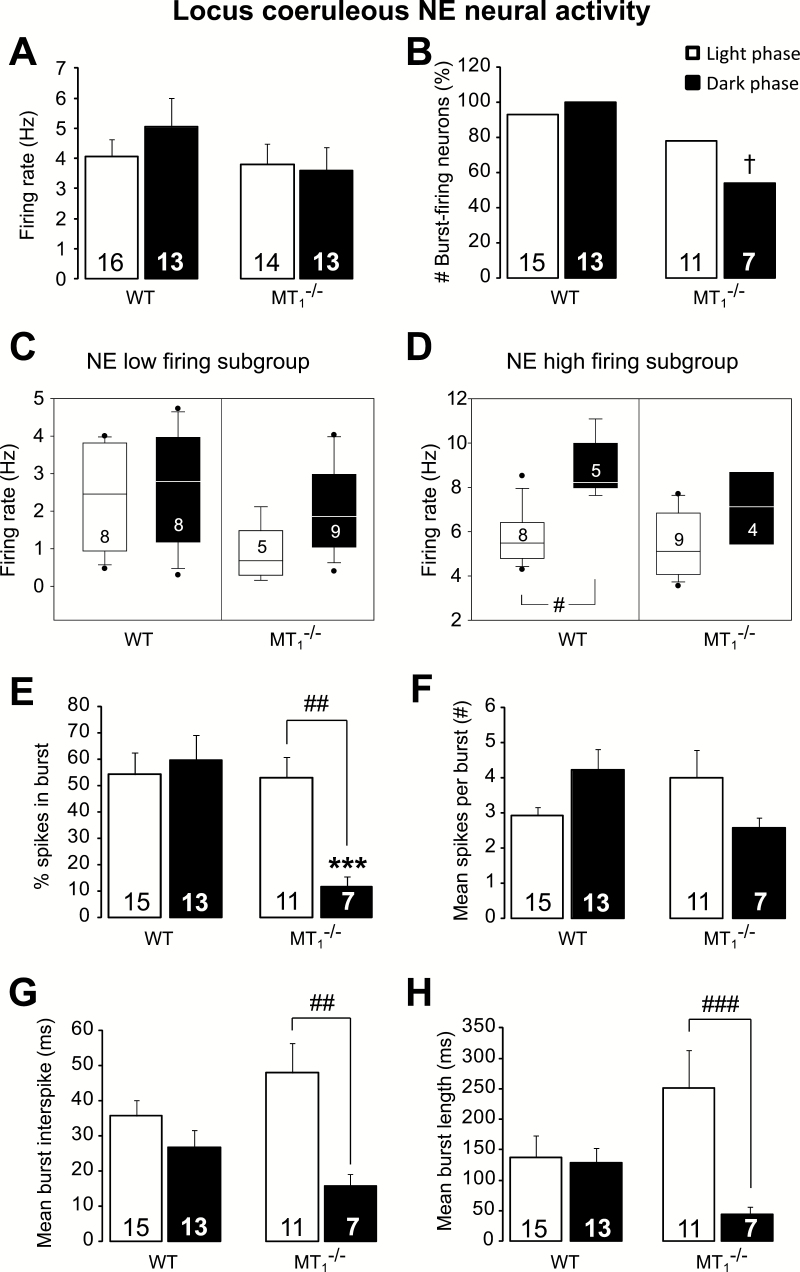
LC NE Neural Activity is Reduced in MT1 -/- Mice
No difference between genotypes was found in the spontaneous firing rate of LC NE neurons (Figure 3A). Conversely, important changes were detected regarding the neural burst activity. Fisher’s exact test showed that during the dark phase, the percentage of bursting-firing neurons is lower in MT1 -/- than in WT mice (p = 0.015, Figure 3B). An example of a LC NE neural recording from WT and MT1 -/- mice is reported in Figure S2 in Supplementary Material.
Figure 3.
Locus coeruleus norepinephrine (NE) burst activity is reduced in MT1 -/- mice. (A) Mean NE firing activity in WT and MT1 -/- mice during the light and the dark phases. (B) The percentage of locus coeruleus NE neurons discharging in bursts is lower in MT1 -/- mice during the dark phase. Boxplot representation of the low (C) and high (D) NE single-spike firing subgroups. Neural activity of the NE high-firing subgroup does not increase during the dark/active phase in MT1 -/- mice (D). Horizontal lines within boxes, boxes, and error bars respectively represent the median, the 25th, and 75th percentiles and the 10th and 90th percentiles. The outliers are displayed as individual points. #p < 0.05, Kruskal–Wallis analyses of variance on ranks followed by Dunn’s method. (E–H) NE neuronal firing pattern in WT and MT1 -/- mice during the light and dark phases. The percentage of spikes in burst (E), the mean burst interspike (G), and the mean burst length (H) are lower during the dark compared to the light phase in MT1 -/- mice only. Results are given as mean ± standard error of the mean and the number of recorded neurons is indicated at the bottom of each column. †p < 0.05 MT1 -/- vs. WT mice, Fisher’s exact test. *p < 0.001 MT1 -/- vs. WT mice; ##p < 0.01 light vs. dark phase, two-way ANOVA, followed by Student-Newman-Keuls post hoc test.
According to our previous study (Bambico et al., 2010), a K-means cluster analysis allowed us to identify the clusters of NE neurons with low and high firing activities for each genotype and phase of the day (Figure 3C and D). No differences were observed regarding the subgroup of LC neurons with low firing activity (Figure 3C) but, interestingly, Kruskal-Wallis one-way ANOVA on ranks [H(3) = 10.33; p = 0.016] showed that the neural activity of LC NE neurons belonging to the high firing subgroup was higher during the dark than during the light phase in WT mice (8.22 Hz [25th/75th percentile, 4.76/6.51 Hz] vs. 5.48 Hz [7.86/10.35]; p < 0.05, q = 3.00; Figure 3D). Importantly, the physiological light/dark difference was abolished in MT1 -/- mice.
Figure 3E–H reports the analysis of the LC NE neuronal burst activity. The percentage of spikes in burst (Figure 3E) was lower in MT1 -/- than in WT mice (p = 0.001) and changed according to the phase of the day in MT1 -/- mice only (interaction: F1,42 = 6.78, p = 0.013; genotype: F1,42 = 7.45, p = 0.009; phase of the day: F1,42 = 3.94, p = 0.05).
A tendency to fewer spikes per burst during the dark phase was found in MT1 -/- compared to WT mice (p = 0.067; interaction: F1,42 = 5.55, p = 0.023; Figure 3F). No genotype differences and an interaction genotype per phase of the day were found for the mean burst interspike (interaction: F1,42 = 3.71, p = 0.060) and the mean burst length (interaction: F1,42 = 4.64, p = 0.037). Very importantly, while the two parameters did not vary according to the phase of the day in WT animals, in MT1 -/- mice they were both higher during the light than during the dark phase (p = 0.002 and p = 0.007, respectively; Figure 3G and H).
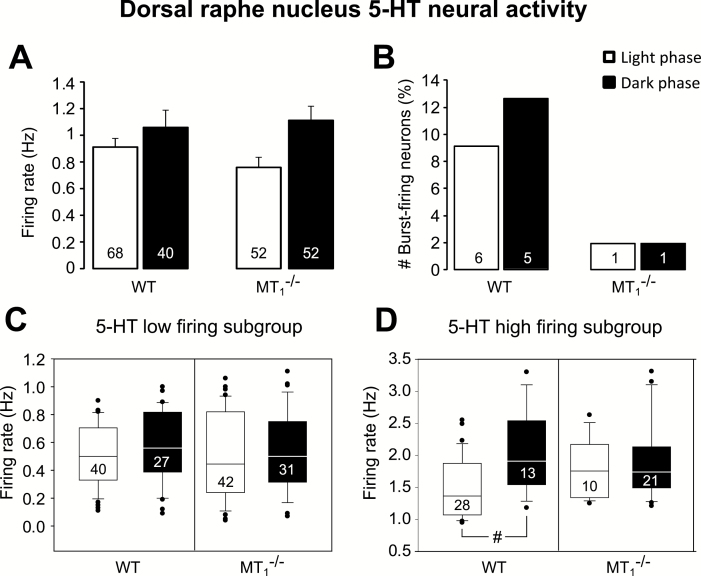
DRN 5-HT Neural Activity is Altered in MT1 -/- Mice
No differences in the DRN 5-HT firing rate between WT and MT1 -/- mice were observed (Figure 4A). Importantly, DRN 5-HT firing activity was higher during the dark than during the light phase (phase of the day: F1,208 = 6.18, p = 0.014). Among all 5-HT neurons recorded in MT1 -/- mice, only one per phase of the day was found to discharge in bursts. On the contrary, in WT mice we found that 8.8% and 12.5% of the neurons were discharging in bursts during the light and the dark phase, respectively (Figure 4B). Due to the limited number of 5-HT bursting neurons in MT1 -/- mice, statistical comparisons versus WT could not be performed. However, a clear reduction in 5-HT neurons discharging in bursts was observed in MT1 -/- mice. An example of a DRN 5-HT neural recording from WT and MT1 -/- mice is reported in Figure S3 in Supplementary Material.
Figure 4.
Dorsal raphe serotonergic high-firing activity in MT1 -/- mice does not change according to the phase of the day. (A) Mean 5-HT firing activity in WT and MT1 -/- mice during the light and the dark phases. (B) Percentage of dorsal raphe nucleus 5-HT neurons discharging in burst. Results are given as mean ± standard error of the mean. The total number of neurons per genotype and phase of the day is reported within the bar. Boxplot representation of the low (C) and high (D) 5-HT single-spike firing subgroups. Neural activity of the 5-HT high firing subgroup does not increase during the dark/active phase in MT1 -/- mice (D). Horizontal lines within boxes, boxes, and error bars respectively represent the median, the 25th, and 75th percentiles and the 10th and 90th percentiles. The outliers are displayed as individual points. #p < 0.05, Kruskal–Wallis analysis of variance on ranks followed by Dunn’s method.
Two subpopulations of 5-HT neurons, one with low and another with high firing activity, could be identified using K-means cluster analysis (Bambico, Cassano, et al., 2010; Figure 4C and D). No difference in the firing rate between the subgroups with low 5-HT firing neurons was found (Figure 4C). Conversely, in analyzing the subgroups with high firing 5-HT neurons (Figure 4D), the median value during the dark phase was higher than during the light phase in WT mice (Kruskal-Wallis one-way ANOVA on ranks: H(3) = 11.03, p = 0.012; 1.91 Hz [25th/75th percentile, 1.51/2.62 Hz] vs. 1.36 Hz [1.04/1.87]; p < 0.05, q = 4.06). Remarkably, such diurnal difference was abolished in MT1 -/- mice (Figure 4D).
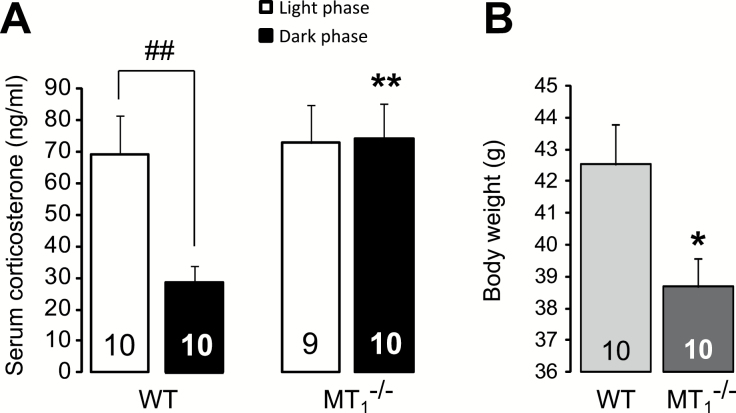
Corticosterone Serum Levels are Higher in MT1 -/- Mice During the Dark Phase
The serum levels of corticosterone (Figure 5A) were found to be higher in MT1 -/- than in WT mice during the dark phase (p = 0.003; interaction: F1,35 = 4.14, p = 0.049; genotype: F1,35 = 5.68, p = 0.023; phase of the day: F1,35 = 3.57, p = 0.067), and more importantly, the physiological diurnal variation of cortisol was disrupted in MT1 -/- mice.
Figure 5.
MT1 -/- mice displayed altered serum corticosterone levels and reduced body weight. (A) Serum corticosterone levels are higher during the dark phase and do not change according to the phase of the day in MT1 -/- mice. (B) 5 month-old MT1 -/- mice showed decreased body weight. **p < 0.01 MT1 -/- vs. WT mice; ##p < 0.01 light vs. dark phase, two-way ANOVA, followed by Student-Newman-Keuls post hoc test. *p < 0.05 MT1 -/- vs. WT mice, student t-test.
MT1 -/- Mice Weigh Less than WT
5 month-old MT1 -/- mice displayed a reduced body weight when compared to similar age WT (-9.07%; student’s t-test, t = 2.50, 18 df, p = 0.022; Figure 5B).
Discussion
We have examined the behavioral characteristics, the serotonergic and norepinephrine neural activities, and the corticosterone levels of MT1 -/- mice. Our results suggest that MT1 -/- mice may be considered an animal model of human melancholic depression. Indeed, they display (1) anhedonia, (2) depressive-like phenotype with diurnal variations, (3) reduced body weight, (4) hyperlocomotion, (5) changes in the monoaminergic neural activity, and (6) altered serum levels of corticosterone with disrupted circadian variation, all features that are core symptoms of melancholia (Table 1). Remarkably, the depressive-like symptoms manifested by MT1 -/- mice were reversed by chronic treatment with desipramine. Similar to melancholic patients (Parker et al., 2010), MT1 -/- mice also exhibited REMS disturbances and reduced power of the delta band of non-REMS, a measure linked to the depth of sleep (Comai et al., 2013).
Table 1.
A Comparison Between the Psychological and Neurobiological Symptoms of Melancholic Depression and the Phenotype of MT1 Receptors Knockout Mice.
| Symptoms of melancholic depression (Parker et al., 2010) | MT1 receptors knockout mice |
|---|---|
| Anhedonia | Anhedonia (↓ sucrose preference) |
| Depression | Depression-like behavior (↑ immobility in FST and TST; ↑ latency to eat in NSFT) |
| Weight loss | ↓ Weight |
| Psychomotor disturbances (agitation or retardation) | Hyperlocomotion (↑ locomotion in OFT), disinhibition (↑ open arm time and entries in EPMT, ↑ head dips in EPMT) |
| Circadian variation of mood | behavioral light/dark differences |
| Hypercortisolemia | ↑ corticosterone serum levels during the dark phase, no light/dark differences in corticosterone levels |
| Disturbance in sleep architecture especially at the level of REMS | ↓ REMS duration, ↓ NREMS EEG delta power and REMS EEG theta power (36) |
| Monoamine activity alterations | ↓ DRN 5-HT and LC NE neuronal bursts activity; altered light/dark firing pattern of DRN 5-HT and LC NE neurons |
| Genetic causes | Genetic inactivation of MT1 receptors |
↑ = increase; ↓ = decrease. DRN, dorsal raphe nucleus; EEG, electroencephalographic; EPMT, elevated plus maze test; FST, forced swim test; LC, locus coeruleus; NE, norepinephrine; NREMS, non-REMS; NSFT, novelty-suppressed feeding test; OFT, open field test; REMS, rapid eye movement sleep; TST, tail suspension test.
Weil et al. (2006) confirmed that MT1 -/- mice had increased immobility in the FST and an impaired prepulse inhibition response, a neurobiological sign often correlated to psychotic-like symptoms. Notably, melancholic depression can also include psychotic symptoms (Caldieraro et al., 2013).
The results obtained in the OFT (decreased thigmotaxis) and in the EPMT (increased % of time spent in the open arm) suggest an “anxiety-resistant” phenotype of MT1 -/- mice, while the increased latency to feed in the NSFT may suggest an anxiogenic-like phenotype. Nevertheless, in the OFT and EPMT, MT1 -/- mice showed increased locomotor activity, which may be a bias when evaluating anxiety-related parameters such as tigmotaxis and percentage of entries into the open arms, respectively (Dawson and Tricklebank, 1995). Therefore, the apparent “anxiety-resistant” phenotype of MT1 -/- mice could instead be the result of a disinhibitory/hyperactive behavior rather than a state of hypo-anxiety. For this reason, the NSFT represents, in this case, the most reliable test for anxiety in MT1 -/- mice. Indeed, even though in the novel arena knockout mice showed increased locomotor activity and decreased thigmotaxis, the latency to feed was significantly longer, further validating their anxiogenic-like phenotype. In keeping with the anxiogenic behavior in MT1 -/- mice, melancholic patients usually display increased levels of anxiety (Day and Williams, 2012). However, Parker et al. (2013) found a lower prevalence of anxiety disorders in melancholic than in non-melancholic patients, and therefore, the issue of anxiety in melancholic depression is complex.
Finally, even if the interpretation of the results obtained in the tests of anxiety may be arguable, the complex phenotype emerging from the EPMT, OFT, and NSFT perfectly reflect the multifaceted manifestations of anxiety observed in melancholic patients (Day and Williams, 2012; Parker et al., 2013). MLT, through MT1 and MT2 receptors, controls the activity of the SCN; in particular, MT1 receptors are involved in the acute inhibitory effect of MLT on the SCN firing rate (Liu et al., 1997), contributing to the control of circadian rhythms. Importantly, SCN projects to the locus coeruleus (Aston-Jones et al., 2001) and dorsal raphe (Deurveilher and Semba, 2005), influencing the rhythms of noradrenaline and serotonin, respectively.
NE and 5-HT are involved in the pathophysiology of depression (Gobbi and Blier, 2005), and our results show that the two monoaminergic neurotransmissions are significantly altered after genetic inactivation of MT1 receptors. In keeping with increased LC NE and DRN 5-HT firing activity during the dark/active phase (Aston-Jones et al., 2001; Ursin, 2002), the spontaneous firing rate of the high-firing subgroup of both monoamines was higher during the dark than during the light phase in control animals. Remarkably, this light/dark difference was abolished in MT1 -/- mice, suggesting a disruption of the diurnal pattern of monoaminergic electrical activity in these animals.
Moreover, MT1 -/- mice showed LC NE decreases in bursts-firing parameters during the dark/active phase and, notably, the increased LC NE burst firing activity is related to the release of the neurotransmitter in the terminal area (Florin-Lechner et al., 1996) and to antidepressant-like activity (Gobbi et al., 2007). One may hypothesize that the decrease in NE burst activity can be related to a depressive-like behavior.
Nonetheless, DRN 5-HT burst activity was also presumably reduced, even if the low number of bursting neurons found in this sample did not allow us to reach a significant statistical difference; a correlation has been well established between 5-HT burst activity, 5-HT release, and antidepressant-like activity (Gartside et al., 2000; Gobbi et al., 2005). NE and 5-HT neurotransmissions are also important regulators of the sleep/wake cycle and behavior (Aston-Jones et al., 2001; Ursin, 2002), and the NE and 5-HT neural impairments observed in knockout mice were paralleled by several behavioral and circadian impairments. Remarkably, the greater behavioral differences between WT and MT1 -/- mice were found during the active/dark phase, when the burst-firing activity of both monoamines was also reduced.
Most of the available treatments for depression target the monoaminergic systems, and melancholic patients have a greater response rate to old tricyclic antidepressants or monoamine oxidase inhibitors than to selective serotonin reuptake inhibitors (Perry, 1996). In agreement, a chronic treatment with desipramine was able to reverse the depressive-like phenotype of MT1 -/- mice, most likely (1) decreasing beta adrenergic neurotransmission in the hippocampus and affecting differentially the sensitivity and the response of somatodendritic and terminal α2-autoreceptors (Lacroix et al., 1991); and (2) increasing the responsiveness of postsynaptic hippocampus and ventral lateral geniculate neurons to 5-HT (Blier and de Montigny, 1994).
Even though desipramine is more selective for NE than for 5-HT (Frazer, 2001), it is not possible to disentangle whether the behavioral recovery observed in these knockout mice was due to NE, 5-HT, or both of them. Further experiments employing pharmacological agents selective for each monoamine (e.g., one selective serotonin reuptake inhibitor and one norepinephrine reuptake inhibitor) may help to address this question.
The other important biological feature that distinguishes melancholic versus non-melancholic depression is the sustained activation of the hypothalamic–pituitary–adrenal axis, which results in hypercortisolism and altered response to the dexamethasone suppression test (Roy et al., 1985; Parker et al., 2010). In keeping with this clinical evidence, MT1 -/- mice showed both increased serum corticosterone levels during the dark/active phase and blunted physiological circadian diurnal fluctuation, confirming the disturbances of the hypothalamic–pituitary–adrenal activity.
The role of MLT and its receptors in mood is not yet elucidated, and treatment with MLT alone does not seem to be an effective antidepressant strategy in humans (Carman et al., 1976; Quera Salva et al., 2011). Although few studies have associated MT2 receptors to depression (for review see Comai and Gobbi, 2014), the present data strongly underlines a role for MT1 receptors in the etiology of depression, in particular of melancholic depression. In agreement with this hypothesis, a specific increase of MT1 receptors in the SCN of depressive patients was recently found in a post-mortem study (Wu et al., 2013). Remarkably, the antidepressant agomelatine has a higher affinity for MT1 (K i = 6.15x10-11 M) than for MT2 (2.68x10-10 M) or 5-HT2c (IC50 = 2.7x10-7 M) receptors (de Bodinat et al., 2010), even though its antidepressant activity seems related to its multi-activity on MT1, MT2, 5-HT2c, and 5-HT2b receptors (Chenu et al., 2014).
Here, we have reported for the first time that genetic inactivation of MT1 receptors in mice yield a phenotype that mimics several of the features of melancholic depression. Animal models of depression have proven to be of considerable value in elucidating pathophysiological mechanisms of disease and for developing novel treatments, but very few were able to reproduce the whole human symptomatology, especially regarding melancholic depression and its diurnal variations. Similarities between the behavioral responses of gamma-aminobutyric acid-type A receptor (Shen et al., 2010), CB1 receptor (Hill and Gorzalka, 2005), or Wfs1 (Kato et al., 2008) knockout mice and symptoms of melancholic depression have been reported. Unfortunately, none of these animal models were able to mimic the light/dark variation of symptoms observed in melancholic patients, and furthermore, they only displayed a few of the core symptoms of the disease. Several studies suggested that an impaired circadian system, regulated by a network of “clock genes,” contributes to the etiology and symptomatology of mood disorders, highlighting an association between mutations at the level of clock genes such us Clock or Per1-3 and variations in mood (McCarthy and Welsh, 2012; Bunney and Bunney, 2013). But clock gene mutant animals also failed in capturing the full spectrum of symptoms shown by patients with mood disorders.
Although more research is needed to further explore the role of the MT1 receptor in melancholic depression, our findings suggest that the MT1 receptor may become a potential novel target for the therapeutics of endogenous depression, and selective MT1 agonists deserve to be tested as antidepressant drugs with chronobiotic effects.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
The authors declare no conflicts of interest.
Acknowledgements
Dr Gobbi was supported by FRSQ (clinical scientist salary program), CIHR (MOP130285), CFI (23381), and MERST (PSR-SIIRI-855). Dr Comai received a fellowship from MUHC; Dr Ochoa-Sanchez from FQRNT, McGill Faculty of Medicine, and CONACYT (Mexico); and Dr Dominguez-Lopez from CONACYT. Authors thank Ms Rebecca Howell and Mr Michael Tau for technical help.
References
- Armitage R. (2007). Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl 115 (s433):104–115. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. (2001). A neural circuit for circadian regulation of arousal. Nat Neurosci 4:732–738. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G. (2010). Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35:2083–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G. (2010). Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis 37:641–655. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. (1994). Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15:220–226. [DOI] [PubMed] [Google Scholar]
- Brown R, Kocsis JH, Caroff S, Amsterdam J, Winokur A, Stokes PE, Frazer A. (1985). Differences in nocturnal melatonin secretion between melancholic depressed patients and control subjects. Am J Psych 142:811–816. [DOI] [PubMed] [Google Scholar]
- Brown WA. (2007). Treatment response in melancholia. Acta Psychiatr Scand Suppl 115(s433):125–129. [DOI] [PubMed] [Google Scholar]
- Bunney BG, Bunney WE. (2013). Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry 73:1164–1171. [DOI] [PubMed] [Google Scholar]
- Caldieraro MA, Baeza FL, Pinheiro DO, Ribeiro MR, Parker G, Fleck MP. (2013). Prevalence of psychotic symptoms in those with melancholic and nonmelancholic depression. J Nerv Ment Dis 201:855–859. [DOI] [PubMed] [Google Scholar]
- Carman JS, Post RM, Buswell R, Goodwin FK. (1976). Negative effects of melatonin on depression. Am J Psych 133:1181–1186. [DOI] [PubMed] [Google Scholar]
- Chenu F, Shim S, El Mansari M, Blier P. (2014). Role of melatonin, serotonin 2B, and serotonin 2C receptors in modulating the firing activity of rat dopamine neurons. J Psychopharmacol 28:162–167. [DOI] [PubMed] [Google Scholar]
- Comai S, Gobbi G. (2014). Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci 39:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai S, Ochoa-Sanchez R, Gobbi G. (2013). Sleep–wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav Brain Res 243:231–238. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD. (1995). Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16:33–36. [DOI] [PubMed] [Google Scholar]
- Day CV, Williams LM. (2012). Finding a biosignature for melancholic depression. Expert Rev Neurother 12:835–847. [DOI] [PubMed] [Google Scholar]
- de Bodinat C, Guardiola-Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ. (2010). Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov 9:743–743. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. (2005). Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience 130:165–183. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. (2007). Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med 8:34–42. [DOI] [PubMed] [Google Scholar]
- Duncan WC., Jr. (1996). Circadian rhythms and the pharmacology of affective illness. Pharmacol Ther 71:253–312. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. (1996). Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res 742:89–97. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Karamouzis M, Iacovides A, Nimatoudis J, Diakogiannis J, Kaprinis G, Demitriadou A, Bech P. (2001). Morning and evening plasma melatonin and dexamethasone suppression test in patients with nonseasonal major depressive disorder from northern Greece (latitude 40–41.5 degrees). Neuropsychobiology 44:113–117. [DOI] [PubMed] [Google Scholar]
- Frazer A. (2001). Serotonergic and noradrenergic reuptake inhibitors: prediction of clinical effects from in vitro potencies. J Clin Psychiatry 62(Suppl 12):16–23. [PubMed] [Google Scholar]
- Gartside SE, Hajos-Korcsok E, Bagdy E, Harsing LG, Jr., Sharp T, Hajos M. (2000). Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience 98:295–300. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Blier P. (2005). Effect of neurokinin-1 receptor antagonists on serotoninergic, noradrenergic and hippocampal neurons: comparison with antidepressant drugs. Peptides 26:1383–1393. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. (2005). Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102:18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, Hen R, Blier P. (2007). Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neuropsychopharmacol 17:328–338. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. (2005). Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol 16:333–352. [DOI] [PubMed] [Google Scholar]
- Jin X, von GC, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. (2003). Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 23:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Ishiwata M, Yamada K, Kasahara T, Kakiuchi C, Iwamoto K, Kawamura K, Ishihara H, Oka Y. (2008). Behavioral and gene expression analyses of Wfs1 knockout mice as a possible animal model of mood disorder. Neurosci Res 61:143–158. [DOI] [PubMed] [Google Scholar]
- Lacroix D, Blier P, Curet O, de Montigny C. (1991). Effects of long-term desipramine administration on noradrenergic neurotransmission: electrophysiological studies in the rat brain. J Pharm Exp Ther 257:1081–1090. [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin XW, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. (1997). Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Parker GB, Greenwood J. (2005). Structural and functional models of depression: from sub-types to substrates. Acta Psychiatr Scand 111:94–105. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Welsh DK. (2012). Cellular circadian clocks in mood disorders. J Biol Rhythms 27:339–352. [DOI] [PubMed] [Google Scholar]
- Parker G, Fink M, Shorter E, Taylor MA, Akiskal H, Berrios G, Bolwig T, Brown WA, Carroll B, Healy D, Klein DF, Koukopoulos A, Michels R, Paris J, Rubin RT, Spitzer R, Swartz C. (2010). Issues for DSM-5: whither melancholia? The case for its classification as a distinct mood disorder. Am J Psych 167:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, McCraw S, Blanch B, Hadzi-Pavlovic D, Synnott H, Rees AM. (2013). Discriminating melancholic and non-melancholic depression by prototypic clinical features. J Affect Disord 144:199–207. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. (2001). The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Perry PJ. (1996). Pharmacotherapy for major depression with melancholic features: relative efficacy of tricyclic versus selective serotonin reuptake inhibitor antidepressants. J Affect Disord 39:1–6. [DOI] [PubMed] [Google Scholar]
- Pier MP, Hulstijn W, Sabbe BG. (2004). Differential patterns of psychomotor functioning in unmedicated melancholic and nonmelancholic depressed patients. J Psychiatr Res 38:425–435. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Quera Salva MA, Hartley S, Barbot F, Alvarez JC, Lofaso F, Guilleminault C. (2011). Circadian rhythms, melatonin and depression. Curr Pharm Des 17:1459–1470. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Linnoila M, Potter WZ. (1985). Plasma norepinephrine level in affective disorders. Relationship to melancholia. Arch Gen Psychiatry 42:1181–1185. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Weissenburger JE. (1994). Melancholic symptom features and DSM-IV. Am J Psych 151:489–498. [DOI] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. (2010). gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry 68:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R. (2002). Serotonin and sleep. Sleep Med Rev 6:55–69. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. (2006). Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull 68:425–429. [DOI] [PubMed] [Google Scholar]
- Wu YH, Ursinus J, Zhou JN, Scheer FA, Ai-Min B, Jockers R, van Heerikhuize J, Swaab DF. (2013). Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord 148:357–367. [DOI] [PubMed] [Google Scholar]